-
氟是人体内重要的微量元素之一,但当过量的氟在人体内累积时,会引起佝偻病、神经系统疾病、韧带骨化和氟斑牙等疾病[1]。据报道,全世界约有23个国家,超过2亿人正面临氟中毒威胁,其中印度和中国受污染影响程度最高[2-3]。在我国地下水体中,氟化物已经被广泛检出。根据《2019中国生态环境状况公报》,全国2 830处浅层地下水水质监测井中,Ⅳ~Ⅴ类占76.1%,其中氟化物是重要污染指标之一[4]。为保障饮水安全,世界卫生组织(WHO)规定饮用水中氟离子(F−)的最大质量浓度为1.5 mg∙L−1,我国《生活饮用水卫生标准》(GB 5749-2006)规定F−质量浓度上限为1.0 mg∙L−1[5]。因此,如何有效去除水体中的F−,将其浓度控制在安全饮用水范围内已成为全球亟待解决的问题。
目前,主要的除氟技术包括电凝聚法[6]、化学沉淀法[7]、膜分离法[8]、离子交换法[9]和吸附法[10]。其中,吸附法具有选择性强、去除率高、成本低、易操作且污泥产量少等优势,已成为当前除氟的研究热点[11-12]。在众多吸附剂中,生物炭因制作简单、成本低廉、环境友好等优点得到广泛应用。但生物炭表面带负电荷,对氟等阴离子作用力弱,因此通过引入金属离子提升生物炭的吸附性能受到国内外研究者的关注[13]。侯笛等[14]先制备生物炭,再采用浸渍法制得铁改性生物炭,其对氟的最大吸附容量为1.55 mg∙g−1。WANG等[15]先制得柚子皮生物炭,再利用La(NO3)3浸渍制得镧改性生物炭,其对氟的最大吸附容量为19.86 mg∙g−1。VENCES-ALVAREZ等[16]将商用活性炭浸渍于不同浓度La3+溶液中1~4 d,制得镧修饰活性炭,其对氟的最大吸附容量为9.96 mg∙g−1。不过,上述方法多为两步法,金属离子沉积于炭材料表面,且制备过程存在浸渍时间长、金属有效负载率低等缺点。
国家统计局已有数据显示,2020 年,我国花生总产量约为 1.8×107 t,同比增长达 2.7%。由于花生壳一类农耕废弃物在我国未形成完整产业链,导致大量的花生壳作为一般垃圾进行焚烧与填埋,增加生活垃圾产量的同时也造成了资源浪费[17]。本研究以花生壳为生物质原料,以LaCl3·7H2O为改性剂,采用简单的一步热解法制备了载镧生物炭(La-BC),在对制备条件进行优化的基础上,通过单因素实验研究了La-BC对F−的吸附性能及其在地下水体中的应用潜能,并结合SEM、XRD和FTIR表征结果,探讨了La-BC的除氟机理,以期为镧改性生物炭处理含氟地下水提供参考。
-
本实验使用的花生壳购自成都市当地农贸市场。首先用自来水清洗花生壳2次以去除表面泥土等杂质,然后在105 °C下干燥24 h;干燥的样品通过破碎和筛分,得到平均粒径小于1.0 mm的花生壳粉末,并储存于密闭容器罐中备用。
-
七水合氯化镧购自阿拉丁化学试剂公司;氟化钠、氢氧化钠、二水合柠檬酸三钠和冰乙酸等购自成都金山化学试剂有限公司,均为分析纯;氟标准溶液(1 000 mg∙L−1)购自国家标准物质中心;溶剂配置和实验用水均为超纯水。
JA2003N电子天平(上海菁海仪器有限公司)、LD-T100破碎机(上海顶帅电器有限公司)、TD-420台式低速离心机(四川蜀科仪器有限公司)、101-3A电热鼓风干燥箱(天津市泰斯特仪器有限公司)、PHS-3C精密pH计(上海雷磁仪器厂)、HNY-100D恒温培养振荡器(天津市欧诺仪器仪表有限公司)、SX-10-13箱式电阻炉(沈阳市节能电炉厂)、PXSJ-216氟离子计(上海仪电科学仪器股份有限公司)。
-
称取5 g花生壳粉,加入适量氯化镧溶液(LaCl3·7H2O),搅拌均匀;混合物在105 °C干燥24 h后,置于箱式马弗炉中进行热解处理;热解产物冷却后用去离子水洗涤2~3次,干燥制得载镧生物炭(La-BC)。采用同样的方法烧制空白生物炭(BC)。
-
将样品粘于导电胶上、喷金以提高其导电率,之后采用Apreo 2C型扫描电镜(SEM)观察材料的表面形貌;利用Ultima IV型X射线衍射仪(XRD)分析样品组成,仪器条件:Cu-Kα为辐射源,电压40 KV,电流40 mA,衍射角2θ=5°~85°;采用Nicolet-iS10型傅里叶变换红外光谱仪(FTIR)对样品表面官能团进行表征,扫描范围为400~4 000 cm−1,分辨率为4 cm−1。
-
取若干锥形瓶,分别配置100 mL不同质量浓度(5~30 mg∙L−1)的F−溶液,调节溶液pH (2~11),加入适量La-BC(0.1~0.6 g∙L−1),在预设温度(15~45 ℃)下振荡反应一定时间(5 min~24 h),取出、过滤,取上清液测定电位,根据电位-氟离子浓度标准曲线求得出水质量浓度。F−去除率和La-BC的吸附容量分别根据式(1)和式(2)进行计算。
式中:η为F−的去除率,%;C0和Ct分别为吸附前和吸附后溶液中的F−质量浓度,mg∙L−1;q为吸附容量,mg∙g−1;V为溶液体积,L;m为La-BC的投加量,g。
-
固定La-BC投加量0.5 g∙L−1,向初始质量浓度为10 mg∙L−1的F−溶液中分批加入100 mg∙L−1和250 mg∙L−1的SO42−、Cl−、NO3−、HCO3−、CO32−和NH4+,然后将溶液置于35 ℃、转速为150 r∙min−1的恒温振荡器中反应6 h,以考察共存离子浓度对La-BC吸附F−的影响。
-
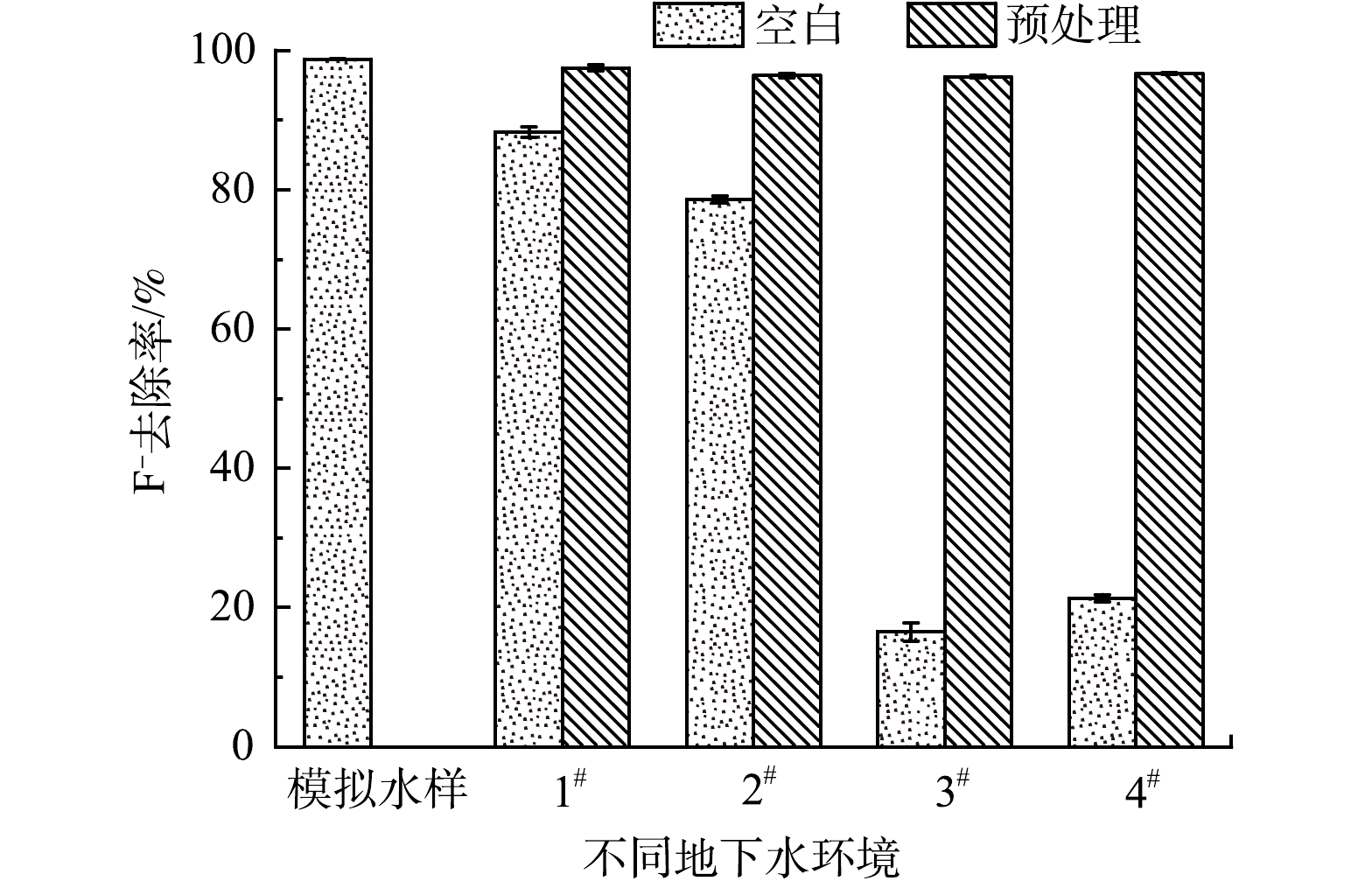
采集真实地下水样进行F−加标实验,以考察La-BC对地下水中F−的去除效果。在取回的地下水样中添加10 mg∙L−1的F−,然后利用0.1 mol∙L−1 HCl调节溶液pH至5.2,去除水样中的HCO3−与CO32-[18],作为酸化预处理实验组,空白实验组不进行任何处理。按照0.5 g∙L−1加入La-BC,将溶液置于35 ℃、转速为150 r∙min−1的恒温振荡器中反应6 h,过滤、取滤液测定F−质量浓度,计算其去除率。真实地下水样分别取自雅安市名山区农村井水(1#和2#)和成都市龙潭寺工业园区地下水(3#和4#),水样中F−背景浓度和水体pH如表1所示。
-
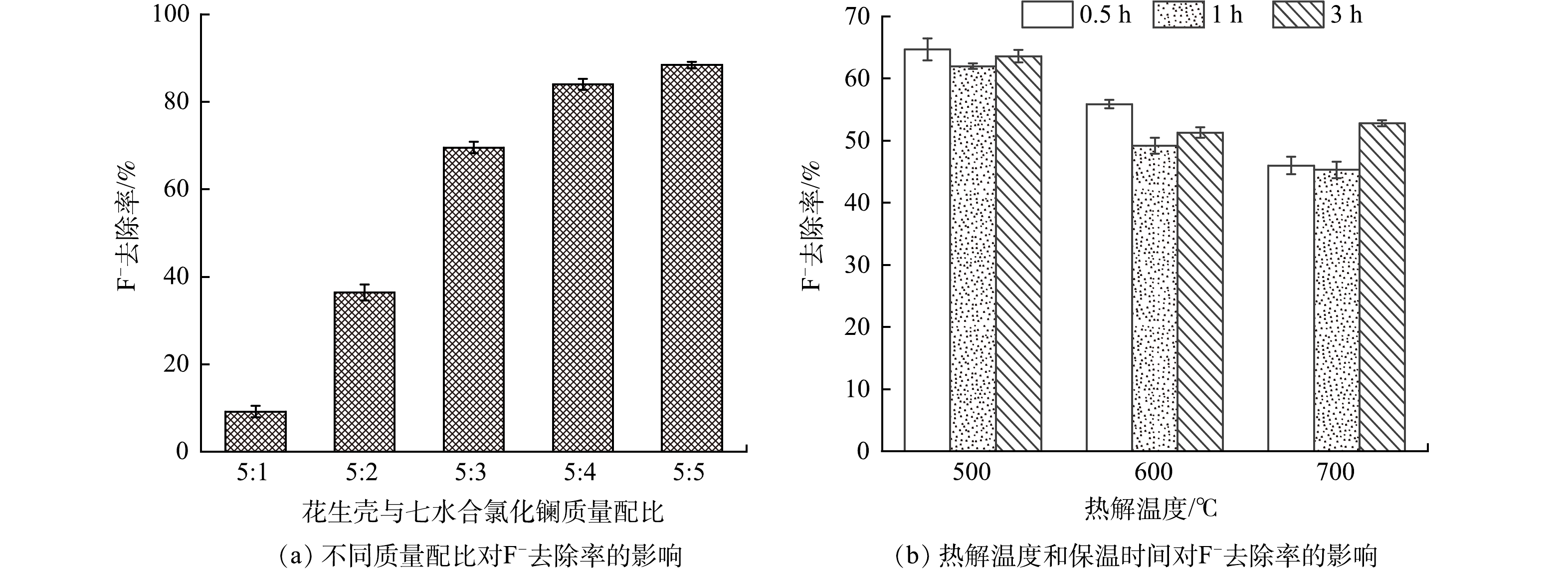
由图1(a)可知,随着花生壳与七水合氯化镧的质量比从5:1增大至5:3,La-BC对F−的去除率从9.2%急剧上升至69.6%;之后再增大质量比,去除率增长变缓。当质量比为5:4与5:5时,F−的去除率分别为84%与88.4%。这是因为生物炭表面有限,过量的镧只能进一步堆叠在已负载的镧物种上,从而降低了可利用的活性位点。为获得含镧化合物的最佳除氟效能,后续实验固定花生壳与七水合氯化镧的最佳质量比为5:3。
由图1(b)可以看到,随着热解温度从500 ℃增加至700 ℃,载镧生物炭对F−的去除率呈现明显下降趋势;在相同热解温度下,延长保温时间,去除率先略微下降再升高。究其原因,在La-BC烧制过程中,热解温度和保温时间会对炭材料表面含镧物种的种类、晶体大小以及氯元素含量产生影响,进而影响除氟活性位点[19]。当热解温度为500 ℃,保温时间分别为0.5、1和3 h时,La-BC对F−的去除率分别为64.7%、62%和63.6%。综合考虑所制样品的稳定性与可重复性,分别选择500 ℃和1 h作为最佳热解温度和最佳保温时间。
-
空白生物炭(BC)、载镧生物炭(La-BC)和吸附氟后的载镧生物炭的SEM表征结果如图2所示。与BC相比,La-BC表面附着了大量形状不规则的颗粒物。吸附氟后,载镧生物炭上的颗粒细化,且密度明显提高,表明La-BC上负载的含镧物种与溶液中的F−发生了反应。
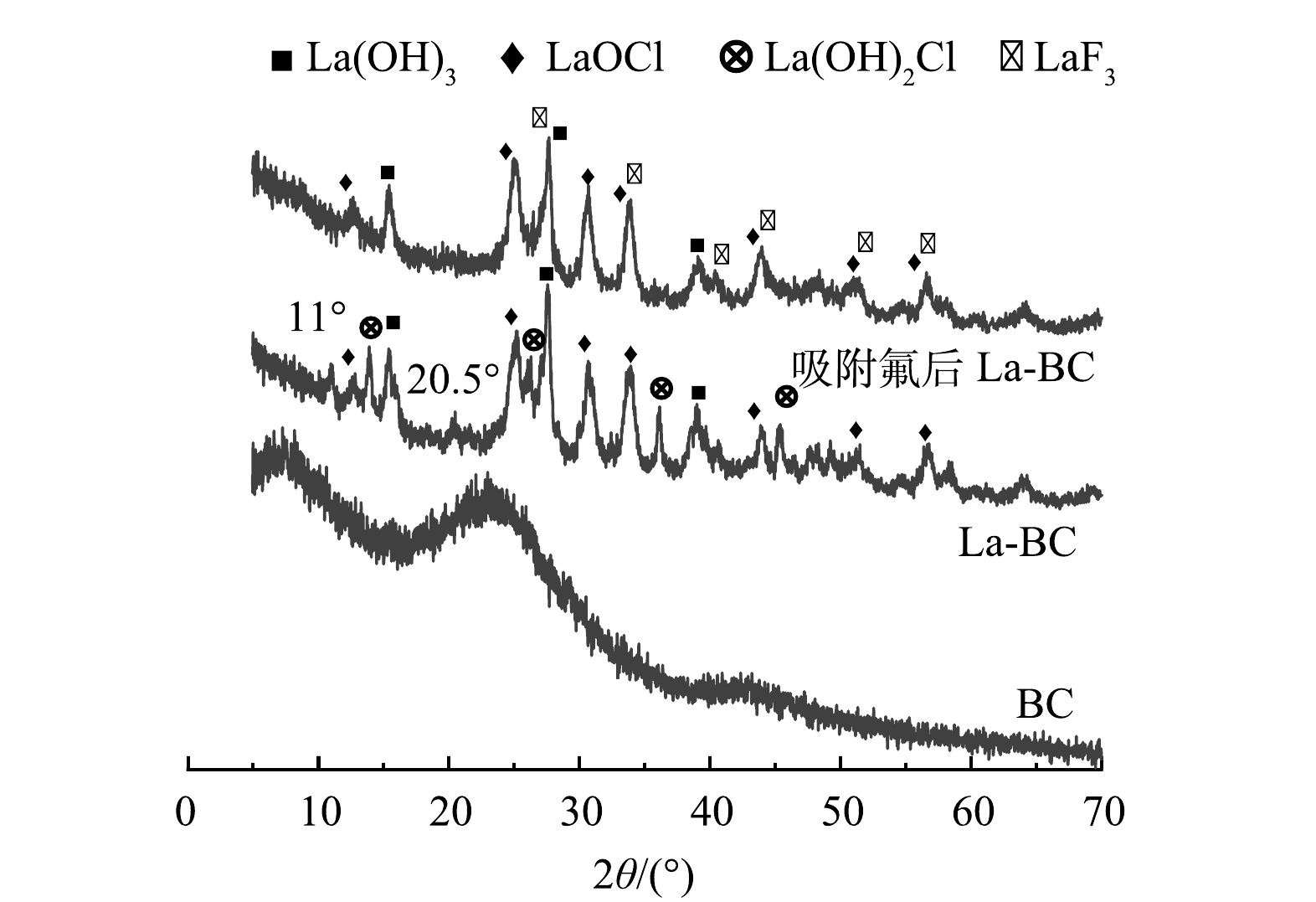
为进一步确认含镧物种与F−之间发生了反应,采用XRD对BC和除氟前后的La-BC进行表征,结果如图3所示。与BC相比,La-BC的XRD图上出现了一系列新的衍射峰,其中12.8°、25.3°、30.7°、34.0°、44.0°、51.4°和56.8°为LaOCl (PDF 01-073-2063)的特征衍射峰;15.4°、27.6°和39.1°为La(OH)3 (PDF 01-073-2034)的特征衍射峰;11.0°和20.5°分别为La2(CO3)3 (PDF 00-025-0423)和LaPO4 (PDF 00-012-0283)的特征衍射峰[20-21]。除此外,14.0°、26.3°、36.1°与45.2°为La(OH)2Cl (PDF 01-085-0839)的特征衍射峰。
La-BC吸附氟后,可以看到11.0°、14.0°、20.5°、26.3°、36.1°和45.2°处的衍射峰均消失,表明La2(CO3)3、LaPO4与La(OH)2Cl都参与了除氟过程,其中La(OH)2Cl为主要的除氟活性物种。与此同时,27.6°、34.0°、43.9°、51.1°与56.8°处的衍射峰强度呈现不同程度增加,位置发生轻微偏移。这是由于除氟过程生成的LaF3 (PDF 01-075-1523)的衍射峰叠加所致。
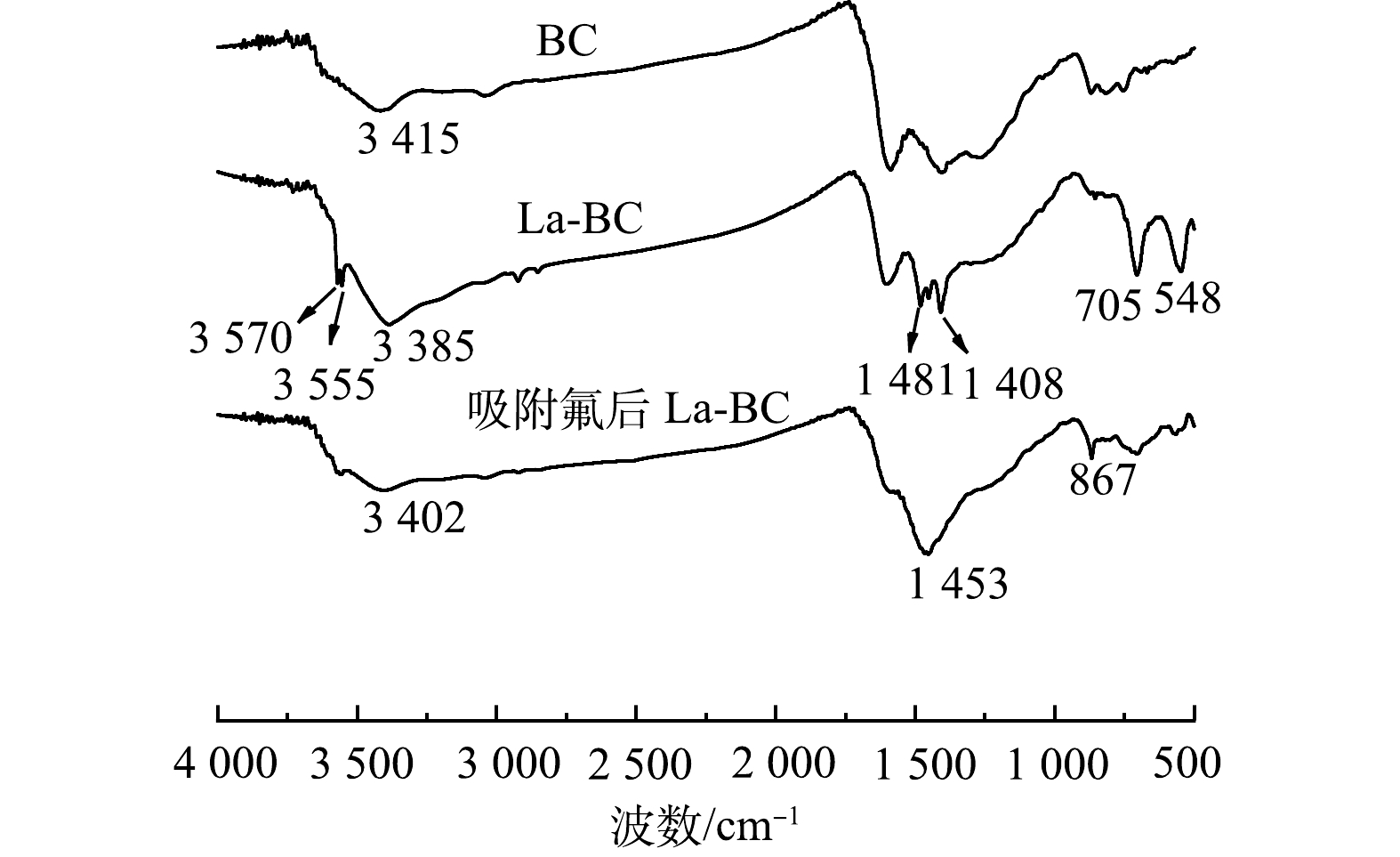
空白生物炭(BC)、载镧生物炭(La-BC)和吸附氟后的载镧生物炭的FTIR表征结果如图4所示。与BC相比,La-BC的FTIR图上出现了一系列新的吸收峰,其中705 cm−1和548 cm−1处的吸收峰属于La-O伸缩振动,3 570 cm−1和3 555 cm−1处的2个弱吸收峰是由于La(OH)2Cl中OH和Cl之间的相互作用所致[22]。另外,1 481 cm−1和1 408 cm−1出现的新吸收峰归因于CO32−的伸缩振动,因为镧系化合物对CO2具有吸收倾向,所以在红外光谱中常可以看到碳酸盐吸收峰[23]。La-BC吸附氟后,3 570 cm−1和3 555 cm−1处的吸收峰消失,705 cm−1和548 cm−1处的吸收峰减弱,表明材料中的La(OH)2Cl与La-O键参与了除氟过程,反应生成的LaF3。由于La-F键振动不在中红外区,无法观察到它的吸收谱带[24]。
-
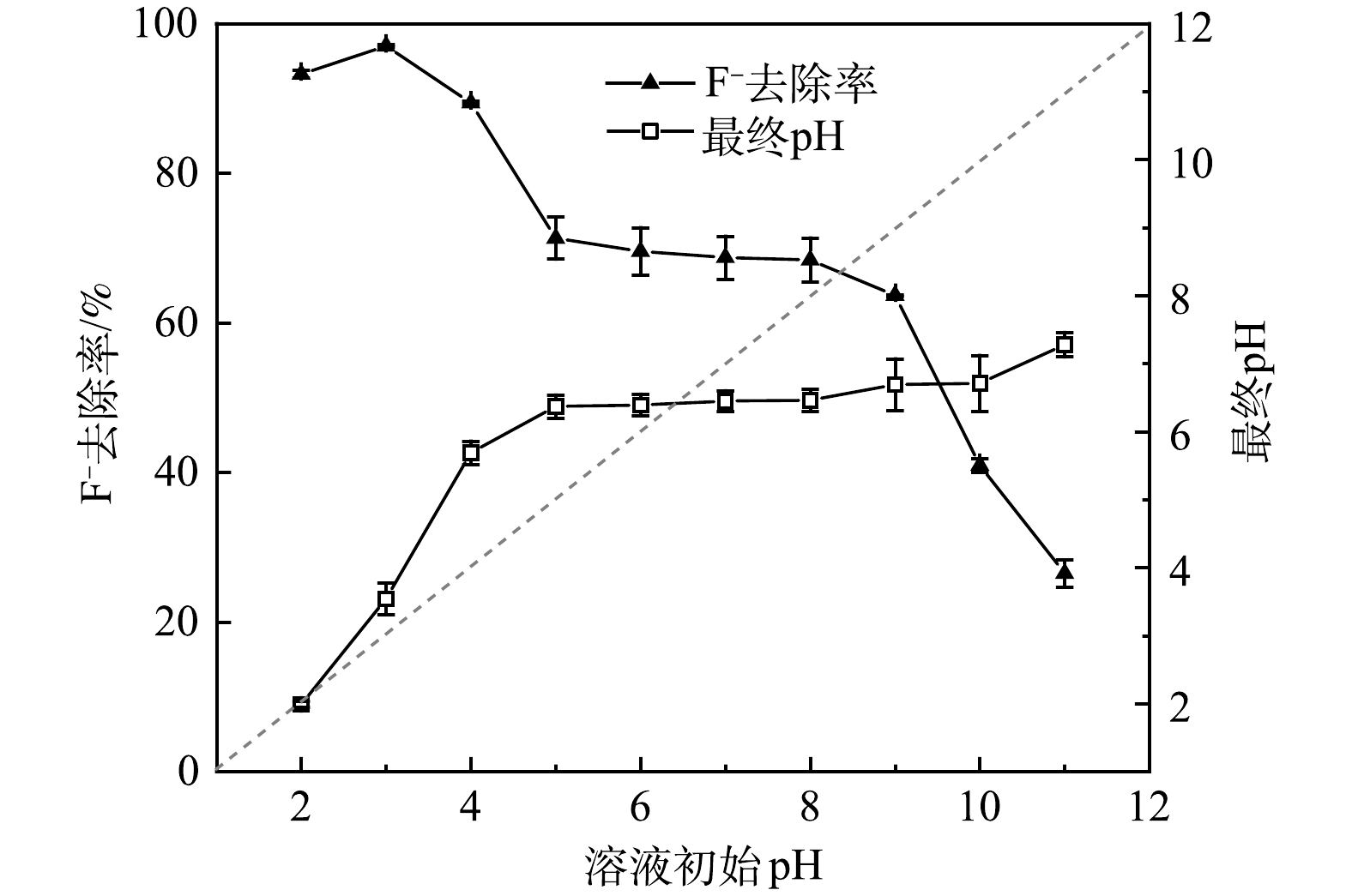
溶液pH是评价吸附剂适用性的关键指标。由图5可知,当溶液pH从2上升至3时,F−去除率增加。这是由于HF的电离常数(pKa)为3.2,当溶液pH低于3.2时,水体中HF的电离受到了抑制。当溶液pH从3上升至5时,F−去除率降低,此时F−去除率主要由材料中La3+的浸出量决定。WANG等[15]的研究表明,当溶液pH低于4.7时,材料中的La3+会浸出与F−发生反应,且浸出浓度随pH减小而增大。当溶液pH为5~8,La3+的浸出量可忽略不计,去除F−完全依赖于La-BC负载的含镧活性物种,此时除氟效率较稳定且保持在68%左右,这也表明La-BC能较好适应受污染地下水的pH (6~8)[25]。当溶液pH从9上升至11时, F−去除率出现显著降低。这归咎于水体pH过高,远远大于La-BC的等电点(pHpzc=6.4),导致吸附材料上的羟基发生去质子化带上负电荷,与水体中F−的作用力由静电引力变成静电斥力;与此同时,溶液碱度增加也使得大量OH−竞争吸附剂表面的活性位点[26]。
-
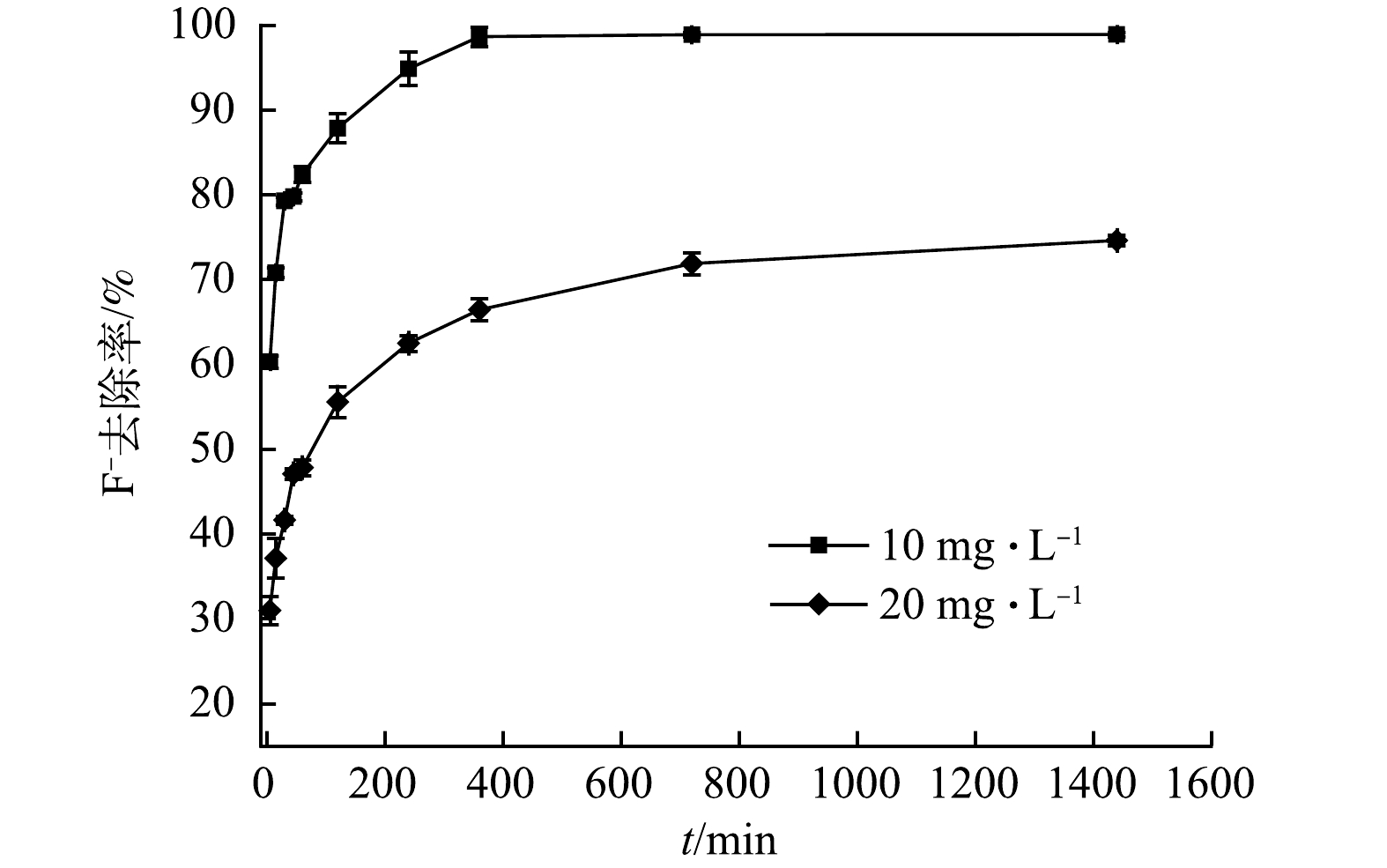
由图6可知,当反应时间为30 min时,初始质量浓度为10 mg∙L−1和20 mg∙L−1的F−溶液,其去除率分别为79.3%和41.6%,分别达到平衡时除氟效率的80.1%和55.7%,与 VENCES-ALVAREZ等[16]报道的现象类似。这是因为反应初期吸附剂表面存在大量活性位点后,能快速捕集溶液中的F−,但随着反应时间的延长,F−去除率的增长趋势逐渐放缓,最终分别在6 h和12 h时趋近平衡,此时F−的去除率分别为98.9%和72.0%。在本研究中,当F−初始质量浓度为10 mg∙L−1,出水F−低于1.0 mg∙L−1,满足我国生活饮用水标准(GB 5749-2006)。
-
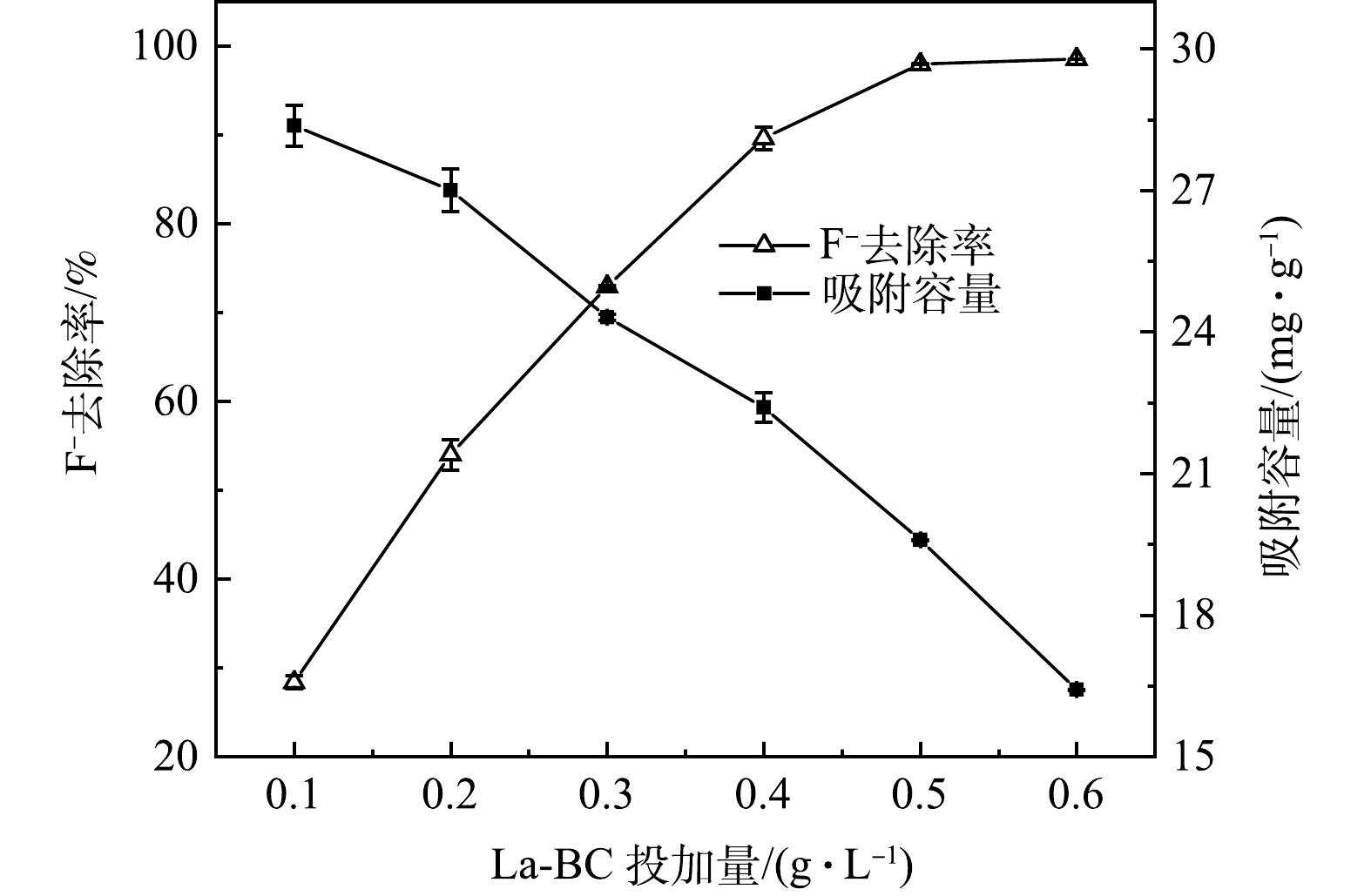
由图7可知,固定F−质量浓度为10 mg∙L−1,当La-BC投加量从0.1 g∙L−1增加至0.5 g∙L−1时,F−的去除率保持线性上升,从最初的28.4%上升至98.0%;之后继续增加La-BC至0.6 g∙L−1,吸附达到平衡。与之相反,随La-BC投加量增加,吸附容量却由28.37 mg∙g−1下降至16.4 mg∙g−1。这是因为溶液中F−数量有限,当La-BC投加量不断增长时,吸附活性位点闲置数量也随之增加。
-
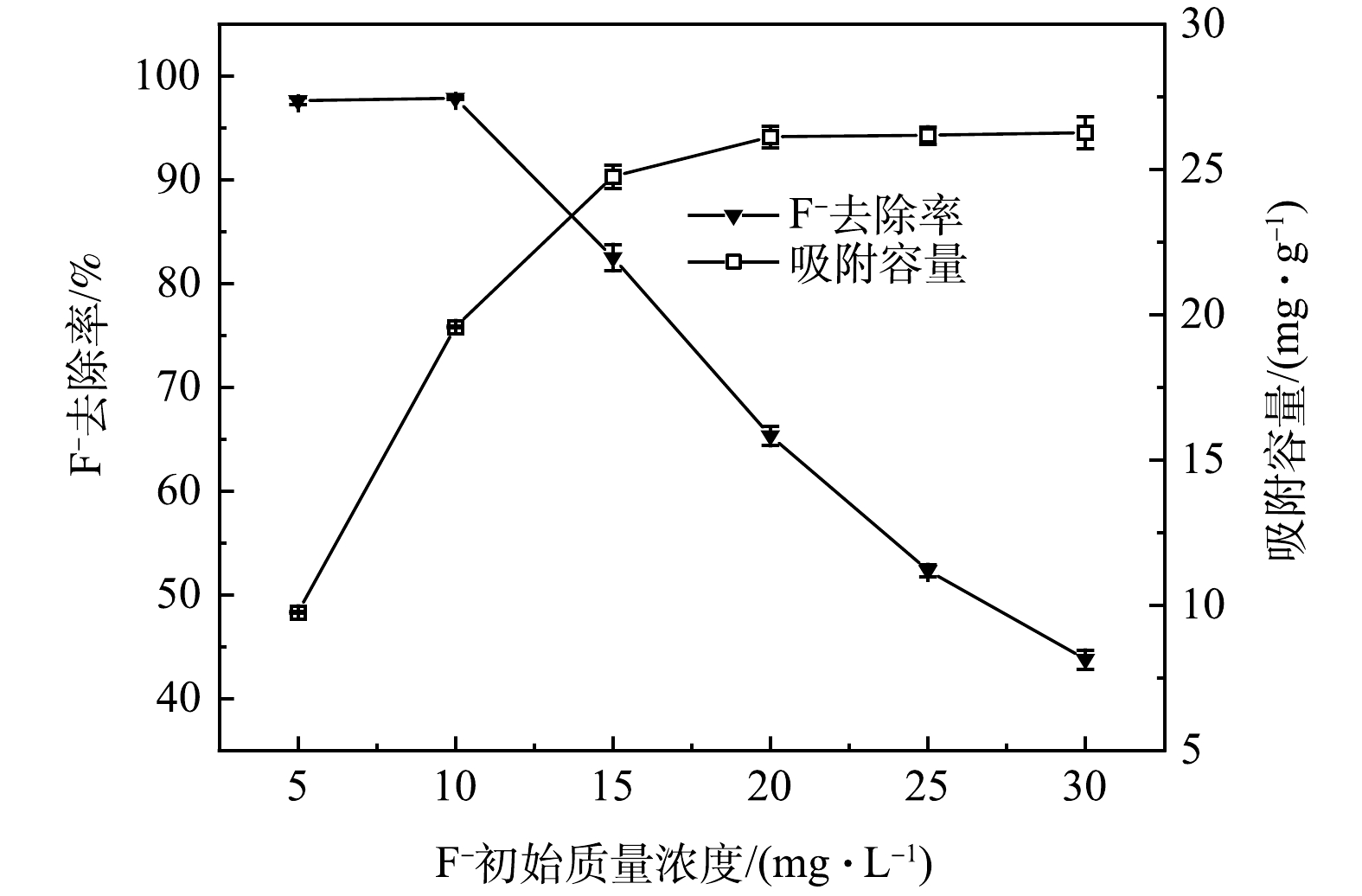
由图8可知,固定 La-BC投加量为0.5 g∙L−1,当F−初始质量浓度由5 mg∙L−1上升至10 mg∙L−1时,其去除率保持不变;当F−质量浓度进一步增加至30 mg∙L−1时,F−去除率由97.6%下降至43.8%。这是因为吸附剂投加量固定时,不断增加的F−数量超过了吸附剂表面活性位点数,导致去除率下降。与此同时,充足的F−使得La-BC表面的吸附活性位点逐渐饱和,因此,吸附容量由9.76 mg∙g−1增加至26.13 mg∙g−1。
-
为了评价La-BC对F−的吸附速率,将F−初始质量浓度为20 mg∙L−1的动力学实验数据,利用准一级动力学方程(式(3))与准二级动力学方程(式(4))进行曲线拟合,所得参数如表2所示。
式中:qt和qe分别为t时刻与平衡时刻的吸附容量,mg∙g−1;t为吸附时间,h;k1为准一级动力学常数,h−1;k2为准二级动力学常数,g∙(mg∙h)−1。
很明显,准二级动力学模型拟合曲线的可决系数( R2=0.914)高于准一级动力学模型(R2=0.814),且其理论吸附容量qe,cal与实验值qe,exp 差异更小,说明La-BC对F−的吸附过程以化学吸附为主导作用。
-
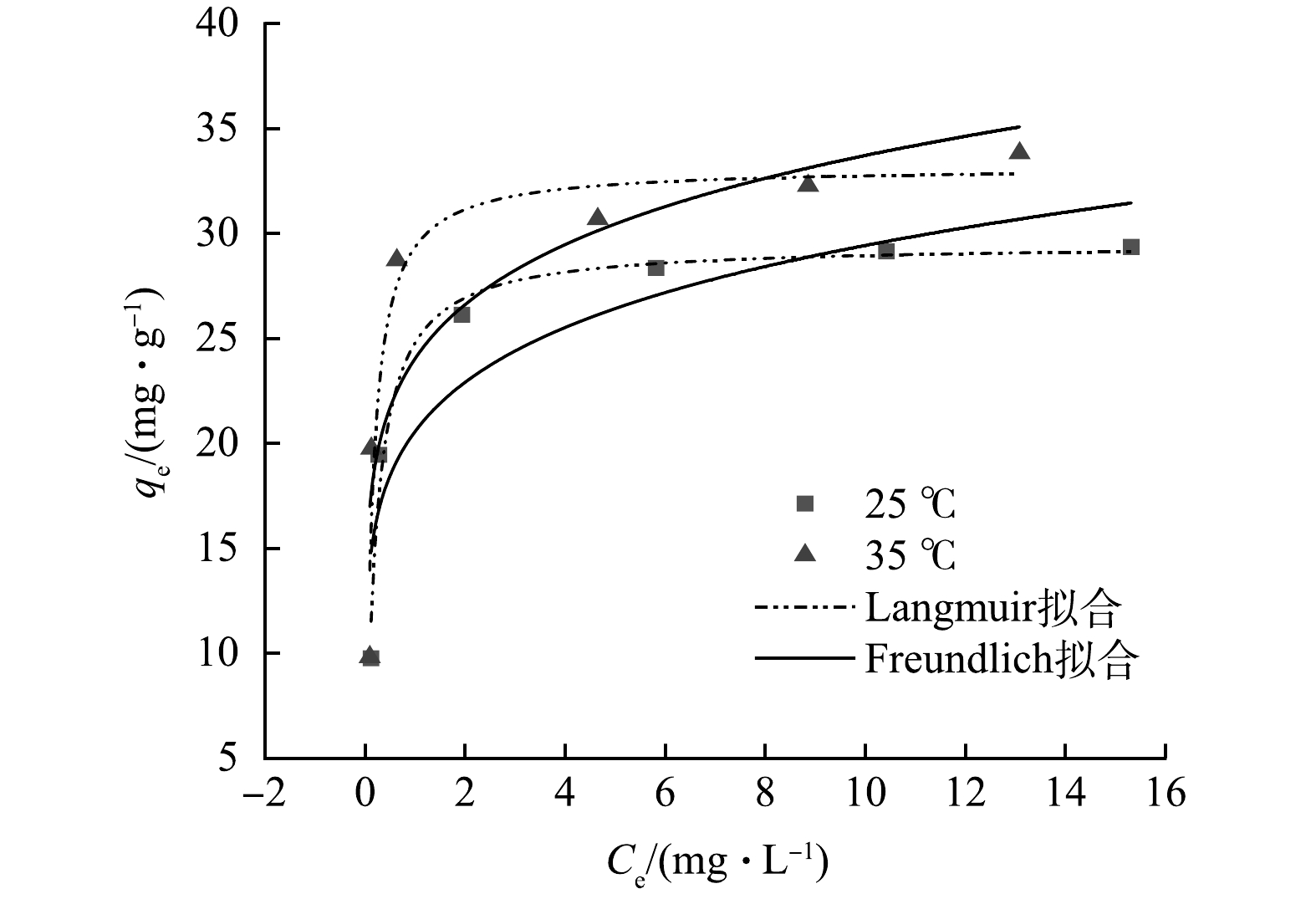
吸附等温线是研究吸附过程的重要依据,其可以用来描述吸附剂与吸附质之间的平衡关系、亲和力及吸附剂的吸附能力。Langmuir模型和Freundlich模型方程分别如式(5)和式(6)所示。在不同温度下,La-BC吸附F−的拟合曲线与参数分别如图9和表3所示。
式中:Ce为吸附平衡时F−质量浓度,mg∙L−1;qmax为最大单层吸附容量,mg∙g−1;KL为Langmuir吸附平衡常数,L∙mg−1;KF为Freundlich吸附平衡常数,(mg∙L1/n)∙(g∙mg1/n)−1;
很明显,Langmuir模型拟合曲线的拟合系数R2均高于Freundlich模型,说明La-BC对F−的去除主要为均匀的单层吸附过程[27]。通过计算,可得到25 ℃和35 ℃下的最大理论吸附容量分别为29.50 mg∙g−1和33.17 mg∙g−1。
-
吉布斯自由能(ΔG0)可以判断吸附过程能否自发进行。根据式(7)和式(8),以lnK0对1/T作图,通过斜率和截距计算F−吸附过程的重要热力学参数,结果见表4。
式中:T为反应温度,K;K0为吸附平衡常数,L∙mol−1;R为理想气体常数,取值为8.314×10−3 kJ∙(mol∙K)−1;
由表4可以看到,焓变(∆H0)为正值,表明La-BC对F−的吸附过程为吸热反应,说明温度升高有利于反应的进行;熵变(∆S0)为正值,表明随着吸附反应的进行,吸附剂固/液界面的混乱度增加。在所有实验温度条件下,吸附自由能(∆G0)均为负值,表明La-BC对F−的吸附行为是自发的。
-
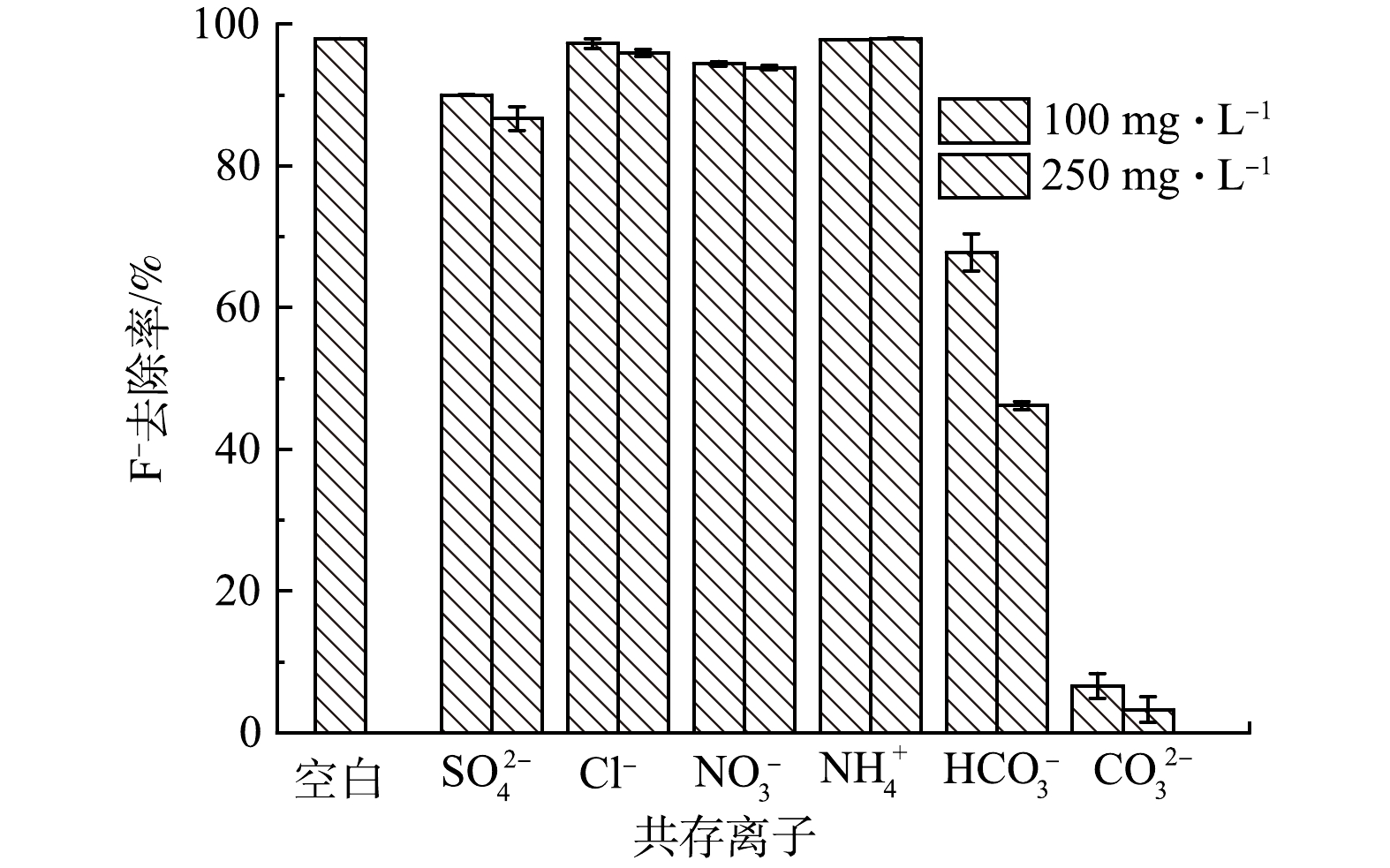
由图10可知,SO42−、HCO3−与CO32− 对F−的去除存在不同程度的影响,且其干扰能力随离子浓度的增加而增大。当SO42−、HCO3−与CO32的质量浓度为250 mg∙L−1时,La-BC对F−去除率分别降为86.71%、46.2%和3.27%。SO42−对除氟效率的负影响可归因于电荷密度高。CO32−的干扰除了与其电荷密度有关,还因为La2(CO3)3 (Ksp=3.98×10−34)具有比LaF3 (Ksp=2.0×10−19)更低的溶解度;同时当CO32− 添加到含氟废水中时,溶液pH上升至10.9,呈现较强碱性也是F−去除率急剧降低的关键[28-29]。HCO3−对除氟效率的影响主要归因于其电离促进溶液中CO32−的形成,从而竞争吸附位点。与上述3种离子相比,Cl−、NO3−和NH4+对F−的去除率影响较小,均不足4%。
-
利用真实地下水加标实验研究La-BC的实际应用潜能,结果如图11所示。由图11可见,加标地下水样1#和2#未经酸化处理时,La-BC对 F−去除率出现小幅度下降,但仍能达到88.2%和78.6%。而未经酸化处理的加标地下水样3#和4#中的F−去除率显著下降,仅为16.5%和21.3%。4个水样经酸化预处理调节溶液pH至5.2后,La-BC对F−去除率均保持在96.2%以上。这是因为地下水体中通常含有不同浓度的HCO3−。
-
1)与空白生物炭(BC)相比,镧改性生物炭(La-BC)的除氟性能得到显著提升。其最佳制备条件为:花生壳与LaCl3∙7H2O的质量比为5:3,热解温度和保温时间分别为500 ℃和1 h。
2) La-BC的等电点pHpzc为6.4,除氟适用pH范围较宽,在水体pH为5~8时表现出稳定的除氟性能。当F−质量浓度为10 mg∙L−1,La-BC投加量为0.5 g∙L−1时,出水F−质量浓度低于1.0 mg∙L−1,满足我国生活饮用水标准(GB 5749-2006)。SO42−、HCO3−和CO32− 对F−的吸附存在不同程度的影响,而Cl−、NO3−和NH4+的影响较小。
3) La-BC对F−的吸附符合准二级动力学和Langmuir等温线模型。在25 ℃和35 ℃下,La-BC的最大理论吸附容量分别为29.50 mg∙g−1和33.17 mg∙g−1。
4)在地下水加标实验中,水样经过酸化预处理调节pH为5.2时,La-BC对工业园区地下水和农村饮用井水均表现出优异的除氟性能。
5) La-BC上的含镧物种包括La(OH)3、LaOCl、La(OH)2Cl以及少量La2(CO3)3和LaPO4,其中La(OH)2Cl为主要的除氟活性物种。在La-BC除氟过程中,主要通过静电作用和化学沉淀将F−转变为LaF3沉淀。
载镧生物炭对水体氟离子的去除性能
Characteristics of fluoride ion removal from waterbody by lanthanum-loaded biochar
-
摘要: 以花生壳为原料、LaCl3∙7H2O为镧前驱体,通过一步热解法制备了载镧生物炭(La-BC),确定了最佳制备条件,考察了溶液pH、吸附时间、La-BC投加量、F−初始质量浓度、共存离子等因素对La-BC吸附性能的影响,评估了La-BC在真实地下水中的应用潜能。结果表明:在pH为5~8时,La-BC表现出稳定的除氟性能。吸附过程符合准二级动力学和Langmuir等温线模型;在25 ℃和35 ℃下,La-BC的最大理论吸附容量分别为29.50 mg∙g−1与33.17 mg∙g−1。SO42-、HCO3−与CO32− 对吸附过程存在不同程度的影响,Cl−、NO3−和NH4+影响较小。加标地下水经酸化预处理后,La-BC对工业园区地下水和农村饮用井水均表现出优异的除氟性能。La-BC上的含镧物种包括La(OH)3、LaOCl、La(OH)2Cl以及少量La2(CO3)3和LaPO4,其中La(OH)2Cl为主要的除氟活性物种,F−主要通过静电作用和化学沉淀转化为LaF3沉淀去除。Abstract: In this work, lanthanum-loaded biochar (La-BC) was prepared by one-step pyrolysis method with peanut shells and LaCl3∙7H2O as precursors, and the optimal preparation conditions were determined. The effects of solution pH, adsorption time, La-BC dosage, F− initial concentration and coexisting ions on F− removal were tested. Meanwhile, the application potential of La-BC in real groundwater was evaluated. Results showed that La-BC exhibited a stable F− removal ability at pH 5~8, and the adsorption process conformed to the pseudo-second-order kinetics and Langmuir isotherm model. At 25 and 35 ℃, the maximum theoretical adsorption capacities of La-BC were 29.50 and 33.17 mg∙g−1, respectively. Except SO42−, HCO3− and CO32−, the influence of the coexisting anions including Cl−, NO3− and NH4+ was negligible. After acidification pretreatment of the spiked groundwater samples, La-BC showed an excellent defluorination performance for both industrial park groundwater and rural drinking well water. XRD results showed that the lanthanum-containing species on the La-BC included La(OH)3, LaOCl, La(OH)2Cl and a small amount of La2(CO3)3 and LaPO4. F− was removed mainly by reaction with La(OH)2Cl and converted to LaF3 by electrostatic interaction and chemical precipitation.
-
Key words:
- Lanthanum-loaded biochar /
- adsorption /
- F- /
- groundwater
-

-
表 1 真实地下水样背景值
Table 1. Background values of real groundwater samples
水体类别 序号 水体pH F−质量浓度/(mg∙L−1) 雅安名山区农村井水 1# 6.85 0.05 2# 6.77 0.05 成都市龙潭寺工业区地下水 3# 6.96 0.47 4# 6.95 0.39 表 2 La-BC吸附F-的动力学模型拟合参数
Table 2. Kinetic fitting parameters of F- adsorption on La-BC
准一级动力学模型 准二级动力学模型 qe,exp/(mg∙g−1) k1/(h−1) qe,cal/(mg∙g−1) R2 k2/(g∙(mg∙h)−1) qe,cal/(mg∙g−1) R2 29.85 2.411 25.69 0.814 0.154 27.20 0.914 表 3 F-在La-BC上的等温吸附模型拟合参数
Table 3. Langmuir and Freundlich fitting parameters of F- adsorption on La-BC
温度/ ℃ Langmuir模型 Freundlich模型 qmax,cal/(mg∙g−1) KL/(L∙mg−1) R2 KF/(mg∙L1/n)·(g∙mg1/n)−1 1/n R2 25 29.50 5.245 0.968 20.559 0.156 0.793 35 33.17 7.674 0.900 24.027 0.147 0.718 表 4 La-BC吸附F-的热力学参数
Table 4. Thermodynamic parameters related to F- adsorption on La-BC
∆G0/(kJ∙mol−1) ∆S0/(kJ∙(mol·k)−1) ∆H0/(kJ∙mol−1) 15 ºC 25 ºC 35 ºC 45 ºC −3.342 −3.921 −4.829 −5.129 0.062 5 14.636 -
[1] DONG S, WANG Y. Characterization and adsorption properties of a lanthanum-loaded magnetic cationic hydrogel composite for fluoride removal[J]. Water Research, 2016, 88: 852-860. doi: 10.1016/j.watres.2015.11.013 [2] WEI L, ZIETZSCHMANN F, RIETVELD L C, et al. Fluoride removal from water by Ca-Al-CO3 layered double hydroxides and simultaneous acidification[J]. Journal of Water Process Engineering, 2021, 40: 101957. doi: 10.1016/j.jwpe.2021.101957 [3] BISWAS G, KUMARI M, ADHIKARI K, et al. A critical review on occurrence of fluoride and its removal through adsorption with an emphasis on natural minerals[J]. Current Pollution Reports, 2017, 3(2): 104-119. doi: 10.1007/s40726-017-0054-8 [4] 中华人民共和国生态环境部. 2019中国生态环境状况公报[EB/OL]. [2020-06-02]. http://www.mee.gov.cn/hjzl/sthjzk/zghjzkgb/202006/P020200602509464172096.pdf. [5] HE Y, ZHANG L, AN X, et al. Enhanced fluoride removal from water by rare earth (La and Ce) modified alumina: Adsorption isotherms, kinetics, thermodynamics and mechanism[J]. Science of the Total Environment, 2019, 688: 184-198. doi: 10.1016/j.scitotenv.2019.06.175 [6] AOUDJ S, KHELIFA A, DROUICHE N, et al. Simultaneous removal of chromium(VI) and fluoride by electrocoagulation–electroflotation: Application of a hybrid Fe-Al anode - ScienceDirect[J]. Chemical Engineering Journal, 2015, 267: 153-162. doi: 10.1016/j.cej.2014.12.081 [7] GONG W X, QU J H, LIU R P, et al. Effect of aluminum fluoride complexation on fluoride removal by coagulation[J]. Colloids & Surfaces A:Physicochemical & Engineering Aspects, 2012, 395(1): 88-93. [8] BOUHADJARS I, KOPP H, BRITHSCH P, et al. Solar powered nanofiltration for drinking water production from fluoride-containing groundwater: A pilot study towards developing a sustainable and low-cost treatment plant[J]. Journal of Environmental Management, 2019, 231: 1263-1269. doi: 10.1016/j.jenvman.2018.07.067 [9] DONG H, TANG H, SHI X, et al. Enhanced fluoride removal from water by nanosized cerium oxides impregnated porous polystyrene anion exchanger[J]. Chemosphere, 2022, 287: 131932. doi: 10.1016/j.chemosphere.2021.131932 [10] GAO Y, LI M, RU Y, et al. Fluoride removal from water by using micron zirconia/zeolite molecular sieve: Characterization and mechanism[J]. Groundwater for Sustainable Development, 2021, 13(6): 100567. [11] SELIMOGLU S, SIMSEK E B, BEKER U. Water defluoridation by alumina modified Turkey clinoptilolite: Equilibrium, kinetic models and experimental design approaches[J]. Water science & technology:Water supply, 2018, 1(18): 14-22. [12] PILLAI P, DHARASKAR S, KHALID M. Optimization of fluoride removal by Al doped ZnO nanoparticles using response surface methodology from groundwater[J]. Chemosphere, 2021, 284: 131317. doi: 10.1016/j.chemosphere.2021.131317 [13] LOGANATHAN P, VIGNESWARAN S, KANDASAMY J, et al. Defluoridation of drinking water using adsorption processes[J]. Journal of Hazardous Materials, 2013, 248-249(1): 1-19. [14] 侯笛, 卫栋慧, 董岁明, 等. 铁改性花生壳生物炭除氟性能及机理研究[J]. 应用化工, 2021, 50(02): 285-289. doi: 10.3969/j.issn.1671-3206.2021.02.003 [15] WANG J, CHEN N, FENG C, et al. Performance and mechanism of fluoride adsorption from groundwater by lanthanum-modified pomelo peel biochar[J]. Environmental Science and Pollution Research, 2018, 25(16): 15326-15335. doi: 10.1007/s11356-018-1727-6 [16] VENCES-ALVAREZ E, VELAZQUEZ-JIMENEZ L H, CHAZARO-RUIZ L F, et al. Fluoride removal in water by a hybrid adsorbent lanthanum-carbon[J]. Journal of Colloid and Interface Science, 2015, 455: 194-202. doi: 10.1016/j.jcis.2015.05.048 [17] 刘延湘, 黄彪, 张丽. 花生壳生物炭对水中重金属Cr6+、Cu2+的吸附研究[J]. 科学技术与工程, 2017, 17(13): 81-85. doi: 10.3969/j.issn.1671-1815.2017.13.015 [18] 王建国. 镧改性柚子皮生物炭除氟性能及机理研究[D]. 北京: 中国地质大学(北京), 2018. [19] 王博, 李梅, 张晓伟. 灼烧过程对稀土氧化物中氯元素的影响[J]. 有色金属(冶炼部分), 2017(8): 47-49. [20] PARK Y, GORMAN C, FORD E. Lanthanum carbonate nanofibers for phosphorus removal from water[J]. Journal of Materials Science, 2020, 55(12): 5008-5020. doi: 10.1007/s10853-019-04324-8 [21] QIU H, LIANG C, YU J, et al. Preferable phosphate sequestration by nano-La(III) (hydr)oxides modified wheat straw with excellent properties in regeneration[J]. Chemical Engineering Journal, 2017, 315: 345-354. doi: 10.1016/j.cej.2017.01.043 [22] HOSOKAWA S, IWAMOTO S, INOUE M. Solvothermal treatment of rare earth chloride hydrates[J]. Cheminform, 2006, 408: 529-532. [23] MOUSAVI-KAMAZANI M, ALIZADEH S, ANSARI F, et al. A controllable hydrothermal method to prepare La(OH)3 nanorods using new precursors[J]. Journal of Rare Earths, 2015, 33: 425-431. doi: 10.1016/S1002-0721(14)60436-1 [24] SRODA M, PALUSZKIEWICZ C. Spectroscopic study of the influence of LaF3 admixture on the crystallization and structure of borosilicate glass[J]. Journal of Molecular Structure, 2007, 834: 302-307. [25] YU Y, WANG C, GUO X, et al. Modification of carbon derived from Sargassum sp. by lanthanum for enhanced adsorption of fluoride[J]. Journal of Colloid & Interface Science, 2015, 441: 113-120. [26] PRABHU S M, ELANCHEZHIYAN S SD, LEE G, et al. Defluoridation of water by Tea-bag model using La3+ modified synthetic resin@chitosan biocomposite[J]. International Journal of Biological Macromolecules, 2016, 91: 1002-1009. doi: 10.1016/j.ijbiomac.2016.05.112 [27] JHA R, JHA U, DEY R K, et al. Fluoride sorption by zirconium (IV) loaded carboxylated orange peel[J]. Desalination & Water Treatment Science & Engineering, 2015, 53(8): 2144-2157. [28] BANSIWALl A, THAKRE D, LABHSHETWAR N, et al. Fluoride removal using lanthanum incorporated chitosan beads[J]. Colloids and Surfaces B:Biointerfaces, 2009, 74(1): 216-224. doi: 10.1016/j.colsurfb.2009.07.021 [29] HAN M, ZHANG J, HU Y, et al. Preparation of novel magnetic microspheres with the La and Ce-bimetal oxide shell for excellent adsorption of fluoride and phosphate from solution[J]. Journal of Chemical & Engineering Data, 2019, 64(8): 3641-3651. -



 下载:
下载: