-
基于硫酸根自由基(
$ {\rm{SO}}_4^{ \cdot - } $ )的高级氧化技术具有选择性小、反应条件温和、高效、快速等优势,已引起国内外学者的广泛关注[1-2]。过硫酸盐(PS)在过渡金属、碱、热等作用下,可激发过硫酸盐中的过氧基(—O—O—)产生硫酸根自由基[3-5]。过渡金属中的Fe2+因来源广泛、环境友好,因此,Fe2+活化是目前研究最多的一种活化方法。Fe2+活化PS也具有一些局限性,在加入少量Fe2+后,Fe2+被快速氧化成Fe3+,无法再生,故不利于反应持续有效地进行;但增加Fe2+的用量,会导致体系中自由基的淬灭反应。因此,Fe2+/PS体系中可引入具有还原性的物质,以保证PS活化过程持续有效进行。近年来,一些含有氧化敏感型官能团结构的有机物如(醌类[6]、酚类[7-8]、醇类[9])被证实对过硫酸盐具有活化作用。而这些有机化合物主要是通过自身含氧官能团电子的得失,形成苯氧自由基,从而引发电子转移激活PS。FANG等[6]的研究表明,在一定条件下,对苯醌与对苯酚之间通过电子的转移,可生成具有活化PS作用的半醌自由基(HQ·)。邻苯醌在水相中有类似于对醌的3种形态:完全还原态(H2Q)、中间氧化态(HQ·)、完全氧化态(Q)[10]。多巴胺(DA)是一种具有邻苯二酚结构的有机化合物,可通过氧化自聚形成具有酚醌结构的聚多巴胺[11-12],其酚醌之间也可进行电子转移[13],可促进PS的活化生成硫酸根自由基。
本研究以PP非织造材料为基体,采用多巴胺(DA)和聚乙烯亚胺(PEI)共沉积法制备了PDA/PEI@PP材料,多巴胺和聚乙烯亚胺可通过交联共沉积作用附着在固体表面[14],制备得到稳定性好、亲水性强且具有酚醌共存结构的复合材料。LENG等[15]的研究表明,具有电子穿梭作用的苯二酚对Fe3+具有还原作用,而PDA/PEI@PP表面也具有邻苯二酚结构[14, 16],对Fe3+可能具有一定的还原作用。因此,本研究将PDA/PEI@PP引入Fe2+活化过硫酸盐体系中,考察了同时具有邻苯醌和邻苯二酚结构的PDA/PEI@PP材料对Fe2+/PS体系的协同催化活化性能及相关的机理,探讨了PDA/PEI@PP材料在过硫酸体系中对Fe3+的还原作用,旨在为提高Fe2+活化PS氧化降解污染物提供一种新途径。
全文HTML
-
主要原料与试剂:聚丙烯非织造布(PP)为工业级;多巴胺(DA)、三羟甲基-氨基甲烷(Tris)、聚乙烯亚胺(PEI)、过硫酸钠(Na2S2O8,PS)、七水合硫酸亚铁、三氯化铁(无水)、酸性红B(acid red B,ARB)、碘化钾(KI)、碳酸氢钠、氢氧化钠、浓硫酸、盐酸、5,5-二甲基-1-吡咯啉-N-氧化物(DMPO)、邻菲罗啉、无水乙醇、叔丁醇均为分析纯,实验用水为超纯水。
-
配制0.01 mmol·L−1的Tris(三羟甲基-氨基甲烷)缓冲溶液,调节pH为8.5,加入一定量的多巴胺和聚乙烯亚胺,按照DA∶PEI=1∶1的质量比,配制成DA/PEI混合液。将用乙醇润湿的非织造布(1.0 g)置于DA/PEI混合溶液中,在恒温水浴中,25 ℃振荡反应12 h。反应后,用蒸馏水洗去非织造布表面未反应的聚合物,并在60 ℃的恒温干燥箱中烘至恒重,得到PDA/PEI@PP样品。通过扫描电镜(SEM)和傅里叶红外光谱(FT-IR)对PDA/PEI@PP材料表面形貌、结构和官能团进行分析。
-
在实验过程中,向200 mL反应体系中分别投加PDA/PEI@PP、FeSO4·7H2O和过硫酸盐(PS)等其他组分,体系中ARB的浓度为0.2 mmol·L−1。将反应液置于恒温水浴中,在25 ℃下均速搅拌,分别在0、2、5、10、20、30、40、50、60、70、80、90 min时移取3 mL待测样于比色皿中,测定ARB浓度。
-
采用分光光度法测定ARB的浓度,采用KI显色法[17]测定反应体系中PS的浓度,采用邻菲罗啉法测定溶液中的Fe2+。以DMPO作为捕捉剂,应用电子自旋共振波谱(ESR)仪对体系中的自由基进行检测和分析。ARB的去除率的计算方法如式(1)所示。ARB催化降解的反应符合动力学一级反应的特征,准一级动力学方程如式(2)所示。PS的分解率的计算方法如式(3)所示。
式中:R为ARB的去除率;C0为ARB的初始浓度,mg·L−1; Ct为t时的ARB浓度,mg·L−1; k为反应速率常数,min−1;y为与反应体系相关的参数。
$R'$ 为PS的分解率;$C_0^{'}$ 为PS的初始浓度,mg·L−1;$C_t^{'}$ 为t时的PS浓度,mg·L−1。
1.1. 实验材料
1.2. PDA/PEI@PP材料的制备及表征
1.3. 过硫酸盐体系催化ARB的降解实验
1.4. 分析与测定方法
-
图1(a)和图1(b)分别为PP非织造布和PDA/PEI@PP的冷场扫描电镜图。由图1(a)和图1(b)可见,PP非织造布由随机堆叠的纤维单丝组成,单个纤维表面比较光滑,材料具有较高的渗透性和孔隙率,这有利于反应分子在材料表面的扩散和传递。而PDA/PEI@PP仍具有非织造布的空间结构,纤维之间有贯穿的孔道,这有利于纤维表面的反应位点参与反应。为能够进一步清晰地观察纤维表面聚合物的复合状态,采用热场扫描电镜对PP非织造布和PDA/PEI@PP的表面纤维进行了分析,结果如图1(c)和图1(d)所示。对比两者可见,PDA/PEI@PP纤维表面出现明显且均匀的复合层,为后续的活化反应提供了更多的反应位点。
-
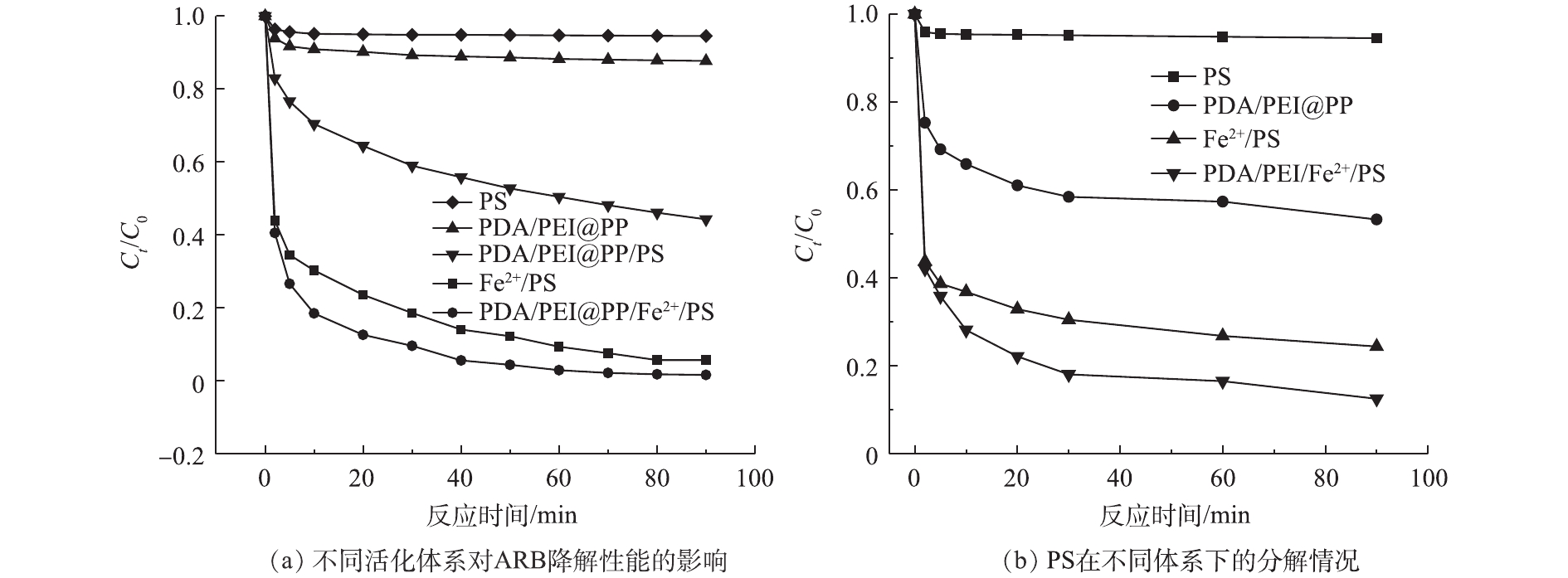
在初始ARB浓度为0.2 mmol·L−1、PS浓度为2 mmol·L−1、Fe2+浓度为0.5 mmol·L−1,PDA/PEI@PP投加量为2 g·L−1的条件下,考察了不同体系(PS、PDA/PEI@PP、PDA/PEI@PP/PS、Fe2+/PS和PDA/PEI@PP/Fe2+/PS)对ARB和PS浓度的影响,实验结果见图2。如图2(a)所示,溶液中只存在PS时,ARB浓度几乎没有变化;当PDA/PEI@PP与ARB共存时,90 min内的ARB减少了12%,这主要是由于复合材料对染料具有一定的吸附作用导致的;在PDA/PEI@PP/PS体系中,ARB可在90 min后降解了56%,这表明PDA/PEI@PP可以活化PS,并产生氧化性物质,其可使得染料被氧化降解。在Fe2+/PS体系中增加PDA/PEI@PP复合材料后,可对ARB的氧化降解速率有所提高。由图2(b)可见,在PDA/PEI@PP反应体系中,PS被分解了47%。Fe2+/PS和PDA/PEI@PP/Fe2+/PS体系中的PS分解率分别为76%和87%,PDA/PEI@PP/Fe2+/PS相比于Fe2+/PS体系PS的消耗量有明显的增加,这说明PDA/PEI@PP也参与了PS的活化,从而加快ARB的氧化降解过程。
由图3可知,Fe2+/PS和PDA/PEI@PP/Fe2+/PS体系中ARB的降解均符合准一级动力学方程。Fe2+/PS和PDA/PEI@PP/Fe2+/PS体系的反应动力学的拟合系数R2分别为0.901和0.906,其反应速率常数k分别为0.026 min−1和0.040 min−1。结果表明,PDA/PEI@PP的引入明显增加了体系的活化降解速率。
-
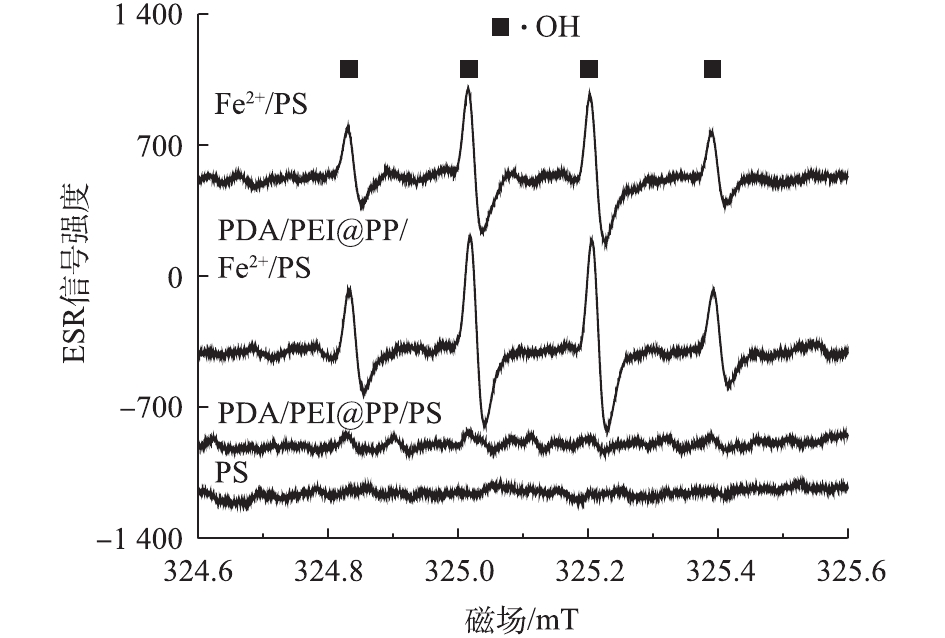
为了进一步证明PDA/PEI@PP可通过活化PS增加Fe2+/PS体系中强氧化自由基的含量,本研究对不同体系(PS、PDA/PEI@PP/PS、Fe2+/PS和PDA/PEI@PP/Fe2+/PS)进行了ESR分析。如图4所示,在初始PS和Fe2+浓度分别为2 mmol·L−1和0.5 mmol·L−1,PDA/PEI@PP投加量为2 g·L−1条件下,当反应进行到20 min时,PDA/PEI@PP/Fe2+/PS体系相比于Fe2+/PS体系,DMPO-OH峰的强度有所增强,且在PDA/PEI@PP/PS体系中也检测到了明显的DMPO-OH峰。结果表明,PDA/PEI@PP可以活化PS产生强氧化自由基。由于硫酸根自由基在一定条件下可以转换成信号较强的羟基自由基[18-19],反应如式(4)所示,因此,检测到的信号主要为羟基自由基。
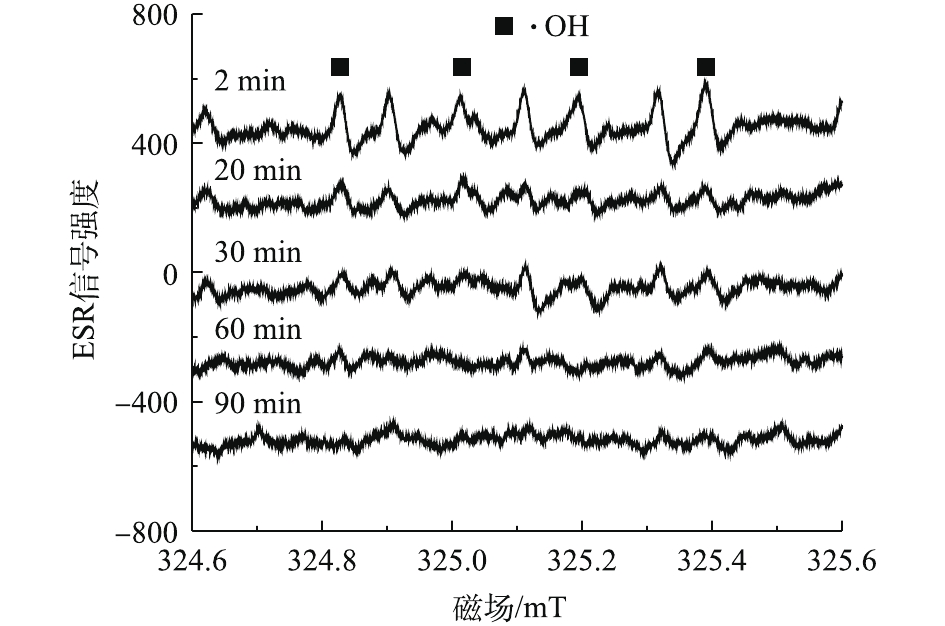
如图5所示,为了对PDA/PEI@PP活化PS的过程做进一步分析,实验对PDA/PEI@PP/PS体系在不同反应时间点的自由基产生情况进行了ESR检测。由于PDA/PEI@PP/PS体系中羟基自由基信号较弱,反应液中干扰物对羟基自由基的强度影响较大,导致PDA/PEI@PP/PS中的羟基自由基的峰型不明显,但随着反应的进行,峰的强度有所变化。当反应进行到2 min时,PDA/PEI@PP/PS溶液中DMPO-OH峰强度相对较强。随着反应时间的增加,PDA/PEI@PP/PS中的自由基信号逐渐减弱,在90 min时自由基信号基本消失。由此可见,PDA/PEI@PP活化PS产生的自由基主要在短时间内快速生成,可对ARB起到氧化降解作用。
-
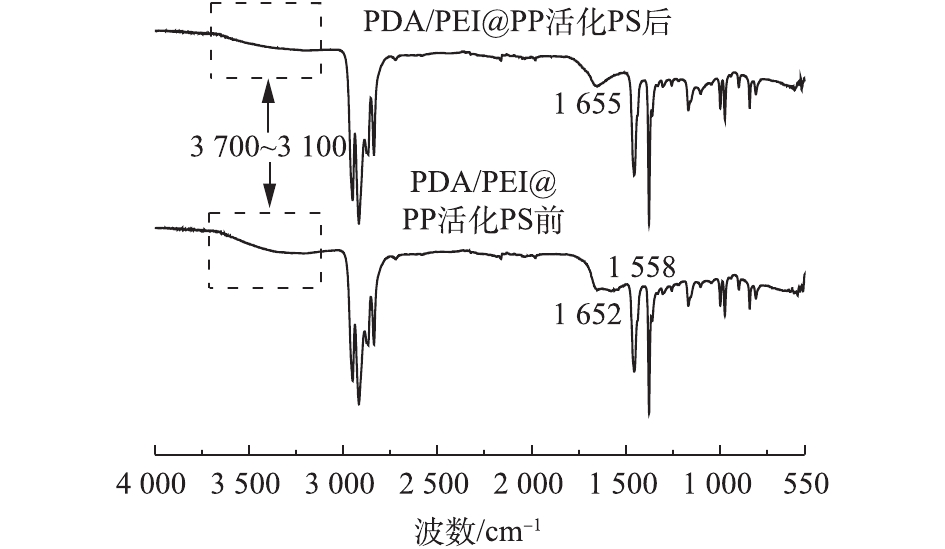
为了进一步证明PDA/PEI@PP上的邻苯二酚基团和邻醌基对PS的活化起主要作用,研究对活化PS前后的PDA/PEI@PP进行FT-IR光谱分析(图6)。如图6所示,在3 700~3 100 cm−1处的宽吸收峰归属于酚羟基的O—H的伸缩振动峰或氨基上的N—H振动峰[20],其峰的强度在活化PS后有所减弱,且在1 655 cm−1处出现了明显的尖峰,其可归属于醌基峰[21]。
由此可以推断,PDA/PEI@PP表面上的邻苯二酚基团(H2Q)可能与邻醌基(Q)在溶液中发生反应,生成具有活化PS的半醌自由基(HQ·)[13, 22],HQ·能够活化PS产生过硫酸根自由基,同时HQ·失电子,转化为邻醌基(Q),如式(5)和式(6)所示。因此,在活化PS后,PDA/PEI@PP上醌基特征峰增强,而酚羟基振动峰有所减弱。
-
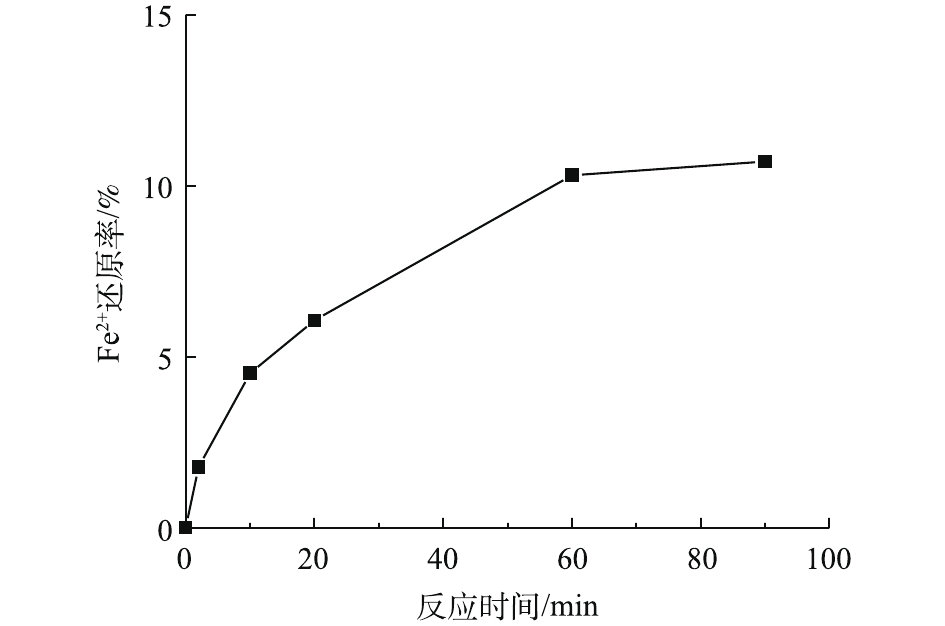
相关研究[15, 23]表明,对苯酚和半醌自由基因具有电子传递的作用,可以将Fe3+还原为Fe2+。而在PDA/PEI@PP表面上含有酚醌共存结构,为了进一步验证PDA/PEI@PP材料对Fe3+的还原作用,对PDA/PEI@PP/Fe3+体系中的Fe2+进行了检测,结果如图7所示。由图7可知,在Fe3+初始浓度为0.5 mmol·L−1,PDA/PEI@PP投加量为2 g·L−1时,反应60 min后,溶液中检测到的Fe2+生成率为10%。结果表明,PDA/PEI@PP材料对Fe3+具有还原作用,可能存在的反应[15]如式(7)和式(8)所示。随着反应的进行,PDA/PEI@PP表面邻苯二酚基团因提供电子,导致酚羟基不断被消耗,从而生成不具有还原作用的醌基,因此,对Fe3+还原效果也随之逐渐减弱。
基于PDA/PEI@PP材料对PS活化作用和对Fe3+还原作用的结果,可以看出,PDA/PEI@PP对Fe2+活化过硫酸盐的促进作用机制主要体现在2个方面(图8):PDA/PEI@PP表面的邻苯二酚基团与邻醌基在溶液中反应,生成具有活化PS的半醌自由基,在短时间内产生硫酸根自由基和羟基自由基;PDA/PEI@PP表面的邻苯二酚基团和半醌自由基对Fe3+具有还原作用又生成了部分Fe2+,从而促进PS的活化。
-
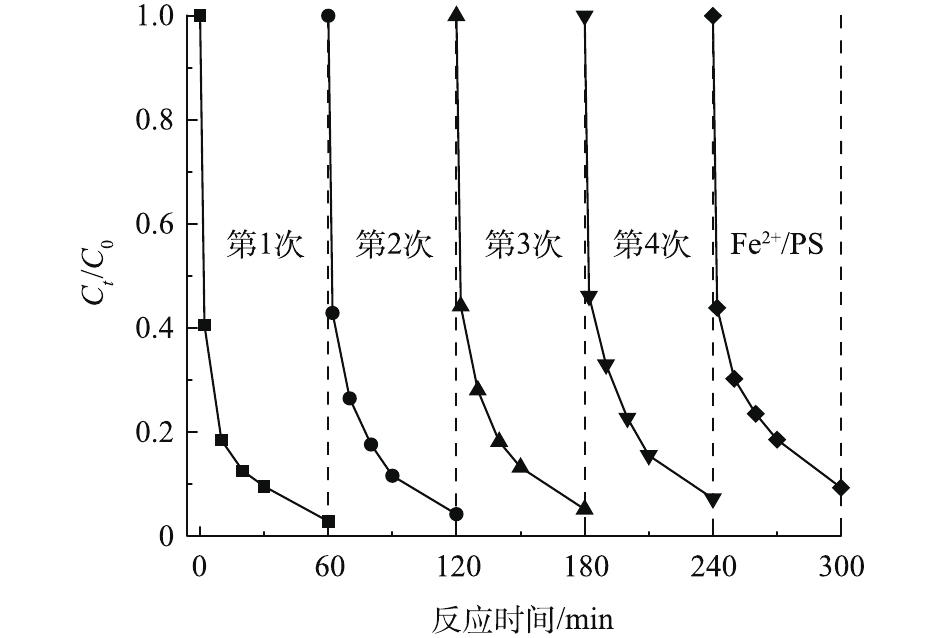
在初始ARB浓度为0.2 mmol·L−1、PS浓度为2 mmol·L−1、Fe2+浓度为0.5 mmol·L−1、PDA/PEI@PP投加量为2 g·L−1的条件下,考察了PDA/PEI@PP在Fe2+/PS体系中的重复利用性,实验结果如图9所示。由图9可知,当PDA/PEI@PP在Fe2+/PS体系中重复使用4次后,体系对ARB的催化活化性能已经接近单纯的Fe2+/PS体系。重复多次使用后,PDA/PEI@PP材料含有的邻苯二酚基团几乎全部转化为醌基基团,无法对PS进行活化以及对Fe3+进行还原,所以对Fe2+/PS体系的促进作用下降明显。
2.1. SEM分析
2.2. PDA/PEI@PP/Fe2+/PS体系中过硫酸盐对ARB的催化特性
2.3. 不同体系下产生的自由基特性
2.4. PDA/PEI@PP活化PS的可能机理
2.5. PDA/PEI@PP对Fe3+的还原作用
2.6. PDA/PEI@PP在Fe2+/PS体系中的可循环利用性
-
1)冷场扫描电镜分析结果表明,PDA/PEI@PP保持PP非织造材料原有的孔道结构。通过热场扫描电镜,对材料纤维表面的分析结果表明,非织造纤维上均匀附着一层聚多巴胺/聚乙烯亚胺复合物。
2)将PDA/PEI@PP引入Fe2+/PS体系可有效促进Fe2+/PS体系对ARB的降解。Fe2+浓度的增加,对PDA/PEI@PP促进Fe2+/PS体系的ARB的降解和反应速率均有影响。
3) PDA/PEI@PP的引入增加了Fe2+/PS体系中羟基自由基的产率,且PDA/PEI@PP可在短时间内活化PS产生硫酸根自由基和羟基自由基。
4) PDA/PEI@PP上的邻苯二酚基团可与邻醌基反应,生成半醌基进而活化PS。通过PDA/PEI@PP对Fe3+的还原作用研究,发现PDA/PEI@PP上的邻苯二酚基对Fe3+也具有还原作用。
5)随着PDA/PEI@PP重复利用次数的增加,PDA/PEI@PP表面的邻苯二酚基团不断被消耗,从而生成醌基,因此,PDA/PEI@PP在Fe2+/PS体系中的促进作用也随之逐渐减弱。






 下载:
下载: