-
原油劣质化、重质化加剧,导致在原油开采与石油炼制过程中,含油废水排放量大、乳化程度高、处理难度大[1-2]。电脱盐废水是最为典型的含油乳化废水,是炼厂需要控制的关键特征污染物质(油与脂、酚类、硫化物、氨氮和总悬浮颗粒物(TSS))的主要来源。通常,在废水中浮油含量较高时,需要根据油水比重差对含油废水进行一级除油处理,如重力分离、气浮等[3]。一级处理后,废水中仍存在大量的乳化油,为高效破乳除油,需要对这类水包油乳化液进行二级处理。现有污水处理工艺难以解决含油废水破乳问题,时常造成污水处理系统出水超标[4]。高含油量、强乳化的电脱盐废水已成为炼厂最难解决的污染问题之一,如何强化电脱盐废水预处理是石油炼制行业面临的巨大挑战。
近年来,人们越来越重视电化学污水处理技术,包括电氧化、电絮凝、电气浮等,所使用的电极涉及不锈钢、铝、铁、铂等常用金属材料[5-8]。部分电极材料处理工业废水的实验对比结果[5,9-13]见表1。铁、铝电极常用于电絮凝工艺。当废水组成复杂且含盐量较高时,常规物理化学技术难以发挥正常作用,此时电絮凝处理技术则能突显优势[8],可通过化学反应、共沉淀或者胶体物质的物理化学附着等作用去除主要污染物[9]。其中,极板溶解出的金属离子可形成多种带电的羟基化形态,吸附于Al(OH)3(s)或Fe(OH)3(s)等表面,发挥活性混凝剂作用[5,9]。阴极产生的氢气起到气浮作用,可强化污染物分离[7,9]。与化学混凝机理相似,在电絮凝过程中,废水体系连续发生着污染物脱稳、颗粒物悬浮以及乳液破乳等反应[10]。
电絮凝处理工艺的运行成本主要包括电极材料、电耗、人工、设备维护、污泥处理等费用,其中电耗和电极材料损耗成本最高[14]。在对电镀废水进行电絮凝处理过程中,降低pH可增加阳极消耗,提高pH则导致能耗升高[15]。利用电絮凝处理机械工业废水时,若达到95%的COD去除率,铁电极与铝电极的成本分别为2.54 欧元·m−3和7.16 欧元·m−3,前者仅为后者的35%[5]。在电絮凝过程中,阳极氧化形成的金属阳离子与OH−发生反应,生成金属氢氧化物胶体,这类物质的胶体性状使氢氧化物吸附于电极表面,形成一层氧化膜,从而降低传质效率而产生电极钝化影响[14]。当反应温度较高时,电极表面的氧化膜被破坏,钝化减弱,反应活性提高,电流效率随之升高[16]。由于电脱盐废水温度通常高于60 ℃,在采用电化学处理时,能够有效减缓极板钝化。此外,利用脉冲电场也可有效控制极板钝化与极化,提高处理效率并降低运行成本[6]。
为解决石油石化含油废液破乳难的问题,本研究以电脱盐废水为研究对象,利用脉冲电场,考察电絮凝技术对电脱盐废水的处理效果,分析电絮凝的破乳分离机制,明确最佳处理条件,旨在为电化学技术高效处理石油石化含油废液提供参考。
-
本实验水样取自某炼厂电脱盐反冲洗废水,pH为7~8,电导率为700~950 μS·cm−1,化学需氧量(COD)为4 500~10 000 mg·L−1,溶解性有机碳(DOC)为300~500 mg·L−1,总油为3 000~5 000 mg·L−1。
实验所用试剂包括正己烷、二氯甲烷等,均为色谱纯;盐酸、无水硫酸钠、无水乙醇、四氯乙烯等,均为分析纯。所用电极材料为铝板和铁板,购自实验反应器加工厂。
-
1) 基本水质分析。采用水质多参数分析仪(美国哈希公司)测定pH值和电导率;采用化学需氧量仪(DR/2400型,美国哈希公司)测定COD;采用总有机碳分析仪(multi N/C 2100 S,德国耶拿公司) 测定DOC;根据国家环境保护标准(HJ-637-2018),采用红外测油仪(Oil480R,北京华夏科创仪器公司)测定总油;采用ICP-OES仪(OPTIMA 7000DV,美国PE公司)测定Ca2+、Mg2+;采用离子色谱仪(ICS-2100,美国戴安公司)测定Cl−质量浓度。
2) 显微镜分析。采用显微镜(BX53M,日本奥林巴斯公司)观察电脱盐废水处理前后的基本性状。分别取少量样品置于载玻片上,在显微镜下观察不同放大倍数时的形态。
3) GC-MS分析。根据《岩石中可溶有机物及原油族组分分析标准》(SYT 5119-2008)对电脱盐废水进行饱和分分离,采用Trace1310气相色谱与TSQ 8000 Evo 三重四级杆质谱仪联用(Thermo,美国)分析饱和分。色谱条件:载气为99.999%的氮气,流速为1 mL·min−1(恒流条件),分流比为20∶1;色谱柱为HP-5MS弹性石英毛细柱(30 m×0.25 mm×0.25 μm),初始柱温为80 ℃,维持3 min后,以6 ℃·min−1的速度升温至280 ℃并维持5 min;进样量为0.2 μL。质谱条件:EI 离子源电压为70 eV,质荷比m/z为35~420。
4) 光谱分析。样品经0.45 μm醋酸纤维膜过滤后,采用紫外可见吸收-三维荧光光谱仪(Aqualog,日本HORIBA公司)扫描紫外可见光谱(UV-vis)和三维荧光光谱(3D-EEMs)。使用1 cm×1 cm四面透光石英比色皿,以超纯水为空白,对水样进行连续扫描。激发波长(Ex)为200~500 nm,发射波长(Em)为200~600 nm,扫描带宽均为5 nm。
采集紫外可见光谱数据并进行分析,计算出250 nm和365 nm处吸光度的比值(E250/E365),可用来反映有机物的芳香度和分子质量。同时,计算出254 nm波长下的吸收系数与DOC质量浓度的比值(SUVA254),可用来表示DOM的芳香度。
采集三维荧光光谱数据,去除拉曼散射和瑞利散射后,获得三维矩阵数列数据与3D谱图,以此分析特征荧光峰和荧光强度。
-
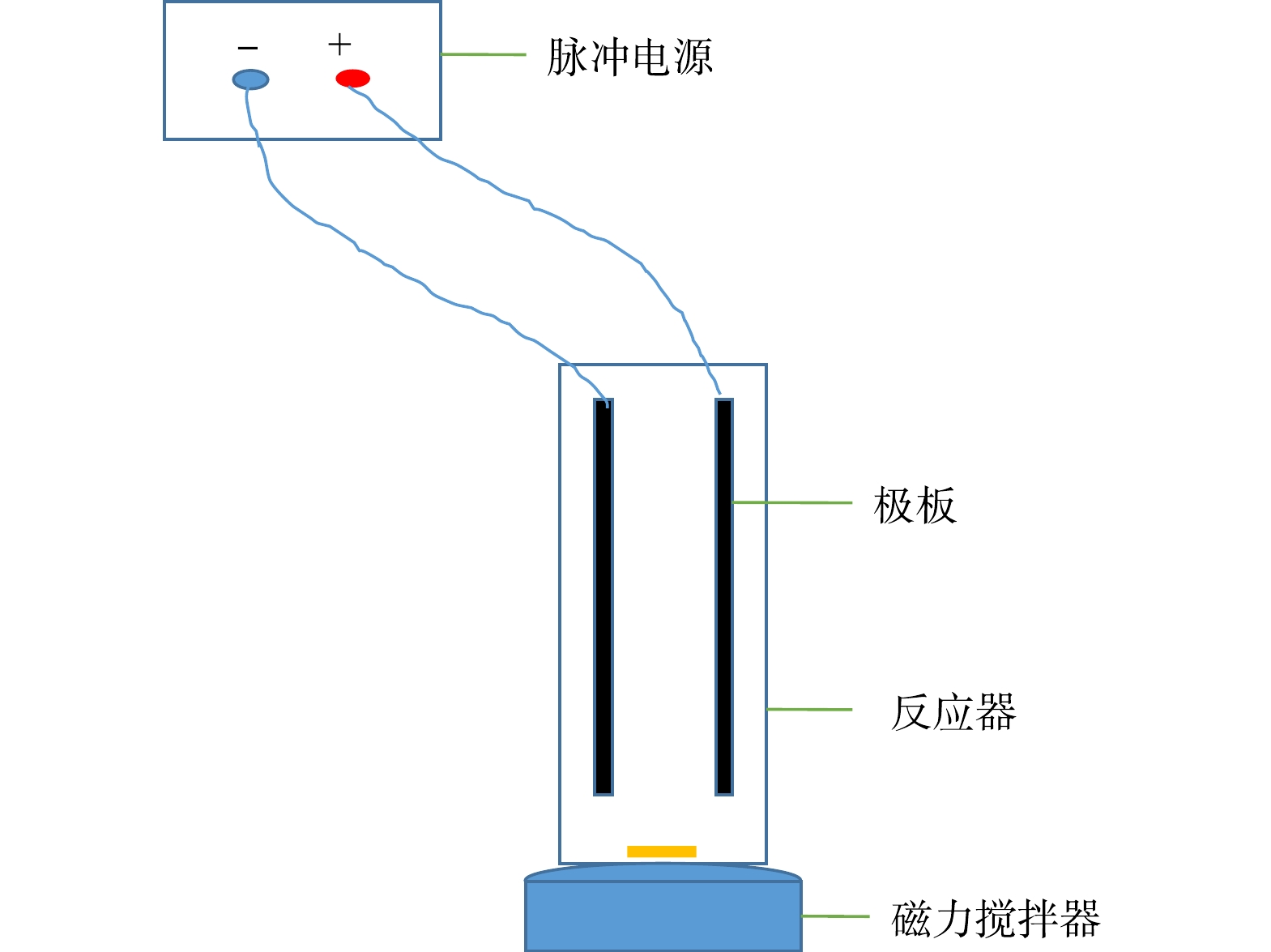
本研究选用板式电化学反应器,电极材料分别为Fe和Al,阳极与阴极均为同种电极材料。极板面积为10.5 cm×14 cm,极板间距为2 cm,每组实验废水500 mL,实验装置流程如图1所示。本研究重点考察极板材料、电流密度、反应时间条件下电化学处理电脱盐废水的效果。在极板使用前,利用丙酮清洗表面。
在Fe-电絮凝处理过程中,采用响应曲面方法(RSM)设计实验方案,对电絮凝处理工艺进行条件优化。根据前期单因素实验确定的变量范围,利用Design-Expert 8.0软件设计了2因素5水平的响应曲面中心复合实验。电流密度和反应时间是电絮凝处理过程中的重要影响因素[12]。CHEN[7]指出,电流密度越高,原位絮凝剂产生量越大,可以产生较好的处理效果,并减小装置体积。因此,本研究重点以反应时间 (X1) 和电流密度 (X2) 为影响电脱盐废水电絮凝处理效果的因变量,以COD去除率 (Y1) 和总油去除率 (Y2) 为响应值,根据RSM分析实验数据获得响应值的预测值(表2)。
对实验结果进行分析后,可以获得与各个影响因素相关的响应值的线性或多项式模型[17](式(1))。
式中:Y为响应值;β0为截距;β1和β2为线性回归系数;β12为交互系数;β11和β22为二次回归系数。
-
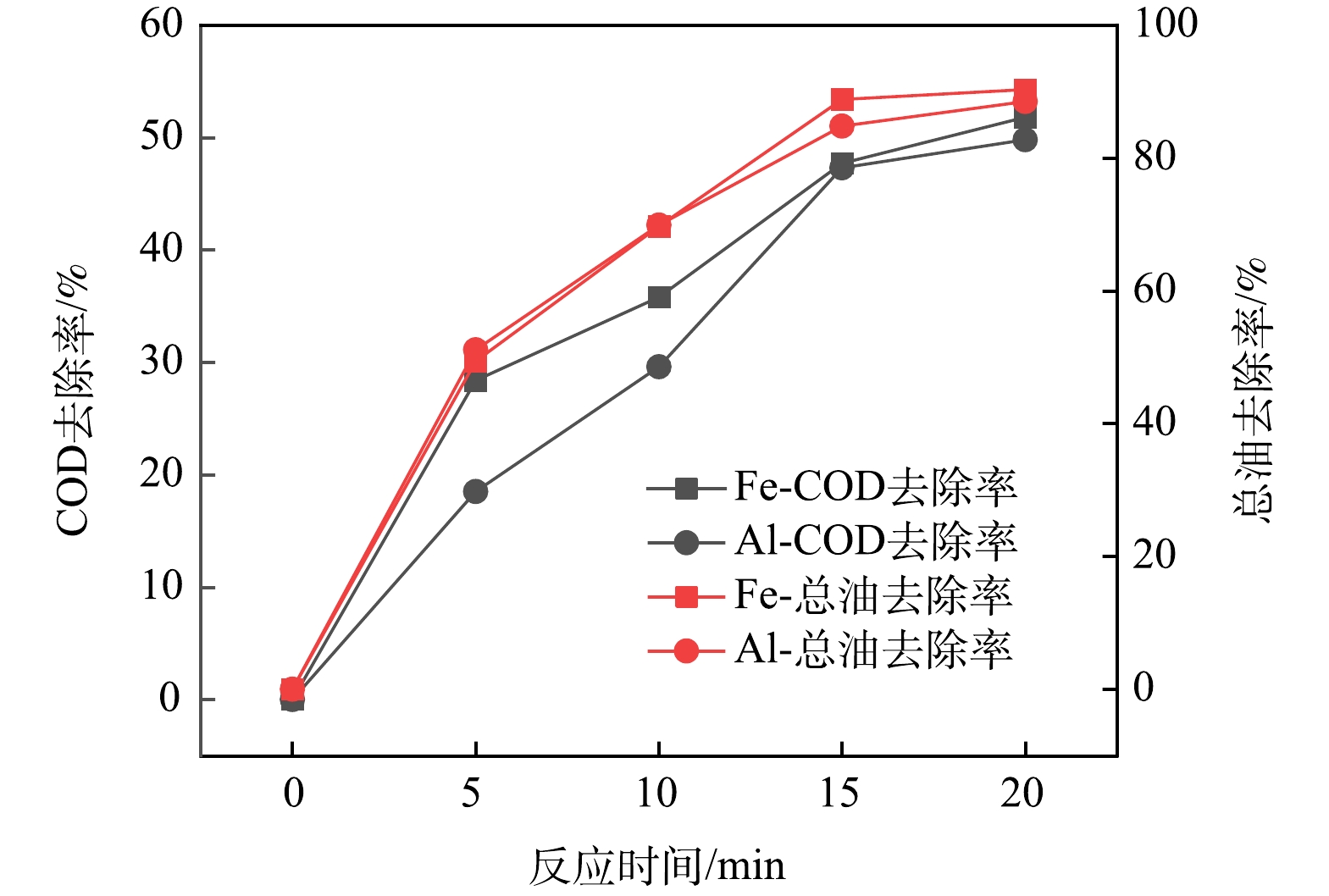
电脱盐废水的COD和总油初始质量浓度平均为5 100 mg·L−1和3 000 mg·L−1。设定电流密度为3 mA·cm−2,检测不同反应时间COD与总油质量浓度,对比Fe电极和Al电极对COD与总油的去除效果,结果如图2所示。可以看出,随着反应时间的增加,COD和总油去除率逐渐升高。当反应20 min时,Fe电极和Al电极的COD去除率分别为52%和50%,总油去除率分别为90.3%和88.5%。综合来看,采用Fe电极时,电脱盐废水的COD与总油去除率略高于Al电极。
进一步利用显微镜观察Fe电极和Al电极处理电脱盐废水前后的样品(放大50倍),结果如图3所示。可以看出,电脱盐废水在显微镜下可见明显的大小不均的油滴(0.5~15 μm),并与黑色的颗粒物及水互相裹夹。采用Fe电极电絮凝处理后,废水中已无明显的油滴,仅可见少量细小的颗粒物悬浮于水相。而以Al电极处理后,水相中颗粒物较多,且可见细微油滴。
综上所述,电絮凝可分离电脱盐废水的油、水、固混合相,其中Fe电极在去除COD和总油时效果较好,材料成本较低[5]。因此,本研究选择Fe电极处理电脱盐废水,进一步优化处理工艺参数,并探究作用机制。
-
1) GC-MS分析。利用Fe电极处理电脱盐废水,电流密度为4 mA·cm−2,取反应时间为5、15、20 min的出水进行饱和分分离,饱和分占总油的比例≥70%。分别对饱和分进行GC-MS分析,并与Al电极反应15 min的谱图进行对比,总离子流如图4所示。可以看出,电脱盐废水饱和分中含有碳数为10~27的烃类化合物及苯酚、甲基苯酚等含氧化合物。采用Fe电极时,电絮凝处理5 min后,主要化合物种类及分布变化不大,但相对丰度降低,即污染物浓度降低,此时对应总油的去除率约为30%;电絮凝15 min后,几乎检测不到碳数>11的有机物。电絮凝20 min出水与15 min出水的谱图相似。而采用Al电极进行电絮凝时,反应15 min后的饱和分中仍残留系列烃类物质,这与图2和图3结果一致。可以推断,Fe电极电絮凝可显著去除电脱盐废水中的石油类物质,但对于酚类等碳数≤10的含氧有机物的去除作用并不明显。
由于GC-MS仅能检测出总油中极性油指标的部分微观组成,因此电絮凝除了难以去除可检测到的酚类物质,对于GC-MS无法检测到的强极性有机物也难以发挥效果。
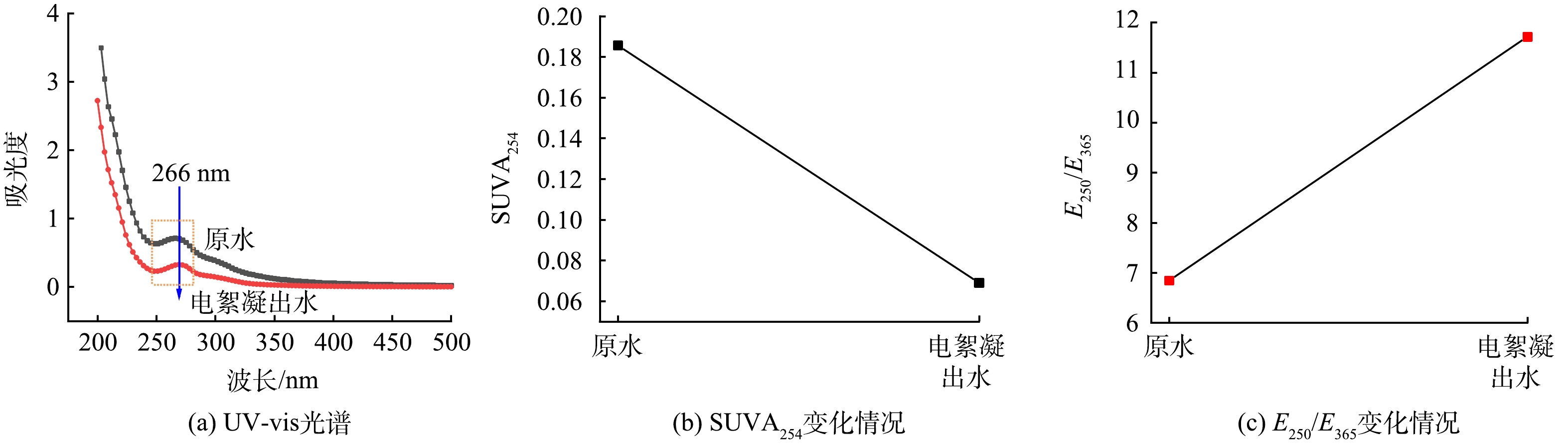
2) 光谱分析。为进一步分析电絮凝对电脱盐废水中有机物的作用,分别取电流密度为4 mA·cm−2、反应时间为20 min前后的电脱盐废水,对其DOM进行UV-vis和3D-EEMs光谱分析。UV-vis可以利用污染物对紫外或者可见光的吸收特性来表征物质浓度和来源,3D-EEMs则能够采用三维激发/发射矩阵对有机物进行特异性识别[18]。
图5为电脱盐废水电絮凝前后DOM的UV-vis谱图以及SUVA254和E250/E365的变化图。由于DOM含有多类不饱和结构,其中“π-π”电子跃迁在250~280 nm之间有明显的紫外吸收[19-20]。由图5可以看出,电脱盐废水反应前后DOM的特征吸收峰为266 nm,电絮凝后DOM的紫外可见吸收明显降低,266 nm处的紫外吸收值由0.711降至0.319,去除率为55%。由图5还可以看出,电脱盐废水电絮凝后E250/E365从6.8升高至11.7,SUVA254则从0.19降至0.07。E250/E365值的升高表明DOM的芳香度和分子量均降低[21],这与SUVA254降低代表芳香结构占比减少相一致。
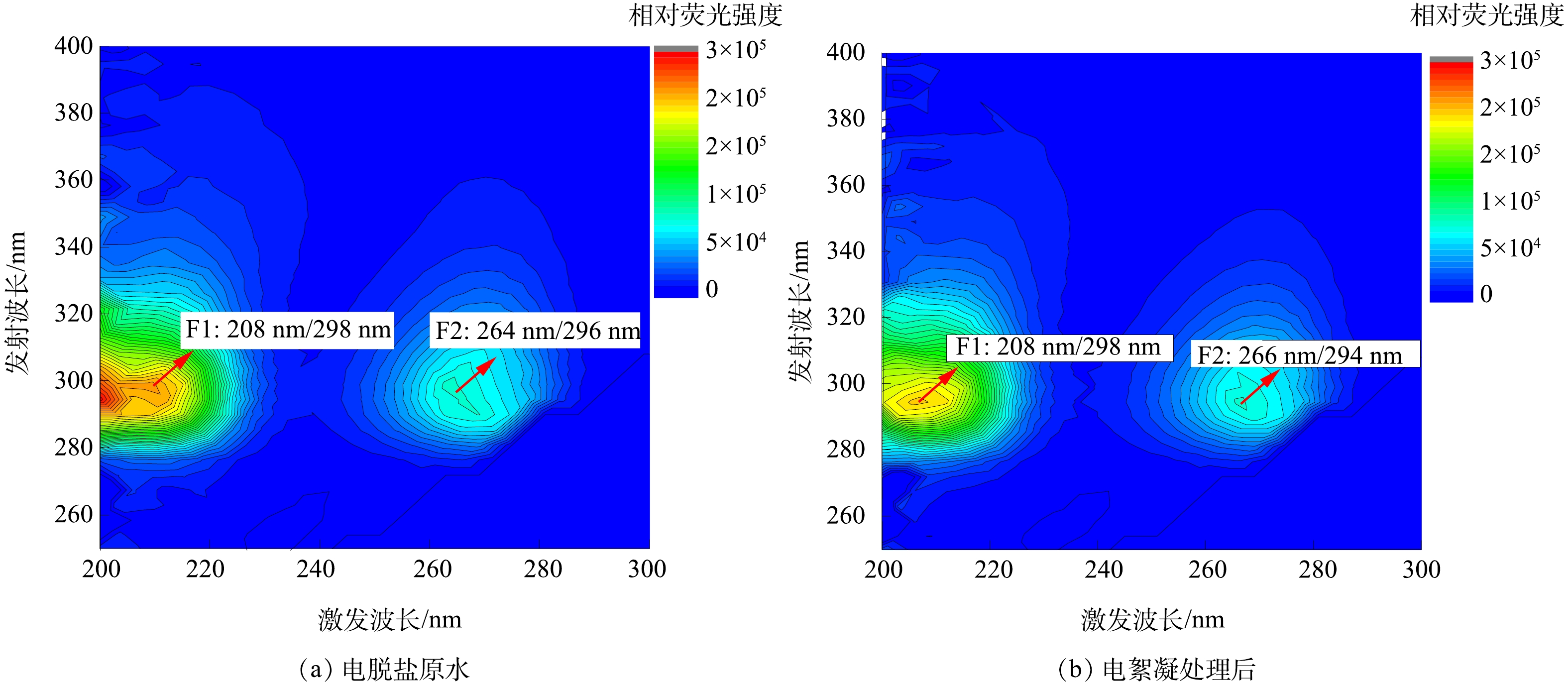
电脱盐废水电絮凝前后三维荧光光谱对比结果如图6所示。可以看出,电脱盐废水DOM主要有2个特征荧光峰,F1为Ex/Em=208 nm/ 298 nm,F2为Ex/Em=264 nm/ 296 nm,均为强激发波长荧光峰。原水中F1和F2的相对荧光强度分别为208 919和77 443。经过电絮凝处理后,2个主要荧光峰的位置未发生很大改变,同时F1和F2的相对荧光强度分别降为194 783和76 833,相对荧光强度的降低幅度不大。据报道[22],强激发波长荧光物质可见于炼油废水,如苯类(Ex/Em=210 nm/ 280 nm,Ex/Em=260 nm/ 285 nm)和酚类(Ex/Em=270 nm/ 300 nm,Ex/Em=220 nm/ 300 nm)等。这类物质分子量较小,极性大、亲水性强,而电絮凝对于该类极性物质的处理效果并不突出,这与图4的分析结果一致。
综上所述,电脱盐废水DOM由一系列不同结构和分子质量的有机物组成,其中芳香性不饱和结构在266 nm处具有较强的紫外吸收,但显现荧光的物质仅为极性较强的酚类、苯类等。电絮凝去除了大部分电脱盐废水中的不溶性物质,包括部分芳香性不饱和有机物,残余在水相的DOM分子质量较低,极性强,且主要为能够显现荧光特性的酚类等物质。
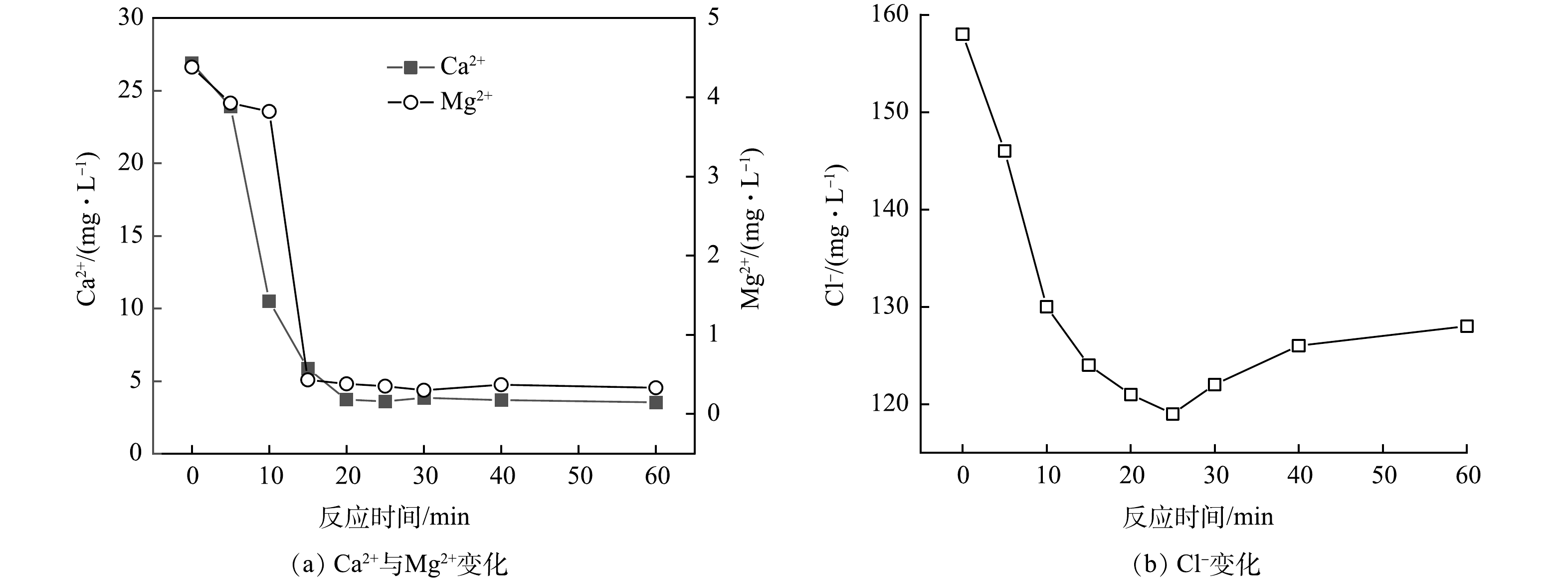
3) 电絮凝过程中主要离子质量浓度变化。电脱盐废水属于含盐含油废水,富含有机物和无机离子,电絮凝处理过程中的多重作用导致无机物质如硬度离子、Cl−等发生变化[9-10],反过来又会影响有机物质的去除[23-25]。为科学阐释电脱盐废水中污染物的迁移转化机制,本研究重点考察了电脱盐废水中共存离子Ca2+、Mg2+和Cl−在电絮凝 (电流密度4 mA·cm−2) 处理过程中的变化,结果如图7所示。电脱盐废水中Ca2+、Mg2+和Cl−的质量浓度分别为26.9、4.38 和158 mg·L−1,经电絮凝处理后浓度逐渐发生变化,其中Ca2+、Mg2+在反应15~20 min时降至最低,去除率分别为86.2%和91.3%,随后质量浓度变化甚微。电絮凝过程中Cl−的变化明显不同,在0~25 min内逐渐降低,达到最低后又有所升高,其在25 min时最高去除率为24.7%,较Ca2+、Mg2+的去除率偏低。
电脱盐废水偏碱性,包含CO32−、HCO3−、OH−等碱性离子以及SO42−。在电絮凝过程中,废水中的Ca2+和Mg2+在阴极附近富集,易与碱性离子形成CaCO3、CaMg(CO3)2、MgCO3、Mg(OH)2等沉淀物,使得废水中硬度离子浓度降低[23]。在同一电流密度下,反应时间增加,硬度离子的去除率也相应升高[24]。但继续延长反应时间,废水体系pH降低,抑制了Ca2+和Mg2+沉淀物的生成[23-24],导致反应时间>20 min的质量浓度变化不大。Cl−的形态变化是导致体系pH降低的一个重要因素[7]。
电脱盐废水中的Cl−不仅是电荷载体,还能通过降低pH减弱HCO3−和SO42−与Ca2+和Mg2+的成垢副作用,抑制电极表面沉淀,形成不导电膜[7]。此外,Cl−在电絮凝过程中产生氧化剂氯气,具有消毒及氧化降解污染物的作用,反应式如式(2)和式(3)所示[13]。氧化剂的生成在一定程度上可导致有机物结构变化,甚至矿化,从而使得部分溶解性COD降低,反应式如式(4)所示,此过程又会增加Cl−质量浓度[26]。因此,在电絮凝过程中,Cl−质量浓度呈现先降低后升高的趋势(图7(b))。但Cl−质量浓度的最大去除率仅为24.7%,这表明,Cl−转化成氧化形态的量较少,对有机物的矿化作用有限。
可以推断,电絮凝过程中产生的活性絮凝剂和H2在去除大部分COD与总油中发挥了重要的混絮凝与气浮分离作用[5,7,9],而Cl−转化为氧化剂Cl2,对提升溶解性有机物的矿化效果也有一定的贡献,同时能够降低pH发挥电极结垢的抑制作用。CHEN[7]通过研究发现,废水中Cl−占总阴离子的20%以上时可以显著改善电絮凝处理效果。
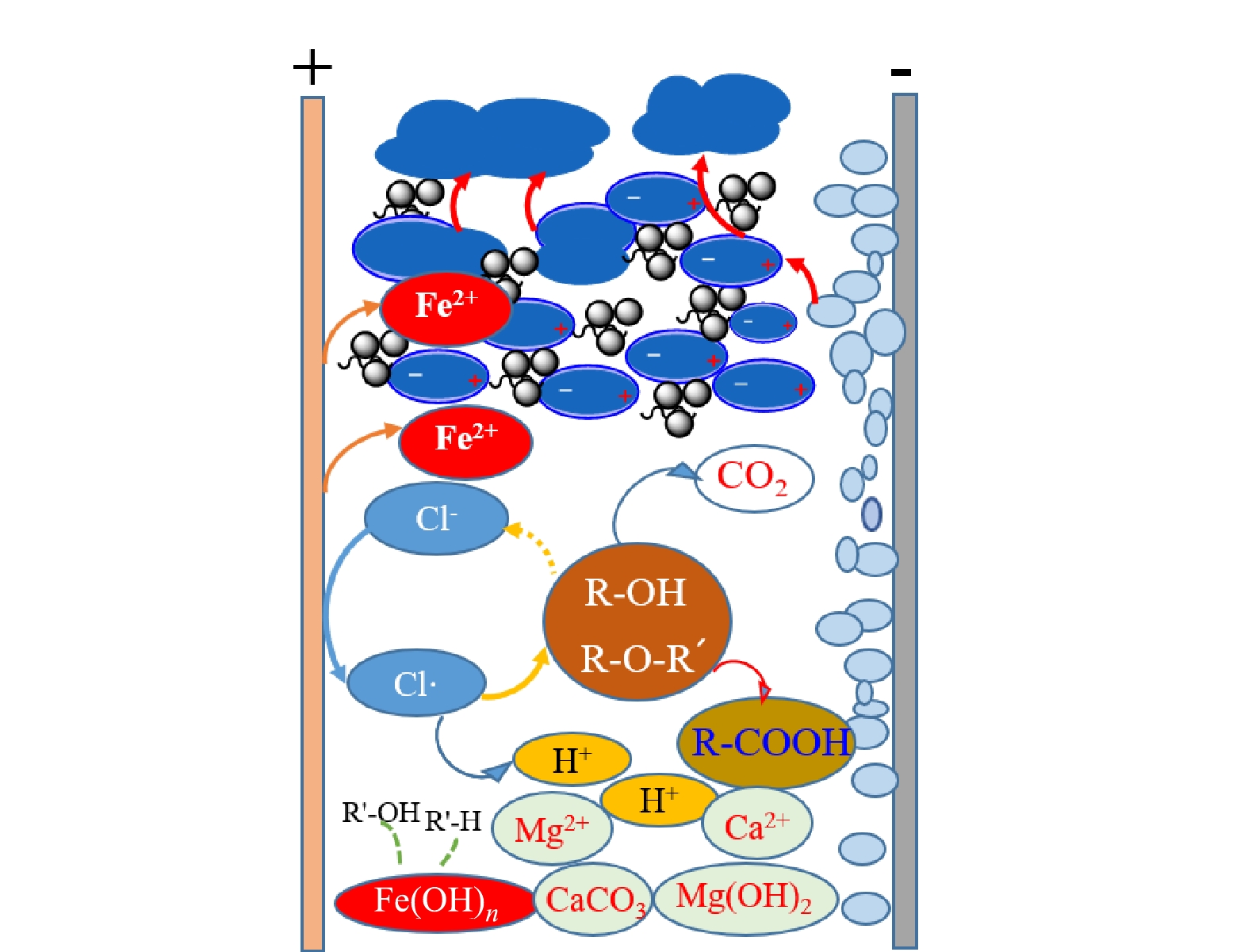
4) 电脱盐废水电絮凝作用机制。电脱盐废水是一种典型的含油废水,由显微镜照片可以看出,电脱盐废水由复杂的油包水与水包油的混合组成。在电絮凝处理过程中,电场破乳与原位絮凝作用使得电脱盐废水中的不溶性物质(如颗粒物、油类等)通过上浮或者沉淀进行分离,结果如图8所示。
电脱盐废水中的乳化物在电场中发生极化作用,表面电荷平衡被破坏,体系脱稳,在水力作用及静电吸引作用下持续破乳融合,小油滴增长为大油滴;溶解性电极在反应过程中还会生成高效的絮凝活性物种(如活性Fe2+),与电场破乳与油滴聚结作用相协同[12,14]。废水中的硬度离子易结垢析出,但共存的Cl−使得体系pH降低,可在一定程度上抑制Ca2+和Mg2+沉淀物的生成。Cl−在电絮凝过程中还会产生氧化剂,可进一步改变溶解性有机物结构,甚至生成CO2,但矿化程度有限。
电场破乳、絮凝、气浮、氧化等作用能够去除电脱盐废水中的油类物质和大部分弱极性、中等极性有机物,COD与总油含量降低效果显著。电脱盐废水经过电絮凝处理后可分离为含油浮渣、溶液与沉淀,在去除上层浮渣与底泥后,溶液中残存的有机物主要为分子质量较低、极性较强的溶解性有机物,且大部分为显现荧光特性的碳数≤10的酚等物质,需经过氧化或者吸附作用进一步降解去除。
-
1) 回归模型分析。对表2的实验结果进行回归分析,可得到响应值(COD去除率与总油去除率)与2个因变量(反应时间和电流密度)之间的基于编码值的二次多项式回归模型,结果如式(5)和式(6)所示。
式中:Y1为COD去除率;Y2为总油去除率。
可以看出,模型中X1与X2的线性回归系数均为正值,表明COD去除率与总油去除率均与反应时间、电流密度呈正相关关系。COD去除率模型与总油去除率模型中电流密度系数分别为11.31和16.82,明显高于反应时间变量的系数,说明2个影响因素中电流密度对响应值的影响程度较大。
根据二次回归模型,对COD去除率模型与总油去除率模型的各项回归系数进行显著性检验和方差分析(ANOVA),结果见表3和表4。
模型的显著性检验结果以F值和P值表示,F值越大,P值<0.05,即表示自变量对响应值的影响具有高度的显著性,具有较高的统计学意义。在表3和表4中,因变量Y1和Y2的F值分别为106.62和106.22,P<0.000 1,表示通过拟合得到的二次回归模型显著性较高。此外,2个因变量模型的多元决定系数R2为0.97,校正决定系数R2(adj)为0.96,同样说明该模型拟合度较好,可用来对实验条件与结果进行分析和预测。
在以COD去除率为响应值的模型中,反应时间和电流密度2个变量的F值分别为135.96和295.97,表明电流密度对电脱盐废水COD去除率的影响最为显著。同样,在以总油去除率为响应值的模型中,反应时间和电流密度的F值分别为158.74和269.16,也体现了电流密度对响应值的显著影响。
2) 工艺参数优化。采用响应曲面法研究2个变量及其交互作用对COD去除率和总油去除率的影响。图9反映了反应时间和电流密度之间交互作用对COD去除率的影响。可以看出,增加电流密度、延长反应时间均能提高COD去除率,且在电流密度为3.5~4.5 mA·cm−2和反应时间为22~33 min时,COD去除率≥80%。
反应时间和电流密度之间交互作用对总油去除率的影响如图10所示。可以看出,在电流密度≥2.2 mA·cm−2、反应时间≥13 min时,总油去除率≥80%,且随着电流密度和反应时间的增加,总油去除率随之升高并达到峰值。
由此可以看出,提高电流密度有利于污染物去除,同时较高的电流密度又增加了处理能耗,反而降低了电流效率[7]。因此,基于处理成本控制,利用RSM对设定的影响因素进行优化设计,得到最佳工艺运行参数:反应时间为25.4 min,电流密度为4.2 mA·cm−2,此时COD去除率与总油去除率的模型预测值分别为80.8%和93.5%。这与实际COD去除率(82.3%)和总油去除率(93.6%)接近。
-
1) 石油炼制电脱盐废水为含油复杂废液体系,采用Fe电极构建的电絮凝系统处理电脱盐废水的效果较好。电絮凝过程中产生的活性絮凝剂和H2在去除大部分COD与总油中发挥了重要的混絮凝与气浮分离作用,而Cl−转化为氧化剂,对提升溶解性有机物的矿化效果也有一定的贡献,同时也能够降低pH以抑制电极结垢。
2) 电场破乳、絮凝气浮与氧化等作用能够去除大部分油类物质和弱极性、中等极性有机物,残存在溶液中的有机物主要为分子质量较低、极性较强且大部分为显现荧光特性的碳数≤10的酚类等。基于处理成本控制,利用RSM对反应时间和电流密度进行优化设计,其中电流密度对电脱盐废水处理效果的影响更为显著,此时COD与总油去除率分别达到80.8%和93.5%。
电脱盐废水电絮凝破乳分离机制及工艺优化
Mechanism and process optimization of electrocoagulation for desalter wastewater demulsification separation
-
摘要: 为降低电脱盐废水污染物含量并去除顽固有机物,采用Fe和Al电极对电脱盐废水进行电絮凝预处理,采用气相色谱-质谱(GC-MS)、紫外可见光谱(UV-Vis)、三维荧光光谱(3D-EEMs)和共存离子检测等方法对电絮凝破乳分离机制进行分析,利用响应曲面方法(RSM)对电絮凝处理工艺进行优化。结果表明,电絮凝处理电脱盐废水15 min后,几乎检测不到碳数>11的有机物;反应20 min后,电脱盐废水在266 nm处紫外特征吸收值降低了55%,而电脱盐废水的2个特征荧光峰并未明显改变;反应20~25 min时,Ca2+、Mg2+、Cl−的去除率达到最高,但Cl−浓度随后又有所升高,显示出体系中发生了氧化还原反应;采用Fe电极处理电脱盐废水时,化学需氧量(COD)去除率和总油去除率均与反应时间、电流密度符合二次回归模型,电流密度对污染物去除率的影响更为显著;电絮凝处理电脱盐废水的最优反应条件为电流密度4.2 mA·cm−2、反应时间25.4 min,此时COD去除率与总油去除率的模型预测值分别为80.8%和93.5%。可以推断,电絮凝以电场破乳、絮凝、气浮、氧化等耦合作用实现了电脱盐废水的高效破乳除油。本研究结果可为电絮凝技术处理电脱盐废水的规模化应用提供参考。Abstract: To lower pollutants contents and remove recalcitrant compounds from desalter wastewater, electrocoagulation with Fe and Al electrodes was employed to treat desalter wastewater. Gas chromatography-mass spectrometry (GC-MS), ultraviolet visible spectroscopy (UV-vis), three-dimensional fluorescence spectroscopy (3D-EEMs) and coexisting ion analysis were used to explore the demulsification and separation mechanism. The operating conditions of electrocoagulation were optimized using a response surface methodology (RSM). Results showed that almost no organics with carbon atom numbers greater than 11 could be detected beyond 15 min treatment. After 20 min, the characteristic absorption value of desalter wastewater at 266 nm decreased by 55%, while the two main fluorescence peaks of desalter wastewater did not change significantly. At 20~25 min, the highest removal rates of Ca2+, Mg2+ and Cl− occurred, but Cl- content increased subsequently, indicating that the oxidation-reduction reactions happened in the system. As desalter wastewater was treated by electrocoagulation with iron electrodes, the removal rates of chemical oxygen demand (COD) and total oil correlated with the variables of current density and reaction time, which followed the quadratic regression model, and current density had a significant effect on the removal rates. The optimal reaction conditions for electrocoagulation were obtained as following: current density of 4.2 mA·cm−2 and reaction time of 25.4 min, where the predictive removal rates of COD and total oil were 80.8% and 93.5%, respectively. It can be concluded that desalter wastewater was efficiently demulsified and oil in it was removed by the coupling effects of electric field demulsification, flocculation, air floatation and oxidation. This study provides a reference for the large-scale application of electrocoagulation technology to treat desalter wastewater.
-
Key words:
- desalter wastewater /
- electrocoagulation /
- demulsification /
- RSM optimization /
- COD removal /
- total oil removal
-

-
表 1 不同电极材料对工业废水的处理效果对比
Table 1. Comparison of treatment effects of different electrode materials on industrial wastewater
电极 污水类型 初始污染物 去除率/% 来源 污染物指标 数值 Fe-Fe 计算机行业生产废水 COD 32 350 mg·L−1 95.72 [5] Fe-Fe 印刷废水 TOC 18 400 mg·L−1 65 [9] Fe-Fe 含氯农药生产废水 COD 90 000 mg·L−1 52 [10] Fe-Fe 含氯农药生产废水 氯离子 34 450 mg·L−1 30 [10] 不锈钢-不锈钢 含氯农药生产废水 COD 90 000 mg·L−1 16 [10] 不锈钢-不锈钢 含氯农药生产废水 氯离子 34 450 mg·L−1 26 [10] Fe-Fe 垃圾渗滤液 COD 11 000 mg·L−1 65.85 [11] Cu-Al 汽车冲洗废水 油与脂 3180~200 mg·L−1 92.5 [12] Cu-Al 汽车冲洗废水 COD 20~1 019 mg·L−1 95.1 [12] Cu-Al 汽车冲洗废水 浊度 118~380 NTU 99 [12] 碳钢-Al 模拟压舱水 油与脂 5 000 mg·L−1 >99 [13] 表 2 响应实验结果
Table 2. Response test results
实验序号 (X1)反应时间/min (X2)电流密度/ (mA·cm-2) (Y1)COD去除率/% (Y2)总油去除率/% 实验值 预测值 实验值 预测值 1 10 1 40.75 40.75 28.046 27.85 2 15 1 51.86 48.98 50.94 45.11 3 20 1 53.02 54.18 58.316 56.47 4 25 1 55.56 56.33 59.57 61.94 5 30 1 58.42 55.45 60.023 61.52 6 10 2 52.23 53.28 41.273 47.39 7 15 2 61.92 61.59 62.732 63.67 8 20 2 64.78 66.86 76.201 74.05 9 25 2 66.23 69.1 77.02 78.54 10 30 5 67.31 68.29 78.553 77.14 11 10 3 59.94 61.12 56.588 60.81 12 15 3 71.11 69.51 76.133 76.11 13 20 3 73.47 74.86 84.49 85.52 14 25 3 74.66 77.18 84.49 89.03 15 30 3 76.34 76.45 87.459 86.65 16 10 4 63.74 64.27 73.894 68.12 17 15 4 78.24 72.74 87.908 82.45 18 20 4 79.58 78.18 92.03 90.88 19 25 4 82.33 80.57 93.67 93.41 20 30 4 83.24 79.92 94.356 90.05 21 10 5 60.67 62.74 67.937 69.32 22 15 5 72.04 71.29 83.305 82.67 23 20 5 75.79 76.8 87.51 90.12 24 25 5 78.26 79.27 86.84 91.68 25 30 5 76.92 78.71 88.565 87.34 表 3 COD去除率模型的ANOVA分析结果
Table 3. ANOVA analysis results for COD removal model
项目 平方和 自由度 均方 F值 P值> F 显著性 模型 2 881.05 5 576.21 106.62 < 0.000 1 显著 X1 734.75 1 734.75 135.96 < 0.000 1 显著 X2 1 599.52 1 1 599.52 295.97 < 0.000 1 显著 X1X2 0.64 1 0.64 0.12 0.735 2 不显著 X12 161.64 1 161.64 29.91 < 0.000 1 显著 X22 384.51 1 384.51 71.15 < 0.000 1 显著 残差 102.68 19 5.4 总差 2 983.73 24 注:COD去除率模型的决定系数R2=0.97,校正决定系数R2(adj)=0.96。 表 4 总油去除率模型的ANOVA分析结果
Table 4. ANOVA analysis results of oil removal model
项目 平方和 自由度 均方 F值 P值> F 显著性 模型 6 981.78 5 1 396.36 106.22 < 0.000 1 显著 X1 2 086.68 1 2 086.68 158.74 < 0.000 1 显著 X2 3 538.14 1 3 538.14 269.16 < 0.000 1 显著 X1X2 95.55 1 95.55 7.27 0.014 3 显著 X12 608.08 1 608.08 46.26 < 0.000 1 显著 X22 653.33 1 653.33 49.7 < 0.000 1 显著 残差 249.76 19 13.15 总差 7 231.54 24 注:总油去除率模型的决定系数R2=0.97,校正决定系数R2(adj)=0.96。 -
[1] BASU S. Impact of opportunity crudes on refinery desalter and wastewater treatment performance-part 1[J]. Hydrocarbon Processing, 2018, 8: 85-89. [2] BASU S. Impact of opportunity crudes on refinery desalter and wastewater treatment performance-part 2[J]. Hydrocarbon Processing, 2018, 9: 97-100. [3] ZHANG H, BUKOSKY S C, RIRTENPART W D. Low-voltage electrical demulsification of oily wastewater[J]. Industrial & Engineering Chemistry Research, 2018, 57(24): 8341-8347. [4] JENKINS D. Manual on the Causes and Control of Activated Sludge Bulking, Foaming, and Other Solids Separation Problems [M]. Boca Raton, Fla. : Lewis Publishers, 2004. [5] ODEN M K. Treatment of CNC industry wastewater by electrocoagulation technology: An application through response surface methodology[J]. International Journal of Environmental Analytical Chemistry, 2020, 100(1): 1-19. doi: 10.1080/03067319.2019.1628955 [6] OZYONAR F, KARAGOZOGLU B. Treatment of pretreated coke wastewater by electrocoagulation and electrochemical peroxidation processes[J]. Separation and Purification Technology, 2015, 150: 268-277. doi: 10.1016/j.seppur.2015.07.011 [7] CHEN G. Electrochemical technologies in wastewater treatment[J]. Separation and Purification Technology, 2004, 38(1): 11-41. doi: 10.1016/j.seppur.2003.10.006 [8] COTILLAS S, LLANOS J, MORALEDA I, et al. Scaling-up an integrated electrodisinfection-electrocoagulation process for wastewater reclamation[J]. Chemical Engineering Journal, 2020, 380: 122415. doi: 10.1016/j.cej.2019.122415 [9] ESCOBAR A, MATEUS A, VASQUEZ A. Electrocoagulation-photocatalytic process for the treatment of lithographic wastewater. Optimization using response surface methodology (RSM) and kinetic study[J]. Catalysis Today, 2016, 266: 120-125. doi: 10.1016/j.cattod.2015.09.016 [10] SRILATHA K, BHAGAWAN D, SHANKARAIAH G, et al. Performance evaluation of different advanced processes for treating chloro-pesticide intermediate industrial wastewater[J]. Sustainable Water Resources Management, 2019, 5(4): 1833-1846. doi: 10.1007/s40899-019-00336-z [11] ORKUN M O, KULEYIN A. Treatment performance evaluation of chemical oxygen demand from landfill leachate by electro‐coagulation and electro‐Fenton technique[J]. Environmental Progress & Sustainable Energy, 2012, 31(1): 59-67. [12] PRIYA M, JEYANTHI J. Removal of COD, oil and grease from automobile wash water effluent using electrocoagulation technique[J]. Microchemical Journal, 2019, 150: 104070. doi: 10.1016/j.microc.2019.104070 [13] RINCON G J, MOTTA E J. Simultaneous removal of oil and grease, and heavy metals from artificial bilge water using electro-coagulation/flotation[J]. Journal of Environmental Management, 2014, 144: 42-50. [14] GHOSH D, MEDHI C R, PURKAIT M K. Treatment of fluoride containing drinking water by electrocoagulation using monopolar and bipolar electrode connections[J]. Chemosphere, 2008, 73(9): 1393-1400. doi: 10.1016/j.chemosphere.2008.08.041 [15] SHARMA D, CHAUDHARI P K, PRAJAPATI A K. Removal of chromium (VI) and lead from electroplating effluent using electrocoagulation[J]. Separation Science and Technology, 2020, 55(2): 321-331. doi: 10.1080/01496395.2018.1563157 [16] KUOKKANEN V. Water Treatment by Electrocoagulation [M]. New York: John Wiley & Sons, Ltd. , 2016. [17] BAHARAKI T, ERKAN N H, ENGIN G. The investigation of shale gas wastewater treatment by electro-Fenton process: Statistical optimization of operational parameters[J]. Process Safety and Environmental Protection, 2017, 109: 203-213. doi: 10.1016/j.psep.2017.04.002 [18] 梁月清, 刘会来, 崔康平, 等. 基于三维荧光光谱-平行因子分析法的工业园区污水溶解性有机物溯源与归趋[J]. 环境工程学报, 2022, 16(4): 1238-1246. doi: 10.12030/j.cjee.202201093 [19] GJESSING E T, KALLQVIST T. Algicidal and chemical effect of UV-radiation of water containing humic substances[J]. Water Research, 1991, 25(4): 491-494. doi: 10.1016/0043-1354(91)90087-7 [20] BACKLUND P. Degradation of aquatic humic material by ultraviolet light[J]. Chemosphere, 1992, 25(12): 1869-1878. doi: 10.1016/0045-6535(92)90026-N [21] AIKEN G R, MCKNIGHT D M, WERSHAW R L. Humic Substances in Soils, Sediments and Water [M]. New York: John Wiley & Sons, Ltd., 1985. [22] 栗则, 张晓飞, 吴百春, 等. 三维荧光光谱技术在石油炼化行业的应用[J]. 分析实验室, 2018, 37(7): 863-868. [23] CHOW H, PHAM A L T. Effective removal of silica and sulfide from oil sands thermal in-situ produced water by electrocoagulation[J]. Journal of Hazardous Materials, 2019, 380: 120880. doi: 10.1016/j.jhazmat.2019.120880 [24] ZHANG H, WU B C, LI X C, et al. Electrocoagulation treatment of shale gas drilling wastewater: Performance and statistical optimization[J]. Science of the Total Environment, 2021, 794: 148436. doi: 10.1016/j.scitotenv.2021.148436 [25] 张华, 罗臻, 张晓飞, 等. 页岩气钻井废水电絮凝预处理实验[J]. 天然气工业, 2019, 39(12): 146-154. doi: 10.3787/j.issn.1000-0976.2019.12.019 [26] 张华, 刘光全, 张晓飞, 等. 电脱盐废水稳定性分析及破乳技术[J]. 化工进展, 2022, 41(9): 5047-5054. -



 下载:
下载: