-
在我国西南地区,岩溶地下水资源是当地社会和经济发展的基石,更是生态文明建设和可持续发展的重要保障[1]。然而,由于岩溶发育强烈,且地表缺少足够厚度的土壤防护层,地表污染物在缺少防渗条件下容易直接侵入含水层导致地下水污染[2]。随着我国石油产业的发展,在石油的开采、运输、储存、加工和使用过程中不可避免会发生泄漏,从而引起地下水遭受石油芳香烃有机污染。其典型污染组分苯、甲苯、乙苯和二甲苯(benzene, toluene, ethyl benzene, xylenes,简称BTEX)具有很强的挥发性、迁移性和致癌性[3-4],一旦发生油品泄漏,会严重危及地下水环境安全。
目前,原位化学氧化(in situ chemical oxidation, ISCO)技术被认为是修复地下水BTEX污染的有效途径[5],其原理是将强氧化剂投注到受污染的地下水中,使之与有机污染物发生氧化反应,促进污染物氧化分解并最终矿化为CO2和H2O[6]。由于过硫酸盐(persulfate, PS)具有氧化还原电位高、在常态下比芬顿试剂和臭氧等氧化剂更稳定、且易溶于水等特点[7-9],常被用来氧化芳香烃和其他石油烃化合物[10-11]。然而,对于广泛分布的岩溶地下河,利用PS修复石油芳香烃污染的研究报道很少见,因此,其应用潜力具有不确定性。为更好地认识这个问题,本研究利用碳酸盐岩管道模型,在实验室开展人工流条件下PS修复汽油BTEX污染的研究,旨在认识并评价岩溶地下河管道中PS稳定性和化学氧化修复效果,且分析其主要的影响因素,可为实际场地修复BTEX污染提供参考。
全文HTML
-
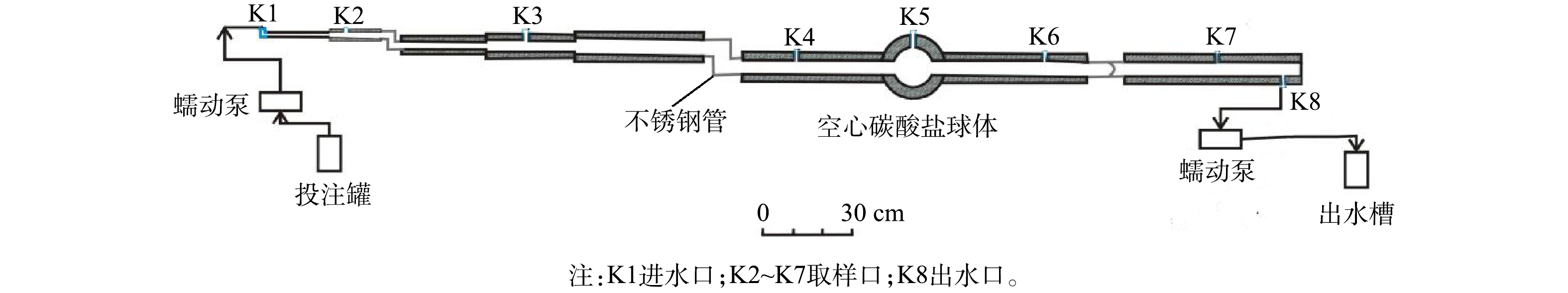
实验使用的碳酸盐岩管道模型主要由扁豆状灰岩制成(图1),以反映自然界岩溶发育的岩性特征。根据X衍射荧光分析(ZSX Primus II, 日本),管道模型的灰岩组分为CaO 44.375%、SiO2 12.219%、MgO 0.672%、Al2O3 1.535%和Fe2O3 0.357%。模型主干由8段内径1~5 cm碳酸盐岩管道、1个内径为10 cm的空心碳酸盐岩球体和3个不锈钢管连接组成,总长367.5 cm,管道内壁表面积5 602 cm2,体积8 672 cm3。进水口K1和出水口K8均连接蠕动泵,以控制管道进出水流量。在进出水口之间设置了6个开口向上的取样孔(K2~K7),他们与进水口之间的距离分别为28、89、180、218、263和320 cm。本次研究利用该模型开展了未污染条件下的PS稳定实验和污染条件下的PS修复实验。
-
实验用水为桂林理工大学雁山校区9栋旁的浅层地下水,其水化学背景值如表1所示。采用的92#汽油购自桂林中国石化良丰加油站,实验试剂均为分析纯,包括过硫酸钠(PS)、氯化钠、碳酸氢钠、碘化钾。
考虑到石油污染源区地下水中BTEX污染物浓度高的特点,实验设计BTEX组分来源于汽油饱和溶液,初始目标浓度为80 mg·L−1。具体配置方法为:在10 L的蜀牛玻璃罐中加入300 mL的92#汽油和9 700 mL的地下水配置成汽油溶液并用木塞密封,之后定时摇晃4~5 d,避光静置24 h后取下清液而得[12]。对于PS修复实验,PS的投注量用PS∶BTEX摩尔比控制,按照PS∶BTEX的摩尔比为18∶1,称取相应的过硫酸钠粉末配置相应浓度过硫酸钠溶液,现用现配。
-
1) PS稳定实验。为了解过硫酸钠在未污染岩溶管道中的迁移特征及其稳定性,在K1点分3次分别投注浓度为5、10和20 g·L−1的PS溶液10 mL,瞬时投注,并在出水口K8监测每次投注后PS浓度变化。每次投注PS前,先向管道注入地下水运行空白实验一周,使水质恢复到背景状况(表1),管道进出水流量稳定在120 mL·h−1。
2) PS修复实验。该实验目的是探查PS去除岩溶管道地下水中汽油污染物BTEX的效果,实验分流水状态和静水状态两个阶段并进行对比,共持续2 158 h。实验开始前,管道中水质维持在表1所示的状态。实验开始后,以120 mL·h−1的流量在K1孔连续投注含BTEX的汽油饱和溶液,待出水中BTEX浓度相对稳定后,于第1 006 h在K2孔以15 mL·h−1的流量连续投注PS溶液,汽油饱和溶液投注流量相应减小为105 mL·h−1,以维持进水总流量为120 mL·h−1不变。根据进水流量比例计算,PS投注浓度为21 g·L−1,与汽油饱和溶液混合后PS浓度约3 000 mg·L−1。在实验后期第1 450 h停止进水和出水,使管道内的水体处于静止状态,目的是对比流水状态和静水状态下水力条件变化对修复效果的影响。
-
1) PS稳定实验取样方案。投注PS之后每8 h在K1和K8孔取样分析进、出水中的
${{\rm{S}}_{\rm{2}}}{\rm{O}}_{\rm{8}}^{{\rm{2 - }}}$ 、${\rm{SO}}_4^{2 - }$ 、和pH等,每天测1次Ca2+和${\rm{HCO}}_3^ - $ ,其他取样孔不定时取样。2) PS修复实验取样方案。每天在K1孔取样分析进水中的BTEX、pH和
${\rm{SO}}_4^{2 - }$ ,在K8号孔取样分析出水中的BTEX、${{\rm{S}}_{\rm{2}}}{\rm{O}}_{\rm{8}}^{{\rm{2 - }}}$ 和${\rm{SO}}_4^{2 - }$ 等;每2 d测1次进出水中Ca2+和${\rm{HCO}}_3^ - $ ,其他取样孔不定时取样。样品分析时,采集水样量20 mL,用气相色谱仪(Agilent 7890 B)检测分析BTEX,色谱柱型号为HP-5MS,30 m×0.25 mm×250 μm,色谱条件参见已有研究[13];用美国哈希HQ30d分析仪检测pH和DO;根据碘量法[14],用紫外可见分光光度计(UV-5800(PC))检测PS浓度,PS浓度和吸光度的可决系数达到0.999 8;用配有高性能阴离子色谱柱的离子色谱(DIONEXICS-1000IC)检测
${\rm{SO}}_4^{2 - }$ ,分析前使用0.45 μm的滤膜对水样进行过滤处理,采用外标法进行含量测定,色谱条件参见已有研究[15];使用滴定法检测${\rm{HCO}}_3^ - $ 和Ca2+。
1.1. 实验装置
1.2. 实验材料
1.3. 实验方案
1.4. 采样与分析
-
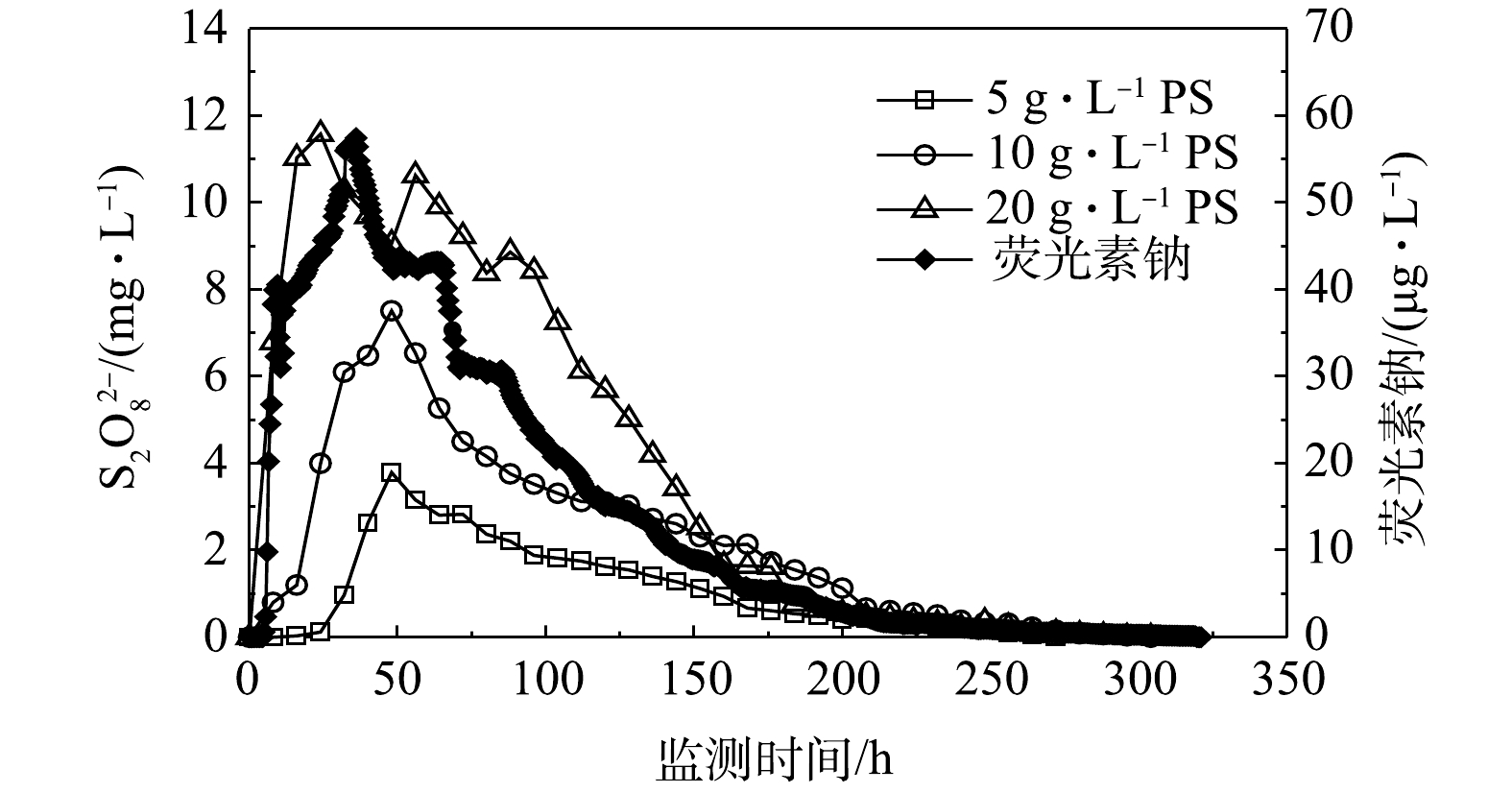
在PS稳定实验中,5、10和20 g·L−13种不同浓度条件下的PS持续监测时长分别为272、328、328 h,出水口K8获得的PS浓度与时间的关系曲线,结果如图2所示。为便于对比分析,图2同时列出了郑民杰[16]在同一装置中相同流量条件下获得的荧光素钠示踪数据曲线。
由图2可知,投注浓度为低浓度(5 g·L−1)和中浓度(10 g·L−1)时,PS在管道中的浓度-时间曲线浓度峰值出现的时间较一致,峰值浓度分别为3.79 mg·L−1和7.51 mg·L−1,后者约是前者的2倍,与进水浓度的倍数关系一致。投注浓度为高浓度(20 g·L−1)时,PS在出水口出现了主峰和次峰,浓度分别为11.58 mg·L−1和10.63 mg·L−1。荧光素钠作为示踪剂,在管道中不容易被岩石吸附,亦不与其他物质发生反应,并且检测精度高,能够精确描述非反应物质在管道中的迁移信息[17]。与荧光素钠对比,不同投注浓度的PS在相同水流速度条件下具有与荧光素钠相似的拖尾曲线,在实验结束时浓度检测均不明显。
1) PS浓度衰减动力学特征。通过对图2中4个浓度与时间曲线下降翼进行指数方程(式(1))拟合,可以反映PS在管道中衰减动力学特征。
式中:Cm为峰值浓度,mg·L−1;Ct为t时刻变化浓度,mg·L−1;k为一级衰减速率常数,h−1;t为监测时间,h。
由表2可以看出,指数方程拟合效果显著(R2>0.90),其中PS一级衰减速率常数为0.011~0.013 h−1,半衰期为120.9~124.9 h,2种参数均与荧光素钠相近,说明PS本身在管道中具有较好的稳定性。
2) PS迁移特征及参数。根据PS在管道出水中的浓度变化,利用QTRACER2程序[18]分析浓度-时间系列数据,可以反映PS在管道中迁移的相关水力特征(表3)。结果表明,不同浓度PS的回收率均比荧光素钠低。由于后者是非反应物质,回收效果较好,而PS回收率下降可能与自身分解有关[19-20]。PS自身分解过程[21]可以用式(2)表示。
由式(2)可知,PS在水体中的分解会产生O2和
${\rm{SO}}_4^{2 - }$ 。由监测结果可知,在3种PS投注浓度下管道出水中的溶解氧和${\rm{SO}}_4^{2 - }$ 浓度变化不大,PS分解现象不明显,这说明PS在管道中具有较好的稳定性。同时也可以看出,随着PS浓度增大,在管道中的迁移速度相对加快,平均滞留时间缩短,这可能与管道中浓度梯度较高、扩散能力较强有关。总体上,PS的平均滞留时间、平均迁移速度和纵向弥散系数都与示踪剂荧光素钠相近,表明PS在管道中具有较强的迁移能力和较好的稳定性。 -
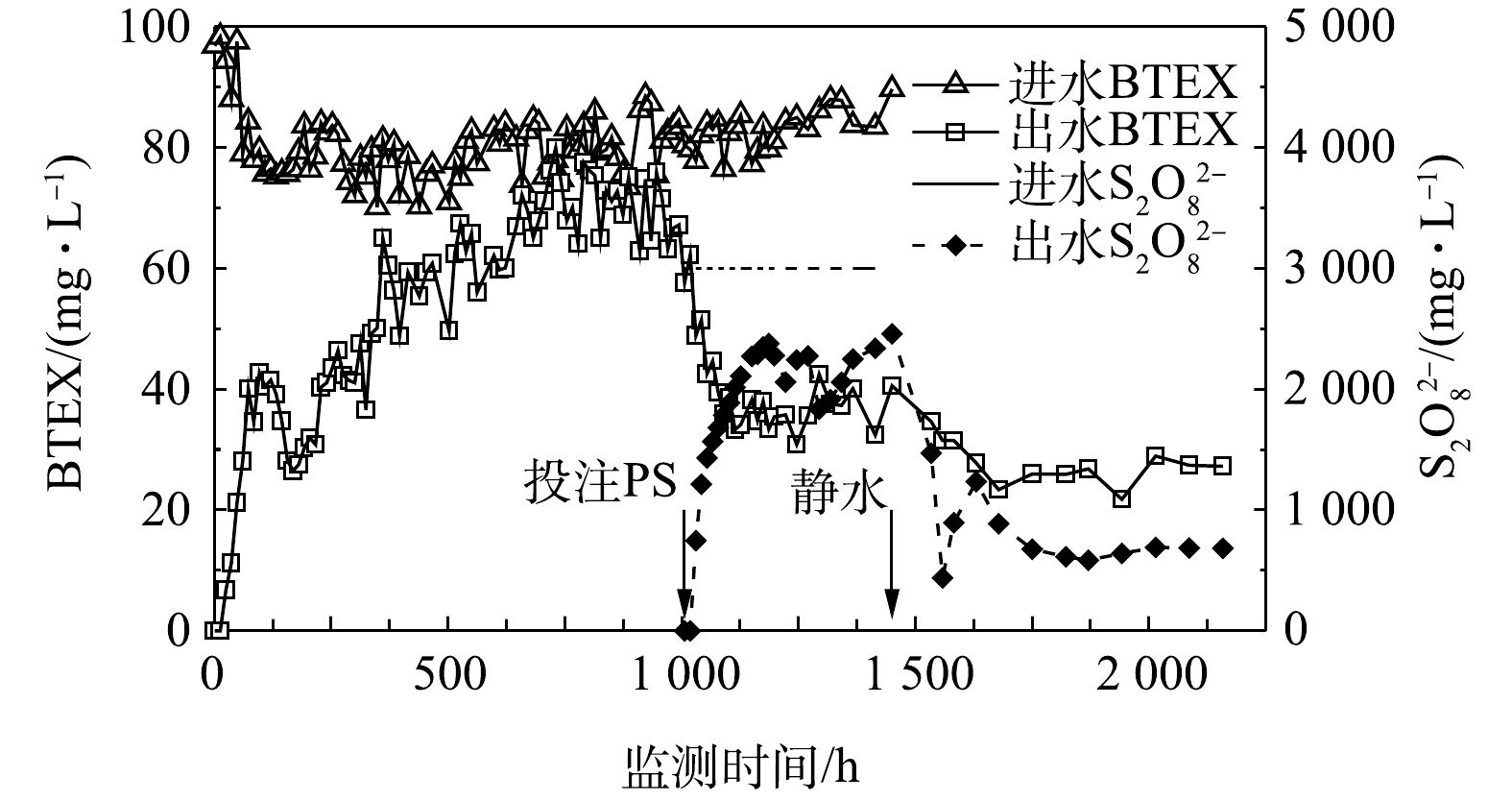
1)化学氧化效果。该组实验首先注入汽油饱和溶液,当出水和进水中的BTEX浓度基本相近后,在第1 006 h开始向K2孔连续添加PS溶液(浓度为21 g·L−1),与汽油饱和溶液混合后PS浓度约3 000 mg·L−1(图3)。随着PS连续注入,管道出水口K8孔PS浓度呈现上升,并趋于平稳。过硫酸盐作为强氧化剂,可以将水中的BTEX污染物有效地去除[22-24]。PS的添加引起出水中的BTEX浓度迅速下降,在1 006~1 114 h内,出水中BTEX浓度由62.3 mg·L−1下降到了33.3 mg·L−1,之后趋于平稳;通过指数方程拟合,BTEX在连续投注PS期间的准一级衰减速率常数为0.006 2 h−1(R2=0.911 4)。在停止进出水时(第1 450 h),K8出水口处的PS和BTEX浓度分别达2 339.3 mg·L−1和32.415 mg·L−1,残余浓度较高。
根据前面的PS稳定实验可知,在没有BTEX存在时,PS具有与非反应示踪剂荧光素钠相似的稳定性,虽然具有一定程度的分解[25],但质量损失较低。在出现汽油BTEX有机物后,不仅BTEX浓度明显下降,而且PS浓度也迅速下降。这表明PS与BTEX接触后发生了化学氧化反应,反应方程[26]如式(3)~(5)所示。
为了解岩溶管道水流静止条件下的PS氧化效果,第1 450 h停止进水和出水,这种状态与枯水季节管道水流停滞的情形相似。结果表明,在1 451~1 678 h内管道内BTEX浓度进一步下降,由31.5 mg·L−1下降到25.9 mg·L−1,之后趋于平稳的态势;通过指数方程拟合得出,BTEX在静水期间的准一级衰减速率常数为0.002 3 h−1(R2=0.978 5)。同时,管道内PS浓度也出现明显下降,实验结束时PS在出水口浓度下降到683.4 mg·L−1,仍然有较高的PS残余量,结果如图3所示。
进出水质量平衡计算表明(表4),在流水化学氧化阶段(1 006~1 449 h),进出水中BTEX和PS在单位时间内质量分别减少4.14 mg·h−1和86.2 mg·h−1,其中BTEX去除效率比投注PS前(0~1 005 h)有明显提升(该期间单位时间内质量减少2.44 mg·h−1)。这表明化学氧化作用能够在短时间内有效去除管道内的BTEX,比自然衰减的效果强。在静水期间(1 450~2 158 h),虽然没有补充PS和BTEX,但管道内残留的BTEX和PS继续反应,BTEX单位时间内质量减少0.60 mg·h−1,PS单位时间内质量减少14.1 mg·h−1。由此,流水条件下BTEX去除效率是静水条件下的9倍,对应的PS消耗效率是6倍。静水条件下化学氧化效果较低,与水体不流动、PS缺乏补充并处于较低浓度水平是有关系的。
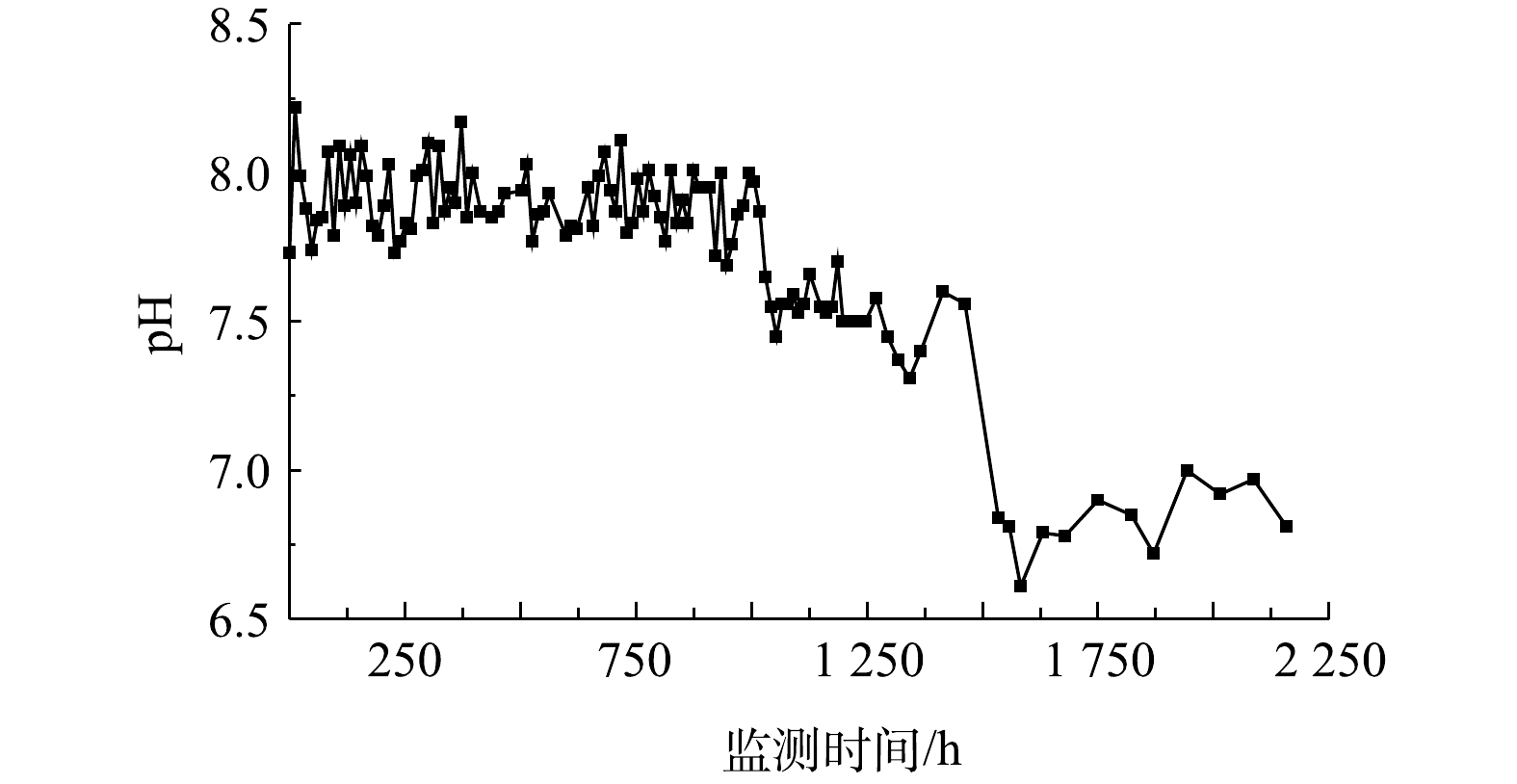
2)化学氧化导致的水化学特征变化。投注PS溶液引起了管道中pH和硫酸盐、钙离子、重碳酸根等组分浓度在出水中不同程度的变化。对于出水中的pH,在投注PS后因受化学氧化作用产酸的影响而呈现下降,但由于碳酸盐岩的缓冲作用[27],pH下降幅度不大(图4)。PS投注前,pH平均值为7.9;PS投注后,流水和静水状态下pH平均值分别为7.6和6.8。在静水状态下,由于缺乏水流交替,因酸的积累导致pH下降较为明显。
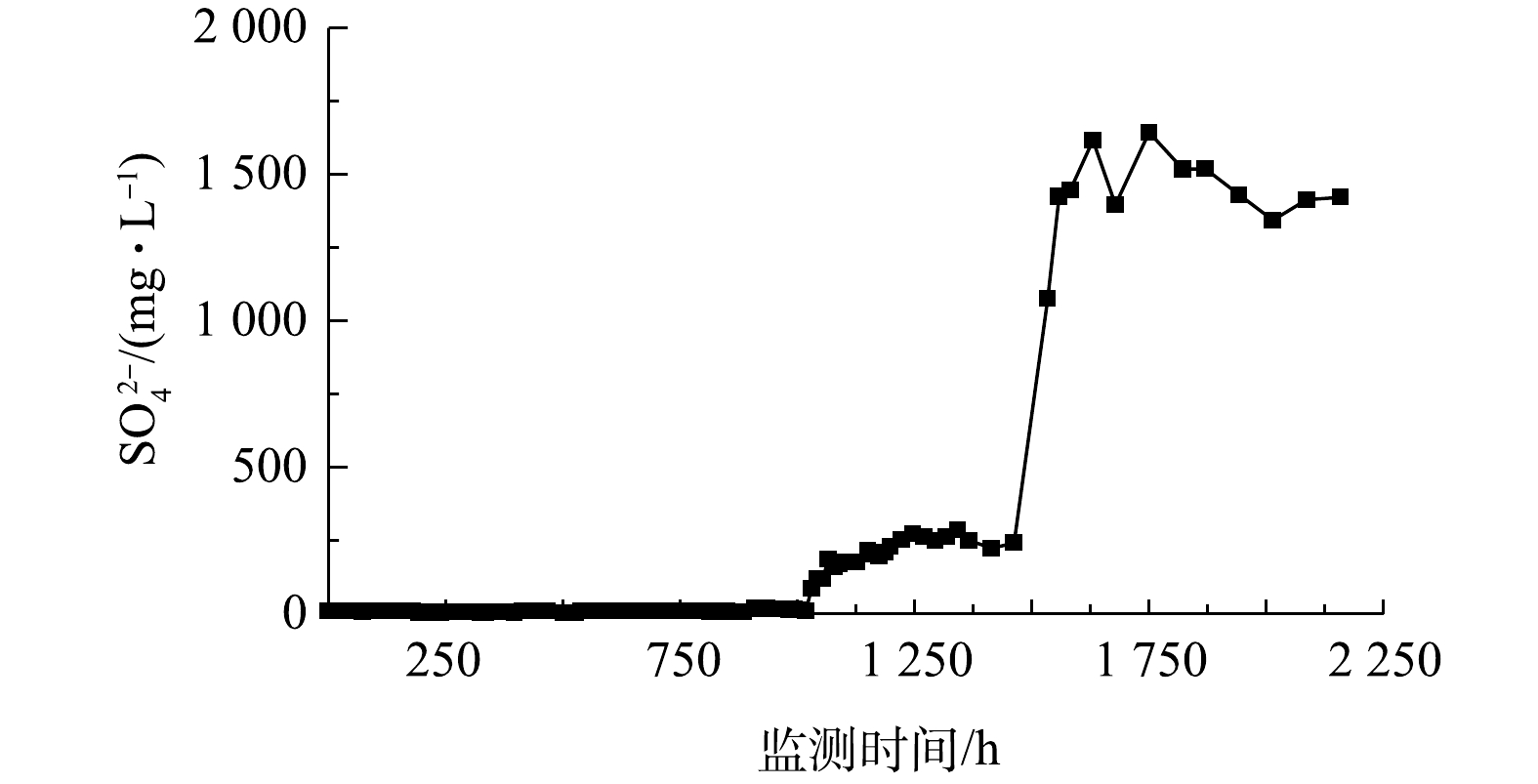
硫酸盐作为PS和BTEX反应后的产物,在投注PS前出水中的硫酸盐浓度稳定,平均浓度为10.1 mg·L−1,进出水中硫酸盐质量稳定(表4);投注PS后流水期间(1 006~1 449 h),出水中的硫酸盐平均浓度上升到243 mg·L−1,单位时间质量增加25.5 mg·h−1;静水期间(1 450~2 158 h),出水口K8处硫酸盐平均浓度上升到1 485.4 mg·L−1,单位时间质量增加11.8 mg·h−1(图5,表4)。由于高浓度硫酸根离子对人体危害较大,世界卫生组织有明确规定,饮用水中的硫酸根离子浓度应低于500 mg·L−1[28],因此,在利用PS氧化降解污染物的同时,也要做好对硫酸根离子相应的处理。
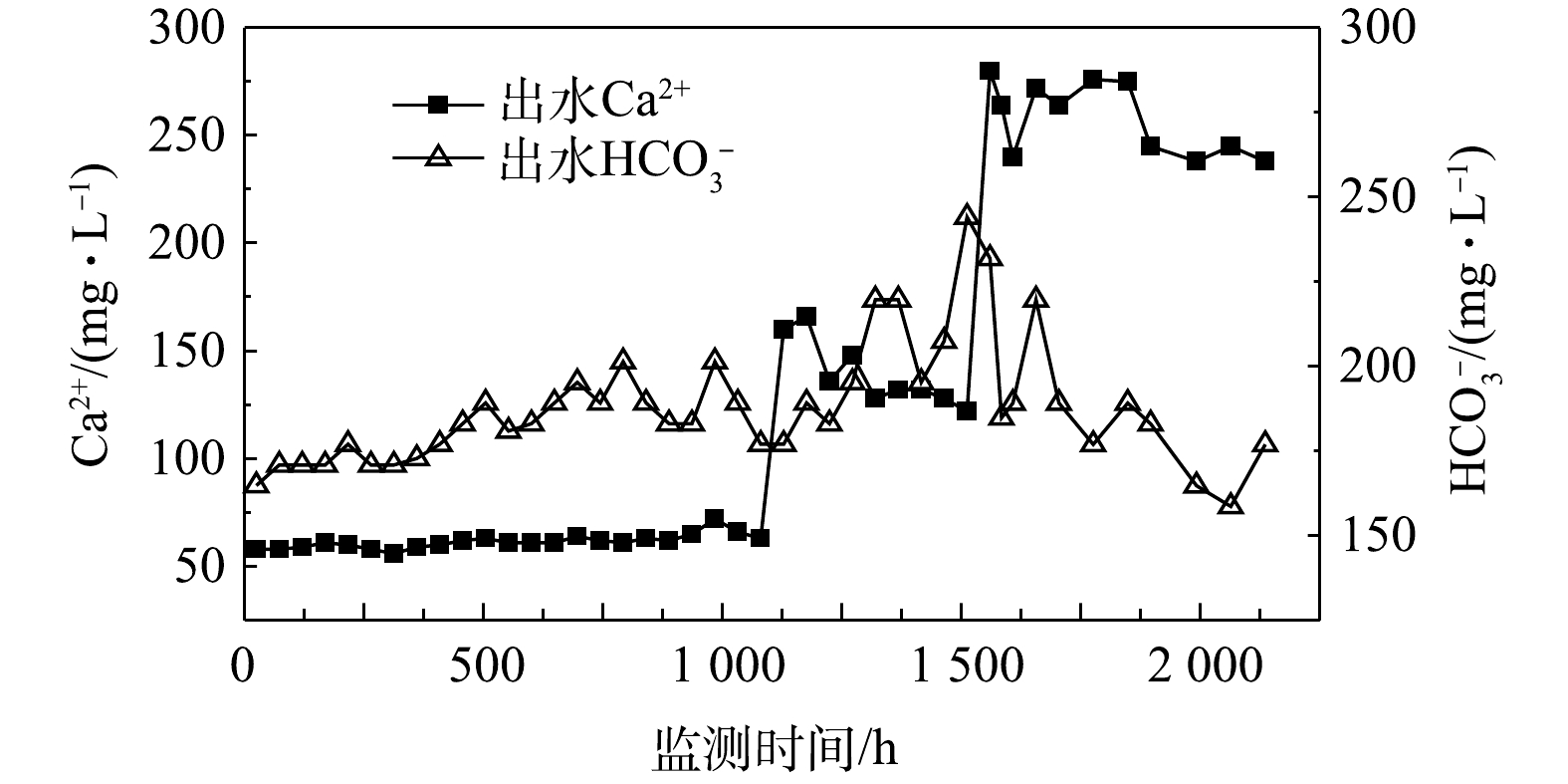
由图6可知,碳酸盐岩在抑制pH下降的同时,也导致出水中Ca2+浓度的持续上升。相应的
${\rm{HCO}}_3^ - $ 浓度在此期间也逐渐上升,最高浓度达到了244.1 mg·L−1。在静水条件下,PS和BTEX反应生成了更多的H+,导致了这期间${\rm{HCO}}_3^ - $ 浓度因为碳酸平衡而下降。3)影响PS化学氧化的因素。首先,水力因素对PS氧化效果具有一定的影响,水体流动有利于PS与污染物的接触、氧化,而静水会导致氧化速率下降。其次,碳酸盐岩对pH的缓冲可能影响PS氧化效果。本实验投注PS与BTEX的摩尔比为18∶1,理论上可以完全氧化管道内的BTEX[29]。在投注PS后,流水状态和静水状态的pH均在6.5~7.5,无法达到PS氧化效果最佳时的pH(3~5)[30]。这可能会影响PS的氧化能力,进而影响BTEX的去除效率。
2.1. PS稳定实验
2.2. 修复实验
-
1)在未污染岩溶管道地下水中,PS具有较好的稳定性和较强的迁移能力,这种特征有利于PS与污染物的接触以及PS对污染物的化学氧化。
2)在汽油污染岩溶管道介质中,PS能够有效去除典型苯系物BTEX,其中流水条件下的去除效率比静水条件下的去除效率高。
3)岩溶管道地下水中的ISCO能够导致水体pH下降,但因碳酸盐岩缓冲作用,其下降幅度较小;pH下降可促进碳酸盐岩溶蚀,并导致水体中钙离子浓度升高。



 下载:
下载: