-
人工湿地是一种环境友好的、可持续的废水处理技术,具有性能稳定、运行和维护成本低等优点。在人工湿地中,微生物活动是实现总氮(TN)去除的主要途径[1]。传统方法主要是采用硝化和反硝化作用来去除TN。然而,生活污水中TN主要由NH4+-N组成,在人工湿地缺氧区域中,溶解氧缺乏限制了硝化过程,导致NH4+-N去除率低,进而导致TN去除效率差。研究人员探索了多种方法来提高NH4+-N去除效率,例如人工曝气[2]、复合人工湿地[3]、潮汐流人工湿地[4]等。然而,这些方法都使系统比传统人工湿地更加复杂或耗能。实际上,在保持传统人工湿地优点的同时,可以通过改变人工湿地基质来提高其脱氮效率。
为提高人工湿地对NH4+-N的去除率,除了填充沸石等对NH4+-N具有吸附作用的基质外[5],还可以选择对NH4+-N具有氧化作用的基质。近年来出现了2种独特的NH4+-N氧化途径,即在缺氧条件下,Fe(III)和Mn(IV)均可氧化去除NH4+-N。这2种技术分别被称为铁氨氧化(Feammox)和锰氨氧化(Mnammox)。热力学研究[6-7]表明,Feammox和Mnammox技术氧化可以将NH4+-N直接氧化为硝态氮(NOx−-N)或N2。一些学者认为Feammox中NH4+-N的直接产物是NOx−-N[8],生成的NOx−-N可以通过反硝化、厌氧氨氧化(anammox)或与Fe2+耦合的方式被还原为N2[6, 9],从而被去除。然而,还有一些学者认为,Feammox更倾向于直接将NH4+-N氧化为N2,这是一种全新的脱氮路径[10]。关于Mnammox技术,锰氧化物具有更低的零电点(1.5~4.6),在微生物代谢中利用效率更高[11],其脱氮性能往往更为优秀。但与Feammox相同,Mnammox的脱氨途径也不明确,其NH4+-N的直接产物倾向于是NOx−-N还是N2,这仍然存在争论[12-14]。总体而言,锰矿和铁矿都具有强化NH4+-N去除的能力。此外,铁矿和锰矿含有丰富的金属离子,这些金属离子可以与磷形成络合物,从而去除水中的可溶性磷酸盐[15]。因此,将铁矿和锰矿作为人工湿地的基质有很好的应用前景。
本文以砾石基质的人工湿地为对照组,另外构建了基质为铁矿和锰矿的2组人工湿地。通过长期的水质监测,以研究铁矿基和锰矿基人工湿地对污染物去除的促进作用,同时比较了不同基质人工湿地中脱氮和除磷效果的差异。最后,结合湿地基质的物化分析及微生物证据,对Feammox和Mnammox技术的差别进行探讨,以期为人工湿地中铁矿和锰矿的应用提供参考。
-
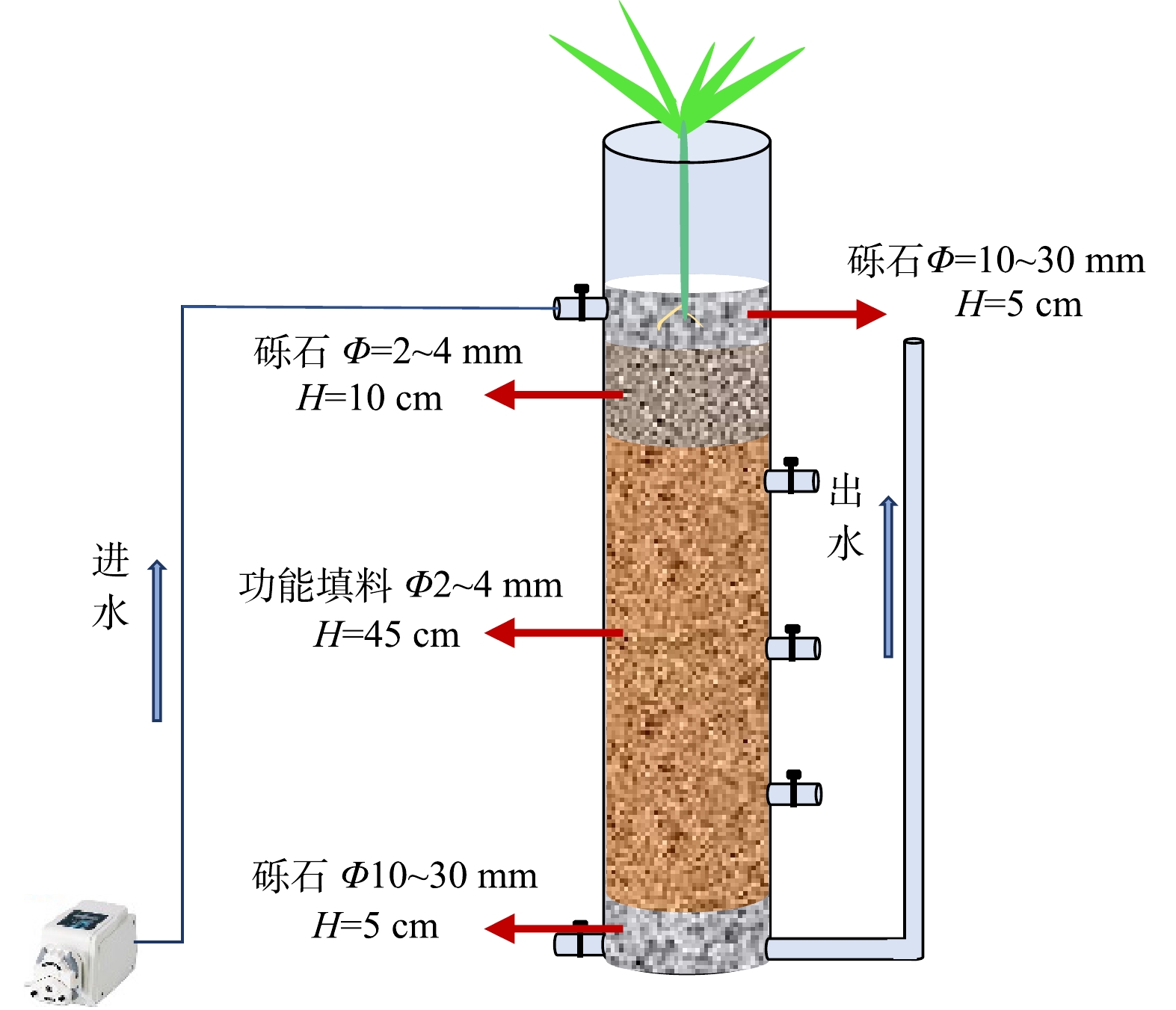
本研究使用直径11 cm的聚氯乙烯管制成3组不同类型的人工湿地。如图1所示,这些人工湿地的工作容积为2 L,高度为65 cm,由下至上分为4层:5 cm的砾石层(粒径1~3 cm)、45 cm的功能基质层(粒径2~4 mm)、10 cm的砾石层(粒径2~4 mm)和5 cm的砾石层(粒径1~3 cm)。根据功能基质的不同被分为3组:铁矿(CW-Fe)、锰矿(CW-Mn)和砾石(CW-C)组人工湿地。铁矿、锰矿和砾石分别来源于河北省石家庄、河南省郑州和江苏省连云港,其体积密度分别为1 794.2、1 069.6和1 388.3 kg·m−3。通过Brunauer–Emmet–Teller(BET,Tristar II 3020,Micromeritics,USA)测试,砾石的比表面积和平均孔隙尺寸分别为2.1 m2·g−1和0.9 nm,铁矿为7.2 m2·g−1和1.2 nm,锰矿为20.3 m2·g−1和1.3 nm。所有人工湿地都种植了30 cm高的健康Cyperus alternifolius L.。
本实验持续运行了180 d,所有人工湿地系统均采用下流方式进水。实验所使用的废水模拟物质包括NH4Cl,KH2PO4,葡萄糖和NaHCO3等,其中NH4+-N的质量浓度为(25±2) mg·L−1,TP的质量浓度为(5±0.5) mg·L−1,耗氧有机污染物的质量浓度(以COD计)为(90±10) mg·L−1,NaHCO3的质量浓度为350 mg·L−1,CaCl2和MgSO4·7H2O的质量浓度均为13 mg·L−1,进水pH为7.0±0.2。实验接种污泥来自重庆市鸡冠石污水处理厂。所有湿地均在20~25 ℃的恒温条件下运行,水力停留时间(hydraulic retention time, HRT)为3 d。
-
1)水样采集和分析。每3 d对人工湿地的进水和出水采样1次。所有样品采样后立刻用孔径为0.45 μm的水系滤膜过滤,然后进行测试。测量TP、NO3−-N、NO2−-N、NH4+-N质量浓度和COD值分别用钼酸铵分光光度法、紫外分光光度法、N-(1-萘基)乙二胺分光光度法、纳氏试剂分光光度法和快速消解分光光度法。pH用便携式分析仪来测定(HACH,HQ40d,美国)。
2)材料特性。分别对3种基质进行了NH4+-N和TP的吸附实验,包括动力学吸附和等温实验。配制了16 mg·L−1 TP溶液和8 mg·L−1 NH4+-N溶液。将3种基质各60 g分别放入盛有1升TP或NH4+-N溶液的细口瓶中(每组3个平行)。为抑制微生物活性,实验加入了3滴氯仿。随后,将细口瓶放入恒温水浴振荡器中(25 ℃,150 r·min−1)。实验在5、10、30、60、120、240、480、780、1 440、2 160 min时 取样。为了拟合动力学,本研究分别采用了准一级动力学方程(式(1))和准二级动力学方程(式(2))。
式中:qe为矿石平衡时的吸附量,mg·g−1;qt为矿石在t时刻的吸附量,mg·g−1;
$ t\mathrm{为}\mathrm{吸}\mathrm{附}\mathrm{时}\mathrm{间} $ ,h;k1为准一级反应速率常数,h−1;k2为准二级反应速率常数,g·(mg·h)−1。实验准备了不同质量浓度的NH4+-N和TP溶液,分别为0、0.5、1、2、4、8、16、32、64 mg·L−1和0、1、2、4、8、16、32、64、128 mg·L−1。将每种基质取3 g,准确地称量到带盖的细口瓶中。接着,加入250 ml不同质量浓度的NH4+-N和TP溶液(每组3个平行),再在每个细口瓶中加入1滴氯仿,然后将样品放置于恒温水浴振荡器中(25 ℃,150 r·min−1)振荡48 h。最后,取样测定滤液中NH4+-N或TP的质量浓度。并使用Langmuir(式(3))和Freundlich(式(4))等温吸附模型进行拟合。
式中:ce为溶液中NH4+-N/TP的平衡质量浓度,mg·L−1;qe为平衡时各基质的吸附量,mg·g−1;qm为各基质最大平衡吸附量,mg·g−1;KL为Langmuir吸附常数,L·mg−1;KF为Freundlich吸附常数,L·mg−1;n为与吸附强度有关的常数。
所有实验前和实验后的铁矿和锰矿都用自来水清洗并干燥。采用堆积法将每种矿石取5克,进行粉碎并过200目筛。然后使用X射线光电子能谱仪(XPS,Thermo Scientific K-Alpha,美国)和扫描电子显微镜结合能谱仪(SEM-EDS,ZEISS Sigma 300,德国)来测试所有样品。用Origin和分峰软件(XPSPEAK)进行数据处理,分析XPS的结果。
3)微生物群落分析。在每个湿地的30 cm高处收集基质并得到浓缩的微生物样品,用于微生物群落分析。所有样品用338F(5′ACTCCTACGGGAGGCAGCAG3′)和806R(5′GGACTACHVGGGTWTCT-AT3′)引物进行扩增[16]。然后,使用QuantiFluorTM-ST(Promega,美国)量化扩增产物,并在MiSeq PE300平台(中国上海)上进行检测。为了分析微生物多样性,将优化后的序列按照97%相似性聚类为操作分类单元(OTU)。然后,使用70%阈值对97%相似度的OTU代表序列进行分类分析物种多样性。同时使用KEGG数据库对氮代谢相关酶的宏基因组进行预测和注释,以探讨CW-Fe和CW-Mn中的氮代谢机理。
-
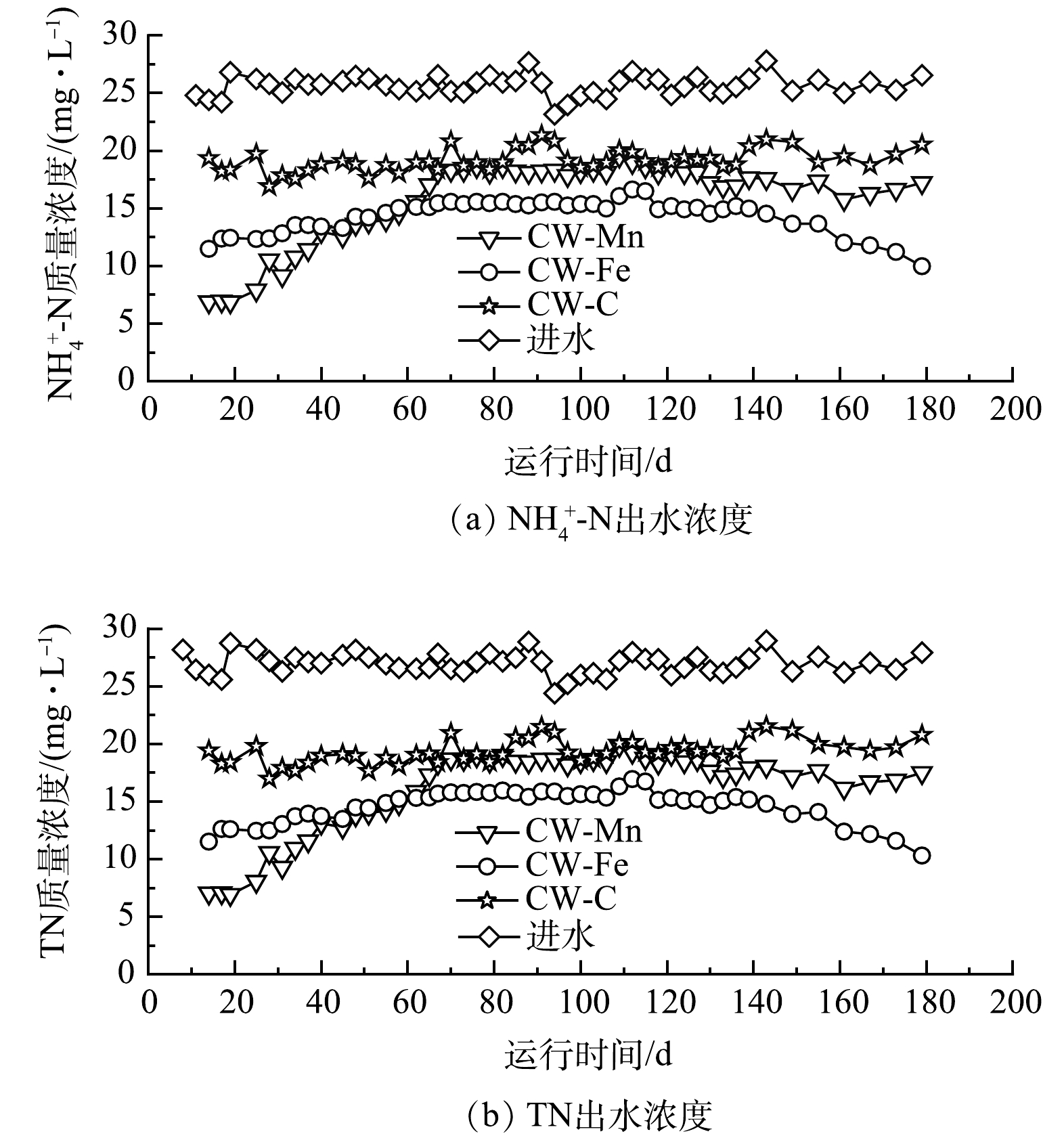
1)氨氮和总氮的去除效果。由图2(a)可知,CW-Mn和CW-Fe的NH4+-N去除效果均优于CW-C。整个实验期间,CW-C的运行相对稳定,其NH4+-N平均出水质量浓度为19.15 mg·L−1,去除率为25.30%(去除速率为0.44 g·(m2·d)−1)。
在实验的第14~67天,CW-Fe和CW-Mn的NH4+-N出水质量浓度分别由11.47 mg·L−1和6.86 mg·L−1逐渐增加到15.42 mg·L−1和18.13 mg·L−1。这可能是因为铁矿和锰矿对NH4+-N的吸附作用在逐渐减弱[17]。其中,在前58 d,CW-Mn的NH4+-N去除效果优于CW-Fe,这应该是由于锰矿对NH4+-N吸附作用比铁矿强[17]。在67~140 d,CW-Fe和CW-Mn中的NH4+-N出水质量浓度保持稳定。CW-Fe的平均NH4+-N出水质量浓度为15.35 mg·L−1,去除率为39.93%(去除速率为0.72 g·(m2·d)−1),CW-Mn中的平均NH4+-N出水质量浓度为18.11 mg·L−1,去除率为29.15%(去除速率为0.52 g·(m2·d)−1)。CW-Fe的NH4+-N去除率比CW-Mn高了10.78%。在实验运行140 d后,CW-Fe中的NH4+-N出水质量浓度逐渐下降,到180 d时其NH4+-N出水质量浓度仅为9.97 mg·L−1,去除率为62.40%(去除速率为1.16 g·(m2·d)−1)。而在140~180 d内,CW-Mn的NH4+-N平均去除率为35.4%(去除速率为0.65 g·(m2·d)−1),比前一阶段高了6.25%。根据上述分析,在整个实验周期内,除了前58 d,CW-Mn的NH4+-N去除效率优于CW-Fe,其余时间CW-Mn低于CW-Fe,即使Mn(IV)的氧化活性比Fe(III)更大。
在整个实验过程中,3组人工湿地中的NO3−-N和NO2−-N出水质量浓度均小于0.2 mg·L−1。其TN出水质量浓度与NH4+-N出水质量浓度的变化趋势十分相似,进出水TN的主要成分均为NH4+-N。
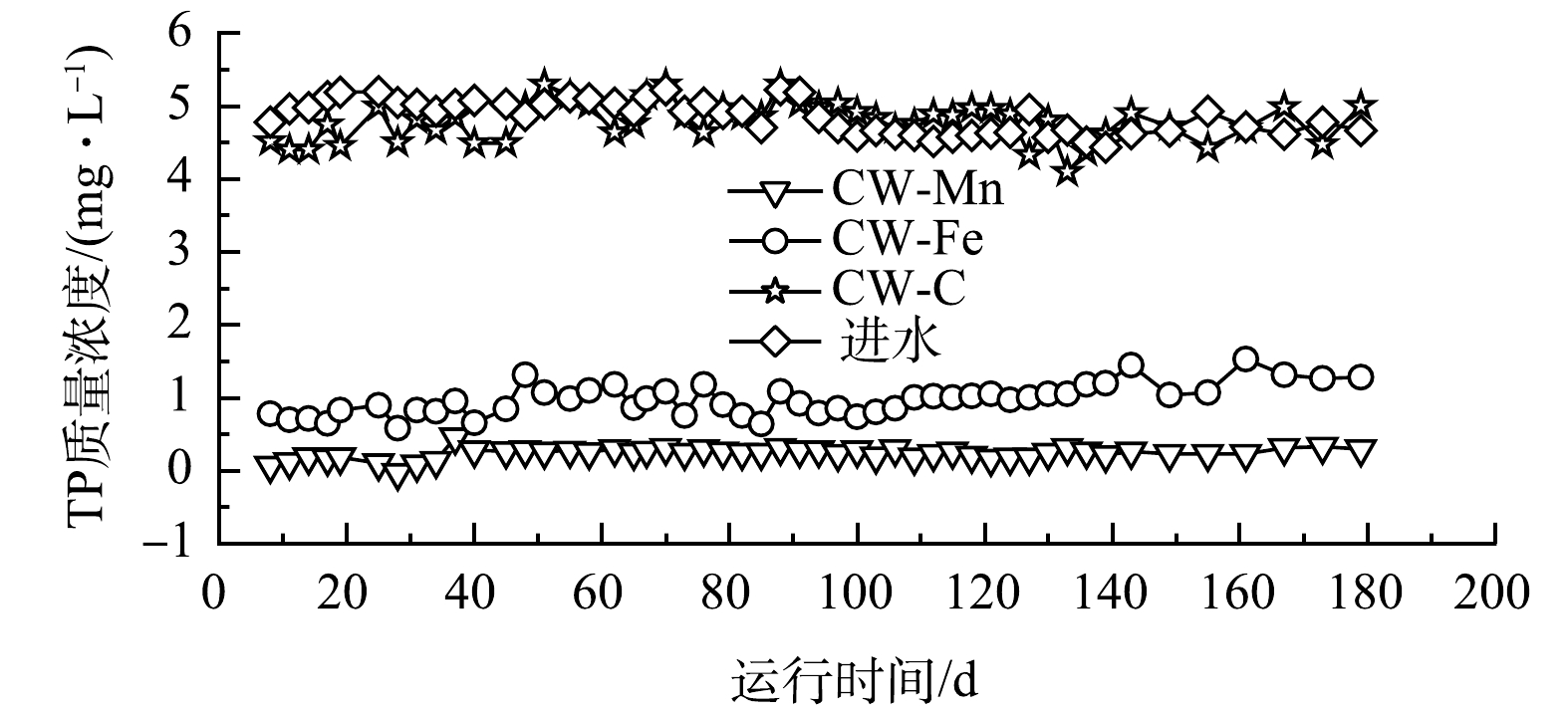
2)总磷的去除效果。由图3可见,3组人工湿地的TP出水质量浓度相对稳定。CW-Fe中平均TP出水质量浓度为0.97 mg·L−1,去除率为79.97%(去除速率为0.27 g·(m2·d)−1);CW-Mn中TP的平均出水质量浓度为0.23 mg·L−1,去除率为95.26%(去除速率为0.32 g·(m2·d)−1);而CW-C的TP平均出水质量浓度为4.77 mg·L−1,去除率为1.4%(去除速率为0.005 g·(m2·d)−1)。结果表明,CW-Mn的TP去除效果最好,比CW-Fe高出15.29%,而CW-C的TP去除效果最差。在废水中,铁矿和锰矿会释放出Mn2+、Fe3+和Ca2+等金属阳离子。这些金属阳离子与磷酸盐结合形成稳定的沉淀,从而可有效地去除TP。相比于砾石对TP的作用,这种去除方式更为有效[17-18]。如果目标出水水质对TP的质量浓度有较高的要求,建议选择CW-Mn。但如果污水的TP质量浓度较低,可以考虑选择CW-Fe,因为CW-Fe的TP去除率也达到了79.97%,而且铁矿比锰矿的价格更实惠,能进一步降低湿地的建设成本。
-
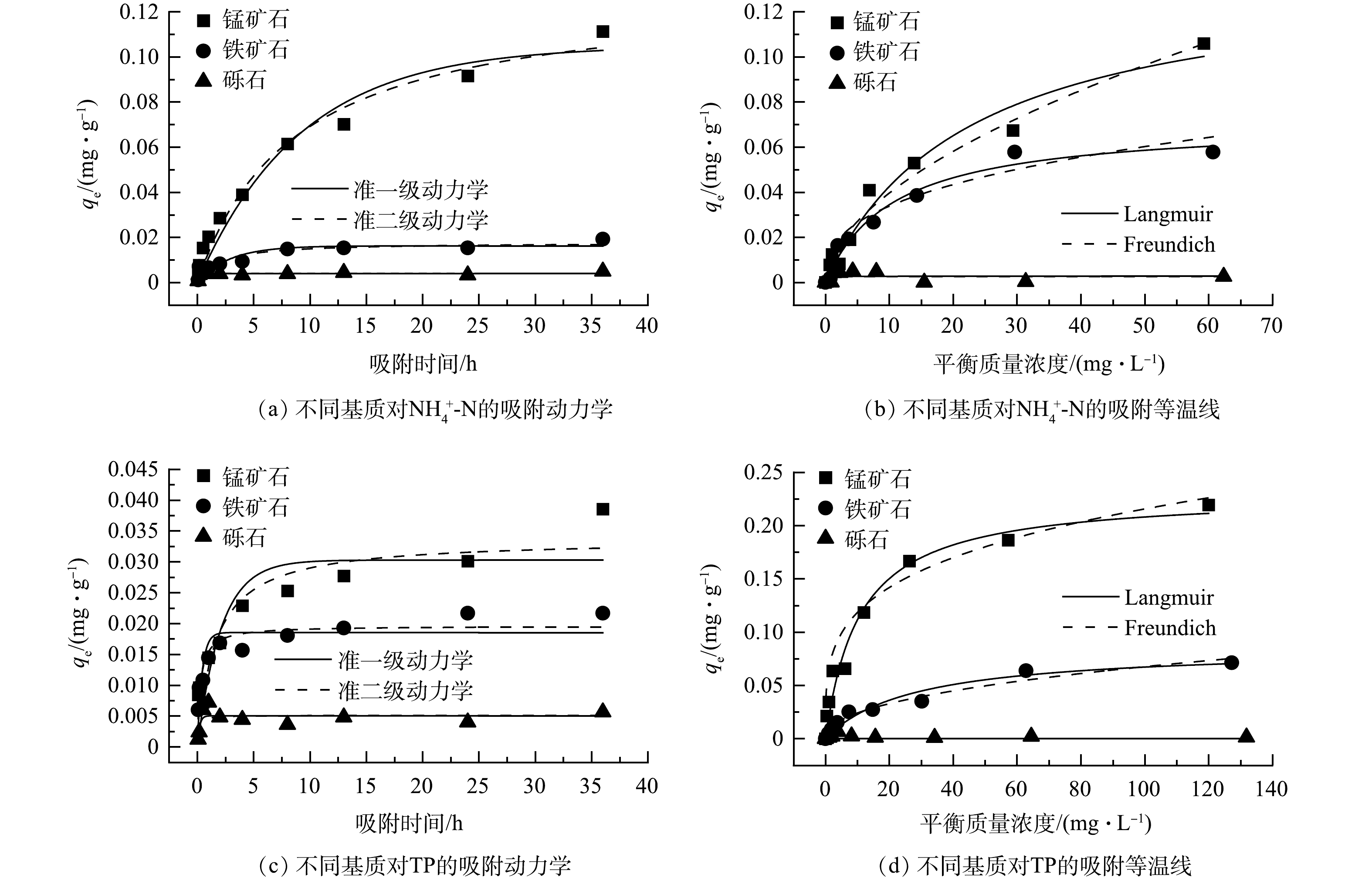
1)材料对氨氮和总磷的吸附动力学。根据图4中的结果,锰矿对TP和NH4+-N吸附性能最好,其次是铁矿,而砾石对TP和NH4+-N几乎没有吸附。这表明在前67 d,CW-Fe和CW-Mn中NH4+-N去除效果的减弱与铁矿和锰矿对NH4+-N的吸附饱和相关。锰矿和铁矿对TP和NH4+-N的吸附均符合准二级动力学模型,其R2均大于准一级动力学模型,说明吸附限制步骤为化学吸附,而不是扩散/离子交换[19]。根据运行期间TP的进水和出水质量浓度,可计算出CW-Fe和CW-Mn在180 d时间内的TP吸收量分别为455.8 mg和544.6 mg。锰矿和铁矿对TP最大平衡吸附量qm分别为0.23 mg·g−1和0.09 mg·g−1,CW-Fe和CW-Mn滤柱最多能吸附TP 657.6 mg和1 034.6 mg。实验运行结束时,CW-Fe中TP吸附量为其最大吸附量的69.3%,而CW-Mn为52.6%。
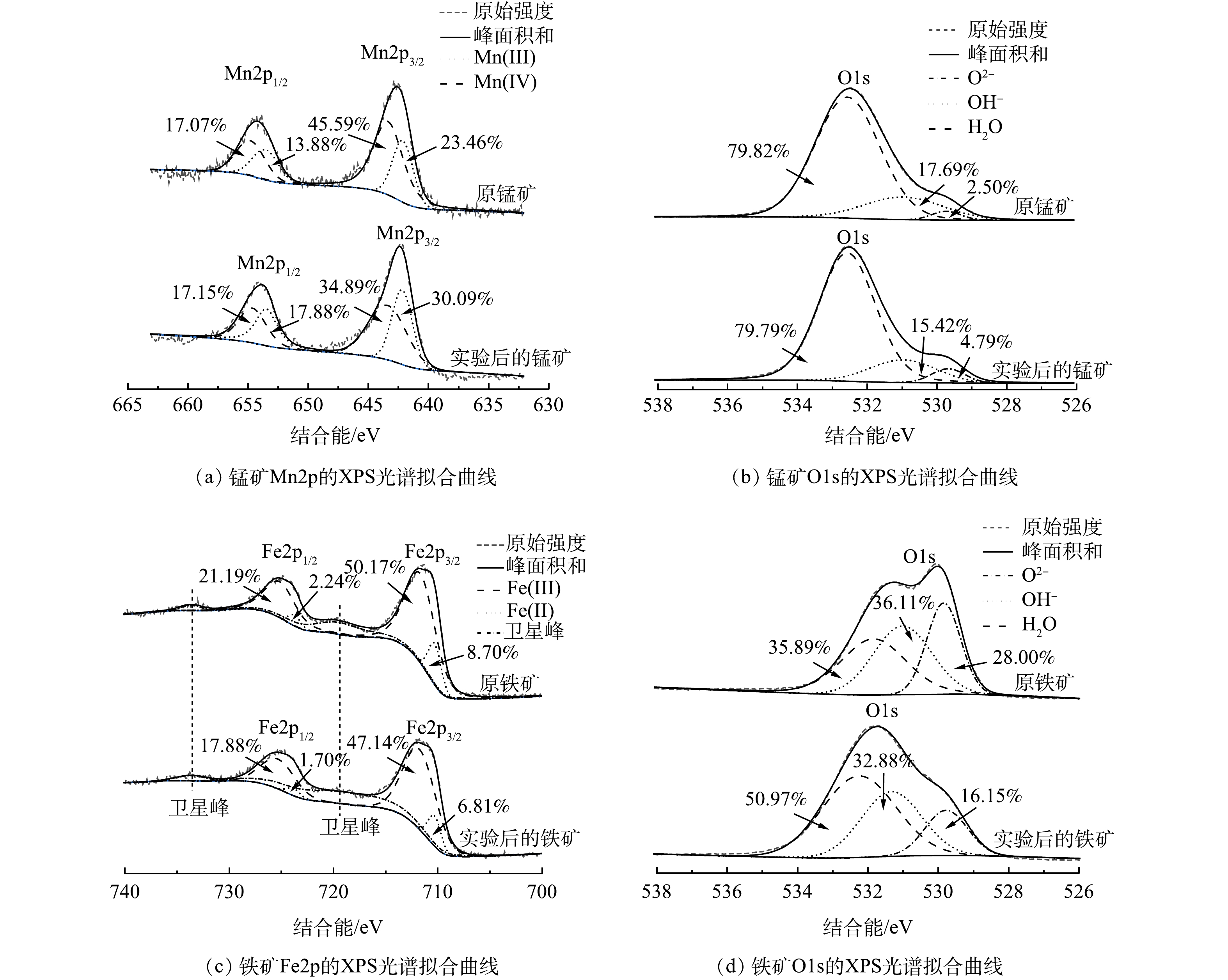
2)湿地运行前后铁/锰矿的特性。XPS分析可以检测铁/锰矿的元素组成和金属氧化状态的变化。由图5(a)可见,2个Mn2p3/2峰分别位于(642.1±0.1) eV和(643.4±0.1) eV,分别代表Mn(III)和Mn(IV)相[20]。原锰矿的Mn(IV)含量(62.6%)高于低价态金属含量。反应后,Mn(IV)的含量略有下降。Fe2p3/2和Fe2p1/2的特征峰的结合能分别为711.7 eV和725.3 eV。Fe2p双峰光谱(2p3/2和2p1/2)之间的结合能差异约为13.6 eV,对应于赤铁矿(Fe2O3)相[21-22]。结合能集中在719.1 eV左右的卫星峰代表典型的Fe2O3氧化态[23],而Fe3O4则没有这个卫星峰[21]。铁矿的此卫星峰在反应后消失,说明Fe(III)向Fe(II)的转化,这与Fe(III)含量减少的结果一致。特征O1s峰的结合能为532 eV。如图5(b)和图5(d)所示,来自不同基底的O1s光谱被分解成3个成分,所有这些成分反应后均有一定程度的变化。XPS表征结果表明,铁矿和锰矿在湿地系统中均发生了变化,高价态的铁和锰氧化物分别可为Feammox和Mnammox反应提供电子受体,并被还原为低价态化合物。
本研究中使用的锰矿和铁矿是天然矿石,它们的元素分布并不均匀,因此没有必要分析材料形态的具体变化。如表1所示,锰矿中的Mn含量在12.5~14.0%之间,而原铁矿和实验后的铁矿的Fe含量分别为58.4%和55.2%。锰矿的Mn含量约为铁矿的20%。铁矿含有较高的Fe(III)含量,这可以为反应提供更多的电子受体。如图2(a)所示,60 d后,当铁矿或锰矿对NH4+-N的吸附变得微弱时,Feammox或Mnammox占NH4+-N去除过程的主导地位,其中Fe(III)或Mn(IV)的含量发挥了主要作用。Fe(III)含量高于Mn(IV)含量,可以提供更多的电子受体,促进NH4+-N的去除。矿石的Mn含量和Fe含量在实验前后没有明显减少。这可能与中性条件下Fe(II)的沉淀和MnOx对Mn离子的吸附有关[24]。
-
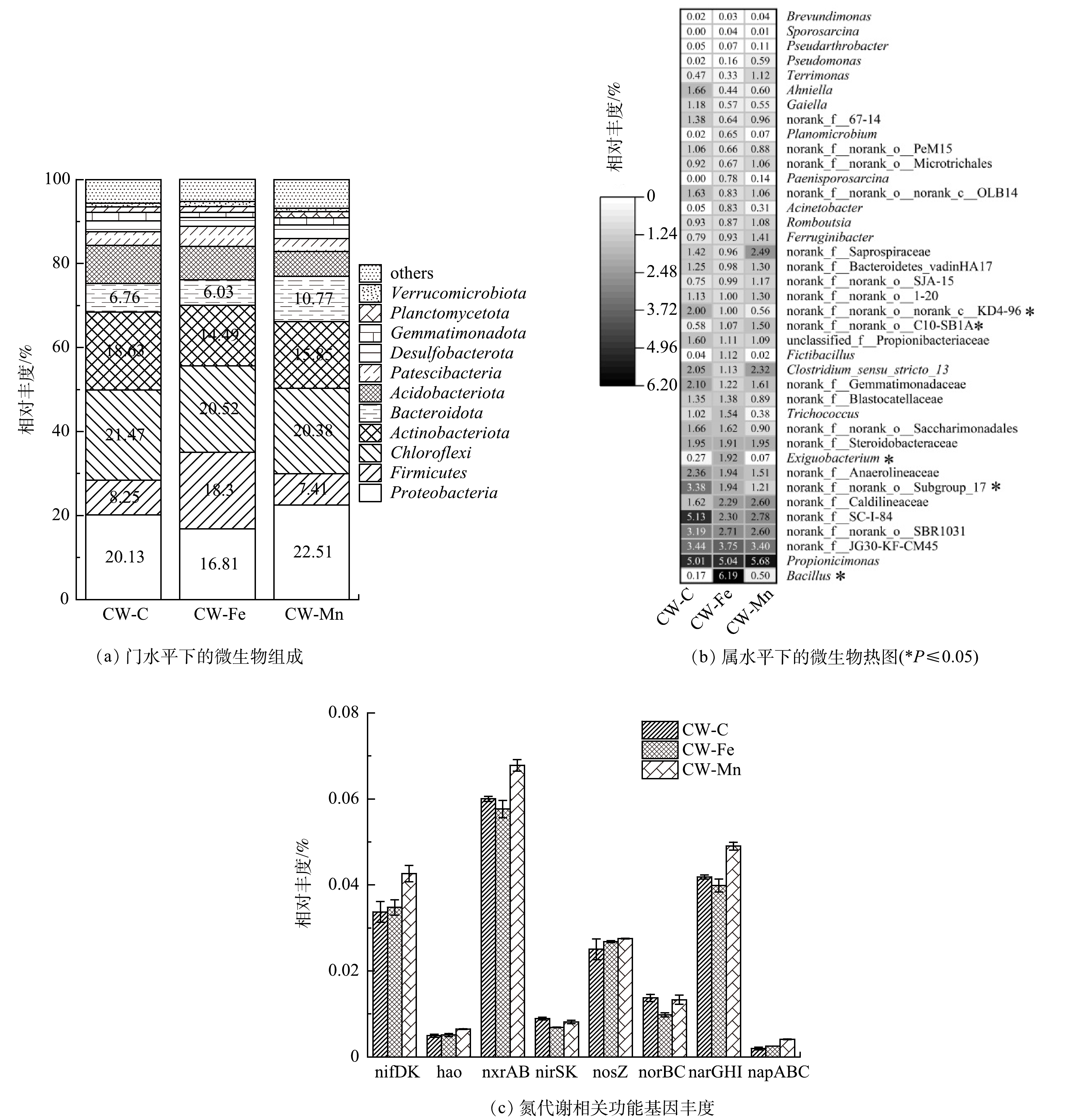
1)微生物群落结构的变化。如图6(a)所示,CW-C、CW-Mn和CW-Fe中门水平下的微生物群落组成中,Proteobacteria,Chloroflexi和Actinobacteriota丰度很高。在CW-Fe中,Firmicutes的丰度(18.3%)为CW-Mn(7.41%)或CW-C(8.25%)的2倍以上。为了进一步研究微生物在不同人工湿地中的作用,在属水平上分析了微生物的群落组成(图6(b))。结果表明,Propionicimonas和norank f JG30-KF-CM45是CW-C、CW-Fe和CW-Mn中共同优势属,且其丰度在3组人工湿地中没有明显差异。norank f JG30-KF-CM45的丰度与TOC含量呈负相关[25],其在3组人工湿地中的丰度为3.4%~3.8%,表明3组人工湿地均在相同的低碳条件下运行。在丰度前15的属中,Firmicutes门下的Bacillus和Exiguobacterium属的丰度有显著差异(P≤0.05)。Bacillus是CW-Fe中丰度最高的属(6.2%),其相对丰度显著高于CW-C(0.2%)和CW-Mn(0.5%)。Exiguobacterium在CW-Fe中的丰度为1.9%,高于CW-C(0.3%)和CW-Mn(0.1%)。KOUKI等[26]曾分离出10个脱氮率大于80%的兼性混养氨氧化细菌,其中4个细菌与Bacillus和Exiguobacterium具有遗传关系。LOVLEY[27]发现一些Bacillus菌株可以使用铁/锰氧化物作为电子受体。Exiguobacterium属下的一种菌株在碱性条件下能通过还原作用有效地溶解赤铁矿[28]。由此可见,Bacillus和Exiguobacterium在还原铁氧化物和氧化NH4+-N方面具有潜力,可能与CW-Fe中的Feammox有关。实际上Bacillus作为铁锰还原菌,其在CW-Mn中也存在富集现象,可能也与Mnammox有关。另一个值得注意的差异是,CW-Fe和CW-Mn的norank f SC-I-84的丰度为2.3%~2.8%,而在CW-C中为5.1%。可能是人工湿地中的铁/锰矿抑制了norank f SC-I-84的富集,但该菌的具体功能尚未被报道[29]。
3组人工湿地中,CW-Mn、CW-Fe和CW-C的反硝化细菌相对丰度分别为5.9%、4.5%和3.9%。使用锰矿作为人工湿地基质会促进反硝化细菌的富集[30],而使用铁矿作为人工湿地基质对反硝化菌富集促进作用较小,甚至会抑制反硝化过程[31]。
2)氮代谢途径。图6(c)反映了与硝化作用(hao,nxrAB)、反硝化作用(nirSK,nosZ,norBC,narGHI,napABC)和固氮作用(nifDK)相关的功能基因在人工湿地中的丰度。结果表明,所有人工湿地中均未检测到与anammox相关的功能基因(hdh和hzsABC),表明湿地系统中未发生anammox反应,因此,Feammox和Mnammox过程均与其无关。与固氮有关的功能基因(nifDK)在CW-Mn中富集,这也是CW-Mn中NH4+-N去除性能不理想的原因之一。CW-C、CW-Fe和CW-Mn中与硝化作用相关的基因丰度分别为0.065%、0.063%和0.074%,与反硝化作用有关的基因丰度分别为0.092%、0.086%和0.102%。CW-Mn中与硝化和反硝化相关的功能基因得到促进,而在CW-Fe中则被抑制。
-
根据吸附实验和系统中TP的去除效果可知,锰矿和铁矿具有良好的TP去除能力,在废水中,铁矿和锰矿会释放出Mn2+、Fe3+和Ca2+等金属阳离子,这些金属阳离子会与磷酸盐结合形成稳定的沉淀,从而有效地去除TP。系统中NH4+-N的去除效果和基质的XPS结果说明,CW-Fe和CW-Mn中分别发生了Feammox和Mnammox反应,促进了NH4+-N的去除。在CW-Mn中,硝化和反硝化相关的功能基因得到了促进,表明Mnammox可以通过常规的氮代谢途径,即功能微生物以Mn(IV)为电子受体,将NH4+-N先氧化为NOx−-N,生成的NOx−-N可以通过传统反硝化或者与Mn2+耦合的反硝化方式生成N2[32]。然而,在CW-Fe中,硝化和反硝化相关的功能基因受到了抑制,而其去除NH4+-N的效果又很显著,表明Feammox中的氮代谢途径跟常规的硝化反硝化的氮代谢途径不一致,即大量NH4+-N并未进行传统的硝化或者反硝化等氮代谢路径,其可能更倾向于直接被氧化为N2。HUANG和JAFFE[8]用乙炔(C2H2)抑制NH4+-N氧化为NO2−-N,N2O还原为N2,和anammox的氮代谢途径,发现Feammox反应没有受到影响,进一步验证了Feammox氧化NH4+-N的直接产物是N2的猜想,与本实验推测结果一致。这是一条全新的氮转化路径,其功能基因也未被报道过。
Feammox和Mnammox具有在人工湿地中广泛应用的潜力,能够在保持传统人工湿地低成本运行和维护等优点的同时,提高其缺氧段的NH4+-N去除效率及TP去除效率。特别是铁氧化物和锰氧化物不仅可以从其他工业和制造废物中回收,也可以利用采矿废物,这使得基质材料来源广泛且成本较低。然而,Feammox和Mnammox的NH4+-N去除效果还有进一步提升的空间。ZHOU等[33]和YANG等[34]的研究表明,添加电子穿梭体可以提高Feammox脱氮性能。同时,WANG等[20]利用锰矿和活性炭作为人工湿地-微生物燃料电池耦合系统的基质,也提高了Mnammox脱氮性能。未来的研究可能会更加专注于探究如何提高Feammox和Mnammox的脱氮性能,并逐渐将这些研究技术转化为中试规模的应用。
-
1)不同类型人工湿地对TP的去除效果顺序为CW-Mn>CW-Fe>CW-C。在基质的NH4+-N吸附能力饱和后,对NH4+-N的去除能力顺序为CW-Fe>CW-Mn>CW-C。
2)铁矿和锰矿填埋在湿地系统后,其金属含量减少,同时部分高价态金属被还原为低价态金属。
3) Bacillus在CW-Mn和CW-Fe中均有富集,而Exiguobacterium只在CW-Fe中富集。
4) Feammox和Mnammox的氮代谢路径存在差异。Feammox倾向于直接将NH4+-N氧化为N2,而Mnammox更倾向于先将NH4+-N氧化为NOx−-N,再进一步还原为N2。
铁/锰矿基人工湿地脱氮除磷性能及机理
Performance and mechanism of nitrogen and phosphorus removal in iron/manganese ore-based constructed wetlands for
-
摘要: 铁氧化物和锰氧化物均可在缺氧条件下介导氨氮(NH4+-N)的氧化去除,这2项技术被称为铁氨氧化(Feammox)和锰氨氧化(Mnammox)。此外,金属氧化物对总磷(TP)也有去除能力,因此,在人工湿地中具有良好的应用前景。为比较铁矿基和锰矿基人工湿地的脱氮除磷效果,本研究建立了铁矿基人工湿地(CW-Fe)、锰矿基人工湿地(CW-Mn)和砾石对照组人工湿地(CW-C)3组人工湿地。结果表明,CW-Fe和CW-Mn的脱氮除磷性能均优于CW-C。尽管锰矿对NH4+-N的吸附作用最强,但CW-Fe却表现出了更优越的NH4+-N长期去除性能。在基质对NH4+-N的吸附饱和后,CW-Fe对NH4+-N的去除率仍有39.93%~62.4%,而CW-Mn只有29.15%~35.4%。由于铁矿和锰矿溶出的金属离子能与磷酸盐结合形成稳定的沉淀,从而有效去除TP,CW-Fe和CW-Mn均有优异的TP去除性能。CW-Mn的TP去除率最高,为95.26%,其次是CW-Fe,为79.97%。在微生物方面,具有还原铁氧化物和氧化NH4+-N潜力的Bacillus和Exiguobacterium在CW-Fe中均得到了显著富集。结合水质数据及脱氮相关功能菌的分析,推测出Feammox中可能更倾向于将NH4+-N直接氧化为N2,而Mnammox则是更倾向于先将NH4+-N氧化为NOx−-N。本研究可为探索同步脱氮除磷的低能耗污水处理工艺及人工湿地中基质的选择提供案例参考。Abstract: Both Fe(III) and Mn(IV) oxides can oxidize and remove ammonia nitrogen (NH4+-N) under anoxic conditions, and these two technologies are known as Feammox and Mnammox. Additionally, metal oxides also have the ability to remove total phosphorus (TP), making them a promising option for use in constructed wetlands (CWs). In order to compare the nitrogen and phosphorus removal efficiency of iron ore-based and manganese ore-based CWs, three groups of CWs were established for this study: iron ore (CW-Fe), manganese ore (CW-Mn), and a control group with gravel (CW-C). The experimental results showed that both CW-Fe and CW-Mn had better nitrogen and phosphorus removal performance than CW-C. Although manganese ore had the strongest adsorption effect on NH4+-N, CW-Fe exhibited a superior performance on long-term removal for NH4+-N. Even after saturated adsorption to NH4+-N by the substrates, the removal efficiency of NH4+-N by CW-Fe remained at 39.93% to 62.4%, while that of CW-Mn only remained 29.15% to 35.4%. Because metal ions dissolved from iron ore and manganese ore can combine with phosphates to form stable precipitates, effectively removing TP, both CW-Fe and CW-Mn showed the excellent TP removal performance. The TP removal efficiency of CW-Mn was the highest, at 95.26%, followed by CW-Fe at 79.97%. In terms of microorganisms, Bacillus and Exiguobacterium, which have the potential to reduce iron oxides and oxidize NH4+-N, were significantly enriched in CW-Fe. Based on the water quality data and analysis of nitrogen-related functional bacteria, it was speculated that Feammox may be more likely to directly oxidize NH4+-N to N2, while Mnammox is more likely to first oxidize NH4+-N to NOx−-N. This study provides a reference for exploring low-energy wastewater treatment processes that achieve simultaneous nitrogen and phosphorus removal, as well as selecting substrates used in constructed wetlands.
-
Key words:
- constructed wetlands /
- Feammox /
- Mnammox /
- nutrient removal
-

-
表 1 基质材料的EDS结果
Table 1. EDS results of substrates
% 基质材料 O Fe Si Mn Al 原锰矿 60.3 5.6 17.6 14.0 2.5 实验后的锰矿 57.4 5.8 20.7 13.3 2.9 原铁矿 29.1 58.4 5.6 5.8 1.1 实验后的铁矿 29.2 55.2 8.0 4.8 2.8 -
[1] TANG S Y, LIAO Y H, XU Y C, et al. Microbial coupling mechanisms of nitrogen removal in constructed wetlands: A review[J]. Bioresource Technology, 2020, 314: 123759. doi: 10.1016/j.biortech.2020.123759 [2] WU Q, XIAO J J, FU L J, et al. Microporous intermittent aeration vertical flow constructed wetlands for eutrophic water improvement[J]. Environmental Science and Pollution Research, 2020, 27(14): 16574-16583. doi: 10.1007/s11356-020-08067-x [3] UNG H T T, LEU B T, TRAN H T H, et al. Combining flowform cascade with constructed wetland to enhance domestic wastewater treatment[J]. Environmental Technology & Innovation, 2022, 27: 102537. [4] ZHANG M P, HUANG J C, SUN S S, et al. Nitrogen removal through collaborativemicrobial pathways in tidal flow constructed wetlands[J]. Science of the Total Environment, 2021, 758: 143594. doi: 10.1016/j.scitotenv.2020.143594 [5] SHI J H, YANG Z X, DAI H L, et al. Preparation and application of modified zeolites as adsorbents in wastewater treatment[J]. Water Science and Technology, 2018, 77: 621-635. [6] DESIREDDY S, CHACKO S P. A review on metal oxide (FeOx/MnOx) mediated nitrogen removal processes and its application in wastewater treatment[J]. Reviews in Environmental Science and Bio-Technology, 2021, 20(3): 697-728. doi: 10.1007/s11157-021-09581-1 [7] WANG Y, BAI Y H, SU J F, et al. Advances in microbially mediated manganese redox cycling coupled with nitrogen removal in wastewater treatment: A critical review and bibliometric analysis[J]. Chemical Engineering Journal, 2023, 461: 141878. doi: 10.1016/j.cej.2023.141878 [8] HUANG S, JAFFE P R. Characterization of incubation experiments and development of an enrichment culture capable of ammonium oxidation under iron-reducing conditions[J]. Biogeosciences, 2015, 12(3): 769-779. doi: 10.5194/bg-12-769-2015 [9] XU B K, SHI L S, ZHONG H, et al. Investigation of Fe(II) and Mn(II) involved anoxic denitrification in agricultural soils with high manganese and iron contents[J]. Journal of Soils and Sediments, 2021, 21(1): 452-468. doi: 10.1007/s11368-020-02776-z [10] DING L J, AN X L, LI S, et al. Nitrogen loss through anaerobic ammonium oxidation coupled to iron reduction from paddy soils in a chronosequence[J]. Environmental Science & Technology, 2014, 48(18): 10641-10647. [11] CHEN S, DING B J, QIN Y B, et al. Nitrogen loss through anaerobic ammonium oxidation mediated by Mn(IV)-oxide reduction from agricultural drainage ditches into Jiuli River, Taihu Lake Basin[J]. Science of the Total Environment, 2020, 700: 134512. doi: 10.1016/j.scitotenv.2019.134512 [12] CHENG C, HE Q, ZHANG J, et al. New insight into ammonium oxidation processes and mechanisms mediated by manganese oxide in constructed wetlands[J]. Water Research, 2022, 215: 118251. doi: 10.1016/j.watres.2022.118251 [13] FERNANDES S O, JAVANAUD C, AIGLE A, et al. Anaerobic nitrification–denitrification mediated by Mn-oxides in meso-tidal sediments: Implications for N2 and N2O production[J]. Journal of Marine Systems, 2015, 144: 1-8. doi: 10.1016/j.jmarsys.2014.11.011 [14] LUTHER G W, SUNDBY B, LEWIS B L, et al. Interactions of manganese with the nitrogen cycle: Alternative pathways to dinitrogen[J]. Geochimica et Cosmochimica Acta, 1997, 61(19): 4043-4052. doi: 10.1016/S0016-7037(97)00239-1 [15] SHEN S T, LI X, CHENG F K, et al. Review: Recent developments of substrates for nitrogen and phosphorus removal in CWs treating municipal wastewater[J]. Environmental Science and Pollution Research, 2020, 27(24): 29837-29855. doi: 10.1007/s11356-020-08808-y [16] FANG D X, ZHAO G, XU X Y, et al. Microbial community structures and functions of wastewater treatment systems in plateau and cold regions[J]. Bioresource Technology, 2018, 249: 684-693. doi: 10.1016/j.biortech.2017.10.063 [17] WANG R G, ZHAO X, WANG T C, et al. Can we use mine waste as substrate in constructed wetlands to intensify nutrient removal? A critical assessment of key removal mechanisms and long-term environmental risks[J]. Water Research, 2022, 210: 118009. doi: 10.1016/j.watres.2021.118009 [18] 曾银金, 许伟斌. 同步脱氮除磷基质应用于人工湿地的研究进展[J]. 湿地科学, 2022, 20(6): 852-858. doi: 10.13248/j.cnki.wetlandsci.2022.06.016 [19] PAP S, KIRK C, BREMNER B, et al. Low-cost chitosan-calcite adsorbent development for potential phosphate removal and recovery from wastewater effluent[J]. Water Research, 2020, 173: 115573. doi: 10.1016/j.watres.2020.115828 [20] WANG Y F, SONG X S, CAO X, et al. Integration of manganese ores with activated carbon granules into CW-MFC to trigger anoxic electron transfer and removal of ammonia nitrogen[J]. Journal of Cleaner Production, 2022, 334: 130202. doi: 10.1016/j.jclepro.2021.130202 [21] OMRAN M, FABRITIUS T, ELMAHDY A M, et al. XPS and FTIR spectroscopic study on microwave treated high phosphorus iron ore[J]. Applied Surface Science, 2015, 345: 127-140. doi: 10.1016/j.apsusc.2015.03.209 [22] DGHOUGHI L, ELIDRISSI B, BERNEDE C, et al. Physico-chemical, optical and electrochemical properties of iron oxide thin films prepared by spray pyrolysis[J]. Applied Surface Science, 2006, 253(4): 1823-1829. doi: 10.1016/j.apsusc.2006.03.021 [23] INGO G M, MAZZONI S, BULTRINI G, et al. Small-area xps and xaes study of the iron-ore smelting process[J]. Surface and Interface Analysis, 1994, 22(1-12): 614-619. doi: 10.1002/sia.7402201131 [24] CHENG Y, ZHANG Y Z, XIONG W Y, et al. Simultaneous removal of tetracycline and manganese (II) ions from groundwater using manganese oxide filters: Efficiency and mechanisms[J]. Journal of Water Process Engineering, 2021, 42: 102158. doi: 10.1016/j.jwpe.2021.102158 [25] JIANG J S, WANG Y, YU D, et al. Garbage enzymes effectively regulated the succession of enzymatic activities and the bacterial community during sewage sludge composting[J]. Bioresource Technology, 2021, 327: 124792. doi: 10.1016/j.biortech.2021.124792 [26] KOUKI S, SAIDI N, M'HIRI F, et al. Isolation and characterization of facultative mixotrophic ammonia-oxidizing bacteria from constructed wetlands[J]. Journal of Environmental Sciences, 2011, 23(10): 1699-1708. doi: 10.1016/S1001-0742(10)60596-7 [27] LOVLEY D. Dissimilatory Fe (III)-and Mn (IV)-reducing prokaryotes[J]. Prokaryotes, 2006, 2: 635-658. [28] ANEKSAMPANT A, NAKASHIMA K, KAWASAKI S. Microbial leaching of iron from hematite: Direct or indirect elution[J]. Materials Transactions, 2020, 61(2): 396-401. doi: 10.2320/matertrans.M-M2019860 [29] ZHANG J L, LIU Q W, LI K, et al. Peanut root exudates suppress fusarium solani and modulate the microbial community structure of rhizosphere in grape replant soil[J]. Horticulturae, 2022, 8(10): 892. doi: 10.3390/horticulturae8100892 [30] XIE H J, YANG Y X, LIU J H, et al. Enhanced triclosan and nutrient removal performance in vertical up-flow constructed wetlands with manganese oxides[J]. Water Research, 2018, 143: 457-466. doi: 10.1016/j.watres.2018.05.061 [31] HUANG X, YAO K, YU J H, et al. Nitrogen removal performance and microbial characteristics during simultaneous chemical phosphorus removal process using Fe3+[J]. Bioresource Technology, 2022, 363: 127972. doi: 10.1016/j.biortech.2022.127972 [32] JAVANAUD C, MICHOTEY V, GUASCO S, et al. Anaerobic ammonium oxidation mediated by Mn-oxides: From sediment to strain level[J]. Research in Microbiology, 2011, 162(9): 848-857. doi: 10.1016/j.resmic.2011.01.011 [33] ZHOU G W, YANG X R, LI H, et al. Electron shuttles enhance anaerobic ammonium oxidation coupled to iron(III) reduction[J]. Environmental Science & Technology, 2016, 50(17): 9298-9307. [34] YANG Y F, PENG H, NIU J F, et al. Promoting nitrogen removal during Fe(III) reduction coupled to anaerobic ammonium oxidation (Feammox) by adding anthraquinone-2, 6-disulfonate (AQDS)[J]. Environmental Pollution, 2019, 247: 973-979. doi: 10.1016/j.envpol.2019.02.008 -



 下载:
下载: