-
我国马铃薯产量约占全球的26%[1],工业化马铃薯加工以生产淀粉为主,然而却有12%~20%的马铃薯以废水形式排出[2]。马铃薯加工废水的排放量大,有机物、悬浮物、有机氮等污染物浓度高[3],厌氧消化是其常用的生物处理方式。但该处理方式存在2个主要问题:废水的易生物降解性导致厌氧消化过程易遭遇VFAs积累而失败[4];该废水在厌氧消化过程中会产生高浓度氨氮,而高浓度氨氮也会对厌氧消化过程产生不利影响。目前,马铃薯加工废水的厌氧生物处理研究多关注运行参数(如HRT[5]、有机负荷[6]等)对处理效果的影响,但处理过程中运行控制优化[7]、产气模拟优化[8]以及三元pH缓冲调控[9]等也日益得到重视。厌氧消化系统中总氨氮(total nitrogen ammonia, TAN)由铵离子(ionic ammonia nitrogen, IAN)和游离氨(free ammonia nitrogen, FAN)共同构成,而FAN浓度受TAN浓度、pH和温度的影响较大[10]。GALLERT等[11]在研究有机废物中温厌氧消化时发现,当FAN浓度为0.22 g·L−1时,沼气产量减半;LI等[12]的研究则表明,TAN为3.0 g·L−1时会抑制丙酸降解,导致甲烷产率从82.91%降至28.09%。
TAN中的IAN可直接抑制产甲烷酶活性,FAN可被动渗透细胞膜[13],导致质子失衡或钾缺乏,从而引起氨氮抑制[14-15],造成VFAs积累;若无充足缓冲能力则pH降低[16],产气下降,影响系统运行稳定性甚至造成系统崩溃。因此,氨氮抑制的调控是实现高浓度有机废水厌氧消化处理稳定运行的关键之一。尽管厌氧微生物氨氮抑制以FAN或TAN为主尚有争议,但人们普遍认为氨氮对产甲烷古菌的影响大于大多数细菌[17]。虽然TAN是微生物生长必须的营养物质,且有利于提高基质缓冲能力,维持稳定的pH[18];但TAN超过一定浓度可能会对厌氧消化产生不利影响。中温厌氧消化过程中因底物类型、功能微生物种类、运行条件(如温度、pH和离子强度等)、微生物驯化和FAN计算方法等因素的不同,FAN和TAN的半抑制浓度变化范围较大,分别为32~1 450 mg·L−1和1 130~11 780 mg·L−1[19]。厌氧消化处理马铃薯加工废水存在遭遇氨氮抑制的风险,但其氨氮抑制阈值尚不清楚,因此,明确马铃薯加工废水厌氧消化处理过程的氨氮抑制阈值是针对性地制定氨氮抑制调控措施的前提。
本研究通过批式中温厌氧消化实验,考察不同氨氮浓度对马铃薯加工废水厌氧消化过程及产甲烷潜势的影响,研究VFAs、SCOD、蛋白质和多糖等有机物对氨氮浓度的响应,明确氨氮抑制阈值,以期为调控马铃薯加工废水厌氧消化处理过程中的氨氮抑制及马铃薯加工废水高效处理与资源化利用提供参考。
全文HTML
-
实验用厌氧消化接种污泥取自广西某制糖厂内循环厌氧反应罐,取回后,4 ℃下保存。使用前,分别用pH=7.17的磷酸盐缓冲液和自来水各淘洗2次,去除残余基质。实验所用的马铃薯加工废水由去皮马铃薯与自来水按质量比1∶1榨汁,取其上清液。实验原料的主要理化指标如表1所示。
-
本实验采用全自动甲烷潜力测试仪(MultiTalent 203,碧普华瑞环境技术(北京)有限公司Nova SKantek)测定马铃薯加工废水厌氧消化过程中的产甲烷数据,该系统由厌氧消化单元、CO2吸收单元、气体计量单元3个部分构成。厌氧消化单元包括15个配备了全自动内置搅拌系统的650 mL血清瓶(有效容积400 mL)和控温水浴槽;CO2 吸收单元包括15个100 mL血清瓶,装有80 mL 3 mol·L−1的NaOH溶液,吸收沼气中的CO2;气体计量单元配备了全自动微量气体体积计量系统及流量数据即时采集、分析功能。
血清瓶中依次放入168 mL种泥、232 mL马铃薯加工废水,充分混匀后,分别加入0、1.53、3.06、4.59和6.11 g氯化铵(分析纯,国药集团化学试剂有限公司),TAN浓度分别为0、1 000、2 000、3 000和4 000 mg·L−1,并依次命名为A组(对照组)、B组(低浓度氨氮投加组)、C 组(中浓度氨氮投加组)、D组(中高浓度氨氮投加组)和E组(高浓度氨氮投加组)。再次搅匀后,氮气吹脱5 min,置换出血清瓶中残余空气,密封确保严格厌氧环境。反应器搅拌速度设定为80 r·min−1,水浴槽温度控制在(37±1) ℃。每组实验均设置3个平行,实验以厌氧消化反应器的日产甲烷量小于5 mL为终点。
-
在实验的第0、1、4、7、17和41天采集样品,每次用注射器采集5~7 mL实时消化液样品,并在37 ℃左右采用梅特勒FE20型pH计测定其pH;pH测定后,取1 mL样品用于常规指标测定,剩余消化液注射回反应器(过程中严格避免空气混入)。留取样品稀释10倍,再经8 000 r·min−1 离心10 min后,取其上清液过0.45 µm水系聚醚砜滤膜(天津市津腾实验设备有限公司),滤液用来测试其他理化指标,其中溶解性化学需氧量(SCOD)通过试剂盒(2125915,HACH)和DR2800分光光度计(HACH,USA)测定;挥发性脂肪酸(VFAs)浓度使用岛津GC-2010气相色谱仪(Shimadzu,Japan)测定,色谱配备FID检测器及DB-FFAP毛细管柱(30 m×0.32 mm×1.00 µm),色谱进样口温度为220 ℃,检测器温度为250 ℃。TSS、VSS根据标准分析方法[20]测定,TAN采用纳氏试剂法进行测定,正磷酸盐采用钼锑抗分光光度法测得,碱度(ALK)通过试剂盒(TNT870,HACH)测定,溶解性蛋白质和多糖分别用分光光度法[21-22]测定。
FAN浓度[23]按照式(1)进行计算。
式中:CFAN为FAN浓度,mg·L−1;CTAN为TAN浓度,mg·L−1;T为厌氧消化反应温度,℃;pH为样品刚采集时的实测pH。
假设厌氧消化过程中有机物降解符合一级反应动力学,累计产甲烷量计算方法[24]见式(2)。
式中:Mt为批式实验在t 时的累计产甲烷量,mL·g−1;M0为产甲烷潜势,mL·g−1;K为产甲烷速率常数,d−1;t为时间,d。
Gompertz 修正模型广泛应用于厌氧消化过程的拟合研究,可用来评价厌氧消化效率和氨氮抑制效果[25],其中停滞时间λ是重要参数之一。模型方程式[26]见式(3)。
式中:Pt为批式实验在t 时的累积产甲烷量(以COD计),mL·g−1;Pm为产甲烷潜势(以COD计),mL·g−1;Rmax为最大日产甲烷速率(以COD计),mL·(g·d)−1;λ为产甲烷停滞时间,d;t为时间,d;e = 2.72。
根据WAN等[27]和CHEN等[28]的理论数据,基于如下参数计算COD值:1 g乙酸约为1.07 g COD,1 g丙酸约为1.51 g COD,1 g丁酸约为1.81 g COD,1 g戊酸约为2.04 g COD,1 g多糖约为1.06 g COD,1 g蛋白质约为1.50 g COD。
-
对照组和各实验组的FAN浓度、TAN浓度及pH用IBM SPSS Statistics 25进行双变量Spearman相关分析,并对结果进行双尾t检验,置信区间α=0.05。
1.1. 实验原料
1.2. 实验装置及方法
1.3. 分析方法
1.4. 统计分析
-
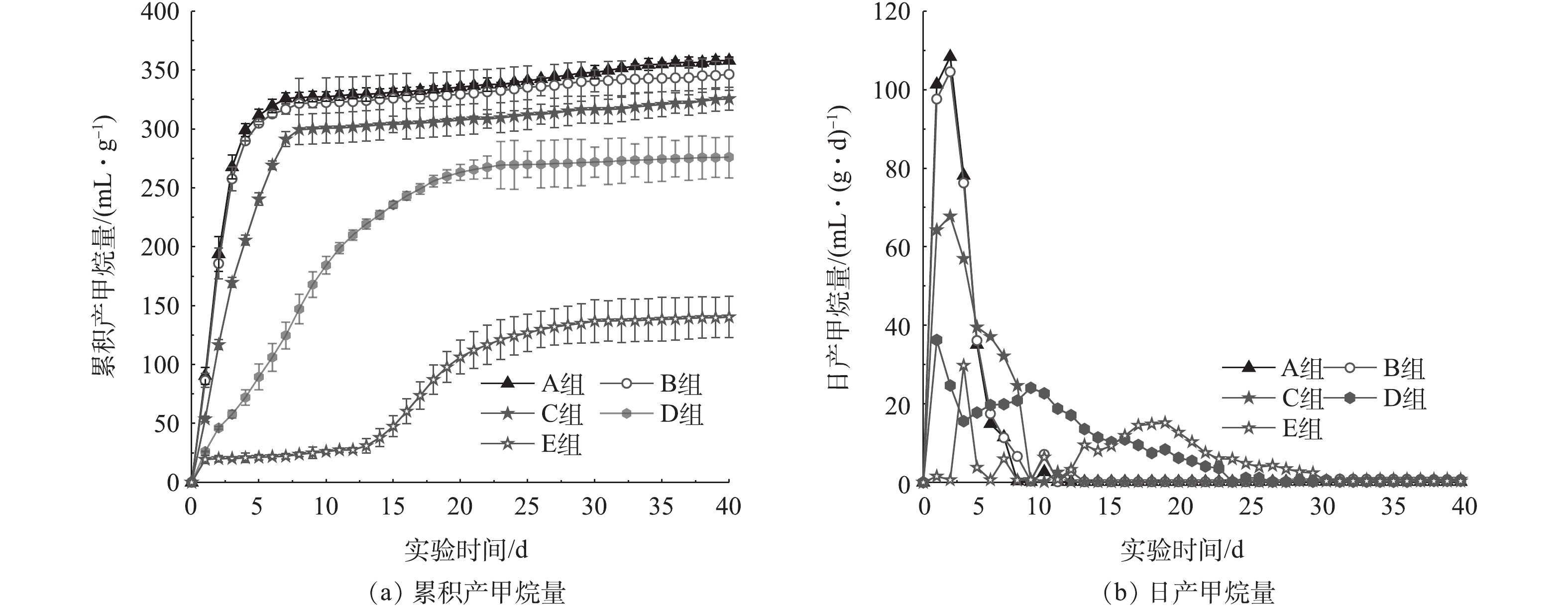
不同氨氮浓度下累积产甲烷量及日产甲烷量的变化如图1所示。由图1(a)可知,与对照组(A组)相比,B组累积产甲烷量变化不明显,表明1 000 mg·L−1以下的氨氮浓度未对马铃薯加工废水厌氧消化的产气量产生不利影响。C组的累积产甲烷量比A组低9.0%,表明该氨氮浓度(2 000 mg·L−1)对厌氧消化产甲烷产生了轻度抑制作用,该结果与孟晓山等[25]的研究结果相似。3 000 mg·L−1氨氮浓度导致D组的累积产甲烷量比对照组低22.9%,且其产甲烷相对滞后,表明氨氮抑制程度进一步增大。当氨氮浓度达到4 000 mg·L−1(E组)时,其累积产甲烷量降比对照组低60.8%,抑制率超过50%,说明厌氧消化过程发生了明显抑制。结束时,基于初始COD值计算的各组甲烷产率分别为358.07、345.47、325.72、276.06和138.41 mL·g−1。
图1(b)为不同氨氮浓度下日产甲烷量曲线,可在一定程度上体现产甲烷菌的活性。A(对照)、B 2组的氨氮并未对产甲烷构成抑制,日产甲烷速率在第2 天分别达到最大值108.40 mL·(g·d)−1和104.57 mL·(g·d)−1。但随着底物的不断消耗,日产甲烷量逐渐降低,并于第11 天左右停止产甲烷。随着氨氮浓度的提高,日产甲烷量降低,其峰值出现时间逐渐滞后,且C、D 2组的峰值分别为对照组的62.5%和33.5%,而E组的峰值仅为对照组的27.5%。由图1(b)明显可见,E组出现约7 d的产甲烷停滞期,表明氨氮抑制不仅影响产甲烷速率,且延长了产甲烷周期。ZHANG等[29]的研究表明,不同的产甲烷峰值对应不同底物的降解,通常第1个产气高峰对应于溶解性有机质的快速酸化与降解,而第2个产甲烷峰值主要对应于粗蛋白、粗脂肪和粗纤维等大分子难溶性有机物的水解、酸化和降解。这是因为反应开始时,马铃薯加工废水中易降解有机物快速分解,氨氮浓度的增加抑制了产甲烷菌活性,降低了产甲烷速率,推迟产甲烷高峰的到来;在不溶性有机物被细菌水解酸化成VFAs的同时,产甲烷菌逐渐适应高氨氮浓度,开始缓慢利用VFAs产甲烷[25]。
由上述结果可知,随着氨氮浓度的增加,产甲烷过程受到了不同程度的抑制。产甲烷模型拟合结果表明,Gompertz修正模型的模拟结果优于一级动力学模型(表2)。马铃薯加工废水的产甲烷潜势Pm随氨氮浓度的升高而逐渐减小,实验组比对照组分别下降2.20%、8.20%、19.10%和56.50%,同实测结果的下降比例相近;产甲烷速率的变化趋势与产甲烷潜势相似,产甲烷迟滞期随氨氮抑制程度的增加而不断增大,抑制最强的E组迟滞期是D组的约14倍,是对照组的50多倍,该模拟结果为量化氨氮浓度对产甲烷过程的抑制程度提供了参考。
-
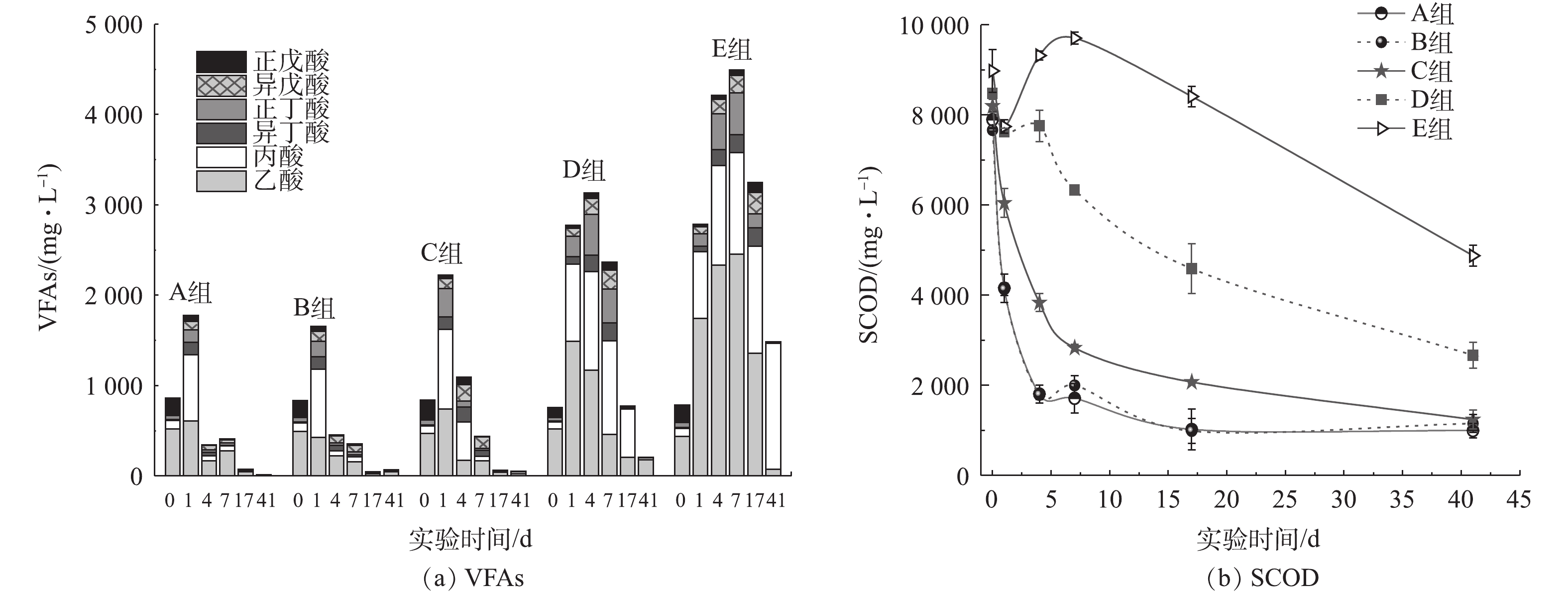
VFAS在厌氧消化复杂的系列反应中充当承上启下的角色,既是有机物水解酸化的产物,同时也是产甲烷菌所利用的底物。VFAs浓度可反映水解酸化和产甲烷2个过程之间的平衡,是评价厌氧消化过程运行稳定性的重要参数[30-31]。由图2(a)可见,与A组相比,B、C 2组的VFAs无明显变化,且反应开始后,乙酸和丙酸快速降解,构成了产甲烷的第1个高峰期,表明氨氮浓度低于2 000 mg·L−1对厌氧消化过程中VFAs降解无不利影响。随着反应的进行,A、B、C 3组的丙酸逐渐被互营型产乙酸菌分解为氢和乙酸,导致第7天乙酸成为总VFAs的主要组分,随着这部分酸被产甲烷菌进一步利用,形成了第2个产甲烷高峰。由于氨氮浓度升高,产甲烷菌比产酸菌对氨氮抑制更敏感[32],打破了体系内产酸与耗酸之间的平衡,产酸菌不断产酸,而产甲烷菌对酸的消耗速率降低,造成VFAs浓度不断提高,D、E 2组总VFAs浓度在第4 天分别达到3 129 mg·L−1和4 209 mg·L−1,VFAs明显积累,这也是公认的除产气速率下降、产气量降低之外氨氮抑制的另一显著特征[33]。在实际的厌氧消化工艺运行中,氨氮抑制会导致VFAs积累,当VFAs值大于4 000 mg·L−1时,应采取积极措施预防氨氮抑制的发生。乙酸转化速率(0.06 h−1)高于丙酸(0.03 h−1)[34],氨氮抑制促使丙酸积累量高于乙酸,D、E 2组趋势相似。E组的VFAs最高积累量达4 494 mg·L−1(约占总SCOD的62.3%),且积累持续时间较长(27 d左右)。实验结束时,高氨氮组仍有约1 500 mg·L−1VFAs(约占残留总SCOD的45.4%)未被产甲烷菌利用,其中丙酸(1 392 mg·L−1)占94.0%,约为2 265 mg·L−1 SCOD。
在厌氧消化过程中,丙酸通常被厌氧微生物代谢转化为乙酸和氢气。在20世纪80年代后期,HILL等[35]提出,以丙酸与乙酸比值作为厌氧消化系统警戒指示因子,并通过实验证实当该比值大于1.4时,厌氧消化系统即将失稳。实验结束时,E组的丙酸与乙酸比值高达26.78,也证实了乙酸被降解后,丙酸并未被充分利用而发生积累。不同程度的丙酸积累也出现在猪粪厌氧消化[25]、餐厨垃圾厌氧消化[36]等系统。本研究中E组氨氮敏感性强的乙酸型产甲烷菌活性受到影响,使乙酸代谢成为限速步骤,之后在第4~7 天,乙酸积累影响上游脂肪酸的转化,进而诱发丙酸积累,此为系统失稳的主要过程。李蕾[37]在研究餐厨垃圾厌氧消化时也发现了类似情况。
SCOD降解情况与厌氧消化程度密切相关。由图2(b)可知,A、B 2组的SCOD在初始阶段降解迅速,且在第17 天达到恒定值,这再次证明1 000 mg·L−1的氨氮浓度对有机物厌氧降解无不利影响。C组初始阶段SCOD降解速率低于A、B 2组,相应地,其产气量、产气速率在前7 d均低于A、B2组(图1)。厌氧消化结束后,E组的残留SCOD浓度(4 877 mg·L−1)高于D组(2 667 mg·L−1),远高于A、B、C组,且D、E 2组的SCOD去除率分别比对照组低21.6%和47.8%,这也表明其有机物降解不完全,并直接导致产气量降低。E组SCOD浓度在经历初始24 h的降解后,呈不断增加的趋势直至第7 天,这可能是由于VFAs的积累和蛋白质浓度(图3(a))以及微生物菌体死亡溶胞释放的溶解性有机物所造成的;自第7 天之后,随着产甲烷菌对高氨氮浓度的逐渐适应,其对VFAs的不断消耗促成了E组SCOD浓度的连续下降(图2(a)),使得产气量缓慢增加(图1(a))。由图2可见,实验结束后,D、E 2组的残留SCOD浓度与VFAs浓度差值仍远大于A、B、C组,表明除了难降解的COD外,其中还可能包含与水解酸化相关的胞外酶、蛋白质和多糖等溶解性微生物产物等[38]。
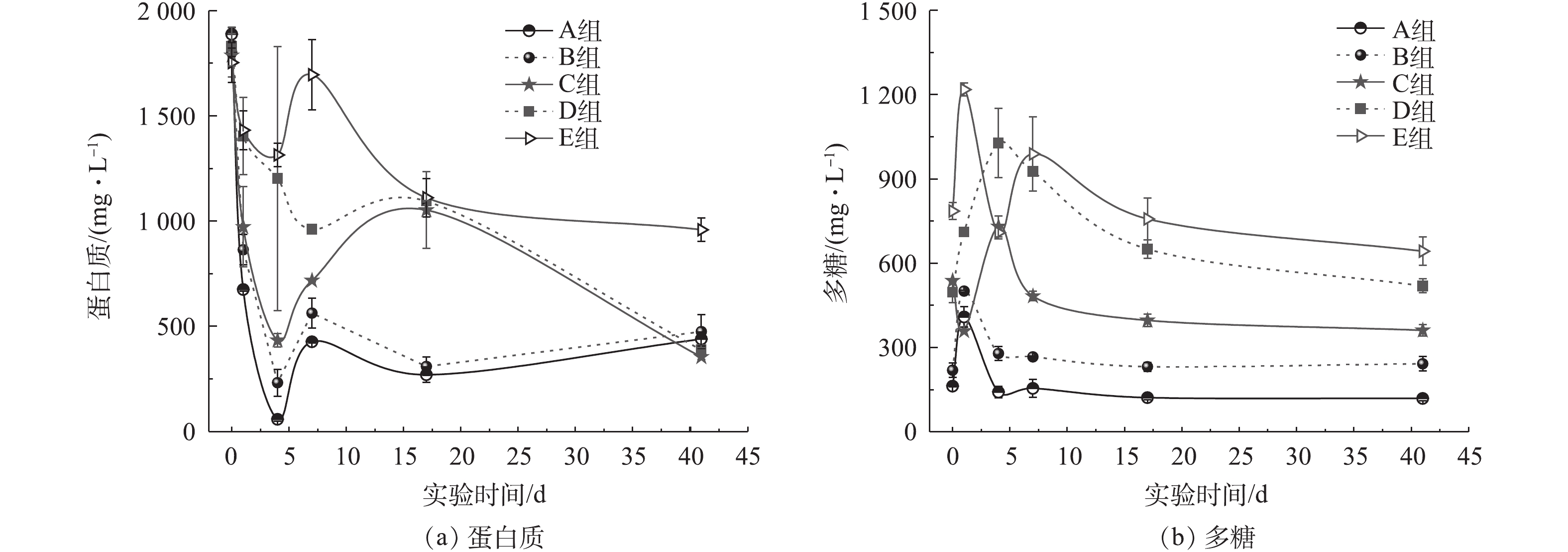
图3分别为不同氨氮浓度下马铃薯加工废水厌氧消化过程中的蛋白质和多糖浓度变化曲线。蛋白质浓度经历了先快速下降、再上升,最后再缓慢下降的过程(图3(a))。反应开始时,溶解性蛋白质经水解过程形成氨氮和小分子有机物,蛋白质浓度降低;随着水解酸化的进行,颗粒态不溶性大分子蛋白质逐步分解为溶解性蛋白质、氨基酸等而被检出,此外,细菌为水解酸化而向胞外释放的酶蛋白也会使蛋白质浓度升高;新增的溶解性蛋白质逐渐被降解,导致蛋白质浓度再次缓慢降低。反应结束时,E组的残余蛋白质浓度为959 mg·L−1,折合成COD约为1 439 mg·L−1,去除率(45.30%)仅为对照组(76.70%)的59.10%。残余蛋白质浓度增高,在一定程度上降低了SCOD的去除率。高浓度氨氮对厌氧消化过程中蛋白质降解的影响,主要通过TAN直接抑制蛋白质降解或抑制蛋白质降解的主要中间产物氨基酸的脱氨作用来实现。詹瑜等[39]在污泥高含固厌氧消化过程中蛋白质的转化研究中发现,1 201 mg·L−1的TAN会抑制厌氧消化过程,尤其是蛋白质降解。PARK等[40] 通过BMP实验考察了氨氮浓度对氨基酸废水厌氧消化过程的影响,结果表明氨氮会抑制脱氨作用。
由图3(b)可见,B组多糖的变化趋势与A组相似,即初始阶段快速释放,再逐渐被降解。而C、D 2组多糖的释放和降解无明显的变化规律,但随着氨氮浓度的增加,多糖释放峰值的出现时间相对更滞后,且降解更不完全。反应结束后,E组的残余多糖浓度为750 mg·L−1,折合成COD的浓度约为795 mg·L−1。A组多糖去除率为26.90%,而C组和E组的多糖去除率分别为32.80%和4.60%,该结果与WANG等[41]的剩余污泥厌氧消化研究结果趋势相似。氨氮抑制作用造成微生物死亡,从而引起有机组分浓度增加,这是厌氧消化过程中蛋白质或多糖浓度增加的原因。
由于蛋白质代谢产物多为乙酸、丁酸和戊酸,而多糖发酵的产物以乙酸、丙酸等小分子有机酸及二氧化碳(部分以碳酸根、碳酸氢根的形式存在)[42]为主,抑制蛋白质和多糖的降解使得体系中有机物不能被充分利用,SCOD去除率低,如E组残余VFAs、蛋白质和多糖分别占SCOD的46.40%、30.80%和17.20%。由于产甲烷菌活性受到抑制,无法充分利用VFAs进行产甲烷,最终降低了产气量。厌氧消化结束时,A、B 2组的残余蛋白质和多糖主要为难降解组分,不易被厌氧微生物利用[43],而E组的残余蛋白质和多糖浓度分别比对照组高780 mg·L−1和550 mg·L−1,该部分则是因氨氮抑制对有机物降解的不利影响产生的。对比蛋白质和多糖在厌氧消化过程中的降解数据,可见蛋白质降解率高于多糖,其先于多糖而被降解,且最终蛋白质去除率高于多糖。
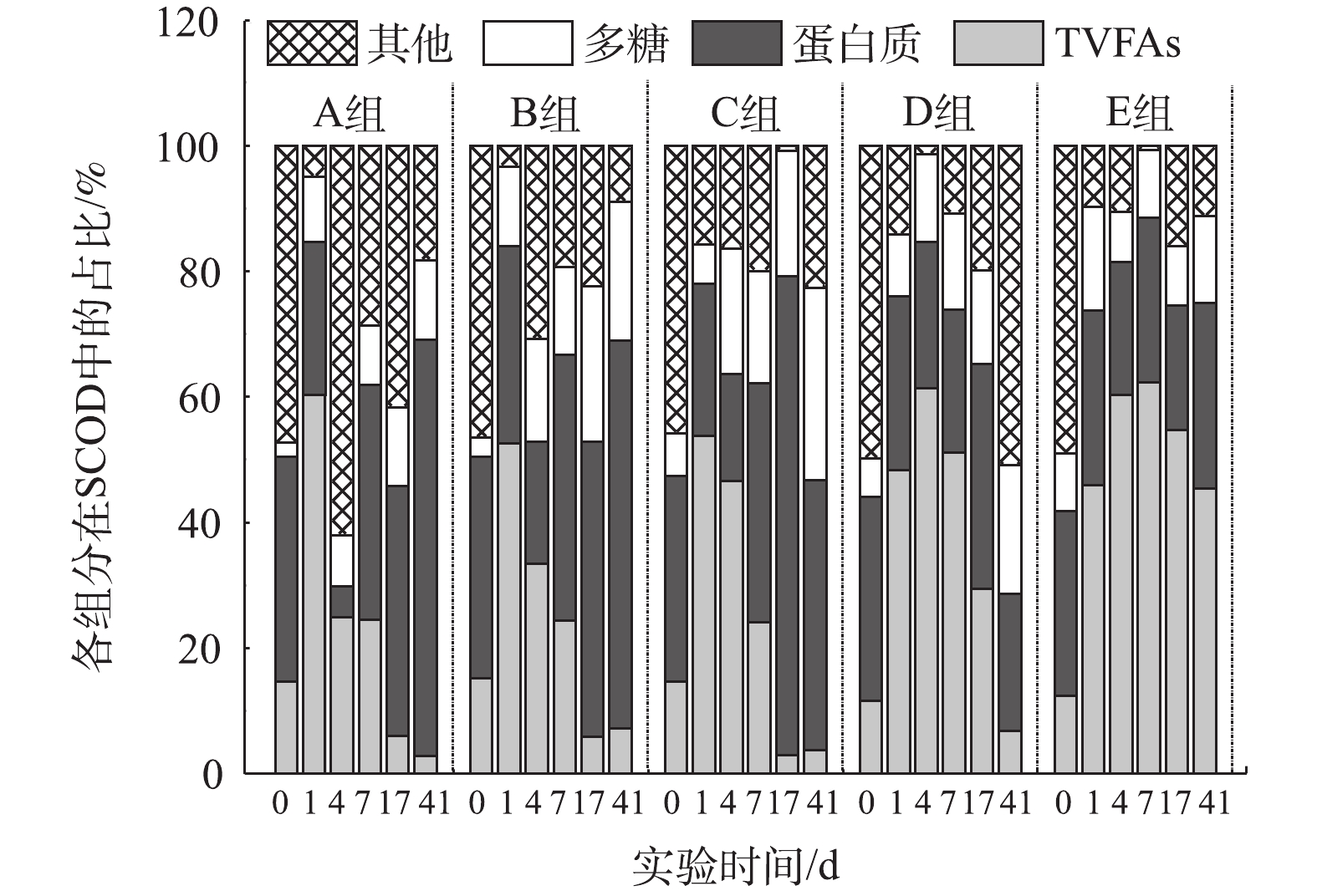
为考察氨氮浓度对马铃薯加工废水厌氧消化过程中不同有机组分的降解情况,本研究对SCOD进行了简化的质量平衡解析(图4)。反应开始时,所有组别中其他组分(未知溶解性化合物)的占比最高,随着反应的不断进行,A、B、C 3组在反应结束时的蛋白质占SCOD比例最高,而D、E 2组分别以其他组分和总VFAs(TVFAs)占比最高。这表明氨氮抑制造成未知溶解性化合物和VFAs积累,使有机物降解不完全。已有研究[44-46]表明,氨氮浓度过高,会直接抑制微生物活性,最终导致厌氧消化的失败,TAN通过抑制乙酸型产甲烷菌活性,破坏产甲烷途径,导致丙酸等中间产物积累,进而影响总体产气效果。抑制所导致的中间产物TVFAs积累亦会导致反应体系酸碱失调,系统因酸化而崩溃。本研究中E组丙酸/乙酸之比异常高则证明其厌氧消化已因酸化而崩溃(图2(a))。
-
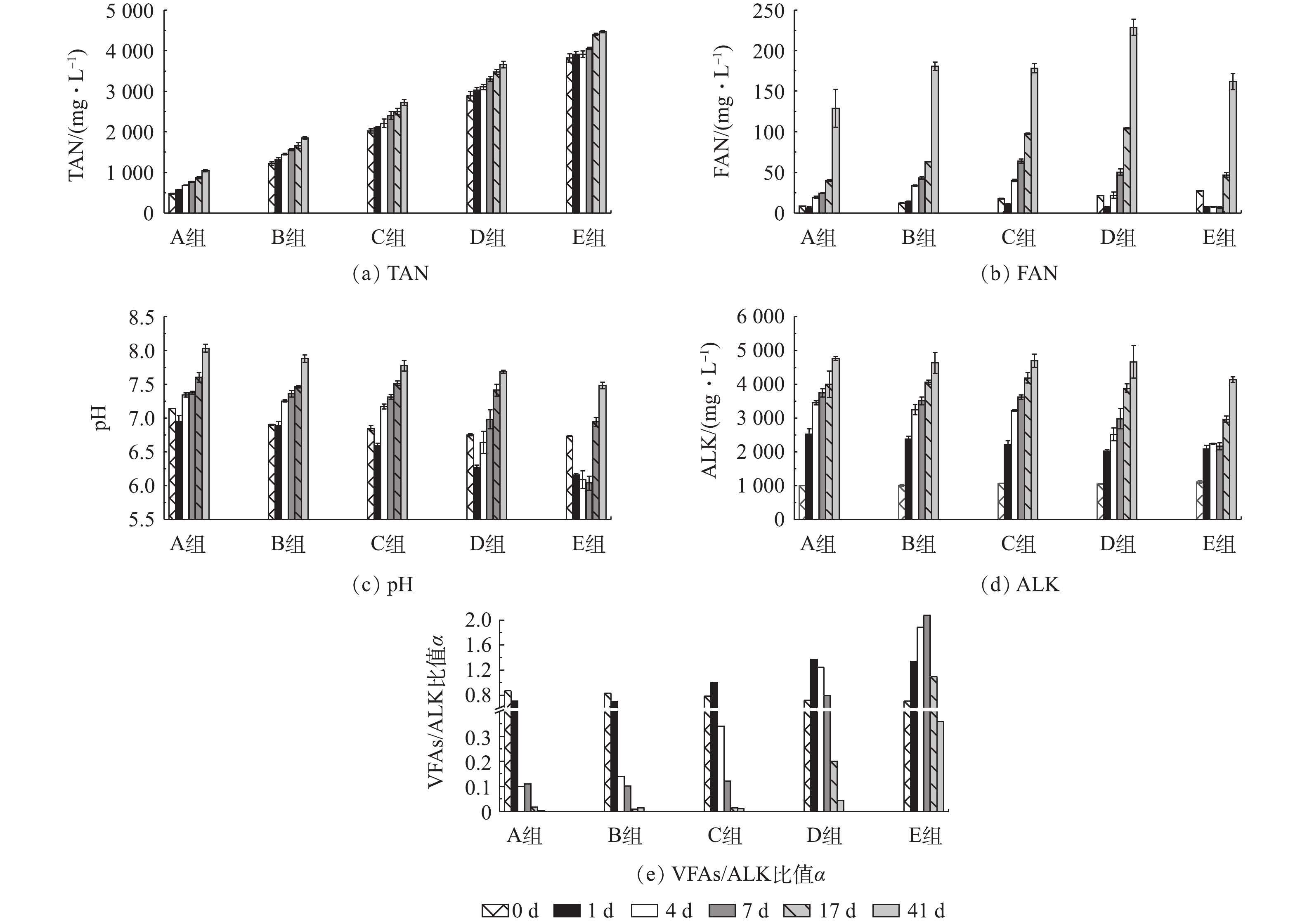
pH和ALK是厌氧消化过程的重要理化参数,可反映厌氧消化运行的稳定性。图5分别展示了厌氧消化过程中TAN、FAN、pH及VFAs/ALK比值变化情况。由图5(a)可见,TAN浓度在A~D组中呈现连续上升趋势,而E组中仅在第7~17 天之间上升趋势明显,各组TAN浓度的绝对增量分别为569.90、628.70、707.30、779.30和649.30 mg·L−1,其增加率分别为120%、52%、35%、27%和17%。A~D组的TAN浓度增加量逐步提高,而D和E组则呈现相反趋势,究其原因,可能是高浓度氨氮抑制了蛋白质分解,降低了有机物释放的氨氮量。E组氨氮抑制造成蛋白质降解不完全,去除率仅为45%(图3(a)),氨氮释放量降低,导致E组的TAN浓度增加量低于D组(图5(a))。
由于本研究厌氧消化反应温度稳定控制在(37±1) ℃,由式(1)可见,其浓度值受TAN浓度变化和pH波动的影响。如图5(b)所示,所有实验组的初始FAN值均高于对照组,随着厌氧消化的进行,A组和B组的FAN浓度不断增加,但C组和D组的FAN浓度呈现先降低再升高的趋势,E组的FAN浓度在初期较低,随后不断升高,但其最终浓度仍低于另外3个实验组。本研究对实验组和对照组氨氮、pH进行Spearman分析并进行双尾t检验,结果见表3。除式(1)外,HANSEN等[47]在对猪粪厌氧消化的氨氮抑制研究中提出FAN浓度计算方法,见式(4)。
由式(4)可知,在温度T一定的条件下,FAN占TAN的比例随pH的升高而增大。因此,在氨氮浓度高的厌氧消化体系中,应重视高pH引起的高FAN抑制。由表3可见,仅高氨氮浓度的E组FAN浓度显著受pH波动影响,而图5(c)中E组在第1~7 天的pH均低于6.2;尽管该组的TAN浓度最高,但TAN变化对FAN的影响不显著(P=0.329),这是导致FAN浓度低的主要原因。表3中A~D组FAN受TAN和pH双重作用的影响,其显著性水平P值均≤0.05。E组,前期受TAN抑制导致产甲烷量较低,后期随着VFAs的不断消耗,pH升高(图5(c)),使得FAN浓度增加(图5(b)),再次抑制厌氧消化过程,主要表现为丙酸积累(图2(a)),可能的原因为FAN抑制互营丙酸降解菌而中断反应[25]。由图5(d)可见,随着TAN增加量的提高,所有组别的ALK均呈现不断增加的趋势,但均低于ALBERTSON等[48]报道的ALK抑制阈值6 500 mg·L−1,且E组由于VFAs积累,其ALK较其他4组略低,推测所有实验组的缓冲能力充足,ALK并非造成厌氧消化抑制的主要原因。VFAs/ALK比值α表示基质的缓冲能力,也被视为反应器稳定性因子[49],ZICKFOOSE等[50]建议,将0.1<α<0.35作为厌氧消化系统的稳定范围,而LI等[51]则认为α<0.3为稳定系统。如图5(e)所示,当厌氧消化结束后,仅E组α值大于0.3,表明该组厌氧消化系统发生酸化,稳定性被破坏。
2.1. 氨氮浓度对产甲烷效果的影响
2.2. 氨氮浓度对有机物降解的影响
2.3. 水质参数及氮化合物的变化
-
1)氨氮浓度低于2 000 mg·L−1时,其对马铃薯加工废水厌氧消化性能无明显不利影响。当氨氮浓度为4 000 mg·L−1时,产甲烷受到明显抑制,累积产甲烷量比对照组下降60.80%,日产甲烷速率仅为对照组的27.50%,产气迟滞期比对照组延长了7.2 d。这些结果表明,马铃薯加工废水厌氧消化处理的氨氮抑制阈值约为3 000 mg·L−1。
2)马铃薯加工废水厌氧消化过程中,氨氮浓度的增加主要导致以丙酸为主的VFAs积累、蛋白质降解率降低,进而造成产气速率降低、产气滞后、总产气量下降。
3) VFAs浓度可作为马铃薯加工废水厌氧消化系统的稳定性判别因子,当其值大于4 000 mg·L−1时,则系统稳定性遭到破坏,须采取措施保证厌氧消化的正常进行。



 下载:
下载: