-
随着中国经济持续高速增长和城市化率的提高,城市生活垃圾的产生量也迅速增加。相比于焚烧、堆肥等处置方法,就开发和建设成本而言,城市生活垃圾的填埋处置最为廉价[1],目前仍然被大规模应用。在我国,采用填埋方式处置的垃圾约占全部处置垃圾的70%[2],填埋所产生的巨量垃圾渗滤液对生态环境和人体健康的危害日益引起广泛关注。随着垃圾渗滤液的污染控制和排放标准日趋严格,对垃圾渗滤液处理工艺技术的改进和开发也提出了更高的要求。
垃圾渗滤液中含有大量难生物降解性有机物(包括酚类化合物、含氮化合物、酯和酮、烯烃、烷烃、醇类、多环芳烃、胺类和邻苯二甲酸类)、氨氮、无机盐以及重金属等[3-7],其成分与诸多因素相关,如降水、气候条件、垃圾类型和组成等,尤其是填埋龄[1]。一般而言,随垃圾填埋龄的增加,垃圾渗滤液的pH由酸性转变为碱性,氨氮浓度逐渐增高,可生物降解性逐渐下降[1, 8]。老龄垃圾渗滤液的填埋龄一般达10 a以上,其有机物以腐殖酸、富里酸类等难降解物质为主,具有可生化性差、氨氮浓度较高等特征[1]。高级氧化技术(advanced oxidation processes, AOPs)可去除传统技术无法处理的难降解有机物,并可提高污水的可生物降解性[9],因此,将其应用于老龄垃圾渗滤液的处理极具推广潜力。
AOPs包括2个过程,即高反应性的自由基的形成及其与有机化合物发生的自由基反应[8]。目前,对基于臭氧(O3)、过氧化氢(H2O2)和过硫酸盐(
S2O2−8 、HSO−5 )的AOPs研究比较广泛。其中O3的氧化性极强,其氧化还原电位达2.07 V,可与大多数有机物发生反应,速度快且无二次污染[10]。在基于O3的AOPs中,H2O2通过提供羟基自由基(·OH)和生成·OH的引发剂(H2O2部分分解产生HO−2 ),来促进O3对污染物的分解[11]。硫酸根自由基(SO⋅−4 )不仅具有更强的氧化性(E0 = 2.5~3.1 V)和更长的半衰期[12],且其对pH的适应范围广(pH=4~9)[13]。而SO⋅−4 一般由过一硫酸盐(HSO−5 , PMS)或过二硫酸盐(S2O2−8 , PS)经紫外光、热、过渡金属、碱或强氧化剂活化生成[14]。各种高级氧化过程产生的自由基攻击目标有机物,从而实现有机物的高效降解。YUAN等[15]比较了O3、PMS、O3/PS、O3/PMS和O3/H2O2体系降解布洛芬(IBP)的结果,指出O3/PMS对IBP的降解能力最强。对于垃圾渗滤液的处理,目前多限于利用某种特定的高级氧化过程评价有机物的降解。ABU AMR等[16]研究了O3/PS体系处理老龄垃圾渗滤液,内容包括处理过程中pH、O3、Na2S2O8的投加量对COD等去除的影响并确定了最优条件,同时还研究了有机污染物的可溶性和可生物降解性的变化情况。贺磊等[17]和胡兆吉等[18]分别考察了O3/H2O2体系深度处理垃圾渗滤液过程中pH、反应时间和H2O2投加量等对处理效果的影响,并确定了最优条件。目前,利用基于O3体系激发自由基的高级氧化过程对老龄垃圾渗滤液有机物的降解及提高其可生化性的研究尚不多,臭氧催化氧化体系在垃圾渗滤液处理实际工程应用的可行性仍有待进一步探讨。本研究针对O3/PS、O3/PMS和O3/H2O2氧化体系处理老龄垃圾渗滤液的过程,基于有机污染物降解动力学,探讨了初始pH、O3、H2O2、Na2S2O8或KHSO5的投加量对处理效果的影响,采用单位数量级能耗(electrical energy per order,EE/O)标准评价其能耗效率,进而比较分析3种高级氧化体系处理能力的差异、可行性及原因,为臭氧高级氧化技术的工程化应用提供参考。
全文HTML
-
实验所用试剂30% H2O2、过二硫酸钠(Na2S2O8)、硫酸、氢氧化钠等均由国药集团化学试剂有限公司生产,级别为分析纯。过硫酸氢钾复合盐(2KHSO5·KHSO4·K2SO4,KHSO5含量≥47%)由上海阿拉丁生化科技股份有限公司生产。
实验装置如图1所示。将1.5 L垃圾渗滤液注入圆柱形夹套式高硼硅玻璃反应器,反应器内径为150 mm、高为200 mm。臭氧由以氧气为气源(内置高纯度制氧机)的臭氧发生器(深圳市飞立电器科技有限公司,FL-810ET)产生,其最大臭氧产量为10 g·h−1,由耐腐蚀转子流量计调节进入反应器的臭氧量,经微孔扩散器进入反应器内。开始反应时,加入药剂(H2O2/Na2S2O8/KHSO5),并开启磁力搅拌器(赛默飞世尔科技,SP131320-33Q)。反应过程中温度由低温恒温槽(宁波天恒仪器厂,THD-06H)调控。
-
垃圾渗滤液收集于福建省厦门市某城市生活垃圾卫生填埋场调节池,该填埋场于1997年7月建成投用,2009年6月封场。其pH、盐度、电导率、碱度(以CaCO3计)、化学需氧量(COD)、五日生化需氧量/化学需氧量(BOD5/COD)、总氮(TN)、氨氮(
NH+4 -N)、总磷(TP)、溶解性磷酸盐(PO3−4 -P)、总固体含量(TS)、氯离子(Cl−)和硫酸根离子(SO2−4 )分别为8.14、0.82%、12.75 mS·cm−1、(4 256.63±16.84) mg·L−1、(760.63±40.48) mg·L−1、0.177±0.01、(1 255.45±48.30) mg·L−1、(1 251.74±9.02) mg·L−1、(9.97±0.55) mg·L−1、(7.72±0.16) mg·L−1、(4.76±0.05) g·L−1、(1 239.76±13.26) mg·L−1和(54.42±0.83) mg·L−1。由此可知,此垃圾渗滤液属于典型的老龄垃圾渗滤液[1]。 -
已有研究[19]指出,基于臭氧的高级氧化过程受体系pH、臭氧通入量、温度和催化剂投加量等因素影响较大。因此,本研究采用单因素实验设计,设定O3/PS体系初始pH分别为3、6、8.14(原液pH)、9,臭氧投加量分别为2、3、4、5、6.5 g·h−1,Na2S2O8投加量分别为300、600、900、2 400 mg·L−1,反应时间为60 min;设定O3/PMS体系初始pH分别为3、6、8.14、9,反应温度分别为25、45、55、65 ℃,KHSO5投加量分别为150、300、600、1 200 mg·L−1,反应时间为60 min;设定O3/H2O2体系初始pH分别为3、6、8.14、9,臭氧投加量为6.5 g·h−1、H2O2投加量分别为1 500、3 000、9 000、12 000 mg·L−1,反应时间为90 min(或60 min)。基于上述实验,考察了3个体系中各种因素对TOC去除的影响。
-
主要水质指标的测定方法如表1所示。因H2O2、PS、PMS对重铬酸钾法测定COD有影响[20-22],所以本研究主要采用测定总有机碳(TOC)的方式,表征O3/PS、O3/PMS以及O3/H2O2氧化体系的处理效果。
能源效率计算参考已有研究中采用的EE/O的计算方法[23-26]。EE/O为单位体积废水中污染物浓度降低一个数量级(90%)所消耗的能量,单位为kWh·m−3。另外,体系加热所需能耗根据比热容公式计算。将本研究中渗滤液pH调节至3、6、9,按投加H2SO4或NaOH的量计算所需能耗,其值分别约为0.43、0.09、0.63 kWh·m−3。
-
有研究[27]指出,O3-AOPs的污染物降解过程符合拟一级反应动力学,其方程见式(1)。
式中:t为处理时间,min;Ct、C0分别为t和0时刻水样中TOC浓度,mg·L−1;k为反应速率常数,min−1。由式(1)可知,ln (C0/Ct)与时间t呈线性关系,本研究中各体系对渗滤液中TOC的降解符合拟一级反应动力学(R2>0.7,P<0.05)。
1.1. 实验装置及试剂
1.2. 垃圾渗滤液来源及性质
1.3. 实验设计
1.4. 分析方法
1.5. 动力学模型拟合
-
有研究[28]表明,利用
SO⋅−4 的高反应活性可有效去除废水中各种难降解有机污染物,包括挥发性有机物、内分泌干扰物、药物、蓝藻毒素和全氟化合物等。在垃圾渗滤液处理领域,它也一直受到关注,包括采用紫外光/过硫酸盐[29]、热/过硫酸盐[30]、微波/过硫酸盐[31]等工艺,都可以达到一定的处理效果。而将PS或PMS与O3相结合,可能同时产生SO⋅−4 和·OH,但却鲜有研究[32]。因此,本研究以O3为基础,探讨投加PS或PMS生成SO⋅−4 处理老龄垃圾渗滤液的效果(图2)。在化学氧化过程中,pH对氧化剂分解生成自由基起重要作用[33]。如图2(a)和表2所示:在初始pH=6时,TOC的去除率最大,为19.4%,反应速率常数为0.003 3 min−1;在初始pH=3或9时,TOC去除率和反应速率都显著下降,分别仅为1.54%、1.37%和0.000 2、0.000 21 min−1。HUANG等[34]和SUN等[35]都指出,在较低pH(<5)时,H+可能捕获
SO⋅−4 和·OH,从而阻碍它们的氧化过程(式(2)和式(3))。相比酸性条件,臭氧分子可直接参与反应,随pH的增加,由OH−催化O3分解生成·OH(式(4)),使有机化合物降解更彻底[9],这也有利于由·OH激发PS产生SO⋅−4 [36](式(5)),因而更有利于对污染物的降解。本研究中的老龄垃圾渗滤液具有碱度高的特点,pH>9时,HCO−3 和CO2−3 浓度增加,而高浓度HCO−3 和CO2−3 会捕获·OH和SO⋅−4 ,生成氧化性较弱的HCO⋅3 和CO⋅−3 [9, 28, 37](式(6)~式(9)),这说明高碱度对污染物降解的负面作用要强于正面作用。上述原因可能导致体系在pH=3或pH=9条件下,TOC去除率和反应速率较低。AMR等[38]发现,增加PS投加量有利于改善渗滤液处理效果,但过量的PS反而导致了处理效果不佳。但与之不同的是,本研究在25 ℃、臭氧投加量3 g·h−1、未调pH条件下,Na2S2O8投加量由300 mg·L−1增加至2 400 mg·L−1,TOC的去除率和反应速率常数也分别由14.8%、0.002 6 min−1略提高至18.2%、0.003 3 min−1(图2(b))。这可能是由于前者实验中Na2S2O8的投加量(3~21.5 g·L−1)远高于本研究,而这意味着本研究条件下氧化过程中
SO⋅−4 的自淬灭反应(式(10))并不显著[39]。由式(4)和式(5)可知,在O3/PS体系中,O3投加量对处理效果具有重要影响。与已有的研究结果[40]相似,随O3投加量由2 g·h−1增加至4 g·h−1,TOC去除率和反应速率常数都逐渐增加,分别由17.8%、0.002 7 min−1增至23.3%、0.004 1 min−1;但O3投加量高于4 g·h−1,其处理效果反而出现了下降(图2(c))。增大投加量,可提高O3由气相进入液相的传质速率,而这有助于增加体系的氧化能力。但随液相中O3趋于溶解饱和,其传质受限[19]。因此,本研究中O3/PS体系处理老龄垃圾渗滤液的较优条件是:温度为25 ℃、pH=6,Na2S2O8、O3投加量分别为600 mg·L−1、4 g·h−1。
-
PS(
−O3S —O—O—SO−3 )中连接2个磺酸基(—SO−3 )的过氧基团(O—O),故很稳定,并不与O3发生反应[41]。而PMS以HSO−5 或SO2−5 (−O—O—SO−3 )形式存在,其中−O—O可与O3反应[37](式(11)~式(15)),这可能意味着相比O3/PS体系,O3/PMS体系处理老龄垃圾渗滤液效果更佳。基于此,本研究进一步探讨了O3/PMS体系的处理效果,结果见图3。初始pH对O3/PMS处理渗滤液的影响如图3(a)和表2所示。在pH值为6时,TOC去除率和反应速率常数都达到最大,分别为22.2%和0.003 6 min−1。其原因可能与O3/PS体系相似。此外,GUAN等[42]指出,在低pH下,几乎没有
SO2−5 (pKa=9.4)存在,所以几乎不发生O3与SO2−5 反应生成SO⋅−4 的情况。在25 ℃、pH=6、O3投加量为6.5 g·h−1条件下,研究了不同KHSO5投加量(150~1 200 mg·L−1)对处理效果的影响。如图3(b)和表2所示,KHSO5投加量在600 mg·L−1时,渗滤液处理效果最佳,TOC的去除率和反应速率常数分别为25.8%和0.004 min−1。一方面,可能是过量PMS与·OH和
SO⋅−4 反应(式(16)和式(17)),减少了自由基种类;另一方面,过量的·OH和SO⋅−4 可能发生自耗反应[20](式(18)~式(20)),从而导致对渗滤液中污染物的降解能力下降。基于上述优化条件,在pH=6,O3、KHSO5投加量分别为6.5 g·h−1、600 mg·L−1时,探究了温度对处理效果的影响。如图3(c)和表2所示,随着反应温度的升高,TOC的去除率和反应速率常数均逐渐增加,其中25、45、55 ℃条件下变化不显著,分别为25.8%、26.5%、28.1%和0.004、0.004 1、0.004 5 min−1;但当温度升至65 ℃时,渗滤液处理效果显著提高,分别达到38.8%和0.006 5 min−1。先前的研究[19]指出,温度以2种方式影响基于臭氧的AOPs过程:一方面,随温度的升高,O3在水中的溶解度降低而浓度下降;另一方面,O3分子平均能量的增加,有利于进行有O3参与的自由基反应。由本研究结果可看出,升温对O3/PMS体系降解污染物的促进作用更重要。须指出的是,PMS中的O—O在较高温度下(一般为30~90 ℃)可发生断裂,从而更易生成
SO⋅−4 [14],即PMS可能实现了热活化。这可能是65 ℃条件下O3/PMS体系处理效果显著提高的原因之一。综合上述结果,O3/PMS体系在温度为65 ℃,pH=6,KHSO5、O3投加量分别为600 mg·L−1、6.5 g·h−1的条件下,处理老龄垃圾渗滤液的效果较好。
-
除O3与H2O2相结合可生成氧化性强且无选择性的·OH(式(21))外,还因其具有成本低、操作简便且基建要求不高等优点,所以,O3/H2O2是目前高级氧化技术中应用于包括垃圾渗滤液在内的各类污水预处理或深度处理最广泛的工艺之一[17-18, 43-46]。O3/H2O2氧化体系去除效果见图4。
pH与·OH的生成与淬灭亦是密切相关的。在25 ℃,O3、H2O2投加量分别为3 g·h−1、2 125 mg·L−1条件下,不同pH(3、6、8.14、9)对处理效果的影响如图4(a)和表2所示。与O3/PS(或PMS)相似,初始pH=6时,TOC的去除率和反应速率常数最大,分别为36.3%和0.005 1 min−1。
H2O2投加量可影响O3/H2O2体系污染物降解能力[19]。如图4(b)所示,在温度为25 ℃、pH=6、O3投加量为3 g·h−1条件下,随H2O2投加量的增加(由1 500 mg·L−1增至12 000 mg·L−1),TOC的去除率和反应速率常数呈现先增加后降低的趋势。在H2O2投加量为9 000 mg·L−1时,TOC的去除率和反应速率常数达到最大,分别为45.4%和0.006 4 min−1;而当H2O2投加量为12 000 mg·L−1时,TOC的去除率和反应速率常数分别下降至42.0%和0.005 7 min−1。先前的研究[19]指出,过量的H2O2可与降解过程中生成的·OH反应(式(22)和式(23)),从而导致处理效果的下降。
基于上述结果可推断,本研究中O3/ H2O2体系在25 ℃、pH=6,O3、H2O2投加量分别为6.5 g·h−1、9 000 mg·L−1条件下,老龄垃圾渗滤液达到了较优的处理效果。
-
从上述实验结果中发现,各体系在反应初始阶段的TOC去除速率提升最快。这可能是因为老龄渗滤液中仍含一定量的易降解有机物,如酚类、醌类和芳香酸[46]。随着反应的进行,较难进一步降解的副产物(如脂肪酸、醛类)大量生成[47],TOC去除速率呈逐渐下降的趋势。
比较O3/PS、O3/PMS和O3/H2O2氧化体系处理老龄垃圾渗滤液的效果可发现,相较于O3体系(在温度为25 ℃、初始pH=6、臭氧投加量为6.5 g·h−1、60 min条件下),优化条件下反应60 min的O3/PS、O3/PMS、O3/H2O2体系的TOC去除率分别提高了111.8%、252.7%和204.5%,其相应的反应速率常数也分别提高了95.2%、209.5%和228.6%。对工业应用而言,比较某一种水处理工艺效益最重要的因素之一是其相应的处理成本[25],因此,本研究引入EE/O值作为能耗效率评价指标,综合考虑处理效果与能源投入的影响。如表2所示,3种氧化体系在不同条件下EE/O值的范围跨度大,O3/PS、O3/PMS和O3/H2O2体系分别为626.8~11 021.2、1 007.5~17 671.7和461.3~2 529.5 kWh·m−3。可见各体系处理条件的优化尤为重要,能有效提高其能耗效率。其中O3/PS、O3/PMS和O3/H2O2体系的优化条件下相应的EE/O值分别为662.6、1 007.5、1 233.7 kWh·m−3。由表2可知,O3/H2O2体系在温度为25 ℃,pH=6,O3、H2O2投加量分别为3 g·h−1、2 125 mg·L−1条件下,其能耗是各体系中最低的,EE/O值为443.9 kWh·m−3,且与O3体系相比,TOC去除率和反应速率常数也分别提高了152.4%和146.4%,优于各体系中绝大部分条件下的处理效果。
优化条件下,O3/H2O2体系的O3、H2O2投加量分别是能源效率最高条件下的2.17倍和4.24倍(表2),而O3/PMS体系需要高温,这些原因分别导致两者能耗都较高。有研究[25]指出,影响EE/O的重要因素主要有反应器设计、水质特性和氧化剂剂量等,由表2可知,O3/PS、O3/PMS和O3/H2O2体系中所采用的氧化剂及其投加量的明显差异导致了不同EE/O的出现。因此,本研究中O3/H2O2可能是最适合的处理工艺。
总之,各条件下的3种氧化体系能耗都较高。TRIPATHY等[48]仅采用微波/过硫酸盐AOPs处理垃圾渗滤液,EE/O高达1 730.7~9 667.0 kWh·m−3,但将AOPs与混凝前处理相结合,其EE/O降至最低,仅为46.0 kWh·m−3。赵丽红等[49]也指出,AOPs与生物处理技术相结合,可缩短AOPs的高耗能过程,并实现对污染物高能效的降解。而本研究也发现,经O3/H2O2处理1 h后的渗滤液BOD5/COD由未经处理原液的0.18增加至0.26(图4(b))。因此,可利用基于臭氧体系的高级氧化法作为预处理手段提高渗滤液可生化性,从而利于后续生物处理工艺对渗滤液中污染物的进一步去除,降低运行成本。
2.1. O3/PS体系
2.2. O3/PMS体系
2.3. O3/H2O2体系
2.4. 不同氧化体系的比较
-
1) O3/PS、O3/PMS和O3/H2O2氧化体系处理老龄垃圾渗滤液,在初始pH为6时,可达到最优的处理效果。在非碱性pH条件下达到最优的处理效果,其原因可能是本研究中老龄渗滤液具有高碱度的特点。
2)优化条件下,O3/PMS氧化体系(65 ℃,pH=6,KHSO5、O3投加量分别为600 mg·L−1、6.5 g·h−1)处理老龄垃圾渗滤液的效果与O3/H2O2氧化体系(25 ℃,pH=6,O3、H2O2投加量分别为6.5 g·h−1、9 000 mg·L−1)相似,且优于O3/PS氧化体系(25 ℃,pH=6,Na2S2O8、O3投加量分别为600 mg·L−1、4 g·h−1)。而O3/PS、O3/PMS和O3/H2O2氧化体系的优化条件下相应的EE/O值分别是662.6、1 007.5、1 233.7 kWh·m−3。
3)综合考虑3种体系各条件下的处理效果与能耗,O3/H2O2氧化体系可能是最适合的工艺。
4) O3/H2O2氧化体系在温度为25 ℃,pH=6,O3、H2O2投加量分别为3 g·h−1、2 125 mg·L−1,反应60 min条件下,TOC去除率和反应速率常数分别可达27.1%和0.005 3 min−1,而EE/O值仅为443.9 kWh·m−3。
5) 3种臭氧催化氧化体系能源效率都不高,其中氧化剂及其投加量以及加热是影响其能耗的重要因素。










 下载:
下载:
 -N
-N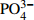 -P
-P











