-
盐酸四环素(TC)是一种常见的抗生素,因其具有生产量大、使用范围广、污染面积大等特点,并且在地表水中的含量达0.07~1.34 μg·L−1,从而引起全社会的高度关注[1-4]。目前,各种技术已被应用于降解和去除水体中抗生素,主要有活性污泥法[5]、吸附[6-7]、氯化[8]、高级氧化[9-10]、光解/光氧化[11-12]、声催化[13]。在上述过程中,由于抗生素的生物活性、极性和持久性,大多数生物过程和高级氧化处理均不足以使其降解和矿化[14]。
传统微电解技术在20世纪首次被用于废水预处理中,因其具有成本低、操作简单、效率高等优点被广泛应用于各种污染物的去除,尤其对含苯环类废水以及重金属有机污染物处理潜力巨大[15-17]。在微电解系统中,将铁(阳极)、碳(阴极)和废水(电解质)混合并接触,在他们之间形成大量的电流微观电池,其中电子转移、凝聚、沉积和吸附的机理同时发生,从而去除污染物[18-20]。不足之处是,在铁碳微电解体系中,常存在电极材料易失活、填料板结堵塞,气液传质效率低等问题。
近年来,微纳米气泡在水处理领域取得了巨大进步[21]。微纳米气泡(MB)是直径在几十nm~几十μm的气泡,而常规气泡(CB)是直径为几十mm~几cm的气泡,与之相比,微纳米气泡具有比表面积大、停留时间长、传质效率高、界面ζ电位高等性质。当微气泡破裂时,气液界面发生剧烈变化会将高浓度离子积蓄的化学能迅速释放,从而激发产生大量羟基自由基[22-23]。微纳米气泡在上浮过程首先是气泡与固态或胶体污染物结合,将污染物带到水面后再被去除,而气泡与污染物结合的过程是非常重要的环节,可以有效提高气液传质效率,促进臭氧分解产生羟基自由基,将微气泡与臭氧氧化技术结合起来有利于废水中有机物的降解[24-25]。微气泡处理苯酚溶液2 h,实验测得苯酚的含量降低了60%,结果表明,微气泡破裂产生的羟基自由基在苯酚的分解过程中起着重要的作用[26]。当用臭氧微纳米气泡处理酸性大红3R废水时,废水的脱色速率和去除率远高于传统气泡系统,且微气泡系统的臭氧分解系数是传统气泡系统的6.2倍[27]。以工业颗粒活性炭(AC)催化微气泡(MB)臭氧化法处理合成酸性大红3R废水,其效果明显优于单独的常规气泡(CB)和微气泡(MB)臭氧化法,这是因为微纳米气泡显著地促进了化学反应、物理吸附、气液界面的传质[28]。然而,将微气泡技术与铁碳微电解处理相结合的研究却鲜见报道。2种技术相结合既发挥了微气泡水中停留时间长、气液传质效率高等优点,一定程度上又解决了铁碳填料易板结堵塞,气液传质效率低的问题,可提高污染物去除率。
为有效处理盐酸四环素制药废水,本研究将微纳米气泡技术与传统铁碳微电解技术相结合,研究了反应时间、铁碳投加量、pH、MB进气量等因素对盐酸四环素去除率的影响。在此基础上,通过紫外可见光谱扫描以及液质联用(LC-MS)分析了盐酸四环素在MB/Fe-C体系的反应机理及可能的降解途径,以期为制药废水的处理提供参考。
全文HTML
-
盐酸四环素(C22H25ClN2O8)购自上海阿拉丁生化科技股份有限公司;浓硫酸(H2SO4)、氢氧化钠(NaOH)、甲醇(CH3OH)均购自成都市科龙试剂厂,实验用水为超纯水。
-
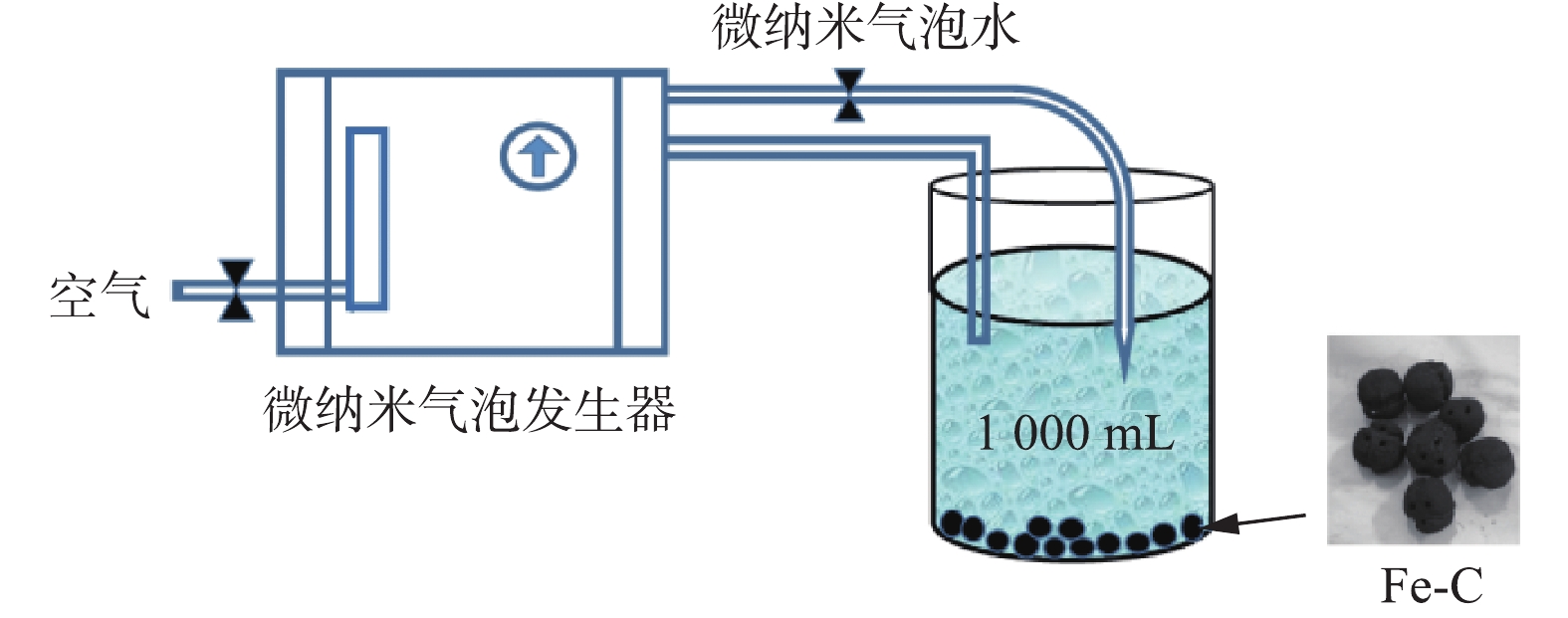
铁碳(Fe-C)材料: 呈圆球状,粒径为1.5 cm×2 cm,比重为1.2 t·m−3,比表面积为1.3 m2·g−1,空隙率为70%,物理强度为1 200 kg·cm−2,化学成分为铁、碳、二氧化硅等其他催化元素(萍乡拓步环保科技有限公司)。用超纯水将Fe-C材料多次冲洗干净。
-
1)实验装置和仪器。实验装置见图1,采用自制的有机玻璃反应器,体积为2.0 L,盐酸四环素溶液经过微纳米气泡发生器产生微纳米气泡,微纳米气泡协同铁碳微电解在反应器中连续循环处理盐酸四环素废水。实验中所用的分析仪器包括:X-射线衍射(XRD,BRUKER D8,德国),傅里叶变换红外分光光度计(FT-IR,岛津,日本),紫外分光度计(UV1102Ⅱ),微纳米气泡发生器(LF-1500,上海行恒科技公司),总有机碳分析仪(TOC-LCPH,岛津,日本),pH计(PHS-3C),液相色谱-质谱联用(Afilent1260-6240)。
2)实验方法。称取一定量的Fe-C材料加入到1 000 mL浓度为20 mg·L−1的盐酸四环素溶液中,调节溶液pH,同时接通微纳米气泡反应器,反应至溶液呈乳白色,每隔15 min取样在离心机中以4 900 r·min−1的转速离心5 min,取上清液置于紫外分光光度计在最大吸收波长为358 nm处测量其吸光度,再根据式(1)计算TC的降解率。
式中:R为TC的降解率;A0为TC溶液的初始吸光度;At为反应t时刻TC溶液的吸光度。
液质联用色谱条件:流动相为65%甲醇和35%去离子水的混合物,流速为1.0 mL·min−1,注射体积为10 μL,柱温为30 ℃,电喷雾离子源(ESI),碰撞电压为135.0 V,毛细管温度为300 ℃,负离子扫描模式,全扫描范围为50~500。
1.1. 实验药品
1.2. 实验材料
1.3. 实验装置、仪器与方法
-
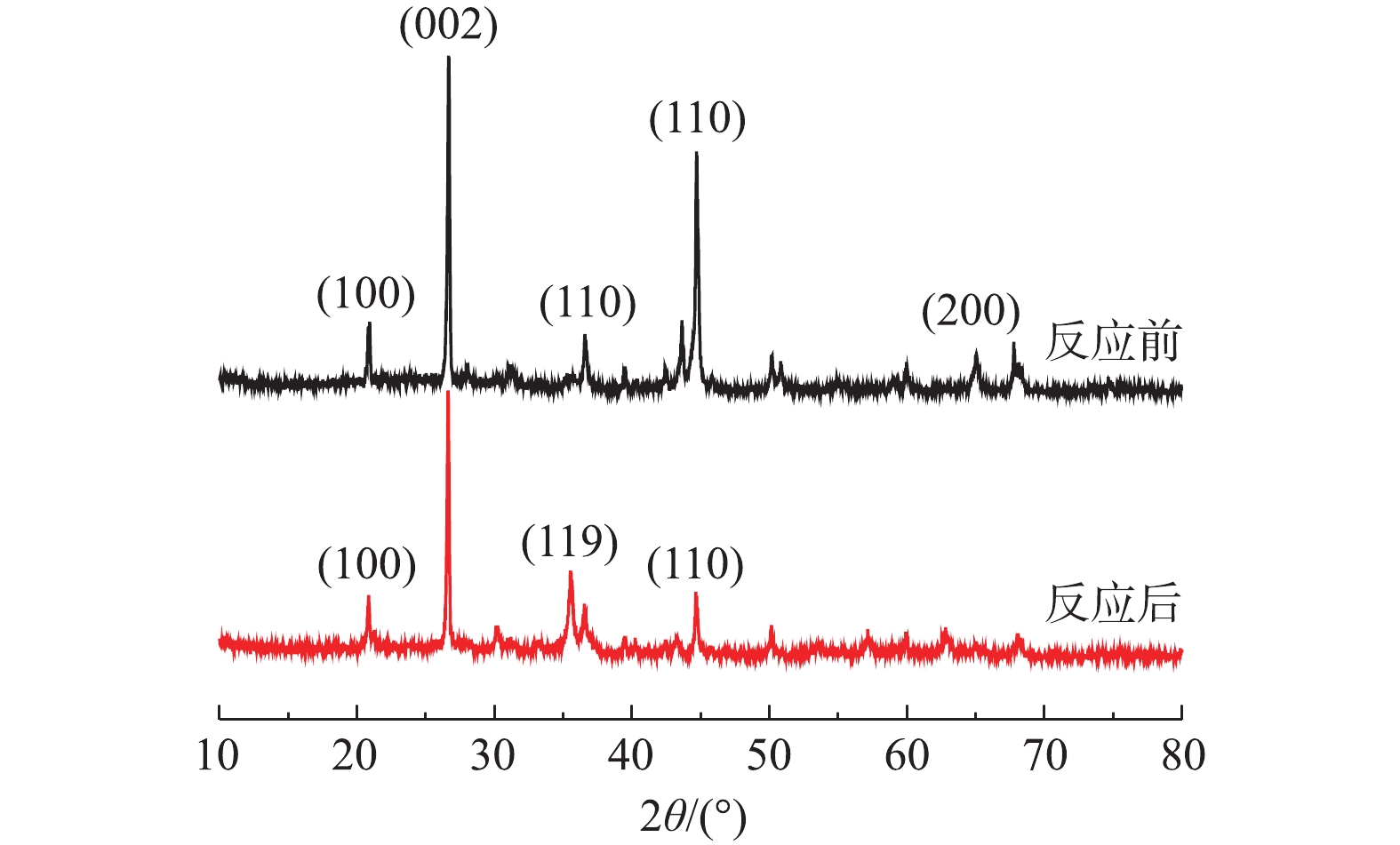
1) XRD分析。图2为铁碳微电解反应前后的XRD图,对比标准PDF卡片,发现Fe-C材料反应前在20.86°、26.55°、44.67°、65.16°等附近有明显尖峰,并且在其他位置没有明显的尖峰存在,其中铁主要集中44.67°(110)及65.16°(200)晶面,碳集中在26.55°(002)晶面,20.86°(100)晶面为SiO2,这说明Fe-C在反应前含有大量的单质铁和碳和少量的SiO2。在MB/Fe-C体系反应后,在20.86°、26.55°、35.55°、44.67°、50.14°的尖峰依然存在,但是其强度均有所减弱,说明酸性条件下微电解反应过程中消耗了铁和碳。但是在反应后的Fe-C中发现了较强的Fe3O4、Fe2O3的尖峰,其分别对应为62.63°(440)和35.64°(119),这说明体系中的铁转化为铁的氧化物。
2) FT-IR分析。在Fe-C投加量为100 g·L−1、初始pH=3、MB进气量为30 mL·min−1的条件下,Fe-C材料FT-IR图谱如图3所示,波长为3 200~3 700 cm−1属于结构—OH或—NH2的拉伸振动,其中1 631 cm−1的谱带归因于被吸附水分子的—OH弯曲振动引起的,1 076 cm−1处的谱带证明了Si—O—Si键的存在, 其对应Si=O的伸缩振动或者是C—N和C—O的伸缩振动[28],565 cm−1的谱带是属于Fe—O的伸缩振动引起的。在MB/Fe-C体系反应后,3 200~3 700、1 631、1 076、565 cm−1等的谱带强度均有所减弱,说明在铁碳微电解处理过程中,可能通过絮凝、共沉淀、吸附或颗粒间桥连等作用与TC发生反应,从而转移到含铁污泥中。
-
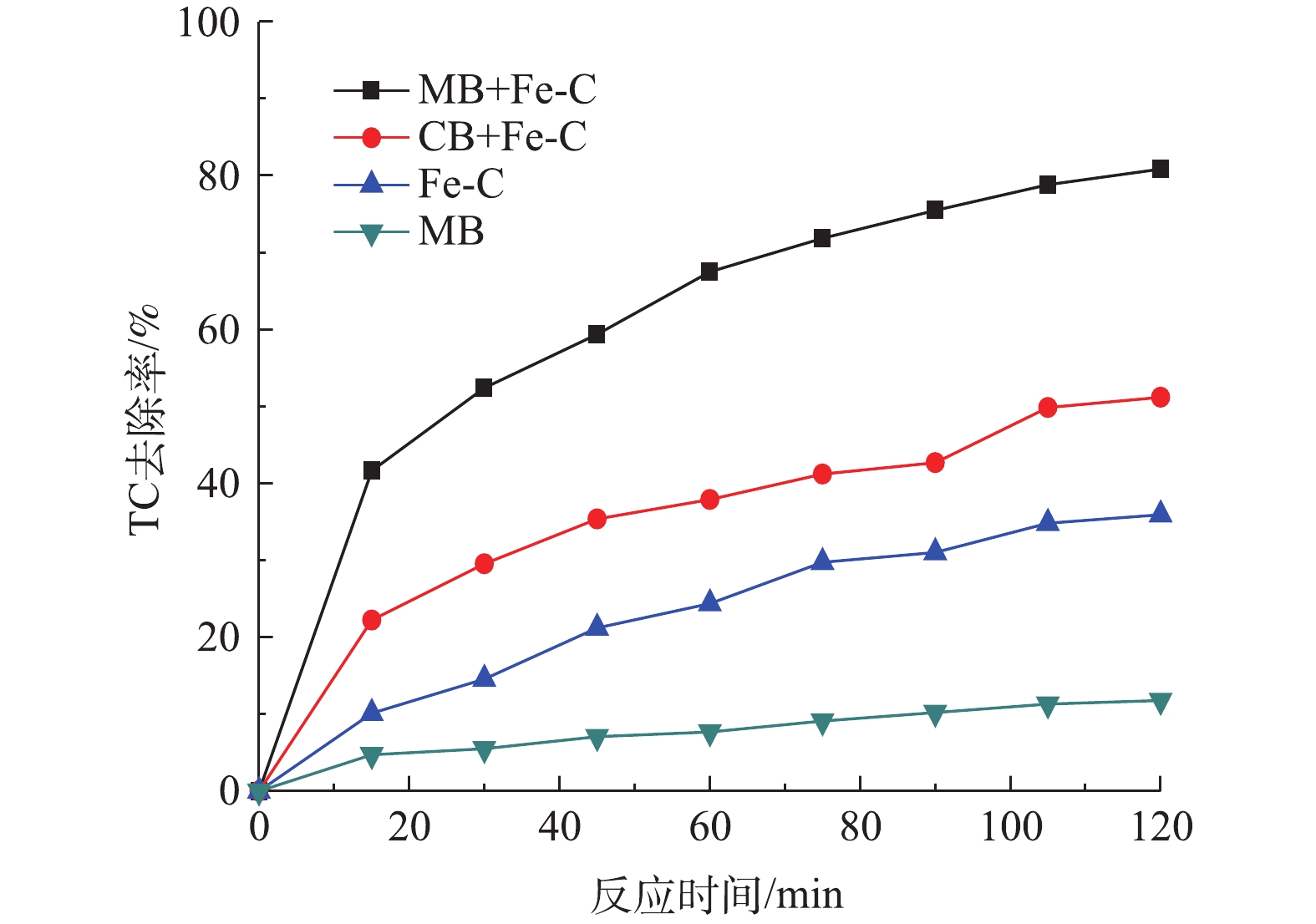
图4为在不同反应体系下TC的降解效果对比。由图4可发现,在单独MB条件下,当MB进气量为30 mL·min−1时,TC去除率为11.79%,变化不明显,这说明TC在MB条件下去除效果较弱。单独Fe-C经2 h反应后对TC的去除率也仅仅为35.92%,说明单独MB或者Fe-C难以降解TC。在常规气泡(CB)的条件下,CB/Fe-C体系对TC的去除率有一定的提升,经2 h常规气泡曝气反应后,CB/Fe-C体系对TC的降解率达到51.19%,这说明通过CB曝气可提高Fe-C对TC的降解,这是由于常规气泡曝气增加了Fe-C与溶液的接触反应效率所致。然而,在TC浓度为20 mg·L−1时,经2 h反应后,MB/Fe-C体系对TC的去除率达到80.84%,远高于相同条件下单独MB和单独Fe-C与CB/Fe-C体系,说明MB协同Fe-C微电解体系具有最优分解TC的能力。
-
控制溶液pH=3.0,Fe-C投加量为100 g·L−1,MB进气量为30 mL·min−1,考察了反应时间(30、60、90、120、150 min) 对TC的去除效果影响,结果如图5所示。随着反应的进行,TC的去除率稳步上升,反应120 min时可达到80.84%,之后趋于稳定。这是因为随着反应时间的延长,溶液中产生[H] (还原氢)、·OH、Fe2+、Fe3+的量增多,不仅有利于氧化还原反应的进行,还能促使Fe2+与·OH更好地发生催化氧化反应,增加对污染物的去除效果,但是,经过一定时间后,铁屑表面会生成一层致密的氧化膜,发生钝化并阻碍反应的进行。因此,确定本实验的最佳曝气反应时间为120 min。
-
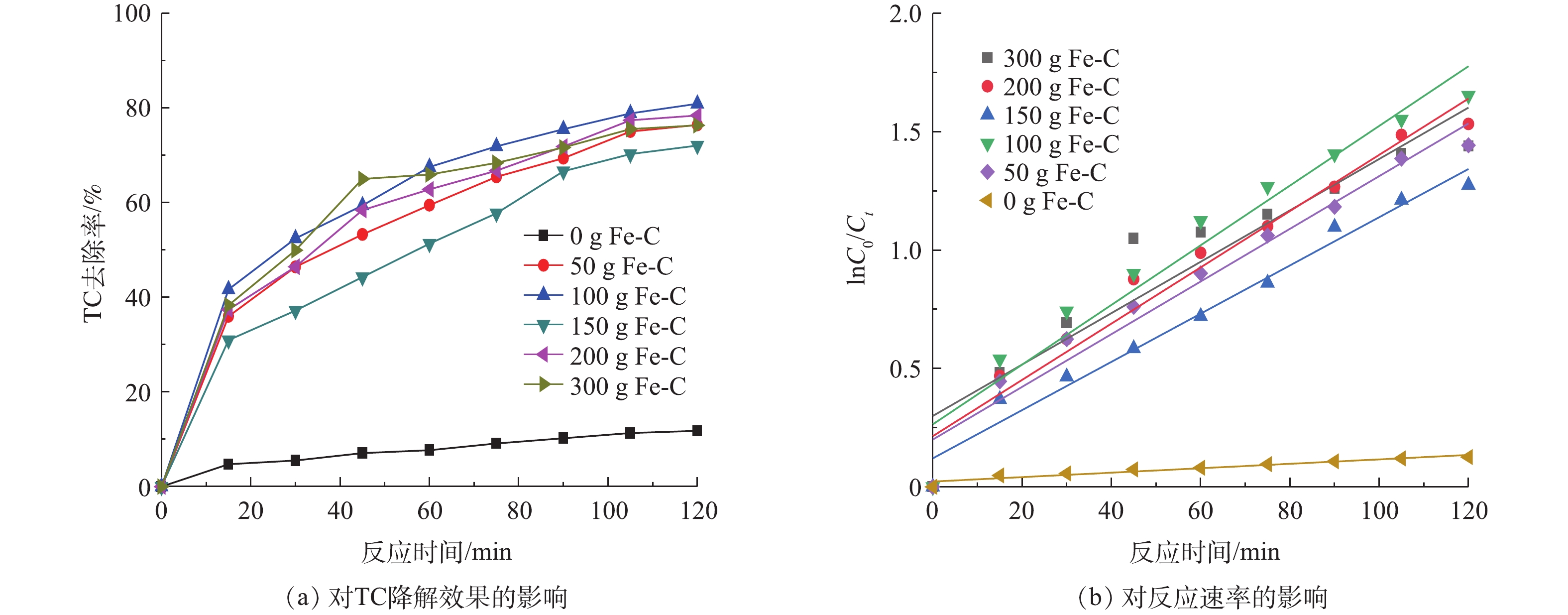
图6(a)为不同Fe-C投加量对TC降解的影响,图6(b)为Fe-C投加量一级反应动力学图谱。随着Fe-C投加量的增大,TC去除率呈现先增加后减弱的趋势。当Fe-C投加量为50 g·L−1时,反应2 h后,TC去除率为76.36%。而当Fe-C投加量增至100 g·L−1时,TC去除率达到80.84%。由图6(b)可知,ln(C0/Ct)与t基本呈线性关系,这表明Fe-C投加量对TC降解过程可用一级反应动力学方程
$ \ln({C_0}/{C_t}) = kt$ 来描述,其结果见表1。当铁碳投加量从0 g·L−1上升到300 g·L−1时,K先从0.009 356上升到0.012 6,然后在降到0.010 8。因为浓度一定时,随着Fe-C投加量的增大,体系反应产生的[H]、Fe2+、Fe3+和·OH量逐渐增加,氧化还原能力逐渐增强;当Fe-C投加量高于100 g·L−1时,TC的去除率反而降低。这是因为体系中Fe过量处于过饱和状态,不仅会造成样品浪费增加成本费用,而且过量的Fe被氧化成Fe2+和Fe3+,出水易出现“返黄”现象,导致Fe(OH)2和Fe(OH)3絮体增多,这不利于电化学反应[29]。 -
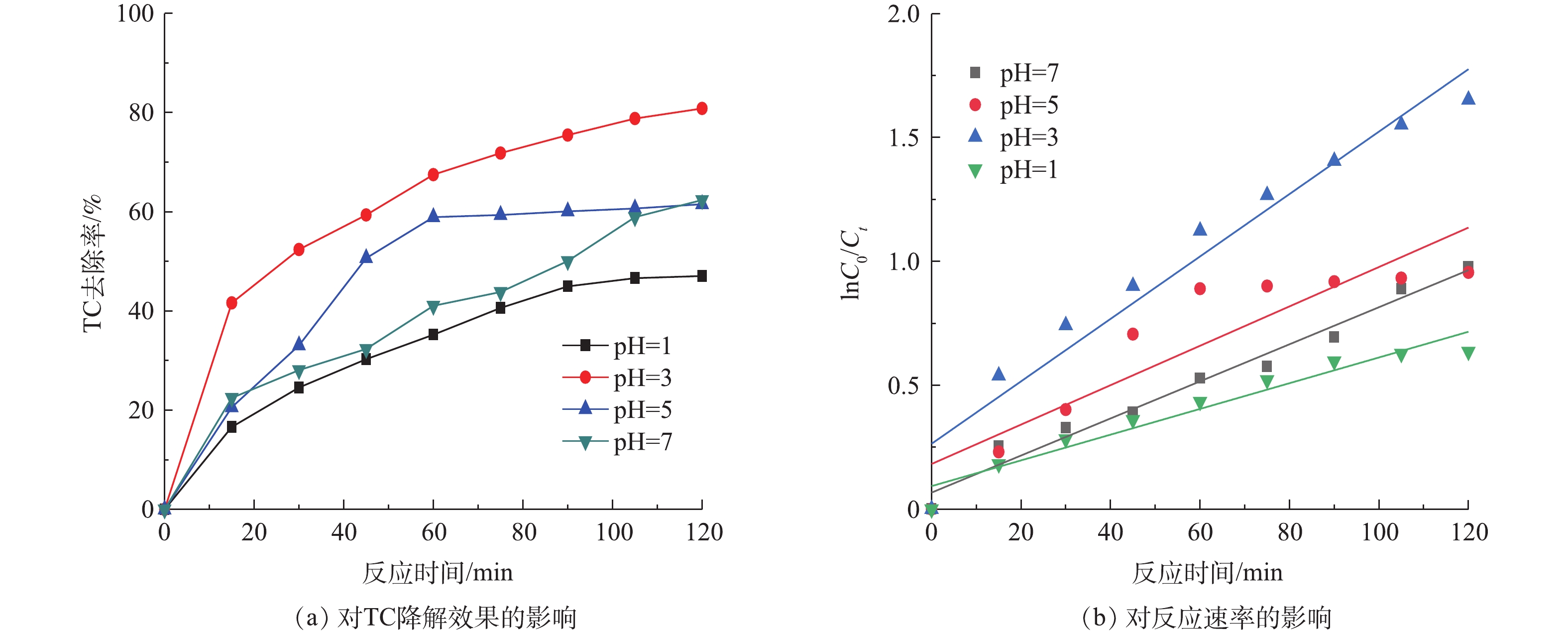
图7(a)为不同初始pH对TC降解的影响,图7(b)为不同pH的一级反应动力学图谱。由图7(a)可知,反应液pH的变化对TC降解有较大影响。其中,当pH=3时,TC去除率最高,可达80.84%。而当pH=1时,TC去除率较低。造成该现象的原因主要是,强酸条件抑制MB/Fe-C体系中Fe2+和Fe3+絮体的形成,减弱铁盐絮凝作用和产生强氧化性·OH的产生量,从而降低TC的去除效果。而当初始pH>3时,随着pH的增加,TC去除率明显减小。pH对TC降解过程用一级反应动力学方程来描述,其结果见表2。当pH由1上升到7时,K先从0.051 9上升到0.012 6,然后降到0.007 48,其半衰期t1/2在pH=3时最低。这是因为pH的升高会导致MB/Fe-C体系中电位差的降低,削弱了微电解能力,也会抑制·OH的产生,从而降低其去除率。综上可知,最佳初始pH为3。
-
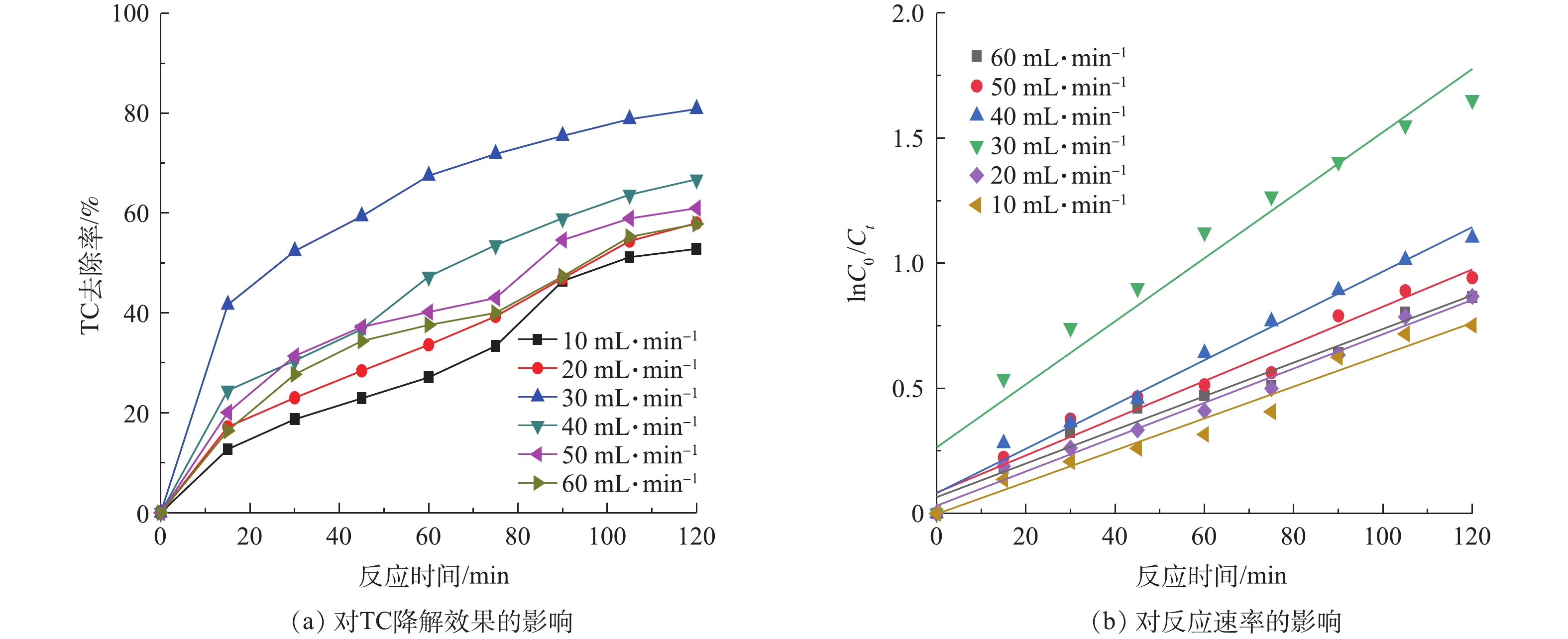
图8(a)为不同微纳米气泡(MB)进气量对TC降解活性的影响,图8(b)为不同MB进气量的一级反应动力学图谱。由图8(a)可明显发现,MB进气量对TC去除有明显影响。在TC浓度为20 mg·L−1条件下,MB进气量对TC的去除率随MB进气量的增加,呈先增强后减弱的变化趋势。其中,当MB进气量由10 mL·min−1增至30 mL·min−1时,降解效果逐渐增强,并在30 mL·min−1时达到最好,TC去除率为80.84%。而随着MB进气量由30 mL·min−1增加到60 mL·min−1时,去除率衰减至57%。MB进气量对TC降解过程用一级反应动力学方程来描述,其结果见表3。随着MB进气量的增加,K先从0.006 37上升到0.012 6,然后下降至0.006 72,其半衰期t1/2在MB进气量30 mL·min−1时为55 min。其主要原因是,产生的微纳米气泡可以增加水样与填料的摩擦作用,减少填料表面的板结和堵塞,从而加快了有机物与填料的充分接触,促进了电极反应的进行。当MB进气量在30 mL·min−1时反应器中微纳米气泡含量最多,呈现白色乳浊状。停止曝气后,由于微纳米气泡上升速度较慢,白色乳浊状可保持2~3 min,气泡破裂时会产生更多的·OH自由基,因此,30 mL·min−1是产生微纳米气泡的最佳进气量。
-
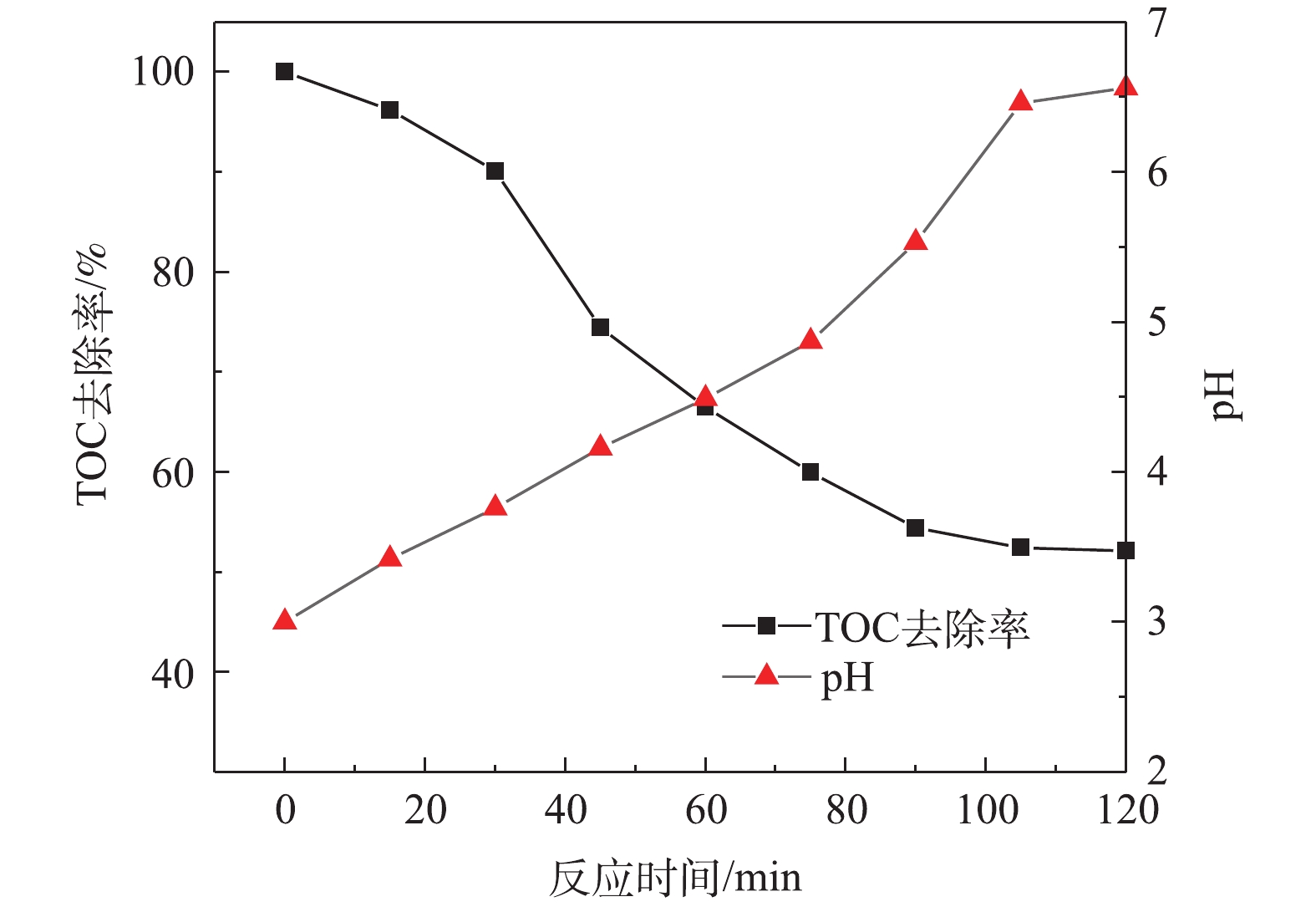
由图9可知,在Fe-C投加量为100 g·L−1、初始pH为3、MB进气量为30 mL·min−1的条件下,随着反应的进行,MB/Fe-C体系中pH逐渐增加,之后溶液pH上升速度减慢,并维持在6.5左右,这是因为Fe2+被氧化(Fe2++ H2O2→Fe3++ OH−+·OH) 反应生成羟基自由基,降解产生小分子有机酸可使微电解体系仍维持偏酸性环境。随着反应的进行,MB/Fe-C体系中TOC的含量迅速降低,反应2 h后,TOC去除率为47.89%,这说明体系中有机物被分解转化为CO2和H2O。
-
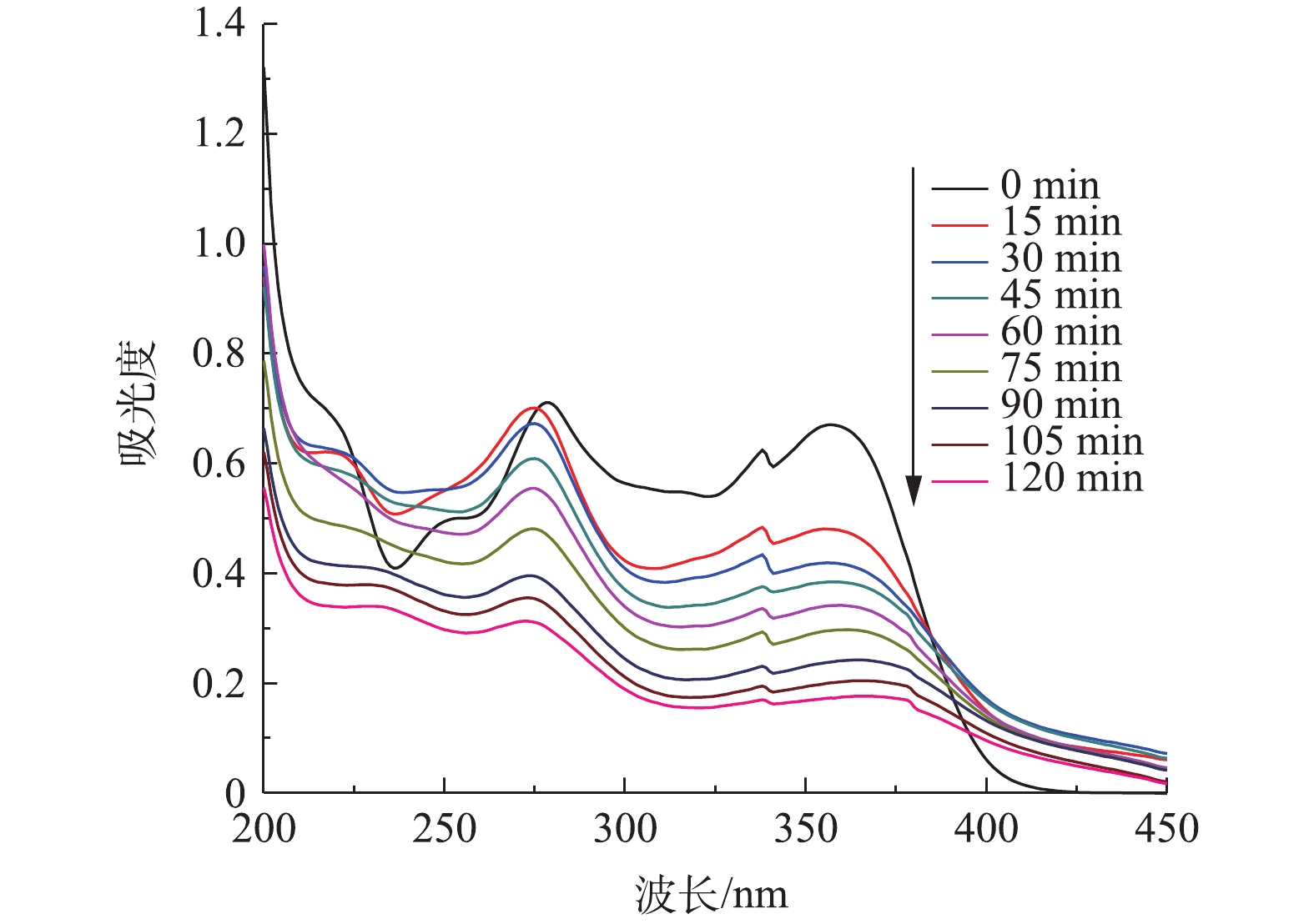
由图10可知,TC在反应前的吸收强度最大,随着反应时间的增加,吸收峰强度逐渐减小,这说明在不同的反应时间下,TC的降解速率不同,在反应开始时,通过电极的混合和电极间的电子转移,形成微观的电池,形成[H]和O·的自由基,其可作为生成其他自由基的前驱体。另一方面,由于铁的氧化和铁羟基的形成,四环素分子和中间化合物被铁羟基捕获沉淀成污泥,并从水溶液中分离出来。随着反应时间的增加,去除过程继续进行,四环素分子和中间化合物通过这些机制被去除。
-
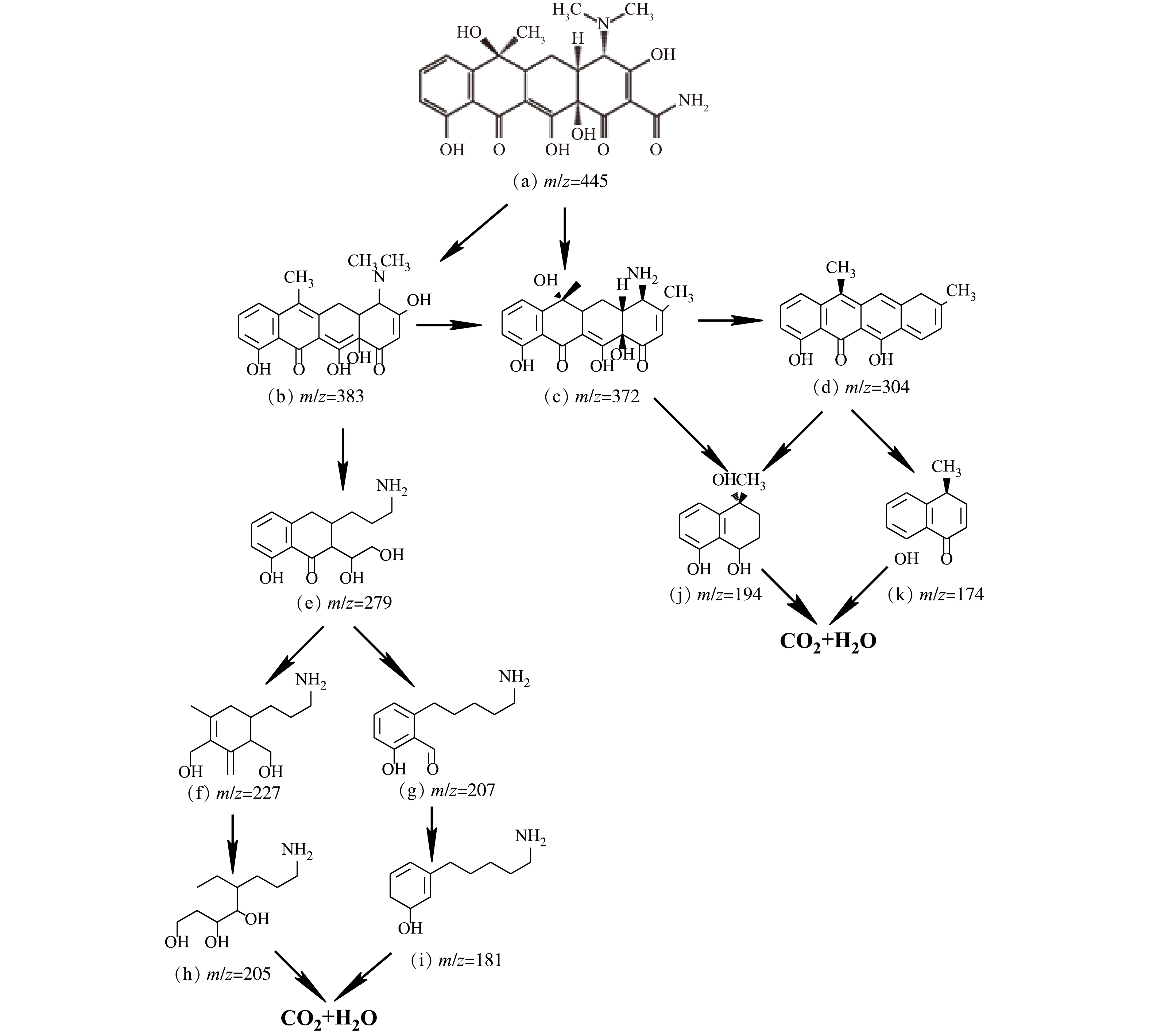
为了进一步研究MB/Fe-C微电解过程的降解机理,通过LC-MS分析确定了最佳条件下MB/Fe-C微电解过程的降解产物,在负离子模式下,经过LC-MS测试可知,TC转化降解的途径如图11所示。由图11可以看出,TC的m/z=445,经过降解后,首先分解成m/z=383(图11 (b))和m/z=372(图11 (c))的产物,再通过去甲基化和碳-碳单键断裂反应分解成m/z=194(图11 (j)),m/z=181(图11 (i))和m/z=174(图11 (k))的产物,最后转化分解为CO2和H2O,其降解机理如图12所示。可以看出,MB破裂产生大量的·OH,而·OH是一种非常有效的有机物氧化剂,由于Fe-C约有1.2 V的电位差,阳极的Fe产生Fe2+,然后发生氧化和絮凝沉淀作用,从而形成亚铁离子氢氧化物(Fe(OH)2+)[30]。阴极的碳产生具有很强化学活性的[H]和O·,MB破裂产生大量的·OH,其与[H]、O·等活性物质相结合可以破坏有机物中的碳链,使难降解物质的形态或结构发生变化,被氧化分解为小的中间体和副产物,四环素分子和中间化合物被铁羟基捕获沉淀成污泥,从水溶液中分离出来,从而有效地清除污染物。
2.1. Fe-C材料表征
2.2. 不同体系下TC的降解效果
2.3. 反应时间的影响
2.4. Fe-C投加量的影响
2.5. 初始pH的影响
2.6. MB进气量的影响
2.7. 最佳条件下pH和TOC的变化
2.8. TC全谱扫描
2.9. HPLC-MS和降解机理分析
-
1)单独微纳米气泡对TC的去除率为11.79%、单独铁碳微电解对TC的去除率为35.92%,而MB/Fe-C体系对TC的去除率可达80.84%,这表明微纳米气泡与铁碳微电解体系具有显著的协同作用。
2)不同反应时间、铁碳投加量、pH、微纳米气泡进气量对盐酸四环素的降解均有较大影响,且符合一级反应动力学方程。当反应时间为120 min、铁碳投加量为100 g·L−1、pH=3、MB进气量为30 mL·min−1时,盐酸四环素的降解率可达80.84%,TOC去除率为47.89%。
3)紫外可见光谱扫描和LC-MS测定分析结果显示,TC经过系列反应转化降解为m/z=194、m/z=181和m/z=174的产物。其中MB破裂产生大量的·OH,其与Fe2+、Fe3+、[H]和O·等活性物质相结合,能破坏有机物中的碳链,使难降解物质的形态或结构发生变化被氧化分解为小的中间体并最终转化分解为CO2和H2O。



 下载:
下载: