-
挥发性有机化合物(volatile organic compounds,VOCs)来源广泛,对大气环境和人体健康均有不利影响[1-3]。常见VOCs控制技术有吸附[4]、吸收[5]、冷凝[6]等回收技术和热氧化[7]、低温等离子体[8]、生物法[9]等销毁技术。工业中较为常用的技术为吸附和热氧化(包括蓄热燃烧、催化燃烧等)[10]。然而,吸附法仅仅将污染物进行了转移,后期存在吸附剂再生问题。而热氧化主要用于高浓度VOCs处理[11],且存在能量效率低的问题。相比之下,低温等离子体因其具有可快速启动和关闭、能耗低、净化效率高等特点而广受研究者关注[12-14]。
低温等离子体的产生方法有介质阻挡放电(DBD)、电晕放电、滑动弧放电、辉光放电等[15]。其中DBD是一种最为常见的低温等离子体产生方法,其放电均匀且稳定[16]。传统的DBD反应器是在高压电极和接地极之间嵌入一层绝缘介质,然而单一的介质阻挡放电低温等离子体在降解VOCs时存在副产物多、能量效率低等问题[17]。有研究[18-19]发现,若在两电极之间加入双层介质,在外加电压下,形成双介质阻挡放电(DDBD),可以有效提高VOCs的去除率并抑制副产物的产生。ZHANG等[18]比较了单双介质阻挡放电对苯乙烯降解的影响,发现DDBD反应器比DBD反应器的CO和CO2选择性提高了40%,同时DBD反应器中生成大量油状有机副产物,而DDBD反应器中则没有。TANG等[19]对比了单双介质阻挡放电反应器对NO的去除,发现DDBD反应器中产生的放电更加均匀稳定,使能量可以得到高效利用,而DBD反应器中的放电强度较大,有利于NO的去除。但李云霞等[20]的研究发现,当外加电压(4.0~6.5 kV)较低时,单介质反应器的CS2去除率高于双介质反应器。因此,双介质阻挡放电反应器对于某种污染物的去除效果并非绝对的优于单介质反应器,而要视具体条件而定。目前,关于双介质和单介质阻挡放电低温等离子体在不同条件下降解VOCs的系统比较研究仍然较少。因此,比较2种反应器在不同条件下的VOCs降解效果,可为实际应用过程中反应器的合理选择提供参考。
本研究首先对比分析了单介质和双介质反应器的放电特征,随后以甲苯为目标污染物,以甲苯去除率、矿化率、CO2选择性为指标,分析了2种反应器在不同电压、不同浓度下对甲苯的去除效果,并对副产物O3、N2O以及反应器的能量效率进行了比较分析。
-
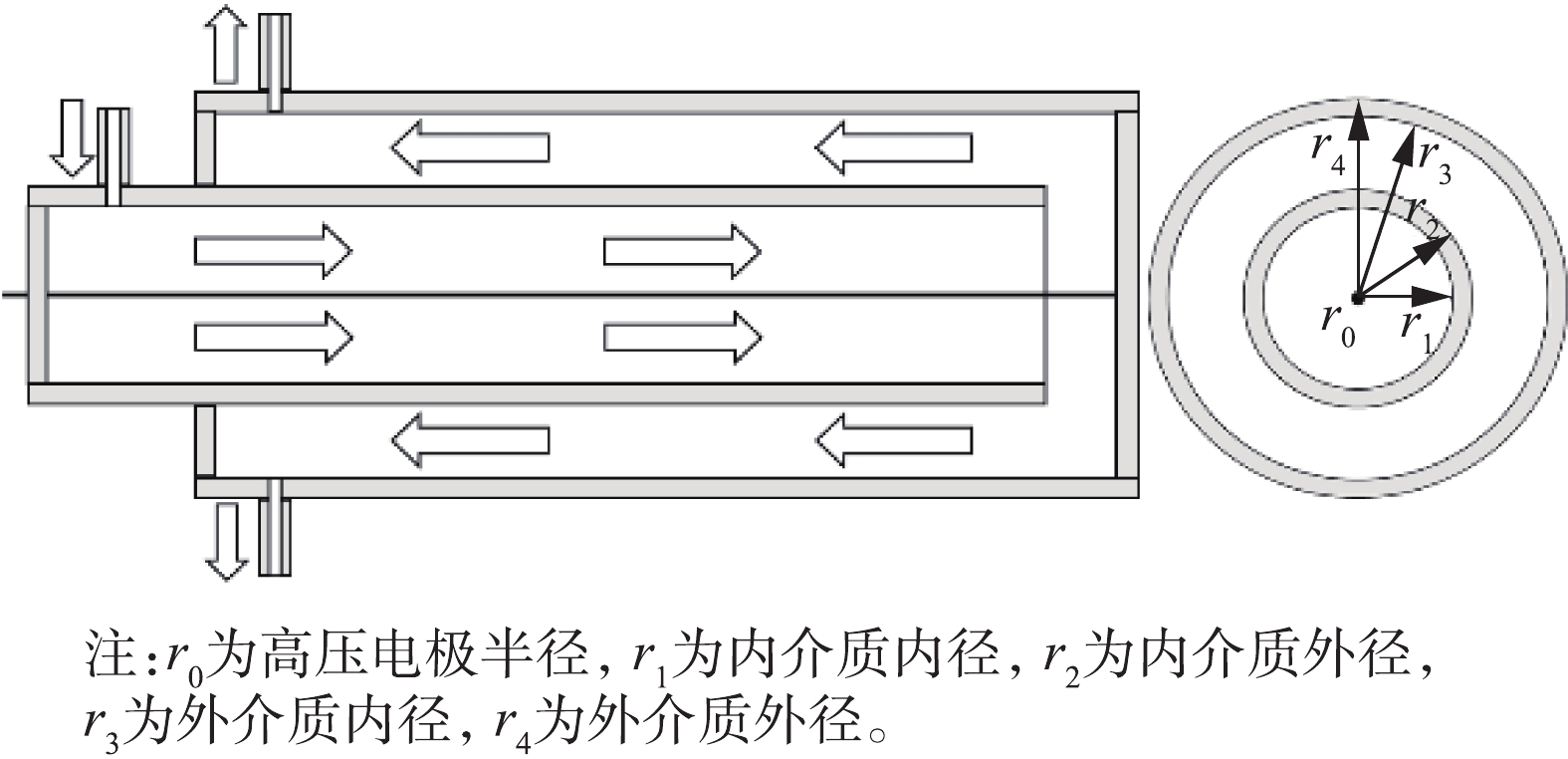
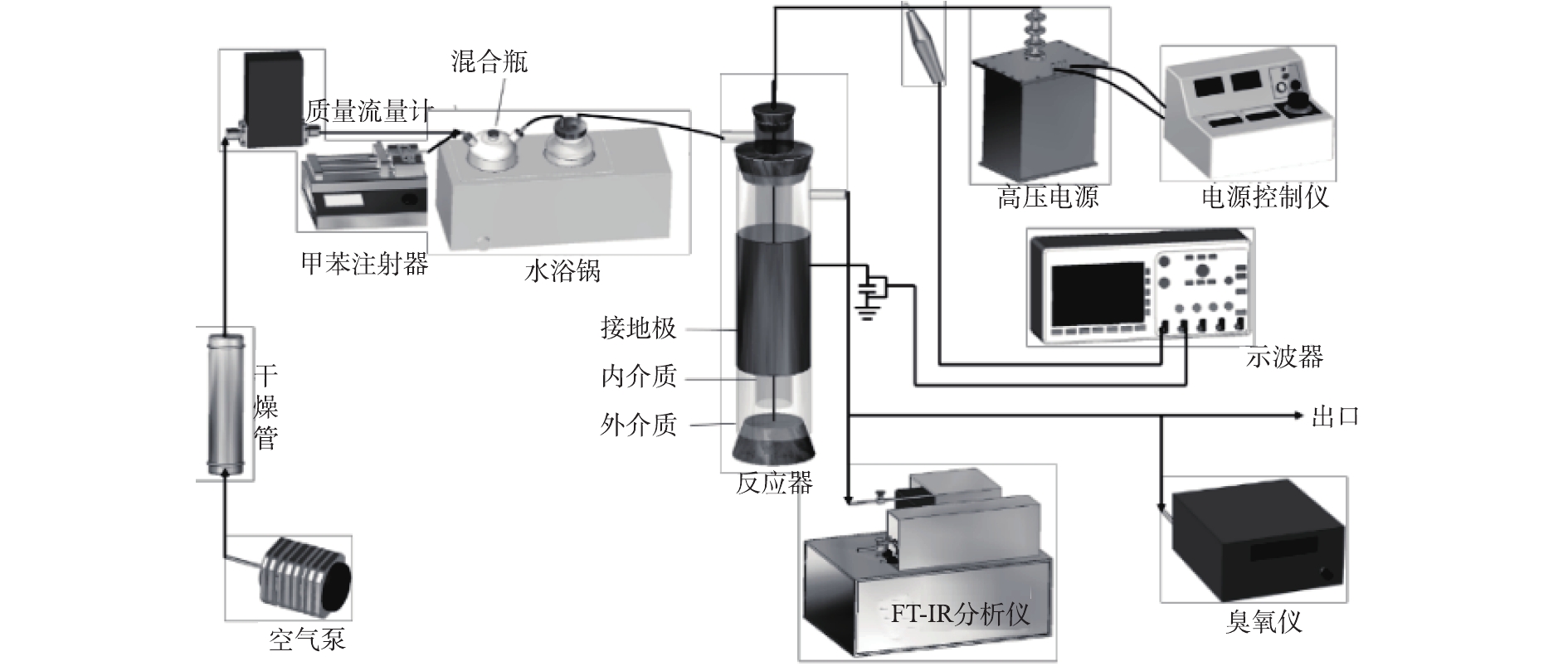
所有实验均在常温常压下进行,实验装置如图1所示。将注射泵注入的液态甲苯与经过干燥后的空气在混合瓶中混合,用来模拟甲苯废气,混合瓶置于恒温水浴锅中以保证甲苯能持续挥发。由质量流量计控制气体流量为2 L·min−1,通过改变注射器的推进速度来调整甲苯浓度,本实验中甲苯浓度分别为616、1 027、1 848 mg·m−3。低温等离子体在自制的线筒式介质阻挡放电反应器中产生,反应管为石英玻璃材质,高压电极为直径1 mm的不锈钢丝,接地极为缠绕在玻璃管外壁的铜皮(厚0.05 mm,长100 mm)。单介质反应器管内径为30 mm,厚1.5 mm,双介质反应器外管尺寸与单介质相同,内管内径为15 mm,厚1 mm。实验所用电源为50 Hz高压交流电源(GJTK-0.01/30K,上海南罡电除尘器有限公司)。使用示波器(DPO3054,Tektronix)通过高压探头(P6015A,Tektronix)测定电流电压波形图。甲苯及其降解过程中产生的H2O、CO2、CO、N2O的浓度由傅里叶变换红外光谱仪(Nicolet Antaris IGS,Thermo Scientific Company)测定,O3浓度通过臭氧检测仪(2B Technologies Model 106-M)测得。
-
反应器的输入功率计算方法[21]见式(1),甲苯的降解效果以甲苯去除率、矿化率、CO2选择性和能量效率作为评价指标,计算方法见式(2)~式(5)。
式中:P为输入功率,W;η为甲苯去除率;MR为矿化率;
$S_{{\rm{CO}}_2} $ 为CO2选择性;f为频率,取值50 Hz;EF为能量效率,g·(kWh)−1;C为电容,取值0.47 μF;A为示波器所测李萨如图的面积;Cin、Cout为反应器进、出口甲苯浓度,mg·m−3;$C_{{\rm{CO}}_2} $ 、CCO为反应器出口CO2、CO浓度,mg·m−3;Q为甲苯气体流量,取值2 L·min−1。 -
图2为单介质和双介质反应器分别在14 kV和24 kV电压下一个正负变换周期内的电流波形图。随着电压的升高,微放电的数量和强度明显增加。在同一电压下,双介质反应器中的微放电数量更少,这是由于双介质反应器中的内层介质阻碍电子的运动,微放电到达内层介质后不易继续向外层介质传播,导致检测到的微放电数量较少。
双介质反应器的结构及气流方向如图3所示。通过理论计算的方法估算2种反应器电场强度,计算方法[22-23]见式(6)和式(7)。
式中:Eg,双和Eg,单分别为双介质和单介质反应器放电区域的电场强度,kV·cm−1;V为放电电压,kV;εd为介质的相对介电常数,取值为4;εg为空气的相对介电常数,取值为1;r为反应器中放电间隙据轴心的距离,cm;r0=0.05 cm;r1=0.75 cm;r2=0.85 cm;r3=1.5 cm;r4=1.65 cm。
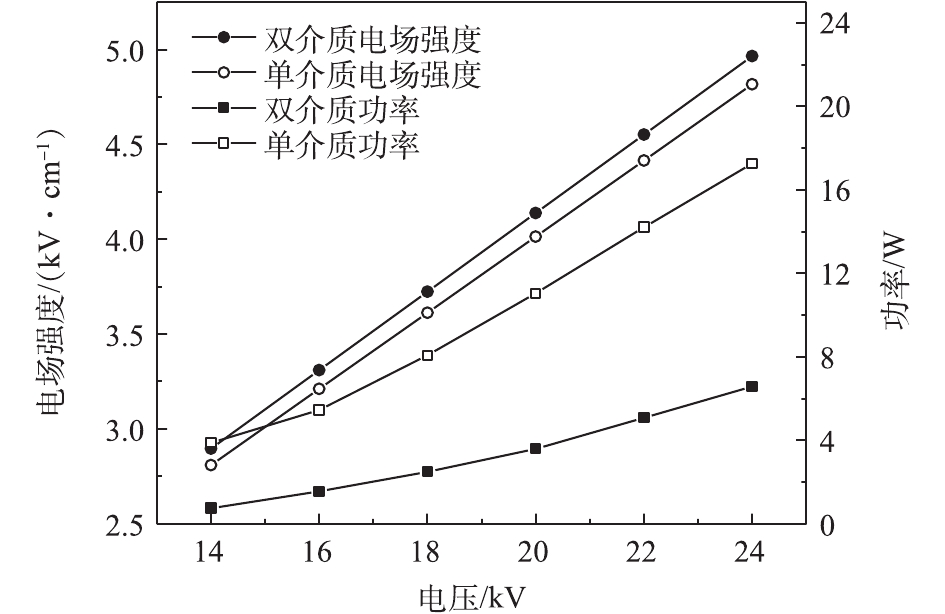
不同反应器的平均电场强度和输入功率比较结果见图4。在相同电压下,双介质反应器的平均电场强度略大于单介质反应器,而双介质反应器的输入功率远小于单介质反应器,与类似研究中的结果[23-24]一致。由于内管的屏蔽作用,在高压电极附近产生的高能电子只有一部分能够运动到外管附近的区域,从而产生更小的电流,以致相同电压下的输入功率更小,这与电流电压波形图(图2)中双介质反应器的微放电数量少于单介质反应器这一现象是一致的。
-
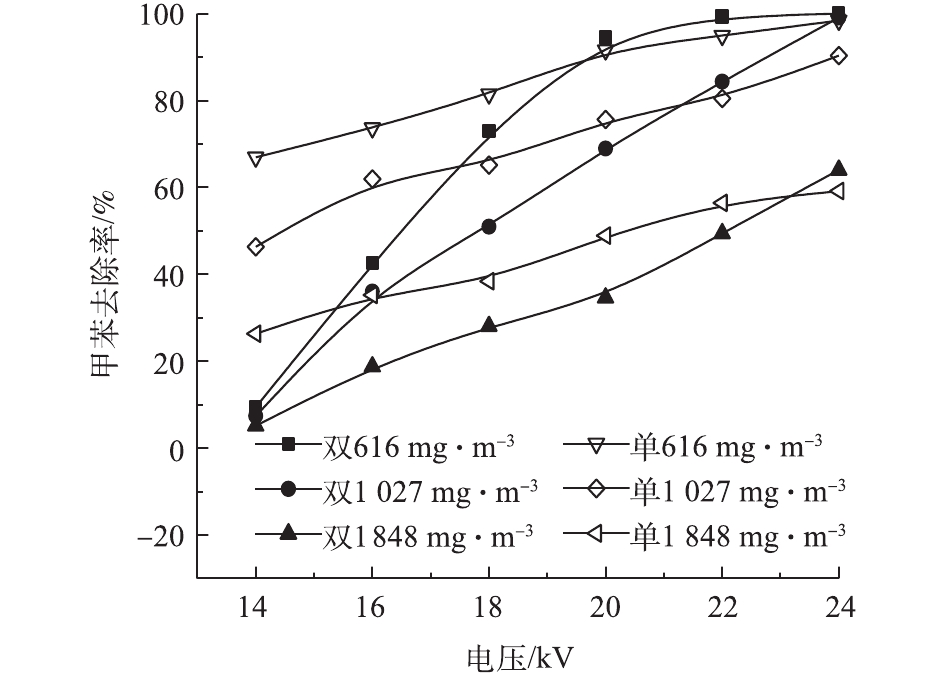
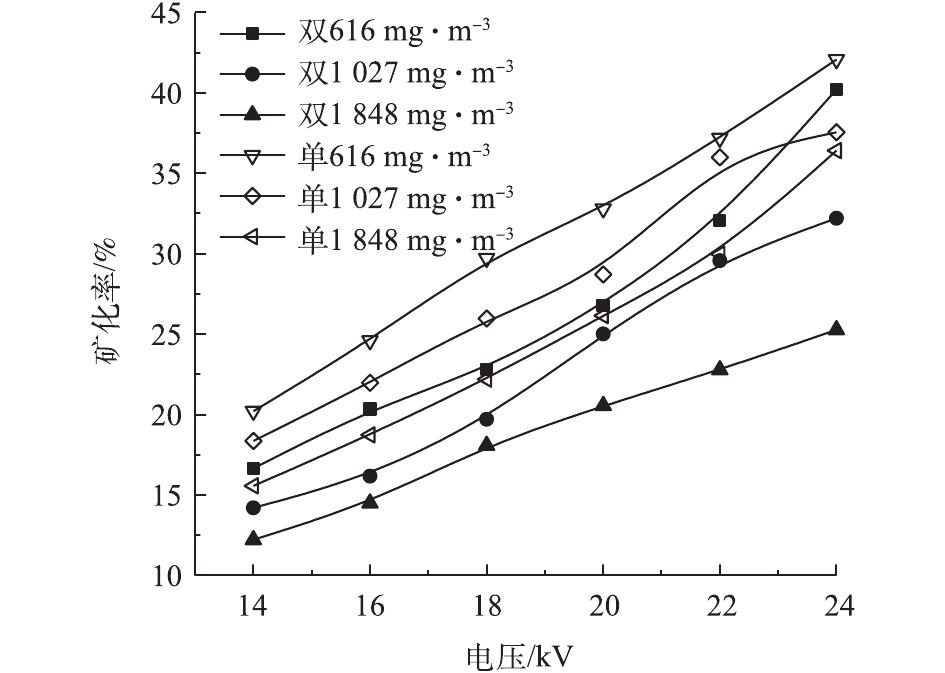
在不同的浓度和电压下,甲苯在2种反应器中的去除率见图5。甲苯浓度分别为616、1 027、1 848 mg·m−3,电压为14~24 kV时,双介质反应器中的甲苯去除率为9.4%~100%、7.4%~99%、5.1%~64%,单介质为67%~98%、46%~90%、26%~59%。随电压的升高和甲苯浓度的降低,甲苯去除率升高。电压升高,放电间隙电场强度增大,电子运动速度加快,使得反应器中的活性粒子数量增加、能量增大,与甲苯分子的碰撞概率增加,使得更多的甲苯被分解[25]。反应器入口浓度升高,甲苯去除率下降。在相同电压下,活性粒子数量和能量一定,浓度升高意味着更多的甲苯分子进入反应器,因此,每一个甲苯分子所能接触到的活性粒子数量减少,导致去除率下降[26]。可以看出,双介质反应器的去除率-电压曲线斜率高于单介质反应器,即双介质反应器的甲苯去除率随电压升高变化更快。电压为20 kV、浓度为616 mg·m−3时,双介质反应器对甲苯的去除率达到了94.51%。李云霞等[20]比较了单双介质反应器对CS2的去除效果,得到了相同的结论。这可能与2种反应器的气体击穿电压和放电间隙电场强度有关。同时,双介质反应器对甲苯去除率高于单介质反应器时的电压的结果并不是固定的,这一电压随甲苯浓度的升高而增加。甲苯在低温等离子体中降解后并非完全生成CO2和H2O,还会生成有机副产物和CO[27]。因此,还须通过矿化率和CO2选择性来评价反应器中的甲苯降解效果。图6和图7反映了不同条件下2种反应器中的甲苯矿化率和CO2选择性。2种反应器的甲苯矿化率均随电压的升高和甲苯浓度的降低而升高,说明高电压、低浓度更有利于甲苯转化为COx。当浓度和放电电压相同时,单介质反应器的矿化率高于双介质反应器,这是由单介质反应器的输入功率更大而导致的。当浓度为616 mg·m−3时,双介质反应器的矿化率为16.66%~40.22%,单介质的矿化率为20.21%~40.27%。
随甲苯浓度的升高,2个反应器中的CO2选择性均降低,说明浓度升高不仅导致矿化率降低,而且使CO2在COx中所占比率下降。在高浓度下,每个甲苯分子所能接触到的活性粒子数目减少,不仅导致其降解率下降,还会发生不充分氧化而生成CO。在单介质反应器中,CO2选择性随电压的升高而降低,在浓度为616、1 027、1 848 mg·m−3时,分别为52.11%~49.28%、50.23%~46.58%、45.75%~43.19%。这说明,随电压的升高,CO对矿化率的贡献逐渐增大。在双介质反应器中,CO2选择性随电压的升高,先降低后升高。这可能是因为电压小于20 kV时,甲苯的降解主要发生在内管中,随电压的升高,外管中有足够的场强促进甲苯的降解和CO的氧化。在相同条件下,双介质反应器的CO2选择性高于单介质。这是因为甲苯在内管中降解时产生的CO随气流进入外管后,可以进一步与臭氧或氧自由基反应生成CO2。而单介质反应器中只有一个放电区域,在各处发生的反应大致相同,不存在类似于双介质中的CO进一步氧化的过程。
-
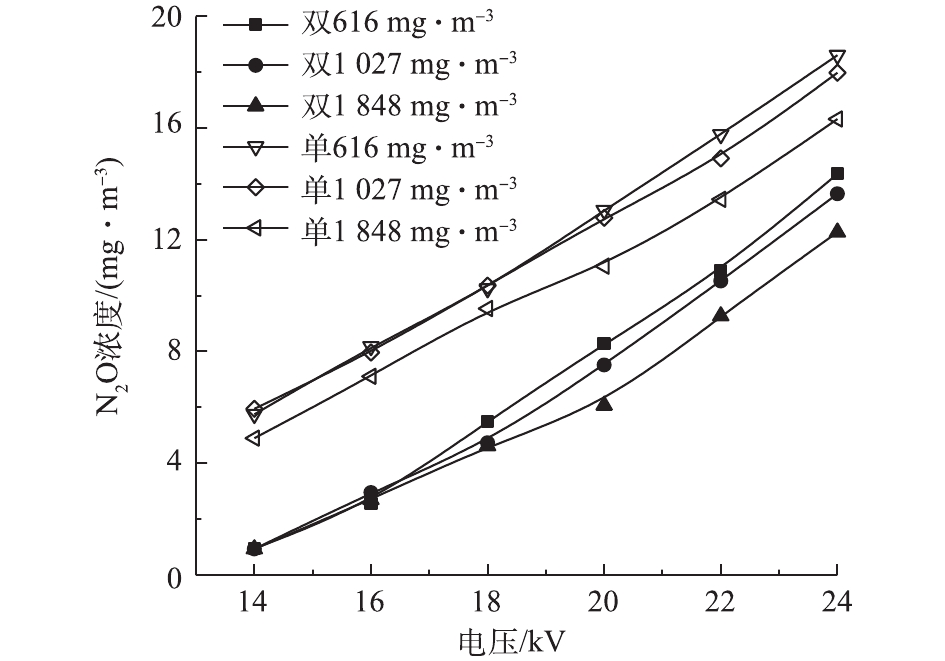
在低温等离子体降解甲苯时,不可避免地会出现O3、N2O等产物[28]。图8和图9反映了2种反应器在不同条件下的O3浓度和N2O浓度。甲苯浓度越高,O3和N2O的浓度越低。在一定放电条件下,反应器中的活性粒子数目和能量一定,甲苯浓度较高时,甲苯分子与活性粒子碰撞的概率增大,更多的活性粒子参与甲苯的降解而非O3和N2O的形成。在双介质反应器中,O3及N2O的浓度均随电压升高而增大。电压升高产生更多的活性粒子,与O2和N2发生一系列反应,生成更多的O3和N2O。在单介质反应器中,随电压的升高,O3浓度先升高后降低。当外加电压过高时,单介质反应器中可能出现火花放电,系统温度升高,使部分臭氧分解[29]。不同于O3,N2O不会因高温而发生分解,其浓度随电压的升高而增大。在同一电压下,单介质反应器具有更高的输入功率,产生更多的活性粒子,因此,N2O浓度高于双介质反应器。双介质反应器的击穿电压更高,不存在单介质反应器中O3因高温而分解的情况,因此,电压超过20 kV后,双介质反应器中的O3浓度更高。相对于N2O,O3很容易被分解,因此,可以在反应器中加入合适的催化剂,来抑制O3的产生[30-31]。
-
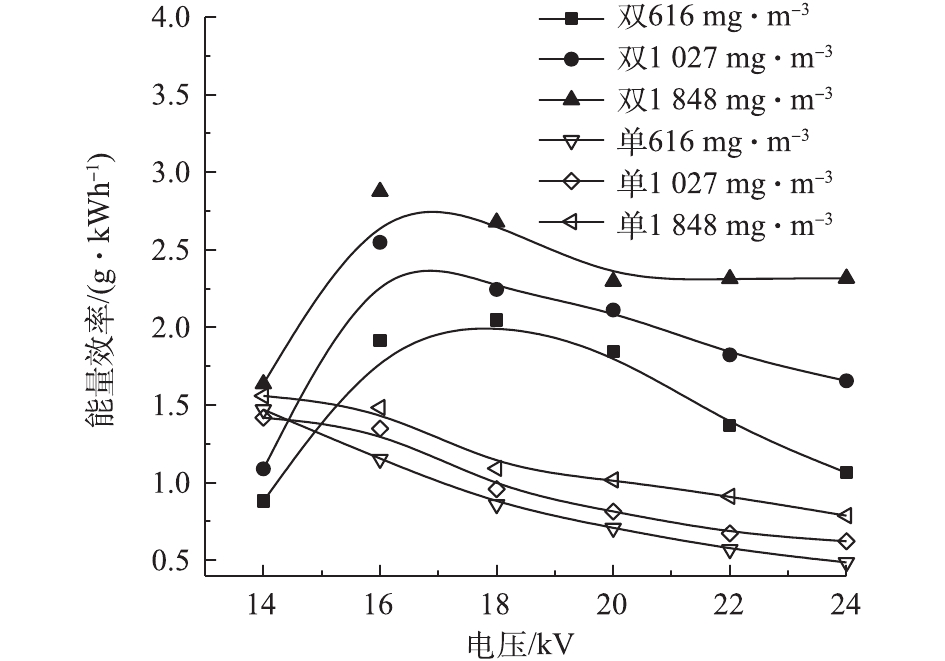
图10反映了2种反应器在不同条件下的能量效率。当甲苯浓度较高时,能量效率较高。虽然高浓度下甲苯的去除率较低,但系统的能量能够被更加有效地利用。随电压的升高,单介质反应器的能量效率逐渐降低[32],而双介质反应器则先升高后下降[33]。电压为14 kV时,双介质反应器对甲苯的去除率不到10%,只有很少的一部分甲苯被降解,导致能量效率较低。电压在16~18 kV时,双介质反应器有最佳的能量效率;电压超过16 kV时,双介质反应器的能量效率高于单介质反应器。这说明甲苯的去除率高并不代表其能量效率也高,因为在相同电压下,2个反应器的输入功率不同。
-
1)在相同电压下,双介质反应器具有更高的平均电场强度,单介质反应器输入功率更高。
2)电压升高、浓度降低,甲苯去除率增加。在浓度为616 mg·m−3,电压为14~20 kV时,单介质反应器对甲苯去除率高;电压为20~24 kV时,双介质反应器对甲苯去除率高。随甲苯浓度的变化,这一电压范围也会发生改变。
3)甲苯矿化率随电压的升高和浓度的降低而升高。在同一条件下,单介质反应器的矿化率高于双介质反应器。单介质反应器的CO2选择性随电压的升高而降低,双介质反应器的CO2选择性随电压先降低后升高。在相同条件下,双介质反应器的CO2选择性更高。
4)在双介质反应器中,O3和N2O浓度均随电压的升高和甲苯浓度的降低而升高。在单介质反应器中,O3浓度随电压的升高先升高后降低,N2O浓度随电压的升高而升高。电压低于20 kV时,单介质反应器中O3浓度较高。双介质反应器中的N2O浓度低于单介质反应器。
5)单介质反应器的能量效率随电压的升高而降低,双介质反应器在电压为16~18 kV时,具有最佳能量效率;电压大于16 kV时,双介质反应器的能量效率高于单介质反应器。
单介质和双介质阻挡放电低温等离子体降解甲苯的比较
Comparison of single and double dielectric barrier discharge non-thermal plasma for toluene removal
-
摘要: 为研究介质阻挡放电(DBD)反应器结构对低温等离子体降解甲苯的影响,设计了具有单层介质和双层介质的DBD反应器。对2种反应器的放电特征、甲苯去除率、矿化率、CO2选择性和能量效率进行了比较,并对施加电压和初始浓度对甲苯降解效果的影响进行了分析。结果表明:在相同电压下,双介质反应器(DDBD)具有更高的电场强度,而单介质反应器(SDBD)的输入功率更高;当甲苯浓度和电压分别为616、1 027、1 848 mg·m−3和14~24 kV时,双介质中的甲苯去除率为9.4%~100%、7.4%~99%、5.1%~64%,单介质为67%~98%、46%~90%、26%~59%。这说明低电压下单介质反应器的甲苯去除率更高,而高电压下则相反,并且,浓度降低、电压升高有利于甲苯的降解。单介质反应器的能量效率随电压升高而降低,双介质反应器则先升高后下降,且双介质反应器的能量效率高于单介质反应器(16~24 kV)。以上研究可为介质阻挡放电在VOCs去除方面的应用提供参考。Abstract: In order to investigate the effect of dielectric barrier discharge (DBD) reactor structure on toluene degradation in non-thermal plasma, reactors with single and double barrier were designed. The discharge characteristics, toluene removal efficiency, mineralization rate, CO2 selectivity and energy efficiency of the two rectors were compared, and the effects of voltage and initial concentration on toluene degradation were analyzed. The results indicated that DDBD reactor had higher electric field strength and SDBD reactor had higher power under the same applied voltage. The removal efficiency of toluene in the DDBD reactor ranged from 9.4%~100%, 7.4%~99%, 5.1%~64% and that in the SDBD reactor ranged from 67%~98%, 46%~90%, 26%~59%, when the initial toluene concentration and the applied voltage were 616, 1 027, 1 848 mg·m−3 and were 14~24 kV, respectively. It showed that SDBD has a higher toluene removal efficiency at low applied voltage, while the removal efficiency is higher in DDBD at a higher applied voltage. Moreover, the decrease of toluene concentration and the increase of applied voltage were favorable for toluene degradation. In SDBD, the energy efficiency decreased with the increase of applied voltage, while that of the DDBD reactor first increased and then decreased. The energy efficiency of the DDBD reactor was higher than that of the SDBD reactor at 16~24 kV. The present study could provide reference for the application of DBD in VOCs abatement.
-

-
-
[1] ZHANG J N, XIAO J F, CHEN X F, et al. Allowance and allocation of industrial volatile organic compounds emission in China for year 2020 and 2030[J]. Journal of Environmental Science, 2018, 69: 155-165. doi: 10.1016/j.jes.2017.10.003 [2] HUI L R, LIU X G, TAN Q W, et al. VOC characteristics, sources and contributions to SOA formation during haze events in Wuhan, Central China[J]. Science of the Total Environment, 2019, 650: 2624-2639. doi: 10.1016/j.scitotenv.2018.10.029 [3] HU R Y, LIU G J, ZHANG H, et al. Levels, characteristics and health risk assessment of VOCs in different functional zones of Hefei[J]. Ecotoxicology and Environmental Safety, 2018, 160: 301-307. doi: 10.1016/j.ecoenv.2018.05.056 [4] ZHANG X Y, GAO B, CREAMER A E, et al. Adsorption of VOCs onto engineered carbon materials: A review[J]. Journal of Hazardous Materials, 2017, 338: 102-123. doi: 10.1016/j.jhazmat.2017.05.013 [5] HARIZ R, RIOSANZ J I, MERCIER C, et al. Absorption of toluene by vegetable oil-water emulsion in scrubbing tower: Experiments and modeling[J]. Chemical Engineering Science, 2017, 157: 264-271. doi: 10.1016/j.ces.2016.06.008 [6] BELAISSAOUI B, MOULLEC Y L, FAVRE E. Energy efficiency of a hybrid membrane/condensation process for VOC (volatile organic compounds) recovery from air: A generic approach[J]. Energy, 2016, 95: 291-302. doi: 10.1016/j.energy.2015.12.006 [7] MUHAMMAD S K, SHAIKH A R, MOHAMMAD M H. Catalytic oxidation of volatile organic compounds (VOCs): A review[J]. Atmospheric Environment, 2016, 140: 117-134. doi: 10.1016/j.atmosenv.2016.05.031 [8] SAVITA K P, VEERAPANDIAN C L, NATHALIE D G, et al. Abatement of VOCs using packed bed non-thermal plasma reactors: A review[J]. Catalysts, 2017, 7: 1-33. [9] ZHANG S H, YOU J P, CHRISTIAN K. Current advances of VOCs degradation by bioelectrochemical systems: A review[J]. Chemical Engineering Journal, 2018, 334: 2625-2637. doi: 10.1016/j.cej.2017.11.014 [10] 席劲瑛, 武俊良, 胡洪营, 等. 工业VOCs气体处理技术应用状况调查分析[J]. 中国环境科学, 2012, 32(11): 1955-1960. doi: 10.3969/j.issn.1000-6923.2012.11.005 [11] 栾志强, 郝郑平, 王喜芹. 工业固定源VOCs治理技术分析评估[J]. 环境科学, 2011, 32(12): 2216-2227. [12] QIN C H, GUO H, LIU P, et al. Toluene abatement through adsorption and plasma oxidation using ZSM-5 mixed with γ-Al2O3, TiO2 or BaTiO3[J]. Journal of Industrial and Engineering Chemistry, 2018, 63: 449-455. doi: 10.1016/j.jiec.2018.03.005 [13] AKIRA M. Generation of non-thermal plasma combined with catalysts and their application in environmental technology[J]. Catalysis Today, 2013, 211: 2-8. doi: 10.1016/j.cattod.2013.03.029 [14] QIN C H, GUO H, BAI W W, et al. Kinetics study on non-thermal plasma mineralization of adsorbed toluene over γ-Al2O3 hybrid with zeolite[J]. Journal of Hazardous Materials, 2019, 369: 430-438. doi: 10.1016/j.jhazmat.2019.01.098 [15] ARNE M V, MORENT R, NATHALIE D G, et al. Non-thermal plasmas for non-catalytic and catalytic VOC abatement[J]. Journal of Hazardous Materials, 2011, 195: 30-54. doi: 10.1016/j.jhazmat.2011.08.060 [16] 王保伟, 王超, 徐艳, 等. 介质阻挡放电等离子体反应器降解盐酸四环素[J]. 化工学报, 2018, 69(4): 1687-1694. [17] KIM H H, YOSHIYUKI T, NOBUAKI N, et al. A multidisciplinary approach to understand the interactions of nonthermal plasma and catalyst: A review[J]. Catalysis Today, 2015, 256: 13-22. doi: 10.1016/j.cattod.2015.04.009 [18] ZHANG H B, LI K, SHU C H, et al. Enhancement of styrene removal using a novel double-tube dielectric barrier discharge (DDBD) reactor[J]. Chemical Engineering Journal, 2014, 256: 107-118. doi: 10.1016/j.cej.2014.06.105 [19] TANG X L, GAO F Y, WANG J G, et al. Comparative study between single- and double-dielectric barrier discharge reactor for nitric oxide removal[J]. Industrial & Engineering Chemistry Research, 2014, 53: 6197-6203. [20] 李云霞, 朱承驻, 陈天虎, 等. 介质阻挡放电反应器中的二硫化碳降解特性[J]. 环境科学研究, 2013, 26(2): 188-193. [21] LEE B, KIM D W, PARK D W. Dielectric barrier discharge reactor with the segmented electrodes for decomposition of toluene adsorbed on bare-zeolite[J]. Chemical Engineering Journal, 2019, 357: 188-197. doi: 10.1016/j.cej.2018.09.104 [22] 赵卫东, 蔡忆昔, 韩文赫, 等. 同轴圆柱结构DBD装置放电功率的模拟计算及实验研究[J]. 高压电器, 2010, 46(6): 25-28. [23] 马天鹏, 钟方川. 估算线筒式介质阻挡放电场强和电子平均动能的方法[J]. 核聚变与等离子体物理, 2017, 37(4): 399-403. [24] 侯世英, 曾鹏, 刘坤, 等. 单介质与双介质结构介质阻挡放电水处理性能的比较[J]. 高电压技术, 2012, 38(7): 1562-1567. [25] YAO X H, ZHANG J, LIANG X H, et al. Plasma-catalytic removal of toluene over the supported manganese oxides in DBD reactor: Effect of the structure of zeolites support[J]. Chemosphere, 2018, 208: 922-930. doi: 10.1016/j.chemosphere.2018.06.064 [26] WANG B W, CHI C M, XU M, et al. Plasma-catalytic removal of toluene over CeO2-MnOx catalysts in an atmosphere dielectric barrier discharge[J]. Chemical Engineering Journal, 2017, 322: 679-692. doi: 10.1016/j.cej.2017.03.153 [27] OSMAN K, MARC A D. A comparative study of dilute VOCs treatment in a non-thermal plasma reactor[J]. Chemical Engineering Journal, 2016, 201: 308-315. [28] KIM H H, OGATA A, SHIGERU F. Oxygen partial pressure-dependent behavior of various catalysts for the total oxidation of VOCs using cycled system of adsorption and oxygen plasma[J]. Applied Catalysis B: Environmental, 2008, 79: 356-367. doi: 10.1016/j.apcatb.2007.10.038 [29] ZHANG H B, LI K, SUN T H, et al. The removal of styrene using a dielectric barrier discharge (DBD) reactor and the analysis of the by-products and intermediates[J]. Research on Chemical Intermediates, 2013, 39(3): 1021-1035. doi: 10.1007/s11164-012-0664-0 [30] ZHENG B, HAN H, YANG S L, et al. Vertically-oriented graphenes supported Mn3O4 as advanced catalysts in post plasma-catalysis for toluene decomposition[J]. Applied Surface Science, 2018, 436: 570-578. doi: 10.1016/j.apsusc.2017.12.081 [31] ZHU R Y, MAO Y B, JIANG L Y, et al. Performance of chlorobenzene removal in a nonthermal plasma catalysis reactor and evaluation of its byproducts[J]. Chemical Engineering Journal, 2015, 279: 463-471. doi: 10.1016/j.cej.2015.05.043 [32] CHANG T, SHEN Z X, HUANG Y, et al. Post-plasma-catalytic removal of toluene using MnO2-Co3O4 catalysts and their synergistic mechanism[J]. Chemical Engineering Journal, 2018, 348: 15-25. doi: 10.1016/j.cej.2018.04.186 [33] 吴萧, 刘盛余, 何廷宇, 等. 介质阻挡放电低温等离子体技术处理3种代表性VOC[J]. 环境工程学报, 2017, 11(10): 5502-5508. doi: 10.12030/j.cjee.201612064 -



 下载:
下载: