-
有机染料是水体中最常见的污染物之一,主要包含胺、芳香化合物、部分重金属等[1]。而高浓度的有机染料污染对环境和人体健康造成极大的危害[2]。生物炭对多种有机物具有高亲和性[3-5],且来源广泛、费用较低[6-7],因此采用生物炭吸附有机染料受到了广泛重视,如YIN等制备的槟榔叶生物炭[3]、ABD-ELHAMID研磨改性的水稻秸秆生物炭[8]、高等制得城镇垃圾生物炭[9]、张等合成的辣椒秸秆生物炭[10]等,均对有机染料吸附表现良好。
此前的研究主要聚焦于生物炭改性及对单一污染物的吸附效能,而对多种污染物的吸附容量差别关注较少。研究生物炭吸附多种污染物的效能差别,能明确污染物影响吸附过程的具体因素,便于预测生物炭对未知污染物的吸附效果。根据过往研究,氢键作用是解释多种污染物在生物炭上吸附的重要机制[11],生物炭上氢键位点的增加能提高对污染物的吸附容量,同时污染物自身的氢键位点对吸附容量也有影响。YANG等以竹生物炭吸附多种典型芳香污染物的研究结果表明,具有氢键受体的污染物更容易被吸附[12]。SONG等选择了5种典型保健品作为污染物,发现污染物的吸附容量随其含有氢键受体数量上升而增加[13]。很多基于线性溶解能关系模型的研究认为污染物氢键供体对其吸附容量没有显著影响[14-16];但有研究[17]表明,污染物氢键供体能与碳基吸附剂的氢键受体形成氢键,从而影响吸附容量。因此,有必要探讨污染物氢键供体对生物炭吸附容量的影响与作用机理。
为了确定污染物氢键供体能否影响生物炭对污染物的吸附容量,本研究制备了以KHCO3和尿素协同活化的玉米秸秆生物炭,并选择了亚甲基蓝与天青B作为目标污染物,通过一系列的吸附实验与计算模拟考察了污染物氢键供体对吸附容量的影响及相关的作用机理,以期为生物炭对不同污染物吸附的实际应用提供参考。
-
玉米秸秆来自汇丰农业加工有限公司。碳酸氢钾(KHCO3)、尿素为分析纯,亚甲基蓝(MB)、天青B(AB)为生物技术级,购自上海麦克林生化科技有限公司。盐酸(HCl,37%)为分析纯,购自天津科密欧化学试剂有限公司。MB与AB的结构如图1所示。
-
取玉米秸秆洗净表面杂质,烘干后粉碎,过150目筛备用。分别称取1.5 g秸秆粉末、3 g尿素、1.5 g KHCO3,在玛瑙研钵内混匀研磨15 min,置于刚玉舟内,在氮气保护的管式炉中热解,热解温度为800 ℃,升温速率为5 ℃·min−1,保持2 h后冷却至室温。得到的产物以1 mol·L−1盐酸浸泡30 min,用纯水洗涤至洗出液呈中性后烘干,得到的生物炭记为KN-BC。此外,作为对照,以同样的方式制备只添加尿素、只添加KHCO3、均不添加的生物炭,分别记为N-BC、K-BC、BC。
-
生物炭的表面形貌使用扫描电子显微镜(SEM,Hitachi SU8010)进行分析。生物炭的比表面积以及孔隙由比表面积与孔径分析仪(Micromeritics ASAP 2460)测定。通过X射线光电子能谱仪(XPS,Thermo Scientific ESCALAB 250Xi)对生物炭表面的元素组成进行分析。傅里叶红外光谱仪(FTIR,IR Nicolet iS10)对生物炭的表面官能团进行测定。拉曼光谱仪(Renishaw inVia)测定了生物炭的缺陷情况。Zeta电位仪(Brookhaven 90 plus PALS)用于测定生物炭表面的Zeta电位。
-
吸附实验在150 mL锥形瓶内进行,溶液体积为50 mL,置于恒温摇床中吸附,温度设定为25 ℃,振荡速度为200 r·min−1。取样后过0.22 μm滤膜,以紫外可见分光光度计(Agilent Varian Cary60)测定样品质量浓度。
对吸附动力学实验,MB或AB溶液初始质量浓度为800 mg·L−1,pH调至7.0,加入10 mg KN-BC,将锥形瓶置于恒温振荡器内,取样时间设定为0、10、20、30、60、120、180、240、360、480 min,测定各时间点的溶液质量浓度并以模型拟合。
对等温吸附实验,加入MB溶液(300~700 mg·L−1)或AB溶液(400~900 mg·L−1),pH调至7.0,加入10 mg KN-BC,振荡8 h后取样,测定溶液质量浓度并以模型拟合。
对考察pH影响的实验, MB或AB溶液初始质量浓度为800 mg·L−1,将溶液pH分别调节为3.0、5.0、7.0、9.0、11.0,此后加入10 mg KN-BC,振荡、过膜、质量浓度测定方式同上。
对考察再生性能的实验, AB溶液初始质量浓度为800 mg·L−1,pH调节为7.0,此后加入10 mg KN-BC,振荡、过膜、质量浓度测定方式同上。随后将吸附后KN-BC加入100 mL乙醇,超声脱附30 min,水洗烘干后重复进行吸附。
-
分别采用准一级(式(1))、准二级动力学模型(式(2))[18-19]对吸附动力学数据进行拟合。
式中:t为时间,min;qt与qe分别为在t时刻与吸附平衡时刻的吸附容量,mg·g−1;k1为准一级动力学速率常数,min−1;k2为准二级动力学速率常数,g·(mg·min)−1。
使用Langmuir[20]、Freundlich[21]、Dubinin-Radushkevich[22]与Redlich-Peterson[23]模型对吸附等温线进行拟合,拟合方程分别如式(3)~式(6)所示。
式中:qe为平衡吸附容量,mg·g−1;Ce为平衡质量浓度,mg·L−1;Qmax与qDR分别为Langmuir与Dubinin-Radushkevich模型的最大吸附容量,mg·g−1;R为气体常数,8.314 J·(mol·K)−1;T为实验温度,298.15 K;KL、KF、KDR与KRP分别为Langmuir、Freundlich、Dubinin-Radushkevich与Redlich-Peterson模型的吸附常数;aRP为Redlich-Peterson模型的另一常数;n与g分别为Freundlich、Redlich-Peterson模型相关系数。
-
使用Gaussian 16第C.01版[24]进行密度泛函理论(DFT)计算。染料、生物炭及其复合物的结构均采用B3LYP交换关联泛函[25-26]与DFT-D3(BJ)色散矫正[27]进行优化(简写为B3LYP-D3(BJ)),选用的基组为def2-SVP[28-29]。在相同等级基组进行了频率计算,以确保得到的结构处于最稳定点。上述优化结构的结合能由B3LYP-D3(BJ)的ma-TZVPP基组[29-30]计算单点能得到。吸附复合物的电荷密度分布使用Multiwfn计算[31]。生物炭结构复杂,这里使用的结构模型(图1(c))对局部区域的性质反应较为准确[32]。
-
图2为各生物炭的SEM表征结果。纯生物炭的表面粗糙,具有一定的孔隙结构,表明生物质已经被炭化分解;而单纯添加氮源的生物炭N-BC的孔隙出现了堵塞。K-BC与KN-BC相比BC产生了更丰富的多孔结构,碎片化结构进一步破碎,同时KN-BC比K-BC具有更完整的孔隙堆叠结构。
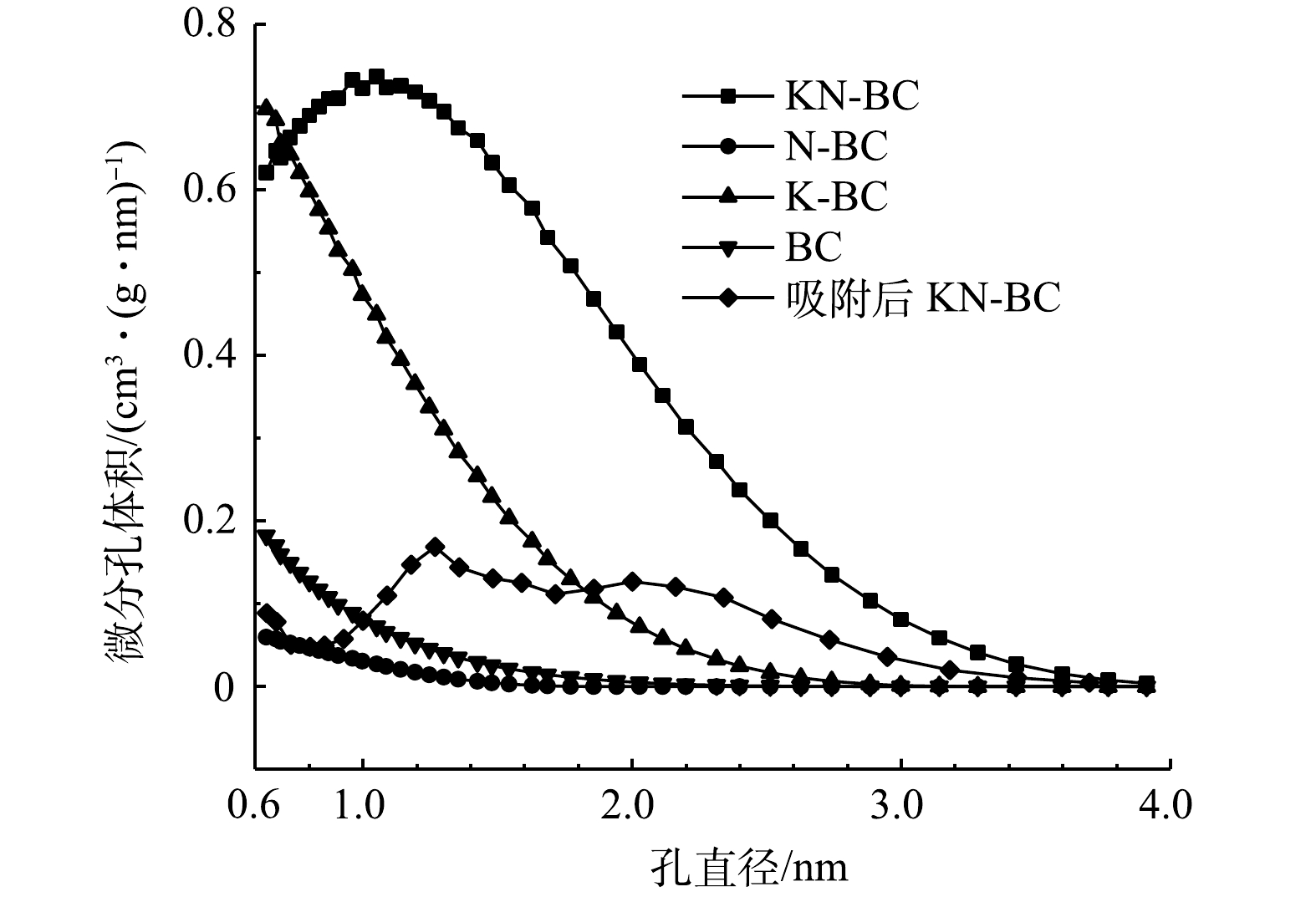
各生物炭的比表面积、孔径与孔径分布情况如图3和表1所示。未改性的BC具有较小的平均孔径(1.74 mm),主要以微孔为主;而N-BC比表面积最小。但当同时添加尿素与KHCO3后,KN-BC表现出最佳的比表面积(2 387.79 m2·g−1),以介孔为主。这是因为尿素与碳酸氢钾在高温下可以协同形成2D网状结构,随后分解能留下更多的层状孔道[33],最终有效的提高了生物炭的孔隙率及其比表面积。
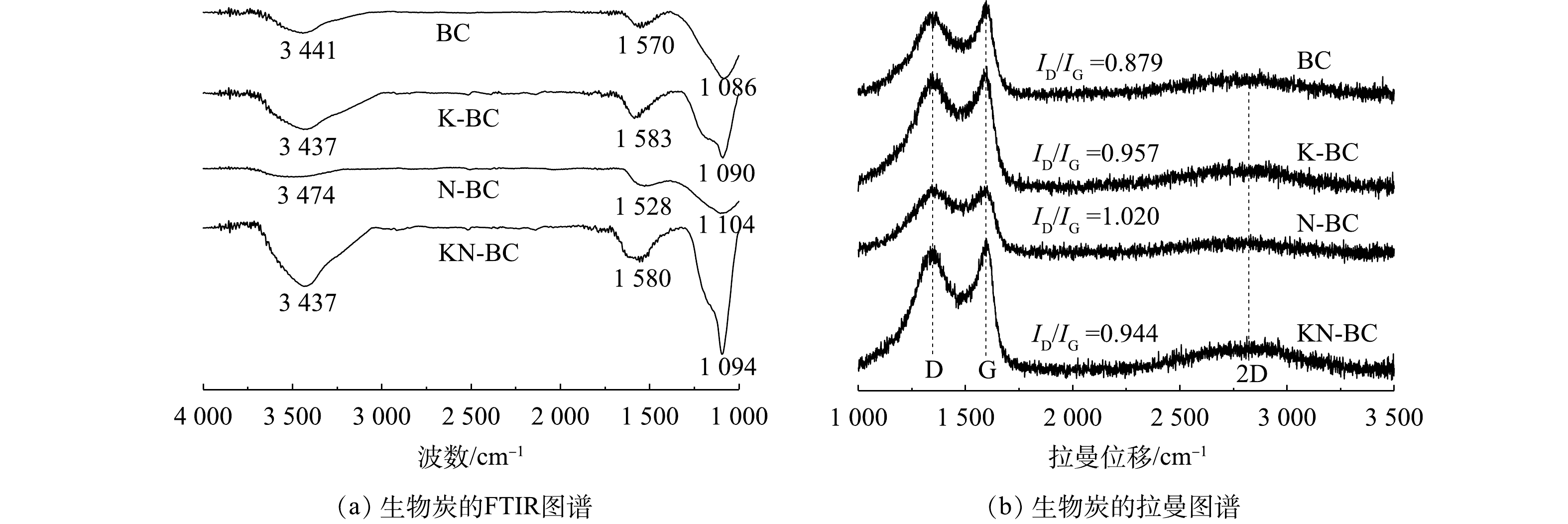
图4(a)为各生物炭的FTIR图谱。BC与KN-BC具有相似的特征峰,但BC的峰强度都相对较弱。KN-BC中主要的峰包括3 437 cm−1处的—OH伸缩振动峰[34]、1 580 cm−1处的苯环中C=C伸缩振动峰[35]和1 094 cm−1处的C—O伸缩振动峰[36]。N-BC在3 474 cm−1处的峰的偏移表明,其对应的除—OH伸缩振动峰外,还有N—H伸缩振动峰[34];1 104 cm−1处峰较宽,包括了C—O与C—N等官能团[37]。在KN-BC中较难确定含氮官能团的存在,进一步表明尿素主要与碳酸氢钾作用,引入到生物炭表面的氮含量较少。
如图4(b)所示,生物炭的拉曼光谱出现了3个主要的特征峰,分别位于1 360、1 590和2 760 cm−1,代表了D峰、G峰与2D峰。D峰和G峰分别反映了生物炭中存在的缺陷结构,以及有序的石墨结构[38-39];2D峰则表明有多层堆叠碳的存在[40]。D峰和G峰的比值(ID/IG)可以用于评估生物炭的缺陷和无序程度,KN-BC的ID/IG值达到了0.944,相比BC的0.879有提升,证明生物炭结构缺陷增加。显著的G峰表明KN-BC中也具有石墨结构。
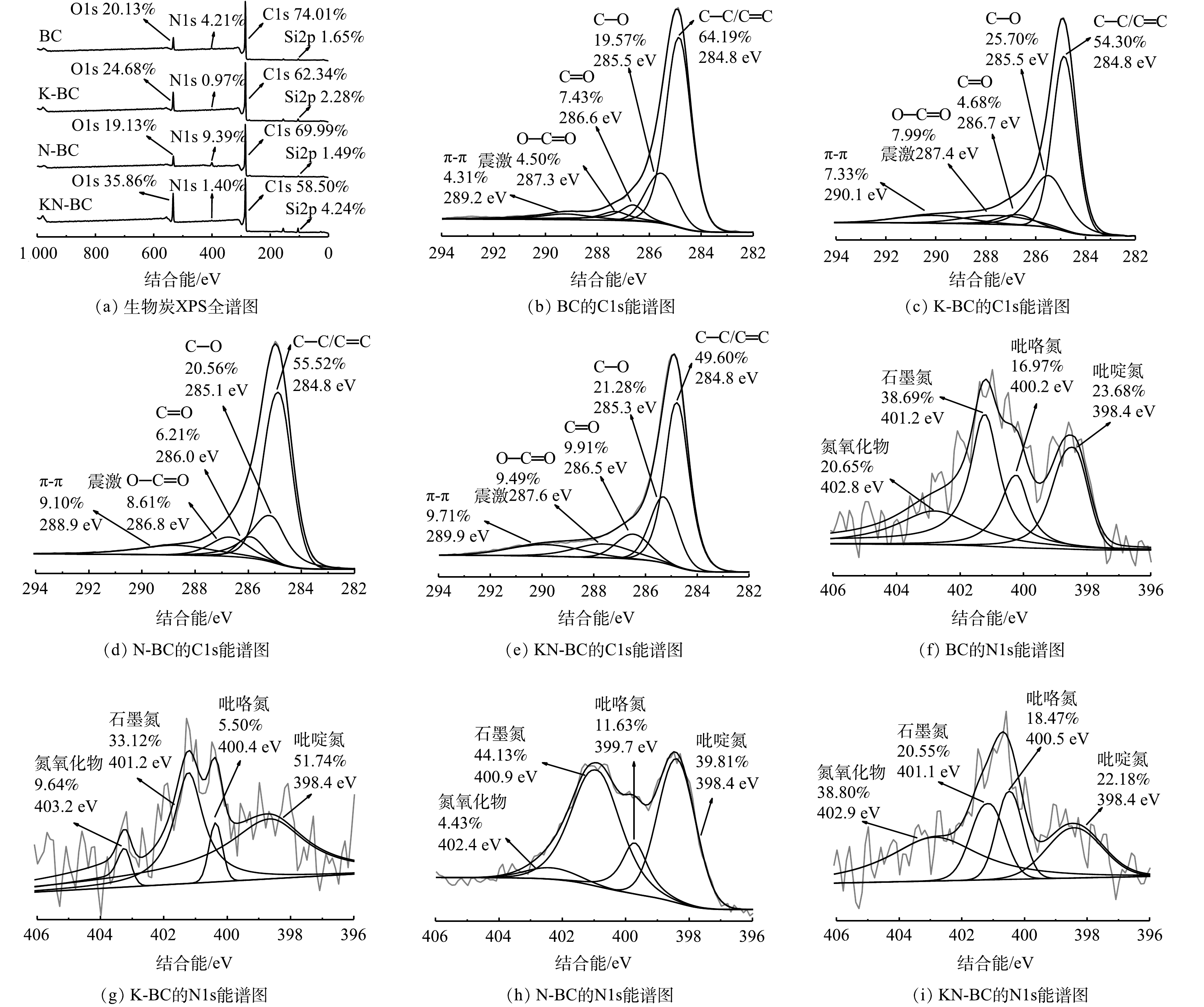
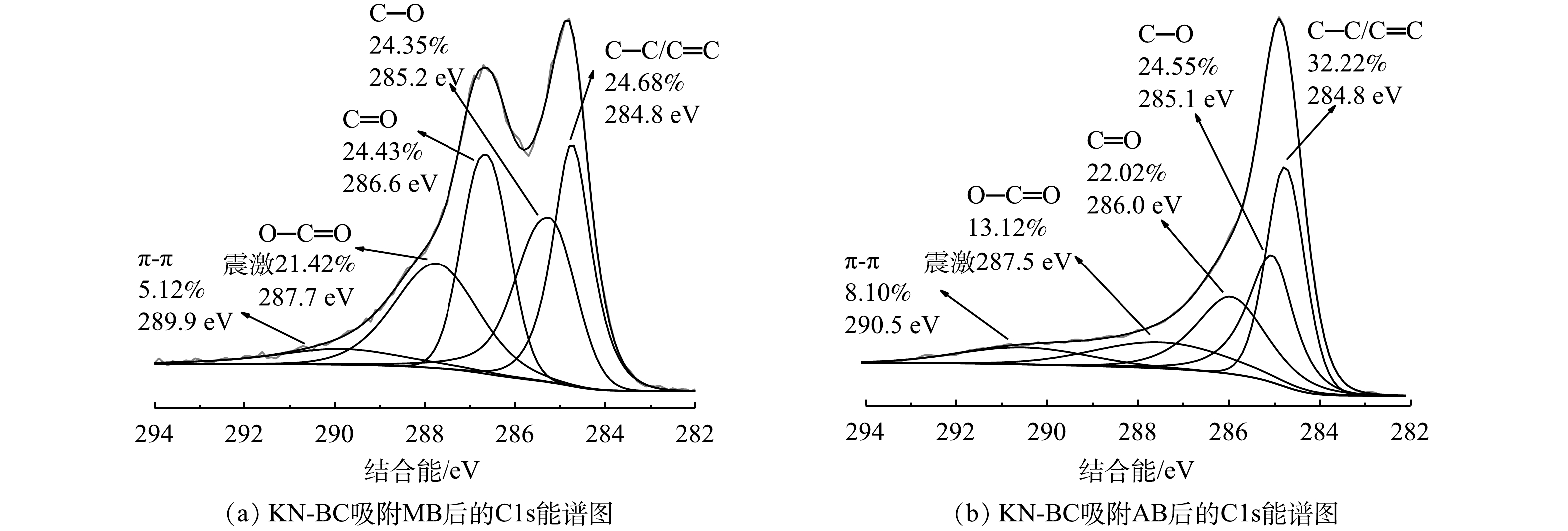
采用XPS对材料表面官能团情况进行表征,结果如图5所示。由图5(a)可以看出,KN-BC表面的N相对含量比BC有减少。表明外源的尿素主要与碳酸氢钾协同作用形成孔径,这与FTIR图谱的结果相符。对KN-BC的C1s峰分峰拟合,主要峰分别位于284.8、285.3、286.5、287.6和289.9 eV,对应C—C/C=C、C—O、C=O、O—C=O和π-π震激[41-42]。N1s中各峰均较弱,主要分为398.4、400.2、401.2和402.8 eV 4个峰,代表吡啶氮、吡咯氮、石墨氮和氮氧化物[43]。在C1s中,KN-BC中总含氧官能团相对含量均相比其他生物炭有所增加,这说明KN-BC表面具有更多含氧基团,可作为活性氢键位点[44],这些位点容易与污染物上的氢键供体结合从而提高吸附能力。
-
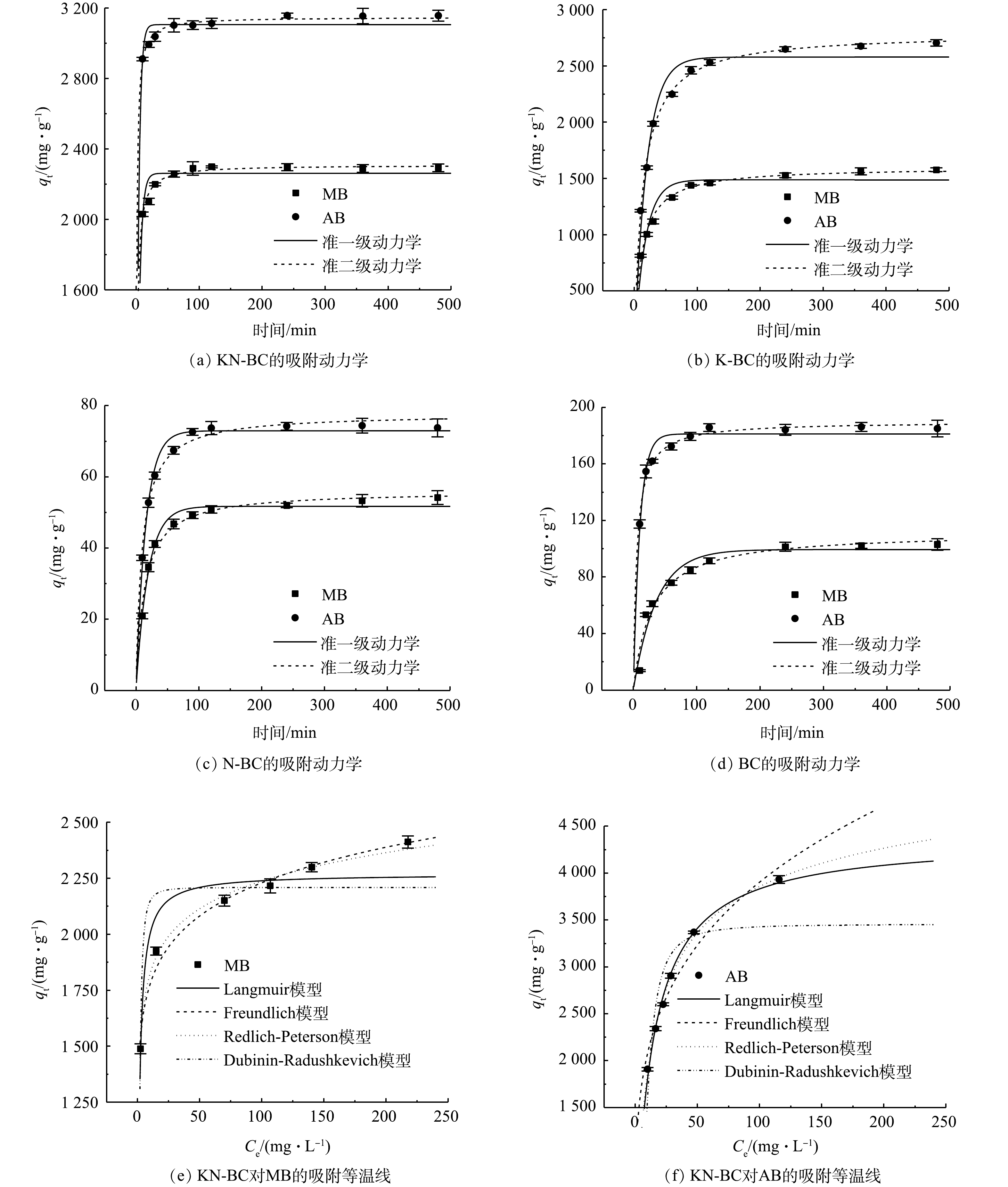
各生物炭对于2种染料MB与AB的吸附动力学如图6(a)~(d)所示。KN-BC表现出了最佳的吸附性能,并且吸附速率更快。这是因为KN-BC相比其他生物炭同时具有更丰富的微孔和介孔存在(图3),而多层次的孔径有利于快速吸附的发生[45]。KN-BC在吸附的前30 min,吸附量快速增加,随后吸附速度显著放缓,并于吸附240 min后趋于稳定。这主要是因为在吸附的初始阶段,材料表面具有充足的吸附位点,有利于快速吸附染料。
准一级与准二级动力学模型的拟合参数见于表2。与准一级动力学模型相比,准二级动力学模型对MB与AB吸附的相关系数R2均更高,准二级动力学能更好的描述这一吸附过程。这表明,各生物炭对MB和AB主要的吸附过程是化学吸附,对染料的吸附首先在表面进行,当表面的吸附位点被占据后,再进行内部扩散[46]。
-
KN-BC对MB和AB的吸附等温线及其拟合参数如图6(e)和图6(f)和表3所示。Redlich-Peterson模型对MB和AB均具有最佳的R2(0.994与0.996),能较好地拟合吸附等温线。Redlich-Peterson模型是基于Langmuir与Freundlich模型的混合情况所提出的模型,其中,g值反映了等温线与Langmuir或Freundlich模型的相近情况,而KN-BC对MB和AB吸附所拟合得到的g值均接近1,表明等温线曲线更多表现出Langmuir模型的特征[47]。KN-BC对于2种染料的吸附机理较为复杂,以均一的单分子层吸附为主。KN-BC对MB与AB的Langmuir模型的最大吸附量Qmax达到了2 268.7 mg·g−1和4 368.5 mg·g−1,具有极强的吸附能力。但是,2种染料的吸附容量差距很大。
-
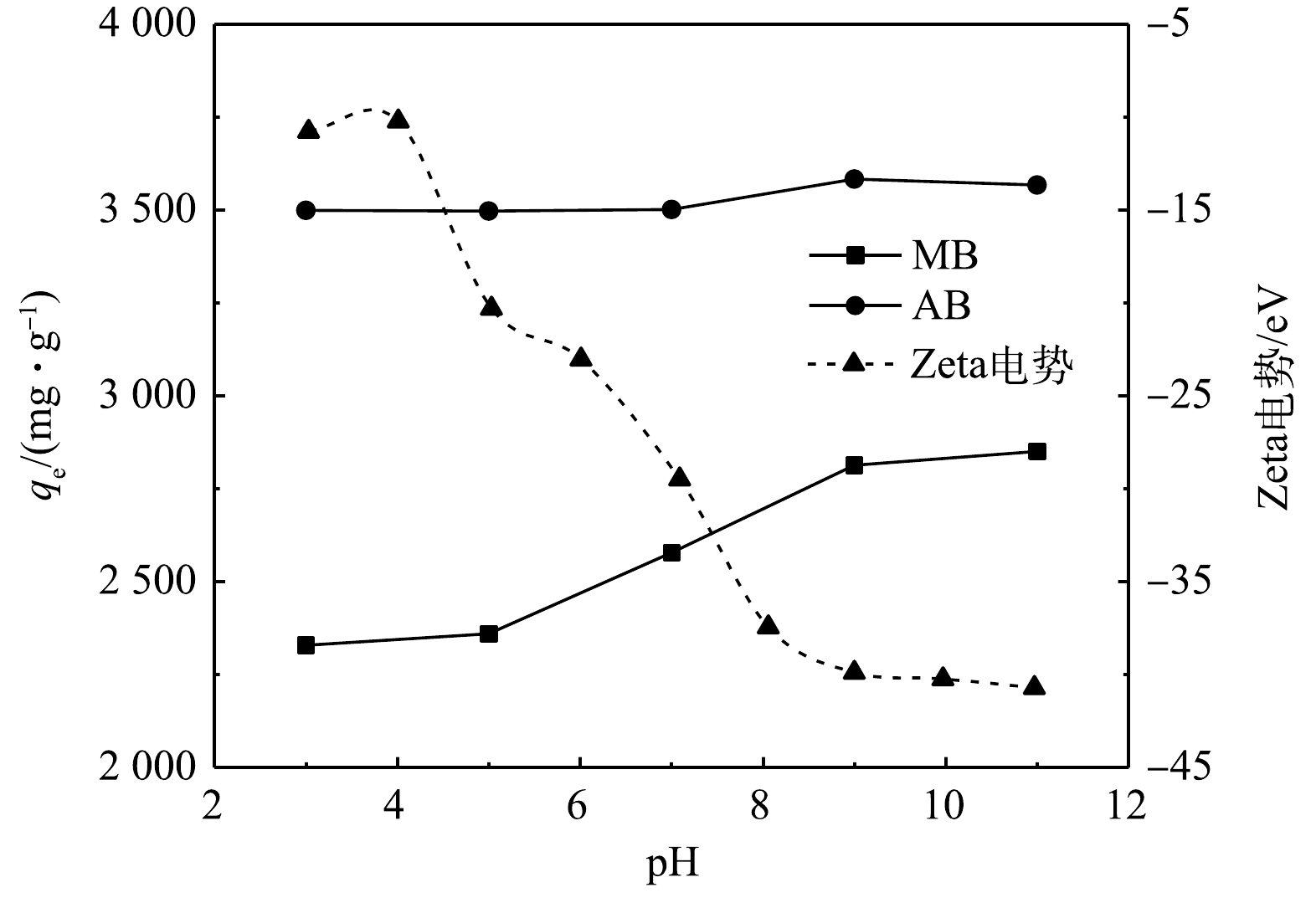
不同的初始pH对吸附影响与KN-BC的Zeta电位列于图7。在实验设定的pH范围内,KN-BC的Zeta电位均小于0,表明生物炭在实验条件下表面均带负电荷,且随pH增大表面负电荷量也有增加。MB与AB均为阳离子染料,因此生物炭表面负电荷的增加可以提升对染料的静电吸引,使得碱性条件下对染料的吸附量稍高于酸性条件下。不过,Zeta电位的快速下降与AB吸附容量轻微提升不匹配,这意味着在设定pH范围内静电引力不是导致吸附的唯一原因[48],KN-BC与AB的吸附受到了其它非静电力相互作用的显著影响[49]。AB相比MB具有氢键供体—NH—,因此单个AB分子可以与KN-BC同时形成氢键作用与π-π相互作用,两者的协同效应对吸附过程的影响更大,使得AB在不同pH下吸附较稳定。
-
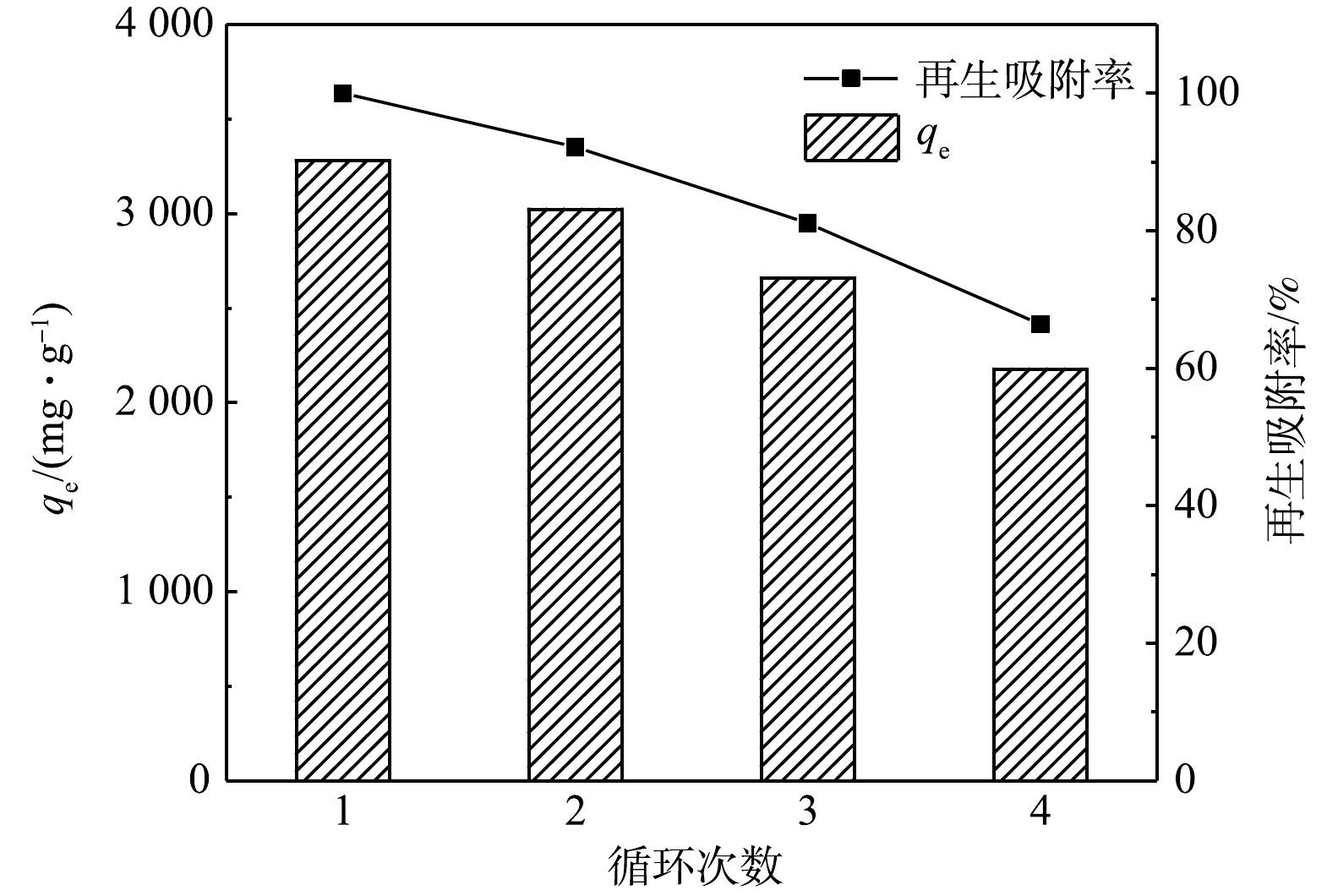
吸附剂再生性能是评价吸附剂用于环境修复潜力的重要指标。KN-BC的再生性能结果如图8所示。在前3轮循环中,KN-BC再生吸附率均维持在80%以上,而第4次实验后仍高于65%。此结果表明,KN-BC有一定的循环使用能力,且吸附容量大,具有应用前景。KN-BC对染料较强的吸附能力使得解吸不完全,导致再生吸附率有所下降。
-
生物炭吸附芳香性有机污染物的主要机理是孔径填充、静电作用、π-π相互作用和氢键作用[50],具体的主导机理受生物炭理化性质以及污染物的影响。如表1所示,KN-BC具有最高的比表面积,吸附后KN-BC的比表面积显著下降,表明污染物扩散进入到了孔隙内。这一结果证明孔径填充作用为KN-BC吸附MB和AB的主要机理之一。此外,在本研究的pH范围内,KN-BC表面均带负电荷(图7),而MB与AB均为阳离子染料,因此,静电作用主要表现为静电引力,有利于吸附。吸附后XPS的C1s能谱如图9所示。对比吸附前的结果(图5(e)),吸附MB或AB后C—C/C=C峰的强度出现显著下降,证明吸附过程中有π-π相互作用的存在[51];同时,C—O峰的峰位相比吸附前的285.3 eV均有偏移,表明吸附过程中在C—O键上有氢键形成[52]。以上结果表明,π-π相互作用和氢键作用也是KN-BC对MB与AB的吸附机理之一。
通过KN-BC对MB与AB在相同初始pH下的吸附效果对比可知,对AB的吸附容量(4 368.5 mg·g−1)明显高于对MB的吸附容量(2 268.7 mg·g−1)。上述的机理阐述不能完全说明这一吸附容量的差别。MB与AB结构上极为相似,电荷数相同,分子大小相近(图1),区别仅在于AB具有亚氨基作为氢键供体,而MB没有氢键供体。这表明污染物氢键供体的有无明显影响了KN-BC对污染物的吸附能力。
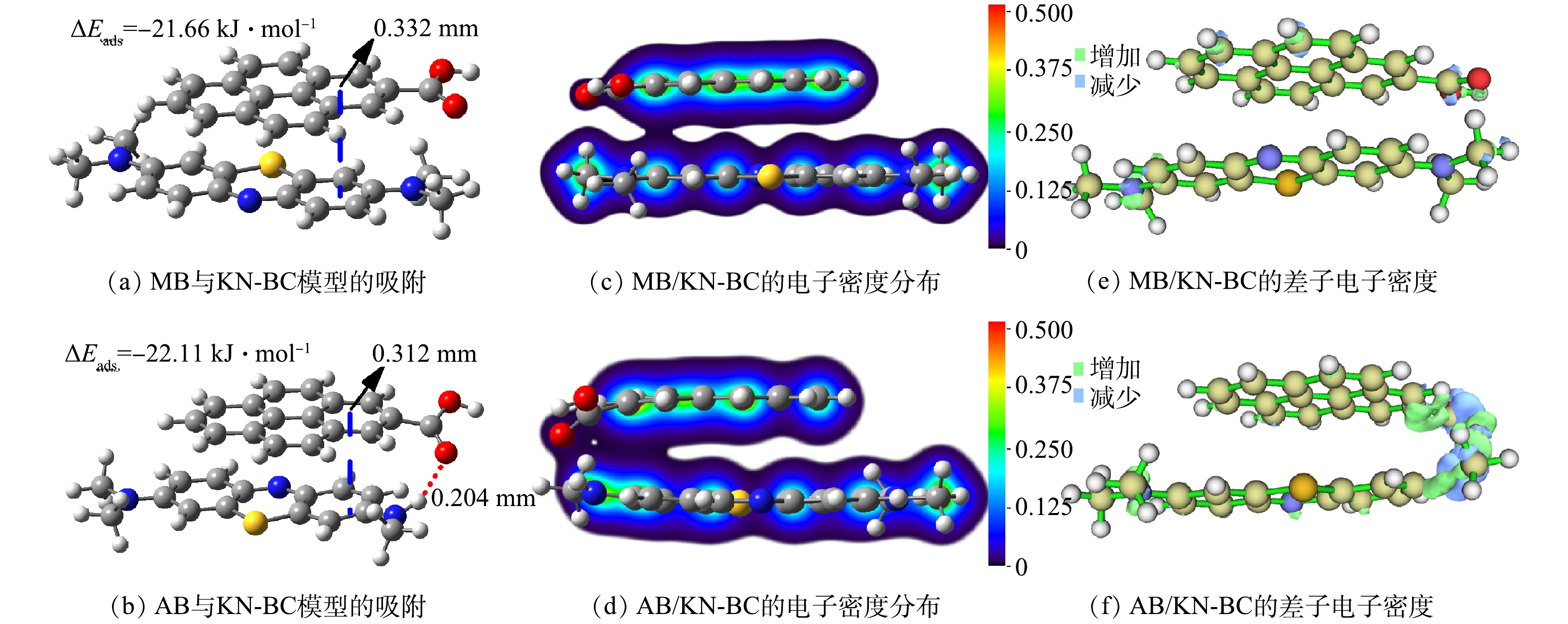
污染物氢键供体对吸附的影响机理可以通过DFT计算进行探究,计算得出的最优化构型与吸附能见图10(a)与图10(b)。由于AB具有氢键供体—NH—,因此,AB与KN-BC可以同时形成氢键作用与π-π相互作用;而MB缺乏氢键供体,因此,在最优化构型中只能单独形成π-π相互作用,导致吸附能绝对值下降。图10(c)与图10(d)反映了复合物的电子密度分布情况。AB/KN-BC复合物的π电子云因氢键作用与π-π相互作用共存而偏移,重叠相比MB/KN-BC复合物增大,增强了π-π相互作用。差分电子密度图(图10(e)与图10(f))也表明氢键作用与π-π相互作用共存增强了π电子密度。两者的协同效应还缩短了污染物与生物炭的苯环间的距离(图10(a)与图10(b))。以上结果表明,氢键作用与π-π相互作用产生协同效应可以明显提高吸附效能,而污染物上具有氢键供体有利于形成这一协同效应。
综上所述,KN-BC对染料的吸附机理主要包括孔径填充、π-π相互作用、氢键作用和静电作用;π-π相互作用和氢键作用的协同效应能极大地增强吸附效能,而具有氢键供体的污染物更易从这一协同效应中受益,因此,KN-BC对有氢键供体的染料吸附容量显著上升。
-
1)使用KHCO3和尿素联合活化制备的玉米秸秆生物炭KN-BC对染料吸附能力较强, Langmuir模型拟合的对MB和AB最大吸附容量达4 368.5 mg·g−1和2 268.7 mg·g−1,在较大的pH范围内能维持吸附能力,再生循环吸附能力良好。
2) KN-BC对于水体中的MB和AB的吸附机理主要包括孔径填充、π-π相互作用、氢键作用和静电作用。
3) AB具有氢键供体,吸附于KN-BC上时可以同时形成氢键作用和π-π相互作用,因此,两者可以产生协同效应,增强π电子密度并缩短AB与KN-BC苯环间的距离,从而增强吸附结合能,明显提高KN-BC对AB的吸附能力。KN-BC对具有氢键供体的污染物的吸附容量有明显提升。
生物炭对含氢键供体染料的吸附性能及机理
Adsorption performance and mechanism of biochar to dyes with H-donor
-
摘要: 为确定染料有无氢键供体对生物炭吸附容量的影响及作用机理,制备了尿素/碳酸氢钾联合活化的玉米秸秆生物炭(KN-BC),考察其对于结构相似的亚甲基蓝(MB)与天青B(AB)的吸附容量差别及具体机制。对KN-BC的表征结果表明,经处理后的生物炭疏松多孔,表面含氧官能团含量显著增加。吸附实验结果表明,Langmuir模型拟合的KN-BC对MB和AB的最大吸附量为2 268.7 mg·g−1和4 368.5 mg·g−1,KN-BC对含有氢键供体的AB吸附性能更好。DFT计算与机理分析结果表明,氢键供体的存在使得单个污染物分子与生物炭可以同时形成氢键和π-π相互作用,两者的协同效应增强了π电子密度,显著提高了吸附效能。以上研究结果为预测生物炭对混合染料污水的吸附提供参考。Abstract: To determine whether the H-donors on dyes affect the adsorption capacity of biochar, the corn straw biochar was prepared by the combined activation of potassium bicarbonate and urea (named KN-BC), and KN-BC was used to adsorb the similar structure dyes of methylene blue (MB) and azure B (AB) to investigate the difference in adsorption capacities and underlying mechanisms. The characterizations revealed that KN-BC was highly porous, and the content of oxygen-containing functional groups increased significantly after activation. The batch experiments indicated that the maximum adsorption capacities to MB and AB in the Langmuir model reached 2 268.7 mg·g−1 and 4 368.5 mg·g−1, respectively. And higher adsorption capacity of KN-BC to AB with the H-donors occurred. DFT computation and analysis implied that the H-donor on dyes allows the co-existence of hydrogen bonding and π-π interaction between single dye molecular and KN-BC. The synergistic effect increased the π-electron density and improved the adsorption capacity. This work can provide a reference for estimating mixed dye adsorption on biochar.
-
Key words:
- biochar /
- adsorption /
- H-donor /
- hydrogen bond /
- π-π interaction /
- synergistic effect
-

-
表 1 生物炭的比表面积与孔径结构
Table 1. Specific surface area and pore structure of biochar
生物炭类型 SBET/(m2·g−1) VPore/(cm3·g−1) Vmic/(cm3·g−1) DP/nm BC 432.53 0.189 0.139 1.74 N-BC 145.11 0.065 0.055 1.80 K-BC 1 898.21 0.905 0.603 1.91 吸附前KN-BC 2 387.79 1.520 0.548 2.55 吸附后KN-BC 687.47 0.448 0.085 2.61 表 2 生物炭对MB与AB吸附的动力学模型拟合参数
Table 2. Kinetics parameters for MB and AB adsorption on biochar
吸附剂 吸附质 准一级动力学 准二级动力学 qe/(mg·g−1) k1/min−1 R2 qe/(mg·g−1) k2/(g·(mg·min)−1) R2 KN-BC MB 2 261.4 0.215 0.666 2 308.9 2.89×10-4 0.956 AB 3 105.5 0.270 0.615 3 148.3 3.56×10-4 0.958 K-BC MB 1 487.3 0.058 0.888 1 599.2 5.77×10-5 0.988 AB 2 577.9 0.050 0.946 2 792.6 2.66×10-5 0.995 N-BC MB 51.7 0.052 0.977 56.0 1.35×10-3 0.980 AB 72.9 0.064 0.976 77.7 1.34×10-3 0.981 BC MB 99.4 0.028 0.949 111.7 3.13×10-4 0.955 AB 181.2 0.097 0.938 190.0 9.54×10-4 0.978 表 3 KN-BC对MB与AB吸附等温线拟合参数
Table 3. Isotherms parameters for MB and AB adsorption on KN-BC
吸附质 Langmuir模型 Freundlich模型 Redlich-Peterson模型 Dubinin-Radushkevich模型 Qmax KL R2 KF n R2 KRP aRP g R2 qDR KDR R2 MB 2 268.7 0.737 0.891 1 411.4 10.07 0.984 5 653.6 3.59 0.92 0.994 2 209.0 5.18×10−7 0.788 AB 4 368.5 0.071 0.992 1 111.7 3.67 0.963 396.8 0.13 0.92 0.996 3 454.9 1.36×10−5 0.780 -
[1] 孙宇. 辣椒秸秆生物炭的制备、改性及对有机染料的吸附性能研究[D]. 邯郸: 河北工程大学, 2021. [2] ANWER H, MAHMOOD A, LEE J, et al. Photocatalysts for degradation of dyes in industrial effluents: Opportunities and challenges[J]. Nano Research, 2019, 12(5): 955-72. doi: 10.1007/s12274-019-2287-0 [3] YIN Z B, LIU N, BIAN S Y, et al. Enhancing the adsorption capability of areca leaf biochar for methylene blue by K2FeO4-catalyzed oxidative pyrolysis at low temperature[J]. RSC Advances, 2019, 9(72): 42343-50. doi: 10.1039/C9RA06592J [4] JIN Z H, WANG B D, MA L, et al. Air pre-oxidation induced high yield N-doped porous biochar for improving toluene adsorption[J]. Chemical Engineering Journal, 2020, 385: 123843. doi: 10.1016/j.cej.2019.123843 [5] ZHOU X H, ZHOU J J, LIU Y C, et al. Preparation of magnetic biochar derived from cyclosorus interruptus for the removal of phenolic compounds: Characterization and mechanism[J]. Separation Science and Technology, 2018, 53(9): 1307-18. doi: 10.1080/01496395.2018.1444056 [6] YU Y, AN Q, JIN L, et al. Unraveling sorption of Cr (VI) from aqueous solution by FeCl3 and ZnCl2-modified corn stalks biochar: Implicit mechanism and application[J]. Bioresource Technology, 2020, 297: 122466. doi: 10.1016/j.biortech.2019.122466 [7] OLIVEIRA F R, PATEL A K, JAISI D P, et al. Environmental application of biochar: Current status and perspectives[J]. Bioresource Technology, 2017, 246: 110-22. doi: 10.1016/j.biortech.2017.08.122 [8] ABD-ELHAMID A I, EMRAN M, EL-SADEK M H, et al. Enhanced removal of cationic dye by eco-friendly activated biochar derived from rice straw[J]. Applied Water Science, 2020, 10(1): 45. doi: 10.1007/s13201-019-1128-0 [9] 高豆豆, 郭敏辉, 王侃, 等. 城镇有机垃圾热解生物炭对水中亚甲基蓝的吸附[J]. 环境工程学报, 2019, 13(05): 1165-74. doi: 10.12030/j.cjee.201810129 [10] 张娟, 孙宇, 黄贵琦, 等. 辣椒秸秆生物炭对考马斯亮蓝染料的吸附性能研究[J]. 工业水处理, 2022, 42(02): 118-23. doi: 10.19965/j.cnki.iwt.2021-0582 [11] FANG Q L, CHEN B L, LIN Y J, et al. Aromatic and hydrophobic surfaces of wood-derived biochar enhance perchlorate adsorption via hydrogen bonding to oxygen-containing organic groups[J]. Environmental Science and Technology, 2014, 48(1): 279-88. doi: 10.1021/es403711y [12] YANG K, YANG J J, JIANG Y, et al. Correlations and adsorption mechanisms of aromatic compounds on a high heat temperature treated bamboo biochar[J]. Environmental Pollution, 2016, 210: 57-64. doi: 10.1016/j.envpol.2015.12.004 [13] SONG J Y, JHUNG S H. Adsorption of pharmaceuticals and personal care products over metal-organic frameworks functionalized with hydroxyl groups: Quantitative analyses of H-bonding in adsorption[J]. Chemical Engineering Journal, 2017, 322: 366-74. doi: 10.1016/j.cej.2017.04.036 [14] ZHAO Y F, LIN S, CHOI J W, et al. Prediction of adsorption properties for ionic and neutral pharmaceuticals and pharmaceutical intermediates on activated charcoal from aqueous solution via LFER model[J]. Chemical Engineering Journal, 2019, 362: 199-206. doi: 10.1016/j.cej.2019.01.031 [15] YU X Q, SUN W L, NI J R. LSER model for organic compounds adsorption by single-walled carbon nanotubes: Comparison with multi-walled carbon nanotubes and activated carbon[J]. Environmental Pollution, 2015, 206: 652-60. doi: 10.1016/j.envpol.2015.08.031 [16] PLATA D L, HEMINGWAY J D, GSCHWEND P M. Polyparameter linear free energy relationship for wood char-water sorption coefficients of organic sorbates[J]. Environmental Toxicology and Chemistry, 2015, 34(7): 1464-71. doi: 10.1002/etc.2951 [17] BHADRA B N, YOO D K, JHUNG S H. Carbon-derived from metal-organic framework MOF-74: A remarkable adsorbent to remove a wide range of contaminants of emerging concern from water[J]. Applied Surface Science, 2020, 504: 144348. doi: 10.1016/j.apsusc.2019.144348 [18] LAGERGREN S K. About the theory of so-called adsorption of soluble substances[J]. Kungliga Svenska Vetenskapsakademiens Handlingar, 1898, 24: 1-39. [19] BLANCHARD G, MAUNAYE M, MARTIN G. Removal of heavy metals from waters by means of natural zeolites[J]. Water Research, 1984, 18(12): 1501-7. doi: 10.1016/0043-1354(84)90124-6 [20] LANGMUIR I. The adsorption of gases on plane surfaces of glass, mica and platinum[J]. Journal of the American Chemical Society, 1918, 40(9): 1361-403. doi: 10.1021/ja02242a004 [21] FREUNDLICH H. Über die adsorption in lösungen[J]. Zeitschrift für Physikalische Chemie, 1907, 57(1): 385-470. [22] DUBININ M I. Physical adsorption of gases and vapors in micropores[J]. Amsterdam:Elsevier, 1975: 1-70. [23] REDLICH O, PETERSON D L. A useful adsorption isotherm[J]. The Journal of Physical Chemistry, 1959, 63(6): 1024. doi: 10.1021/j150576a611 [24] FRISCH M J, TRUCKS G W, SCHLEGEL H B, et al. Gaussian 16, Revision C. 01[CP/DK]. Wallingford CT: Gaussian Inc. , 2019. [25] LEE C, YANG W, PARR R G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density[J]. Physical Review B, 1988, 37(2): 785-9. doi: 10.1103/PhysRevB.37.785 [26] BECKE A D. Density-functional thermochemistry. III. The role of exact exchange[J]. The Journal of Physical Chemistry, 1993, 98(7): 5648-52. doi: 10.1063/1.464913 [27] GRIMME S, EHRLICH S, GOERIGK L. Effect of the damping function in dispersion corrected density functional theory[J]. Journal of Computational Chemistry, 2011, 32(7): 1456-65. doi: 10.1002/jcc.21759 [28] TREUTLER O, AHLRICHS R. Efficient molecular numerical integration schemes[J]. Journal of Chemical Physics, 1995, 102(1): 346-54. doi: 10.1063/1.469408 [29] WEIGEND F, AHLRICHS R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy[J]. Physical Chemistry Chemical Physics, 2005, 7(18): 3297-305. doi: 10.1039/b508541a [30] ZHENG J J, XU X F, TRUHLAR D G. Minimally augmented Karlsruhe basis sets[J]. Theoretical Chemistry Accounts, 2011, 128(3): 295-305. doi: 10.1007/s00214-010-0846-z [31] LU T, CHEN F W. Multiwfn: A multifunctional wavefunction analyzer[J]. Journal of Computational Chemistry, 2012, 33(5): 580-92. doi: 10.1002/jcc.22885 [32] WANG H Z, GUO W Q, LIU B H, et al. Edge-nitrogenated biochar for efficient peroxydisulfate activation: An electron transfer mechanism[J]. Water Research, 2019, 160: 405-14. doi: 10.1016/j.watres.2019.05.059 [33] TIAN W J, ZHANG H Y, SUN H Q, et al. Heteroatom (N or N-S)-doping induced layered and honeycomb microstructures of porous carbons for CO2 capture and energy applications[J]. Advanced Functional Materials, 2016, 26(47): 8651-61. doi: 10.1002/adfm.201603937 [34] XIAO X, CHEN B L, ZHU L Z. Transformation, morphology, and dissolution of silicon and carbon in rice straw-derived biochars under different pyrolytic temperatures[J]. Environmental Science and Technology, 2014, 48(6): 3411-9. doi: 10.1021/es405676h [35] LENG L J, XU S Y, LIU R F, et al. Nitrogen containing functional groups of biochar: An overview[J]. Bioresource Technology, 2020, 298: 122286. doi: 10.1016/j.biortech.2019.122286 [36] CHEN B L, ZHOU D D, ZHU L Z. Transitional adsorption and partition of nonpolar and polar aromatic contaminants by biochars of pine needles with different pyrolytic temperatures[J]. Environmental Science and Technology, 2008, 42(14): 5137-43. doi: 10.1021/es8002684 [37] SHAFEEYAN M S, DAUD W W, HOUSHMAND A, et al. Ammonia modification of activated carbon to enhance carbon dioxide adsorption: Effect of pre-oxidation[J]. Applied Surface Science, 2011, 257(9): 3936-42. doi: 10.1016/j.apsusc.2010.11.127 [38] RAMESHA G K, VIJAYA K A, MURALIDHARA H B, et al. Graphene and graphene oxide as effective adsorbents toward anionic and cationic dyes[J]. Journal of Colloid and Interface Science, 2011, 361(1): 270-7. doi: 10.1016/j.jcis.2011.05.050 [39] VOGGU R, ROUT C S, FRANKLIN A D, et al. Extraordinary sensitivity of the electronic structure and properties of single-walled carbon nanotubes to molecular charge-transfer[J]. Journal of Physical Chemistry C, 2008, 112(34): 13053-6. doi: 10.1021/jp805136e [40] KIM S J, SONG Y J, WRIGHT J, et al. Graphene bi- and trilayers produced by a novel aqueous arc discharge process[J]. Carbon, 2016, 102: 339-45. doi: 10.1016/j.carbon.2016.02.049 [41] PEREIRA R C, ARBESTAIN M C, SUEIRO M V, et al. Assessment of the surface chemistry of wood-derived biochars using wet chemistry, Fourier transform infrared spectroscopy and X-ray photoelectron spectroscopy[J]. Soil Research, 2015, 53(7): 753-62. doi: 10.1071/SR14194 [42] TIAN S Q, WANG L, LIU Y L, et al. Enhanced permanganate oxidation of sulfamethoxazole and removal of dissolved organics with biochar: Formation of highly oxidative manganese intermediate species and in situ activation of biochar[J]. Environmental Science and Technology, 2019, 53(9): 5282-91. doi: 10.1021/acs.est.9b00180 [43] LI Y C, XING B, WANG X L, et al. Nitrogen-doped hierarchical porous biochar derived from corn stalks for phenol-enhanced adsorption[J]. Energy & Fuels, 2019, 33(12): 12459-68. [44] GAO F, XU Z X, DAI Y J. Removal of tetracycline from wastewater using magnetic biochar: A comparative study of performance based on the preparation method[J]. Environmental Technology & Innovation, 2021, 24: 101916. [45] YU J F, FENG H P, TANG L, et al. Insight into the key factors in fast adsorption of organic pollutants by hierarchical porous biochar[J]. Journal of Hazardous Materials, 2021, 403: 123610. doi: 10.1016/j.jhazmat.2020.123610 [46] WANG H L, TANG H Q, LIU Z T, et al. Removal of cobalt(II) ion from aqueous solution by chitosan–montmorillonite[J]. Journal of Environmental Sciences, 2014, 26(9): 1879-84. doi: 10.1016/j.jes.2014.06.021 [47] TONG D S, WU C W, ADEBAJO M O, et al. Adsorption of methylene blue from aqueous solution onto porous cellulose-derived carbon/montmorillonite nanocomposites[J]. Applied Clay Science, 2018, 161: 256-64. doi: 10.1016/j.clay.2018.02.017 [48] ZHOU N, GUO X X, YE C Q, et al. Enhanced fluoride removal from drinking water in wide pH range using La/Fe/Al oxides loaded rice straw biochar[J]. Water Supply, 2021, 22(1): 779-94. [49] LIU S J, PAN M D, FENG Z M, et al. Ultra-high adsorption of tetracycline antibiotics on garlic skin-derived porous biomass carbon with high surface area[J]. New Journal of Chemistry, 2020, 44(3): 1097-106. doi: 10.1039/C9NJ05396D [50] CHENG N, WANG B, WU P, et al. Adsorption of emerging contaminants from water and wastewater by modified biochar: A review[J]. Environmental Pollution, 2021, 273: 116448. doi: 10.1016/j.envpol.2021.116448 [51] WU J, YANG J W, FENG P, et al. High-efficiency removal of dyes from wastewater by fully recycling litchi peel biochar[J]. Chemosphere, 2020, 246: 125734. doi: 10.1016/j.chemosphere.2019.125734 [52] XU C H, NIE J D, WU W C, et al. Self-healable, recyclable, and strengthened epoxidized natural rubber/carboxymethyl chitosan biobased composites with hydrogen bonding supramolecular hybrid networks[J]. ACS Sustainable Chemistry and Engineering, 2019, 7(18): 15778-89. doi: 10.1021/acssuschemeng.9b04324 -




 下载:
下载: