-
草甘膦 (N-phosphonomethylglycine, PMG)[1-2]是一种广谱灭生性、内吸传导性的典型溶解态有机磷 (dissolved organic phosphorus DOP) 除草剂,具有高效、广谱、低毒的优点[3]。然而,PMG分子高度的流动性和水溶性[4]使其在施用后以城市径流、雨水冲刷[5]、过度喷洒等形式潜入水循环系统,广泛污染于各类水体环境中[6-7]。当前,价廉且高效的吸附材料研发已成为吸附去除有机磷的主要研究之一。利用废弃物制备新型、廉价、“绿色”吸附剂是研究热点[8-9],牡蛎壳是一种产量高且含有丰富CaCO3和大量微孔的天然壳体。研究发现,对牡蛎壳粉改性后其比表面积更大,可高效吸附水体中的有机磷[10]。然而,牡蛎壳粉只能将有机磷从水相转移至固相,并未实现降解,其毒害作用仍将对环境造成长期潜在风险。
光催化技术可以将有机磷分解为溶解态无机磷、CO2和H2O等小分子[11]。其中金属离子和非金属离子掺杂改性TiO2不仅具备化学稳定性高、成本低、无毒等优势,同时能有效弥补TiO2的缺陷,实现在可见光波长范围内响应,降低光生载流子复合率[12]。其中,铈离子 (Ce3+/Ce4+) 是4种最丰富的稀土金属之一,价格便宜且无毒,被认为是修饰TiO2晶体和电子结构、改变光学性质,提高量子产率的理想掺杂剂[13-15]。其次,在TiO2晶格中掺杂氮元素可以减小禁带宽度[16],光响应范围向可见光区域红移[17]。因此,将Ce和N掺杂于TiO2并将其负载在改性牡蛎壳粉上,研究其在模拟太阳光下吸附-光催化协同降解PMG的降解性能显得尤为必要。
本研究采用水热法制备改性牡蛎壳粉/Ce-N-TiO2 (CeNT@Oys)复合光催化剂,借助SEM、BET、XPS等手段对其表面的形貌和结构进行了表征分析;以PMG为目标去除物,研究了CeNT@Oys吸附-光催化协同降解PMG的性能,进行协同作用评价与分析,并考察不同pH、温度、复合光催化剂投加量等影响因素对协同作用的影响,以期为水体中有机磷类农药的高效、彻底去除提供参考。
-
主要试剂包括:过硫酸钾、钼酸铵、抗坏血酸、酒石酸锑钾、浓硫酸、盐酸;PMG (质量分数≥95%) ;实验用水均为超纯水。制备光催化剂时选取无水乙醇作为溶剂,六水合硝酸铈(Ce(NO3)3-6H2O)和尿素分别作为铈和氮的来源,废弃牡蛎壳取自中国湖南省。所有的化学品纯度均为分析纯,可以直接使用,无需进一步处理。
主要仪器与装置:模拟日光氙灯光源 (CEL-S500型) ,多头恒温磁力搅拌器 (HJ-6型) ,紫外可见分光光度计 (UV-1800型) ,高速台式冷冻离心机 (H2050R-1型) ,马弗炉 (KSY-14-16型) ,微波闭式消解仪 (GZ-WXJ-Ⅲ型) ,电热恒温鼓风干燥箱 (DHG-9030A型) 。
-
将废弃牡蛎壳表面残渣用洗涤剂和清洁球清洁,用切割机将其表面打磨至通体白色并切割成小块,以65 ℃鼓风干燥箱烘干后密封式制样粉碎机粉碎并以200目过筛粒径过筛,然后在0.1%稀盐酸中浸泡1 h,真空干燥后在马弗炉中以900 ℃煅烧2 h制备成改性牡蛎壳粉,密封保存备用。
-
通过水热法制备CeNT@Oys,其制备步骤如下:将36 mL无水乙醇和定量改性牡蛎壳粉进行超声振荡混合,然后逐滴加入钛酸四丁酯匀速搅拌制得混合物A;再次量取相同体积的无水乙醇、3.0 mL超纯水和一定量盐酸充分搅拌混合后,投加2.0 g尿素和一定量的六水合硝酸铈,在超声振荡作用下制得混合物B。在快速搅拌且不产生气泡的条件下,将混合物B以30 滴·min−1速率缓慢滴加至混合物A中,在室温下继续搅拌1.5~2 h。
在水热处理阶段,将上述混合物转移至聚四氟乙烯内衬高温高压反应釜中,将其保存至120 ℃的真空干燥箱中16 h后取出冷却,用去离子水将沉淀混合物过滤数次,60 ℃烘干过夜。为使材料结晶完全,在马弗炉中以一定温度煅烧3 h制备成CeNT@Oys复合光催化剂。另外,在不加入改性牡蛎壳粉的基础上,Ce-N-TiO2同样采用上述程序制备。
-
1)协同作用研究方法。PMG水溶液的光催化降解在CEL-S500型模拟日光氙灯光源照射下进行。此外,为减少光源对温度影响并保持反应环境温度平衡,光催化反应器由双层套杯连接低温恒温循环器组成。实验步骤如下:量取100 mL质量浓度50 mg·L−1的PMG水溶液,加入磁力搅拌子,分别向水溶液中投加5 mg改性牡蛎壳粉、95 mg Ce-N-TiO2和100 mg CeNT@Oys复合光催化剂并超声混合15 min。
在磁力搅拌器运作的同时打开冷却水循环系统,然后启动模拟日光氙灯光源。光照开始后用取样器分别于20、40、60、80、100、120、140、160、180 min时量取少量悬浮液,在高速离心机中离心15 min后吸取1 mL上清液待测定磷酸根质量浓度。同时采用上述实验方法分析温度、pH、磁力搅拌器转速、复合光催化剂投加量等影响因素对吸附-光催化协同作用的影响。
2)磷酸根质量浓度确定方法。磷酸根质量浓度的测定方法采用钼酸铵分光光度法,具体操作步骤如下:吸取上清液后定容至25 mL刻度线,添加1 mL抗坏血酸溶液,摇匀,30 s后再添加2 mL钼酸盐溶液,在25~30 ℃水浴锅中静置15 min,移取上述比色管中部分溶液于比色皿中,在波长700 nm的紫外可见分光光度计中测定吸光度,根据磷酸根标准曲线确定磷酸根质量浓度,以此计算PMG溶液的有机磷降解率(式(1))。
式中:η为PMG中有机磷的降解率,%;Ct为t时刻PMG中PO43-含量;C0为初始时刻PMG中总磷含量;测定总磷时需将PMG水溶液中加入过硫酸钾溶液,然后在闭式消解仪中消解后再根据上述程序测定。
-
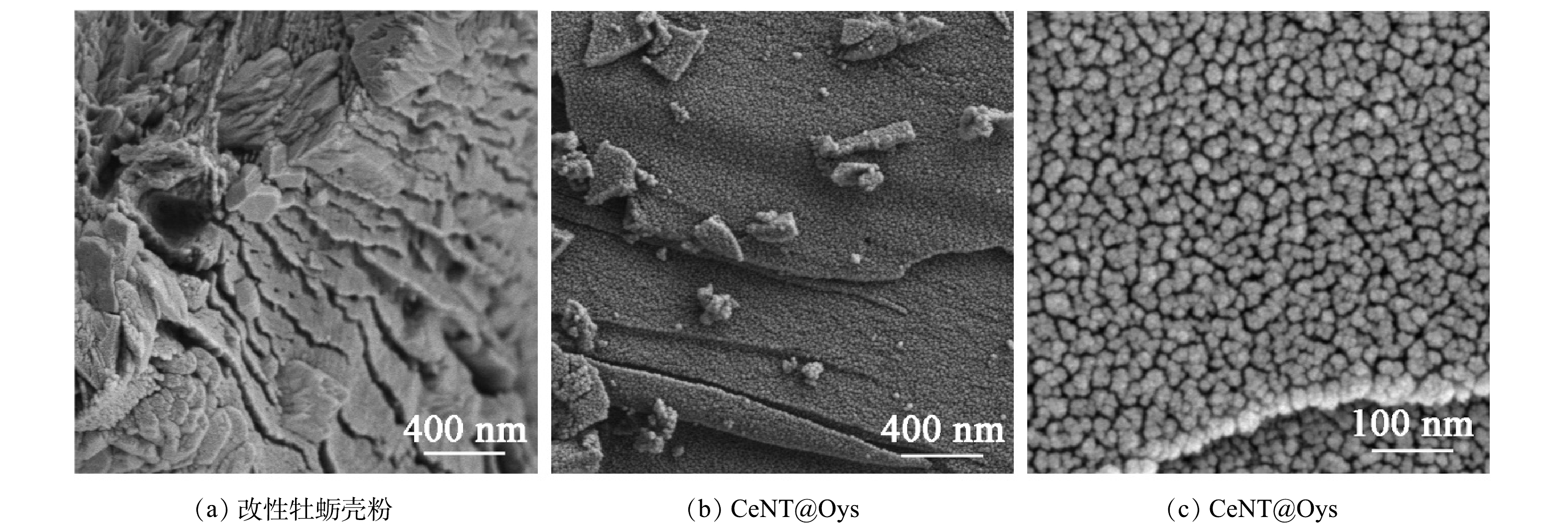
1)表面形貌分析。图1(a)为改性牡蛎壳粉扫描电镜图,图1(b)和图1(c)为不同放大倍数下CeNT@Oys复合光催化剂扫描电镜图。如图1(a) 所示,改性牡蛎壳粉呈现出分层板块状微观结构。图1(b) 表明Ce-N-TiO2纳米颗粒均匀分布在改性牡蛎壳粉的板块结构表面,并在个别区域存在颗粒轻微聚集现象。由图1(c) 可以看出,再次放大后CeNT@Oys纳米颗粒呈现粒径大小约为20~30nm的均匀纳米颗粒状态。
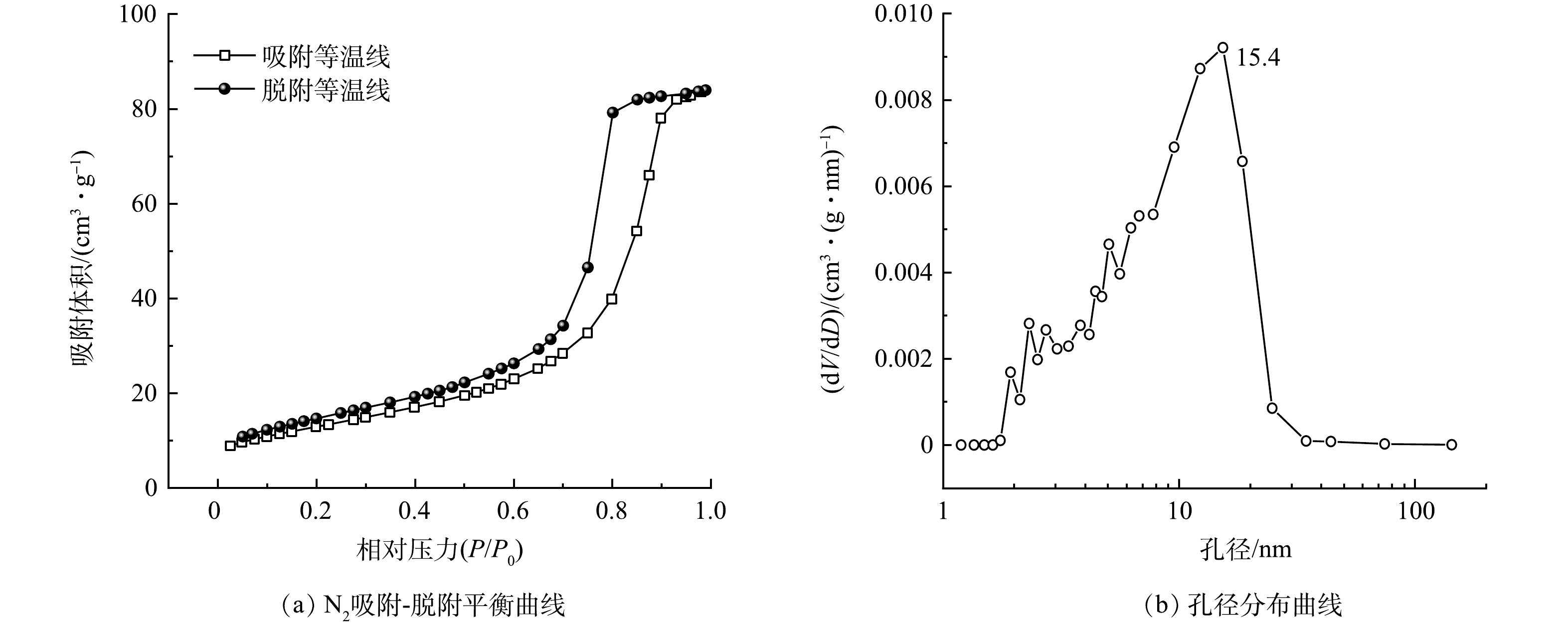
2)比表面积、孔径分析。图2和图3分别 为Ce-N-TiO2和CeNT@Oys的 N2吸附-解吸等温曲线和孔径分布曲线。根据IUPAC分类分析,Ce-N-TiO2和CeNT@Oys均为IV型等温吸附曲线,并且在相对压力为0.6~0.9内具有吸附滞后现象,存在H2型回滞环,表明其存在介孔结构[18]。Ce-N-TiO2在掺杂改性牡蛎壳粉前后其比表面积分别为61.649 m2·g−1和75.301 m2·g−1,在相对压力大于0.9时吸附趋于饱和,表明2种光催化剂孔径分布较均匀。基于BJH方法,由氮气等温线的吸附分支估算孔径分布。可见,在掺杂改性牡蛎壳粉前后Ce-N-TiO2平均孔径中心分别为15.4 nm和12.2 nm。这再次证明催化剂表面孔洞大部分为介孔结构。
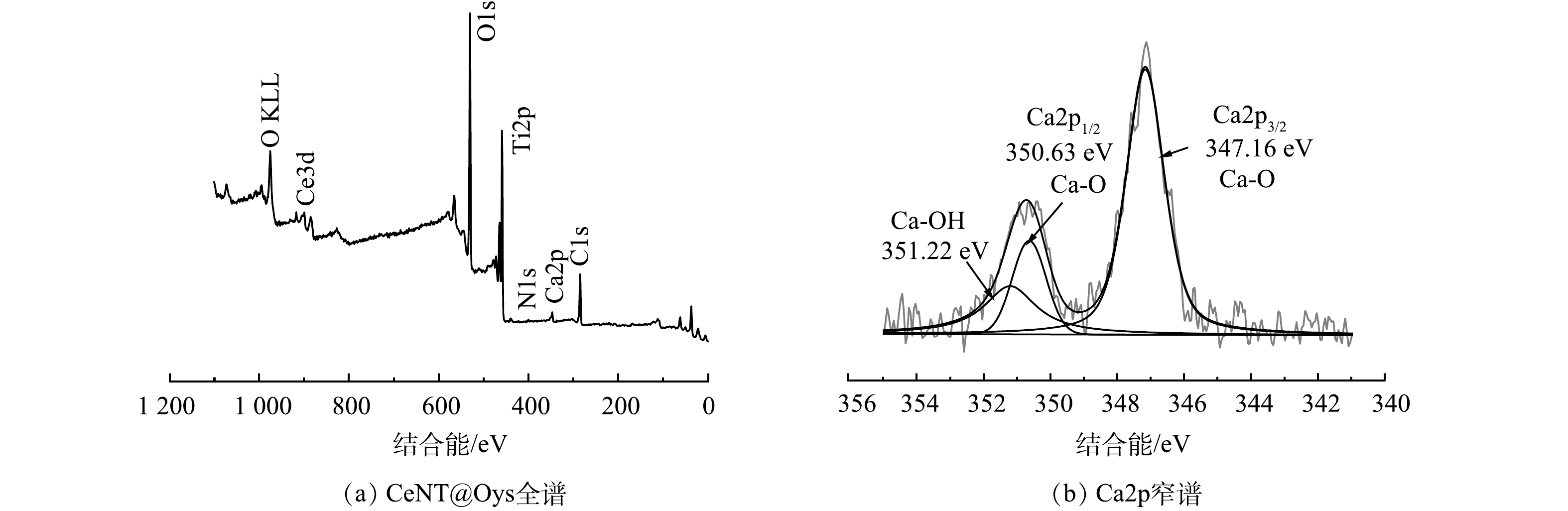
3)XPS表征分析。图4(a)为CeNT@Oys的XPS全谱图。可见,样品中含有元素Ti、O、Ca、N和Ce,其中Ti和O为主要元素,Ca元素来源于改性牡蛎粉的掺杂,而Ce和N的弱峰分别来自于元素前驱体的引入。不同元素的高分辨率XPS谱图以C1s主峰结合能(284.80 eV) 为基准作电荷校正。图4(b)为Ca2p高分辨谱图。Ca2p3/2中位于347.16 eV的Ca—O键、Ca 2p1/2中位于350.63 eV的Ca—O键和351.22 eV的Ca—OH键,三峰面积比为4:2:1,Ca—OH键的存在表明在一定程度上改性牡蛎壳粉的存在有利于对羟基的吸附。
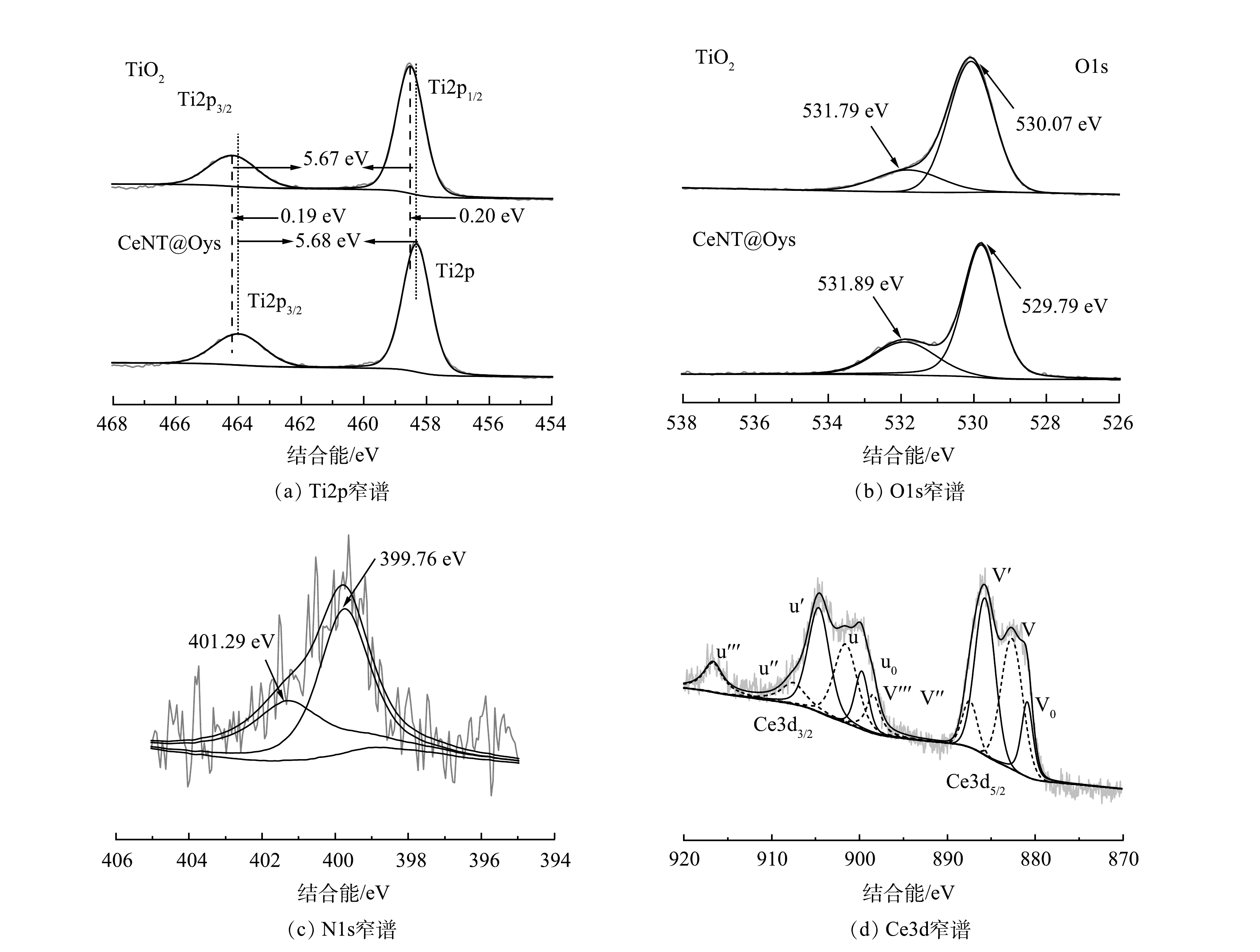
图5(a)为Ti2p高分辨谱图。458.51 eV和458.31 eV的结合能均代表Ti2p1/2,结合能为464.18 eV和463.99 eV的谱峰代表Ti2p3/2,双峰之间差值约5.6 eV,这与Ti4+(锐钛矿TiO2)一致,表明Ti只以Ti4+形式存在。图5(b)为O1s被去卷积为双峰,Ce-N-TiO2负载于改性牡蛎壳粉前后,一组特征峰(529.79 eV、530.07 eV) 代表晶格氧 (O—Ti—O) ,另一组特征峰 (531.79 eV、531.89 eV) 归属于化学吸附氧或羟基的存在。CeNT@Oys在531.89 eV处的峰值高于TiO2,显然,制备的复合光催化剂中的羟基含量更高,这表明与Ce和N的掺杂共掺杂使催化剂表面羟基含量增多,这更有利于捕获光生电子以提高电子-空穴对分离效率,优化光催化性能[19-20]。此外,CeNT@Oys中的Ti元素的双峰均向较低结合能处移动约0.2 eV,这主要是由于N的电负性小于O[21],N掺杂导致Ti和O周围电子云密度增大,结合能减小,峰位发生偏移。
关于N掺杂TiO2中N1s能谱图分析略有不同。大多数研究者认为,N1s结合能主要与N在TiO2掺杂方式或N-TiO2合成方法相关[22]。TiN特征峰在396 eV附近,因此396 eV~398 eV的特征峰归因于氮物种置换晶格氧形成的O—Ti—N键或N—Ti—N键,398 eV–401 eV的特征峰则为间隙N物种的形成。如图5(c) 所示,N1s拟合曲线在396-398 eV未检测到Ti—N的N1s峰,显而易见,399.76 eV的峰值是N以间隙形式构成Ti—N—O或N—O—Ti的结构特征所形成[23-24]。而401.29 eV则可能与N—H键的存在有关[25]。
图5(d) 为Ce3d分峰拟合的XPS图,由于Ce4f轨道和O2p轨道的不同杂化情况以及Ce3+和Ce4+电子价态不同,Ce3d光谱分裂为自旋轨道多重态,即3d5/2自旋轨道态(标记为v)和3d3/2自旋轨道态 (标记为u) 。根据BURROUGHS等[26]构建的规则进行反褶积运算。图中实线拟合峰代表Ce4+,虚线拟合峰代表Ce3+。以Ce3d5/2为例,v'、v''、v'''为3d5/2自旋轨道中代表Ce4+的特征峰,其中v和v''特征峰的存在归因于O2p轨道分配2个或1个电子到Ce4f轨道上,即 (5d6s)04f2 O2p4和 (5d6s)04f1 O2p5混合构型。v'''特征峰为Ce4+的初级光电发射引起,即 (5d6s)04f0 O2p6。v0和v'为构建Ce3+的3d5/2自旋轨道态的特征峰。其中v'代表未杂化构型,即 (5d6s)04f1 O2p6,v0代表 (5d6s) 04f2 O2p5混合构型。由此推断,催化剂样品中存在Ce3+和Ce4+,并且Ce存在于晶界间隙或表面,形成Ce—O—Ti键。
-
在实验环境25 ℃、初始pH=7,催化剂投加量1.0 g·L−1,磁力搅拌器转速300 r·min−1,氙灯光功率400 W的条件下,分别考察改性牡蛎壳粉对PMG单独吸附、TiO2单独光催化、Ce-N-TiO2纳米颗粒对PMG的单独光催化降解及CeNT@Oys对PMG吸附与光催化协同作用的降解率,其结果如图6所示。
与TiO2单独光降解比较,复合催化剂对有机磷降解率明显更高,这是由于纯TiO2禁带较宽,仅能吸收紫外光导致其光响应能力在模拟日光下远远低于改性催化剂。将改性牡蛎壳粉的单独吸附去除率和Ce-N-TiO2单独光催化降解率叠加,得到理论叠加降解率曲线,从而分析CeNT@Oys复合光催化剂对PMG吸附-光催化降解是否具有协同作用。由图6可以看出,在前120 min内复合材料的降解率和降解速率明显高于理论叠加曲线的降解速率,PMG中有机磷降解速率常数由1.179×10−2 min−1升至2.441×10−2 min−1,这表明将改性牡蛎壳粉与Ce-N-TiO2复合后对于降解PMG具有明显的吸附-光催化协同作用。这主要是因为掺入改性牡蛎壳粉有利于将PMG吸附至Ce-N-TiO2光催化剂表面,在光催化反应中产生浓度效应[27],加速PMG分子与电子-空穴对作用,从而增强光催化降解活性;通过分析Ce-N-TiO2负载于改性牡蛎壳粉前后的比表面积可知,掺入改性牡蛎壳粉在一定程度上使Ce-N-TiO2比表面积增大,对吸附PMG分子起到正向促进作用。然而,在光催化降解120 min后,虽然理论叠加值曲线始终位于复合光催化剂协同降解曲线之下,但两者差距逐渐缩小,这表明CeNT@Oys对PMG的吸附-光催化降解协同作用逐渐降低,主要原因是随着反应的进行,溶液中有机污染物分子逐渐减少,降解中起到明显控制作用的过程逐渐由光降解过程转变为吸附过程[28],因此,当PMG质量浓度较低时,接触到CeNT@Oys复合光催化剂的概率下降,使得吸附-光催化协同作用明显减弱。
-
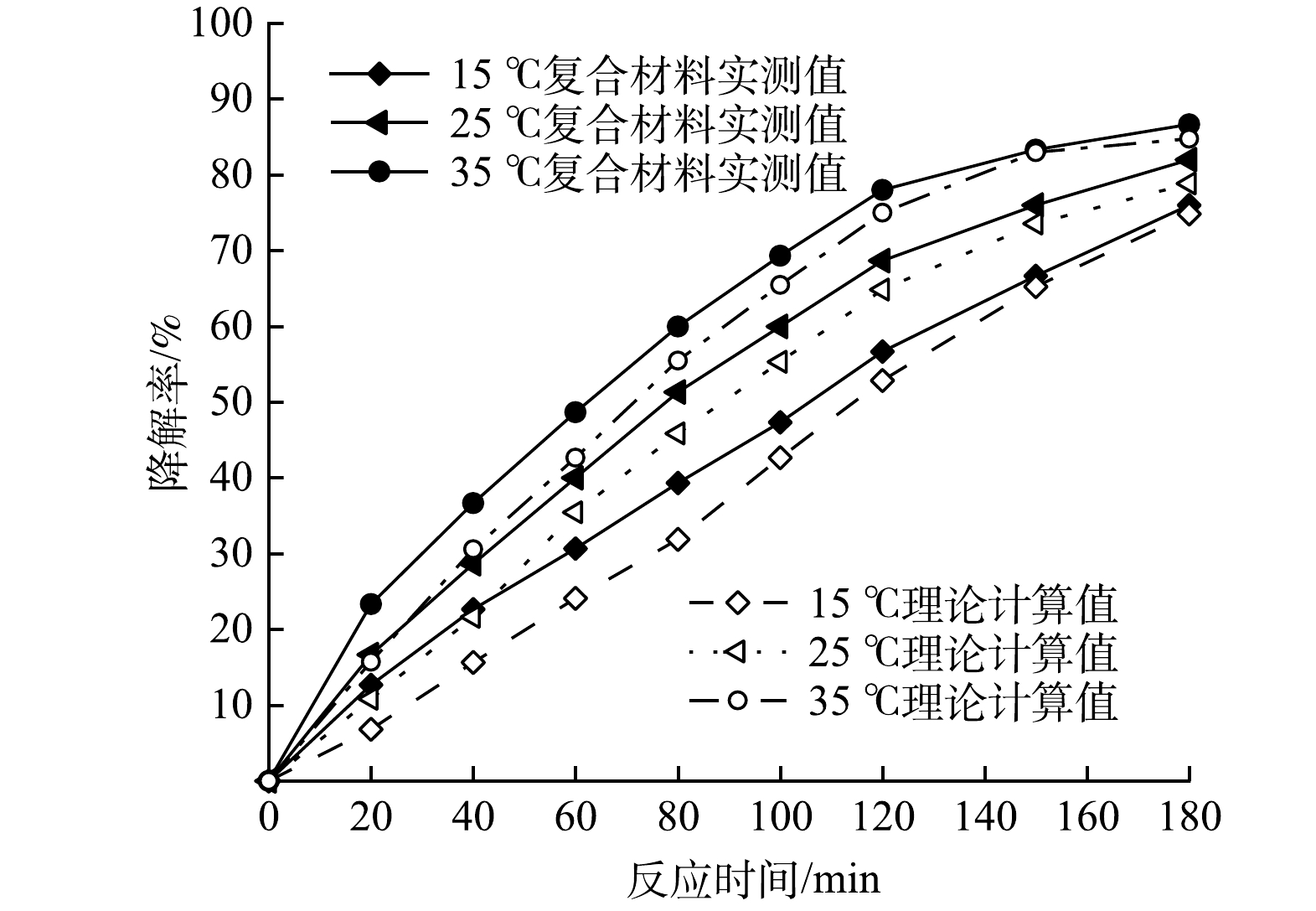
1) 温度对复合光催化剂协同作用影响。在100 mL初始pH=3.0的PMG溶液中投加100 mg的复合光催化剂,氙灯光功率400 W,磁力搅拌器转速200 r·min−1的条件下,考察反应温度对CeNT@Oys光催化剂吸附-光催化降解PMG影响,结果如图7所示。由图7可知,随着反应体系温度变化,叠加理论值曲线均位于复合光催化剂吸附-光催化协同降解曲线下方,充分体现了复合光催化剂对PMG的吸附-光催化协同作用。另外,温度升高,光催化降解效率增大,这表明在一定温度范围内,温度升高对光催化降解具有促进作用。首先,这是因为随着温度升高,PMG分子热运动速度加快,使光生活性基团与PMG分子之间有效碰撞率提高。另外,根据对PMG吸附热力学研究表明[29],CeNT@Oys复合光催化剂对PMG的吸附过程为放热反应,表明温度升高有利于PMG分子降解生成的中间体脱附,为PMG分子更高效地提供活性位点支持光催化反应进行。因此,在一定温度范围内升高温度将更有利于协同作用的进行。
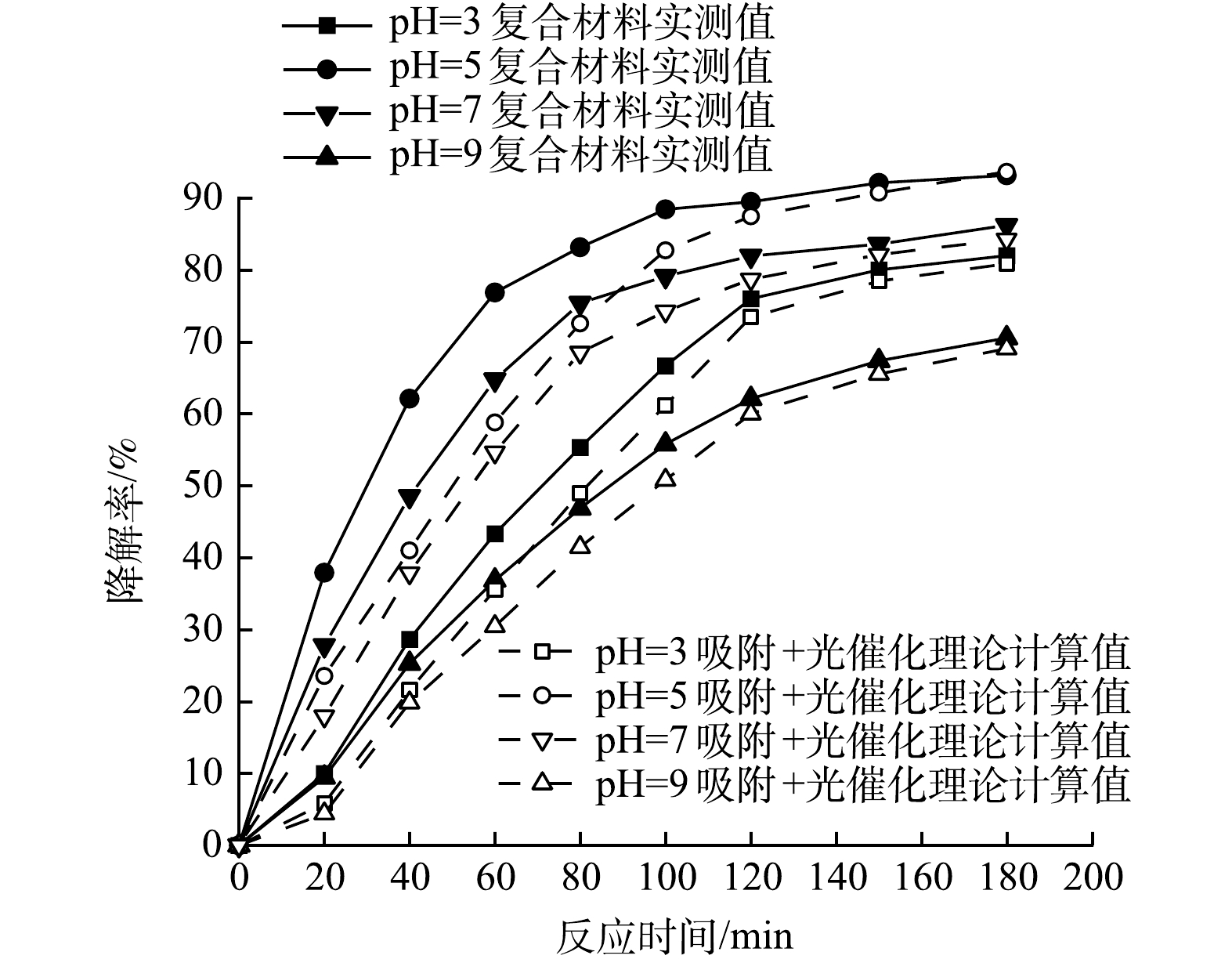
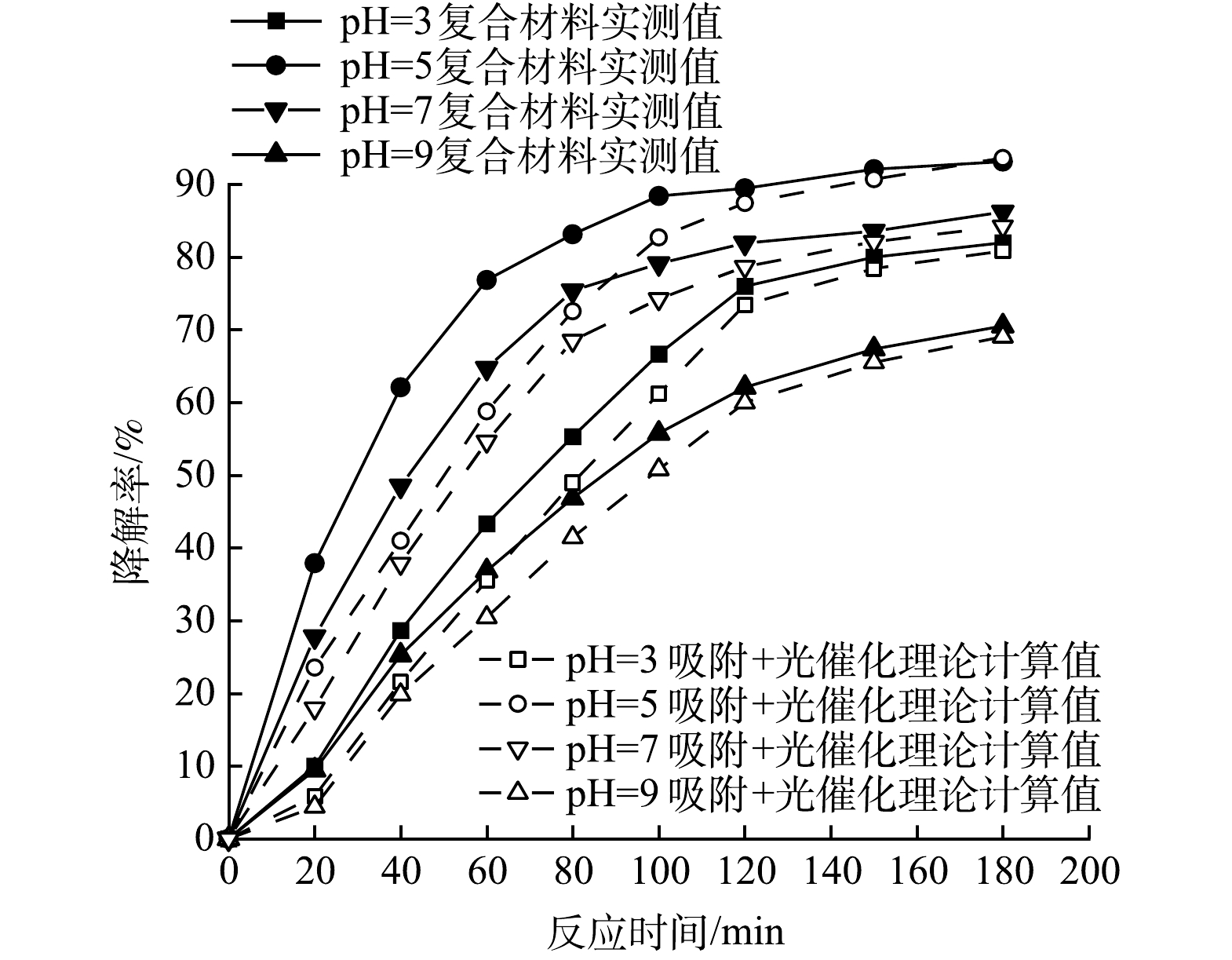
2) 初始pH对复合光催化剂协同作用的影响在100 mL初始pH分别为3.0、5.0、7.0和9.0的PMG溶液中投加100 mg的复合光催化剂,氙灯光功率400 W,反应体系温度35 ℃,磁力搅拌器转速200 r·min−1的条件下,考察PMG初始pH分别为3.0、5.0、7.0和9.0时对CeNT@Oys光催化剂对PMG吸附-光催化协同作用的影响,实验结果如图8所示。由图8可知,无论在酸性、中性和碱性条件下CeNT@Oys对PMG光降解均具有吸附-光催化协同效应。当PMG水溶液初始pH分别为3.0、5.0、7.0、9.0时,复合光催化剂对其降解率呈现先增大后减小的趋势,在pH=5时有机磷降解率达到最大值,并且PMG在降解前期的协同作用更明显。
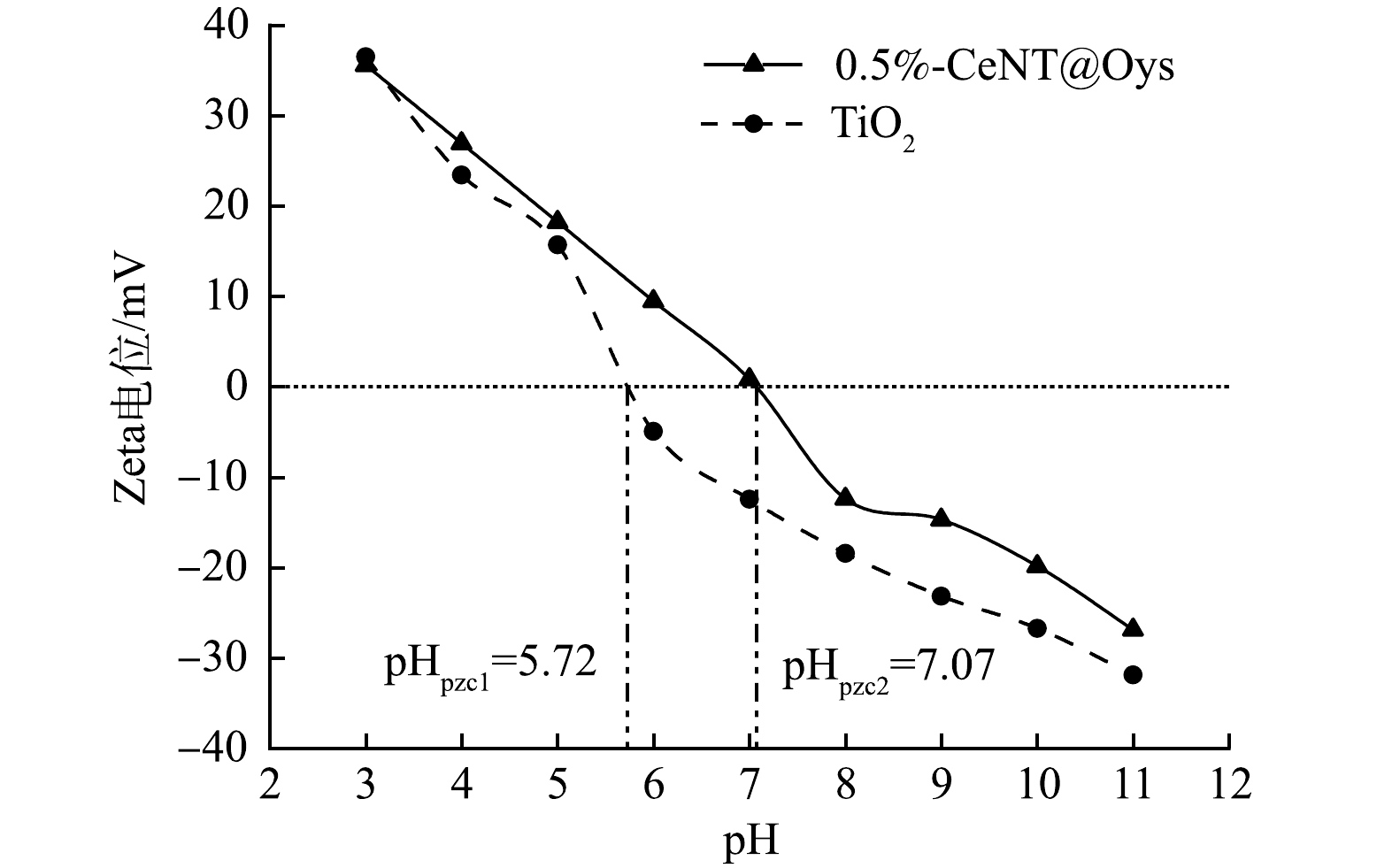
随着pH的增大,有机磷降解率出现先增大后减小趋势的原因是pH影响电荷存在状态和形式,导致催化剂和PMG分子之间的吸附力强弱发生变化。为确定在不同pH下复合光催化剂表面带电状态,通过Zeta电位仪测定其零点电位,如图9所示。CeNT@Oys光催化剂的pHpzc为7.07,即当PMG溶液pH>7.07时,催化剂表面带负电荷,当溶液pH<7.07时其表面带正电荷,而且pH越小表面正电荷越多。据研究表明,PMG在水溶液中主要以H2PMG1-和HPMG2-负电荷形式存在,并且PMG水溶液pH越大其负电荷越多[30]。
根据静电吸附-排斥作用,在pH<7.07时,PMG与催化剂产生静电吸引力相互吸附促进光催化降解PMG,并且随着pH增大PMG溶液负电荷增多、催化剂表面正电荷减少,在pH=5时两者间吸附力最强,因此,在pH=5时PMG中有机磷降解率最高。另外,有研究[31]表明,PMG在高酸性条件下更不易降解,而且光生电子会被增加的H+吸附捕获。而在pH>7.07时,两者之间存在静电互斥力导致吸附性能下降导致活性基团难以发挥作用,因此在pH=9时有机磷降解率最低。
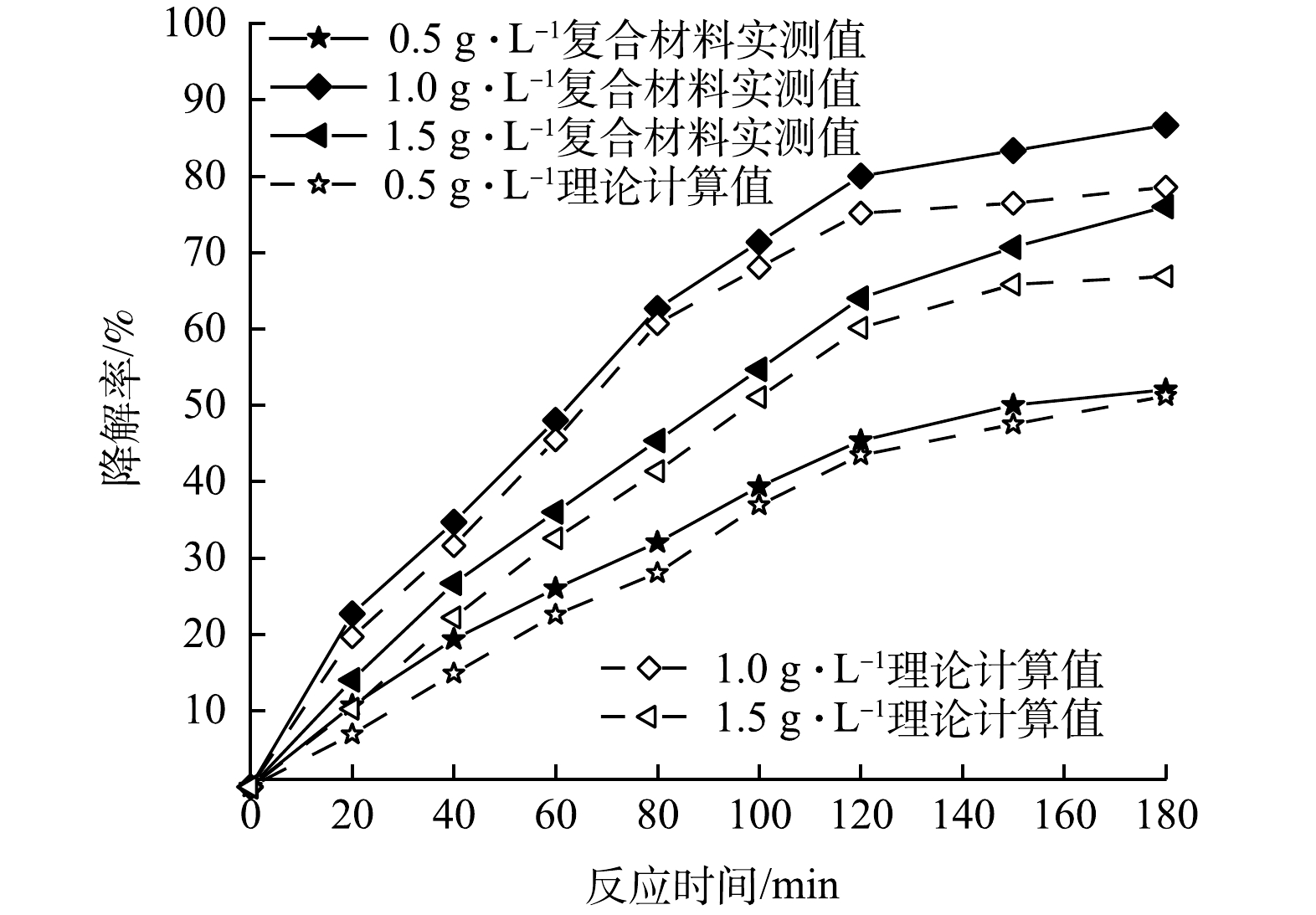
3)复合光催化剂投加量对协同作用的影响。在100 mL初始pH=5.0的PMG溶液中分别投加50、100和150 mg的复合光催化剂,氙灯光功率400 W,反应体系温度35 ℃,磁力搅拌器转速200 r·min−1的条件下,考察CeNT@Oys光催化剂投加量分别为0.5、1.0、1.5 g·L−1对PMG吸附-光催化协同作用的影响,结果如图10所示。由图10可知,改性牡蛎壳粉单独吸附与Ce-N-TiO2单独光催化降解率叠加值曲线均位于复合光催化剂吸附-光催化协同降解曲线下方,表明在不同复合光催化剂投加量条件下CeNT@Oys对PMG光降解均具有吸附-光催化协同效应。另外,分析图9可知,有机磷降解率随光催化剂投加量增加呈现出先增大后减小的趋势,出现该趋势的主要原因是当光催化剂质量浓度过高会产生光散射和屏蔽效应,光催化剂不能充分被光能激发,最终反而会降低光催化效率。另外,溶液中活性位点数量随着催化剂增多而增加,当光催化剂用量过多时,颗粒与颗粒之间的团聚趋势也会加速,逐渐使其表面的活性位点减少从而降低光降解率。
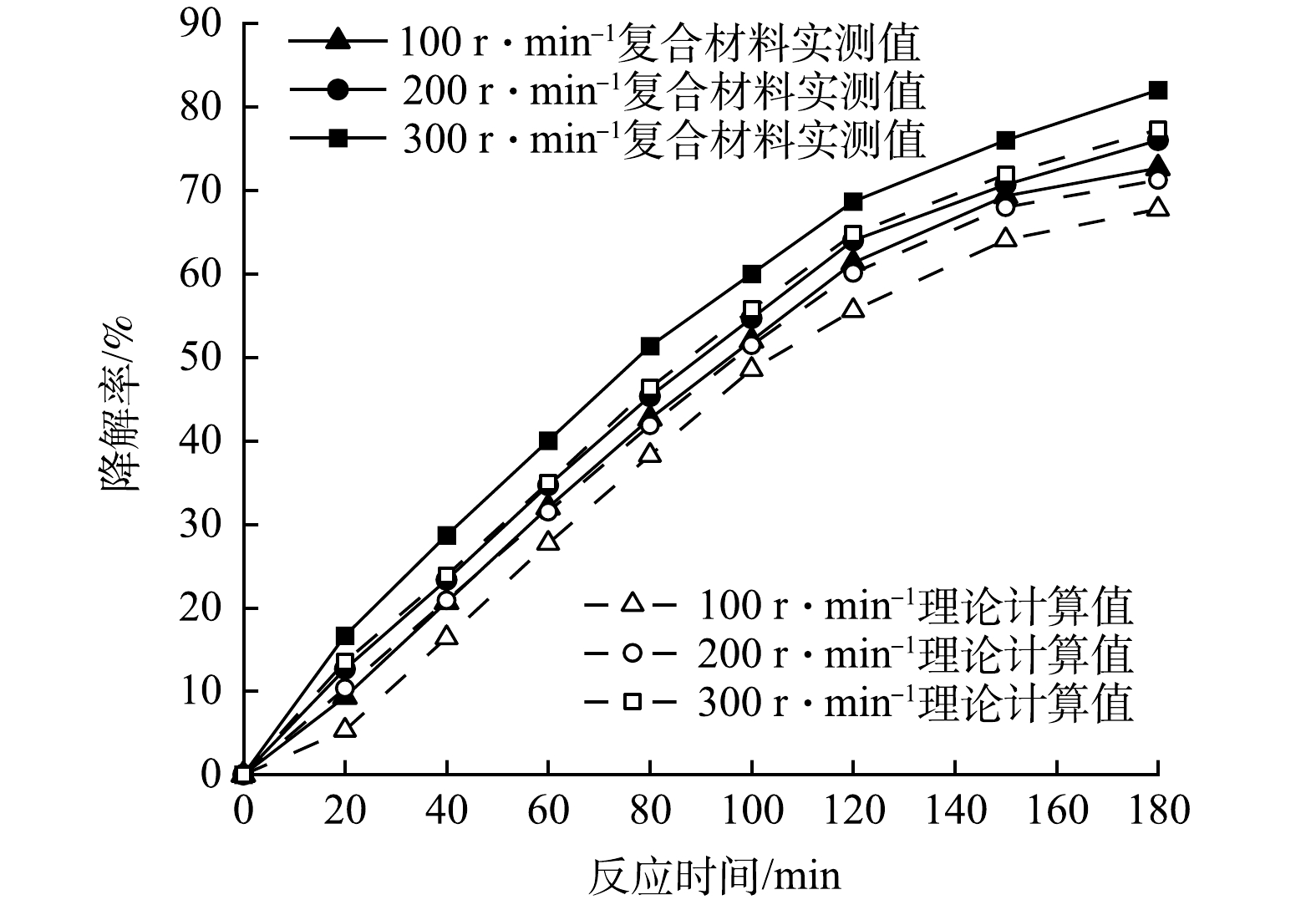
4)磁力搅拌器转速对复合光催化剂协同作用的影响。在100 mL初始pH=5.0的PMG溶液中投加100 mg的CeNT@Oys复合光催化剂,模拟日光氙灯光功率设定400 W,反应温度35 ℃,考察磁力搅拌器转速对CeNT@Oys复合光催化剂吸附-光催化降解PMG的性能,结果如图11所示。由图11可知,在不同转速下,改性牡蛎壳粉单独吸附与Ce-N-TiO2单独光催化降解率叠加值曲线均位于复合光催化剂吸附-光催化协同降解曲线下方。表明在不同搅拌转速条件下CeNT@Oys对PMG光降解均具有吸附-光催化协同效应。PMG中有机磷降解率随磁力搅拌器转速增加逐渐提高,这主要是因为转速影响PMG水溶液中溶解氧的含量,转速越大,溶解氧量越多,其与光生电子(e−)反应产生更多超氧自由基,提高有机磷降解率。此外,铈离子可以利用溶解氧产生超氧自由基,其反应过程如式(2)和式(3)所示。
另外,磁力搅拌器转速加快在一定程度上会使颗粒间碰撞概率增大,有利于催化剂和PMG分子之间吸附与脱附过程的进行,但转速过快会导致反应容器出现明显的中心漩涡,导致粉末光催化剂无法均匀分散于PMG溶液中,因此,磁力搅拌器转速也不宜过大。
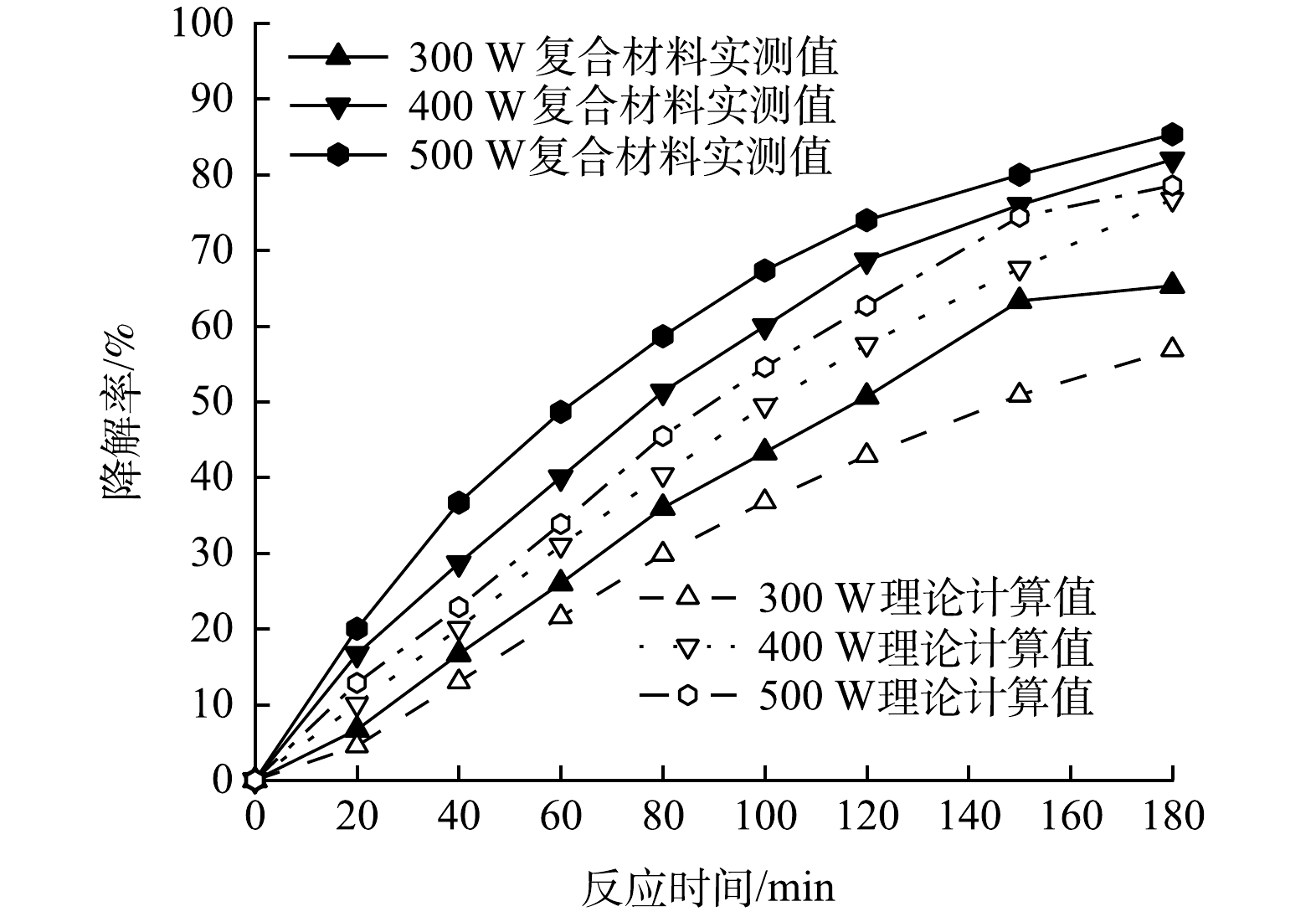
5)氙灯光功率对复合光催化剂协同作用的影响在100 mL初始pH=5.0的PMG溶液中投加100 mg的CeNT@Oys复合光催化剂,磁力搅拌器转速设为300 r·min−1,反应温度35 ℃,考察模拟日光氙灯光功率分别设定300、400、500 W时,CeNT@Oys复合光催化剂对PMG吸附-光催化协同作用的影响,结果如图12所示。由图12可知,氙灯光功率改变,改性牡蛎壳粉单独吸附与Ce-N-TiO2单独光催化降解率叠加值曲线均位于吸附-光催化协同降解曲线下方,表明在不同光照功率条件下CeNT@Oys对PMG光降解均具有吸附-光催化协同效应。如图12所示,随着氙灯光功率的提高,PMG中有机磷降解率呈现出逐渐增大的稳定趋势,相比于光功率由300 W升高至400 W,当400 W升高至500 W时,有机磷降解率提升较少。
主要原因是光照强度取决于光功率的大小,而光照强度决定CeNT@Oys复合光催化剂在给定波长下的光吸收程度。光催化反应中光生电子-空穴形成的起始速率依赖于光照强度。光催化反应器内的光强分布始终决定着污染物的总转化率和降解效率。在同一波长下,光强越强,在单位时间和单位面积上产生更多光子,光催化剂表面的光子活化几率也增加,PMG中有机磷降解效率越高。有研究[32-33]表明,在低光强下,光催化降解速率与光照强度呈正比关系;当光强超出一定范围后,光催化降解速率与光强的平方根呈线性关系,因此,基于氙灯用电增加量和草甘膦降解提升率性价比考虑,光功率设定为400 W。
-
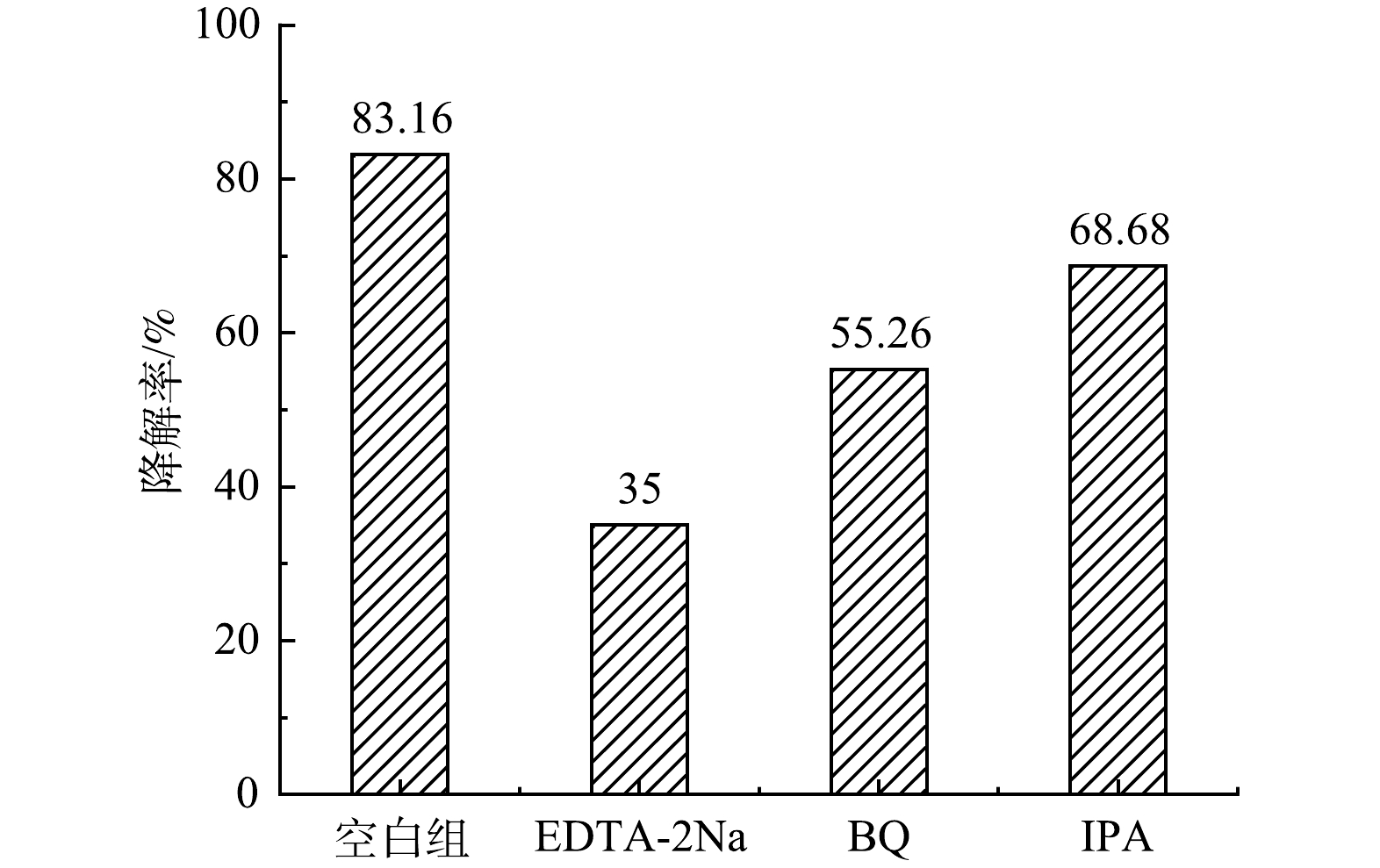
为分析CeNT@Oys复合光催化剂光催化降解PMG水溶液时发挥作用的活性物质种类,在最佳反应条件下,使用异丙醇(IPA)、乙二胺四乙酸二钠(EDTA-2Na)和1,4-苯醌(BQ)分别作为·OH、h+和·O2−的淬灭剂,初步确定活性物种以及其作用相对大小。
在最佳反应条件下,同时设置四组实验,分别为空白组、在空白组的基础上分别投加摩尔浓度为10 mmol·L−1的IPA、EDTA-2Na和0.2 mmol·L−1BQ溶液的3组实验,淬灭实验结果如图13所示。加入EDTA-2Na后严重影响CeNT@Oys复合光催化剂对PMG中有机磷的降解速率,这说明光生空穴或与空穴反应生成的活性基团是PMG中有机磷降解的主要活性物种。而对苯醌和EDTA-2Na的加入使PMG中有机磷降解率分别降低了27.9%和14.48%。这可能是在超氧和羟基自由基被淬灭后,空穴消耗减少,电子-空穴复合率增大所导致,同时也说明超氧自由基和羟基自由基均在降解PMG过程中起到氧化作用。
-
1)改性牡蛎壳粉的掺入使Ce-N-TiO2比表面积由61.649 m2·g−1增加至75.301 m2·g−1, 其中Ce以表面附着和间隙形式存在,而N则以间隙形式掺杂。
2)相比Ce-N-TiO2单独光催化,复合光催化剂的降解速率常数由1.179×10−2 min−1提升至2.441×10−2 min−1,充分体现了复合光催化剂对PMG的吸附-光催化降解的协同作用。改性牡蛎壳粉的掺入有助于Ce-N-TiO2吸附PMG且可提高光催化降解速率,加速催化剂表面吸附位点再生速率,继而对PMG分子进行下一轮吸附-光催化降解。
3)在不同影响因素下,单独吸附和单独光催化理论叠加值均低于复合光催化剂降解率,证明CeNT@Oys复合光催化剂在不同影响因素下同样具备吸附-光催化协同作用。在研究范围内,CeNT@Oys复合光催化剂吸附-光催化协同降解PMG的最佳反应条件为:pH为 5、实验温度为35 ℃、催化剂投加量为1.0 g·L−1、磁力搅拌器转速为300 r·min−1、光功率设定400 W。
4)通过淬灭实验检测活性基团种类,光生空穴及与空穴反应生成的活性基团是有机磷降解过程的主要活性物种,·OH和·O2−同时作用于PMG降解过程中。
改性牡蛎壳粉/Ce-N-TiO2吸附-光催化降解草甘膦
Adsorption-photocatalysis degradation of glyphosate by the modified oyster shell powder/Ce-N-TiO2
-
摘要: 通过水热法成功制备了改性牡蛎壳粉/Ce-N-TiO2复合光催化剂,采用扫描电子显微镜、比表面积测试和X射线光电子能谱分析对其微观形貌、物化性质进行表征分析,并研究其在模拟太阳光下吸附-光催化协同降解草甘膦的降解性能。结果表明:改性牡蛎壳粉单独吸附与Ce-N-TiO2单独光催化叠加理论降解率低于复合光催化剂协同作用降解率,充分证明两者复合后具有良好的吸附-光催化协同作用;在前120 min内复合材料的降解速率明显高于理论叠加曲线的降解速率,光催化降解草甘膦中有机磷的速率常数由1.179×10−2 min−1提升至2.441×10−2 min−1;在实验范围内,改性牡蛎壳粉/Ce-N-TiO2吸附-光催化协同降解草甘膦的最佳反应条件为:pH为5、实验温度为35 ℃、催化剂投加量为1.0 g·L−1、磁力搅拌器转速为300 r·min−1、光功率设定为400 W。Abstract: The modified oyster shell powder/Ce-N-TiO2(CeNT@Oys) composite photocatalyst was successfully prepared by the hydrothermal method. Its micro-morphology and physicochemical properties were characterized and analyzed by scanning electron microscopy (SEM), specific surface area test (BET) and X-ray photoelectron spectroscopy (XPS), and its degradation performance of glyphosate under the simulated sunlight was studied. The results showed that the sum of the theoretical degradation rates of modified oyster shell powder adsorbed alone and Ce-N-TiO2 photocatalyst alone was lower than that of composite photocatalyst, which fully proved that the composite photocatalyst had a good adsorption-photocatalysis synergistic effect; The degradation rate of the composite in the first 120 min was obviously higher than the sum of the theoretical degradation curves. The photocatalysis rate constant for organic phosphorus during photocatalysis degradation of glyphosate process increased from 1.179×10−2 min−1 to 2.441×10−2 min−1. Within the test range, the optimal reaction conditions for the synergistic degradation of glyphosate by CeNT@Oys adsorption photocatalysis were: pH=5, 35 ℃, catalyst dosage of 1.0 g·L−1, rotating speed of magnetic stirrer of 300 r·min−1 and the set optical power of 400 W.
-
Key words:
- composite photocatalysts /
- glyphosate /
- adsorption /
- photocatalytic degradation /
- synergistic reaction
-

-
-
[1] GILL J P K, SETHI N, MOHAN A, et al. Glyphosate toxicity for animals[J]. Environmental Chemistry Letters, 2017, 16(2): 401-426. [2] 孟秀柔, 宋青梅, 王飞, 等. 草铵膦和草甘膦在水环境中的行为和毒性效应研究进展[J]. 生态毒理学报, 2021, 16(3): 144-154. [3] ESPINOZA-MONTERO P J, VEGA-VERDUGA C, ALULEMA-PULLUPAXI P, et al. Technologies Employed in the Treatment of Water Contaminated with Glyphosate: A Review[J]. Molecules, 2020, 25(23): 5550-5576. doi: 10.3390/molecules25235550 [4] BONANSEA R I, FILIPPI I, WUNDERLIN D A, et al. The Fate of Glyphosate and AMPA in a Freshwater Endorheic Basin: An Ecotoxicological Risk Assessment[J]. Toxics, 2017, 6(1): 3-16. doi: 10.3390/toxics6010003 [5] VILLAMAR-AYALA C A, CARRERA-CEVALLOS J V, VASQUEZ-MEDRANO R, et al. Fate, eco-toxicological characteristics, and treatment processes applied to water polluted with glyphosate: A critical review[J]. Critical Reviews in Environmental Science and Technology, 2019, 49(16): 1476-1514. doi: 10.1080/10643389.2019.1579627 [6] BATTAGLIN W A, MEYER M T, KUIVILA K M, et al. Glyphosate and Its Degradation Product AMPA Occur Frequently and Widely in U. S. Soils, Surface Water, Groundwater, and Precipitation[J]. JAWRA Journal of the American Water Resources Association, 2014, 50(2): 275-290. doi: 10.1111/jawr.12159 [7] RUIZ-TOLEDO J, CASTRO R, RIVERO-PEREZ N, et al. Occurrence of glyphosate in water bodies derived from intensive agriculture in a tropical region of southern Mexico[J]. Bulletin of Environmental Contamination and Toxicology, 2014, 93(3): 289-293. doi: 10.1007/s00128-014-1328-0 [8] JIANG N, SHANG R, HEIJMAN S G J, et al. High-silica zeolites for adsorption of organic micro-pollutants in water treatment: A review[J]. Water Res, 2018, 144: 145-161. doi: 10.1016/j.watres.2018.07.017 [9] ABBAS M N. Phosphorus removal from wastewater using rice husk and subsequent utilization of the waste residue[J]. Desalination and Water Treatment, 2014, 55(4): 970-977. [10] OLADOJA N A, ADELAGUN R O A, AHMAD A L, et al. Phosphorus recovery from aquaculture wastewater using thermally treated gastropod shell[J]. Process Safety and Environmental Protection, 2015, 98: 296-308. doi: 10.1016/j.psep.2015.09.006 [11] 水博阳, 宋小三, 范文江. 光催化技术在水处理中的研究进展及挑战[J]. 化工进展, 2021, 40(S2): 356-363. doi: 10.16085/j.issn.1000-6613.2021-0756 [12] 王家恒, 宫长伟, 付现凯, 等. TiO2光催化剂的掺杂改性及应用研究进展[J]. 化工新型材料, 2016, 44(1): 15-18. [13] MAARISETTY D, BARAL S S. Defect-induced enhanced dissociative adsorption, optoelectronic properties and interfacial contact in Ce doped TiO2: Solar photocatalytic degradation of Rhodamine B[J]. Ceramics International, 2019, 45(17): 22253-22263. doi: 10.1016/j.ceramint.2019.07.251 [14] ALIPANAHPOUR DIL E, GHAEDI M, ASFARAM A, et al. Synthesis and application of Ce-doped TiO2 nanoparticles loaded on activated carbon for ultrasound-assisted adsorption of Basic Red 46 dye[J]. Ultrason Sonochem, 2019, 58: 104702. doi: 10.1016/j.ultsonch.2019.104702 [15] MAKDEE A, UNWISET P, CHAYAKUL CHANAPATTHARAPOL K, et al. Effects of Ce addition on the properties and photocatalytic activity of TiO2, investigated by X-ray absorption spectroscopy[J]. Materials Chemistry and Physics, 2018, 213: 431-443. doi: 10.1016/j.matchemphys.2018.04.016 [16] 赵文霞, 刘帅, 王蕊, 等. 煅烧氛围对N-TiO2可见光催化性能的影响[J]. 环境工程学报, 2019, 13(12): 2907-2914. [17] JIN C-Z, YANG Y, YANG X-A, et al. Visible photocatalysis of Cr(VI) at g/L level in Si/N-TiO2 nanocrystals synthesized by one-step co-hydrolysis method[J]. Chemical Engineering Journal, 2020, 398: 125641. doi: 10.1016/j.cej.2020.125641 [18] SHAARI N, TAN S H, MOHAMED A R. Synthesis and characterization of CNT/Ce-TiO2 nanocomposite for phenol degradation[J]. Journal of Rare Earths, 2012, 30(7): 651-658. doi: 10.1016/S1002-0721(12)60107-0 [19] MENG Z, WAN L, ZHANG L, et al. One-step fabrication of Ce–N-codoped TiO2 nano-particle and its enhanced visible light photocatalytic performance and mechanism[J]. Journal of Industrial and Engineering Chemistry, 2014, 20(6): 4102-4107. doi: 10.1016/j.jiec.2013.12.111 [20] NASIR M, BAGWASI S, JIAO Y, et al. Characterization and activity of the Ce and N co-doped TiO2 prepared through hydrothermal method[J]. Chemical Engineering Journal, 2014, 236: 388-397. doi: 10.1016/j.cej.2013.09.095 [21] SINGARAM B, VARADHARAJAN K, JEYARAM J, et al. Preparation of cerium and sulfur codoped TiO2 nanoparticles based photocatalytic activity with enhanced visible light[J]. Journal of Photochemistry and Photobiology A:Chemistry, 2017, 349: 91-99. doi: 10.1016/j.jphotochem.2017.09.003 [22] SENTHILNATHAN J, PHILIP L. Photocatalytic degradation of lindane under UV and visible light using N-doped TiO2[J]. Chemical Engineering Journal, 2010, 161(1/2): 83-92. [23] CHEN Y, LIU K. Fabrication of Ce/N co-doped TiO2/diatomite granule catalyst and its improved visible-light-driven photoactivity[J]. J Hazard Mater, 2017, 324(Pt B): 139-150. [24] BIAN Z, FENG Y, LI H, et al. Adsorption-photocatalytic degradation and kinetic of sodium isobutyl xanthate using the nitrogen and cerium co-doping TiO2-coated activated carbon[J]. Chemosphere, 2021, 263: 128254. doi: 10.1016/j.chemosphere.2020.128254 [25] ERAIAH R K, MADRAS G. Metal–metal charge transfer and interfacial charge transfer mechanism for the visible light photocatalytic activity of cerium and nitrogen co-doped TiO2[J]. Journal of Sol-Gel Science and Technology, 2014, 71(2): 193-203. doi: 10.1007/s10971-014-3350-4 [26] BURROUGHS P, HAMNETT A, ORCHARD A F, et al. Satellite structure in the X-ray photoelectron spectra of some binary and mixed oxides of lanthanum and cerium[J]. Journal of the Chemical Society, Dalton Transactions, 1976, 1(17): 1686-1698. [27] XUE G, LIU H, CHEN Q, et al. Synergy between surface adsorption and photocatalysis during degradation of humic acid on TiO2/activated carbon composites[J]. J Hazard Mater, 2011, 186(1): 765-772. doi: 10.1016/j.jhazmat.2010.11.063 [28] LUO Y, WEI X, GAO B, et al. Synergistic adsorption-photocatalysis processes of graphitic carbon nitrate (g-C3N4) for contaminant removal: Kinetics, models, and mechanisms[J]. Chemical Engineering Journal, 2019, 375: 122019. doi: 10.1016/j.cej.2019.122019 [29] 张伟, 梁哲, 汪爱河, 等. 改性牡蛎壳粉优化制备及其对草甘膦的吸附性能[J]. 工业水处理, 2022, 42(03): 90-97. doi: 10.19965/j.cnki.iwt.2021-0670 [30] LIN L, ZHANG X, XIAO G, et al. Photocatalytic Degradation Glyphosate with Cerium and Nitrogen Co-Doped TiO2 under Visible Irradiation[J]. Advanced Materials Research, 2012, 430-432: 1048-1051. doi: 10.4028/www.scientific.net/AMR.430-432.1048 [31] RAJORIYA S, BARGOLE S, GEORGE S, et al. Synthesis and characterization of samarium and nitrogen doped TiO2 photocatalysts for photo-degradation of 4-acetamidophenol in combination with hydrodynamic and acoustic cavitation[J]. Separation and Purification Technology, 2019, 209: 254-269. doi: 10.1016/j.seppur.2018.07.036 [32] HERRMANN J M. Photocatalysis fundamentals revisited to avoid several misconceptions[J]. Applied Catalysis B:Environmental, 2010, 99(3/4): 461-468. [33] 万燕, 魏任星, 卢挺, 等. 二氧化钛多相光催化降解有机污染物影响因素研究进展[J]. 现代化工, 2018, 38(5): 53-56. doi: 10.16606/j.cnki.issn0253-4320.2018.05.012 -



 下载:
下载: