-
随着现代印染工业的发展,染料废水的经济、高效处理愈发重要。亚甲基蓝(MB),化学名为氯化3,7-双(二甲氨基)噻吩嗪-5-鎓,常用于棉、麻、蚕丝织物、纸张的染色和竹、木等的着色,是印染废水中具有代表性的阳离子有机污染物之一。目前,对MB废水的处理方法主要有化学絮凝、微生物降解、离子交换、膜分离、电化学/光催化氧化及吸附法等[1-5]。其中吸附法由于操作简单、处理效率高,被认为是染料废水处理中最有效的方法[6]。吸附法中常用的吸附剂主要有碳纳米管[7]、黏土矿物[8]、生物质碳[9]及各类合成聚合物[10-13]等,尤其是合成的多孔聚合物材料由于其具有高比表面积、高孔隙率和高渗透性等特点,且对污染物的吸附速率快、饱和吸附量高、易回收再利用等而被广泛关注[14-15]。多孔聚合物的制备途径有多种,按成孔机制主要有冷冻干燥法、相分离法、模板法、生孔剂法等[16],其中Pickering乳液模板法因可方便调节模板种类、大小、形状等来控制制备材料的孔结构和形貌,而被认为是制备多孔聚合物最简便有效的方法[17-18]。已被用于稳定Pickering乳液做模板制备多孔材料的天然高分子主要有纤维素[19]、淀粉[20]、蛋白质[21]、果胶[22]、细菌[23]等。LI等[24]首次使用牛血清蛋白(BSA)的纳米胶体粒子稳定的O/W型Pickering乳液为模板,化学交联BSA粒子,制备了多层次的纯蛋白质多孔支架材料。CAPRON等[25]利用纤维素纳米晶的胶体粒子制备了水包十六烷的Pickering乳液,冷冻干燥下除去水相和油相,得到了多孔纤维素泡沫。LIU等[26]以壳聚糖胶体粒子稳定的Pickeirng乳液为模板,制备了纯的壳聚糖多孔洞结构的支架材料,研究了其对Cu2+的吸附能力,发现支架材料中的孔洞可以提供更多吸附空间和吸附位点,提高吸附能力。ZHU等[27]也以羟丙基纤维素大分子胶体溶液稳定的Pickering乳液为模板,自由基引发聚合制得多孔水凝胶,并将其用于废水中Rb+和Cs+的去除,其最高平衡吸附量可达232.5 mg·g−1和239.9 mg·g−1。
小麦麸质蛋白(wheat gluten,WG)是小麦淀粉加工的副产物,是多形化的多肽混合物,主要由醇溶蛋白和麦谷蛋白组成,其分子间的非共价键(如氢键、离子键与疏水键等)可以促进醇溶蛋白与麦谷蛋白团聚,影响WG的结构与物理性质。WG具有大量的活性基团,可以通过物理、化学和生物等方法进行各种改性,制备出许多具备优良性能和多种功能的新型材料,如包装材料、医用材料,食品替代材料等[28-29]。LIU等[30]用乳化-溶剂蒸发法成功制备了WG稳定的高内相乳液,可用于食品中替代蛋黄酱。CHIOU等[31]利用柠檬酸交联WG制备水凝胶,但此类凝胶机械强度低,吸附量小。本研究以WG中醇溶蛋白稳定的O/W型Pickering乳液滴为孔模板,WG与丙烯酸(AA)原位自由基接枝聚合,制备多孔小麦麸质蛋白-g-聚丙烯酸钠(WG-g-PNaA)水凝胶,以改善纯WG凝胶作为吸附材料的机械强度低和吸附容量小的缺点,重点考察了其对水体中亚甲基蓝(MB)的吸附和去除性能,以期为拓展WG在印染废水处理方面的应用提供参考。
全文HTML
-
小麦麸质蛋白(WG,蛋白含量75%~80%)购自美国鲍勃红磨坊天然食品公司,丙烯酸(AA,分析纯)购自天津市凯信化学工业有限公司,过硫酸铵(APS,分析纯)和N,N′-亚甲基双丙烯酰胺(MBA,化学纯)购自阿拉丁试剂有限公司,液体石蜡购自天津市百世化工有限公司,亚甲基兰(MB,分析纯)购自天津市凯通化学试剂有限公司。其他试剂均为分析纯,所有溶液均用去离子水配制。
-
电子微量天平(精度为0.001 mg,Sartorius BP211D型,瑞士);电热恒温鼓风干燥箱(GZX-GF 101-1-BSP-/H型,上海跃进医疗器械有限公司);电动搅拌机(D60-型,4 000 r·min−1,杭州仪表电机厂);恒温振荡器(SHA-B型,常州国华电器有限公司);pH计(Mettler Toledo 320型,梅特勒-托利多上海有限公司);傅里叶红外光谱仪(FT-IR,Thermo Nicolet NEXUS TM Spectrophotometer型,美国);场发射扫描电子显微镜(FESEM,JSM-6701F型),日本精工(JEOL),测试前样品经喷金处理;BET吸附仪(3H-2000PS4型,贝士德仪器科技(北京)有限公司);紫外可见分光光度计(UV-vis,北京莱伯泰科原仪器有限公司)。
-
称取WG 2.00 g,置于100 mL烧杯中,加入V水∶V乙醇=2∶1的混合液24 mL后,充分搅拌,制得WG悬浮液。在此悬浮液中加入中和度为65%的AA 3.6 g,再补加水,使溶液总体积为30 mL。高速搅拌下,逐滴加入一定体积的石蜡油,形成WG稳定的O/W型Pickering乳液。室温静置12 h,乳液不分层。将此乳液转入一连接有搅拌棒、滴液漏斗、N2导管和回流冷凝管的四口瓶中,通入N2,以去除反应体系中O2。体系升温至60 ℃,加入含0.1 g APS的水溶液1 mL,恒温0.5 h后,加入0.018 g MBA。观察四口瓶内反应物的状态,待瓶内的乳液成胶团后,停止搅拌,N2氛中继续恒温3 h,反应结束后,取出合成的凝胶,置于甲醇中浸泡,洗除凝胶网络中的石蜡油,常压恒温下干燥,研碎,过40~80目网筛,备用。此凝胶标记为HA。
无Pickeirng乳液致孔的WG-g-PNaA水凝胶制备和处理方法同上,只是在反应中不需要加入石蜡油。此凝胶标记为HB。
-
在150 mL锥形瓶中,加入0.05 g多孔WG-g-PNaA干凝胶和浓度分别为10、50、100、300、500、1 000 mg·L−1的MB溶液25 mL,用0.1 mol·L−1 HCl (NaOH)调节pH为2~10,于25 ℃下以150 r·min−1振荡3 h。吸附后,使用紫外分光光度计在665 nm(最大吸收波长)下测定吸光度,从而计算吸附平衡时溶液中MB的剩余浓度Ce,根据式(1)和式(2)分别计算平衡吸附量和去除率。
式中:qe为平衡吸附量,mg·g−1;R为去除率;C0和Ce分别为MB的初始浓度和吸附平衡时的浓度,mg·L−1;V为溶液体积,L;m为吸附剂用量,g。
等温动力学吸附和等温热力学吸附实验的测定方法同上。取300 mg·L−1的MB溶液,调节pH至9.0,吸附时间为1~240 min,吸附温度分别为25、35、45 ℃,相应条件的吸附量根据式(1)进行计算。
等温热力学吸附过程用Langmuir和Frenudlich吸附模型进行拟合,模型方程[32]分别如式(3)和式(4)所示。
式中:qe为平衡吸附容量,mg·g−1;qm为饱和吸附容量,mg·g−1;KL为Langmuir吸附平衡常数,L·mg−1;RL是Langmuir吸附等温线的一个重要参数,0<RL<1,有利吸附;RL>1,不利吸附;RL=1,为线性吸附;RL=0,吸附过程为不可逆;KF为Freundlich吸附容量的参数,L·g−1;1/n是吸附强度或表面不均匀性量度。
等温动力学吸附过程分别采用准二级吸附动力学模型(式(6))和粒子内扩散模型(式(7))进行拟合[32]。
式中:qt和qe分别为t时刻和吸附平衡时的吸附容量,mg·g−1;k2为准二级动力学方程的速率常数,g·(mg·min)−1;kid为粒子内扩散速率常数;t为吸附时间,min;c为边界层的厚度。
-
称取吸附MB后的WG-g-PNaA水凝胶0.10 g,加入100 mL浓度为0.1 mol·L−1的HCl溶液中,在室温下,恒温振荡3 h (150 r·min−1),完成脱附。脱除率根据式(2)计算,将C0和Ce换为q0和qe,分别为脱除前吸附于凝胶上的MB量和脱附平衡后残留在凝胶上的MB量。
1.1. 材料
1.2. 分析测试仪器
1.3. 多孔WG-g-PNaA水凝胶的制备
1.4. 多孔WG-g-PNaA水凝胶对MB的吸附性能测试
1.5. 脱附实验
-
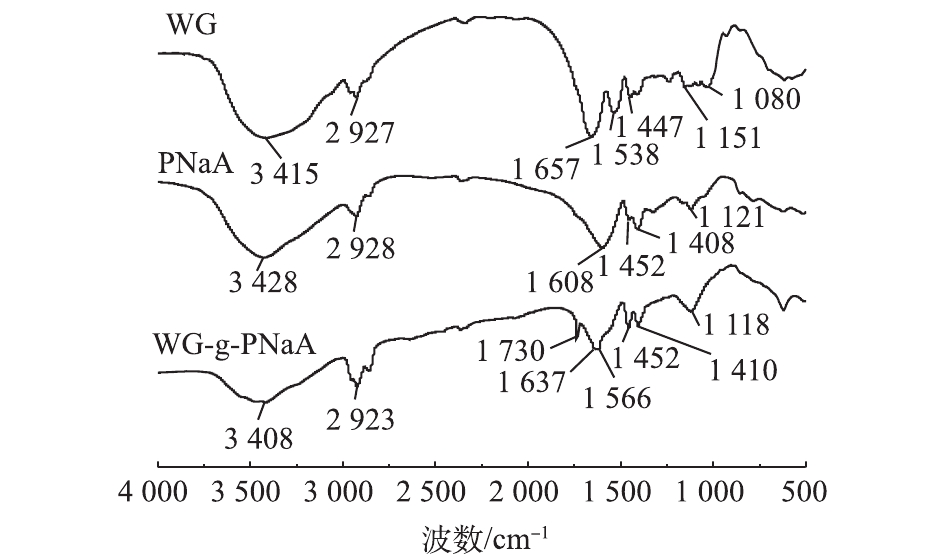
WG稳定的Pickering乳液模板多孔WG-g-PNaA水凝胶的形成过程如图1所示。WG中醇溶蛋白包裹加入的石蜡油使其均匀地分散于AA水相中,形成稳定的O/W型乳液[30]。在此体系中,APS热解产生
${\rm{SO}}_4^ - $ ·自由基,从而引发WG中麦谷蛋白等链上的—NH2、—OH与部分中和的AA烯键接枝聚合,同时交联剂MBA以其末端双乙烯基也参与聚合交联,形成将石蜡油包裹其内的三维网络体系。在反应结束后,洗涤除去作为孔模板的Pickering乳液滴,即得多孔WG-g-PNaA水凝胶。图2为WG、PNaA凝胶和WG-g-PNaA水凝胶的FT-IR谱图。比较WG和WG-g-PNaA水凝胶的光谱可知,1 657 cm−1和1 538 cm−1处对应的WG链上酰胺(I)和酰胺(II)的特征吸收峰在与丙烯酸—COOH接枝聚合后,位移至1 637 cm−1和1 566 cm−1处;而WG-g-PNaA凝胶谱图中1 452 cm−1和1 410 cm−1处的PNaA链上羧基的对称伸缩振动吸收峰,与纯PNaA凝胶上吸收峰位置一致;最后,在WG-g-PNaA水凝胶谱图中,1 730 cm−1处新出现的吸收峰为WG链上—OH与丙烯酸—COOH成酯接枝的羧酸酯C=O伸缩振动吸收峰。上述信息与此前报道的以酵母做致孔剂合成的多孔WG-g-PNaA水凝胶FT-IR谱图信息[33]一致。
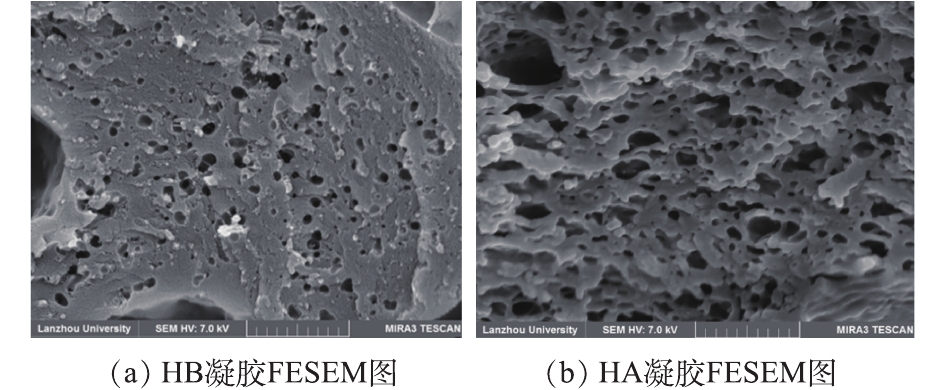
图3为HA和HB干凝胶表面形貌的FESEM图。由图3(b)可清楚地看到,相对于无Pickering乳液的WG-g-PNaA水凝胶网络体系(图3(a)),由WG醇溶蛋白做乳化剂的丙烯酸水相包裹石蜡油的Pickering乳液滴,在丙烯酸与WG链接枝聚合及与MBA交联时被包裹在其中,反应结束后除去石蜡油滴,在WG-g-PNaA凝胶三维网络中留下较为规整的贯穿网孔结构,此贯穿孔有利于吸附质传质扩散,从而提高吸附分离速率及效率。
为进一步验证Pickering乳液滴在WG-g-PNaA凝胶网络中的致孔作用,分别测定了HA和HB干凝胶的BET比表面积和平均孔径。HA的比表面积为18.04 m2·g−1,相对于HB样(8.16 m2·g−1)增大了2.2倍;HA的平均孔径为200~210 nm,HB的平均孔径为160~180 nm,此BET比表面积和平均孔径数据与FESEM呈现的凝胶网孔形貌结果一致,即Pickering乳液滴可作为有效致孔模板,大幅提高WG-g-PNaA水凝胶的BET比表面积和平均孔径。
-
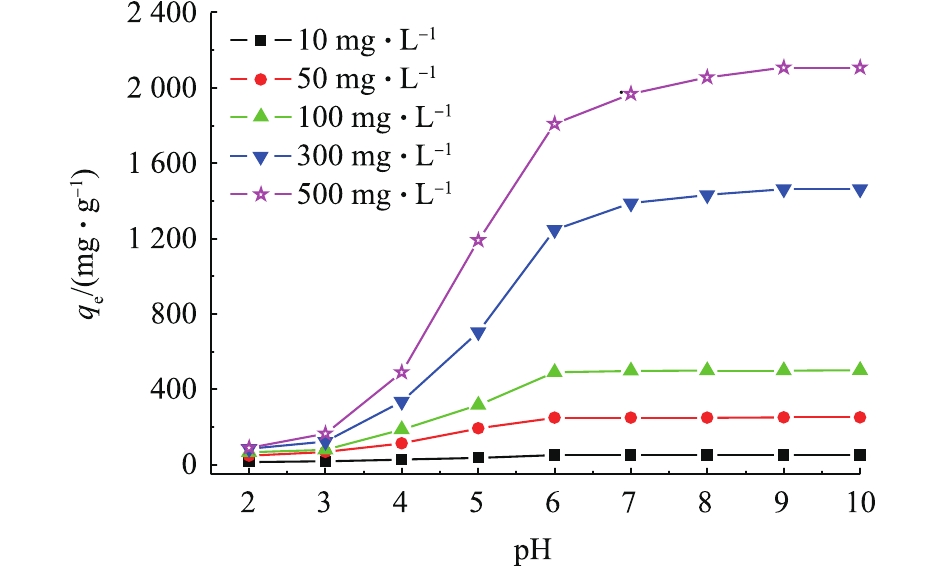
图4为pH=2~10时,多孔WG-g-PNaA水凝胶对不同起始浓度MB溶液的吸附曲线。可以看出,WG-g-PNaA水凝胶对MB的平衡吸附量qe随溶液pH的增大而逐渐增大,C0为10、50、100 mg·L−1的MB溶液,当溶液pH=6时,qe达到最大,去除率接近100%;而当C0为300、500 mg·L−1时,qe在pH=6~9时继续保持缓慢增长趋势,当pH=9时达到最大,后保持不变。这主要是因为WG-g-PNaA水凝胶网络中的—COO−在较低pH环境下,可与H+结合,从而以—COOH形式存在。一方面,其可降低与阳离子染料MB的静电吸引;另一方面,—COOH之间的氢键作用使聚合物链溶胀受限,MB分子传质阻力增大,吸附量较低。当pH≥6时,羧基基团离子化,有利于与带正电的MB+静电吸引,吸附量增大。对MB初始浓度C0≤100 mg·L−1的溶液,在pH=6环境下,吸附率近达100%,再增大溶液pH,吸附量不变。但对MB初始浓度为300、500 mg·L−1的溶液,WG-g-PNaA网络中内层WG链上的—SH需要较高pH才能解离出—S−而进一步与MB+结合,故随着pH的增大,qe仍保持增大的趋势,直至pH=9时全部—SH解离,吸附平衡后,qe保持不变。以此可以推断,WG-g-PNaA水凝胶对MB的吸附机理主要以静电吸附为主。
-
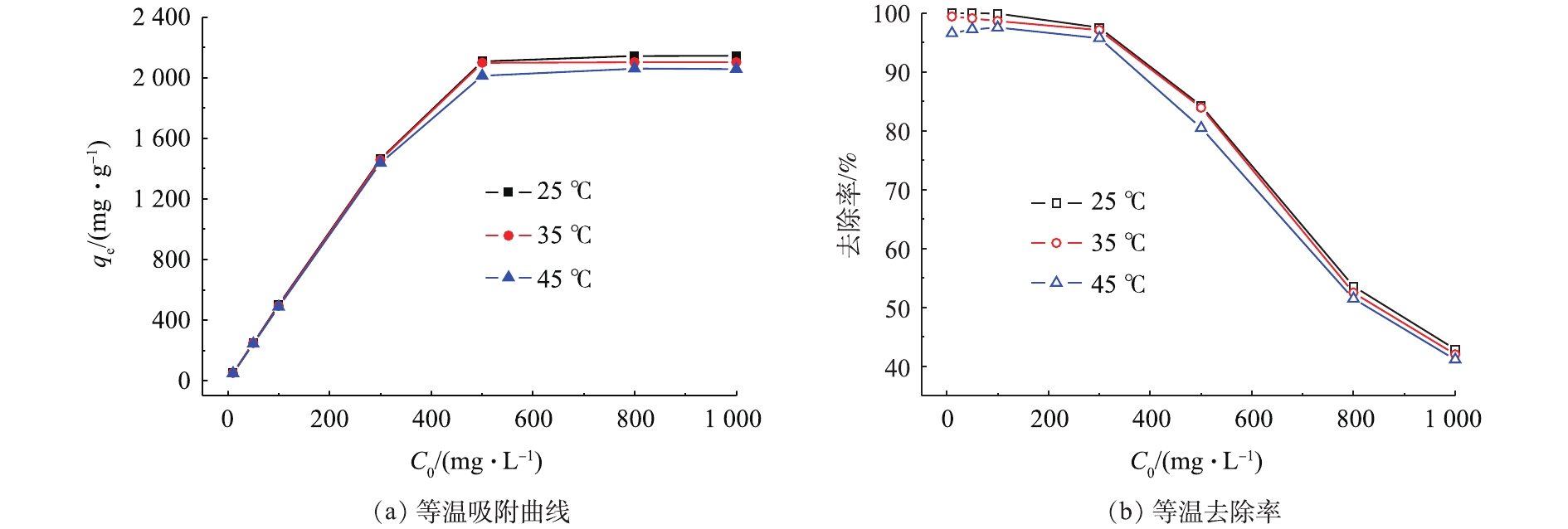
图5为25、35、45 ℃环境下多孔WG-g-PNaA水凝胶对不同起始浓度MB的吸附曲线及去除率(溶液pH=9.0)。由图5(a)可知,qe随MB浓度的增大而增大,当C0≥800 mg·L−1时,qe不再增大,这表明吸附已达到饱和。另外,根据图5(b),当C0较大时,去除率下降较多,原因可能是:一方面,由于较高浓度的MB分子间由于范德华力和氢键作用易形成二聚体或多聚体,影响了其在WG-g-PNaA 凝胶网络中的扩散,不利于静电吸附作用[13];另一方面,在较高初始浓度C0下,吸附剂吸附基本达到饱和,故去除率急剧降低。
运用Langmuir和Freundlich吸附等温线模型(式(3)~式(5))对上述等温热力学数据进行拟合分析,结果见表1。可以看出,由Langmuir吸附等温线模型计算得到的理论吸附量qm与实验平衡吸附量qe接近,R2高于Freundlich吸附等温线模型对应的数值,说明Langmuir吸附等温线模型能较好地描述该吸附过程,MB在多孔WG-g-PNaA水凝胶网络中的吸附过程符合单层吸附模式。另外,随着温度的升高,qe略有降低,但影响不大。在测定温度范围内,0<RL<1,这说明吸附是有利的。
-
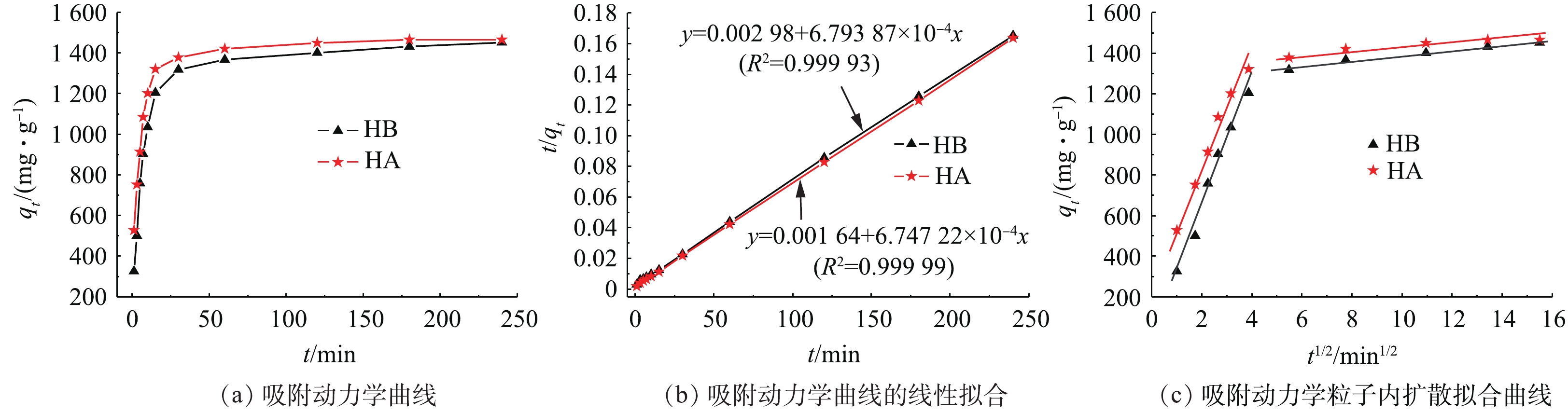
图6探讨了吸附时间对吸附MB性能及吸附动力学的影响。如图6(a)所示(起始浓度C0为300 mg·L−1,溶液pH=9.0,吸附温度为25 ℃),HA对MB的吸附量在15 min内即可达到平衡吸附量的90%,20 min后,基本达到吸附平衡;而HB达90%吸附量时,约需30 min。
采用准二级动力学方程和粒子内扩散模型(式(6)和式(7))对上述吸附行为进行拟合,拟合曲线如图6(b)、图6(c)所示,相应拟合参数见表2。可以看出,其准二级动力学拟合线性可决系数R2几乎接近1,理论计算平衡吸附量qe, cal与实验测定平衡吸附量qe接近,说明其吸附行为符合准二级动力学模型,化学吸附为速率控制步骤。
图6(c)为该类复合水凝胶吸附MB的粒子内扩散模型拟合图。由图6(c)可知,吸附分2个阶段:第1阶段为大孔扩散,即MB分子从溶液扩散到WG-g-PNaA凝胶三维网络孔隙外表面;第2阶段为微孔扩散,即MB分子进入WG-g-PNaA交联网络链中。比较HA和HB的K1d可知,K1d(HA)(28.59)>K1d(HB)(12.89),说明凝胶网络中的孔隙在吸附初始阶段有利于MB分子扩散传质,较多接触复合凝胶表面的大部分吸附位点,提高吸附速率。此部分分析结果与准二级动力学模型拟合的k2(HA)(2.775 9)>k2(HB)(1.548 9)显示结果一致。但是由于粒子内扩散模型拟合线性可决系数R2低于准二级动力学模型的,而且粒子内扩散模型拟合2个阶段都不过原点,这说明粒子内扩散不是速控步骤。
-
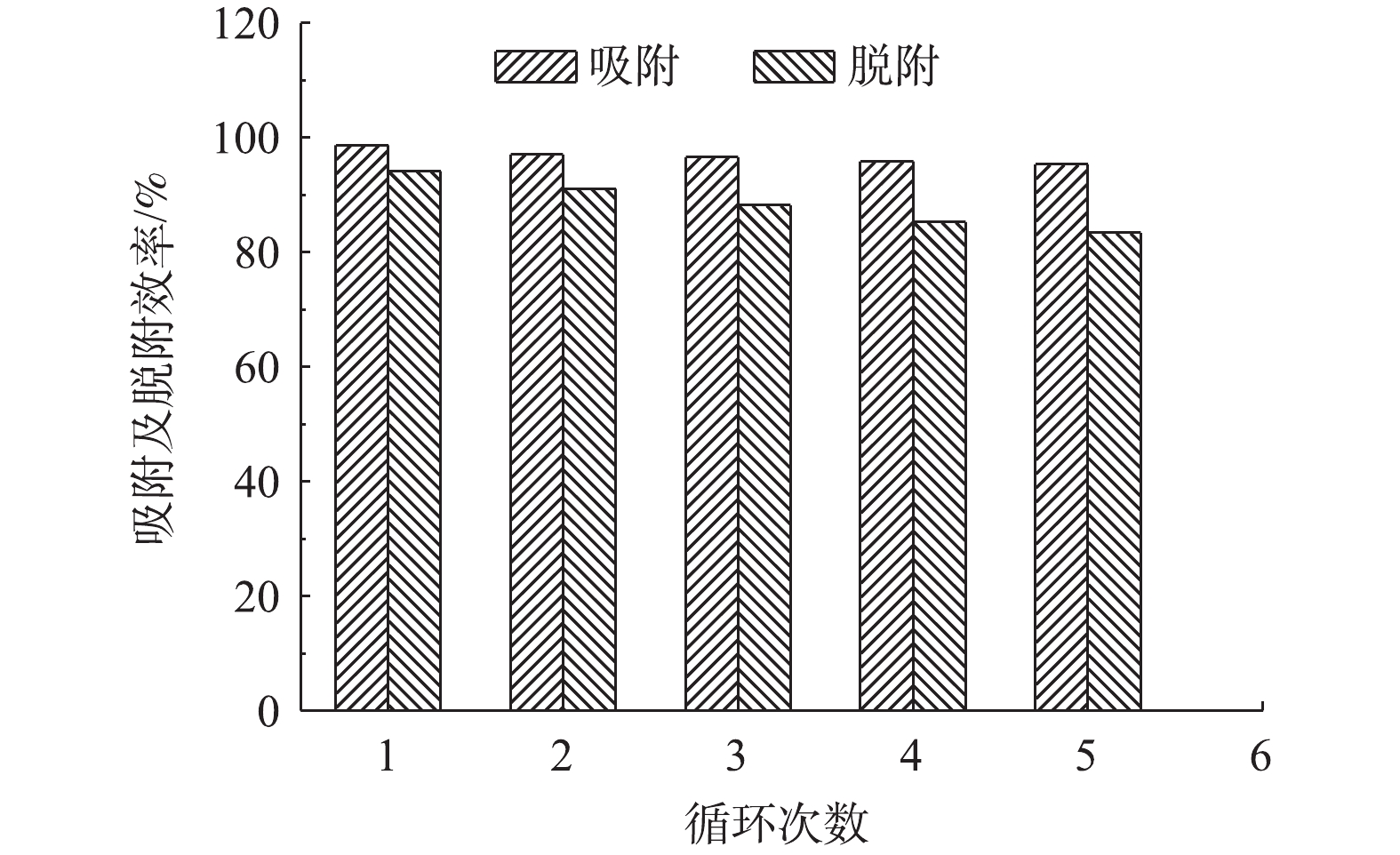
图7为多孔WG-g-PNaA水凝胶对MB的吸附-脱附循环效果。由图7可知,在第1次脱附后,其吸附量相对于新凝胶下降5.8%,后续循环吸附量逐渐缓慢降低,经过5次脱附-吸附后,其吸附量仍可保持初始吸附量的83.4%。在每次吸附后,0.1 mol·L−1 HCl对MB的脱除率均可达到95.3%以上,这说明WG-g-PNaA水凝胶是一种高效的、可多次循环使用的脱除水体中MB的吸附剂。
2.1. Pickering乳液模板多孔WG-g-PNaA水凝胶的制备过程和表征分析
2.2. 溶液pH对MB去除率的影响
2.3. MB起始浓度对去除率的影响及吸附热力学研究
2.4. 吸附时间对吸附MB的影响及吸附动力学研究
2.5. 多孔WG-g-PNaA水凝胶对MB的循环吸附性能
-
1) WG醇溶蛋白稳定O/W型Pickering模板法制备了多孔WG-g-PNaA水凝胶。FESEM表征表明,Pickering乳液滴在WG-g-PNaA水凝胶三维网络中形成较为规整的连续贯通的网孔结构,BET比表面积相对于无Pickering乳液致孔样品增大了2.2倍。
2)多孔WG-g-PNaA水凝胶对MB最佳吸附起始浓度C0=300 mg·L−1、溶液pH=9.0、吸附温度25 ℃时,平衡吸附量qe可达1 463.2 mg·g−1,去除率为98.5%。吸附动力学符合准二级动力学模型,吸附等温线符合Langmuir模型,属于单层吸附模式,饱和吸附量为2 144.2 mg·g−1。吸附受温度影响不大,在25~45 ℃时,0<RL<1,有利于吸附。
3) 0.1 mol·L−1 HCl溶液可使MB从吸附后的WG-g-PNaA水凝胶上有效解吸,实现对MB的循环吸附,而且经过5次吸附-脱附循环后,仍可保持初始吸附量的83.4%。由此可见,WG-g-PNaA水凝胶是一种经济、高效的MB吸附材料,可望用于印染废水的处理。




 下载:
下载: