-
废水中重金属离子往往会与一些有机配体(EDTA、有机酸等)形成可溶性络合物,对微生物和水生动植物产生毒害作用[1]。由于络合物稳定性较强,因此,在对重金属进行调碱沉淀去除之前,需要对络合物进行氧化破络,使其释放出重金属离子[2-3]。高级氧化技术目前已被广泛应用于络合物的破络处理,如光催化法、电化学法、芬顿法及类芬顿法[4-6]。光催化氧化法反应条件要求较高,在实际应用中局限性较大。电化学法受电极材料限制较大,对于高浓度络合态重金属的去除效果较差。芬顿法的氧化能力较强且反应速度快,但是传统芬顿法对过氧化氢(H2O2)利用率低,所需氧化剂和催化剂的浓度较高,增加了处理成本。PS作为类芬顿法中的一种氧化剂,廉价易得,能在高效催化剂的活化的作用下,快速高效地催化降解污染物[7]。
研究表明,结构对称的PS易被过渡金属类催化剂活化[8]。然而,单独的过渡金属类催化剂具有金属离子较易溶出和碱性条件下催化性能偏低等缺陷,将金属类催化剂与BC结合,能够提高催化剂整体的稳定性和石墨化程度,进而提升催化性能。目前碳基金属负载型材料被广泛应用于水中有机污染物的降解。YAN等[9]将零价铁和BC负载活化PS降解水中的三氯乙烯,负载后材料整体的抗氧化性和催化活性得以提升;OUYANG等[10]将磁性Fe3O4和BC负载,使催化材料的活性点位增多,从而更有效地活化PS降解水中的1,4-二恶烷。在多种过渡金属材料中,CuO由于具有优异的物理和化学性质而被广泛应用在催化,传感和电极材料等领域,尤其是在活化PS的过程中,具有较强导电性的CuO材料可以充当电子中间体,加快电子转移,通过非自由基途径加速污染物的降解,这使得CuO负载型材料在催化性能方面脱颖而出[11-12]。目前关于碳基CuO负载型材料活化PS的研究大多集中在降解水中有机污染物方面,而在处理EDTA-Cu/Ni/Zn络合物方面的研究鲜有报道,同时研发出快速高效去除水中络合态污染物新技术尤为重要。本研究利用CuO@BC活化PS对EDTA-Cu废水进行氧化破络处理,实现对有机物的降解,再进行调碱沉淀实现对重金属的去除,研究CuO@BC-PS体系对EDTA-Cu、TOC和铜的去除效果及影响因素,通过淬灭实验和EPR图谱初步阐明了该体系降解EDTA-Cu的机理。
全文HTML
-
1) CuO@BC的制备。生物炭来源于木质燃料在450~550 ℃条件下热解后的副产物,破碎研磨后,利用0.1 mol·L−1硝酸对其进行浸渍改性,再采用水热法和高温煅烧法将CuO负载至生物炭表面,清洗干燥后得到CuO@BC[12-13],以进行相关表征和降解实验。
2) CuO@BC的表征。使用扫描电子显微镜(SEM,S-3400)对负载前后材料的形貌进行观察。
-
1)主要试剂:乙二胺四乙酸二钠(分析纯),三水合硝酸铜(优级纯),过硫酸钠(99%),六水合三氯化铁(优级纯),四丁基溴化铵(分析纯),乙酸铵(分析纯),乙醇(分析纯),叔丁醇(分析纯),2,5-二甲基呋喃(分析纯)。
2)主要仪器:高效液相色谱仪(Alliance e2695, 美国Wastes公司),火焰原子吸收分光仪(Z2000,日本日立公司),总有机碳分析仪(TOC-L CPH,日本岛津公司),恒温水浴振荡器(PHS-3C,上海仪电科学仪器股份有限公司)和pH计(FE28-Standard,瑞士梅特勒托利多公司),电化学工作站(Multi Autolab/204,瑞士Metrohm Autolab B.V.公司)。
-
1)模拟废水的配制。按照EDTA与Cu2+ 摩尔比为1∶1称取适量的三水合硝酸铜(Cu(NO3)2·3H2O)和乙二胺四乙酸二钠(EDTA-2Na)溶解于去离子水中,保持搅拌24 h使其充分络合[14-15]。EDTA-Cu模拟废水贮备液中含110 mg·L−1的铜和500 mg·L−1的EDTA。
2) EDTA-Cu的降解。将EDTA-Cu贮备液稀释25倍,取200 mL稀释后的使用液至500 mL锥形瓶内,调节pH至3.0、5.0、7.0、9.0和11.0后,分别按照0.2、0.5、1、2和4 mmol·L−1加入PS和按照0.2、0.5、0.8、1.0和1.5 g·L−1加入CuO@BC,在298、308、318和328 K条件下于恒温水浴振荡器内以180 r·min−1分别匀速反应5、15、30、60和120 min,反应结束后,加入乙醇并摇匀,待络合处理。
-
1)络合。在进行上机测定前,取5 mL样品于10 mL比色管内,加入0.3 mL 0.5%(体积分数)稀盐酸和1 mL 230 mg·L−1氯化铁溶液使溶液中剩余EDTA及其化合物全部转化为EDTA-Fe以供测定,定容至刻度线,混匀后黑暗条件下络合16 h以保证转化完全[16]。
2)高效液相色谱(HPLC)的测定。测定条件[16]:色谱柱为C18(150 mm×4.6 mm×5 μm);检测器为Waters 2998 PDA;柱温为30 ℃;流动相A为四丁基溴化铵-乙酸铵溶液(乙酸,pH=4.00),流动相B为乙腈,A与B的体积比为97.5∶2.5;测定波长为254 nm;流速为0.5 mL·min−1;进样量为10 μL。
3)标准曲线的测定。分别准确移取1.0、2.0、3.0、4.0和5.0 mL EDTA-Cu使用液至10 mL容量瓶内并定容,配制浓度为2.0、4.0、6.0、8.0和10.0 mg·L−1的EDTA-Cu标准溶液。按照上述条件进行处理与测定。
4)铜的测定。使用浓度为0.05 mol·L−1的NaOH和H2SO4将待测样品的pH调节至6.0~12.0,充分搅拌均匀后,取上清液并通过0.45 μm滤膜,使用火焰原子吸收光谱仪对溶液中剩余的铜含量进行测定。
5)TOC的测定。使用660 ℃燃烧法对废水TOC进行测定,以研究该反应体系对污染物的矿化能力。
-
1)淬灭实验。为了研究硫酸根自由基(
$ {\rm{SO}}_4^{ \cdot - }$ )、羟基自由基(·OH)和单线态氧(1O2)在本催化氧化体系中的作用,反应开始前分别在反应液中添加50 mmol·L−1的乙醇、叔丁醇和2,5-二甲基呋喃作为自由基淬灭剂,其余步骤按照1.3中的EDTA-Cu的降解方法进行处理。2)自由基捕获。采用电子顺磁共振技术(EPR)技术对
$ {\rm{SO}}_4^{ \cdot - }$ 、·OH和1O2进行捕获。向含EDTA-Cu溶液中加入适量的PS与CuO@BC,向体系中加入一定量的5,5-二甲基-1-吡咯啉-N-氧化物(DMPO)作为$ {\rm{SO}}_4^{ \cdot - }$ 和·OH的捕获剂,2,2,6,6-四甲基哌啶(TEMP)作为1O2的捕获剂,保持2种捕获剂的浓度均为0.5 mol·L−1以上以保证捕获效率,保持振荡反应10 min后制样并进行上机测定[17]。
1.1. CuO@BC的制备及表征方法
1.2. 主要试剂与仪器
1.3. EDTA-Cu废水处理
1.4. 分析检测方法
1.5. 机理探索
-
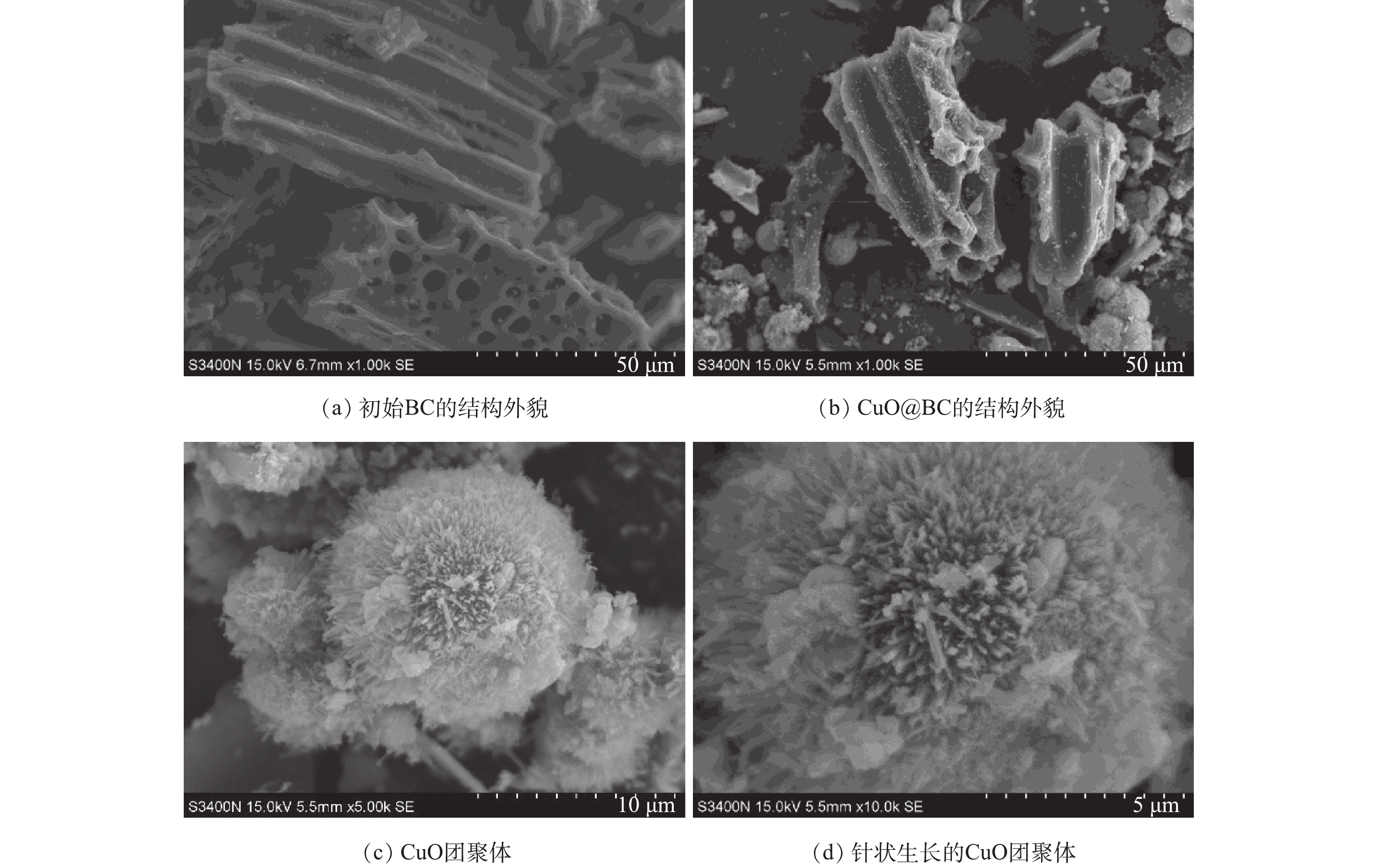
图1为材料的SEM图。通过负载后的CuO@BC的SEM结果(图1(a)、图1(b))可以看出,CuO成功负载至BC表面和结构内部,可见负载后CuO的稳定性和分散程度都有明显的提高。图1(c)、图1(b)显示了CuO团聚体以针状生长,这种形状可能带来更大的比表面积和更多的活性位点有利于后续的催化降解反应的进行[12]。对催化材料的深入表征及分析已在本课题组先前研究中报道,并指出,BC表面的纳米CuO结晶度低,稳定性高[18]。由于本研究重点在于CuO@BC在催化PS降解络合污染物的性能研究,具体表征本文不再过多分析。
-
根据预实验结果,调节EDTA-Cu络合废水pH至7.0,分别加入不同的摩尔浓度的PS,混合均匀后按0.5 g·L−1加入CuO@BC,在298 K条件下EDTA-Cu的降解情况如图2所示。当体系中PS浓度达到0.5 mmol·L−1时,30 min时可降解74.6%的EDTA-Cu,并且反应60 min后基本达到完全降解。随着PS浓度进一步地提高,EDTA-Cu的降解速率提升甚微,这可能是因为体系中CuO@BC提供的活性位点是一定的,当CuO@BC与PS之间的反应达到平衡状态时,体系的活性维持将在一定的水平[18]。
-
在温度为298 K条件下,向pH为7.0的EDTA-Cu模拟废水中加入摩尔浓度为0.5 mmol·L−1的PS,分别按不同投加量加入CuO@BC。由图3可见,当反应进行5 min时,投加0.5 g·L−1的CuO@BC仅可降解29.1%的EDTA-Cu,而投加1.5 g·L−1的CuO@BC便可降解71.9%的EDTA-Cu,此时CuO@BC的投加量直接决定了EDTA-Cu的降解速率。随着反应时间的增加,反应进行60 min时,0.5 g·L−1的CuO@BC投加量便可降解97.1%的EDTA-Cu,与投加量为0.8、1.0和1.5 g·L−1时的降解效率基本保持一致,所以在60 min后,CuO@BC投加量的继续增加对EDTA-Cu的降解率的提升效果并不明显。这可能是因为在反应刚开始时,CuO@BC投加量的增加可以提供更多的活性位点,进一步活化PS而产生更多的反应活性物质,从而大大地提升了反应速率;随着反应时间的增加,污染物浓度降低,而CuO@BC的投加量达到一定程度后对EDTA-Cu的降解效果差异不大[19]。
-
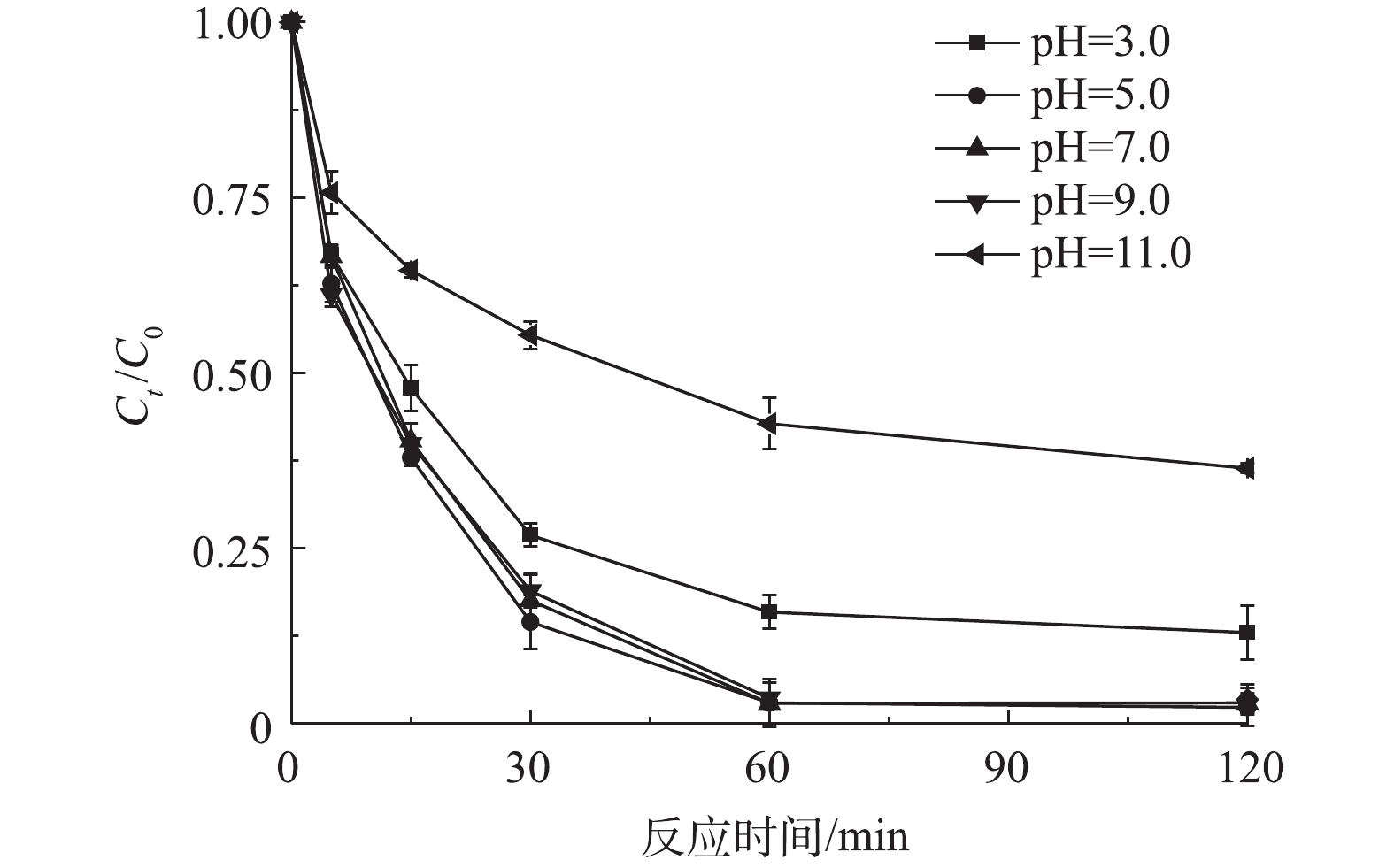
反应开始前,将反应液分别调节至不同的pH,然后加入0.5 mmol·L−1的PS和0.5 g·L−1的CuO@BC,在298 K条件下EDTA-Cu的降解趋势如图4所示。当反应液的pH为5.0、7.0和9.0时,反应体系对EDTA-Cu均具有较好的降解效果,60 min时降解效率分别为97.0%、97.1%和96.3%。然而在pH为3.0时,降解效率有所减弱,这可能是因为当溶液中H+浓度高时,CuO@BC表面的CuO会更容易被转化成Cu2+,而游离态的Cu2+并不能有效地活化PS,从而减弱降解效果[12]。此外,当溶液pH为11.0时,高浓度的OH−会消耗体系中PS,减少了活性物质,不利于污染物的降解[20]。因此,在非强酸或强碱的环境中(pH为5.0~9.0),CuO@BC-PS体系对EDTA-Cu均具有良好的降解效果。
将初始反应液调节至7.0,然后加入0.5 mmol·L−1的PS和0.5 g·L−1的CuO@BC,分别在温度为298、308、318和328 K条件下进行降解实验,由图5可见,随着反应温度的升高,体系对污染物的降解速率有明显的提升。328 K条件下,降解94.1%的污染物仅需15 min,相比于常温条件下缩短近1倍的时间。一方面,该体系对EDTA-Cu的降解反应为吸热反应,因此,升高反应温度有利于反应的进行[21-22]。另一方面,升高温度可在CuO@BC活化PS的同时起到热活化的作用,加快活性物质的产生,从而加速了污染物的降解[23]。
-
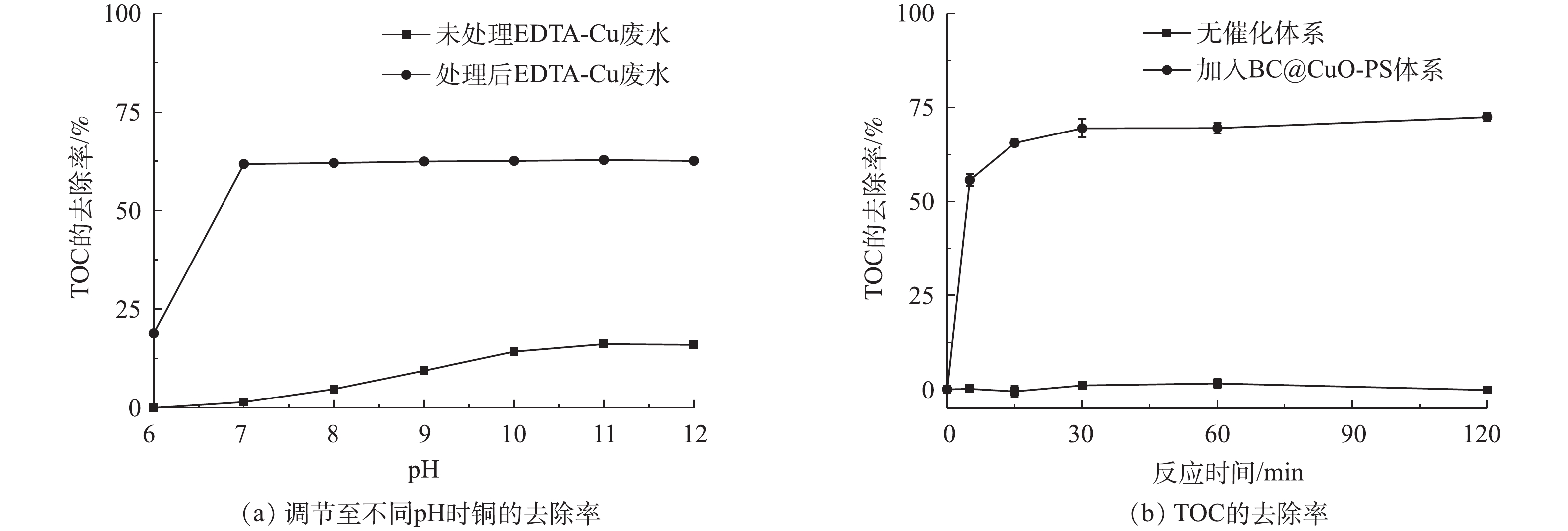
图6反映了废水中铜和TOC的去除情况。将未经处理的和在PS浓度为0.5 mmol·L−1、CuO@BC投加量为0.5 g·L−1、pH为7.0和298 K条件下处理120 min后的EDTA-Cu废水调节至不同的pH(6.0~12.0),铜的去除效果如图6(a)所示。对于未经高级氧化处理的EDTA-Cu废水,当调节pH至11.0时,铜的去除率仅有16.2%。将经过高级氧化处理后的络合废水进行调碱,当pH调节至7.0时,可去除61.8%的铜,继续提升pH,去除效果几乎没有变化。这一结果说明CuO@BC-PS体系能够有效地对EDTA-Cu进行破络,并且释放出的游离态Cu2+在pH不低于7.0的环境中较易被沉淀去除。图6(b)显示了EDTA-Cu降解过程中溶液TOC的去除情况,可以看出,在反应至120 min时,TOC的去除率达到最高,为72.5%,低于同时间EDTA-Cu的降解率。结合图6(a)中可以发现,当将处理高级氧化120 min后的废水调节pH至11.0时,铜的去除率达到最高,为62.8%,同样低于反应120 min时EDTA-Cu的降解效率。这可能是因为在EDTA-Cu的分解过程中,一部分降解中间产物无法被完全矿化,比如氨基乙酸、乙二酸和氨等,这些中间产物能与Cu2+形成稳定的配合物,从而降低了TOC和铜的去除效率,因此反应体系中EDTA-Cu的矿化率与铜的去除密切相关[16, 21]。
-
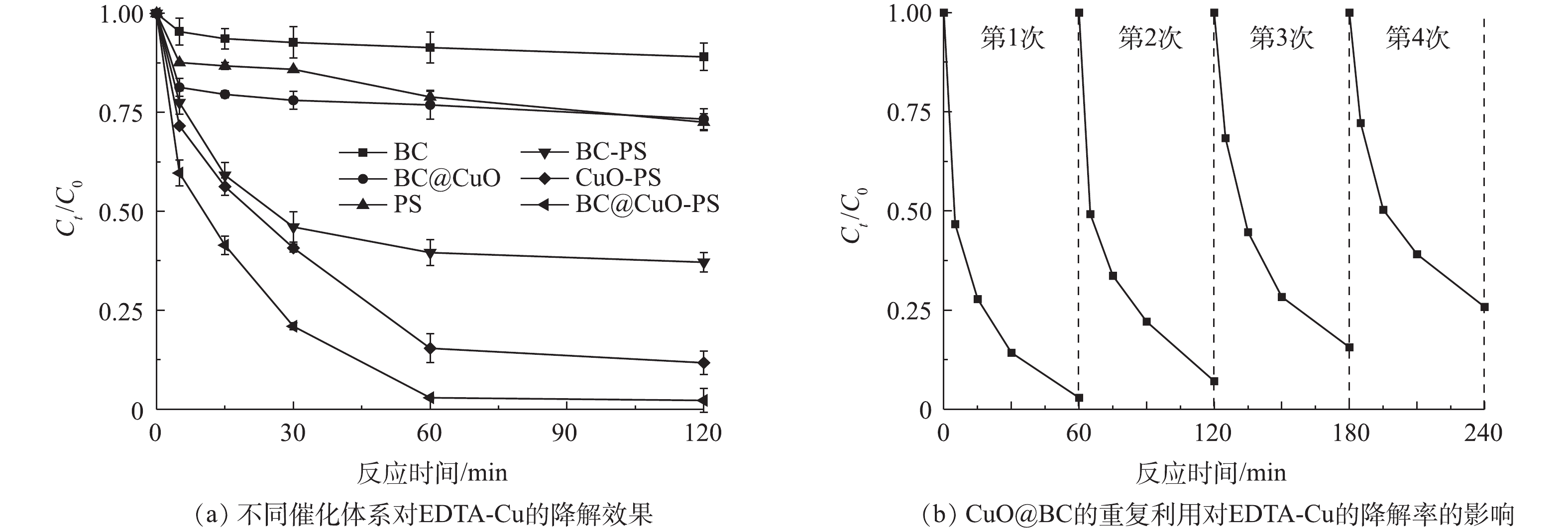
图7反映了不同体系对EDTA-Cu的去除效果及CuO@BC的重复性利用性能。在PS浓度为0.5 mmol·L−1、CuO@BC投加量为0.5 g·L−1、pH为7.0和室温为298 K条件下进行了不同催化体系的对照实验和重复性实验以分析CuO@BC-PS体系对EDTA-Cu的降解效能以及CuO@BC的稳定性。如图7(a)所示,单独的BC和CuO@BC对EDTA-Cu去除效果非常有限,充分反应120 min后降解率分别仅有11.0%和26.7%,说明单独炭基材料的物理吸附作用难以有效去除EDTA-Cu。无催化剂时,单独PS在120 min时仅可降解27.5%的EDTA-Cu;未经负载的BC对PS的活化程度也非常有限,30 min时可降解54.0%的EDTA-Cu,且随着反应时间增加,降解效果并没有明显的提升;相对于BC,CuO能够相对更加有效的活化PS,在60 min时可降解84.6%的EDTA-Cu,但随着反应时间增加,降解效率的提升并不明显;而CuO@BC可以快速高效地活化PS,在60 min时基本上可完全降解EDTA-Cu。实验结果说明CuO是活化PS的主要物质,且当CuO与BC负载后,相对于单一的催化剂,复合催化剂的性能得到进一步提升。由图7(b)还可以看出,将CuO@BC在与首次反应参数相同的条件下循环使用4次后,仍可有效降解74.2%的EDTA-Cu,说明此反应体系中CuO@BC的活化性能衰减比较缓慢。这可能是因为BC能够提高复合材料的石墨化程度和表面官能团种类,与CuO结合后使CuO@BC整体的稳定性和转移电子能力进一步增强,有利于对污染物的高效降解[11, 24]。
-
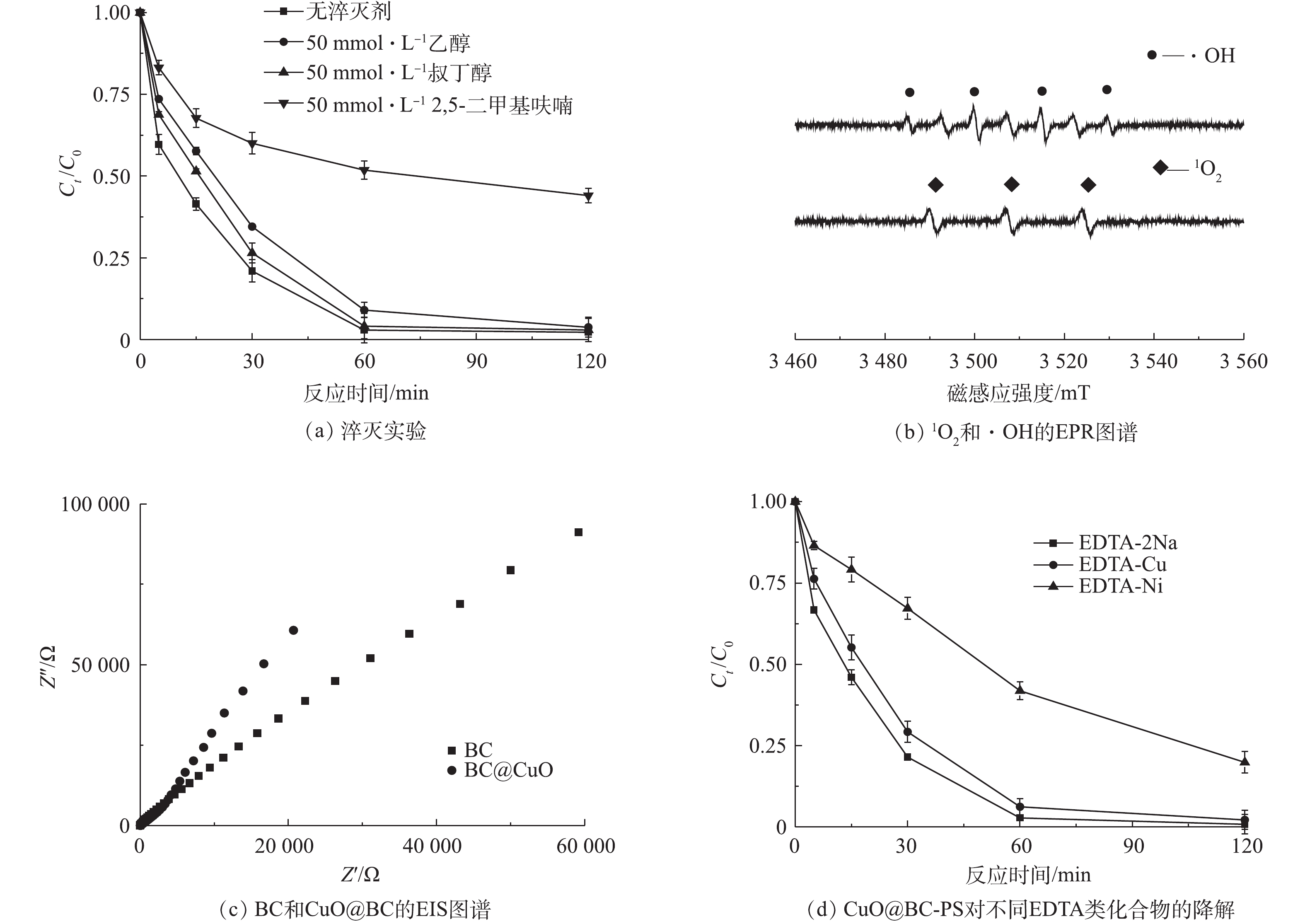
探索CuO@BC-PS体系对EDTA-Cu的降解机理,分别使用乙醇、叔丁醇和2,5-二甲基呋喃进行淬灭实验,以鉴别体系中的活性物质种类。根据表1中不同淬灭剂对活性物质的淬灭能力,可以发现乙醇和叔丁醇能够淬灭体系中
$ {\rm{SO}}_4^{ \cdot - }$ 和·OH,2,5-二甲基呋喃能够淬灭体系中的1O2。图8反映了EDTA-Cu的降解途径和Cu协同作用。由图8(a)可以看出,加入乙醇和叔丁醇对EDTA-Cu的降解影响不大,在60 min时均可达到91.0%以上的降解率,这说明反应体系中$ {\rm{SO}}_4^{ \cdot - }$ 和·OH的作用甚微。同时还可以发现,加入2,5-二甲基呋喃后,EDTA-Cu的降解受到一定程度的抑制,反应120 min后,仅有55.9%的被降解。为了准确分析反应体系中活性物质的种类和作用,采用EPR技术对自由基和1O2进行捕获并分析,结果如图8(b)所示。图谱中1∶2∶2∶1和1∶1∶1的特征峰分别证实了体系中·OH和1O2的存在,结合淬灭实验和EPR图谱的响应程度,可以看出该体系中1O2的作用要高于·OH[25-26]。此外,利用电化学阻抗谱(EIS)对CuO@BC转移电子能力进行分析,结果如图8(c)所示,可以发现,在EIS曲线中,相比于原始BC,负载后CuO@BC的阻抗曲线斜率明显增大,表明电子较易在复合材料和溶液中进行转移[27]。综上可得,CuO@BC-PS体系在对EDTA-Cu进行解络的过程中,自由基的作用甚微,CuO@BC主要通过非自由途径活化PS,1O2和电子转移是促进EDTA-Cu降解的主要原因。这一结论与先前关于苯环类污染物的降解研究结果较为相似[12]。此外,在保持降解参数相同的情况下,利用CuO@BC-PS体系分别对EDTA-2Na、EDTA-Cu和EDTA-Ni进行降解去除,以分析溶液中不同重金属对CuO@BC-PS的协同作用。通过图8(d)可见,CuO@BC-PS对EDTA-2Na和EDTA-Cu的降解效率稍高于EDTA-Ni,可能原因是EDTA-Cu中的Cu以及溶液中游离态的Cu2+能够在一定程度上对PS进行活化,但是活化作用并不明显[18]。 -
收集利用EDTA-草酸对Cu、Ni和Zn污染土壤淋洗处理后的淋洗废液,适当稀释后淋洗废液使用液中EDTA、Cu、Ni和Zn的浓度分别为253.6、20.35、4.65和1.56 mg·L−1。根据污染物浓度对氧化剂和催化剂用量进行适当调整,在5 g·L−1的CuO@BC、5 mmol·L−1的PS和初始pH为7.0的室温条件下,EDTA和多种金属的络合物(EDTA-Cu/Ni/Zn)、COD和TOC的降解情况如图9所示。在反应开始后的10 min内,EDTA-Cu/Ni/Zn被快速分解,当反应至30 min时,EDTA-Cu/Ni/Zn的去除率可达到67.9%,当反应60 min后,该体系可去除67.9%的EDTA-Cu/Ni/Zn、60.0%的COD和54.1%的TOC,随着反应的继续进行,EDTA-Cu/Ni/Zn、COD和TOC的去除率变化不大。可以发现,CuO@BC-PS对实际废水的处理效能较好,对EDTA-Cu/Ni/Zn、COD和TOC的去除规律与模拟废水较为相似,这是因为EDTA与常见二价重金属络合方式大致相同,产物为五元环结构配合物。另一方面,相比于模拟废水中的EDTA-Cu,CuO@BC-PS体系对EDTA-Cu/Ni/Zn的降解效率偏低,这可能是因为实际淋洗废水中同时存在多种重金属和小分子有机物,从而影响CuO@BC-PS体系对EDTA-Cu/Ni/Zn的去除效果[5]。
2.1. CuO@BC的表征结果
2.2. PS浓度对EDTA-Cu降解的影响
2.3. CuO@BC的投加量对EDTA-Cu降解的影响
2.4. 初始pH和温度对EDTA-Cu降解的影响
2.5. 铜和TOC的去除
2.6. 不同体系对EDTA-Cu的去除效果及CuO@BC的重复性能评估实验
2.7. EDTA-Cu的降解途径和协同机制分析
2.8. CuO@BC-PS对实际废水的处理效能
-
1) CuO@BC-PS体系对EDTA-Cu废水具有高效的破络和降解效率,在同时考虑成本和去除效率的情况,最佳降解条件为:PS浓度为0.5 mmol·L−1、CuO@BC投加量为0.5 g·L−1、初始pH=7.0。在室温和上述优化条件下反应60 min后,可降解97.1%的EDTA-Cu,TOC去除率为69.6%,调碱后铜的去除率可达到62.2%。
2) CuO@BC在该反应体系内较为稳定,经重复使用4次后,对EDTA-Cu的降解率仍可达到74.2%,材料重复性和稳定性良好。
3)淬灭反应结果和EPR图谱阐明了CuO@BC-PS体系作用的机理,体系
$ {\rm{SO}}_4^{ \cdot - }$ 、·OH的作用较弱,CuO@BC以非自由基途径活化PS,主要通过1O2和电子转移实现EDTA-Cu的氧化降解。



 下载:
下载: