-
苯酚是一种典型且不易降解的有机污染物,广泛用作合成色素、农药和杀虫剂的中间体[1],近年来引起了公众的极大关注。许多技术,如物理吸附[2]、生物降解[3]和化学氧化[4]等,已被用于去除苯酚和酚类有污染物。虽然这些方法具有可行性,但它们也存在一些缺点,如成本高、降解不彻底、生成比原始污染物更具毒性的副产物等。因此,需要开发一些可有效处理难降解污染物的技术。其中,基于Fenton反应的电化学高级氧化技术(电-Fenton技术,EF)被认为是一种高效且环保的降解方法[5],其可直接在阴极还原O2生成H2O2[6],避免了H2O2的运输和储存[7],而后加入Fe2+催化生成的H2O2进一步分解为·OH,有机物(R)最终被·OH氧化降解[8]。
但传统电-Fenton技术的一些缺点限制了其被广泛应用,如严格的pH范围(3~4)[9]、铁泥的产生、Fe2+的再生效率低以及催化剂不能循环使用等[10]。使用非均相电-Fenton催化剂[11]则能克服这些缺点,一方面,其可以拓宽降解有机污染物的pH范围,使其在接近中性的环境中进行[12];另一方面,非均相电-Fenton催化剂将活性组分(主要是Fe)直接负载于催化剂载体上,可以减少铁泥的形成,重复利用性好。因为活性炭具有来源广泛、孔隙率高和比表面积大等特点,故其被视为负载催化剂的理想载体[13]。另外,已有研究[14]表明,电-Fenton技术中污染物的降解效率与阴极H2O2的生成量有着密切联系,因此,阴极材料的选择至关重要,具有导电性能好、多孔性和稳定性好等特点的阴极材料更有利于阴极H2O2的生成。
本研究利用非均相电-Fenton技术去除水中的苯酚。首先,利用等体积浸渍法将铁负载在活性炭上,制备了非均相电-Fenton催化剂Fe/AC;然后,以具有良好导电性和较大3D活性表面的石墨毡作为阴极材料,构建非均相电-Fenton反应器,考察了该技术对水中苯酚的去除效果,探讨了催化剂用量、电流密度和电解液的初始pH对苯酚去除率的影响,通过循环实验,测定Fe/AC的催化性能和铁渗出量,进而考察了催化剂的稳定性和可重复使用性,并进一步研究了该去除过程的作用机理,以期为非均相电-Fenton技术处理实际废水提供参考。
全文HTML
-
活性炭AC(卡尔冈碳素,粒径为0.5~1 mm);石墨毡GF(北京晶龙特碳石墨厂);九水合硝酸铁Fe(NO3)3·9H2O(元立化工,AR);苯酚(阿拉丁,AR);硫酸H2SO4(元立化工,AR);氢氧化钠NaOH(元立化工,AR);甲醇(元立化工,HPLC)。实验室用水为去离子水。
-
使用硝酸铁水溶液对AC采用等体积浸渍法制备非均相电-Fenton催化剂Fe/AC[15]。首先,用去离子水洗涤AC 3次,并在105 ℃下烘干8 h;然后,将6 g AC置于自封袋中,加入3.3 mL 0.52 g·mL−1的Fe(NO3)3·9H2O溶液,立即将自封袋封口,摇晃使其浸渍均匀,于室温放置2 h后,在60 ℃干燥12 h;最后,在200 ℃马弗炉中热处理4 h,制备得到Fe/AC催化剂。
-
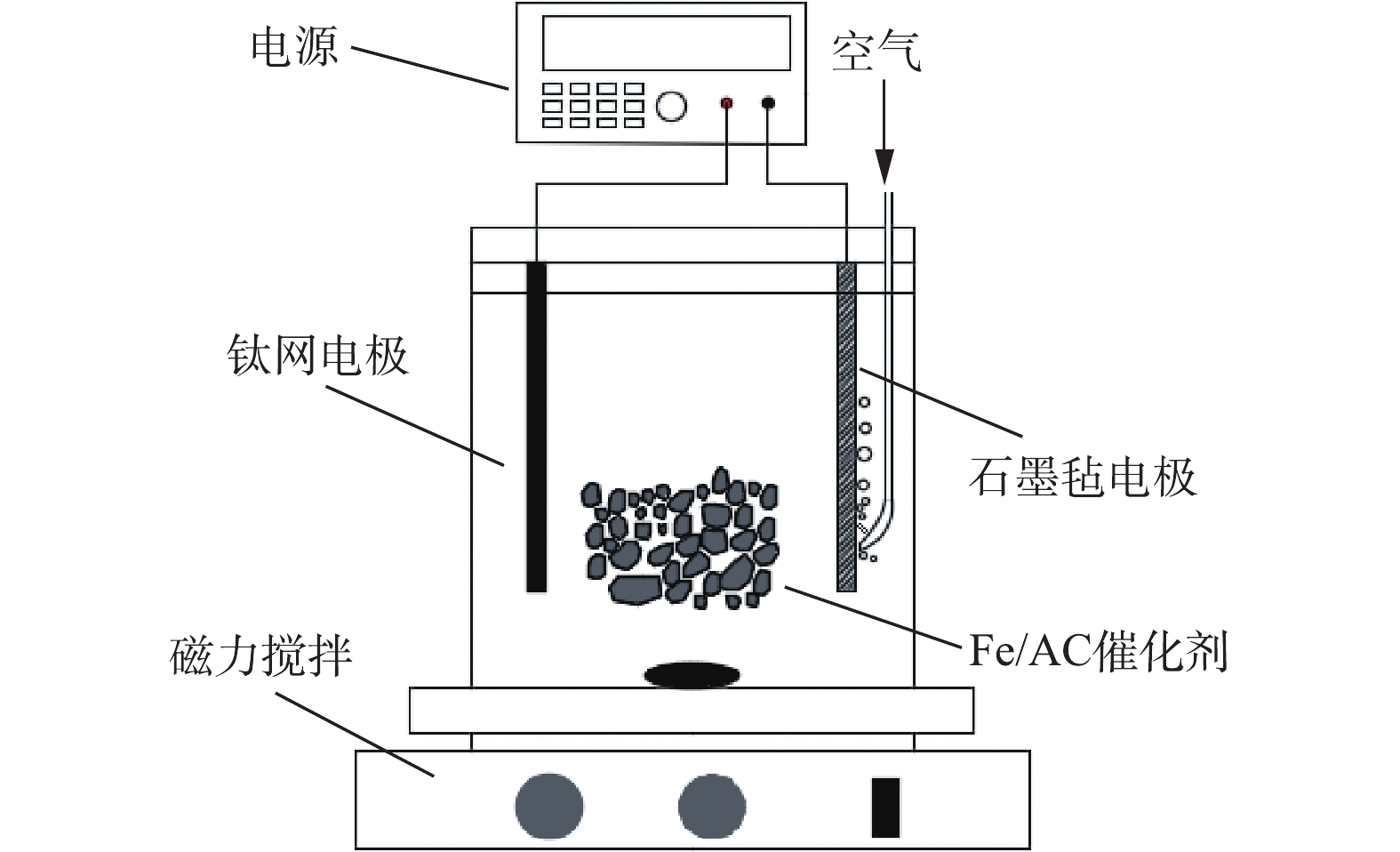
在体积为200 mL的非均相电-Fenton反应器中进行苯酚去除实验,反应器结构示意图如图1所示。阴极为石墨毡电极[16](5 cm×3 cm),相同尺寸的钛网用作阳极。电极垂直放置,电极间距为2 cm。将苯酚溶解在含有0.05 mol·L−1 Na2SO4的去离子水中制备含有100 mg·L−1苯酚的电解液。向阴极底部附近鼓入0.1 L·min−1空气,通电并在阴极上直接还原O2产生H2O2。电流由直流电源(Itech Electronics-IT 6800A)提供。在常规操作中,将一定量的Fe/AC催化剂分散到150 mL电解液中,该电解液的初始pH由H2SO4或NaOH调节。另外,通过磁力搅拌(540 r·min−1)确保电解溶液的浓度均匀。在特定的时间取样,并用0.45 μm的微孔滤膜过滤后进行分析。
分别考察了催化剂用量、电流密度和电解液的初始pH对非均相电-Fenton技术去除水中苯酚的影响。非均相电-Fenton催化剂的稳定性和可重复使用性是评估催化剂性能的重要指标,在本研究中进行连续运行实验以评估Fe/AC催化剂的循环性能。
-
利用比表面积及孔径分析仪(Quantachrome Autosorb-iQ2,康塔公司,美国)和汞孔隙率法测定材料的比表面积和孔体积。分别利用电感耦合等离子体发射光谱(ICP-OES,Varian-VISTA-MPX,瓦里安公司,美国)和X射线光电子能谱(XPS,Thermo ESCALAB 250XI,赛默飞世尔科技公司,美国)对催化剂中总铁含量和Fe的种类等进行分析。
苯酚去除率
$\alpha $ 通过式(4)进行计算。式中:
${C_0}$ 为苯酚的初始浓度,mg·L−1;$C$ 为反应过程中某一时刻的苯酚浓度,mg·L−1。利用紫外分光光度计(UV-Vis,岛津公司,日本),采用草酸钛钾法测定样品中H2O2的浓度,检测波长为400 nm。阴极生成H2O2的电流效率β[16]由式(5)进行计算。
式中:n为将氧气还原成H2O2所转移的电子数;F为法拉第常数,取值96 486 C·mol−1;
${C_{{{\rm{H}}_{\rm{2}}}{{\rm{O}}_{\rm{2}}}}} $ 为生成H2O2的浓度,mol·L−1;V为溶液体积,L;I为电流,A;t为时间,s。利用高效液相色谱仪(HPLC,岛津公司,日本)测定样品中的苯酚浓度。色谱条件为:Shim-pack GIST C18分析柱(5 μm,250 mm×4.6 mm),色谱柱温30 ℃,流动相为甲醇和水(70∶30,体积比),流速1 mL·min−1,紫外检测波长222 nm。利用总有机碳分析仪(TOC-5000,岛津公司,日本)测试样品的TOC值。采用邻菲咯啉法,利用紫外分光光度计,在波长510 nm处测定样品中铁的浓度[17]。
1.1. 实验药品与材料
1.2. Fe/AC催化剂的制备
1.3. 实验方法与设备
1.4. 分析方法
-
利用ICP-OES分析测定了Fe/AC中铁负载量,结果为3.94%,基本达到铁的理论负载量。对AC载体和Fe/AC催化剂的比表面积和孔体积进行了表征,结果如表1所示。由表1可知,硝酸铁溶液的浸渍导致了活性炭比表面积和孔体积的减小,这可能是由于Fe进入了AC的孔道中所致。
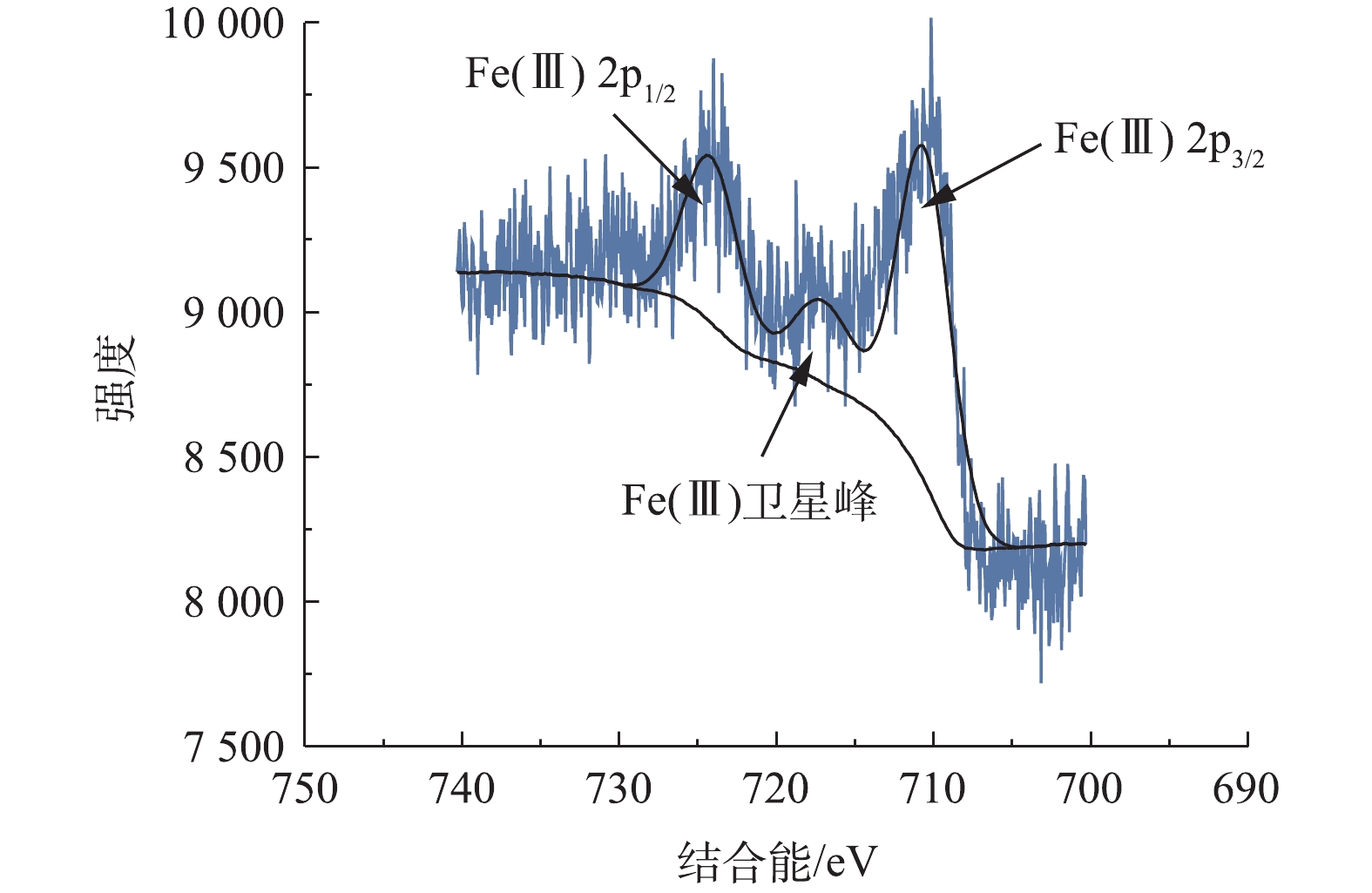
为了确定Fe/AC催化剂中Fe的价态和存在形式,使用X射线光电子能谱(XPS)对Fe/AC进行表征,如图2所示。根据XPS光谱图,观察到Fe(Ⅲ)的Fe2p3/2峰和Fe2p1/2峰分别位于结合能711.0 eV和724.6 eV处,面积比为1∶0.5,除此之外,在结合能为717.0 eV处存在Fe(Ⅲ)的卫星峰,因此,推测Fe/AC催化剂中铁的价态主要为三价,即Fe基本上以Fe2O3的形式存在。
-
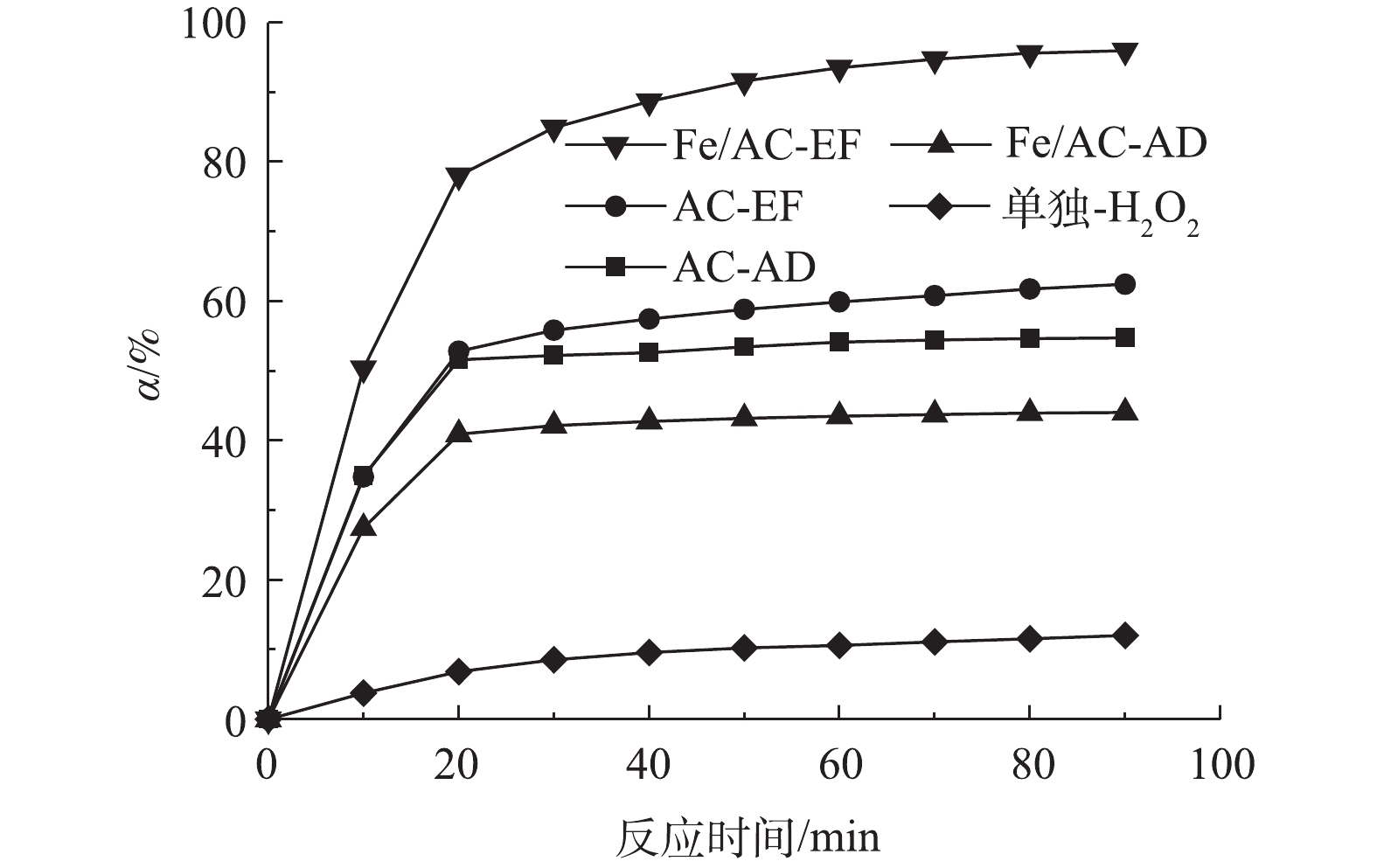
为了测试非均相电-Fenton催化剂Fe/AC对水中苯酚的去除效果并区分吸附和催化氧化的影响,在电流密度9 mA·cm−2和电解液初始pH=5的条件下,分别进行了AC吸附(AC-AD)、Fe/AC吸附(Fe/AC-AD)、以AC为非均相电-类Fenton催化剂(AC-EF)、以Fe/AC为非均相电-Fenton催化剂(Fe/AC-EF)和不加催化剂仅通电(单独-H2O2)5种实验条件下去除水中苯酚的实验,结果如图3所示。由图3可知,对未浸渍Fe的AC来说,在通电和不通电的条件下,苯酚的去除率基本相同。这表明苯酚的去除基本上是通过吸附实现的,AC的催化氧化作用几乎可以忽略不计。对于在不加催化剂仅通电的过程,在反应90 min后,水中苯酚的去除率为11.37%,远低于其他几种情况。这是因为在该过程中未加入催化剂,无吸附作用,且不能将阴极生成的H2O2催化分解为·OH,而H2O2的氧化还原电位(E0(H2O2/OH−)=0.88 V(SHE))低于·OH(E0(·OH/H2O)=2.80 V(SHE)),故不能实现苯酚的有效去除。利用Fe/AC进行反应,当不通电时,其对苯酚的去除率稍低于AC吸附的去除率。这说明在这个去除过程中仍是吸附起主要作用,但由于Fe/AC的比表面积低于未负载Fe的AC,从而造成前者吸附去除率更低。当通电时的90 min后,苯酚的去除率达到了95.94%,这归因于Fe/AC催化氧化和吸附的共同作用。因此,非均相电-Fenton催化剂Fe/AC可实现对水中苯酚的有效去除。
-
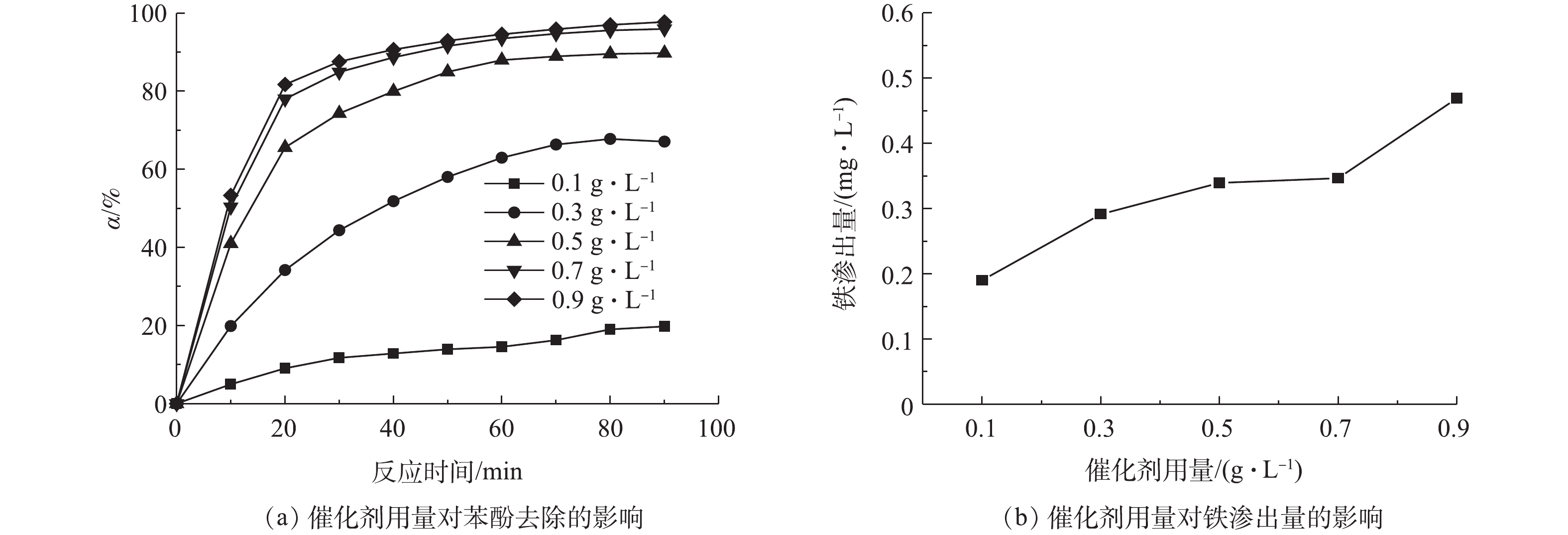
1)催化剂用量的影响。在电流密度为9 mA·cm−2和电解液初始pH为5的条件下,研究了Fe/AC用量对苯酚去除的影响,结果如图4所示。随着Fe/AC用量的增加,苯酚的去除率有所升高。但当催化剂用量从0.7 g·L−1增加到0.9 g·L−1时,苯酚的去除率几乎没有发生变化。这说明当催化剂用量为0.7 g·L−1时,催化剂上进行反应的活性位点已经足够,所以不能通过增加催化剂用量来提高反应速率。同时,过量催化剂的使用反而可能会导致消耗·OH的副反应(式(6))的发生[18]。除此之外,由图4(b)可知,电解液中铁的渗出量随催化剂用量的增加而增加。因此,选择0.7 g·L−1的催化剂用量用于后续实验。
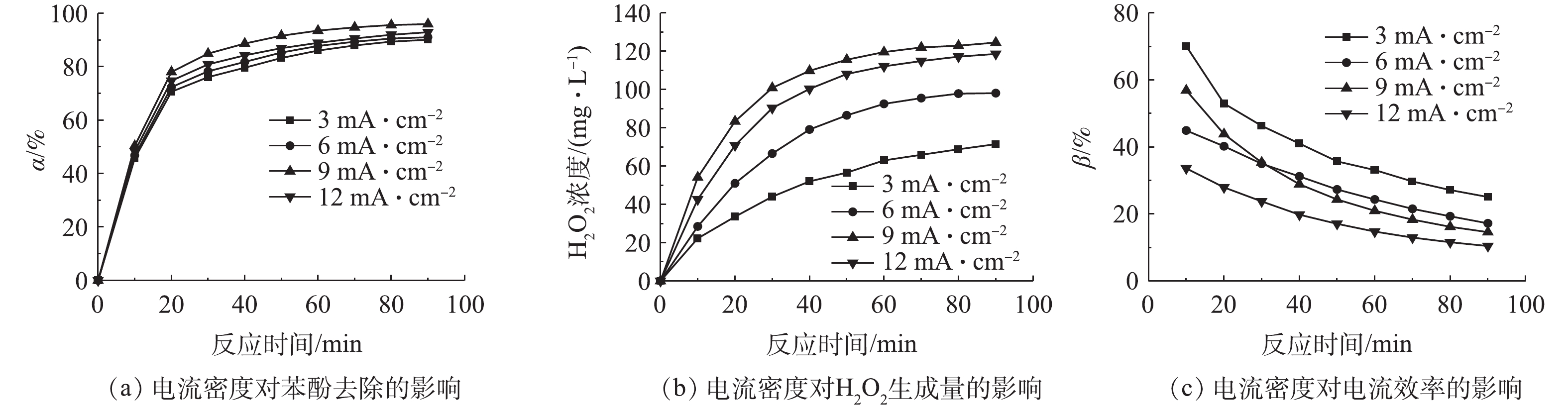
2)电流密度的影响。在催化剂用量为0.7 g·L−1和电解液初始pH为5的条件下,考察了电流密度对苯酚去除的影响,结果如图5所示。由图5(a)可知,在电流密度为3~9 mA·cm−2时,苯酚去除率随电流密度的增加而升高,但当电流密度从9 mA·cm−2增加到12 mA·cm−2时,苯酚的去除率没有继续增加,反而有所下降。这是由于电流密度影响H2O2的生成量所致,在不加催化剂且仅通电的条件下,测定H2O2浓度随时间的变化情况,结果如图5(b)所示。当电流密度为3~9 mA·cm−2时,电流密度的增加使H2O2生成量升高;而当电流密度大于9 mA·cm−2时,阴极H2O2的生成量不再明显增加,反而有所下降,这可能是由于电流密度过大所引起的如式(7)~式(9)所示副反应的发生[19-20],造成H2O2生成量下降,从而降低了非均相电-Fenton技术去除苯酚的效率。通过式(5)计算电流密度随时间的变化,结果如图5(c)所示。在反应90 min后,在电流密度为3、6、9和12 mA·cm−2时,电流效率分别为25%、17%、14%和10%。综上可知,当电流密度(3~6 mA·cm−2)较低时,虽然体系有较高的电流效率,但H2O2生成量不高,苯酚的去除率也较低;当电流密度(12 mA·cm−2)过高时,H2O2生成量和苯酚去除率较高,而电流效率却很低,因此,可选择9 mA·cm−2作为最佳电流密度。
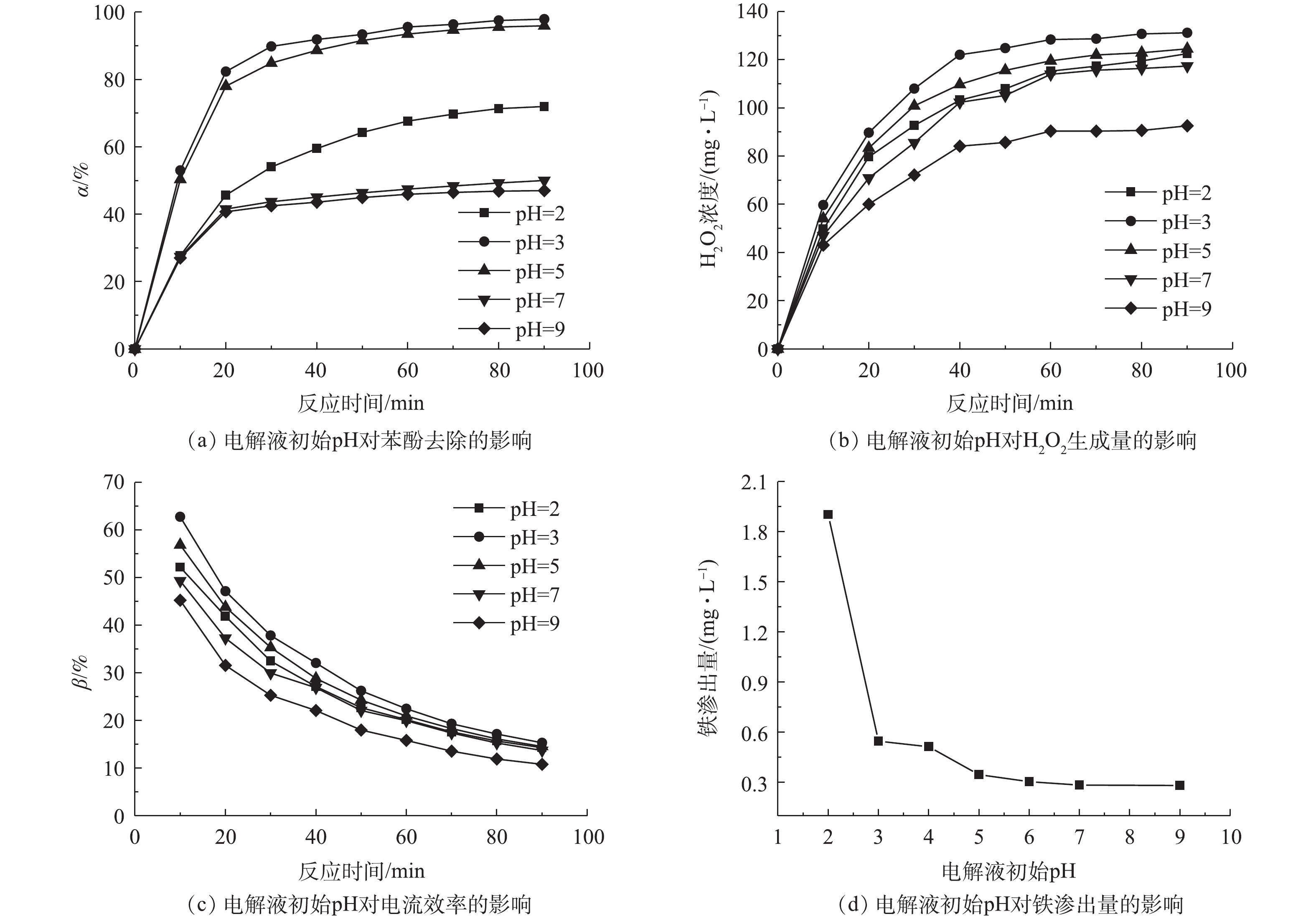
3)电解液初始pH的影响。在催化剂用量为0.7 g·L−1和电流密度为9 mA·cm−2的条件下,考察了电解液初始pH对非均相电-Fenton催化剂Fe/AC去除水中苯酚的影响,结果如图6所示。由图6可知,在电解液初始pH为2、3和5时,苯酚去除率可分别达到71.98%、97.92%和95.94%,在pH为3时,苯酚去除率达到最高,与已有报道结果[21]一致。这是因为酸性条件有利于电-Fenton反应的进行和H2O2的生成,在Fe/AC作用下可以催化生成更多的·OH,从而实现苯酚的有效降解。在中性(pH为7)和碱性(pH为9)条件下,反应90 min后,苯酚的去除率仅为50.02%和47.02%,其效果略高于Fe/AC对苯酚吸附的效果。
为了进一步验证电解液初始pH对苯酚去除率可能是由于影响到系统中H2O2的生成量所致,实验考察了不加催化剂仅通电的条件下,不同电解液初始pH时H2O2的生成量和电流效率,结果如图6(b)和图6(c)所示。由图6(b)可以看出,当电解液初始pH为3和5时,酸性增强,H2O2的生成量升高;pH由3变为2时,由于酸性过强,造成了H2O2生成量下降。这可能由于在过强的酸性条件下,H2O2会捕获质子形成
$ {{\rm{H}}_{\rm{3}}}{\rm{O}}_{\rm{2}}^{\rm{ + }}$ ,后者非常稳定,其反应如式(10)所示。此外,过量H+也可以与·OH结合并终止反应(式(11)),反应会不利于H2O2的生成[22]。在碱性条件下,H+与OH−反应生成水,因此,在碱性条件下,H2O2的生成量较低。可以看出,电解液初始pH也影响阴极上H2O2的生成。图6(c)比较了不同pH条件下的电流效率,在pH分别为3和5的条件下,电-Fenton系统均有较高的电流效率。本研究考虑了电解液初始pH对Fe/AC催化剂的稳定性的影响,结果如图6(d)所示。电解液初始pH越小,Fe/AC催化剂的铁渗出量越大。虽然酸性条件有利于苯酚的去除,但具有更高的铁渗出量。综合苯酚去除率、电流效率和铁渗出量,并考虑实际生产情况,选择pH=5作为最佳电解液初始pH。
-
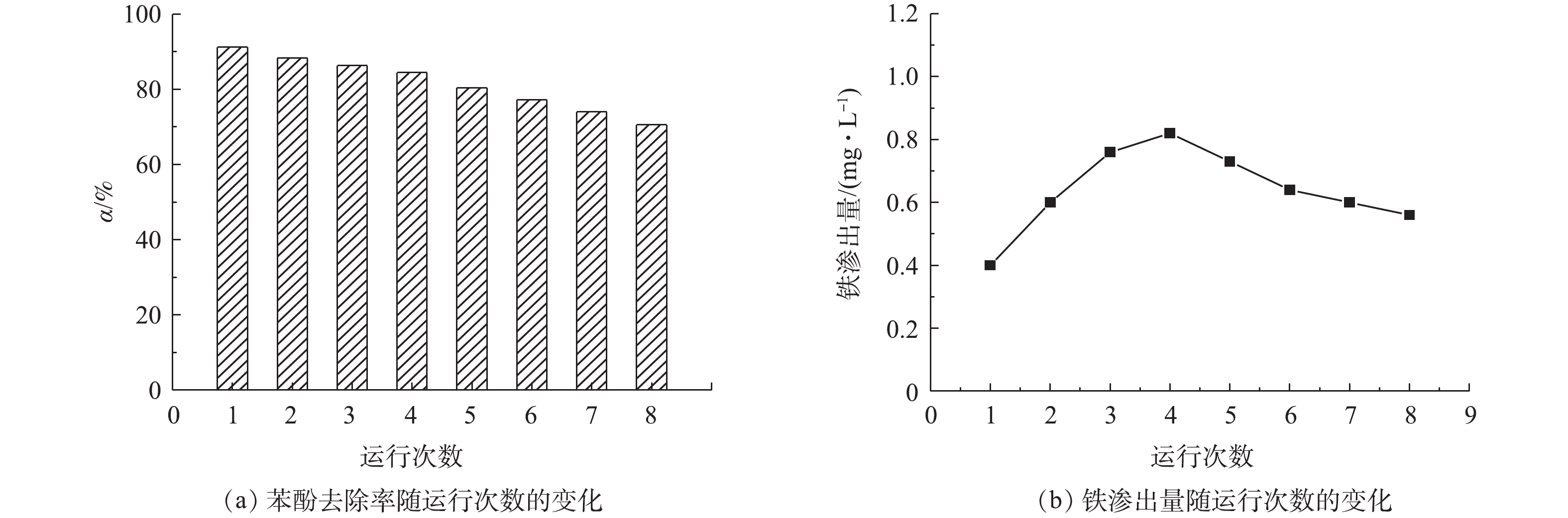
在催化剂用量0.7 g·L−1、电流密度9 mA·cm−2和电解液初始pH=5的条件下,连续进行了8次苯酚去除实验,结果如图7所示。由图7可知,随着运行次数的增加,非均相电-Fenton催化剂Fe/AC去除苯酚的效率呈现下降趋势,苯酚的去除率从91.18%降低至70.55%,主要是由于Fe/AC催化剂中铁的渗出、活性位点被覆盖和孔堵塞等所造成的。经过8次连续运行实验后,浸出铁的总量约占总铁量的18%。因此,非均相电-Fenton催化剂Fe/AC具有良好的稳定性和可重复使用性。
-
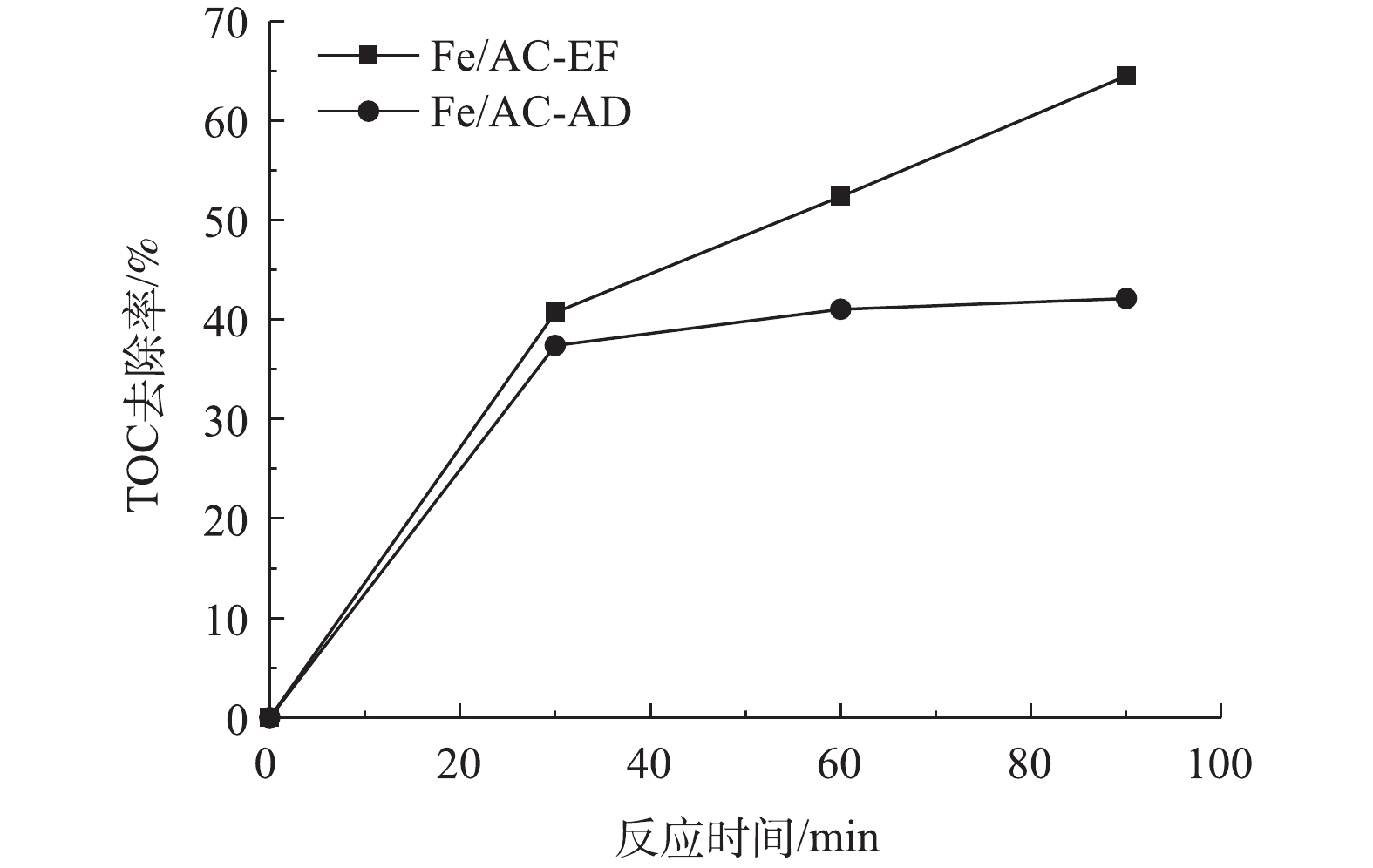
为了确定非均相电-Fenton过程中苯酚的矿化效果,测试了Fe/AC-EF过程的TOC去除率,并与Fe/AC吸附过程进行了比较,结果如图8所示。在Fe/AC-AD过程中,苯酚未被氧化降解,反应90 min后的TOC去除率为42.13%,这是通过Fe/AC的吸附作用实现的。在Fe/AC-EF过程中,反应90 min后的TOC去除率为64.51%,这归因于电-Fenton反应对苯酚的氧化作用和Fe/AC的吸附作用。使用甲醇对反应后的Fe/AC进行洗涤后,再检测脱附的物质,几乎检测不到残余的苯酚,这表明吸附在催化剂上的苯酚已被氧化降解。为了获知苯酚的降解中间产物,对反应90 min后的电解液进行了HPLC检测,将所得到的色谱图与可能存在的芳香类物质和短链酸的液相色谱图进行了比较[23-24],经过对比物质的停留时间,发现反应后的电解液中可能存在苯醌、对苯二酚、马来酸、乙酸和甲酸等中间产物。
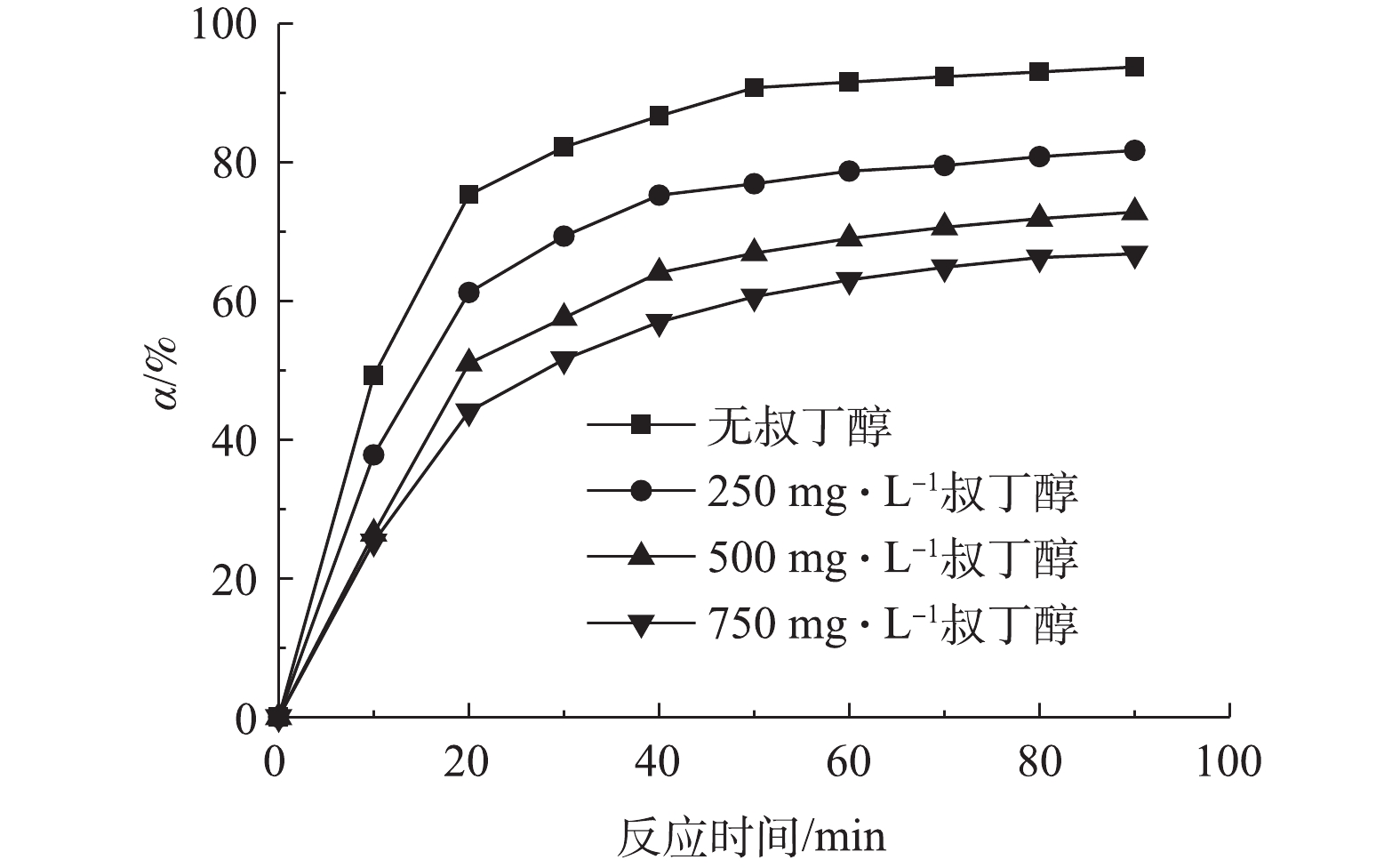
在反应前,将·OH淬灭剂——叔丁醇[25]添加到溶液中,测定非均相电-Fenton过程中苯酚的去除率,从而确定反应过程中·OH的存在,结果如图9所示。由图9可知,随着叔丁醇浓度从0 mg·L−1增加到750 mg·L−1,反应90 min后苯酚的去除率从95.94%降低至66.82%,这说明Fe/AC可催化H2O2分解产生·OH,其为降解苯酚的主要自由基。
在反应过程中,Fe/AC会向电解液中渗出铁,这可能会导致均相电-Fenton反应的发生,故本研究考察了催化剂渗出的铁引发的均相电-Fenton反应对于苯酚去除的贡献。首先,将0.7 g·L−1的Fe/AC加入到150 mL 含有0.05 mol·L−1 Na2SO4的溶液中,鼓入0.1 L·min−1空气,在540 r·min−1的转速下搅拌90 min;然后,过滤除去Fe/AC,得到含有渗出铁的溶液;最后,向溶液中加入100 mg·L−1苯酚并通电反应90 min。结果表明,渗出的铁引发的均相电-Fenton过程仅能实现6.58%的苯酚去除率。因此,非均相电-Fenton反应在苯酚降解过程中占据主导地位。
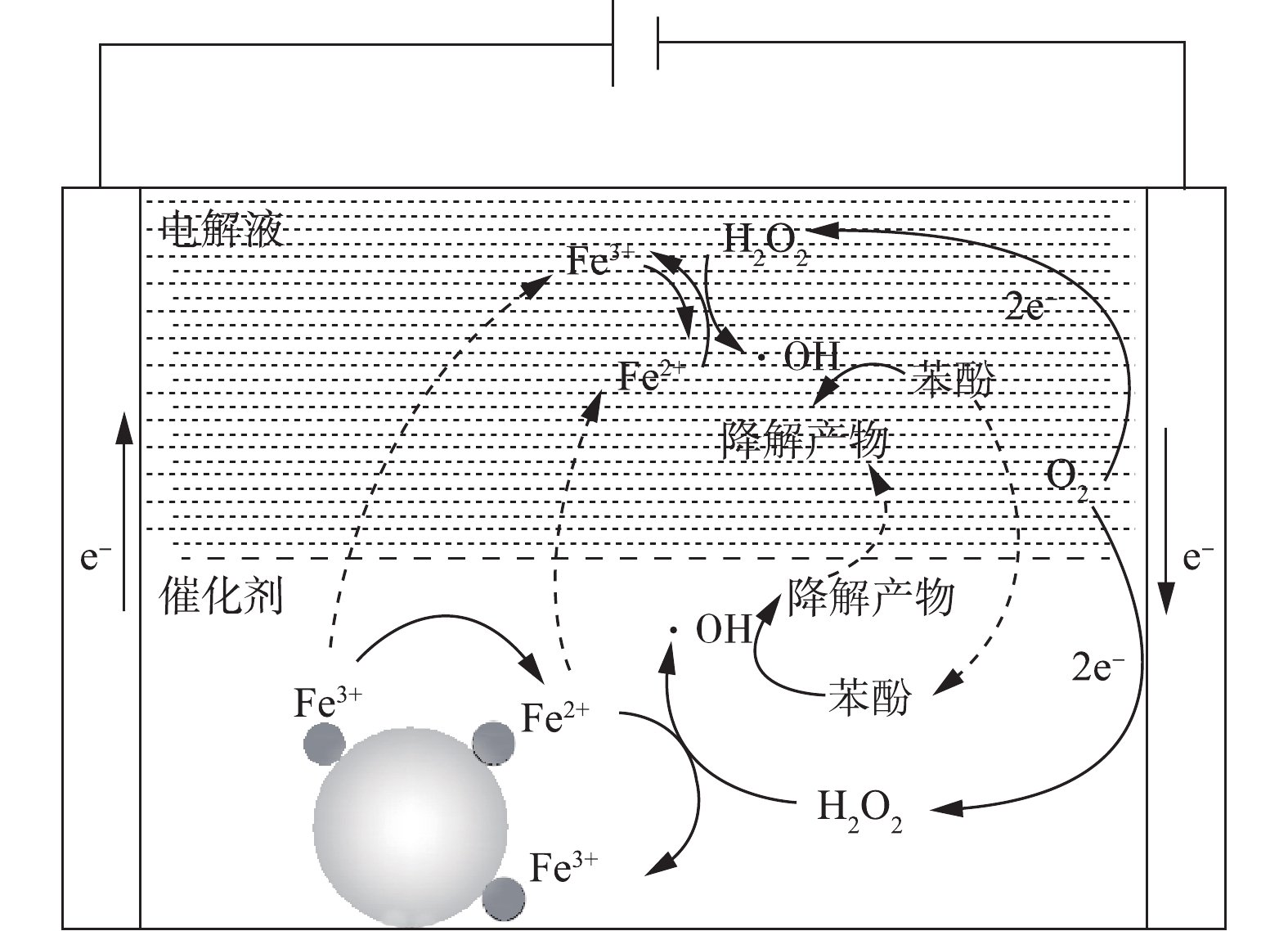
根据上面的研究结果,提出了以Fe/AC为催化剂的非均相电-Fenton技术去除水中苯酚的可能反应机理(图10)。在通电条件下,石墨毡阴极经过2个电子氧还原反应生成H2O2。据相关研究[26-27]可知,≡Fe3+与H2O2(式(12))和HO2·(式(13))的反应生成≡Fe2+,其中≡Fe3+和≡Fe2+代表催化剂表面上的Fe(Ⅲ)和Fe(Ⅱ)活性位点,生成的≡Fe2+可与H2O2反应生成·OH(式(14))。溶解的Fe3+是从催化剂中渗出而分散在电解液中的,并通过相关反应(式(15)~式(17))分解H2O2,生成·OH。此外,还有可能发生不利于氧化过程的竞争性反应,反应如式(18)~式(22)所示。由于Fe/AC的吸附作用,苯酚主要吸附在催化剂表面,进而被生成的·OH分解(式(23)),少量苯酚的分解在电解液中进行。
2.1. Fe/AC催化剂的表征
2.2. 非均相电-Fenton催化剂Fe/AC对苯酚的去除效果
2.3. 苯酚去除率的影响因素分析
2.4. Fe/AC催化剂的循环性能
2.5. 苯酚去除机理的分析
-
1)在催化剂用量为0.7 g·L−1、电流密度为9 mA·cm−2和电解液初始pH为5的条件下,经Fe/AC非均相电-Fenton反应90 min后,苯酚去除率和TOC去除率可分别达到95.94%和64.51%。
2) 在非均相电-Fenton反应过程中,苯酚的高效去除归因于吸附和催化氧化共同作用的结果。
3) 8次连续去除实验结果说明Fe/AC具有良好的循环性能。
4)在该催化反应中,·OH是降解苯酚的主要自由基。



 下载:
下载: