-
厌氧氨氧化(anaerobic ammonia oxidation,ANAMMOX)是一种公认的经济有效的污水脱氮工艺。该工艺比传统的硝化-反硝化工艺可节约60%的氧气需求和100%的有机碳源,并且污泥产量和温室气体排放大大减少[1-2]。然而,功能菌厌氧氨氧化菌(anammox bacteria,AAOB)由于生长速度慢,倍增时间长并且容易随反应器出水而流失,导致ANAMMOX工艺启动期较长,故影响了该工艺的推广应用[3]。为此,研究者采用各种方法缩短ANAMMOX反应器的启动时间。为加强体系中细菌的滞留效果,经常采取添加载体或者设计合适的反应器的方法[4-6]。例如,附着式生长的移动床生物膜反应器(moving bed biofilm reactor,MBBR)可以创建一个稳定的系统[7-8]。生物滤池同样具有良好的生物截留能力[9]。刘雪娇等[10]采用生物滤池反应器,45 d 即成功启动了ANAMMOX反应,总氮容积去除速率(nitrogen removal rate,NRR)可达到1.41 kg·(m3·d)−1
此外,选择合适的接种污泥亦可加快ANAMMOX启动[11-12]。尽管现有研究证明,常规污泥(活性污泥、厌氧消化污泥、反硝化污泥和硝化污泥)在适当的条件下经过驯化培养可以启动ANAMMOX反应。但常规污泥中AAOB数量较少,导致培养过程耗时较长[1]。接种成熟的厌氧氨氧化颗粒污泥(anammox granule sludge, AnGS)是实现ANAMMOX快速启动的捷径[13-14]。LIU等[11]将厌氧消化和AnGS分层接种,缩短了细胞裂解期和滞后期,实现了反应器快速启动。TANG等[15]通过加入少量ANAMMOX污泥启动了ANAMMOX中试反应器。实验结果表明,ANAMMOX污泥的添加促进了反应器内AAOB的代谢和活性的表达。具体来说,这归因于AAOB之间存在的群体感应(quorum sensing,QS)现象[16]。
细菌QS由分泌的化学信号组成,当他们积累到一定水平时,调节特定基因的激活和转录[17]。例如,在ANAMMOX过程中,N-酰基高丝氨酸内酯(N-acyl-homoserine lactones,AHLs)调节的QS参与了ANAMMOX系统的细菌活性、基因表达和代谢途径[18]。一方面,细菌可以产生信号分子并将他们释放到周围的环境中[19]。更重要的是,外源微量信号分子被证明可以通过诱导AAOB内源信号分子的产生,进一步激活AAOB的功能表达[16]。TANG等[16]证实了ANAMMOX反应器上清液中存在AHLs,其中包括C6-HSL、C8-HSL和C12-HSL。这意味着,除了采用外围技术手段营造更利于AAOB生长的环境,利用AAOB的QS机制,通过外加ANAMMOX上清液产生的AHLs诱导AAOB基因表达,从而提高AAOB代谢活性,是加速AAOB生长的直接策略。
基于上述考虑,本研究构建了两级ANAMMOX串联污泥系统,将一级反应器(R1)出水引入二级反应器(R2)。一方面有效利用R1随出水流失的ANAMMOX污泥,另一方面获取R1上清液有效成分,以通过简单的操作,实现后置反应器R2的快速启动;通过考察启动过程中的脱氮性能、污泥理化性质和菌群结构特征,以验证两级串联启动ANAMMOX的可行性。最后,分析了快速启动过程的相关机理,以期为ANAMMOX反应器的快速启动提供参考。
-
接种污泥分为AnGS和悬浮污泥。AnGS取自实验室稳定运行1a的上流式厌氧污泥床反应器(UASB)反应器(R1)。反应器有效容积31.8 L,内径0.17 m,顶部0.4 m为沉淀区。反应器运行温度在 (32±1) ℃,连续泵入(WS-50-01-L,中国)合成废水,不特别除氧。进水NH4+-N质量浓度为200~220 mg·L−1,NO2−-N与NH4+-N配比保持在1.1~1.25。MLSS为13 700 mg·L−1,NRR稳定在(2.65±0.2) kg·(m3·d)−1。 悬浮污泥在室温(18±2) ℃下间歇运行,MLSS为3 100 mg·L−1,NRR为0.12 kg·(m³·d)−1。
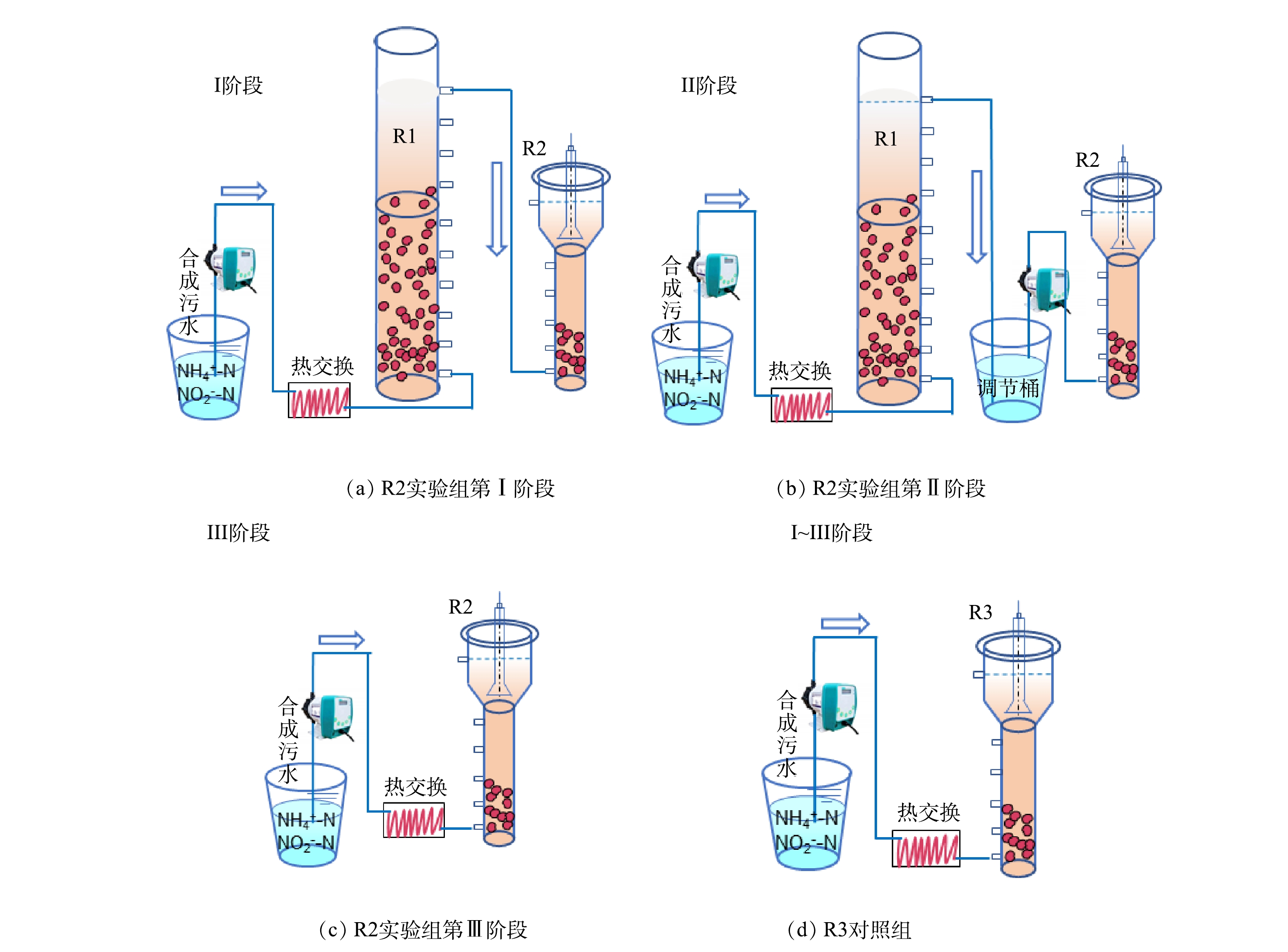
为比较两级串联启动ANAMMOX反应器的运行效果,构建了2个相同的UASB反应器(R2和R3)。如图1(c)所示,反应器的底部直径是0.1 m,高度是0.8 m,有效容积是8 L。R2和R3中接种等量的AnGS(300 mL)和悬浮污泥(1 500 mL),并通过换热器及加热带进行加热,柱体外加保温棉,使温度保持在(32±1) ℃。
-
1)实验用水。实验进水分为R1出水、R1出水上清液和合成废水。通过调节水力停留时间(HRT)控制R1出水NH4+-N稳定在40~55 mg·L−1,NO2−-N为45~60 mg·L−1。合成废水底物由(NH4)2SO4和NaNO2组成,无机碳源由NaHCO3(0.6 g·L−1)提供。同时向其中补充0.025 g·L−1 KH2PO4, 0.15 g·L−1 CaCl2·2H2O,0.3 g·L−1 MgSO4·7H2O,0.1 g·L−1 FeSO4·7H2O,0.008 g·L−1 FeCl3矿物质,以及1 mL·L−1的微量元素[20]以满足营养要求。此外,1%的硫酸用于调节进水pH为7.6±0.2。
2)运行策略。运行流程示意图如图1所示。其中,R2以稳定运行的一级反应R1出水为进水进行启动,并逐步替换为R1出水上清液和合成废水。实验总共运行90 d,根据进水特征不同分为3个阶段。第Ⅰ阶段为1~30 d,第Ⅱ阶段为31~60 d,第Ⅲ阶段为61~90 d。
第Ⅰ阶段,R1出水直接引入R2,随R1出水流失的污泥一并进入R2,给R2带来生物量补充。然而,R2也不可避免地存在污泥流失。通过测定,R1每天污泥流失量稳定在 135~210 mg·L−1,R2每天污泥流失量为60~115 mg·L−1(表3)。因此,这一时期R2每天的污泥净补充量约为20~150 mg·L−1。第Ⅱ阶段,R1出水在调节桶沉淀并调节pH后,将上清液泵入R2。即认为这一阶段R2没有污泥补充,但其他组分与第Ⅰ阶段保持一致。第Ⅲ阶段,将无机合成污水泵入R2。此外,1~40 d 的水力停留时间(hydraulic retention time,HRT)为5 h,之后缩短HRT为4 h。具体运行参数参见表1。以R3作为对照组,全程以人工合成污水进行启动并运行。进水底物浓度和HRT与R2保持一致。
-
水质测定采用国家标准方法[21]。NH4+-N:纳氏试剂分光光度法;NO2−-N:N-(1-萘基)-乙二胺光度法;NO3−-N:紫外分光光度法。此外,采用重量法测定反应器出水MLSS以确定污泥流失量,并用PHS-3C型pH计测量pH。以蛋白质(PN)和多糖(PS)总量表征胞外聚合物(extracellular polymeric substance,EPS)。PN采用考马斯亮蓝法,PS采用苯酚-硫酸法[22]。EPS的提取步骤参考WANG等的描述[23]。采用高通量Illumina MiSeq测序平台(Illumina, San Diego, USA)分析了接种污泥R1和R2运行结束时(第90天)的微生物群落组成。
样本用经过液氮碾磨预处理后,利用土壤 DNA 快速提取试剂盒(Bio101, Vista, CA)从样本中提取 DNA。采用 NanoDrop 2 000 分光光度计测量 DNA浓度,对细菌16S rRNA基因V3~V4区进行扩增,引物分别为341F(CCTACGGGNGGCWGCAG)和805R (GACTACHVGGGTATCTAATCC)。采用Illumina HiSeq 2 500 PE250进行测序,测序数据使用 MEGAN 软件进行微生物的16S分析,按照99%的识别阈值分组为序列聚类操作分类单元(OTUs)。得到的结果使用R软件的工具包进行分类学分析,主要包括α多样性分析、物种多样性分析和基于COG的功能预测分析。最后,利用Hemi软件将结果可视化。
-
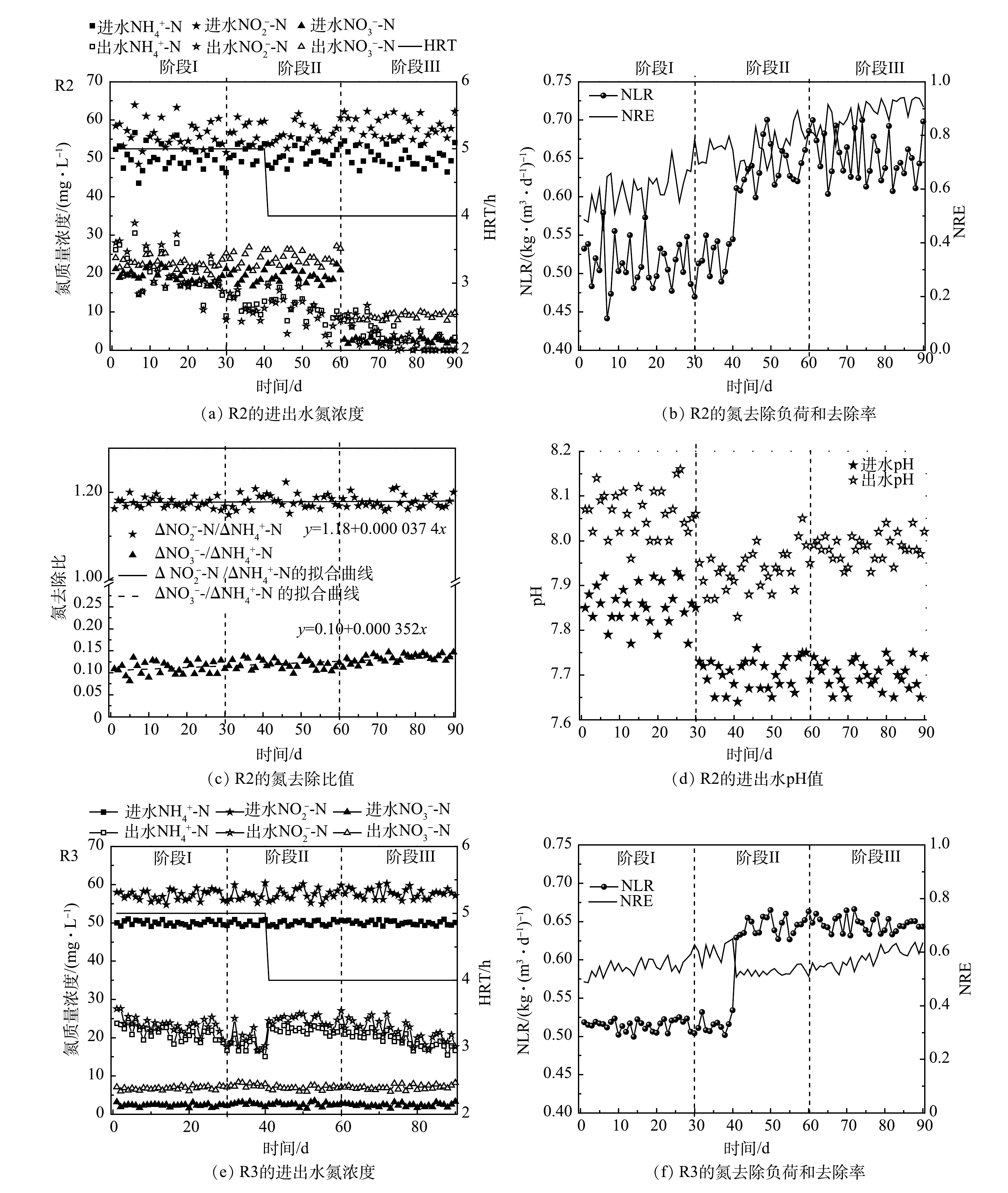
R2和R3在启动和稳定的运行过程中脱氮效果变化如图2所示。由图2(a)可见,第Ⅰ阶段在进水底物和HRT保持一定的条件下,R2的出水NH4+-N和NO2−-N逐渐减小,分别由最初的26.2 mg·L−1和28.0 mg·L−1下降为14.3 mg·L−1和15.3 mg·L−1左右。总氮去除率(nitrogen removal efficiency,NRE)由第1天的49%上升为第30 天的67%,显著高于直接使用合成废水进水的对照组(R3,58.2%)。这说明以R1出水为进水加速了R2的启动。其原因为:一方面由于R2每天有来自于一级反应器(R1)流失的生物量补充(135~210 mg·L−1),弥补了启动初期的生物量损失(60~115 mg·L−1),通过功能菌积累提高了ANAMMOX活性;另一方面,得益于成熟的ANAMMOX系统释放出的活性因子,在一定程度上促进了R2中功能菌的代谢和活性表达。
第Ⅱ阶段,以R1出水上清液作为R2进水,尽管没有了R1出水的生物量补充,但观察到R2出水底物浓度比第Ⅰ阶段的速率下降更快,并且出水NO3−-N升高,意味着ANAMMOX活性的快速提高。可见,第Ⅰ阶段的生物量积累为第Ⅱ阶段活性的快速提高奠定了基础,并且R1上清液中存在的AHLs可以积极地刺激AAOB代谢功能和活性提高。LIU等[18]在15 ℃下通过添加外源C6-HSL,使ANAMMOX反应器的NRE提高到74.6%,显著高于对照组(22.2%),同时基因表达水平也得到提高。张晶等[24]在研究信号分子对高负荷 UASB 反应器中AnGS的特性时发现,C8-HSL能够对颗粒污泥的EPS含量、PN/PS值及表面疏水性产生长期影响,并推测外源添加AHLs信号分子可诱发污泥颗粒持续内源释放[16]。这些结果表明信号分子有助于ANAMMOX性能的全面提升。由图2(c)可见,△NO3−-N/△NH4+-N远低于ANAMMOX反应理论比值0.26。这可归因于进水COD的存在,其可促进体系中的反硝化作用,从而导致更多的NO3−-N去除。此外,这一时期通过中间调节桶将R2的进水pH由R1出水的较高值(约7.85)降低为7.68左右,由于ANAMMOX反应消耗H+并产生碱度,更合适的pH可促进ANAMMOX反应的进行,因此,R2的进、出水pH差值进一步增加(图2(d))。第41天,将HRT由5 h缩短为4 h,出水NH4+-N和NO2−-N在波动上升之后继续下降。相反,R3出水水质恶化,NH4+-N质量浓度达到23.2 mg·L−1,NRE下降为51%。由此可见,生物量的积累和QS调节在提高R2处理效果的同时,也增加了系统的抗冲击负荷能力。第56天,R2的NLR达到0.54 kg·(m3·d)−1,高于研究中普遍认为的启动标准(0.5 kg·(m3·d)−1) [25],即视为已实现ANAMMOX反应器的快速启动。PENG等[26]通过在UASB反应器中添加聚氨酯海绵对低温存储后的AAOB进行活化,将ANAMMOX启动时间缩短28%。这也说明生物量保持对ANAMMOX的初期启动时十分有效的。
第Ⅲ阶段,将R2进水替换为人工合成污水。这一操作阻断了进水有机物和 NO3−-N,潜在地削弱了反硝化作用。并且,更严格的控制进水△NO2−-N/△NH4+-N在1.1~1.2,可进一步优化ANAMMOX反应条件。R2的脱氮性能随着运行时间的延长不断提高,第90 天,在进水NH4+-N和NO2−-N分别为50.4 mg·L−1和58.1 mg·L−1时,出水NH4+-N和NO2−-N低于检出限,NRE达到93%以上。相比之下,R3的NRE增长缓慢,最终稳定在61%左右。显然,R2的运行策略更快地提高了处理效果。此外,由于副产物NO3−-N的产生,ANAMMOX反应的NRE理论值最高为89%。这说明,R2在ANAMMOX活性不断加强的同时,体系中仍存在不可忽视的反硝化作用,从而将ANAMMOX过程产生的NO3−-N转化为NO2−-N和N2,为NRE的提高做出贡献。据报道[27],反硝化菌(Denitrifying bacteria,DNB)不可避免地存在于ANAMMOX体系中,其特定菌属和AAOB之间具有营养共生关系。此外,图2(c)显示,R2的△NO2−-N/△NH4+-N和△NO3−-N/△NH4+-N从第1天到第90天呈现出逐渐上升的趋势,更接近ANAMMOX反应的理论化学计量比(1.32,0.26);特别是△NO3−-N/△NH4+-N,其值由0.1上升为0.135,预示着ANAMMOX主导趋势。ANAMMOX系统中NO2−-N和NO3−-N去除比例低于理论值的情况在其他研究中也有报道[28-29]。除了反硝化作用,这还可能与系统中存在的氨氧化细菌(ammonia oxidizing bacteria,AOB)有关,该细菌在DO为0.12 mg·L−1时仍能进行短程硝化反应[30]。多种氮转化途径可以为ANAMMOX反应提供底物补充,并提高脱氮效率。
-
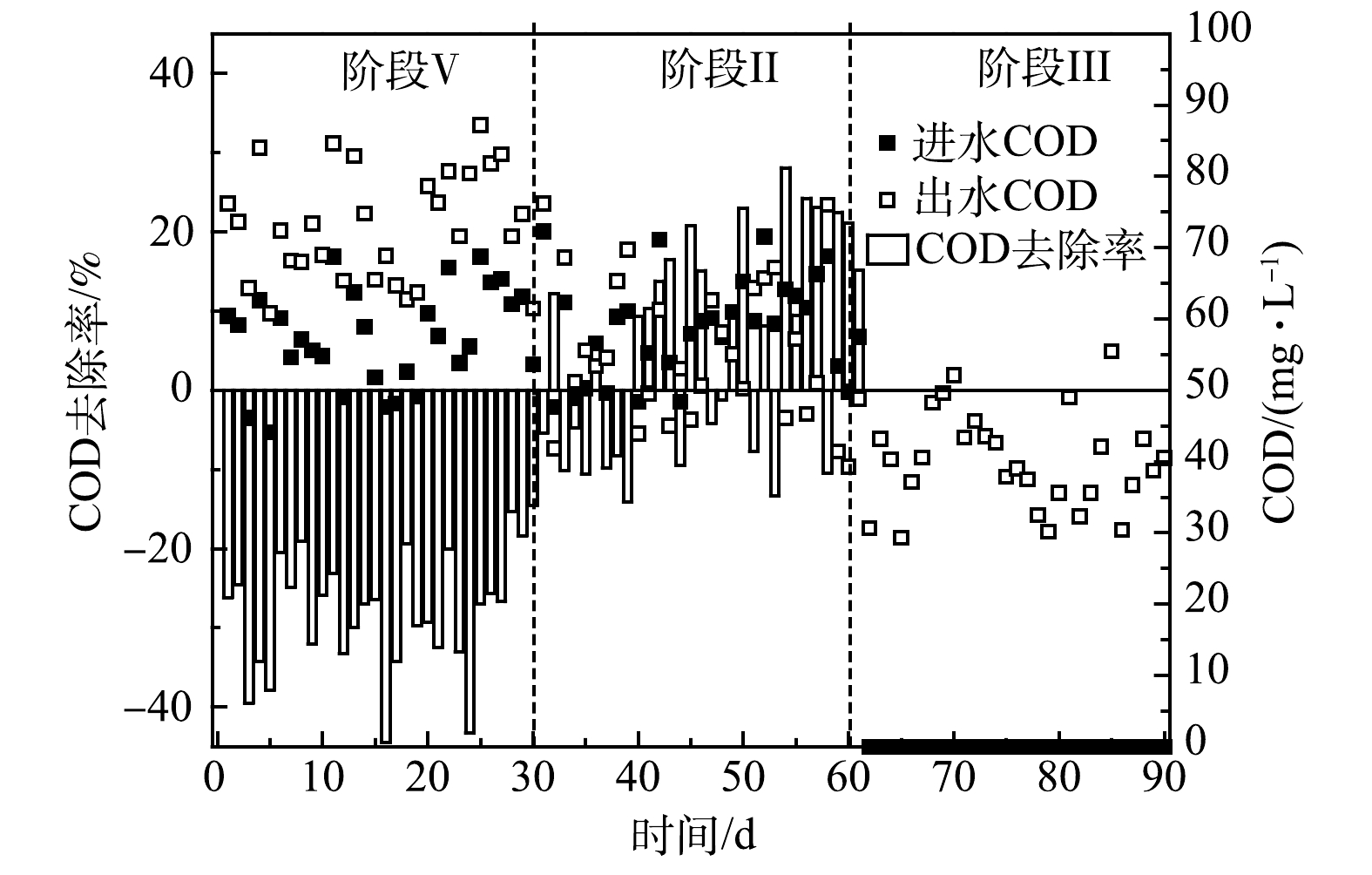
R2系统中COD的变化如图3所示。R1中微生物可溶性代谢产物(soluble microbial products,SMP)和细胞凋亡分解的有机物导致其出水含有一定的有机物,因此,R2在第Ⅰ阶段的进水中引入了COD。由于接种了ANAMMOX污泥,R2属于ANAMMOX反应占主导的自养脱氮体系,因此,对COD的利用很少。此外,R2不断产生代谢产物,进一步增加了出水COD值,导致在第Ⅰ阶段COD的去除率为负值。值得注意的是,第Ⅱ阶段R2的出水COD值逐渐下降,表现出低于进水的趋势。这导致COD的去除率由负值变为正值,最高达到28.1%。有研究[29]表明,SMP包含的PN、PS和腐殖酸等是潜在的电子供体,可被DNB原位利用。据此推测,来自于R1出水的NO3−-N和有机物为R2体系中DNB的增殖提供条件,表现为COD的去除。此外,相比AnGS,DNB更多的存在于ANAMMOX系统的悬浮污泥中[23],这意味着随R1出水流失的悬浮污泥相比保留在系统内的颗粒污泥含有更多的DNB,也潜在地增加了R2中DNB丰度。然而,通过2.1.1节的分析可知,有限的NO3−-N和COD值并未影响ANAMMOX脱氮效果,反而强化了系统对氮和碳的去除。这说明,两级ANAMMOX反应器串联进行启动是可行的,R1带给R2的积极影响大于负面效应。在第Ⅲ阶段,由于进水被替换为合成废水,避免了NO3−-N和有机物的摄入,因此,反硝化作用被削弱。此时出水COD平均值为40.4 mg·L−1,反映了R2系统中微生物的代谢和衰亡。
-
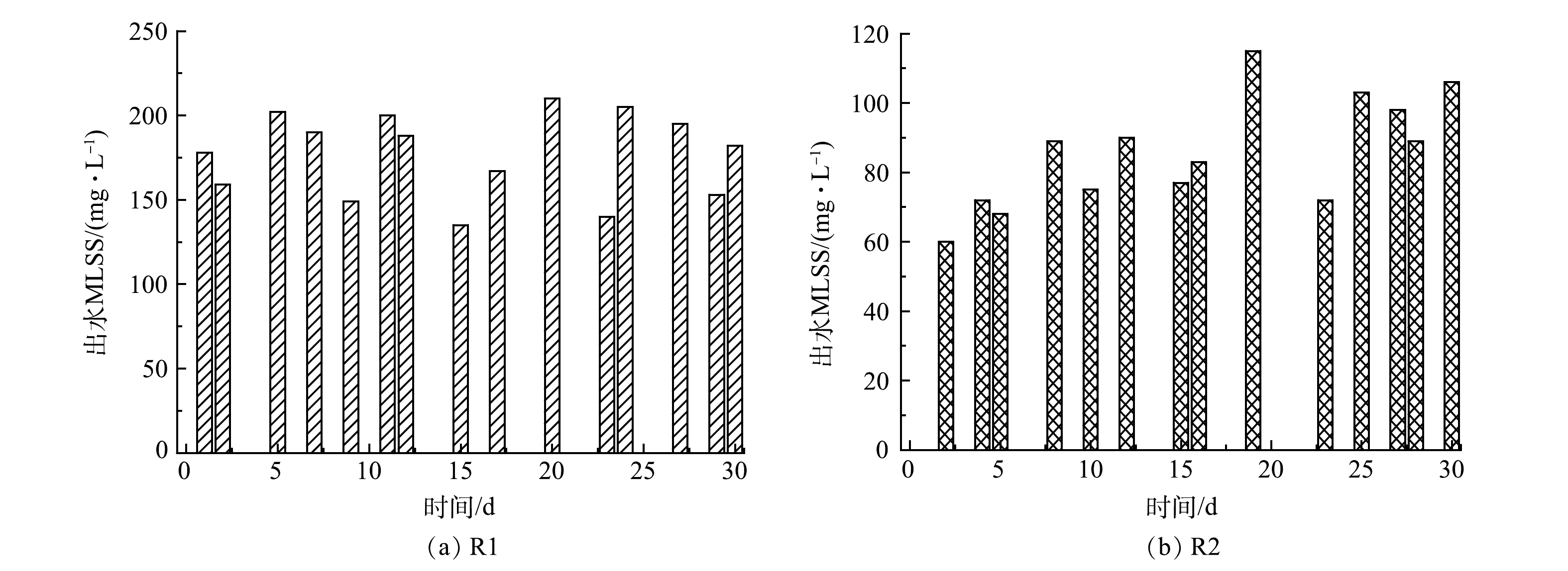
为进一步分析R2和R3启动过程中的污泥性能变化,分别在第1 、15 、30 、60 和90天测定它们的污泥浓度和EPS含量变化如图4所示。前30 d,由于不断有来自R1的流加污泥补充,R2的MLSS由3 610 mg·L−1增加至5 880 mg·L−1。之后,停止污泥补给,伴随着AAOB活性的提高,其生长速度加快,即在有利条件下倍增时间缩短,更接近14 d的理论值[31]。因此,R2的MLSS在第60天达到6 350 mg·L−1,第90天达到6 220 mg·L−1。由此可见,启动过程中生物量的积累和活性提高是相互促进的。R3的 MLSS前期增长较缓,在第Ⅱ~Ⅲ阶段才表现出升高趋势,最终为4 460 mg·L−1,但仍远低于R2。其可能原因是:由于缺乏生物量和AHLs活性因子的补充,R3较低的污泥浓度和代谢水平限制了其生长。这说明ANAMMOX快速启动过程中生物量的原始积累是必要的。
EPS可以反应污泥微生物活性,也会影响处理性能[32]。通过测量运行过程中的EPS变化发现(图4(b)),0~30 d,R2的EPS浓度明显增加(228 mg·g−1到255.5 mg·g−1),但PN/PS却呈现下降趋势。这可归因于PS以比PN更快的速率增加,也说明补充的悬浮污泥EPS组分中含有更多的PS。已报道的研究结果表明,含AHLs的上清液可以诱导ANAMMOX系统中EPS的分泌[33],并且EPS含量的升高会促使AnGS黏附更多的絮体和小颗粒,从而进一步完善颗粒结构,扩大颗粒尺寸,促进其活性提高。31~60 d,其EPS由255.5 mg·g−1增加至265.2 mg·g−1,浓度变化不大,仍保持较高水平。PS的浓度下降和PN浓度的上升导致PN/PS的比值由3.19增加到为3.44。这证实了我们之前的分析,即悬浮污泥中含有更多的PS,停止悬浮污泥摄入后,R2污泥的PN占比相对上调。第90天,PN和PS浓度都下降导致EPS值降低为217.7 mg·g−1,PN/PS的值为3.15。李海玲等[34]也报道过系统稳定运行后,污泥PN和PS值趋于稳定。这说明EPS产量和细菌活性变化具有一致性,可以反映系统的运行状态:活性提高期,功能菌释放更多的EPS,进入稳定期后,EPS产量也会相对减少。相比R2,R3的EPS变化较为平稳,并且总体水平低于R2,这可归因于R3活性增长滞后于R2。同时,上述结果也支持了添加含AHLs的上清液可增加EPS分泌的结论[32]。
-
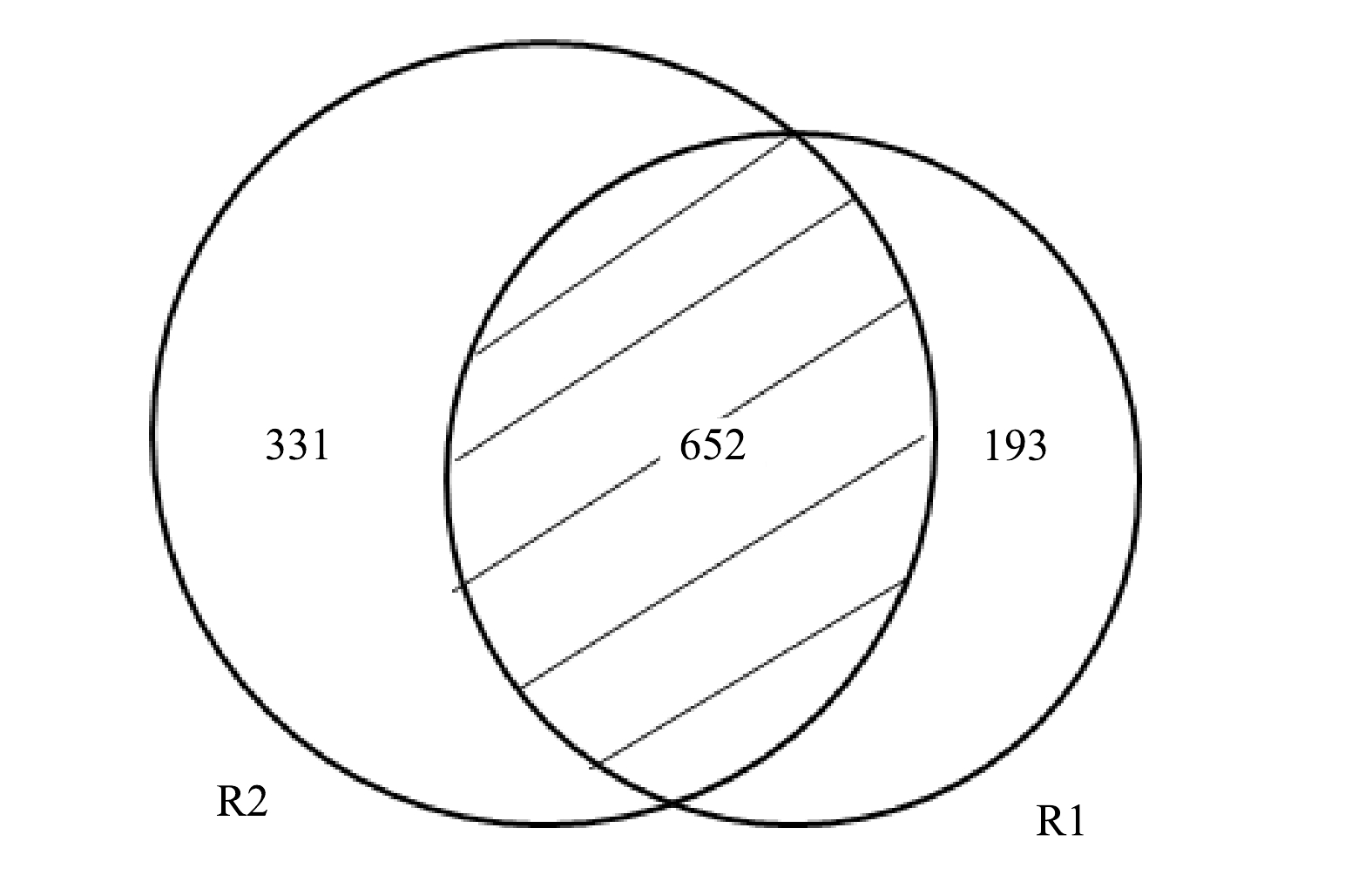
R1和R2共产生67 867个序列,表2列出了二者的OTUs的数量及多样性指数。相比R1,R2的ACE、Chao1和Shannon指数均有所上升。ACE指数和Chao1指数代表物种丰富度,而Shannon指数反映了物种多样性,这意味着R2的物种丰富度和多样性均高于R1。这是由于COD的引入为异养菌(heterotrophic bacteria,HB)的繁殖提供了条件,丰富了R2的微生物组成。从2个样本基于OTU的Venn图中也可以得到类似的结果(图5)。Venn图更直观的反映了各样本共有和特有的OTU数目,以了解其组成的相似性。 由图可知,R1和R2共有652个OTUs,R2包含更多特有序列,预示着其他菌属的繁殖。
-
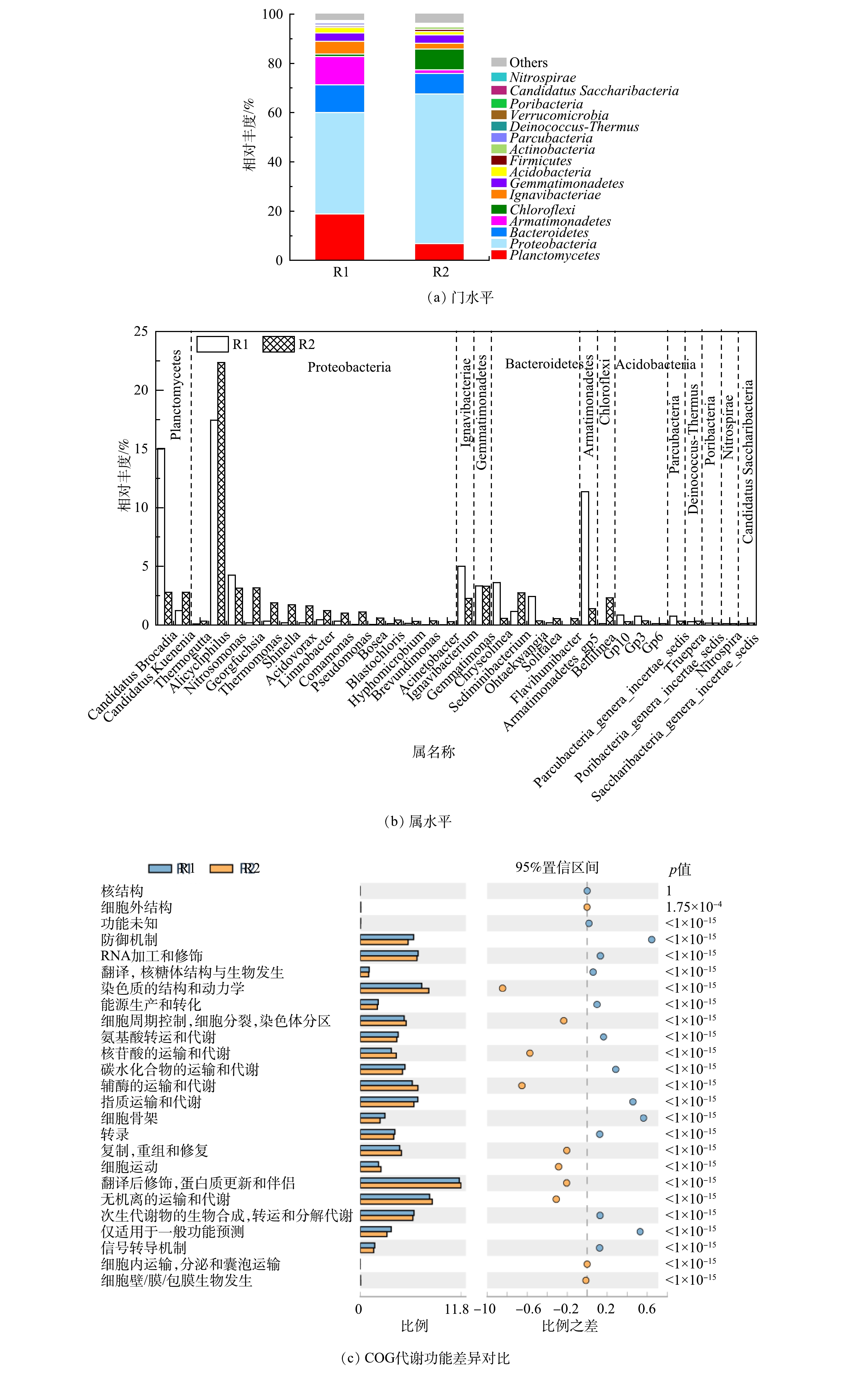
图6(a)显示了R1和R2在门水平的分类组成。浮霉菌门(Planctomycetes)、变形菌门(Proteobacteria)、拟杆菌门(Bacteroidetes)、装甲菌门(Armatimonadetes)和绿弯菌门(Chloroflexi)等相对丰度≥1%的8种细菌在门水平占主导,占比达到菌群总数的92%以上[35-36]。和许多研究一致,变形菌门的相对丰度高于AAOB所属的浮霉菌门。特别是在R2中,其占比达到60.8%。R1中浮霉菌门占比为18.85%,仅次于变形菌门,R2中浮霉菌门占比为6.81%,位列第3。变形菌门拥有丰富的菌群种类,并且其可以参与信号分子的合成和降解,这些信号分子可能通过QS与其他细菌相互作用[37-38]。这证实了上文的分析,R1出水的引入一方面促进了R2系统的群体感应,另一方面,SMP和凋亡细菌产生的COD促进了R2中DNB生长。同时,R2中绿弯菌门增加。绿弯菌门在自养脱氮系统中较为常见,可以清除ANAMMOX产生的代谢产物并降解胞外蛋白[39],更多的有机物积累解释了其升高原因。
属水平物种组成的对比分析如图6(b)所示。R1和R2中均存在Candidatus Kuenenia和Candidatus Brocadia2种ANAMMOX功能菌。R1中Candidatus Brocadia(15.03%)显著高于Candidatus Kuenenia(1.23%),而R2中二者相差不大(2.79%和2.8%)。这说明由R1到R2的环境,更适合Candidatus Kuenenia的生存。R2中观察到丰富的DNB,包括Alicycliphilus、Thermomonas、Limnobacter、Comamonas和Pseudomonas等。之前的研究表明,Candidatus Brocadia在絮体污泥中更为丰富,而Candidatus Kuenenia聚集在颗粒中[20]。并且Candidatus Brocadia是对底物亲和力低,生长速度快的R“策略者”[40],Candidatus Kuenenia则对氨和亚硝酸盐有很高的亲和力[41]。据此推测,由于R2较低的进水底物不利于Candidatus Brocadia生长优势,导致其占比下降。相反,R2中引入的SMP等有机组分刺激其中AnGS在运行初期产生了更多的EPS,使AnGS尺寸进一步增加的同时也改变了其组成:新招募的DNB在颗粒表面开始了定居生长,而Candidatus Kuenenia被包裹在颗粒中,获得了稳定的生存条件,因此得到富集。
DNB的广泛存在解释了R2的△NO3−-N/△NH4+-N低于理论值的原因。值得注意的是,DNB的繁殖并没有影响到R2的脱氮效果,并且还观察到其中△NO3−-N/△NH4+-N升高的趋势(图2(c))。这可能是由于AAOB和其他菌属间的交流互动,在一定程度上提高了其代谢水平。ZHAO等[42]发现,装甲菌门和变形菌门可以为 AAOB 提供生长因子叶酸和钼辅因子。R1中属于装甲菌门的Armatimonadetes_gp5的占比(11.35%)显著高于R2(1.4%),说明R1和R2中分别形成以Armatimonadetes_gp5和多种DNB主导的其他细菌和AAOB之间不同的共生关系,也潜在解释了R2中DNB增加的积极作用。此外,由于进水并未严格厌氧,R1和R2中还含有少量属于AOB的亚硝化单胞菌(Nitrosomonas;4.25%,3.14%)。其将NH4+-N转化为NO2−-N,是导致△NO2−-N/△NH4+-N低于理论值的主要原因。并且亚硝化单胞菌在R2中的比例低于R1,一方面说明R2厌氧环境的更好保持,另一方面可能归因于有机物对自养菌AOB的抑制。可见,菌属间的迁移与转化直接反映微生物的生存环境。
图6(c)反映了R1和R2基于COG 代谢功能差异。COG 代谢功能主要包括细胞结构和能动性、运输和代谢、信号传导、防御机制等相关细胞生化过程。R2在次生代谢物的生物合成、转运和分解代谢、无机离子的运输和代谢、脂质运输和代谢、碳水化合物的运输和代谢和氨基酸转运和代谢方面显著优于R1。R1则在细胞运动、复制,重组和修复、翻译,核糖体结构与生物发生、细胞壁,膜,包膜生物发生和细胞内运输,分泌和囊泡运输方面更胜一筹。以上结果在微生物代谢水平验证了上文的推测,即QS积极地提高了R2菌群的代谢水平,促进了AAOB活性。这是其相对丰度低于DNB等HB,但在系统脱氮中发挥主导作用的原因。
综上所述,运行条件可显著改变ANAMMOX污泥系统菌群结构,并产生不同的基因功能表达水平。以R1出水启动导致R2中DNB等HB占比上升,但也在一定程度上促进了AAOB代谢水平提高。实际运行中,把握二者的平衡至关重要,为避免有机物持续引入导致DNB过度生长对AAOB产生威胁,应在AAOB活性提高后改用无机合成废水继续培养。
-
1)水质和底物。以一级ANAMMOX出水为进水启动二级ANAMMOX,进水底物浓度较低,有利于避免底物过剩对运行初期活性较低的AAOB的抑制作用。STROUS等[43]发现,大于0.5%空气饱和度的氧浓度会降低AAOB 的活性,使系统内脱氮性能恶化。一级反应器出水DO<0.2 mg·L−1,可为对DO敏感的AAOB提供良好的生长环境。
2) AHLs活性因子的补充。ANAMMOX反应器上清液中存在AHLs,如C6-HSL或C8-HSL可以促进微生物中EPS的产生,并提高基因表达水平和代谢途径[16-18]。TANG等[16]通过批次实验证明外源性添加C6-HSL可使ANAMMOX活性比对照组高出近20%。同样,R1出水AHLs积极地刺激了R2中AAOB活性和代谢水平的提高,进而加速了其启动过程。
3)流加菌量补充。图7反映了测量的R1和R2前30 d的污泥流失情况。R1的污泥流失量直接补给R2,因此,R1损失的污泥量和R2损失污泥量的差值为R2每日净增的污泥质量浓度(20~150 mg·L−1)。潜在的生物量补充提高了R2的处理效果和抗冲击负荷能力。同时,悬浮污泥在EPS和水力冲击的作用下不断黏附在颗粒污泥上,有利于AnGS粒径增长。此外,一级ANAMMOX出水引入二级反应器的策略充分利用了一级反应器的流失生物量,减少了细菌损失。综上所述,稳定的细菌含量和信号分子诱导的QS调控是加速ANAMMOX启动的关键。
-
1)两级ANAMMOX串联可以实现后置反应器的快速启动,运行90 d后NRE达93%以上,显著高于对照组(61%)。
2)快速启动关键在于一级反应器R1出水流失污泥为后置反应器R2提供了稳定的生物量补充,并且R1上清液包含的AHLs可通过激发QS促进R2中功能菌的代谢活性。
3) R1引入的有机物可导致R2中AAOB功能菌Candidatus Brocadia占比下降,DNB占比提高,但并未影响R2的ANAMMOX性能。相反,菌群间相互作用可促进与运输和代谢相关功能基因的表达水平和NRE的提高。
4)为保证AAOB生长优势,应避免COD的持续输入。因此,在AAOB活性增长之后,可改用人工配置的模拟污水继续培养,以实现ANAMMOX系统的长期稳定运行。
两级ANAMMOX串联快速启动后置反应器的性能及机理
Performance and mechanism analysis of two-stage ANAMMOX in series to realize fast start-up of post reactor
-
摘要: 外源添加酰基高丝氨酸内酯类信号分子(AHLs)可以提高厌氧氨氧化菌(AAOB)的活性,有助于厌氧氨氧化(ANAMMOX)的快速启动,然而,AHLs的获取会增加操作成本。基于此,本研究构建了两级ANAMMOX串联反应器,以充分利用一级反应器(R1)出水有效成分实现后置反应器(R2)的快速启动。以R1的出水为进水,并逐渐过渡到以R1出水上清液和合成废水为进水的策略运行R2;通过脱氮性能、污泥特性和微生物群落变化来验证该策略的可行性,并进行了相关的机理分析。结果表明,R2在第56天实现了快速启动,运行90 d后,在总氮容积负荷为0.6~0.7 kg·(m3·d)-1时,总氮去除率达到93%以上,显著高于直接以合成污水启动的对照组(R3,61%)。这可归因于R1流失污泥为R2提供了稳定的生物量补充,并且其上清液包含的群体感应信号分子促进了R2中AAOB的活性提高。高通量测序结果表明,R1出水引入的有机物导致R2群落多样性升高,反硝化菌等异养菌相对丰度增加,ANAMMOX功能菌Candidatus Brocadia占比下降,然而,与R1相比,R2物质运输和代谢相关功能基因表达水平上调。以上结果说明,通过两级ANAMMOX串联实现后置反应器的快速启动是可行的。
-
关键词:
- 厌氧氨氧化(ANAMMOX) /
- 两级串联 /
- 快速启动 /
- 群体感应 /
- 微生物群落
Abstract: Exogenous addition of acylhigh serine lactones (AHLs) can improve the activity of anaerobic ammonia oxidation bacteria (AAOB), which is conducive to the rapid start-up of anaerobic ammonia oxidation (ANAMMOX). However, the acquisition of AHLs increases the operating cost. Based on this, a two-stage ANAMMOX series reactor was constructed in this study to make full use of the effluent active components of the primary reactor (R1) to realize the rapid start-up of the post-reactor (R2). In the experiment, R1 effluent was used as the influent, and gradually transferred to the R1 effluent clear liquid and synthetic wastewater as the influent. The feasibility of the strategy was verified by nitrogen removal performance, sludge characteristics and microbial community changes, and the relevant mechanism was analyzed. The results showed that R2 achieved rapid start-up on the 56th day, its total nitrogen volume load was 0.6-0.7 kg·(m3·d)-1 after operation for 90 days, and its total nitrogen removal efficiency could reach higher than 93%, which was significantly higher than that of the control group (R3, 61%) started with synthetic sewage directly. This was attributed to the fact that the sludge lost from R1 provided a stable biomass supplement for R2, and the quorum-sensing signal molecules contained in its supernatant promoted the activity of AAOB in R2. High-throughput sequencing results showed that the organic matter introduced into R1 effluent led to an increase in R2 community diversity and the proportion of heterotrophic bacteria such as denitrification, and a decrease in the proportion of ANAMMOX functional bacteria Candidatus Brocadia. However, compared with R1, the expression levels of transport and metabolity-related functional genes in R2 were up-regulated. These results indicate that it is feasible to realize the rapid start-up of the post-reactor through two-stage ANAMMOX series.-
Key words:
- anaerobic ammonia oxidation (ANAMMOX) /
- two-stage series /
- quick start-up /
- quorum sensing /
- microbiome
-

-
表 1 R2的运行条件及进水特征
Table 1. Operating conditions and inflow characteristics of R2
运行阶段 时间/d 进水来源 NH4+−N/
(mg·L−1)NO2−−N/
(mg·L−1)NO3−−N/
(mg·L−1)COD/
(mg·L−1)DO/
(mg·L−1)pH HRT/h NLR/
(kg·(m3·d)−1)Ⅰ 1~30 R1出水 40~55 45~65 16~25 40~70 <0.2 7.77~7.93 5 0.45~0.58 Ⅱ 31~60 R1出水上清液 40~55 45~65 16~25 40~70 <0.6 7.65~7.75 5/4 0.49~0.70 Ⅲ 61~90 合成废水 40~55 45~65 1.5~3.5 — 3~5 7.65~7.75 4 0.60~0.70 表 2 ANAMMOX污泥菌群的多样性指数变化
Table 2. Diversity index changes of ANAMMOX sludge microbial community
反应器 序列数 OTUs ACE指数 Chao1指数 Shannon指数 R1 32 366 845 1 037.31 963.21 3.73 R2 35 501 983 1 098.51 1 042.12 4.36 -
[1] CHEN H, HU H Y, CHEN Q Q, et al. Successful start-up of the anammox process: Influence of the seeding strategy on performance and granule properties[J]. Bioresource Technology, 2016, 211: 594-602. doi: 10.1016/j.biortech.2016.03.139 [2] ZEKKER I, RIKMANN E, TENNO T, et al. Anammox bacteria enrichment and phylogenic analysis in moving bed biofilm reactors[J]. Environmental Engineering Science, 2012, 29: 946-950. doi: 10.1089/ees.2011.0146 [3] KARTAL B, NIFTRIK L V, KELTJENS J T, et al. Anammox-growth physiology, cell biology and metabolism[J]. Advances in Microbial Physiology, 2012, 60(60): 211-262. [4] CHEN W, CHEN S, HU F, et al. A novel anammox reactor with a nitrogen gas circulation: Performance, granule size, activity, and microbial community[J]. Environmental Science and Pollution Research, 2020, 27(15): 1-11. [5] TAO Y, GAO D W, FU Y, et al. Impact of reactor configuration on anammox process start-up: MBR versus SBR[J]. Bioresource Technology, 2012, 104(1): 73-80. [6] ADAMS M, XIE J X, CHANG Y F, et al. Start-up of anammox systems with different biochar amendment: Process characteristics and microbial community[J]. Science of the Total Environment, 2021, 790: 148242. doi: 10.1016/j.scitotenv.2021.148242 [7] KOWALSKI M S, DEVLIN T R, Oleszkiewicz J A. Start-up and long-term performance of anammox moving bed biofilm reactor seeded with granular biomass[J]. Chemosphere, 2018, 200: 481-486. doi: 10.1016/j.chemosphere.2018.02.130 [8] LIU Y, NIU Q G, WANG S P, et al. Upgrading of the symbiosis of Nitrosomanas and anammox bacteria in a novel single-stage partial nitritation-anammox system: Nitrogen removal potential and Microbial characterization[J]. Bioresource Technology, 2017, 244: 463-472. doi: 10.1016/j.biortech.2017.07.156 [9] JIANG H, LIU G H, Ma Y, et al. A pilot-scale study on start-up and stable operation of mainstream partial nitrification-anammox biofilter process based on online pH-DO linkage control[J]. Chemical Engineering Journal, 2018, 350: 1035-1042. doi: 10.1016/j.cej.2018.06.007 [10] 刘雪娇, 田智勇, 杨宏, 等. 厌氧氨氧化(ANAMMOX)生物滤池反应器的启动特性[J]. 环境工程学报, 2012, 6(12): 4299-4304. [11] LIU Y, LIU W, LI Y Y, et al. Layered inoculation of anaerobic digestion and anammox granular sludges for fast start-up of an anammox reactor[J]. Bioresource Technology, 2021, 339: 125573. doi: 10.1016/j.biortech.2021.125573 [12] CHEN J B, ZHOU X, CAO X W, et al. Optimizing anammox capacity for weak wastewater in an AnSBBR using aerobic activated sludge as inoculation[J]. Journal of Environmental Management, 2020, 280: 111649. [13] WANG H, PENG L, MAO N, et al. Effects of Fe3+ on microbial communities shifts, functional genes expression and nitrogen transformation during the start-up of Anammox process[J]. Bioresource Technology, 2021, 320: 124326. doi: 10.1016/j.biortech.2020.124326 [14] YIN X, QIAO S, ZHOU J, et al. Fast start-up of the anammox process with addition of reduced graphene oxides[J]. Chemical Engineering Journal, 2016, 283: 160-166. doi: 10.1016/j.cej.2015.07.059 [15] TANG C J, ZHENG P, CHEN J W, et al. Start-up and process control of a pilot-scale Anammox bioreactor at ambient temperature[J]. Chinese Journal of Biotechnology, 2009, 25: 406-412. [16] TANG X, LIU S T, ZHANG Z T, et al. Identification of the release and effects of AHLs in anammox culture for bacteria communication, Chemical Engineering Journal, 2015, 273: 184-191. [17] PAPENFORT K, BASSLER B L. Quorum sensing signal–response systems in Gram-negative bacteria[J]. Nature Reviews Microbiology, 2016, 14: 576-588. doi: 10.1038/nrmicro.2016.89 [18] LIU L J, Xu S H, Wang F, et al. Effect of exogenous N-acyl-homoserine lactones on the anammox process at 15℃: Nitrogen removal performance, gene expression and metagenomics analysis[J]. Bioresource Technology, 2021, 314: 125760. [19] ZHANG H M, CAI L, ZHANG F, et al. Vacuum lyophilization preservation and rejuvenation performance of anammox bacteria[J]. Journal of Bioscience and Bioengineering, 2020, 129: 519-527. doi: 10.1016/j.jbiosc.2019.10.007 [20] 王晓曈, 杨宏. 基于粒径分化的厌氧氨氧化污泥性能与微生物多样性分析[J]. 环境科学, 2021, 42: 1930-1938. [21] 国家环境保护总局. 水和废水监测分析方法[J]. (第四版). 北京:中国环境科学出版社, 2002: 323-334. [22] MA C, JIN R C, YANG G F, et al. Impacts of transient salinity shock loads on Anammox process performance[J]. Bioresource Technology, 2012, 112: 124-130. doi: 10.1016/j.biortech.2012.02.122 [23] WANG X T, YANG H, SU Y, et al. Effects of sludge morphology on the anammox process: Analysis from the perspectives of performance, structure, and microbial community[J]. Chemosphere, 2022, 288: 132390. doi: 10.1016/j.chemosphere.2021.132390 [24] 张晶, 张林华, 姜冬冬, 等. 信号分子对高负荷UASB中ANAMMOX颗粒特性的影响[J]. 中国环境科学, 2018, 38: 10. [25] 唐崇俭. 厌氧氨氧化工艺特性与控制技术的研究[D]杭州: 浙江大学, 2011. [26] PENG Z H, LEI Y F, LIU Y M, et al. Fast start-up and reactivation of anammox process using polyurethane sponge[J]. Biochemical Engineering Journal, 2022, 177: 108249. doi: 10.1016/j.bej.2021.108249 [27] CHU Z R, WANG K, LI X K, et al. Microbial characterization of aggregates within a one-stage nitritation–anammox system using high-throughput amplicon sequencing[J]. Chemical Engineering Journal, 2015, 262: 41-48. doi: 10.1016/j.cej.2014.09.067 [28] CHEN Z, ZHANG X J, MA Y P, et al. Anammox biofilm system under the stress of Hg(II): Nitrogen removal performance, microbial community dynamic and resistance genes expression[J]. Journal of Hazardous Materials, 2020, 395: 122665. doi: 10.1016/j.jhazmat.2020.122665 [29] ZHANG X N, SUN Y L, MA F, et al. Role of soluble microbial product as an intermediate electron station linking C/N and nitrogen removal performance in sequencing batch reactor[J]. Environmental Research, 2020, 183: 109248. doi: 10.1016/j.envres.2020.109248 [30] BLACKBURNE R, YUAN Z, KELLER J. Partial nitrification to nitrite using low dissolved oxygen concentration as the main selection factor[J]. Biodegradation, 2008, 19: 303-12. doi: 10.1007/s10532-007-9136-4 [31] STROUS M, HEIJNEN J J, KUENEN J G, et al. The sequencing batch reactor as a powerful tool for the study of slowly growing anaerobic ammonium-oxidizing microorganisms[J]. Applied Microbiology Biotechnology, 1998, 50: 589-596. doi: 10.1007/s002530051340 [32] BOLEIJ M, KLEIKAMP H, PABST M, et al. Decorating the anammox house: Sialic acids and sulfated glycosaminoglycans in the extracellular polymeric substances of anammox granular sludge[J]. Environmental Science &Technology, 54: 5218-5226. [33] ZHAO R, ZHANG H M, ZHANG F, et al. Fast start-up anammox process using Acyl-homoserine lactones (AHLs) containing supernatant[J]. Journal of Environmental Sciences, 2018, 65: 127-132. doi: 10.1016/j.jes.2017.03.025 [34] 李海玲, 李冬, 张杰, 等. 调控温度和沉降时间实现ANAMMOX颗粒快速启动及其稳定运行[J]. 环境科学, 2019, 40: 837-844. [35] WANG G P, ZHANG D, XU Y, et al. Comparing two start up strategies and the effect of temperature fluctuations on the performance of mainstream anammox reactors[J]. Chemosphere, 2018, 209: 632-639. doi: 10.1016/j.chemosphere.2018.06.134 [36] WANG S P, LIU YUAN, NIU Q G, et al. Nitrogen removal performance and loading capacity of a novel single-stage nitritation-anammox system with syntrophic micro-granules[J]. Bioresource Technology, 2017, 236: 119-128. doi: 10.1016/j.biortech.2017.03.164 [37] FENG Z L, WU Y H, SHI Y H, et al. Successful startup of one-stage partial nitritation and anammox system through cascade oxygen supply and potential ecological network analysis[J]. Science of the Total Environment, 2019, 696: 134065. doi: 10.1016/j.scitotenv.2019.134065 [38] LI A J, HOU B L, LI M X. Cell adhesion, ammonia removal and granulation of autotrophic nitrifying sludge facilitated by N-acyl-homoserine lactones[J]. Bioresource Technology, 2015, 196: 550-558. doi: 10.1016/j.biortech.2015.08.022 [39] LAWSON C E, WU S, BHATTACHARJEE A S, et al. Metabolic network analysis reveals microbial community interactions in anammox granules[J]. Nature Communications, 2017, 8: 15416. doi: 10.1038/ncomms15416 [40] LI X, TAO R J, TIAN, M J, et al. Recovery and dormancy of nitrogen removal characteristics in the pilot-scale denitrification-partial nitrification-Anammox process for landfill leachate treatment[J]. Journal of Environmental Management, 2021, 300: 113711. doi: 10.1016/j.jenvman.2021.113711 [41] DE COCKER P, BESSIERE Y, HERNANDEZ-RAQUET G, et al. Enrichment and adaptation yield high anammox conversion rates under low temperatures[J]. Bioresource Technology, 2018, 250: 505-512. doi: 10.1016/j.biortech.2017.11.079 [42] ZHAO Y P, LIU S F, Jiang B, et al. Genome-centered metagenomics analysis reveals the symbiotic organisms possessing ability to cross-feed with anammox bacteria in anammox consortia[J]. Environmental Science & Technology, 2018, 52: 11285-11296. [43] STROUS M, VAN GERVEN, KUENEN J G, et al. Effect of aerobic and microbial aerobic condition on anaerobic ammonium-oxiding (Anammox) sludge[J]. Applied and Environmental Microbiology, 1997, 63: 2446-2448. doi: 10.1128/aem.63.6.2446-2448.1997 -



 下载:
下载: