-
油田采出水(oilfield produced wastewater, OPW)指地下开采出的含水原油经油水分离技术处理后得到的油田污水[1]。随着我国大多数油田进入中、后期高含水开采阶段,各油田为稳定产量而不断加大开采力度,导致油田采出水产量也随之增加,自2010年起,我国每年约需处理的油田采出水就已超过10×109 m³,且呈逐年增加趋势[2-3]。扣除采出水回注后数据,近年来我国油田采出水的年外排量需求均超3 000×104 t,占油田生产废水处理总量的90%以上[4]。油田采出水主要含有以下种类的物质:溶解油和分散油、溶解矿物质、化学药剂、溶解气、固体颗粒物质,其中溶解油和分散油主要为各种烃类混合物,如苯、甲苯、乙苯、二甲苯、酚类、有机酸类化合物、多环芳烃(polycyclic aromatic hydrocarbons, PAHs)等[5]。PAHs指具有2个及以上的苯环结构的持久性有机污染物(persistent organic pollutants, POPs),对生物体具有致畸、致癌、致突变的三致效应,其中的萘、菲、芴、苊等16种PAHs属于美国环境保护署(environmental protection agency, EPA)认定的对人类和生态健康具有潜在影响的优先控制PAHs污染物[6-10]。为满足处理量大且低成本油田生产需求,目前国内大多数油田采出水的外排处理工艺普遍采用传统的物理分离联合生化处理法。有研究表明,生物处理工艺能够有效地去除绝大多数有机污染物,不能去除油田采出水中所含相对较高浓度的PAHs,且由于PAHs对微生物产生直接毒害作用,抑制了微生物的生长代谢,并具有较低的致死浓度,导致各水处理厂的生化工艺处理效能大大降低,外排水质波动大,外排水抽查达标率仅为50%左右[11-13]。
当前,石油开采生产面临环保排放压力日益增加,迫切需求对现有采油污水处理工艺进行升级增效以及开发应用高效率深度水处理新技术。对油田采出水中的PAHs类污染物的彻底去除成为了亟待解决的关键瓶颈问题之一,相关研究单位均致力于在该方向取得突破。
针对废水中PAHs的处理方法主要包括膜过滤、物理吸附、化学氧化、光催化氧化降解等[13-18]。光催化氧化法相对于上述其他PAHs降解技术具有反应条件温和、处理效率高、无二次污染、矿化彻底且适于处理低浓度污染物的优势,极具发展前景[19-21]。然而现有研究开展的工作大多集中在针对油田采出水中萘、菲、芴等低环PAHs物质中的单组分或双组分的光催化去除,实验水样主要为实验室人工自配模拟采油废水,鲜有针对实际油田采出水中多组分PAHs污染物共存条件下的光催化氧化竞争行为及降解效率的研究[22-23]。
本研究首先表征了所采用的g-C3N4/TiO2复合薄膜光催化剂的物理化学性质;后利用紫外光辐照g-C3N4/TiO2复合薄膜光催化剂,对取自采油现场的采出水水样进行降解,研究多组分PAHs污染物共存条件下的不同环数PAHs发生光催化氧化反应的规律及其降解动力学,以期为今后解决油田采出水中PAHs生物毒害问题探索新工艺方法并积累理论参考数据做贡献,从而推动我国石油开采废水处理行业早日实现技术升级。
-
实验采用的试剂:实验所用的水样为呼和浩特某油田采出水。钛酸正四丁酯(C16H36O4Ti)、乙酰丙酮(C5H8O2)、正丙醇(C3H8O)、正己烷(C6H14)、甲醇(CH3OH/CH4O)、草酸(H2C2O4)、二氯甲烷(CH2Cl2)、异丙醇(C3H8O)、罗丹明B(C28H31CIN2O3)、乙二胺四乙酸二钠(C10H14N2Na2O8)、对苯醌(C6H4O2)、氯化钠(NaCl)、PAHs标准液(SS TCL Polynuclear Aromatic Hydrocarbons Mix 2 000 μg·mL−1,美国SUPELCO公司)。
实验所用的水样为呼和浩特某油田三元采出水现场污水。对其进行水质化验,具体参数为含油质量浓度为193 mg·L−1,悬浮物质量浓度为112 mg·L−1,聚合物质量浓度为296.5 mg·L−1,COD值为193 mg·L−1,氯离子质量浓度为7 148 mg·L−1,总硬度为2 152 mg·L−1,悬浮物粒径中值为5.6 μm,pH为9.89。
-
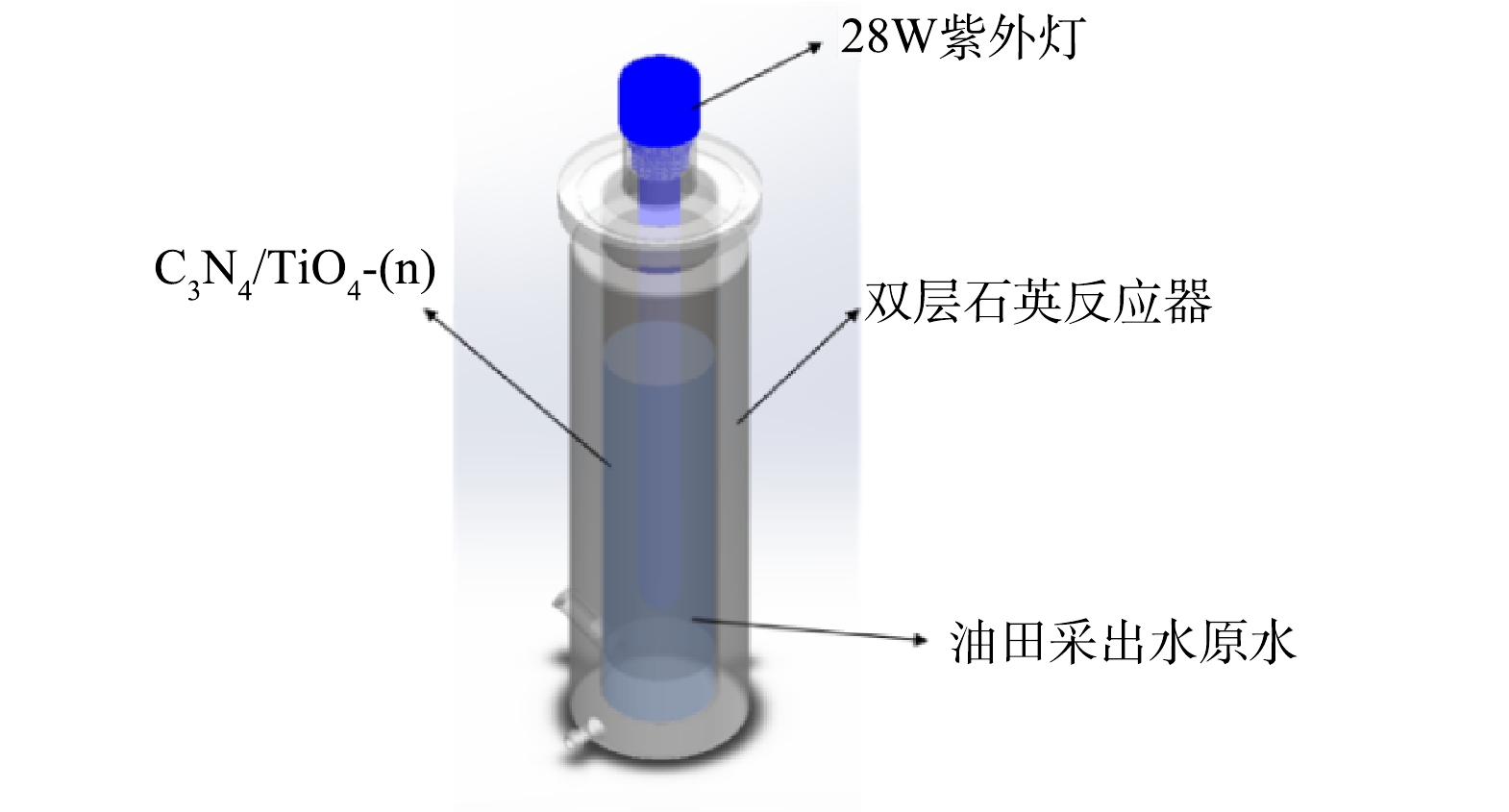
实验采用的仪器:气相色谱-质谱联用仪 (Agilent 7890B/5977B,美国安捷伦科技有限公司);固相微萃取仪(美国SUPELCO公司);氮吹仪(TTL-DCI,北京同泰科技发展有限公司);马弗炉(LE 14/11,德国纳贝瑟姆有限公司);集热式磁力搅拌器(DF-101S,上海力辰邦西仪器科技有限公司);真空干燥烘箱(DHG-9143A,上海精其有限公司);无油隔膜真空泵(XZ-1,上海仪昕科学仪器有限公司);紫外可见分光光度计(U4100,日本株式会社日立制作所);扫描电子显微镜(S4800,日本株式会社日立制作所)观察样品表面形貌, X射线衍射仪(D8 ADVANCE,德国布鲁克有限公司),PLS-SXE300/300UV型氙灯(北京泊菲莱科技有限公司),CHI660D型电化学工作站(上海辰华仪器公司)。光催化降解油田采出水实验装置为自制,其结构示意图如下图1所示。
-
将钛酸正四丁酯、乙酰丙酮、正丙醇等按一定比例混合均匀后向混合溶液中滴加少量去离子水,在室温环境下静置5 d,再向其中加入质量分数为0.5%的g-C3N4,磁力搅拌器和超声波分散仪依次对溶胶体系中的g-C3N4进行10 min分散处理,形成均匀的悬浊液体系。使用浸润提拉法对TA1钛箔基材分别进行1次和2次涂覆,涂覆后的基体钛箔在真空干燥箱下高温焙烧,400 ℃下焙烧2 h,得到g-C3N4/TiO2复合薄膜光催化剂材料,记为g-C3N4/TiO2-(1)和g-C3N4/TiO2-(2)。
-
水样采集:使用1 000 mL棕色玻璃采样瓶对油田采出水原水(直接取自内蒙某油田采油现场水处理站的进水口)进行收集,为防止空气干扰,采样瓶在水样完全排出空气后进行密封,并将密封采样瓶于4 ℃下避光运输及保存,并在7 d内完成后续测试。
-
在图1中所示的实验装置中,量取900 mL油田采出水原水于定制石英反应器中进行实验。g-C3N4/TiO2光催化膜有效反应面积为281.6 cm2,紫外光源为28 W的UVC灯,紫外辐照强度为5.7 μW·cm−²,转速550 r·min−1,分别在0、20、30、45以及60 min时取样100 mL,依次标记为0、20、30、45、60 min,后续处理同下述水样和滤膜的前处理过程。使用GC-MS对样品上机测试。
-
采用扫描电子显微镜(scanning electron microscope, SEM)和X射线衍射仪(X-ray diffraction, XRD)对所制备的光催化剂样品的表面微观形貌及物相组成进行表征。采用电化学三电极法对薄膜光催化剂的光电响应活性进行表征,具体为50 mm×50 mm大小的g-C3N4/TiO2复合薄膜光催化剂经封装后作为工作电极,饱和甘汞电极为参比电极,铂片电极为对电极,3.5%的氯化钠溶液为支持电解质,以电流-时间曲线(I-t)法测试经紫外光辐照的处于不同服役时间的g-C3N4/TiO2复合薄膜光催化剂,表征其光电响应活性。采用自由基猝灭实验可对本文光催化降解反应中的有效自由基的种类进行分析,从而便于进一步分析推测PAHs的降解路径。以5 mg·L−1的罗丹明B溶液作为模型污染物,0.3 mmol的乙二胺四乙酸二钠(EDTA-2Na)、异丙醇或对苯醌作为不同自由基的淬灭剂,经对模型污染物进行光催化反应60 min后取样,测试比较模型污染物的降解率。
采用固相微萃取-气相色谱/质谱联用方法(gas chromatography-mass spectrometry,GC-MS)对水样中16种优先控制的PAHs进行检测分析。其中固相微萃取方法如下。 1)抽滤:使用真空抽滤装置搭配0.45 μm滤膜对100 mL处理后的水样进行过滤,滤膜留存。2)活化:5 mL二氯甲烷,10 mL甲醇以及10 mL水依次流过C18固相微萃取柱。3)过样:无油真空泵以1~15 mL·min−1的流速抽取处理后的水样进入固相萃取柱,并进行水样富集。4)抽干:富集后对固相萃取柱继续抽空气干燥30 min,后用氮气干燥20 min。5)洗脱:用10 mL体积比为1:1的正己烷和二氯甲烷混合溶液作为洗脱剂对固相萃取柱中的PAHs进行洗脱,洗脱速率为1~5 mL·min−1,棕色顶空瓶收集洗脱溶剂。6)氮吹:高纯氮气对洗脱后液体氮吹,吹至洗脱液剩余0.2~0.4 mL。7)定容:微量进样针对棕瓶中的样品进行转移,使用正己烷对棕瓶进行3次润洗,将润洗液一同转移至GC进样瓶中,定容至1 mL。8)加标:向定容后的进样瓶中添加20 µL 10 mg·L−1的标准液。上述水样固相微萃取过程中得到的滤膜在室温条件下干燥后,使用洁净的手术剪将滤膜裁剪为2 mm×1 mm小条状并放置于50 mL的离心管中,并加入10 mL体积比为1:1的正己烷和二氯甲烷混合溶液,使用漩涡振荡器对离心管中的滤膜进行萃取。萃取得到的萃取剂使用针式过滤器(PTFE,0.45 µm)过滤后转移至棕色顶空瓶中。其余步骤同水样固相微萃取过程6~8。
色谱条件为:HP-5MS column(30 m×0.25 mm×0.25 µm)色谱柱,载气为高纯氦气(纯度>99.99%),流量为1 mL·min−1。进样口温度280 ℃,不分流模式进样,吹扫流量为3 mL·min−1。程序升温条件为:初始温度 80 ℃,保持2 min,以20 ℃·min−1的速度升温至180 ℃,保持5 min,再以10 ℃·min−1的速度升至300 ℃,保持8 min。质谱条件为:电子轰击离子源(electron impact ion source)电离方式,电离源70 eV,采用离子扫描方式,离子源温度280 ℃,传输线温度280 ℃,四极杆温度150 ℃,溶剂延迟时间4 min,采用多反应监测模式(MRM)。
-
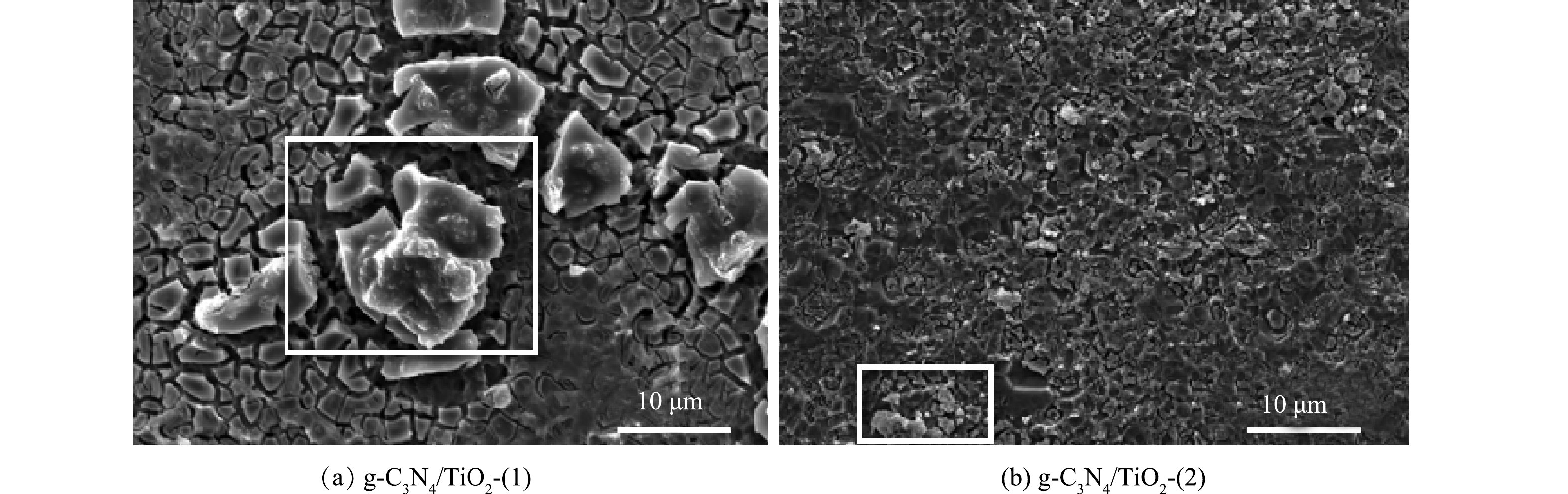
使用扫描电子显微镜对所制备的g-C3N4/TiO2薄膜光催化剂的表面形貌进行表征,结果如图2所示。可见,所制备单层g-C3N4/TiO2-(1)薄膜即可将负载基体表面完整覆盖,无明显缺陷,表面的裂纹间隙相对较大。氮化碳组分呈孤岛式分布于薄膜表面(如图2(a));负载制备2 层g-C3N4/TiO2-(2)的薄膜表面相对更致密,裂纹间隙更细碎。如图2(b)白框内区域所示,g-C3N4呈更细小块状形态零星分布,与TiO2薄膜结嵌合程度更高。这有助于g-C3N4/TiO2共同形成Z型半导体,使得光生电子集中于较负的导带,而光生空穴则集中于较正的价带,可提升光生电子和光生空穴的分离能力,提高光催化反应驱动力,同时还提高了对太阳光光谱波长的吸收范围,能够保障g-C3N4/TiO2薄膜具有较高的光催化反应活性[24-26]。因此,后续研究均采用负载2层g-C3N4/TiO2-(2)的薄膜光催化剂。
-
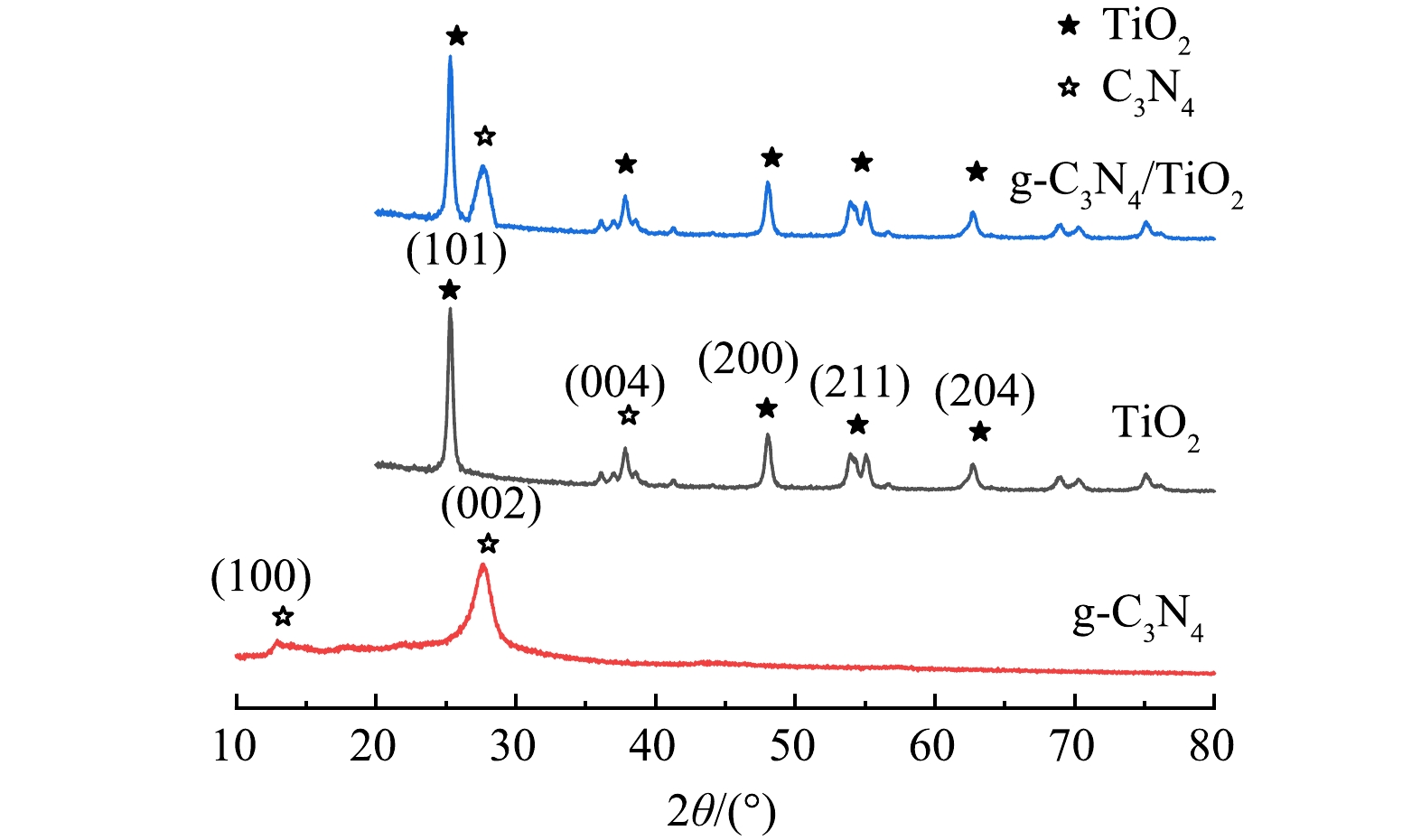
图3为所采用的g-C3N4/TiO2复合薄膜光催化剂与纯g-C3N4和TiO2光催化剂的X射线衍射谱图的对照图。纯g-C3N4在2θ为13.1°和27.5°处出现的特征衍射峰对应于(100)和(002)六方晶面(JCPDS 066-0813);纯TiO2在2θ为25.1°、37.9°、47.9°、54.7°和62.5°处的特征衍射峰分别对应于(101)、(004)、(200)、(105)、(211)和(204)锐钛矿相TiO2晶体平面(JCPDS 021-1272)。本文所制备的g-C3N4/TiO2复合薄膜光催化剂具有与纯g-C3N4和TiO2相同的特征衍射峰,表明其为含有锐钛矿相TiO2和g-C3N4的复合组分。
-
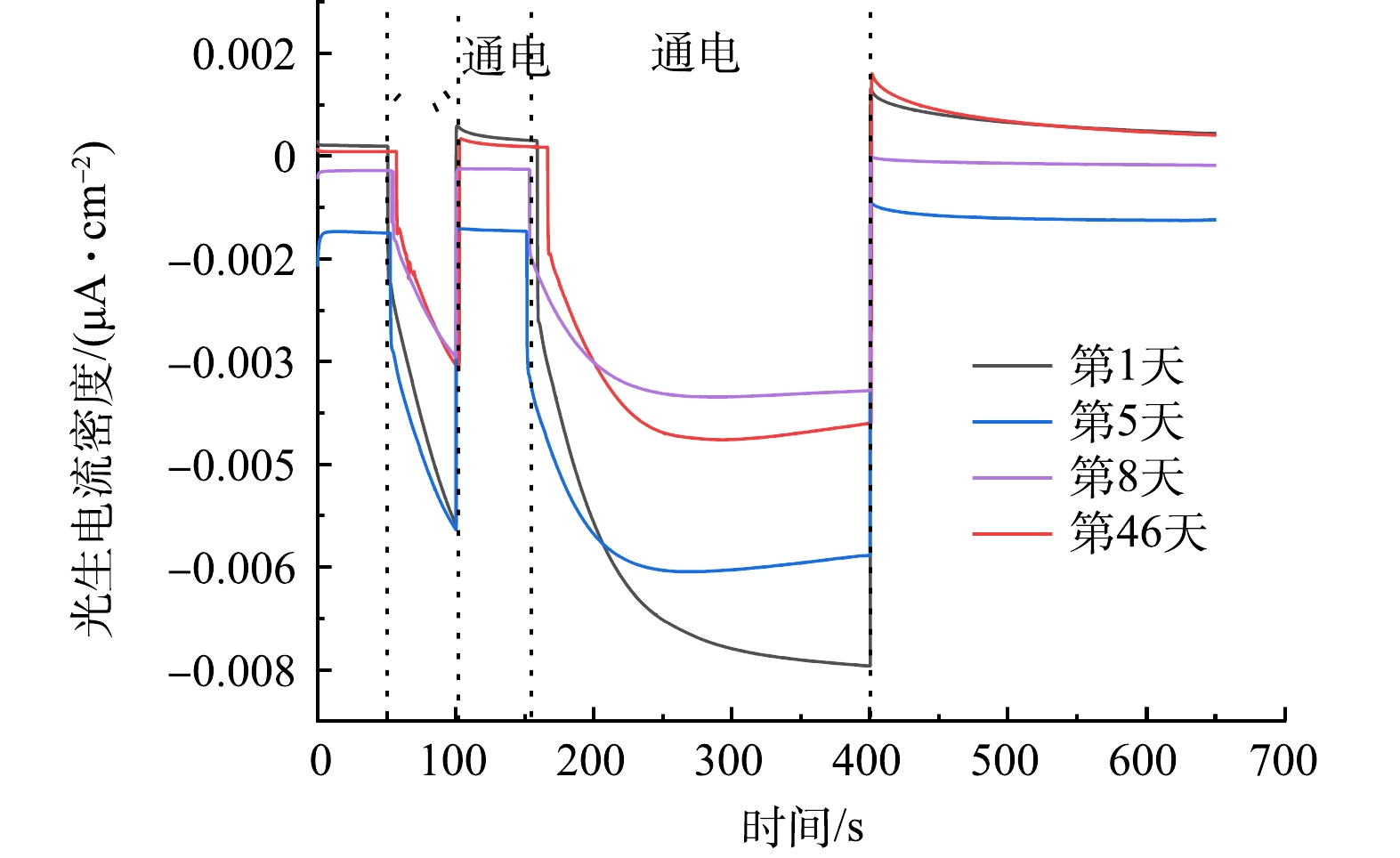
图4为处于不同服役时间的g-C3N4/TiO2的光催化活性监测的结果,随着紫外灯的开启与关闭,测得的g-C3N4/TiO2的光生电流密度呈有规律的周期性波动,表明该g-C3N4/TiO2复合薄膜光催化剂具有良好的光电转换能力。对暴露于油田采出水中第1、5、8和46天的g-C3N4/TiO2-(2)复合薄膜光催化剂的光生电流密度监测中发现,新制备的催化剂材料在第1天的光生电流密度可达0.80×10−2 μA·cm−2,随着服役时间的延长其光生电流密度略有降低,但在8~46 d其光生电流密度逐渐稳定,约为0.47×10−2 μA·cm−2,表明其在实际油田采出水的应用中具有相对较可靠、稳定的光催化活性。
-
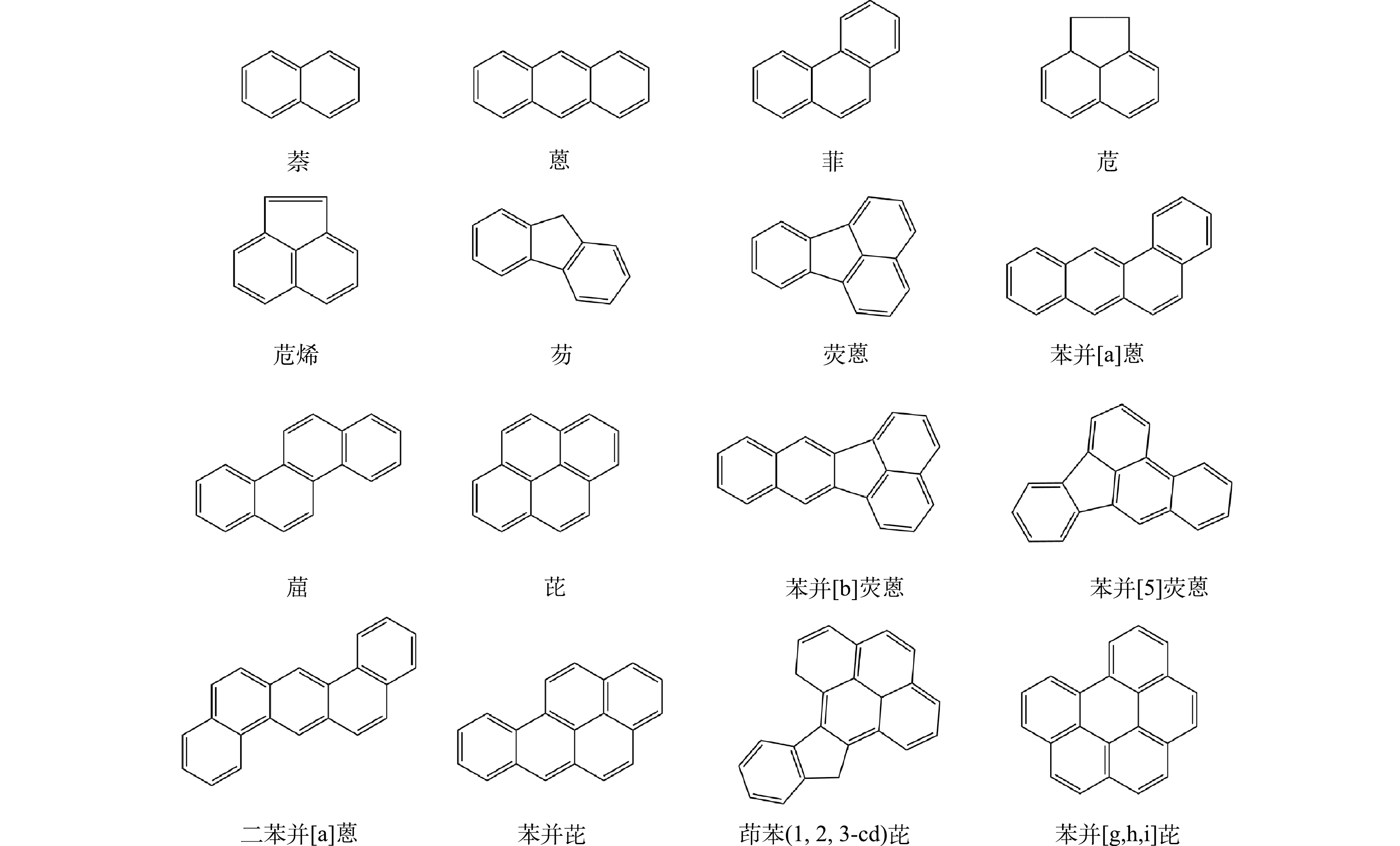
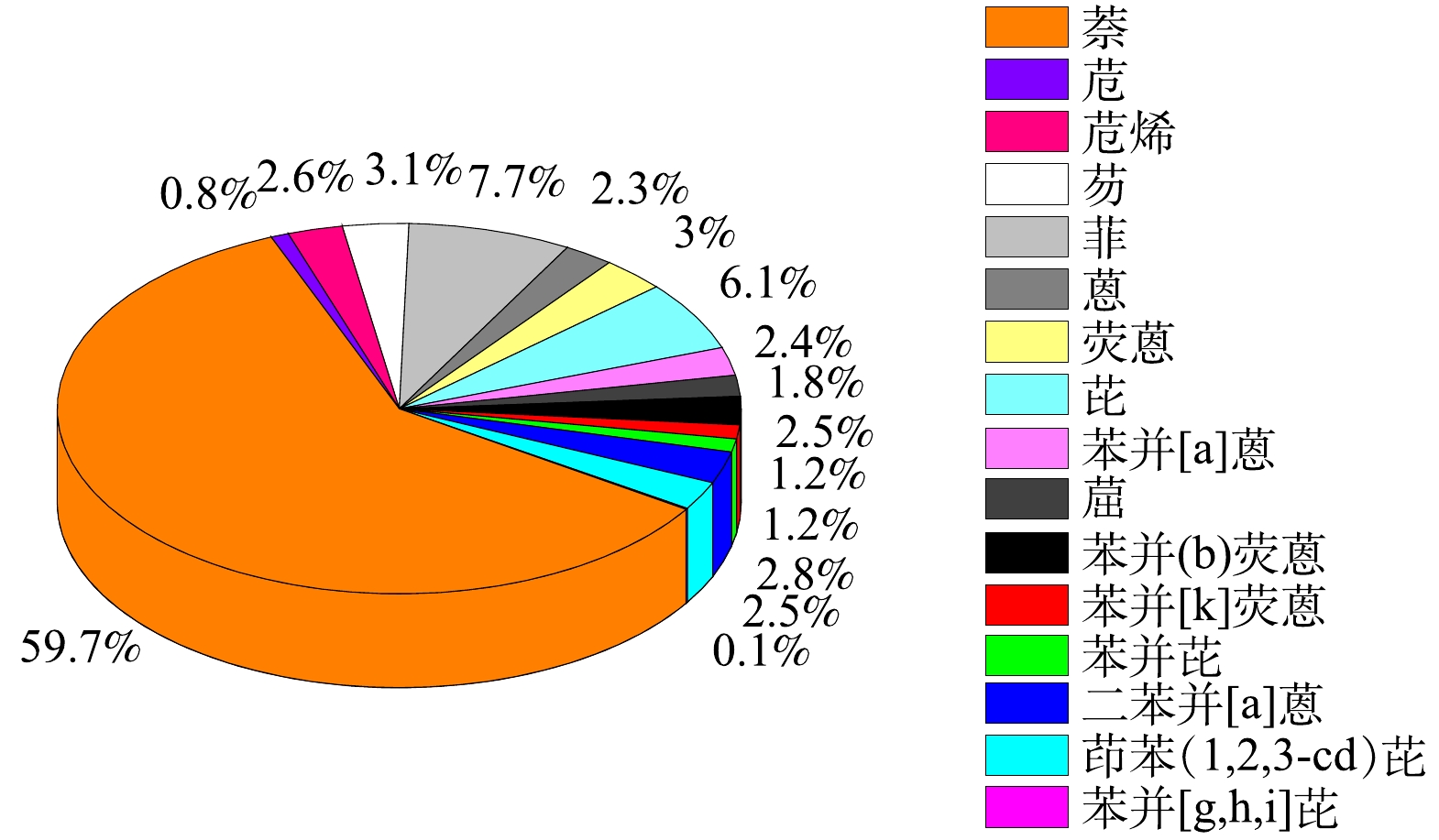
图5为16种优先控制的PAHs分子结构式,表1为油田采出水原水(0 min)中的PAHs各组分含量。可以看出,该石油采出水中的不溶总PAHs (记为颗粒态ΣPAHs)的含量远高于溶解的总PAHs (记为溶解态ΣPAHs),且表1中的苯并[a]蒽相对其他15中需要优先控制的PAHs类物质,对环境以及生物的毒性相对更强,属于世界卫生组织国际癌症研究机构公布的2B类致癌物。
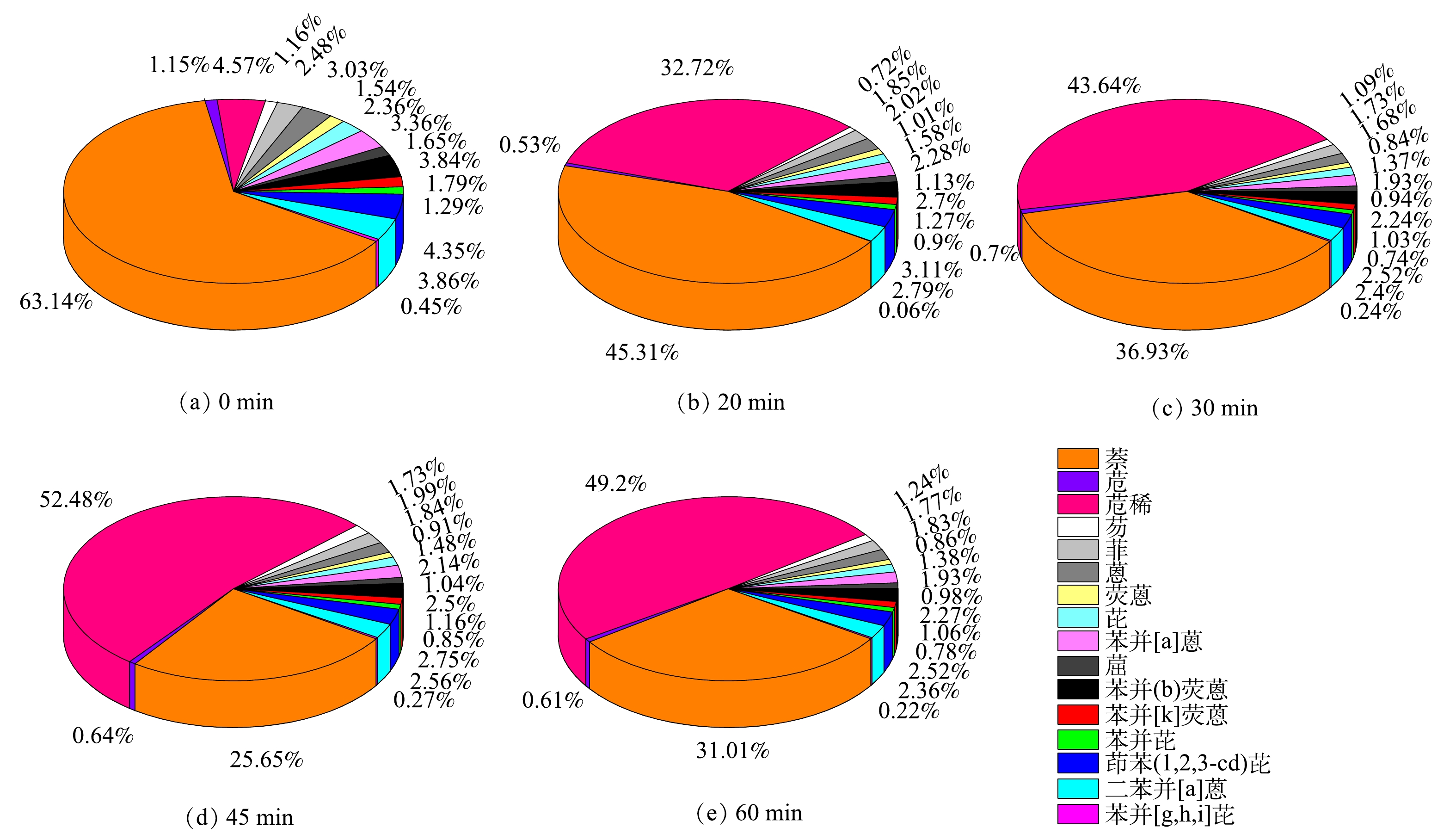
图6为表1中相应各ΣPAHs的含量百分比分布。可以得出,本次现场采出水的原水中萘的分布最广,含量占比超过50%;而苯环数超过3的PAHs占比大于20%,其中的5环苯并[a]蒽含量占比2.4%,因其高生物毒性的特点而需要在开展PAHs物质的降解行为研究中进行关注。本文选用该石油采出水作为处理水样,相对于众多仅开展萘和菲等污染物的研究,能更有助于探明光催化降解工艺对真实油田采出水的降解效果。
-
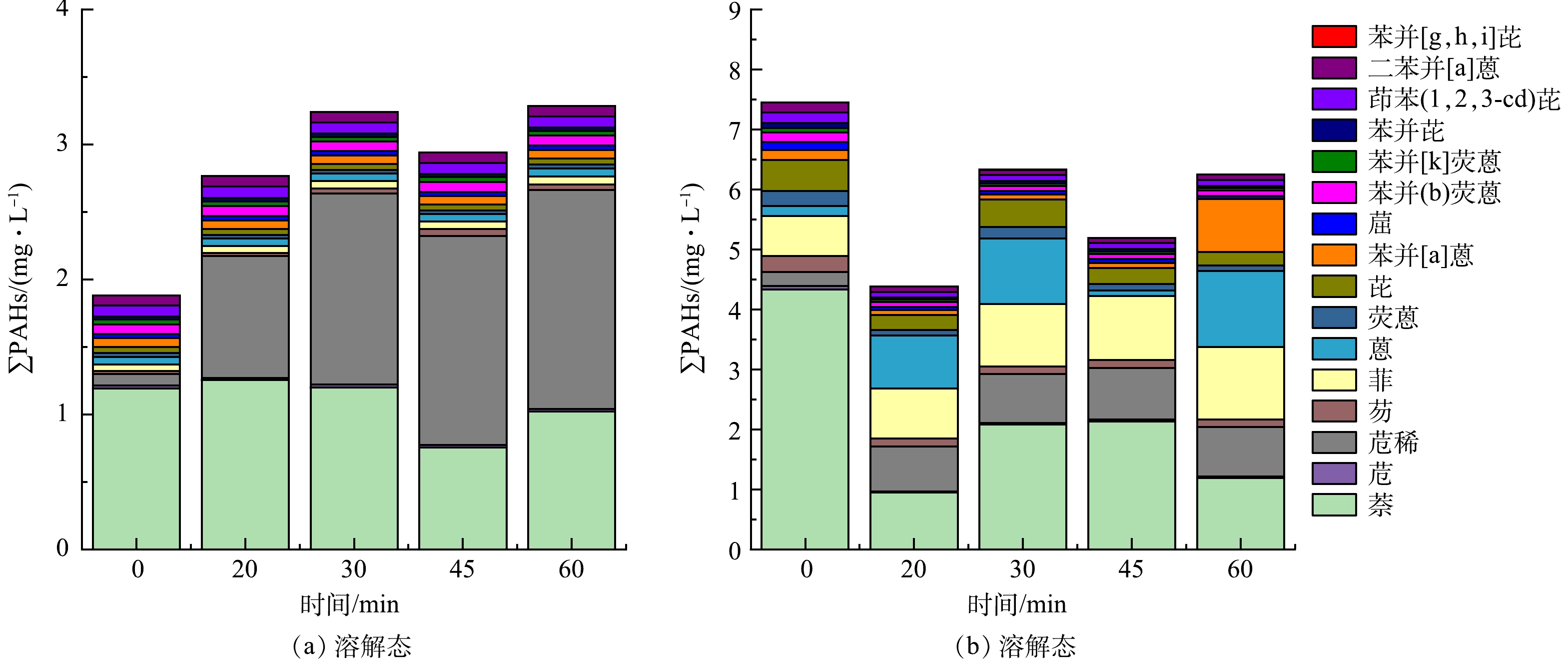
经UV/g-C3N4/TiO2光催化氧化反应后得到的各水样过滤分离处理,可得到溶解态与颗粒态的ΣPAHs含量分布随光催化反应时间的变化,结果如图7所示。可以发现,石油采出水原水中不溶性ΣPAHs(图(a))的含量明显高于溶解态ΣPAHs(图(b)),随着光催化氧化反应时间延长,不溶性ΣPAHs的含量趋于降低,而溶解态ΣPAHs的含量略有升高。原水中含量占比最多的2环萘,无论是其溶解态还是不溶的颗粒态的含量均随着光催化反应时间的增加而降低。这与李贞燕等[22-23]的研究结果相吻合,进一步证明光催化反应可以高效率降解萘,即使在有多组分PAHs共存的条件下,萘的降解优先级仍然最高,尤其是水样中不溶的颗粒态萘可被迅速的去除。采出水原水经光催化反应处理后,由图8中的溶解态和颗粒态萘的含量可计算得到萘在60 min总去除率可达61.13%。
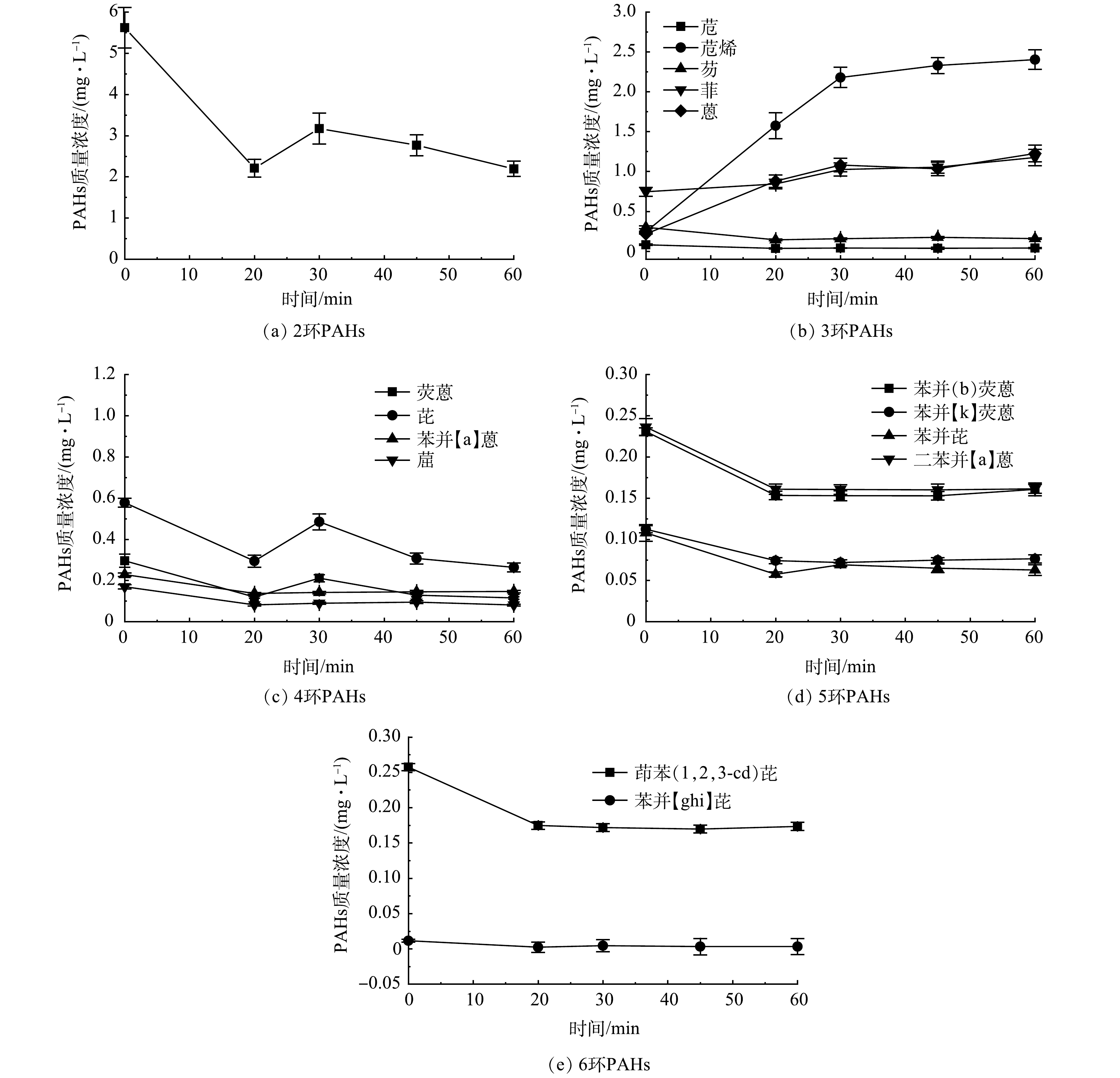
图8为含不同苯环数的ΣPAHs含量随光催化反应时间的变化情况,可以看出,伴随着原水中的萘含量占比的迅速降低,苊烯、蒽、菲的含量有明显升高;同时,4环、5环和6环ΣPAHs的含量均呈下降趋势,包括高生物毒性的苯并[a]蒽、苯并芘等污染物。这可大大降低采出水的生物毒性。
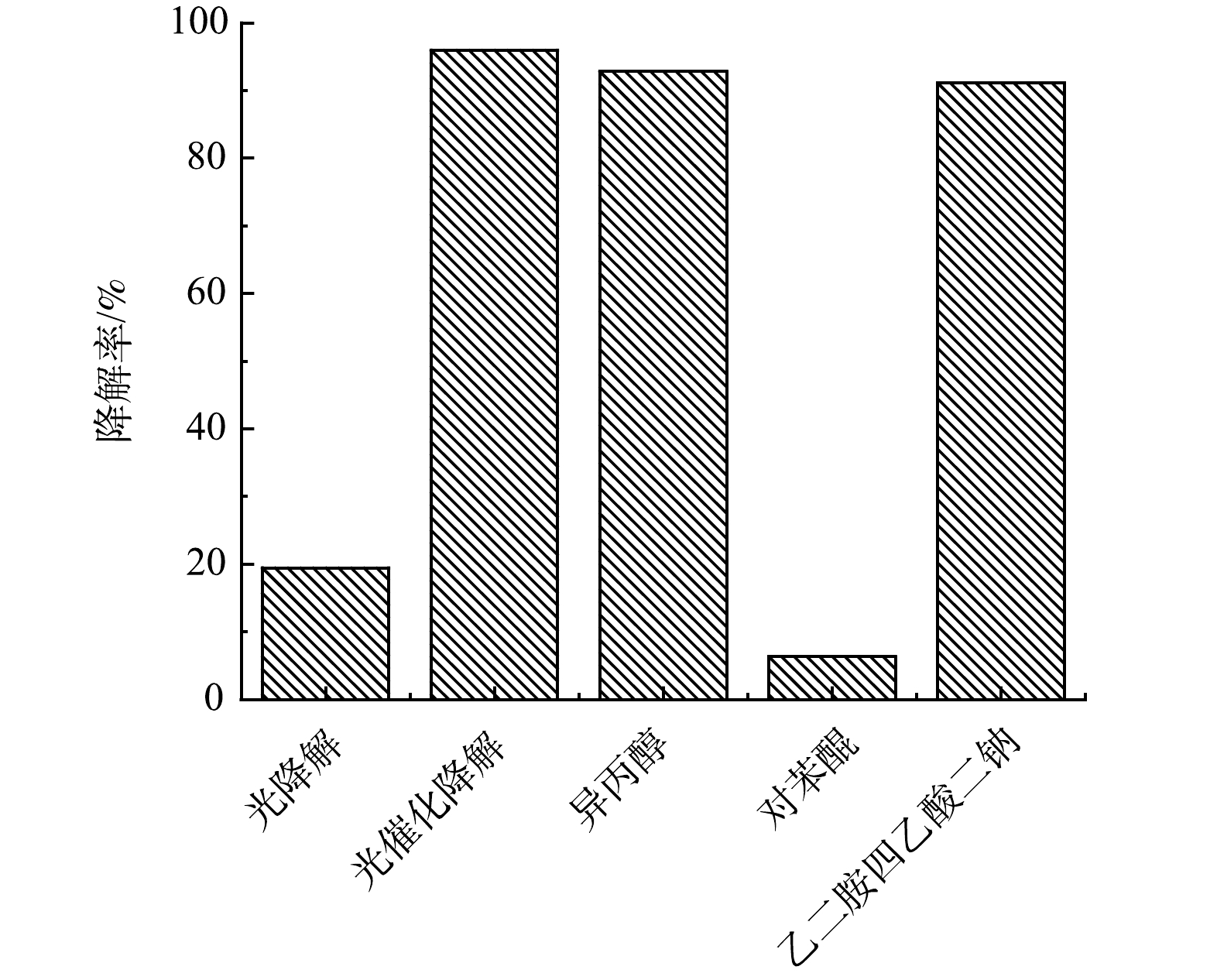
由图9中对g-C3N4/TiO2光催化反应中有效自由基的测定结果可见,在与油田采出水的弱碱性条下,EDTA-2Na、异丙醇和苯醌这3种自由基淬灭剂的加入均对g-C3N4/TiO2光催化降解模型污染物罗丹明B产生了一定负面影响,其中·O2−自由基淬灭剂—对苯醌对罗丹明B的降解抑制作用最显著:反应停留时间为60 min时罗丹明B降解率仅为6.34%,远低于各对照组,因此,可以确定在油田采出水的弱碱性条件下,g-C3N4/TiO2对污染物的光催化降解过程中的有效自由基为·O2−,进一步根据NZILA等[26]和QIN等[27]的研究,可初步推测采出水水样中高环PAHs发生的降解过程。
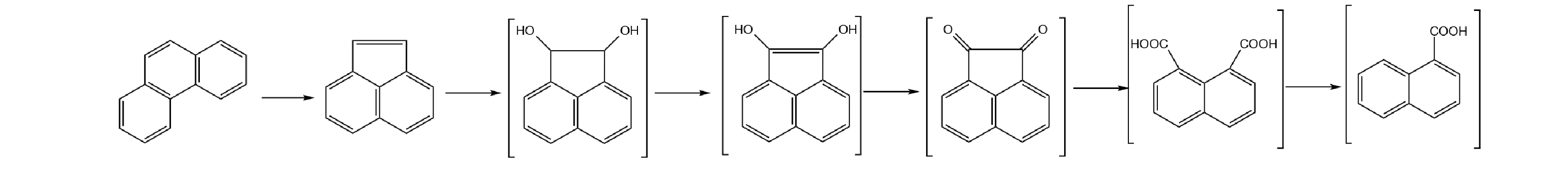
以苯并芘的降解过程为例,其在降解过程中碳碳单键断裂开环,在氧化过程中形成的中间产物涉及的PAHs及其衍生物可包含4环芘、䓛、3环菲、2环萘以及大量的羰基、胺基和羟基以及相对应的烷基化PAHs衍生物[26-28]。如图10所示,在高环PAHs降解至菲的过程中,菲仍具有向苊烯进一步降解的可能性。因此,在高环PAHs降解开环的过程中,逐级降解得到菲,部分菲易被降解得到苊烯。这可能是图8中的苊烯含量增加的原因。待高环PAHs的降解趋于稳定后,菲和苊烯的质量浓度有所增加也趋于平稳,随光催化反应时间的进一步增加,菲可逐步转化为萘。因此,苊烯的含量变化在含多组分高环PAHs的降解处理过程中具有指示作用,可将苊烯作为指向污染物而加以监测。上述结果也支持今后在实际油田水处理厂应用光催化反应工艺过程中辅助以微滤技术,将对于提升PAHs的去除效率有所帮助。
图11为含不同苯环数的溶解态PAHs含量随光催化反应时间的变化。可以发现,随着光催化反应时间的增加,溶解态PAHs的含量相应增加。这要归因于3环的苊烯含量显著增加。而苊烯增加的原因可参考前文中对颗粒态高环PAHs的降解途径,可知各种高环PAHs被光催化降解所产生的较多苊烯溶解进入到了水样中。因此,虽然光催化反应使得溶解于采出水样中的PAHs含量略有增加,但主要增加的是低生物毒性的苊烯,而高生物毒性的各种高环PAHs类的含量占比仍然降低,这对改善水样的生物毒性非常有利。
-
1)制备的g-C3N4/TiO2复合薄膜光催化剂表面致密,g-C3N4与TiO2薄膜结嵌合程度高,光电响应明显,稳定性突出,克服了粉态型光催化剂易流失、可重复利用性差的缺点,更具应用优势,有助于提升光催化技术未来在油田水处理领域的产业化应用可靠性。
2)对油田采出水中优先控制的16种PAHs进行检测的结果表明,该石油采出水中的不溶颗粒态ΣPAHs的含量远高于溶解态ΣPAHs,且其中2~3环的萘、菲、芴等低环芳烃含量占比约70%,苯环数超过3的相对较高生物毒性的PAHs浓度占比超20%。
3)在多组分高环PAHs共存的条件下,不溶的颗粒态萘的降解优先级仍然最高,尤其是水样中被更迅速的去除,在60 min内对萘的总去除率可达61.13%。随光催化反应时间的增加,高毒性的 PAHs可被降解生成苊烯、蒽、菲、萘等,可大大降低采出水的生物毒性。因此,可以考虑在油田采出水的处理工艺中增加PAHs脱毒工艺,对保障原有水厂中的生化工艺段高效率、稳定运行极具价值。
g-C3N4/TiO2复合薄膜光催化降解石油采出水中多环芳烃
Degradation of polycyclic aromatic hydrocarbons in oilfield produced wastewater based on g-C3N4/TiO2 composite thin film photocatalyst
-
摘要: 针对亟待解决的石油开采废水中的多环芳烃(polycyclic aromatic hydrocarbons, PAHs)污染物的生物毒害性问题,利用紫外光辐照g-C3N4/TiO2复合薄膜光催化剂,对取自内蒙某采油现场的采出水水样进行降解处理,研究多组分PAHs污染物共存条件下的不同环数PAHs发生光催化氧化反应的降解规律及降解动力学。采用扫描电子显微镜(scanning electron microscope, SEM)表征光催化剂表面微观形貌,采用固相微萃取法富集和萃取水样中的PAHs,采用气相色谱-质谱联用法(gas chromatography-mass spectrometry, GC-MS)检测分析PAHs含量。经UV/g-C3N4/TiO2光催化反应处理,采出水中不溶的颗粒态萘优先于溶解态萘被降解去除,经60 min后对萘的总去除率可达61.13%。大于4环不溶性高环PAHs,可被优先光催化降解,从而高毒性的PAHs污染物被逐级转化为相对较低生物毒性的苊烯、蒽、菲、萘等,其中苊烯的含量增长最显著。结论石油采出水的外排处理工艺中增加PAHs光催化脱毒工艺具有一定可行性,对于保障原有水厂中的生化工艺段高效率、稳定运行将极具价值。Abstract: Aiming at the urgent problem regarding the biological toxicity of polycyclic aromatic hydrocarbons(polycyclic aromatic hydrocarbons, PAHs)pollutants in oilfield-produced wastewater, original wastewater samples from an oil production site in Inner Mongolia were degraded by using UV irradiation g-C3N4/TiO2 composite film photocatalyst. The photocatalytic oxidation behavior and degradation kinetics of PAHs with different benzene ring numbers were studied under the coexistence of multiple pollutants. The surface morphology of the photocatalyst was characterized by scanning electron microscopy (scanning electron microscope, SEM). PAHs in water samples were enriched and extracted by solid-phase microextraction. The content of PAHs was quantitatively analyzed by gas chromatography/mass spectrometry (gas chromatography-mass spectrometry, GC-MS). The results showed that after UV/g-C3N4/TiO2 photocatalytic reaction treatment, insoluble granular naphthalene was removed preferentially than dissolved naphthalene. The total removal rate of naphthalene could reach 61.13% after 60 min photocatalytic reaction. Insoluble PAHs with more than 4 benzene rings could be preferentially degraded by photocatalysis. Then the highly toxic PAHs were gradually converted into acenaphthene (Ace), anthracene (Ant), phenanthrene (Phe), naphthalene (Nap) and so on, of which the content of Ace increased significantly. The application of PAHs photocatalytic "detoxification" process in the efflux treatment of oilfield-produced water has a certain feasibility. More extensive research is worth carrying out in the future. It will be of great value to ensure the highly efficient and stable operation of the existing biochemical process in petroleum production wastewater treatment plants.
-
Key words:
- produced wastewater /
- PAHs /
- photocatalytic degradation /
- solid-phase microextraction /
- GC-MS.
-

-
表 1 油田采出水原水水样(0 min)中所含的PAHs质量浓度
Table 1. PAHs concentration in 0 min
PAHs
0 min水样(过滤分离) PAHs含量总和/(mg·L−1) 溶解态
/(mg·L−1)颗粒态
/(mg·L−1)萘(Nap) 1.194 07 4.337 38 5.531 45 苊(Acy) 0.021 742 0.056 49 0.078 232 苊稀(Ace) 0.010 052 0.230 318 0.240 37 芴(Fl) 0.021 92 0.267 536 0.289 456 菲(Phe) 0.046 805 0.669 296 0.716 101 蒽(Ant) 0.057 235 0.159 895 0.217 13 荧蒽(Flu) 0.029 166 0.251 054 0.280 22 芘(Pyr) 0.044 552 0.519 69 0.564 242 苯并[a]蒽(BaA) 0.063 517 0.163 323 0.226 84 䓛(Chr) 0.031 157 0.138 712 0.169 869 苯并(b)荧蒽(BbF) 0.072 649 0.155 904 0.228 553 苯并[k]荧蒽(BkF) 0.033 369 0.078 795 0.112 164 苯并芘(BaP) 0.024 312 0.082 777 0.107 089 茚苯(1,2,3-cd)芘(InP) 0.082 25 0.175 794 0.258 044 二苯并[a]蒽(DahP) 0.072 927 0.158 395 0.231 322 苯并[g,h,i]芘(BghiP) 0.008 501 0.001 767 0.010 268 ΣPAHs 1.814 224 7.447 127 9.261 351 -
[1] 邓述波, 周抚生, 余刚, 等. 油田采出水的特性及处理技术[J]. 工业水处理, 2000(7): 10-12. [2] 马虹, 李婷, 陈冰, 等. UV/H2O2/TiO2催化氧化法对油田采出水中多环芳烃的处理效果[J]. 环境化学, 2012, 31(12): 1874-1877. [3] 刘建兴, 袁国清. 油田采出水处理技术现状及发展趋势[J]. 工业用水与废水, 2007(5): 20-23. doi: 10.3969/j.issn.1009-2455.2007.05.006 [4] 丁鹏元, 党伟, 王莉莉, 等. 油田采出水回注处理技术现状及展望[J]. 现代化工, 2019, 39(3): 21-25. [5] 李凌波, 闫松, 曾向东, 等. 油田采出水中有机物组成分析[J]. 石油化工, 2002(6): 472-475. [6] IMAM A, SUMAN S K, KANAUJIA P K, et al. Biological machinery for polycyclic aromatic hydrocarbons degradation: A review[J]. Bioresource Technology, 2022, 343: 126121. doi: 10.1016/j.biortech.2021.126121 [7] 马自俊. 乳状液与含油污水处理技术[M]. 北京: 中国石化出版社, 2006. [8] PATEL A B, SHAIKH S, JAIN K R, et al. Polycyclic aromatic hydrocarbons: sources, toxicity, and remediation approaches[J]. Frontiers in Microbiology, 2020, 11: 562813. doi: 10.3389/fmicb.2020.562813 [9] MALLAH M A, CHANGXING L, MALLAH M A, et al. Polycyclic aromatic hydrocarbon and its effects on human health: an updated review[J]. Chemosphere, 2022: 133948. [10] ABDEL-SHAFY H I, MANSOUR M S. A review on polycyclic aromatic hydrocarbons: source, environmental impact, effect on human health and remediation[J]. Egyptian Journal of Petroleum, 2016, 25(1): 107-123. doi: 10.1016/j.ejpe.2015.03.011 [11] 姚翔. CY含油污水生化处理站的污水处理技术选择研究[D]. 昆明: 昆明理工大学, 2015. [12] 魏香婷, 李欢, 刘海琴, 等. 土壤中有机污染物多环芳烃的微生物降解[J]. 山东化工, 2021, 50(13): 251-252. [13] MOJIRI A, ZHOU J L, OHASHI A, et al. Comprehensive review of polycyclic aromatic hydrocarbons in water sources, their effects and treatments[J]. Science of the Total Environment, 2019, 696: 133971. doi: 10.1016/j.scitotenv.2019.133971 [14] ADEOLA A O, FORBES P B. Advances in water treatment technologies for removal of polycyclic aromatic hydrocarbons: Existing concepts, emerging trends, and future prospects[J]. Water Environment Research, 2021, 93(3): 343-359. doi: 10.1002/wer.1420 [15] PENG X, XU P, DU H, et al. Degradation of polycyclic aromatic hydrocarbons: A review[J]. Applied Ecology and Environmental Research, 2018, 16: 6419-6440. doi: 10.15666/aeer/1605_64196440 [16] GONG C, HUANG H, QIAN Y, et al. Integrated electrocoagulation and membrane filtration for PAH removal from realistic industrial wastewater: Effectiveness and mechanisms[J]. RSC Advances, 2017, 7(83): 52366-52374. doi: 10.1039/C7RA09372A [17] KUPPUSAMY S, THAVAMANI P, VENKATESWARLU K, et al. Remediation approaches for polycyclic aromatic hydrocarbons (PAHs) contaminated soils: Technological constraints, emerging trends and future directions[J]. Chemosphere, 2017, 168: 944-968. doi: 10.1016/j.chemosphere.2016.10.115 [18] 王旺阳, 刘聪, 袁珮. 吸附法去除环境中多环芳烃的研究进展[J]. 化工进展, 2017, 36(1): 355-363. [19] 戴菀萱, 刘颖, 丁珊珊, 等. 光催化降解水环境中多环芳烃的研究进展[J]. 水资源保护, 2018, 34(5): 63-68. [20] NGUYEN V-H, SMITH S M, WANTALA K, et al. Photocatalytic remediation of persistent organic pollutants (POPs): A review[J]. Arabian Journal of Chemistry, 2020, 13(11): 8309-8337. doi: 10.1016/j.arabjc.2020.04.028 [21] SUN B, LI Q, ZHENG M, et al. Recent advances in the removal of persistent organic pollutants (POPs) using multifunctional materials: A review[J]. Environmental Pollution, 2020, 265: 114908. doi: 10.1016/j.envpol.2020.114908 [22] 李贞燕, 陈冰. ·OH对光催化臭氧降解油田采出水中多环芳烃影响的研究[J]. 水处理技术, 2015, 41(1): 77-80. [23] 李贞燕, 陈冰. 油田采出水中萘和芴的紫外光催化和·OH氧化降解过程影响因素与条件优化分析[J]. 环境工程, 2015, 33(10): 31-34. [24] NI S, FU Z, LI L, et al. Step-scheme heterojunction g-C3N4/TiO2 for efficient photocatalytic degradation of tetracycline hydrochloride under UV light[J]. Colloids and Surfaces A:Physicochemical and Engineering Aspects, 2022, 649: 129475. doi: 10.1016/j.colsurfa.2022.129475 [25] XU Z, ZHUANG C, ZOU Z, et al. Enhanced photocatalytic activity by the construction of a TiO2/carbon nitride nanosheets heterostructure with high surface area via direct interfacial assembly[J]. Nano Research, 2017, 10: 2193-2209. doi: 10.1007/s12274-017-1453-2 [26] NZILA A, MUSA M M. Current status of and future perspectives in bacterial degradation of benzo[a] pyrene[J]. International Journal of Environmental Research and Public Health, 2021, 18(1): 262. [27] QIN W, ZHU Y, FAN F, et al. Biodegradation of benzo (a) pyrene by Microbacterium sp. strain under denitrification: Degradation pathway and effects of limiting electron acceptors or carbon source[J]. Biochemical Engineering Journal, 2017, 121: 131-138. doi: 10.1016/j.bej.2017.02.001 [28] WOO O, CHUNG W, WONG K, et al. Photocatalytic oxidation of polycyclic aromatic hydrocarbons: Intermediates identification and toxicity testing[J]. Journal of Hazardous Materials, 2009, 168(2/3): 1192-1199. -



 下载:
下载: