-
目前,水污染仍是一个影响经济和生态系统的全球性问题,特别是重金属引发的水污染已成为备受关注的问题之一[1-3]。化学上将密度大于4.5 g·cm−3的金属称为重金属,如铬、铜、铅、锌、钴、镍等[4]。重金属离子(Cu(Ⅱ)、Pb(Ⅱ)、Cd(Ⅱ)、Zn(Ⅱ)等)具有毒害性、长期累积性和难降解性等特性,造成的水污染具有隐蔽性和不可逆性[5-6]。而砷(As)是一种类金属元素,在自然界中分布广泛。有色金属矿山的开采和冶炼、电镀等化工业的不合规排放以及过量使用化肥、农药是含砷废水的主要来源[7-8]。其中,冶炼厂和化工厂所产生的含砷废水主要含有As、Cu、Pb、Zn、Cd等重金属离子和氟、硫等元素。根据冶炼原料中砷含量的不同,废水中砷含量一般从几十毫克每升到几十克每升不等,其含量均高于《污水综合排放标准》(GB 18466-2005)中规定的0.5 mg·L−1标准限值,因此,需经过处理达标后才能排放[9]。
现阶段,用于处理重金属废水的方法主要有吸附法、化学沉淀法、离子交换法、膜滤法和溶剂萃取法等[10-13]。其中,吸附法具有操作简单、吸附高效和成本低等特点,被广泛应用于重金属废水治理[10, 14-16]。在吸附剂的选择上,使用易获取和可降解天然高分子材料也逐渐成为趋势。氧化石墨烯(graphene oxide, GO)作为一种新型纳米材料,因其比表面积较大,含氧官能团(如—OH、—C=O和—COOH)丰富和易表面修饰等优点,在处理重金属废水中备受关注[17-19]。刘伟等[18]通过制备磁改性GO,发现磁改性GO在pH=2时对5 mg·L−1的Cr(VI)去除率达82.9%。李仕友等[20]成功制备出GO/SiO2复合材料,但GO/SiO2在pH=2.5时对5 mg·L−1 Cd(Ⅱ)的去除率仅为45.2%。再者,有学者利用不同浓度NaOH对GO进行水热处理后,对重金属的吸附量比改性前提高了3倍,但改性后的GO亲水性较强,难以回收利用[21]。壳聚糖(chitosan, CS)是一种具有大量羟基和高活性氨基的天然可降解高分子材料,能通过螯合反应与重金属离子进行配位,也是具有潜力的新型吸附材料[22-23]。此外,CS作为脱乙酰化甲壳素产品,来源广泛,无毒、易降解和抗菌,使其在重金属废水处理中比其他材料更具优势[24-25],但CS在酸性条件下易流失。因此,将GO与CS组合后制备出的GO/CS复合材料不仅可以降低GO/CS的亲水性[26-28],也能进一步改善对水中重金属的去除效果。龚育等[29]研究发现,添加5% GO的CS对铌(Nb)的去除效果优于CS。罗肃霜等[30]制备的TiO2/CS微粒在紫外光催化下对As(Ⅲ)的最大吸附容量虽有4.97 mg·g−1,但在微粒中加入GO后,吸附容量达到12.43 mg·g−1。ZHANG等[31]通过制备EDTA-2Na改性氧化石墨烯壳聚糖(GEC)复合材料,发现对Cr(VI)的吸附容量大小依次为GEC>GOCS>CS。
此外,将GO或CS复合材料负载铁或铁的(氢)氧化物后,能进一步增强复合材料的抗酸性能和热稳定性[32-33]。PARASTAR等[34]将GO和CS先后加入到Fe2S3和H2O2混合溶液中,制备成GO/FeOOH/CS复合材料并用于去除水中的Pd(Ⅱ)和Cd(Ⅱ),发现在pH=6时复合材料对5 mg·L−1的Pd(Ⅱ)和Cd(Ⅱ)最高去除率分别为29.6%和43.07%。AHMAD等[35]利用共沉淀法制备壳聚糖-氧化铁(CS-Fe2O3)用于去除水中的Pb(Ⅱ)和Cd(Ⅱ),发现在pH=5时CS-Fe2O3对50 mg·L−1 Pb(Ⅱ)和Cd(Ⅱ)的去除率均在85%以上。SHERLALAC等[27]通过共沉淀法制备的磁性壳聚糖氧化石墨烯(CMGO)纳米复合材料在pH=7.3时对10 mg·L−1 As(Ⅲ)的去除率为61%。HOSSEINZADEH等[36]同样通过共沉淀法制备的磁性壳聚糖/氧化石墨烯(MCGONs)纳米复合材料在pH=8时对100 mg·L−1 Cu(Ⅱ)的去除率为38%。赵超然等[37]通过包埋法制备不同载铁量的GOCS球形材料用于吸附As(Ⅲ),发现As(Ⅲ)的吸附容量随pH增加呈下降趋势,在pH=3时吸附量达到3.53 mg·g−1。SHAN等[23]对比研究了浸渍蒸发法和共沉淀法制备FeOx修饰的氧化石墨烯壳聚糖复合材料对Cr(VI)的去除效果,发现浸渍蒸发法制备的复合材料具有更好的吸附性能。上述研究表明:铁改性GO或CS复合材料对水体中不同重金属离子均有去除效果,但复合材料中的载铁形态及其改性方法会明显影响去除效果。这将进一步影响其在不同水环境条件下对重金属离子(如As(Ⅴ)、Cu(Ⅱ)、Pb(Ⅱ)和Cd(Ⅱ))的去除效果。然而,较少有研究对比讨论载铁形态或改性方法对复合材料在不同溶液pH中对重金属离子的去除影响。
因此,本研究基于常用的浸渍蒸发法和共沉淀法分别制备2种不同铁改性氧化石墨烯壳聚糖复合材料微球,设计并开展2种微球在不同水环境中(pH分别为3、7和11)对As(Ⅴ)、Cu(Ⅱ)、Pb(Ⅱ)和Cd(Ⅱ)的吸附实验,对比分析去除效果。同时,结合扫描电镜(scanning electron microscope,SEM)、傅里叶红外光谱(fourier transform infrared, FTIR)、X射线衍射(X-ray diffraction, XRD)和比表面积(BET surface area)表征技术揭示吸附机制,为不同吸附剂应用于多种重金属废水的治理提供参考。
-
氧化石墨烯(GO, 95%)购于苏州碳丰石墨烯科技有限公司。壳聚糖(CS, 脱乙酰度>90%)购于上海蓝季科技发展有限公司。七水合砷酸钠(Na2HAsO4·7H2O, AR, 成都贝斯特)。二水合氯化铜(CuCl2·2H2O, AR),硝酸铅(Pb(NO3)2, AR),四水合硝酸镉(Cd(NO3)2·4H2O, AR),六水合三氯化铁(FeCl3·6H2O, AR),四水合氯化亚铁(FeCl2·4H2O, AR),盐酸(HCl, GR),氢氧化钠(NaOH, GR),冰醋酸(CH3COOH, GR),甲醇(CH3OH, AR),无水乙醇(C2H5OH, AR),硝酸(HNO3, AR)均采购于西陇化工股份有限公司。戊二醛(C5H8O2, 50 %),溴化钾(KBr, GR)购自于阿拉丁试剂(上海)有限公司。实验用水均为超纯水。
使用浸渍蒸发法制备Fe(Ⅱ)改性氧化石墨烯壳聚糖复合微球(Fe-GOCS)。首先将氧化石墨烯和壳聚糖粉末按照一定比例混合,加入配置好的200 mL 1.5%的醋酸溶液,超声搅拌直至二者混合均匀,得到GOCS混合液;将混合液滴入至2% NaOH溶液中,常温静置固化成球,用超纯水将洗涤液洗至接近中性,过滤;在过滤后的材料中加入200 mL 5%戊二醛-甲醛溶液后置于摇床振荡2~3 h,用50%乙醇溶液洗涤至无醛味后,过滤,放置45 ℃的鼓风干燥箱中烘干,得到GOCS复合微球;将微球放入0.1 mol的FeCl2·4H2O溶液中,放置于200 ℃的电热板上加热蒸干,冷却后洗涤至电导率低于25 μs·cm−1,烘干,得到Fe-GOCS复合微球。使用共沉淀法制备Fe(Ⅲ)改性氧化石墨烯壳聚糖复合微球(Fe@GOCS),在上述配置好的GOCS混合液中加入0.1 mol FeCl3·6H2O,继续超声搅拌直至固体完全溶解,得到Fe@GOCS混合溶液;将该溶液滴入至5% NaOH溶液中,静置24 h后,洗涤至中性,过滤,置于200 mL 5%戊二醛-甲醛溶液中摇床振荡2~3 h后,用50%乙醇溶液洗涤至无醛味,过滤,烘干,得到Fe@GOCS复合微球。
-
1)实验设计。采用批实验对比研究在不同pH条件下 GOCS、Fe-GOCS和Fe@GOCS分别对As(Ⅴ)、Cu(Ⅱ)、Pb(Ⅱ)和Cd(Ⅱ)的去除效果。分别称取50 mg GOCS、Fe-GOCS和Fe@GOCS置于若干100 mL离心管中,分别添加50 mL 25 mg·L−1 As(Ⅴ)、Cu(Ⅱ)、Pb(Ⅱ)和Cd(Ⅱ)模拟液,再使用1 mol·L−1 HCl和1 mol·L−1 NaOH调节模拟液pH分别为3±0.1、7±0.1和11±0.1,然后将模拟液置于恒温水浴振荡器中,在298.15 K、170 r·min−1条件下,振荡48 h。使之充分反应后,取上清液过0.45 μm滤膜后测定重金属离子浓度。
在固液比为1.0 g·L−1、As(Ⅴ)初始质量浓度为25 mg·L−1、温度为298.15 K和pH分别为7和3的条件下,收集首次吸附As(Ⅴ)后的Fe-GOCS和Fe@GOCS,并用50 mL 1.5 mol·L−1 NaOH和超纯水洗脱,直到洗脱液电导率稳定,然后将这些复合材料干燥后再次用于As(Ⅴ)的吸附。解吸和吸附过程重复5次,每次吸附后收集上清液和脱吸液用于As(Ⅴ)浓度的测定,结果将用于分析多次解吸和吸附对As(Ⅴ)去除效果的影响,以及吸附剂的再生性能。
2)样品表征分析。吸附前后As(Ⅴ)、Cu(Ⅱ)、Pb(Ⅱ)和Cd(Ⅱ)的浓度均利用电感耦合等离子体发射光谱仪(ICP-OES, Optima 7000 DV)测试,测试数据误差小于5%。对GOCS和吸附反应前后的Fe-GOCS和Fe@GOCS进行表征分析。其中,复合材料的官能团使用溴化钾压片法由傅里叶红外光谱仪(FTIR, iS 10, 美国赛默飞世尔公司)测试完成;样品结构测定采用X射线衍射仪(XRD,X'Pert3 Powder,荷兰帕纳科公司),测试扫描2θ步长0.026 26°,扫描速度为0.656 5(°)·s−1,扫描2θ为5°~90°;形貌微观特征由场发射电子扫描显微镜(SEM, JSM-7900F, 日本)测定;样品的比表面积和孔结构采用全自动比表面及孔隙度分析仪(BET, Micromeritics ASAP 2020, 美国)测试。
3)数据处理。GOCS、Fe-GOCS和Fe@GOCS对As(Ⅴ)、Cu(Ⅱ)、Pb(Ⅱ)和Cd(Ⅱ)平衡吸附量(qe)和去除率(re)分别由式(1)和式(2)计算。
式中:qe是吸附平衡时吸附剂对重金属离子的吸附量,mg·g−1;C0和Ce分别是重金属离子初始和吸附平衡时的质量浓度,mg·L−1;V是含重金属离子模拟液的体积,mL;m是吸附剂的投加量,mg;re是吸附平衡时重金属离子的去除率,%。
-
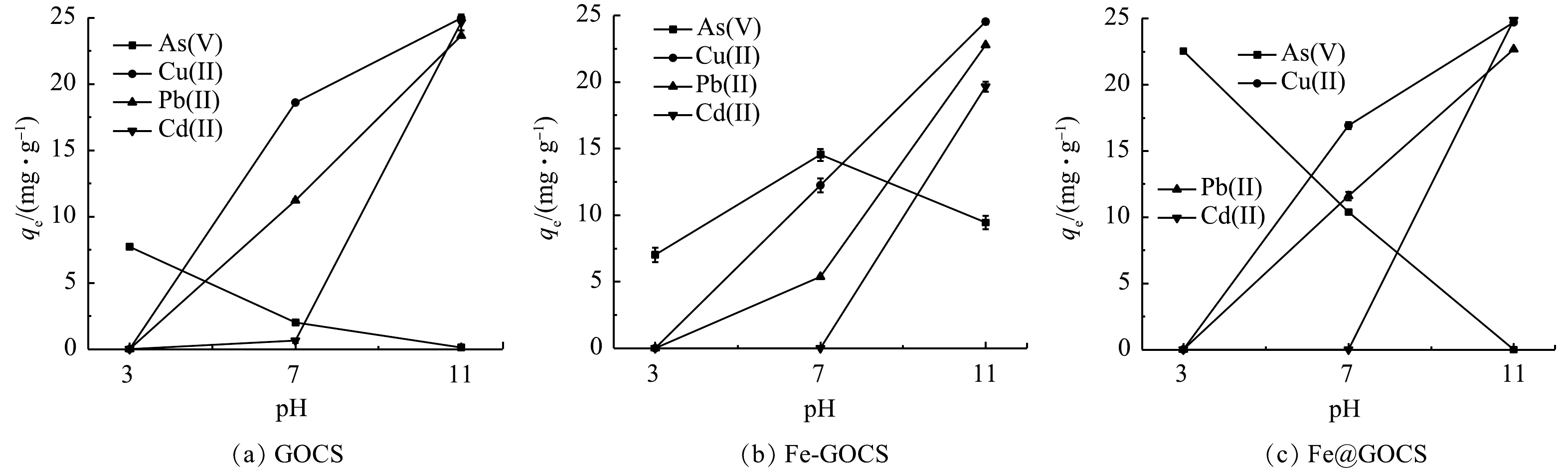
不同pH条件下,GOCS、Fe-GOCS和Fe@GOCS对As(Ⅴ)、Cu(Ⅱ)、Pb(Ⅱ)和Cd(Ⅱ)的去除结果分别如表1和图1所示。可以发现:改性前后的复合材料对不同重金属的平衡吸附量(qe)和去除率(re)存在明显差异。其中,GOCS和Fe@GOCS对As(Ⅴ)的qe均随pH升高而逐渐降低,qe均在pH=3时最大,分别为7.72 mg·g−1和22.54 mg·g−1。在pH=11时GOCS对As(Ⅴ)的qe虽有0.12 mg·g−1,但Fe@GOCS却为0(图1(a)和图1(c))。而Fe-GOCS对As(Ⅴ)的qe随pH升高先显著增加后略微降低。当pH=3时,qe最小为7.02 mg·g−1;pH=7时,qe达到最大为14.54 mg·g−1(图1(b))。结合表1可知:酸性条件下,Fe@GOCS对As(Ⅴ)去除率显著高于GOCS和Fe-GOCS,三者对As(Ⅴ)的去除率分别为87.99%、30.20%和27.40%。同时,除As(Ⅴ)过程中Fe平衡质量浓度仅为0.04 mg·L−1,远低于《生活饮用水卫生标准》(GB 5749-2022)所规定的0.3 mg·L−1。而Fe-GOCS的Fe平衡质量浓度为0.4 mg·L−1,存在二次污染风险,说明酸性条件下Fe@GOCS比Fe-GOCS更稳定。中性和碱性条件下,Fe-GOCS对As(Ⅴ)的re则显著高于GOCS和Fe@GOCS,Fe平衡质量浓度分别为0和0.08 mg·L−1,均低于0.3 mg·L−1的限值。相比之下,Fe@GOCS更适用于酸性条件下对As(Ⅴ)的去除,而在中性和碱性条件下Fe-GOCS更适用。同时,在最佳适用pH条件下,不同铁改性后的GOCS对As(Ⅴ)的去除效果均优于GOCS。
由图1可知,3种材料对Cu(Ⅱ)和Pb(Ⅱ)的去除规律相似,qe均随着pH的增大呈上升趋势。其中,pH=3时,GOCS、Fe-GOCS和Fe@GOCS均未去除Cu(Ⅱ)和Pb(Ⅱ)。pH=7时,GOCS、Fe-GOCS和Fe@GOCS对Cu(Ⅱ)的qe分别为18.59、12.24和16.92 mg·g−1,对Pb(Ⅱ)的qe分别为11.21、5.37和11.60 mg·g−1。而pH=11时,3种材料对Cu(Ⅱ)和Pb(Ⅱ)的qe相近,均达到最大。其中,GOCS、Fe-GOCS和Fe@GOCS对Cu(Ⅱ)的qe分别为24.97、24.53和24.71 mg·g−1,对Pb(Ⅱ)的qe分别为23.63、22.77和22.68 mg·g−1。在中性和碱性条件下均未发现Fe溢出。由此可知,3种材料均不适用于去除酸性条件下的Cu(Ⅱ)和Pb(Ⅱ)。中性条件下,GOCS更适用去除Cu(Ⅱ),其次是Fe@GOCS;而对于去除Pb(Ⅱ)的结果则相反。碱性条件下考虑到Cu(Ⅱ)和Pb(Ⅱ)存在水解或共沉淀作用[38],3种材料均不适用于对Cu(Ⅱ)和Pb(Ⅱ)的去除。
从表1 Cd(Ⅱ)的去除结果中可知,3种材料在pH=3均未吸附Cd(Ⅱ)。pH=7时,仅GOCS具有微弱的去除效果,qe和re分别为0.64 mg·g−1和2.57%。当pH=11时,Fe@GOCS的去除效果均优于GOCS和Fe-GOCS,qe分别为24.89、24.65和19.65 mg·g−1。由此可知,GOCS更适用于中性条件下去除Cd(Ⅱ)。而在碱性条件下,Cd(Ⅱ)存在较强的水解或共沉淀作用[38],表明2种材料均不适用于去除Cd(Ⅱ)。
-
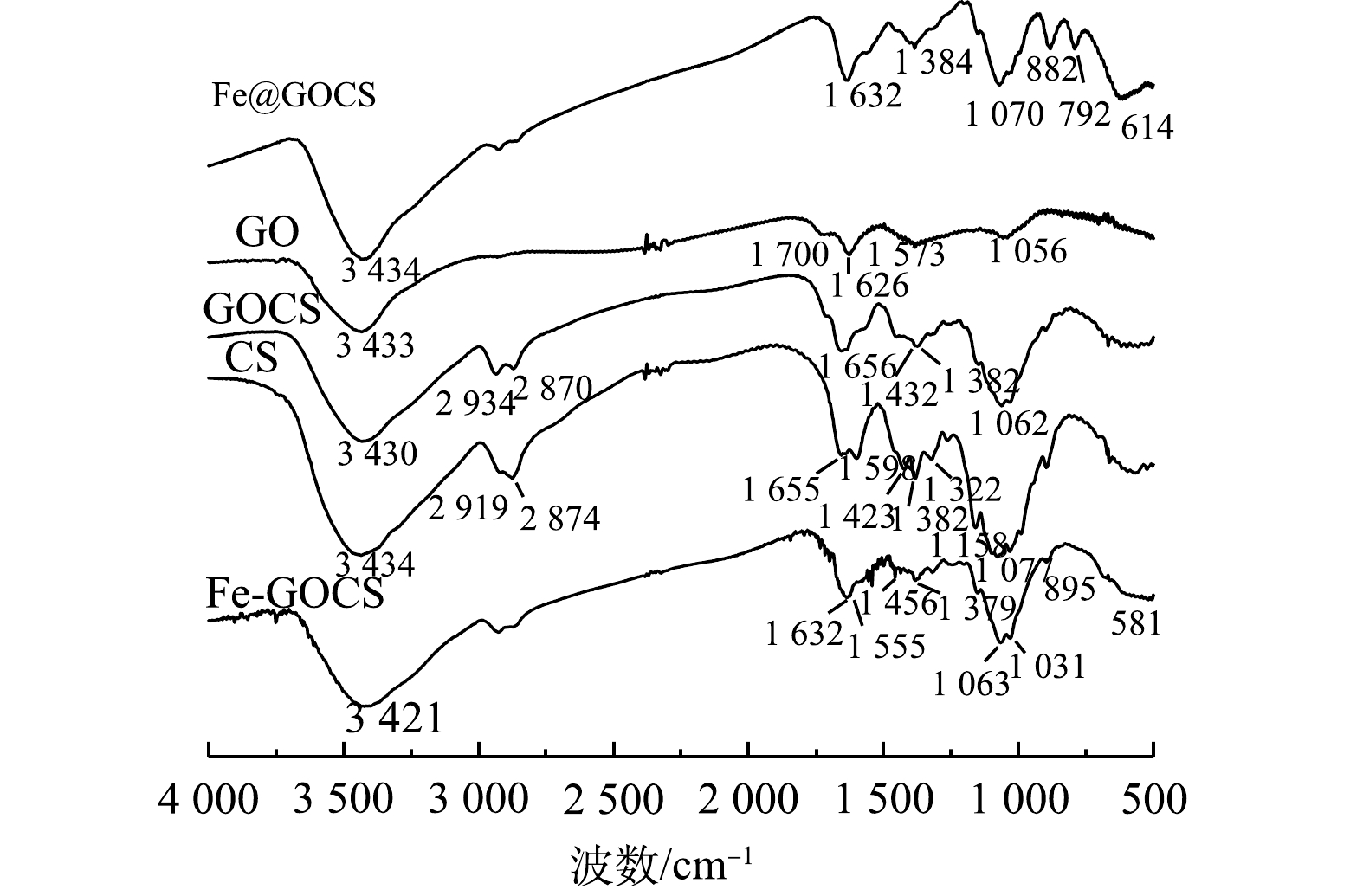
1) FTIR表征。吸附前复合材料的FTIR表征结果如图2所示。由图2可知,GO、CS和GOCS具有多种官能团。其中,GO图谱中1 700 cm−1附近是—COOH的C=O伸缩振动峰,1 626 cm−1附近是水分子中的O—H弯曲振动峰,1 573 cm−1附近是—NH2面内变形特征峰和1 056 cm−1附近的C—O—C弯曲振动峰。CS图谱中3 434 cm−1附近是N—H伸缩振动峰,2 919 cm−1和2 874 cm−1附近是CH3和CH2中C—H伸缩振动峰,1 655 cm−1和1 598 cm−1附近是酰胺I键C=O的伸缩振动峰,1 432~1 322 cm−1附近是CH3和CH2中C—H弯曲振动峰,1 158 cm−1附近是C—O—C弯曲振动峰以及1 077 cm−1的碳环弯曲振动峰[39]。将GO与CS混合后,图谱显示GOCS在3 430 cm−1附近是水分子中O—H和CS中N—H伸缩振动峰,在2 919 cm−1和2 870 cm−1附近保留了CS中的C—H伸缩振动峰,在1 062 cm−1附近形成较强的环氧基C—O—C的C—O弯曲振动峰。此外,GOCS在1 656 cm−1附近形成酰胺键—CONH—,该特征峰说明GO中的—COOH与CS中的—NH2组装成功[37]。在经过不同铁改性后,GOCS图谱出现不同特征。其中,Fe@GOCS在1 070 cm−1附近出现碳环弯曲振动峰,在1 384 cm−1附近出现C—OH弯曲振动峰。882 cm−1和792 cm−1附近出现α-FeO(OH)中O—H的面内和面外弯曲振动峰,是针铁矿的特征吸收峰,614 cm−1为Fe—O的特征峰。而Fe-GOCS在3 421 cm−1出现的宽峰是其表面—OH与水分子形成的氢键有关[40],1 063 cm−1和1 031 cm−1附近是C—O弯曲振动峰,1 555 cm−1附近为峰值增强的—NH2面内变形特征峰,在581 cm−1附近形成赤铁矿的Fe—O弯曲振动峰[41]。此外,Fe@GOCS和Fe-GOCS在1 632 cm−1附近均保留了GOCS的酰胺键—CONH—,这与赵超然等[37]和李仕友等[42]的研究结果一致。
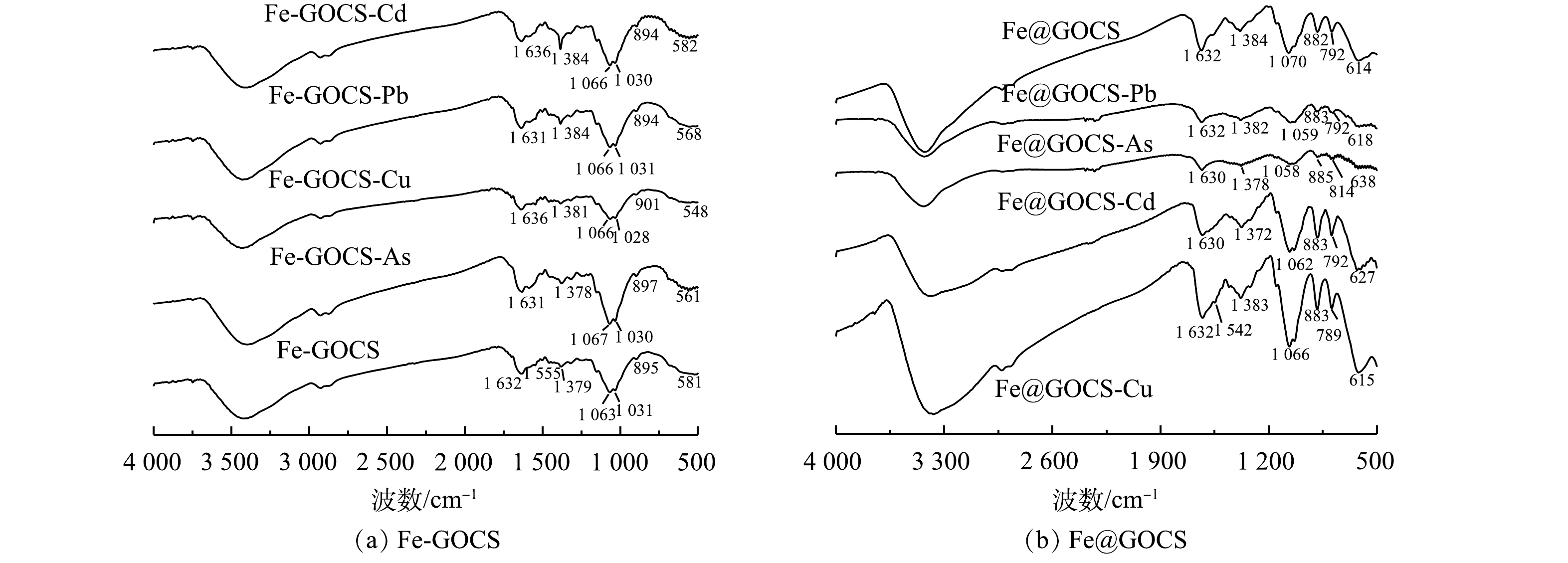
Fe-GOCS和Fe@GOCS在最优条件下去除As(Ⅴ)、Cu(Ⅱ)、Pb(Ⅱ)和Cd(Ⅱ)后的FTIR图谱如图3所示。由图3(a)可见,Fe-GOCS在去除As(Ⅴ)后,1 063 cm−1和1 031 cm−1附近的C—O弯曲振动峰增强,而581 cm−1处对应的Fe—O特征峰偏移至561 cm−1。这说明C—H、C—O和Fe—O均可能参与到As(Ⅴ)去除中。在去除Cu(Ⅱ)和Pb(Ⅱ)后,Fe-GOCS中的Fe—O分别偏移至548 cm−1和568 cm−1且峰强减弱,这说明Fe—O可能参与去除。Fe-GOCS-Cd图谱显示,Fe-GOCS在1 456~1 379 cm−1的C—H多组伸缩振动峰数量减少,仅在1 384 cm−1处均在C—H单峰。同时,在去除As(Ⅴ)、Cu(Ⅱ)和Pb(Ⅱ)后,该处的C—H峰值增强,说明C—H均可能参与到As(Ⅴ)、Cu(Ⅱ)和Pb(Ⅱ)去除。
与Fe-GOCS吸附前后表征结果类似,Fe@GOCS在去除重金属后,官能团也发生不同程度的变化(图3(b))。其中,在去除As(Ⅴ)和Pb(Ⅱ)后,614 cm−1附近对应的Fe—O特征峰偏移至638 cm−1。此外,多组官能团特征峰均出现不同程度减弱,包括882 cm−1和792 cm−1附近的α-FeO(OH)中O—H的面内和面外弯曲振动峰、1 070 cm−1附近的碳环弯曲振动峰、1 384 cm−1附近的C—OH弯曲振动峰以及1 632 cm−1的—CONH—。由此可知,Fe@GOCS去除As(Ⅴ)和Pb(Ⅱ)是多种官能团共同作用的结果。与去除As(Ⅴ)和Pb(Ⅱ)结果相反,Fe@GOCS在去除Cu(Ⅱ)后,多组官能团特征峰均显著增强。这可能是因为Fe@GOCS中的Fe为+3价,Fe—O体系中的氧带有负电荷,而Cu(Ⅱ)的电负性较其他3种重金属离子强[43],与Fe—O上的氧以及O—H、C—OH和—CONH—之间的相互作用较强,因而对分子筛的骨架振动改变较为明显。Fe@GOCS与Cd(Ⅱ)反应后,特征峰位置未出现明显变化,说明Fe@GOCS去除Cd(Ⅱ)后化学结构没有改变,表明Fe@GOCS的主要官能团并未与Cd(Ⅱ)发生显著作用。这一结果与范先媛等[44]使用孔径为4×10−10 m的分子筛去除Cu(Ⅱ)和Cd(Ⅱ)的结果相似。
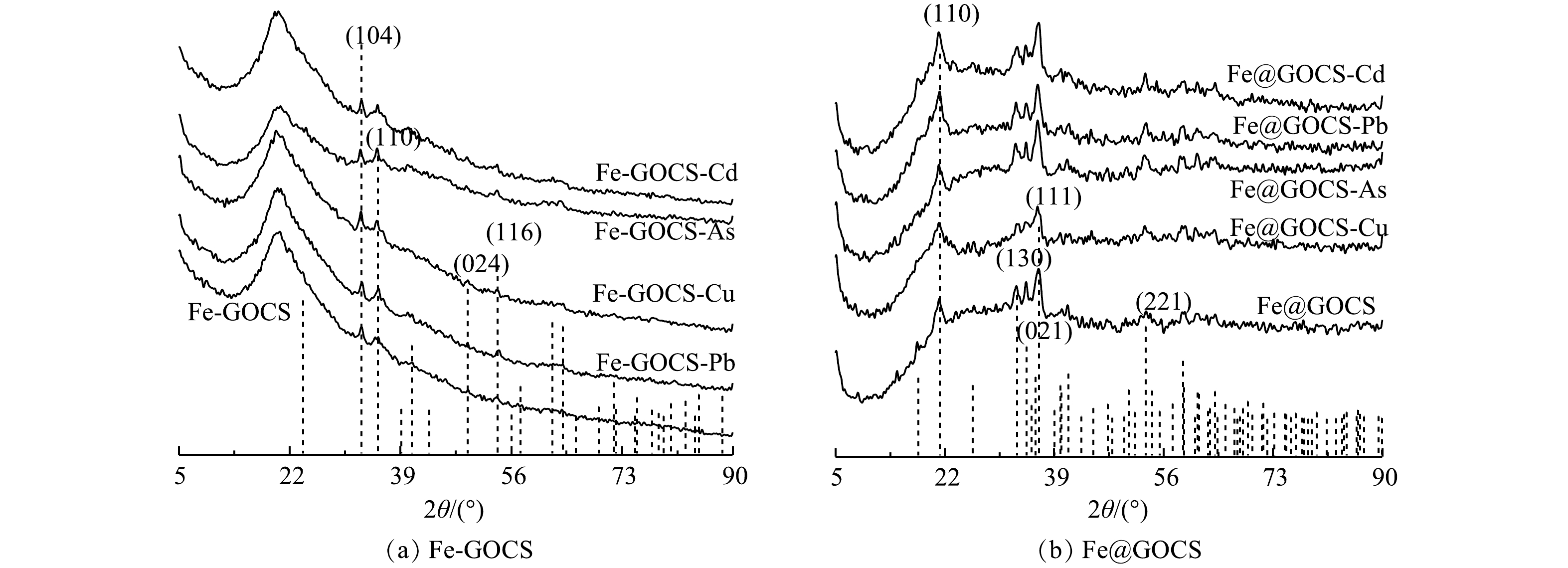
2) XRD表征。由图4可以看出,GO和CS分别在2θ为12°和42°附近以及20°附近有明显衍射峰,为无定型结构,GOCS仅在2θ=20°附近存在衍射峰,且峰型变窄,峰面积减小。这说明GO和CS的成功组装[32]。Fe-GOCS在2θ为33°和35°附近出现新的衍射峰。但35°附近的衍射峰峰值较弱,这可能受到制备材料中一些杂质的影响。与标准PDF卡片(ICDD PDF 72-0469)进行对照比较,新出现的衍射峰为赤铁矿,以α-Fe2O3矿物形态存在,同时也意味着FeCl2成功将GOCS改性[37]。而Fe@GOCS在2θ为17°、21°、33°、34°、36°以及53°附近均出现新的衍射峰,对比标准PDF卡片(ICDD PDF 81-0462)后确定为针铁矿,以α-FeO(OH)矿物形态存在[45]。
Fe-GOCS和Fe@GOCS在最优条件下去除As(Ⅴ)、Cu(Ⅱ)、Pb(Ⅱ)和Cd(Ⅱ)后的XRD结果分别如图5(a)和图5(b)所示。由图5可知,反应前后Fe-GOCS和Fe@GOCS的衍射峰位置未发生明显改变且未出现新衍射峰。这表明Fe-GOCS和Fe@GOCS稳定性良好,在去除重金属过程中并没有生成新的物相。此外,由图5(a)可知,Fe-GOCS位于2θ=33°和35°的衍射峰出现不同程度的增强。这说明Fe-GOCS上的α-Fe2O3参与As(Ⅴ)、Cu(Ⅱ)和Pb(Ⅱ)的去除。而对于Cd(Ⅱ),α-Fe2O3衍射峰并未出现明显变化,说明α-Fe2O3并未参与去除Cd(Ⅱ)。这些结果与2.1.1 FTIR分析的结果一致,也再次表明碱性条件下Cd(Ⅱ)的去除主要与离子水解或共沉淀作用有关[44, 46]。由图5(b)中也可以看出,Fe@GOCS衍射峰强度出现不同程度的变化。其中Fe@GOCS在吸附As(Ⅴ)和Pb(Ⅱ)后,位于2θ=17°、21°、36°和53°处的α-FeO(OH)衍射峰峰值明显增强。这可能受到了吸附剂中其他官能团的影响,如O—H、C—OH和—CONH—等。在吸附Cu(Ⅱ)后,α-FeO(OH)衍射峰峰值明显降低,说明α-FeO(OH)参与去除Cu(Ⅱ)。与Fe-GOCS去除Cd(Ⅱ)后的结果类似,Fe@GOCS-Cd的衍射峰并未出现明显变化,表明Fe@GOCS中的α-FeO(OH)没有参与反应。
3) SEM表征。GOCS、Fe-GOCS和Fe@GOCS的SEM表征结果如图6所示。可以看出,改性前GOCS表面光滑且富有光泽(图6(a)),堆叠着许多片状结构(图6(b))。经FeCl2浸渍改性后,Fe-GOCS表面变得黯淡且有裂隙出现(图6(c)),同时GOCS的片状层结构被排列紧密的球状物质所代替且有聚集成斑块状物质的现象(图6(d)),结合第2.1.2节XRD物相分析确定该球状物质为赤铁矿;经FeCl3改性后,Fe@GOCS表面出现大量且层次分明的褶皱(图6(e))。同时,褶皱之间充填有针刺状或块状物质(图6(f)),结合第2.1.2节的XRD物相分析确定该针刺状物质为针铁矿。由此可知,GOCS经不同载铁方式后微观表面形貌发生显著变化,同时证实GOCS均被成功改性。这一结果与FTIR和XRD的分析一致。
Fe-GOCS和Fe@GOCS分别吸附As(Ⅴ)、Cu(Ⅱ)、Pb(Ⅱ)和Cd(Ⅱ)后的微观形貌如图7所示。Fe-GOCS吸附As(Ⅴ)后表面较未吸附之前球状物质虽没有明显变化,但成块面积明显减少(图7(a));吸附Cu(Ⅱ)后,表面的球状物质明显减少且分布不均,暴露出大量针刺状物质(图7(b));吸附Pb(Ⅱ)后的Fe-GOCS表面球状物质形貌发生改变,斑块状物质面积增加,少许的针刺状物质掺杂其中(图7(c));Fe-GOCS在与Cd(Ⅱ)反应后斑块状物质面积与未反应前并未发生明显变化,仅斑块状物质较为分散(图7(d))。这表明Fe-GOCS虽未能够去除Cd(Ⅱ),但Cd(Ⅱ)可以修饰材料表面形貌。Fe@GOCS在去除As(Ⅴ)后,原先的褶皱变得光滑,但有明显萎缩迹象且变得杂乱无序(图7(e));吸附Cu(Ⅱ)后的Fe@GOCS表面褶皱消失,变得粗糙且凹凸不平(图7(f));在去除Pb(Ⅱ)后,褶皱间的针刺状或块状物质明显减少,褶皱表面出现大小不等的凹陷(图7(g));与Cd(Ⅱ)反应后的Fe@GOCS表面褶皱几乎消失殆尽,仅一些针刺状物质残留在表面(图7(h))。
4) BET表征。GOCS、Fe-GOCS和Fe@GOCS的BET比表面积和孔结构参数列于表2。由表2可见,GOCS的比表面积和孔体积分别为4.22 m2·g−1和2.25×10−4 cm3·g−1。经FeCl3改性后,Fe@GOCS比表面积增至42.27 m2·g−1,孔体积增至6.58×10−4 cm3·g−1,这使得酸性条件(pH=3)下Fe@GOCS对As(Ⅴ)的吸附量比GOCS高出14.82 mg·g−1。同时,结合SEM表征结果,Fe@GOCS表面出现大量且层次分明的褶皱有利于进一步提高吸附量。经FeCl2改性后,相较于GOCS,Fe-GOCS的比表面积降低2.12 m2·g−1,孔体积降低0.38×10−4 cm3·g−1,这使得Fe-GOCS在酸性条件下对As(Ⅴ)的吸附量减少0.70 mg·g−1。但在中性和碱性条件下,3种材料对于As(Ⅴ)、Cu(Ⅱ)、Pb(Ⅱ)和Cd(Ⅱ)的去除效果也存在差异,这说明除材料本身的空间结构外,材料表面的电荷、载铁形态以及离子形态也会影响去除效果[37, 47-48]。
-
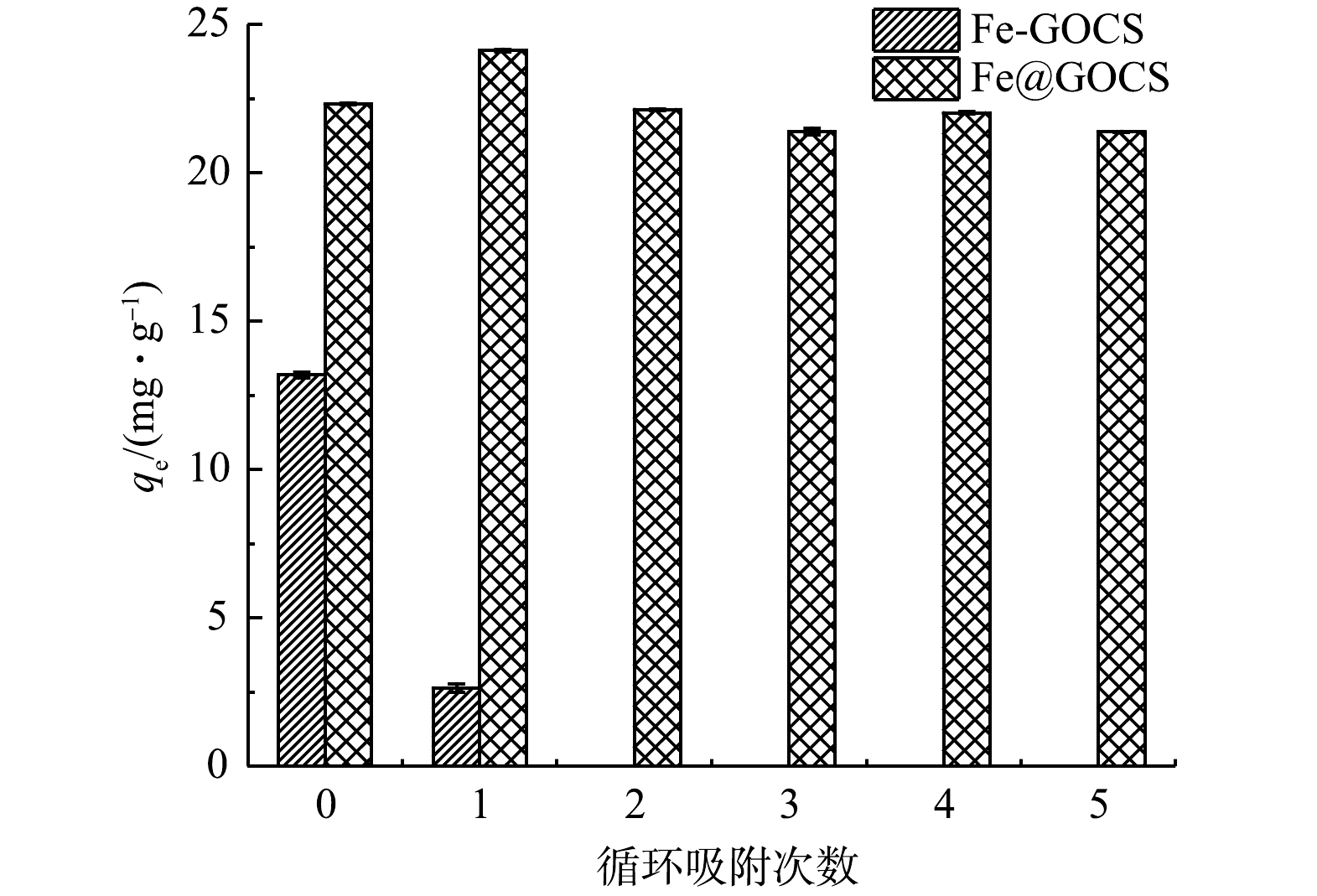
As(Ⅴ)分别在Fe-GOCS和Fe@GOCS上重复解吸和吸附5次后,平衡吸附容量(qe)的变化如图8所示。Fe-GOCS在对As(Ⅴ)的初始qe虽有13.19 mg·g−1,但在循环吸附1次后,Fe-GOCS并未展现出良好的吸附效果。而Fe@GOCS对As(Ⅴ)的qe随着解吸和吸附循环次数的增加先上升后略有降低,这可能与材料表面上铁的溶解损失有关。在第5次解吸后,Fe@GOCS对As(Ⅴ)的qe仍高达22.02 mg·g−1,仅比初始qe减少0.31 mg·g−1,说明Fe@GOCS再生性良好,可重复用于酸性含砷废水中。同时在整个实验过程中,虽然Fe-GOCS和Fe@GOCS中的部分Fe溶解在水溶液中,但Fe平衡质量浓度分别为0~0.22 mg·L−1和0~0.04 mg·L−1,仍未超过《生活饮用水卫生标准》(GB 5749-2022)铁的0.3 mg·L−1的标准限值。这不仅进一步说明本研究制备的Fe@GOCS比Fe-GOCS具有更加稳定且良好的环保再生能力,也使Fe@GOCS可进一步用于吸附处理As(Ⅴ)脱吸液(C0=0.95~17.16 mg·L−1),降低脱吸液对环境的二次污染风险。
-
将所制备的Fe-GOCS和Fe@GOCS与目前所制备的复合材料对As(Ⅴ)、Cu(Ⅱ)和Pb(Ⅱ)去除效果进行对比,其结果如表3所示。一方面,在pH=3时Fe@GOCS对As(Ⅴ)的re为87.99%,均高于AMD污泥复合材料[49]和含铁锰污泥壳聚糖-海藻酸吸附剂[50]等。GO和CS来源广泛和价格低廉[17, 24-25],同时Fe@GOCS制备方法简便且具有良好的环保再生能力,这也为Fe@GOCS在As(Ⅴ)去除提供广阔的应用前景。此外,Fe-GOCS和Fe@GOCS分别在pH=7和3时对25 mg·L−1 As(Ⅴ)吸附达到平衡后,溶液中分别仍有11.08 mg·L−1和3.08 mg·L−1的As(Ⅴ),超出《污水综合排放标准》(GB 18466-2005)中砷的标准限定值0.5 mg·L−1。因此,可使用Fe@GOCS对酸化后仍超标的As(Ⅴ)废液进一步吸附处理。另一方面,在pH=7时,Fe-GOCS和Fe@GOCS对Cu(Ⅱ)的re分别为49.45%和68.38%,均高于磁性GOCS复合材料和纯CS,说明Fe-GOCS和Fe@GOCS对Cu(Ⅱ)去除效果良好。而对于Pb(Ⅱ)和Cd(Ⅱ)的去除,Fe-GOCS和Fe@GOCS却没有表现出良好的去除效果。
总的来说,Fe@GOCS能够兼顾多种重金属离子高效率去除且不会因材料中Fe溢出造成环境二次污染问题,是一种环保型可再生吸附剂。对于Fe-GOCS,能在不同的溶液环境有效去除多种重金属离子,但应进一步提升去除效果。
-
结合pH、FTIR、XRD和BET分析结果,推测了Fe-GOCS和Fe@GOCS对As(Ⅴ)、Cu(Ⅱ)、Pb(Ⅱ)和Cd(Ⅱ)的去除机理。Fe-GOCS和Fe@GOCS的零点电荷pHpzc分别是7.5[47]和8.2[37]。当溶液环境为弱酸性(pH=3)时,As(Ⅴ)主要以HAsO42-存在[48]。Fe-GOCS中的α-Fe2O3在水溶液中可形成羟基化表面(Fe—OH+2、Fe—OH、FeO−)[53],使材料表面基团质子化,在静电吸引作用下去除HAsO42-形态的As(Ⅴ)。而Fe@GOCS中的α-FeO(OH)本身具有大量的-OH,在pH=3时,Fe—OH质子化形成Fe—OH+2,在静电吸引作用下去除As(Ⅴ)。具体的去除机理可解释为分式(3)和式(4)及总反应(式(5))。
当溶液环境为中性时,Cu(Ⅱ)和Pb(Ⅱ)均会发生不同程度的水解。其中,Cu(Ⅱ)的水解常数pKCu(pK实测值和pK计算值分别为6.5和7.0)小于Pb(Ⅱ)的水解常数(pK实测值和pK计算值分别为7.2和11.7)[38],说明Cu(Ⅱ)的水解程度大于Pb(Ⅱ),Cu(Ⅱ)的σ杂化轨道接受氧的非键电子对的能力更强,形成Cu-OH配键的键能更大[54],这也证明在中性条件下,2种材料均对Cu(Ⅱ)的去除效果最好。除Cu(Ⅱ)和Pb(Ⅱ)自身水解外,结合FTIR和XRD分析结果,在其他官能团(Fe-GOCS中的C—H和Fe@GOCS中的C—OH、O—H及—CONH—)提供的键能帮助下,Fe-GOCS和Fe@GOCS中的Fe—O分别通过与Cu(Ⅱ)和Pb(Ⅱ)形成Fe—O—Cu和Fe—O—Pb表面络合物实现对其的去除。而Cd(Ⅱ)(pK实测值和pK计算值分别为9.7和9.5[38])主要通过自身水解被去除。
-
1) Fe-GOCS对As(Ⅴ)的去除率在pH=7时达到最大,为56.74%。而Fe@GOCS对于As(Ⅴ)的去除率在pH=3时达到最大,为87.99%。酸性条件下,应优先考虑Fe@GOCS。
2)溶液pH的改变对Cu(Ⅱ)和Pb(Ⅱ)的去除效果影响较大。Fe-GOCS、Fe@GOCS对Cu(Ⅱ)和Pb(Ⅱ)的去除率在pH=11时均达到最大。除GOCS外,在弱酸性~中性条件下,对于Cu(Ⅱ)和Pb(Ⅱ)的去除应优先考虑Fe@GOCS。
3) Fe-GOCS和Fe@GOCS对Cd(Ⅱ)的吸附在酸性和中性时效果较差。碱性条件下2种复合材料对Cd(Ⅱ)的re均达到最大,但这与复合材料中丰富的官能团无关,主要与Cd(Ⅱ)自身水解有关。
4)再生吸附循环5次后,Fe@GOCS对As(Ⅴ)的qe仍高达22.02 mg·g−1,仅低于初始qe的0.31 mg·g−1,说明说明Fe@GOCS再生性良好,可重复用于酸性含砷废水中,而Fe-GOCS的再生性能较差。
5) Fe-GOCS中的C—H、Fe—O和C—O等官能团,Fe@GOCS中的Fe—O、O—H、C—OH和—CONH—等官能团在As(Ⅴ)、Cu(Ⅱ)和Cd(Ⅱ)去除过程中发挥着主要作用;参与吸附的主要物质分别为Fe-GOCS中的赤铁矿(α-Fe2O3),Fe@GOCS中的针铁矿[α-FeO(OH)]。
铁改性氧化石墨烯壳聚糖对重金属的去除效果
Heavy metals removal by iron modified graphene oxide chitosan
-
摘要: 为揭示改性方式对复合材料去除重金属离子的影响,利用浸渍蒸发法和共沉淀法制备铁改性氧化石墨烯壳聚糖复合微球,分别命名为Fe-GOCS和Fe@GOCS。通过批实验,对比分析其在不同水环境(pH=3、7和11)下对As(Ⅴ)、Cu(Ⅱ)、Pb(Ⅱ)和Cd(Ⅱ)的去除效果,并结合FTIR、XRD、SEM和BET表征技术揭示去除机理。结果表明,随pH增加,Fe-GOCS和Fe@GOCS对As(Ⅴ)的去除率(re)呈不同的变化趋势,前者先增加后降低,pH=7时最高re为56.74%,而后者则逐渐降低,pH=3时re最大,为87.99%。表征结果证实2种材料对As(Ⅴ)的去除均与Fe—O键有关,去除效果存在差异的主要原因与其不同的载铁形态有关(前者为α-Fe2O3,后者为α-FeO(OH))。随pH增加,2种材料对Cu(Ⅱ)和Pb(Ⅱ)的re均逐渐增大,但去除效果在中性条件下最好,分别为49.45%和23.52%(Fe-GOCS),68.38%和50.85%(Fe@GOCS),但稍差于GOCS;对Cd(Ⅱ)的re也逐渐增大,但Fe-GOCS的最大re低于Fe@GOCS,分别为78.30%和99.16%。Cu(Ⅱ)和Pb(Ⅱ)的去除一方面与其离子水解作用有关,另一方面主要通过形成Fe—O—Cu和Fe—O—Pb配合物去除。而Cd(Ⅱ)的去除主要与水解作用有关。循环吸附5次后,Fe@GOCS对As(Ⅴ)的re仍在80%以上,而Fe-GOCS的再生能力较差。整体上,Fe@GOCS对重金属的去除效果略优于Fe-GOCS,而Fe-GOCS适用的pH范围相对较广。Abstract: To reveal the influence of modification methods on heavy metal ions removal by composites, Fe-modified graphene oxide chitosan composite microspheres were prepared by immersion evaporation and coprecipitation, which were named Fe-GOCS and Fe@GOCS, respectively. Batch experiments were conducted to investigate the removal effects of As (V), Cu (II), Pb (II) and Cd (II) in different water environments (pH=3, 7 and 11), and the removal mechanism was revealed by FTIR, XRD, SEM and BET characterization techniques. Results showed that: with the increase of pH value, the removal efficiency (re) of As(V) by Fe-GOCS and Fe@GOCS showed different trends. The former increased first and then decreased, and the re reached the maximum value of 56.74% at pH=7, while the latter gradually decreased, and the re reached the maximum value of 87.99% at pH=3. The characterization results showed that As(V)removal by the two materials was related to the Fe-O bond, and the main reason for the difference in the removal effect was related to the different morphology of the iron carrier (α-Fe2O3 in the former and α-FeO(OH) in the latter). With the increase of pH, the re of Cu(II) and Pb(II) increased gradually, but the removal effect was the best under neutral conditions, which was 49.45% and 23.52% (Fe-GOCS), 68.38% and 50.85% (Fe@GOCS), respectively, while they were slightly worse than those of GOCS. The re of Cd(II) also increased gradually, but the maximum re of Fe-GOCS was lower than Fe@GOCS, which was 78.30% and 99.16%, respectively. The removal of Cu(II) and Pb(II) was related to ionic hydrolysis on the one hand and mainly through the formation of Fe-O-Cu and Fe-O-Pb complex on the other hand. The removal of Cd(II) was mainly related to hydrolysis. After five cycles of adsorption, the re of As(V) by Fe@GOCS was still above 80%, but the regeneration ability of Fe-GOCS was poor. On the whole, the removal effect of Fe@GOCS was slightly better than that of Fe-GOCS, and Fe-GOCS was suitable for a relatively wide pH range.
-
Key words:
- iron form /
- graphene oxide /
- chitosan /
- heavy metals /
- removal
-
目前,水污染仍是一个影响经济和生态系统的全球性问题,特别是重金属引发的水污染已成为备受关注的问题之一[1-3]。化学上将密度大于4.5 g·cm−3的金属称为重金属,如铬、铜、铅、锌、钴、镍等[4]。重金属离子(Cu(Ⅱ)、Pb(Ⅱ)、Cd(Ⅱ)、Zn(Ⅱ)等)具有毒害性、长期累积性和难降解性等特性,造成的水污染具有隐蔽性和不可逆性[5-6]。而砷(As)是一种类金属元素,在自然界中分布广泛。有色金属矿山的开采和冶炼、电镀等化工业的不合规排放以及过量使用化肥、农药是含砷废水的主要来源[7-8]。其中,冶炼厂和化工厂所产生的含砷废水主要含有As、Cu、Pb、Zn、Cd等重金属离子和氟、硫等元素。根据冶炼原料中砷含量的不同,废水中砷含量一般从几十毫克每升到几十克每升不等,其含量均高于《污水综合排放标准》(GB 18466-2005)中规定的0.5 mg·L−1标准限值,因此,需经过处理达标后才能排放[9]。
现阶段,用于处理重金属废水的方法主要有吸附法、化学沉淀法、离子交换法、膜滤法和溶剂萃取法等[10-13]。其中,吸附法具有操作简单、吸附高效和成本低等特点,被广泛应用于重金属废水治理[10, 14-16]。在吸附剂的选择上,使用易获取和可降解天然高分子材料也逐渐成为趋势。氧化石墨烯(graphene oxide, GO)作为一种新型纳米材料,因其比表面积较大,含氧官能团(如—OH、—C=O和—COOH)丰富和易表面修饰等优点,在处理重金属废水中备受关注[17-19]。刘伟等[18]通过制备磁改性GO,发现磁改性GO在pH=2时对5 mg·L−1的Cr(VI)去除率达82.9%。李仕友等[20]成功制备出GO/SiO2复合材料,但GO/SiO2在pH=2.5时对5 mg·L−1 Cd(Ⅱ)的去除率仅为45.2%。再者,有学者利用不同浓度NaOH对GO进行水热处理后,对重金属的吸附量比改性前提高了3倍,但改性后的GO亲水性较强,难以回收利用[21]。壳聚糖(chitosan, CS)是一种具有大量羟基和高活性氨基的天然可降解高分子材料,能通过螯合反应与重金属离子进行配位,也是具有潜力的新型吸附材料[22-23]。此外,CS作为脱乙酰化甲壳素产品,来源广泛,无毒、易降解和抗菌,使其在重金属废水处理中比其他材料更具优势[24-25],但CS在酸性条件下易流失。因此,将GO与CS组合后制备出的GO/CS复合材料不仅可以降低GO/CS的亲水性[26-28],也能进一步改善对水中重金属的去除效果。龚育等[29]研究发现,添加5% GO的CS对铌(Nb)的去除效果优于CS。罗肃霜等[30]制备的TiO2/CS微粒在紫外光催化下对As(Ⅲ)的最大吸附容量虽有4.97 mg·g−1,但在微粒中加入GO后,吸附容量达到12.43 mg·g−1。ZHANG等[31]通过制备EDTA-2Na改性氧化石墨烯壳聚糖(GEC)复合材料,发现对Cr(VI)的吸附容量大小依次为GEC>GOCS>CS。
此外,将GO或CS复合材料负载铁或铁的(氢)氧化物后,能进一步增强复合材料的抗酸性能和热稳定性[32-33]。PARASTAR等[34]将GO和CS先后加入到Fe2S3和H2O2混合溶液中,制备成GO/FeOOH/CS复合材料并用于去除水中的Pd(Ⅱ)和Cd(Ⅱ),发现在pH=6时复合材料对5 mg·L−1的Pd(Ⅱ)和Cd(Ⅱ)最高去除率分别为29.6%和43.07%。AHMAD等[35]利用共沉淀法制备壳聚糖-氧化铁(CS-Fe2O3)用于去除水中的Pb(Ⅱ)和Cd(Ⅱ),发现在pH=5时CS-Fe2O3对50 mg·L−1 Pb(Ⅱ)和Cd(Ⅱ)的去除率均在85%以上。SHERLALAC等[27]通过共沉淀法制备的磁性壳聚糖氧化石墨烯(CMGO)纳米复合材料在pH=7.3时对10 mg·L−1 As(Ⅲ)的去除率为61%。HOSSEINZADEH等[36]同样通过共沉淀法制备的磁性壳聚糖/氧化石墨烯(MCGONs)纳米复合材料在pH=8时对100 mg·L−1 Cu(Ⅱ)的去除率为38%。赵超然等[37]通过包埋法制备不同载铁量的GOCS球形材料用于吸附As(Ⅲ),发现As(Ⅲ)的吸附容量随pH增加呈下降趋势,在pH=3时吸附量达到3.53 mg·g−1。SHAN等[23]对比研究了浸渍蒸发法和共沉淀法制备FeOx修饰的氧化石墨烯壳聚糖复合材料对Cr(VI)的去除效果,发现浸渍蒸发法制备的复合材料具有更好的吸附性能。上述研究表明:铁改性GO或CS复合材料对水体中不同重金属离子均有去除效果,但复合材料中的载铁形态及其改性方法会明显影响去除效果。这将进一步影响其在不同水环境条件下对重金属离子(如As(Ⅴ)、Cu(Ⅱ)、Pb(Ⅱ)和Cd(Ⅱ))的去除效果。然而,较少有研究对比讨论载铁形态或改性方法对复合材料在不同溶液pH中对重金属离子的去除影响。
因此,本研究基于常用的浸渍蒸发法和共沉淀法分别制备2种不同铁改性氧化石墨烯壳聚糖复合材料微球,设计并开展2种微球在不同水环境中(pH分别为3、7和11)对As(Ⅴ)、Cu(Ⅱ)、Pb(Ⅱ)和Cd(Ⅱ)的吸附实验,对比分析去除效果。同时,结合扫描电镜(scanning electron microscope,SEM)、傅里叶红外光谱(fourier transform infrared, FTIR)、X射线衍射(X-ray diffraction, XRD)和比表面积(BET surface area)表征技术揭示吸附机制,为不同吸附剂应用于多种重金属废水的治理提供参考。
1. 材料与方法
1.1 试剂与材料
氧化石墨烯(GO, 95%)购于苏州碳丰石墨烯科技有限公司。壳聚糖(CS, 脱乙酰度>90%)购于上海蓝季科技发展有限公司。七水合砷酸钠(Na2HAsO4·7H2O, AR, 成都贝斯特)。二水合氯化铜(CuCl2·2H2O, AR),硝酸铅(Pb(NO3)2, AR),四水合硝酸镉(Cd(NO3)2·4H2O, AR),六水合三氯化铁(FeCl3·6H2O, AR),四水合氯化亚铁(FeCl2·4H2O, AR),盐酸(HCl, GR),氢氧化钠(NaOH, GR),冰醋酸(CH3COOH, GR),甲醇(CH3OH, AR),无水乙醇(C2H5OH, AR),硝酸(HNO3, AR)均采购于西陇化工股份有限公司。戊二醛(C5H8O2, 50 %),溴化钾(KBr, GR)购自于阿拉丁试剂(上海)有限公司。实验用水均为超纯水。
使用浸渍蒸发法制备Fe(Ⅱ)改性氧化石墨烯壳聚糖复合微球(Fe-GOCS)。首先将氧化石墨烯和壳聚糖粉末按照一定比例混合,加入配置好的200 mL 1.5%的醋酸溶液,超声搅拌直至二者混合均匀,得到GOCS混合液;将混合液滴入至2% NaOH溶液中,常温静置固化成球,用超纯水将洗涤液洗至接近中性,过滤;在过滤后的材料中加入200 mL 5%戊二醛-甲醛溶液后置于摇床振荡2~3 h,用50%乙醇溶液洗涤至无醛味后,过滤,放置45 ℃的鼓风干燥箱中烘干,得到GOCS复合微球;将微球放入0.1 mol的FeCl2·4H2O溶液中,放置于200 ℃的电热板上加热蒸干,冷却后洗涤至电导率低于25 μs·cm−1,烘干,得到Fe-GOCS复合微球。使用共沉淀法制备Fe(Ⅲ)改性氧化石墨烯壳聚糖复合微球(Fe@GOCS),在上述配置好的GOCS混合液中加入0.1 mol FeCl3·6H2O,继续超声搅拌直至固体完全溶解,得到Fe@GOCS混合溶液;将该溶液滴入至5% NaOH溶液中,静置24 h后,洗涤至中性,过滤,置于200 mL 5%戊二醛-甲醛溶液中摇床振荡2~3 h后,用50%乙醇溶液洗涤至无醛味,过滤,烘干,得到Fe@GOCS复合微球。
1.2 实验方法
1)实验设计。采用批实验对比研究在不同pH条件下 GOCS、Fe-GOCS和Fe@GOCS分别对As(Ⅴ)、Cu(Ⅱ)、Pb(Ⅱ)和Cd(Ⅱ)的去除效果。分别称取50 mg GOCS、Fe-GOCS和Fe@GOCS置于若干100 mL离心管中,分别添加50 mL 25 mg·L−1 As(Ⅴ)、Cu(Ⅱ)、Pb(Ⅱ)和Cd(Ⅱ)模拟液,再使用1 mol·L−1 HCl和1 mol·L−1 NaOH调节模拟液pH分别为3±0.1、7±0.1和11±0.1,然后将模拟液置于恒温水浴振荡器中,在298.15 K、170 r·min−1条件下,振荡48 h。使之充分反应后,取上清液过0.45 μm滤膜后测定重金属离子浓度。
在固液比为1.0 g·L−1、As(Ⅴ)初始质量浓度为25 mg·L−1、温度为298.15 K和pH分别为7和3的条件下,收集首次吸附As(Ⅴ)后的Fe-GOCS和Fe@GOCS,并用50 mL 1.5 mol·L−1 NaOH和超纯水洗脱,直到洗脱液电导率稳定,然后将这些复合材料干燥后再次用于As(Ⅴ)的吸附。解吸和吸附过程重复5次,每次吸附后收集上清液和脱吸液用于As(Ⅴ)浓度的测定,结果将用于分析多次解吸和吸附对As(Ⅴ)去除效果的影响,以及吸附剂的再生性能。
2)样品表征分析。吸附前后As(Ⅴ)、Cu(Ⅱ)、Pb(Ⅱ)和Cd(Ⅱ)的浓度均利用电感耦合等离子体发射光谱仪(ICP-OES, Optima 7000 DV)测试,测试数据误差小于5%。对GOCS和吸附反应前后的Fe-GOCS和Fe@GOCS进行表征分析。其中,复合材料的官能团使用溴化钾压片法由傅里叶红外光谱仪(FTIR, iS 10, 美国赛默飞世尔公司)测试完成;样品结构测定采用X射线衍射仪(XRD,X'Pert3 Powder,荷兰帕纳科公司),测试扫描2θ步长0.026 26°,扫描速度为0.656 5(°)·s−1,扫描2θ为5°~90°;形貌微观特征由场发射电子扫描显微镜(SEM, JSM-7900F, 日本)测定;样品的比表面积和孔结构采用全自动比表面及孔隙度分析仪(BET, Micromeritics ASAP 2020, 美国)测试。
3)数据处理。GOCS、Fe-GOCS和Fe@GOCS对As(Ⅴ)、Cu(Ⅱ)、Pb(Ⅱ)和Cd(Ⅱ)平衡吸附量(qe)和去除率(re)分别由式(1)和式(2)计算。
stringUtils.convertMath(!{formula.content}) (1) stringUtils.convertMath(!{formula.content}) (2) 式中:qe是吸附平衡时吸附剂对重金属离子的吸附量,mg·g−1;C0和Ce分别是重金属离子初始和吸附平衡时的质量浓度,mg·L−1;V是含重金属离子模拟液的体积,mL;m是吸附剂的投加量,mg;re是吸附平衡时重金属离子的去除率,%。
2. 结果与讨论
2.1 不同pH条件下重金属的去除效果
不同pH条件下,GOCS、Fe-GOCS和Fe@GOCS对As(Ⅴ)、Cu(Ⅱ)、Pb(Ⅱ)和Cd(Ⅱ)的去除结果分别如表1和图1所示。可以发现:改性前后的复合材料对不同重金属的平衡吸附量(qe)和去除率(re)存在明显差异。其中,GOCS和Fe@GOCS对As(Ⅴ)的qe均随pH升高而逐渐降低,qe均在pH=3时最大,分别为7.72 mg·g−1和22.54 mg·g−1。在pH=11时GOCS对As(Ⅴ)的qe虽有0.12 mg·g−1,但Fe@GOCS却为0(图1(a)和图1(c))。而Fe-GOCS对As(Ⅴ)的qe随pH升高先显著增加后略微降低。当pH=3时,qe最小为7.02 mg·g−1;pH=7时,qe达到最大为14.54 mg·g−1(图1(b))。结合表1可知:酸性条件下,Fe@GOCS对As(Ⅴ)去除率显著高于GOCS和Fe-GOCS,三者对As(Ⅴ)的去除率分别为87.99%、30.20%和27.40%。同时,除As(Ⅴ)过程中Fe平衡质量浓度仅为0.04 mg·L−1,远低于《生活饮用水卫生标准》(GB 5749-2022)所规定的0.3 mg·L−1。而Fe-GOCS的Fe平衡质量浓度为0.4 mg·L−1,存在二次污染风险,说明酸性条件下Fe@GOCS比Fe-GOCS更稳定。中性和碱性条件下,Fe-GOCS对As(Ⅴ)的re则显著高于GOCS和Fe@GOCS,Fe平衡质量浓度分别为0和0.08 mg·L−1,均低于0.3 mg·L−1的限值。相比之下,Fe@GOCS更适用于酸性条件下对As(Ⅴ)的去除,而在中性和碱性条件下Fe-GOCS更适用。同时,在最佳适用pH条件下,不同铁改性后的GOCS对As(Ⅴ)的去除效果均优于GOCS。
表 1 不同pH条件下复合材料对重金属的去除结果Table 1. Removal results of heavy metals by composite materials under different pH conditions离子 pH GOCS Fe-GOCS Fe@GOCS qe/(mg·g−1) re/(%) qe/(mg·g−1) re/(%) Fe平衡质量浓度/(mg·L−1) qe/(mg·g−1) re/(%) Fe平衡质量浓度/(mg·L−1) As(Ⅴ) 3 7.72 30.20 7.02 27.40 0.40 22.54 87.99 0.04 7 2.01 7.86 14.54 56.74 0 10.38 40.53 0.04 11 0.12 0.47 9.45 36.87 0.08 — — 0.15 Cu(Ⅱ) 3 — — — — 0.26 — — 0.13 7 18.59 74.27 12.24 49.45 0 16.92 68.38 0 11 24.97 99.74 24.53 99.09 0 24.71 99.83 0 Pb(Ⅱ) 3 — — — — 0.15 — — 0.06 7 11.21 47.15 5.37 23.52 0 11.60 50.85 0 11 23.63 99.38 22.77 99.78 0 22.68 99.39 0 Cd(Ⅱ) 3 — — — — 0.13 — — 0.05 7 0.64 2.57 — — 0.05 — — 0 11 24.65 98.31 19.65 78.30 0 24.89 99.16 0 注: “-”表示未吸附。 由图1可知,3种材料对Cu(Ⅱ)和Pb(Ⅱ)的去除规律相似,qe均随着pH的增大呈上升趋势。其中,pH=3时,GOCS、Fe-GOCS和Fe@GOCS均未去除Cu(Ⅱ)和Pb(Ⅱ)。pH=7时,GOCS、Fe-GOCS和Fe@GOCS对Cu(Ⅱ)的qe分别为18.59、12.24和16.92 mg·g−1,对Pb(Ⅱ)的qe分别为11.21、5.37和11.60 mg·g−1。而pH=11时,3种材料对Cu(Ⅱ)和Pb(Ⅱ)的qe相近,均达到最大。其中,GOCS、Fe-GOCS和Fe@GOCS对Cu(Ⅱ)的qe分别为24.97、24.53和24.71 mg·g−1,对Pb(Ⅱ)的qe分别为23.63、22.77和22.68 mg·g−1。在中性和碱性条件下均未发现Fe溢出。由此可知,3种材料均不适用于去除酸性条件下的Cu(Ⅱ)和Pb(Ⅱ)。中性条件下,GOCS更适用去除Cu(Ⅱ),其次是Fe@GOCS;而对于去除Pb(Ⅱ)的结果则相反。碱性条件下考虑到Cu(Ⅱ)和Pb(Ⅱ)存在水解或共沉淀作用[38],3种材料均不适用于对Cu(Ⅱ)和Pb(Ⅱ)的去除。
从表1 Cd(Ⅱ)的去除结果中可知,3种材料在pH=3均未吸附Cd(Ⅱ)。pH=7时,仅GOCS具有微弱的去除效果,qe和re分别为0.64 mg·g−1和2.57%。当pH=11时,Fe@GOCS的去除效果均优于GOCS和Fe-GOCS,qe分别为24.89、24.65和19.65 mg·g−1。由此可知,GOCS更适用于中性条件下去除Cd(Ⅱ)。而在碱性条件下,Cd(Ⅱ)存在较强的水解或共沉淀作用[38],表明2种材料均不适用于去除Cd(Ⅱ)。
2.2 吸附介质前后变化特征
1) FTIR表征。吸附前复合材料的FTIR表征结果如图2所示。由图2可知,GO、CS和GOCS具有多种官能团。其中,GO图谱中1 700 cm−1附近是—COOH的C=O伸缩振动峰,1 626 cm−1附近是水分子中的O—H弯曲振动峰,1 573 cm−1附近是—NH2面内变形特征峰和1 056 cm−1附近的C—O—C弯曲振动峰。CS图谱中3 434 cm−1附近是N—H伸缩振动峰,2 919 cm−1和2 874 cm−1附近是CH3和CH2中C—H伸缩振动峰,1 655 cm−1和1 598 cm−1附近是酰胺I键C=O的伸缩振动峰,1 432~1 322 cm−1附近是CH3和CH2中C—H弯曲振动峰,1 158 cm−1附近是C—O—C弯曲振动峰以及1 077 cm−1的碳环弯曲振动峰[39]。将GO与CS混合后,图谱显示GOCS在3 430 cm−1附近是水分子中O—H和CS中N—H伸缩振动峰,在2 919 cm−1和2 870 cm−1附近保留了CS中的C—H伸缩振动峰,在1 062 cm−1附近形成较强的环氧基C—O—C的C—O弯曲振动峰。此外,GOCS在1 656 cm−1附近形成酰胺键—CONH—,该特征峰说明GO中的—COOH与CS中的—NH2组装成功[37]。在经过不同铁改性后,GOCS图谱出现不同特征。其中,Fe@GOCS在1 070 cm−1附近出现碳环弯曲振动峰,在1 384 cm−1附近出现C—OH弯曲振动峰。882 cm−1和792 cm−1附近出现α-FeO(OH)中O—H的面内和面外弯曲振动峰,是针铁矿的特征吸收峰,614 cm−1为Fe—O的特征峰。而Fe-GOCS在3 421 cm−1出现的宽峰是其表面—OH与水分子形成的氢键有关[40],1 063 cm−1和1 031 cm−1附近是C—O弯曲振动峰,1 555 cm−1附近为峰值增强的—NH2面内变形特征峰,在581 cm−1附近形成赤铁矿的Fe—O弯曲振动峰[41]。此外,Fe@GOCS和Fe-GOCS在1 632 cm−1附近均保留了GOCS的酰胺键—CONH—,这与赵超然等[37]和李仕友等[42]的研究结果一致。
Fe-GOCS和Fe@GOCS在最优条件下去除As(Ⅴ)、Cu(Ⅱ)、Pb(Ⅱ)和Cd(Ⅱ)后的FTIR图谱如图3所示。由图3(a)可见,Fe-GOCS在去除As(Ⅴ)后,1 063 cm−1和1 031 cm−1附近的C—O弯曲振动峰增强,而581 cm−1处对应的Fe—O特征峰偏移至561 cm−1。这说明C—H、C—O和Fe—O均可能参与到As(Ⅴ)去除中。在去除Cu(Ⅱ)和Pb(Ⅱ)后,Fe-GOCS中的Fe—O分别偏移至548 cm−1和568 cm−1且峰强减弱,这说明Fe—O可能参与去除。Fe-GOCS-Cd图谱显示,Fe-GOCS在1 456~1 379 cm−1的C—H多组伸缩振动峰数量减少,仅在1 384 cm−1处均在C—H单峰。同时,在去除As(Ⅴ)、Cu(Ⅱ)和Pb(Ⅱ)后,该处的C—H峰值增强,说明C—H均可能参与到As(Ⅴ)、Cu(Ⅱ)和Pb(Ⅱ)去除。
与Fe-GOCS吸附前后表征结果类似,Fe@GOCS在去除重金属后,官能团也发生不同程度的变化(图3(b))。其中,在去除As(Ⅴ)和Pb(Ⅱ)后,614 cm−1附近对应的Fe—O特征峰偏移至638 cm−1。此外,多组官能团特征峰均出现不同程度减弱,包括882 cm−1和792 cm−1附近的α-FeO(OH)中O—H的面内和面外弯曲振动峰、1 070 cm−1附近的碳环弯曲振动峰、1 384 cm−1附近的C—OH弯曲振动峰以及1 632 cm−1的—CONH—。由此可知,Fe@GOCS去除As(Ⅴ)和Pb(Ⅱ)是多种官能团共同作用的结果。与去除As(Ⅴ)和Pb(Ⅱ)结果相反,Fe@GOCS在去除Cu(Ⅱ)后,多组官能团特征峰均显著增强。这可能是因为Fe@GOCS中的Fe为+3价,Fe—O体系中的氧带有负电荷,而Cu(Ⅱ)的电负性较其他3种重金属离子强[43],与Fe—O上的氧以及O—H、C—OH和—CONH—之间的相互作用较强,因而对分子筛的骨架振动改变较为明显。Fe@GOCS与Cd(Ⅱ)反应后,特征峰位置未出现明显变化,说明Fe@GOCS去除Cd(Ⅱ)后化学结构没有改变,表明Fe@GOCS的主要官能团并未与Cd(Ⅱ)发生显著作用。这一结果与范先媛等[44]使用孔径为4×10−10 m的分子筛去除Cu(Ⅱ)和Cd(Ⅱ)的结果相似。
2) XRD表征。由图4可以看出,GO和CS分别在2θ为12°和42°附近以及20°附近有明显衍射峰,为无定型结构,GOCS仅在2θ=20°附近存在衍射峰,且峰型变窄,峰面积减小。这说明GO和CS的成功组装[32]。Fe-GOCS在2θ为33°和35°附近出现新的衍射峰。但35°附近的衍射峰峰值较弱,这可能受到制备材料中一些杂质的影响。与标准PDF卡片(ICDD PDF 72-0469)进行对照比较,新出现的衍射峰为赤铁矿,以α-Fe2O3矿物形态存在,同时也意味着FeCl2成功将GOCS改性[37]。而Fe@GOCS在2θ为17°、21°、33°、34°、36°以及53°附近均出现新的衍射峰,对比标准PDF卡片(ICDD PDF 81-0462)后确定为针铁矿,以α-FeO(OH)矿物形态存在[45]。
Fe-GOCS和Fe@GOCS在最优条件下去除As(Ⅴ)、Cu(Ⅱ)、Pb(Ⅱ)和Cd(Ⅱ)后的XRD结果分别如图5(a)和图5(b)所示。由图5可知,反应前后Fe-GOCS和Fe@GOCS的衍射峰位置未发生明显改变且未出现新衍射峰。这表明Fe-GOCS和Fe@GOCS稳定性良好,在去除重金属过程中并没有生成新的物相。此外,由图5(a)可知,Fe-GOCS位于2θ=33°和35°的衍射峰出现不同程度的增强。这说明Fe-GOCS上的α-Fe2O3参与As(Ⅴ)、Cu(Ⅱ)和Pb(Ⅱ)的去除。而对于Cd(Ⅱ),α-Fe2O3衍射峰并未出现明显变化,说明α-Fe2O3并未参与去除Cd(Ⅱ)。这些结果与2.1.1 FTIR分析的结果一致,也再次表明碱性条件下Cd(Ⅱ)的去除主要与离子水解或共沉淀作用有关[44, 46]。由图5(b)中也可以看出,Fe@GOCS衍射峰强度出现不同程度的变化。其中Fe@GOCS在吸附As(Ⅴ)和Pb(Ⅱ)后,位于2θ=17°、21°、36°和53°处的α-FeO(OH)衍射峰峰值明显增强。这可能受到了吸附剂中其他官能团的影响,如O—H、C—OH和—CONH—等。在吸附Cu(Ⅱ)后,α-FeO(OH)衍射峰峰值明显降低,说明α-FeO(OH)参与去除Cu(Ⅱ)。与Fe-GOCS去除Cd(Ⅱ)后的结果类似,Fe@GOCS-Cd的衍射峰并未出现明显变化,表明Fe@GOCS中的α-FeO(OH)没有参与反应。
3) SEM表征。GOCS、Fe-GOCS和Fe@GOCS的SEM表征结果如图6所示。可以看出,改性前GOCS表面光滑且富有光泽(图6(a)),堆叠着许多片状结构(图6(b))。经FeCl2浸渍改性后,Fe-GOCS表面变得黯淡且有裂隙出现(图6(c)),同时GOCS的片状层结构被排列紧密的球状物质所代替且有聚集成斑块状物质的现象(图6(d)),结合第2.1.2节XRD物相分析确定该球状物质为赤铁矿;经FeCl3改性后,Fe@GOCS表面出现大量且层次分明的褶皱(图6(e))。同时,褶皱之间充填有针刺状或块状物质(图6(f)),结合第2.1.2节的XRD物相分析确定该针刺状物质为针铁矿。由此可知,GOCS经不同载铁方式后微观表面形貌发生显著变化,同时证实GOCS均被成功改性。这一结果与FTIR和XRD的分析一致。
Fe-GOCS和Fe@GOCS分别吸附As(Ⅴ)、Cu(Ⅱ)、Pb(Ⅱ)和Cd(Ⅱ)后的微观形貌如图7所示。Fe-GOCS吸附As(Ⅴ)后表面较未吸附之前球状物质虽没有明显变化,但成块面积明显减少(图7(a));吸附Cu(Ⅱ)后,表面的球状物质明显减少且分布不均,暴露出大量针刺状物质(图7(b));吸附Pb(Ⅱ)后的Fe-GOCS表面球状物质形貌发生改变,斑块状物质面积增加,少许的针刺状物质掺杂其中(图7(c));Fe-GOCS在与Cd(Ⅱ)反应后斑块状物质面积与未反应前并未发生明显变化,仅斑块状物质较为分散(图7(d))。这表明Fe-GOCS虽未能够去除Cd(Ⅱ),但Cd(Ⅱ)可以修饰材料表面形貌。Fe@GOCS在去除As(Ⅴ)后,原先的褶皱变得光滑,但有明显萎缩迹象且变得杂乱无序(图7(e));吸附Cu(Ⅱ)后的Fe@GOCS表面褶皱消失,变得粗糙且凹凸不平(图7(f));在去除Pb(Ⅱ)后,褶皱间的针刺状或块状物质明显减少,褶皱表面出现大小不等的凹陷(图7(g));与Cd(Ⅱ)反应后的Fe@GOCS表面褶皱几乎消失殆尽,仅一些针刺状物质残留在表面(图7(h))。
4) BET表征。GOCS、Fe-GOCS和Fe@GOCS的BET比表面积和孔结构参数列于表2。由表2可见,GOCS的比表面积和孔体积分别为4.22 m2·g−1和2.25×10−4 cm3·g−1。经FeCl3改性后,Fe@GOCS比表面积增至42.27 m2·g−1,孔体积增至6.58×10−4 cm3·g−1,这使得酸性条件(pH=3)下Fe@GOCS对As(Ⅴ)的吸附量比GOCS高出14.82 mg·g−1。同时,结合SEM表征结果,Fe@GOCS表面出现大量且层次分明的褶皱有利于进一步提高吸附量。经FeCl2改性后,相较于GOCS,Fe-GOCS的比表面积降低2.12 m2·g−1,孔体积降低0.38×10−4 cm3·g−1,这使得Fe-GOCS在酸性条件下对As(Ⅴ)的吸附量减少0.70 mg·g−1。但在中性和碱性条件下,3种材料对于As(Ⅴ)、Cu(Ⅱ)、Pb(Ⅱ)和Cd(Ⅱ)的去除效果也存在差异,这说明除材料本身的空间结构外,材料表面的电荷、载铁形态以及离子形态也会影响去除效果[37, 47-48]。
表 2 GOCS和Fe-GOCS和Fe@GOCS的比表面积和孔结构参数Table 2. Specific surface area and pore structure parameters of GOCS, Fe-GOCS and Fe@GOCS吸附材料 比表面积/(m2·g−1) 孔体积/(cm3·g−1) GOCS 4.22 2.25×10−4 Fe-GOCS 2.10 1.87×10−4 Fe@GOCS 42.27 6.58×10−4 2.3 再生实验
As(Ⅴ)分别在Fe-GOCS和Fe@GOCS上重复解吸和吸附5次后,平衡吸附容量(qe)的变化如图8所示。Fe-GOCS在对As(Ⅴ)的初始qe虽有13.19 mg·g−1,但在循环吸附1次后,Fe-GOCS并未展现出良好的吸附效果。而Fe@GOCS对As(Ⅴ)的qe随着解吸和吸附循环次数的增加先上升后略有降低,这可能与材料表面上铁的溶解损失有关。在第5次解吸后,Fe@GOCS对As(Ⅴ)的qe仍高达22.02 mg·g−1,仅比初始qe减少0.31 mg·g−1,说明Fe@GOCS再生性良好,可重复用于酸性含砷废水中。同时在整个实验过程中,虽然Fe-GOCS和Fe@GOCS中的部分Fe溶解在水溶液中,但Fe平衡质量浓度分别为0~0.22 mg·L−1和0~0.04 mg·L−1,仍未超过《生活饮用水卫生标准》(GB 5749-2022)铁的0.3 mg·L−1的标准限值。这不仅进一步说明本研究制备的Fe@GOCS比Fe-GOCS具有更加稳定且良好的环保再生能力,也使Fe@GOCS可进一步用于吸附处理As(Ⅴ)脱吸液(C0=0.95~17.16 mg·L−1),降低脱吸液对环境的二次污染风险。
2.4 性能评价
将所制备的Fe-GOCS和Fe@GOCS与目前所制备的复合材料对As(Ⅴ)、Cu(Ⅱ)和Pb(Ⅱ)去除效果进行对比,其结果如表3所示。一方面,在pH=3时Fe@GOCS对As(Ⅴ)的re为87.99%,均高于AMD污泥复合材料[49]和含铁锰污泥壳聚糖-海藻酸吸附剂[50]等。GO和CS来源广泛和价格低廉[17, 24-25],同时Fe@GOCS制备方法简便且具有良好的环保再生能力,这也为Fe@GOCS在As(Ⅴ)去除提供广阔的应用前景。此外,Fe-GOCS和Fe@GOCS分别在pH=7和3时对25 mg·L−1 As(Ⅴ)吸附达到平衡后,溶液中分别仍有11.08 mg·L−1和3.08 mg·L−1的As(Ⅴ),超出《污水综合排放标准》(GB 18466-2005)中砷的标准限定值0.5 mg·L−1。因此,可使用Fe@GOCS对酸化后仍超标的As(Ⅴ)废液进一步吸附处理。另一方面,在pH=7时,Fe-GOCS和Fe@GOCS对Cu(Ⅱ)的re分别为49.45%和68.38%,均高于磁性GOCS复合材料和纯CS,说明Fe-GOCS和Fe@GOCS对Cu(Ⅱ)去除效果良好。而对于Pb(Ⅱ)和Cd(Ⅱ)的去除,Fe-GOCS和Fe@GOCS却没有表现出良好的去除效果。
表 3 材料去除性能对比Table 3. Comparison of material removal performance总的来说,Fe@GOCS能够兼顾多种重金属离子高效率去除且不会因材料中Fe溢出造成环境二次污染问题,是一种环保型可再生吸附剂。对于Fe-GOCS,能在不同的溶液环境有效去除多种重金属离子,但应进一步提升去除效果。
2.5 去除机理
结合pH、FTIR、XRD和BET分析结果,推测了Fe-GOCS和Fe@GOCS对As(Ⅴ)、Cu(Ⅱ)、Pb(Ⅱ)和Cd(Ⅱ)的去除机理。Fe-GOCS和Fe@GOCS的零点电荷pHpzc分别是7.5[47]和8.2[37]。当溶液环境为弱酸性(pH=3)时,As(Ⅴ)主要以HAsO42-存在[48]。Fe-GOCS中的α-Fe2O3在水溶液中可形成羟基化表面(Fe—OH+2、Fe—OH、FeO−)[53],使材料表面基团质子化,在静电吸引作用下去除HAsO42-形态的As(Ⅴ)。而Fe@GOCS中的α-FeO(OH)本身具有大量的-OH,在pH=3时,Fe—OH质子化形成Fe—OH+2,在静电吸引作用下去除As(Ⅴ)。具体的去除机理可解释为分式(3)和式(4)及总反应(式(5))。
stringUtils.convertMath(!{formula.content}) (3) stringUtils.convertMath(!{formula.content}) (4) stringUtils.convertMath(!{formula.content}) (5) 当溶液环境为中性时,Cu(Ⅱ)和Pb(Ⅱ)均会发生不同程度的水解。其中,Cu(Ⅱ)的水解常数pKCu(pK实测值和pK计算值分别为6.5和7.0)小于Pb(Ⅱ)的水解常数(pK实测值和pK计算值分别为7.2和11.7)[38],说明Cu(Ⅱ)的水解程度大于Pb(Ⅱ),Cu(Ⅱ)的σ杂化轨道接受氧的非键电子对的能力更强,形成Cu-OH配键的键能更大[54],这也证明在中性条件下,2种材料均对Cu(Ⅱ)的去除效果最好。除Cu(Ⅱ)和Pb(Ⅱ)自身水解外,结合FTIR和XRD分析结果,在其他官能团(Fe-GOCS中的C—H和Fe@GOCS中的C—OH、O—H及—CONH—)提供的键能帮助下,Fe-GOCS和Fe@GOCS中的Fe—O分别通过与Cu(Ⅱ)和Pb(Ⅱ)形成Fe—O—Cu和Fe—O—Pb表面络合物实现对其的去除。而Cd(Ⅱ)(pK实测值和pK计算值分别为9.7和9.5[38])主要通过自身水解被去除。
3. 结论
1) Fe-GOCS对As(Ⅴ)的去除率在pH=7时达到最大,为56.74%。而Fe@GOCS对于As(Ⅴ)的去除率在pH=3时达到最大,为87.99%。酸性条件下,应优先考虑Fe@GOCS。
2)溶液pH的改变对Cu(Ⅱ)和Pb(Ⅱ)的去除效果影响较大。Fe-GOCS、Fe@GOCS对Cu(Ⅱ)和Pb(Ⅱ)的去除率在pH=11时均达到最大。除GOCS外,在弱酸性~中性条件下,对于Cu(Ⅱ)和Pb(Ⅱ)的去除应优先考虑Fe@GOCS。
3) Fe-GOCS和Fe@GOCS对Cd(Ⅱ)的吸附在酸性和中性时效果较差。碱性条件下2种复合材料对Cd(Ⅱ)的re均达到最大,但这与复合材料中丰富的官能团无关,主要与Cd(Ⅱ)自身水解有关。
4)再生吸附循环5次后,Fe@GOCS对As(Ⅴ)的qe仍高达22.02 mg·g−1,仅低于初始qe的0.31 mg·g−1,说明说明Fe@GOCS再生性良好,可重复用于酸性含砷废水中,而Fe-GOCS的再生性能较差。
5) Fe-GOCS中的C—H、Fe—O和C—O等官能团,Fe@GOCS中的Fe—O、O—H、C—OH和—CONH—等官能团在As(Ⅴ)、Cu(Ⅱ)和Cd(Ⅱ)去除过程中发挥着主要作用;参与吸附的主要物质分别为Fe-GOCS中的赤铁矿(α-Fe2O3),Fe@GOCS中的针铁矿[α-FeO(OH)]。
-
表 1 不同pH条件下复合材料对重金属的去除结果
Table 1. Removal results of heavy metals by composite materials under different pH conditions
离子 pH GOCS Fe-GOCS Fe@GOCS qe/(mg·g−1) re/(%) qe/(mg·g−1) re/(%) Fe平衡质量浓度/(mg·L−1) qe/(mg·g−1) re/(%) Fe平衡质量浓度/(mg·L−1) As(Ⅴ) 3 7.72 30.20 7.02 27.40 0.40 22.54 87.99 0.04 7 2.01 7.86 14.54 56.74 0 10.38 40.53 0.04 11 0.12 0.47 9.45 36.87 0.08 — — 0.15 Cu(Ⅱ) 3 — — — — 0.26 — — 0.13 7 18.59 74.27 12.24 49.45 0 16.92 68.38 0 11 24.97 99.74 24.53 99.09 0 24.71 99.83 0 Pb(Ⅱ) 3 — — — — 0.15 — — 0.06 7 11.21 47.15 5.37 23.52 0 11.60 50.85 0 11 23.63 99.38 22.77 99.78 0 22.68 99.39 0 Cd(Ⅱ) 3 — — — — 0.13 — — 0.05 7 0.64 2.57 — — 0.05 — — 0 11 24.65 98.31 19.65 78.30 0 24.89 99.16 0 注: “-”表示未吸附。 表 2 GOCS和Fe-GOCS和Fe@GOCS的比表面积和孔结构参数
Table 2. Specific surface area and pore structure parameters of GOCS, Fe-GOCS and Fe@GOCS
吸附材料 比表面积/(m2·g−1) 孔体积/(cm3·g−1) GOCS 4.22 2.25×10−4 Fe-GOCS 2.10 1.87×10−4 Fe@GOCS 42.27 6.58×10−4 表 3 材料去除性能对比
Table 3. Comparison of material removal performance
-
[1] KARA I, TUNC D, SAYIN F, et al. Study on the performance of metakaolin based geopolymer for Mn(II) and Co(II) removal[J]. Applied Clay Science, 2018, 161(9): 184-193. [2] OGATA F, UETA E, KAWASAKI N. Characteristics of a novel adsorbent Fe-Mg-type hydrotalcite and its ad-sorption capability of As(III) and Cr(VI) from aqueous solution[J]. Journal of Industrial and Engineering Chemistry, 2018, 59(3): 56-63. [3] VARDHAN K H, KUMAR P S, PANDA R C. A review on heavy metal pollution, toxicity and remedial measures: Current trends and future perspectives[J]. Journal of Molecular Liquids, 2019, 290(9): 111197. [4] 郝君. 所有重金属超标都有毒[J]. 西部资源, 2014(4): 68-69. [5] SARI A, TUZEN M. Cd(II) adsorption from aqueous solution by raw and modified kaolinite[J]. Applied Clay Science, 2014, 88-89(2): 63-72. [6] CAROLIN C F, KUMAR P S, SARAVANAN A, et al. Efficient techniques for the removal of toxic heavy metals from aquatic environment: A review[J]. Journal of Environmental Chemical Engineering, 2017, 5(3): 2782-2799. doi: 10.1016/j.jece.2017.05.029 [7] 雷鸣, 曾敏, 郑袁明, 等. 湖南采矿区和冶炼区水稻土重金属污染及其潜在风险评价[J]. 环境科学学报, 2008, 28(6): 1212-1220. doi: 10.3321/j.issn:0253-2468.2008.06.029 [8] 易爽, 刘牡丹, 宋卫锋, 等. 高效重金属捕集剂TDTC对络合铜的去除性能[J]. 环境工程学报, 2021, 15(12): 3844-3853. doi: 10.12030/j.cjee.202108152 [9] 邢慧琳, 黄海辉. 含砷酸性废水处理工艺现状与展望[J]. 中国资源综合利用, 2019, 37(9): 34-38. doi: 10.3969/j.issn.1008-9500.2019.09.011 [10] LIU Y Y, SOHI S P, LIU S Y, et al. Adsorption and reductive degradation of Cr(VI) and TCE by a simply synthesized zero valent iron magnetic biochar[J]. Journal of Environmental Management, 2019, 235(4): 276-281. [11] LIU G F, LIAO L, DAI Z M, et al. Organic adsorbents modified with citric acid and Fe3O4 enhance the removal of Cd and Pb in contaminated solutions[J]. Chemical Engineering Journal, 2020, 395(9): 125108. [12] KARA I, YILMAZER D, TUNALI AKAR S. Metakaolin based geopolymer as an effective adsorbent for adsorption of zinc(II) and nickel(II) ions from aqueous solutions[J]. Applied Clay Science, 2017, 139(9): 54-63. [13] YANG J, MA Y J, WENG Y L, et al. Solvent extraction of Fe3+ from the hydrochloric acid route phosphoric acid by D2EHPA in kerosene[J]. Journal of Industrial and Engineering Chemistry, 2014, 20(5): 3446-3452. doi: 10.1016/j.jiec.2013.12.033 [14] ZHANG X, LIU Y. Concurrent removal of Cu(II), Co(II) and Ni(II) from wastewater by nanostructured layered sodium vanadosilicate: Competitive adsorption kinetics and mechanisms[J]. Journal of Environmental Chemical Engineering, 2021, 9(5): 105945. doi: 10.1016/j.jece.2021.105945 [15] LASHEEN M R, AMMAR N S, IBRAHIM H S. Adsorption/desorption of Cd(II), Cu(II) and Pb(II) using chemically modified orange peel: Equilibrium and kinetic studies[J]. Solid State Sciences, 2011, 14(2): 202-210. [16] WANG P, DING F J, HUANG Z B, et al. Adsorption behavior and mechanism of Cd(II) by modified coal-based humin[J]. Environmental Technology & Innovation, 2021, 23(8): 101699. [17] 栗雯绮, 陈文革, 崔晓娟, 等. 氧化石墨烯膜的制备、改性及应用研究进展[J]. 表面技术, 2021, 50(2): 199-210. doi: 10.16490/j.cnki.issn.1001-3660.2021.02.020 [18] 刘伟, 杨琦, 李博, 等. 磁性石墨烯吸附水中Cr(VI)研究[J]. 环境科学, 2015, 36(2): 537-544. [19] KUMAR A S K, JIANG S J. Chitosan-functionalized graphene oxide: A novel adsorbent an efficient adsorption of arsenic from aqueous solution[J]. Journal of Environmental Chemical Engineering, 2016, 4(2): 1698-1713. doi: 10.1016/j.jece.2016.02.035 [20] 李仕友, 熊凡, 王亮, 等. 氧化石墨烯/SiO2复合材料对Cd(Ⅱ)的吸附[J]. 复合材料学报, 2017, 34(6): 1205-1211. [21] 曹宝月, 高歌, 徐珊, 等. 表面亲水改性GO的制备及其对铬离子的吸附性能[J]. 工业水处理, 2018, 38(3): 50-53. doi: 10.11894/1005-829x.2018.38(3).050 [22] 李莹, 杨欣悦, 王雪羽, 等. 壳聚糖基复合膜的成膜机理和特性研究进展[J]. 食品工业科技, 2022, 43(7): 430-438. [23] SHAN H M, ZENG C Y, ZHAO C R, et al. Iron oxides decorated graphene oxide/chitosan composite beads for enhanced Cr(VI) removal from aqueous solution[J]. International Journal of Biological Macromolecules, 2021, 172(10): 197-209. [24] 刘珊, 赵春朋, 李涛, 等. 改性壳聚糖凝胶球对Cu(II)的吸附[J]. 环境化学, 2020, 39(7): 2013-2021. [25] 陈潇, 张浩宇, 霍神焕, 等. 壳聚糖改性地聚合物的力学及吸附性能[J]. 复合材料学报, 2019, 36(12): 2959-2967. doi: 10.13801/j.cnki.fhclxb.20190402.003 [26] 赵恒, 焦体峰, 张乐欣, 等. 氧化石墨烯-壳聚糖复合水凝胶的制备与吸附性能研究(英文)[J]. Science China Materials, 2015, 58(10): 811-818. [27] SHERLALAC A I A, RAMAN A A A, BELLO M M, et al. Adsorption of arsenic using chitosan magnetic graphene oxide nanocomposite[J]. Journal of Environmental Management, 2019, 246(9): 547-556. [28] 郭明媛, 马永宁, 陈腾飞. 还原氧化石墨烯-壳聚糖复合膜的制备及其性能[J]. 复合材料学报, 2022, 39(3): 1291-1299. doi: 10.13801/j.cnki.fhclxb.20210819.001 [29] 龚育, 孔亚飞, 马真真, 等. 氧化石墨烯/壳聚糖复合微球的制备及其对铌的吸附性能[J]. 冶金分析, 2017, 37(8): 15-20. doi: 10.13228/j.boyuan.issn1000-7571.010119 [30] 罗肃霜, 杨春平, 何慧军, 等. TiO2/壳聚糖/氧化石墨烯复合微粒的制备及吸附As(Ⅲ)[J]. 环境工程学报, 2016, 10(9): 4873-4878. doi: 10.12030/j.cjee.201504092 [31] ZHANG L, LUO H J, LIU P P, et al. A novel modified graphene oxide/chitosan composite used as an adsorbent for Cr(VI) in aqueous solutions[J]. International Journal of Biological Macromolecules, 2016, 87(6): 586-596. [32] 曾春芽, 单慧媚, 赵超然, 等. 纳米铁-氧化石墨烯/壳聚糖复合材料的制备及其力学性能[J]. 复合材料学报, 2022, 39(4): 1739-1747. doi: 10.13801/j.cnki.fhclxb.20210601.003 [33] 黄文涛, 邓呈逊, 吉宇尘, 等. 壳聚糖功能化磁性氧化石墨烯复合材料的制备及对甲基橙的吸附[J]. 复合材料学报, 2021, 38(4): 1262-1271. doi: 10.13801/j.cnki.fhclxb.20200723.003 [34] PARASTAR M, SHESHMANI S, SHOKROLLAHZADEH S. Cross-linked chitosan into graphene oxide-iron(III) oxide hydroxide as nano-biosorbent for Pd(II) and Cd(II) removal[J]. International Journal of Biological Macromolecules, 2020, 166(1): 229-237. [35] AHMAD R, MIRZA A. Facile one pot green synthesis of chitosan-iron oxide (CS-Fe2O3) nanocomposite: Re-moval of Pb(II) and Cd(II) from synthetic and industrial wastewater[J]. Journal of Cleaner Production, 2018, 186(6): 342-352. [36] HOSSEINZADEH H, RAMIN S. Effective removal of copper from aqueous solutions by modified magnetic chitosan/graphene oxide nanocomposites[J]. International Journal of Biological Macromolecules, 2018, 113(7): 859-868. [37] 赵超然, 单慧媚, 曾春芽, 等. Fe@GOCS的制备及其对水中As(III)的吸附[J]. 环境科学, 2020, 41(8): 3665-3674. [38] 温元凯, 邵俊. 离子极化和金属离子水解规律性[J]. 科学通报, 1977, 22(6): 267-268. [39] 董炎明, 王勉, 吴玉松, 等. 壳聚糖衍生物的红外光谱分析[J]. 纤维素科学与技术, 2001, 9(2): 42-56. doi: 10.3969/j.issn.1004-8405.2001.02.007 [40] 王倩. 针铁矿中Al、Cd同晶共替代的竞争关系及其Cd的配位[D]. 武汉: 华中农业大学, 2017. [41] 刘永红, 叶发兵, 岳霞丽, 等. 铁氧化物的合成及其表征[J]. 化学与生物工程, 2006, 23(7): 10-12. doi: 10.3969/j.issn.1672-5425.2006.07.004 [42] 李仕友, 史冬峰, 唐振平, 等. 壳聚糖/氧化石墨烯复合材料吸附U(VI)的特性与机理[J]. 环境科学学报, 2017, 37(4): 1388-1395. [43] 冯长君, 唐自强, 沈莹. 金属离子的电负性与其水解性、配合物稳定性的探讨[J]. 徐州师范学院学报(自然科学版), 1991, 9(2): 39-43. [44] 范先媛, 谢升昌, 刘红, 等. 4A分子筛去除水中Pb2+、Cd2+、Zn2+、Cu2+性能和机理[J]. 环境科学与技术, 2019, 42(5): 46-52. [45] SHAN H M, PENG S X, ZHAO C R, et al. Highly efficient removal of As(III) from aqueous solutions using goethite/graphene oxide/chitosan nanocomposite[J]. International Journal of Biological Macromolecules, 2020, 164(12): 13-26. [46] 严潞, 方炜炳, 王迁, 等. 氧化石墨烯-聚合羟基铝复合材料的合成及其对Cd(II)的去除效果[J]. 复合材料学报, 2016, 33(3): 613-617. [47] 赵超然, 单慧媚, 彭三曦, 等. 载铁氧化石墨烯壳聚糖对水中Cr(VI)的吸附研究[J]. 水处理技术, 2021, 47(4): 45-51. [48] 刘喜, 敖鸿毅, 刘剑彤. 铁改性竹炭去除水中的As(III)和As(V)[J]. 环境工程学报, 2012, 6(9): 2958-2962. [49] 张亚辉, 张瑞雪, 吴攀, 等. AMD污泥复合材料吸附As(V)的机制及其影响因素[J]. 环境科学, 2022, 43(5): 2673-2684. [50] 曾辉平, 王繁烁, 于亚萍, 等. 壳聚糖海藻酸钠铁锰泥吸附剂制备与除As(V)研究[J]. 中国环境科学, 2020, 40(3): 1146-1155. doi: 10.3969/j.issn.1000-6923.2020.03.026 [51] JALU R G, CHAMADA T A, KASIRAJAN R. Calcium oxide nanoparticles synthesis from hen eggshells for removal of lead (Pb(II)) from aqueous solution.[J]. Environmental Challenges, 2021, 4(8): 100193. [52] NAFISUR R, MOHD N, POORNIMA V, et al. Efficient removal of Pb(II) from water using silica gel functionalized with thiosalicylic acid: Response surface methodology for optimization[J]. Journal of King Saud University - Science, 2021, 33(1): 101232. doi: 10.1016/j.jksus.2020.101232 [53] 梁美娜, 刘海玲, 刘树深, 等. 纳米氧化铁的制备及其对砷的吸附作用[J]. 应用化学, 2007, 24(12): 1418-1423. [54] 李静明, 吴根华. 金属离子水解机理与水解常数计算[J]. 安庆师范学院学报(自然科学版), 1999, 5(1): 94-95. -






 DownLoad:
DownLoad: