-
据报道,2050年全球塑料行业产量将达到330×109 t[1],而原生或次生的MPs早已被发现广泛存在于在地表水、地下水、土壤和生物体中[2],成为塑料生产和使用衍生的重要环境问题。这些具有粒径小、比表面积大等特点的MPs[3],极易在环境中吸附和累积污染物[4],对生态环境及生物体健康产生威胁。有研究[5-7]表明,MPs在迁移过程中会受酸碱侵蚀、氧化作用等环境因素发生老化,表面粗糙度和比表面积增大,表面含氧官能团和吸附位点增加,从而表现出对有机污染物更强的吸附能力,间接引发更大的生态环境风险。
随着我国多轮“限塑令”的下达,可降解塑料产业迎来了井喷式发展阶段。以PGA为代表,一些原先可降解材料中的“贵族”,已蜂拥成为各地产业转型布局中的备选[8]。作为降解最快的脂肪族聚酯类高分子材料,PGA主要应用于高端医学植入,因其良好的生物相容性,最终可以在人体中降解成无毒无害的乙醇酸[9]。近年来,PGA也开始以农用薄膜、包装材料等形式,逐渐在民用领域有所应用。可降解塑料虽然最终会被完全降解,但由于其自身易降解的特点,环境迁移中的老化过程会有所加快,一定程度可能会比传统塑料具有更大的环境风险。范秀磊等[10]研究表明,脂肪族聚酯类高分子材料聚乳酸(poly lactic acid,PLA)作为可降解塑料,老化后对磺胺甲恶唑的最大吸附量(14.7 mg·g−1)是老化前(1.63 mg·g−1)的9.02倍,而不可降解塑料聚乙烯(poly ethylene,PE)老化后对磺胺甲恶唑的最大吸附量(5.20 mg·g−1)仅为老化前的3.01倍。因此,对于PGA等产量即将攀升的新型可降解材料,有必要进行相关的前期环境风险研究。
以TCH作为典型代表的四环素类抗生素是世界上生产和使用数量第二多的抗生素,在临床医疗和畜牧业中被广泛使用[11]。大量残留在水体、土壤等介质中的TCH可能被MPs吸附,带来更大的生态毒性[12],目前关于环境中MPs对抗生素类污染物的吸附研究主要集中于典型的不可降解MPs上,而对于正处于市场培育阶段的可降解PGA更为稀缺。
本研究采用微米级PGA颗粒作为目标MPs,TCH作为目标污染物,通过SEM、FT-IR、XPS、接触角表征以及吸附动力学、吸附等温线实验,探究了老化对PGA-MPs吸附TCH性能的影响。研究结果可为进一步探究PGA作为污染物载体具有的环境风险提供参考,同时有助于丰富和完善可降解MPs环境风险研究的基础数据。
-
材料与试剂:实验所需PGA-MPs购买于广东省东莞旺达塑胶原料有限公司,平均粒径0.5 mm。实验所用试剂均为AR级,其中TCH购自麦克林生化有限公司,H2O2(质量分数为30%)购自天津欧博凯化工有限公司,H2SO4购自天津大茂试剂。实验所用溶液均由超纯水(电阻率≥18.2 Ω)配制。PGA与TCH的理化性质见表1。
仪器:DZF真空干燥箱(上海坤天实验仪器有限公司)用于PGA的预处理。BS-2F数显振荡培养箱(常州国宇仪器制造有限公司)用于PGA的老化及吸附实验。采用UV-5200紫外-分光光度计(上海元析仪器有限公司)在357 nm波长下测定TCH浓度。采用扫描电子显微镜(scanning electron microscope,SEM,ZEISS Sigma 300,德国)观察PGA老化前后表面形态的变化。采用傅里叶红外光谱仪(fourier transform infrared spectroscopy,FT-IR,美国)分析PGA老化前后表面官能团的变化。采用X射线光电子能谱(X-ray photoelectron spectroscopy,XPS,美国)分析PGA老化前后表面元素的组成变化。采用接触角测量仪(contact angle measuring,承德鼎盛JY-82C视频接触角测定仪)对PGA表面进行接触角测量。采用全自动比表面积及孔隙度分析仪BET(automated surface area and porosity analyz,Micromeritics ASAP 2460,美国)对PGA的比表面积进行测量。
-
老化实验。分别将3 g PGA与100 mL 1 mol·L−1 H2SO4、质量分数为30%的H2O2溶液在250 mL锥形瓶中混合,置于恒温振荡器中,在150 r·min−1、25 ℃条件下振荡老化15 d,所得PGA即为酸处理(PGA-H2SO4)、碱处理(PGA-H2O2)和氧化处理的PGA。
-
吸附动力学实验。分别称取老化前后PGA各0.5 g与100 mL初始质量浓度15 mg·L−1的TCH溶液放置于100 mL玻璃瓶中,并置于恒温振荡器中在150 r·min−1下进行吸附实验。预实验发现PGA在12 h内可达到吸附平衡。因此,分别在1、2、3、4、5、6、7、8、9、10、11、12 h避光吸附后取样,经过0.22 μm有机系滤膜过滤后,测定TCH质量浓度。所有吸附实验均进行一组平行实验,以未加PGA的TCH溶液进行空白实验。
吸附等温线实验。在初始质量浓度为6、9、12、15、18 mg·L−1的TCH溶液中进行,恒温振荡12 h后取样,其余步骤均与吸附动力学实验相同。实验均在25 ℃条件下进行。
-
PGA对TCH的吸附量根据式(1)进行计算。对PGA吸附TCH的过程进行伪一级动力学(式(2))、伪二级动力学(式(3))、Weber-Morris模型(式(4))拟合。采用Herry(式(5))、Freundlich(式(6))和Langmuir(式(7))等温吸附模型模拟合吸附等温线实验数据。
式中:C0和Ct分别为初始时刻和t时刻TCH的质量浓度,mg·L−1;V为TCH体积,L;m是PGA质量,g;Qt为t时刻的吸附量,mg·g−1。
式中:Qe为平衡时单位质量PGA对TCH的吸附量,mg·g−1;K1为伪一级动力学速率常数,h−1;K2为伪二级动力学速率常数,g·(mg·h)−1;Kp为W-M模型速率常数,mg·(g·h0.5)−1;C为常数。
式中:Qm为单位质量PGA对TCH的最大吸附量,mg·g−1;Ce为平衡时TCH的质量浓度,mg·L−1;Kh为Herry方程分配系数;Kf为Freundlich吸附速率常数,L·mg−1;Kl为Langmuir吸附速率常数,(mg·g−1)·(L·mg−1)1∕n;n无量纲。
-
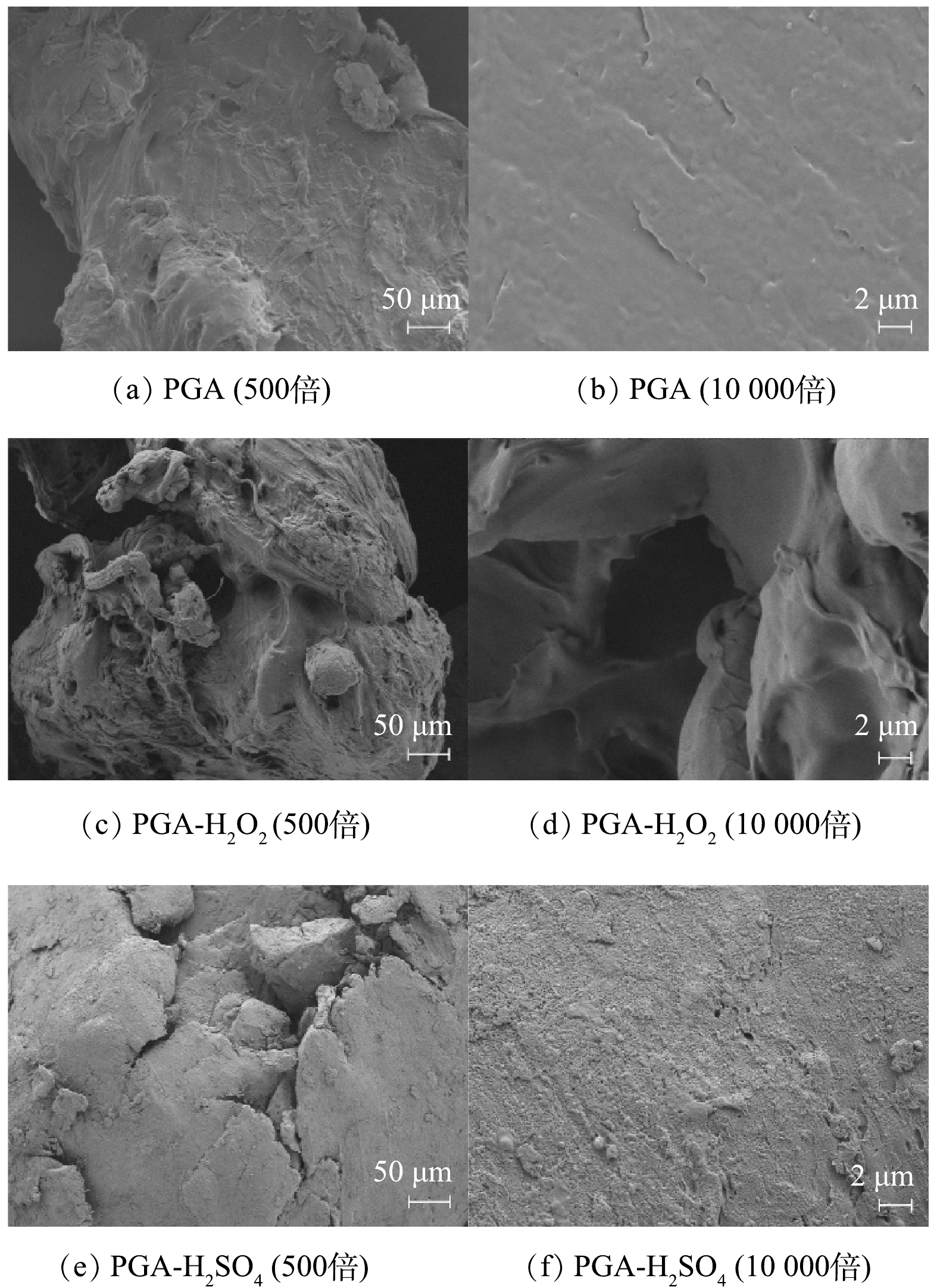
1)PGA老化前后表面形态。老化前后PGA的表面形态如图1所示。可以看出,老化前PGA表面较为光滑,而经过两种老化方式后表面变得异常粗糙,皆出现了大量的褶皱、裂纹,但表面形态略有不同,经H2O2老化后PGA表面出现高低不平的凸起,而经H2SO4老化后,在10 000倍率下可清晰观察到表面有许多孔状结构。孔凡星等[13]发现可降解PLA经硫酸钾热活化老化之后,比表面积从2.556 m2·g−1增加至15.806 m2·g−1。对老化前后PGA的比表面积进行测量,结果表明,经H2O2与H2SO4老化后,PGA比表面积由0.017 m2·g−1分别增至0.327 m2·g−1和0.467 m2·g−1。老化后PGA粗糙的颗粒表面可能产生更大的比表面积和更多的污染物吸附位点[14]。
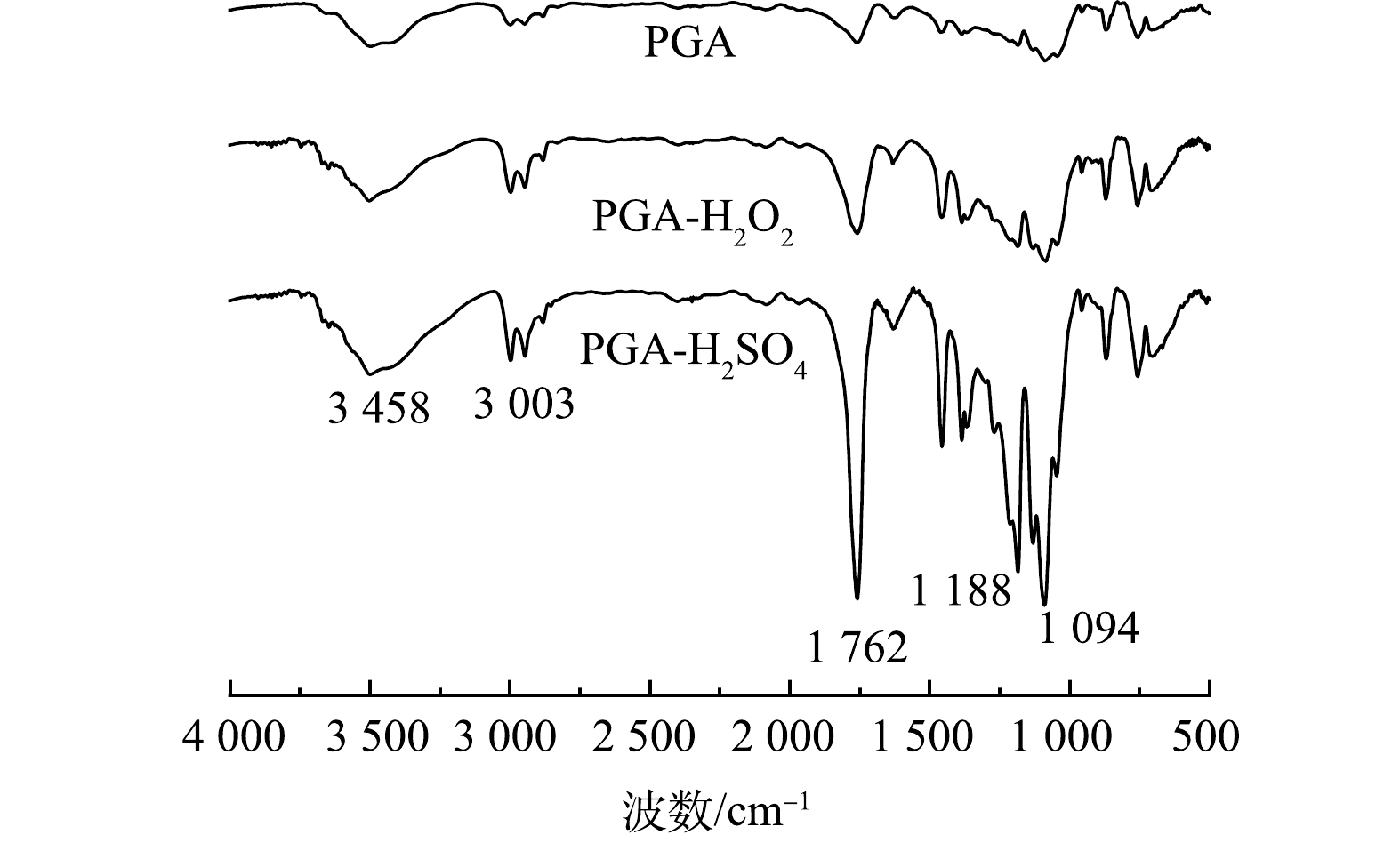
2) PGA老化前后表面官能团及元素表征。如图2所示,根据PGA分子结构判断,在3 458、3 003、1 762、1 188~1 094 cm−1处的振动分别是由—OH、—CH2、C=O、C—O引起的吸收峰。2种老化后的PGA均未出现新的吸收峰或发生峰位偏移,但3 458、3 003、1 762、1 188~1 094 cm−1处的特征峰强度均明显增强,这表明PGA在老化过程中未产生新的基团,分子链由长变短[15]。进一步用羰基指数[16]来量化PGA的老化程度(式(8))。
式中:R为羰基指数;A0为以1 762 cm−1为中心的峰区面积;A为波长在600~2 000 cm−1的峰区面积。
根据式(8)计算出PGA、PGA-H2O2、PGA-H2SO4的羰基指数分别为0.128、0.180、0.199,老化后PGA的羰基指数值均大于老化前PGA。
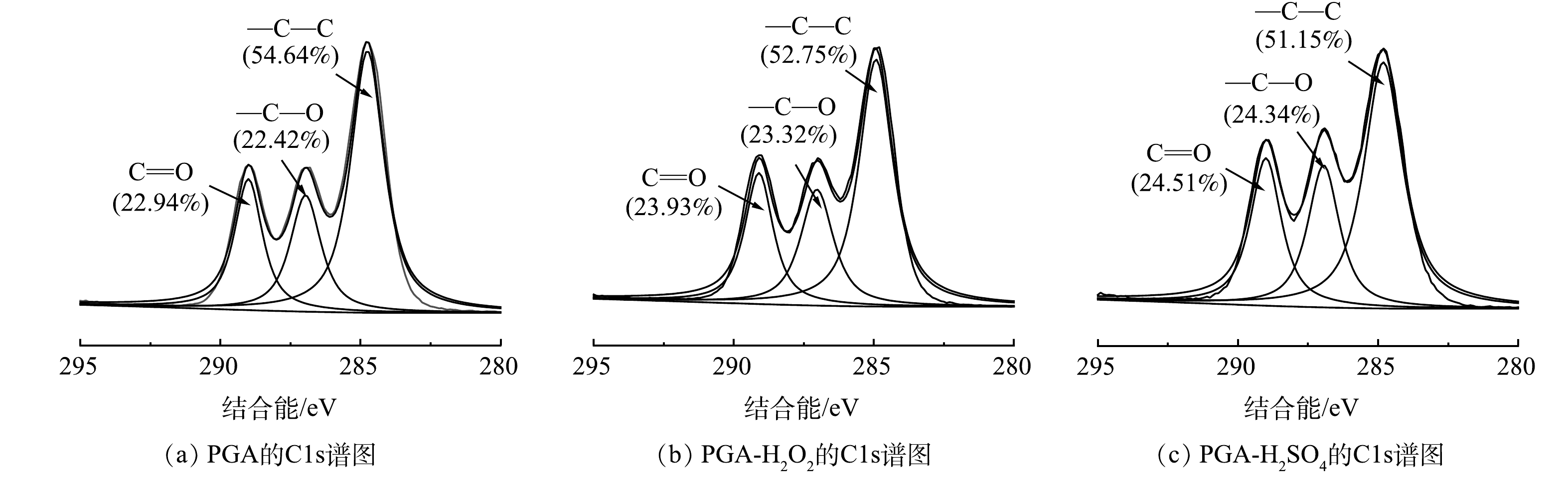
利用XPS定量比较老化前后PGA的组成。如图3所示,老化前后的XPS图谱中均有3个明显的峰,分别对应—C—C、—C—O、C=O。可以看出老化后PGA的含氧官能团含量较老化前均有一定程度增加,且PGA-H2SO4含氧官能团的总增量(3.49%)大于PGA-H2O2(1.89%)。
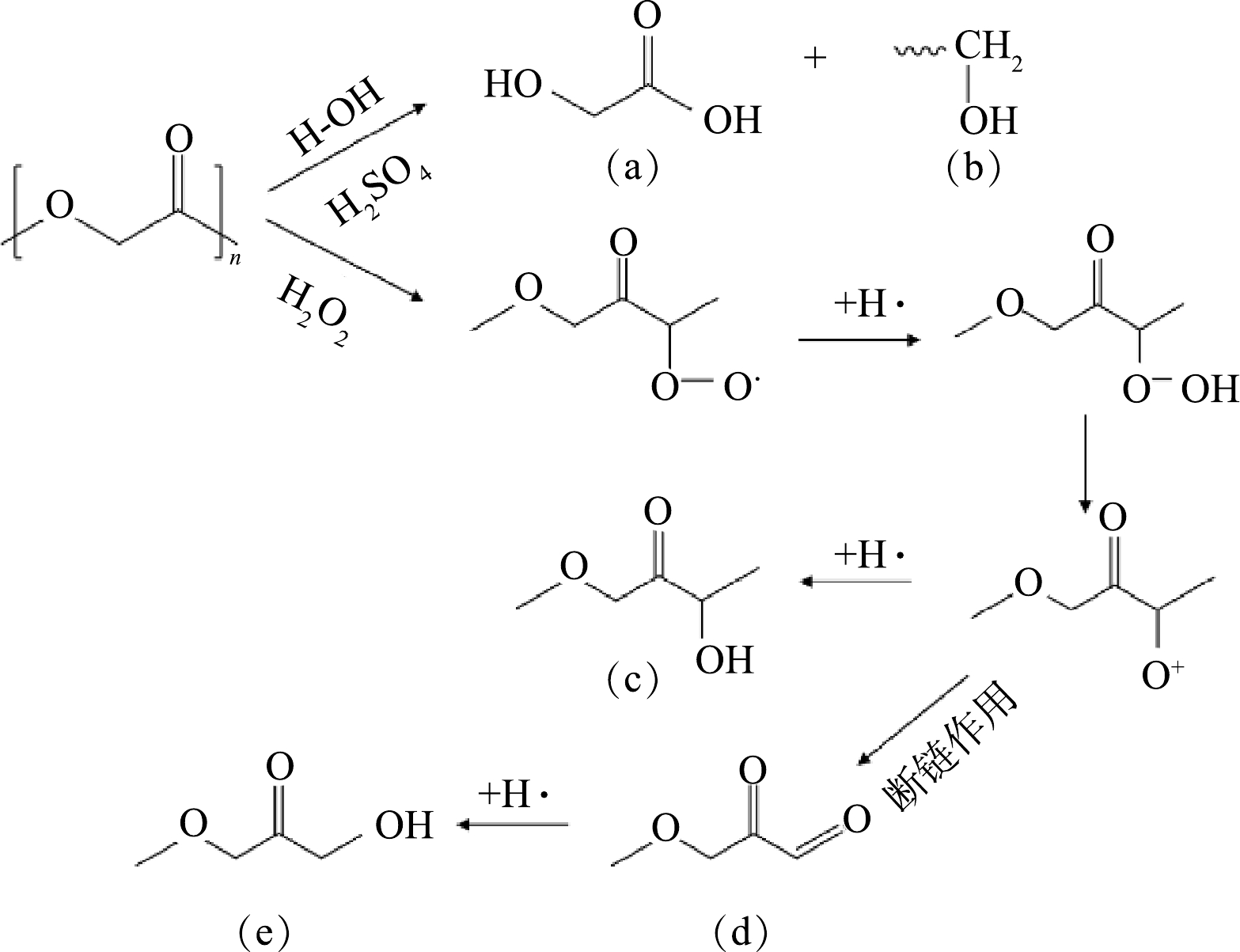
一般来说,高分子塑料在老化过程中会发生链断裂、氧化和重排[17]。含氧官能团的变化被认为与聚合物表面部分C—H键和C—C键受到氧化导致链断裂有关[18-19]。H2O2可通过直接氧化或者吸收光子产生过氧自由基[20],因为水环境中H+较多,过氧自由基会与H+生成-OH[21],导致C—H键摩尔比减少,C—O键摩尔比增加。在酸性环境中,PGA中的COO键首先发生水解断裂[22],形成羧基端与羟基端,通过自由基进行进一步的断链反应[2]。因此,H2O2与H2SO4对PGA老化机理可能不同。如图4所示,PGA-H2SO4老化水解主要破坏C—H键产生乙醇酸(a)以及羟基端短链(b),PGA-H2O2在水环境中不仅产生c,还可通过断链作用产生d与e,主要破坏C—H键与C—O键,致使PGA-H2O2中羰基相对含量多于PGA-H2SO4,这与XPS谱图里相对应。由于反应所需键能不同,PGA可能更易与H2SO4反应,这可能导致PGA-H2SO4的比表面积与含氧官能团含量大于PGA-H2O2。
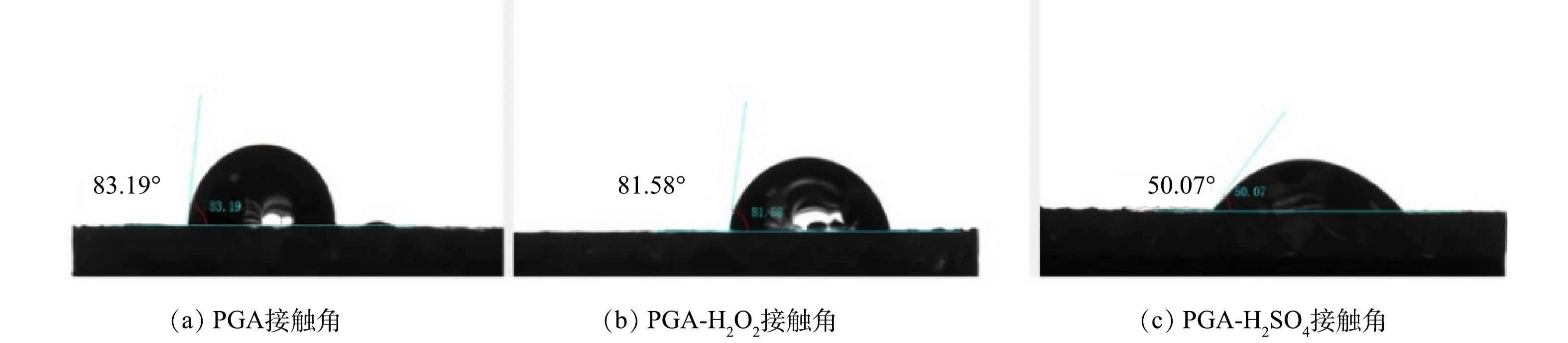
3) PGA老化前后接触角。接触角的大小常用来表征材料的亲疏水性。如图5所示,老化前PGA接触角为83.19°。由于PGA分子链中含有可与水反应的酯基,因此,具有一定的亲水性。经H2O2和H2SO4老化后接触角下降为81.58°和50.07°,表明H2O2和H2SO4老化均可以增加PGA表面的亲水性[23],且PGA-H2SO4的老化程度大于PGA-H2O2。这与红外光谱和XPS分析结果一致。此外,有研究[10]表明,老化过程中MPs亲水性增强有利于吸附亲水性有机物。
-
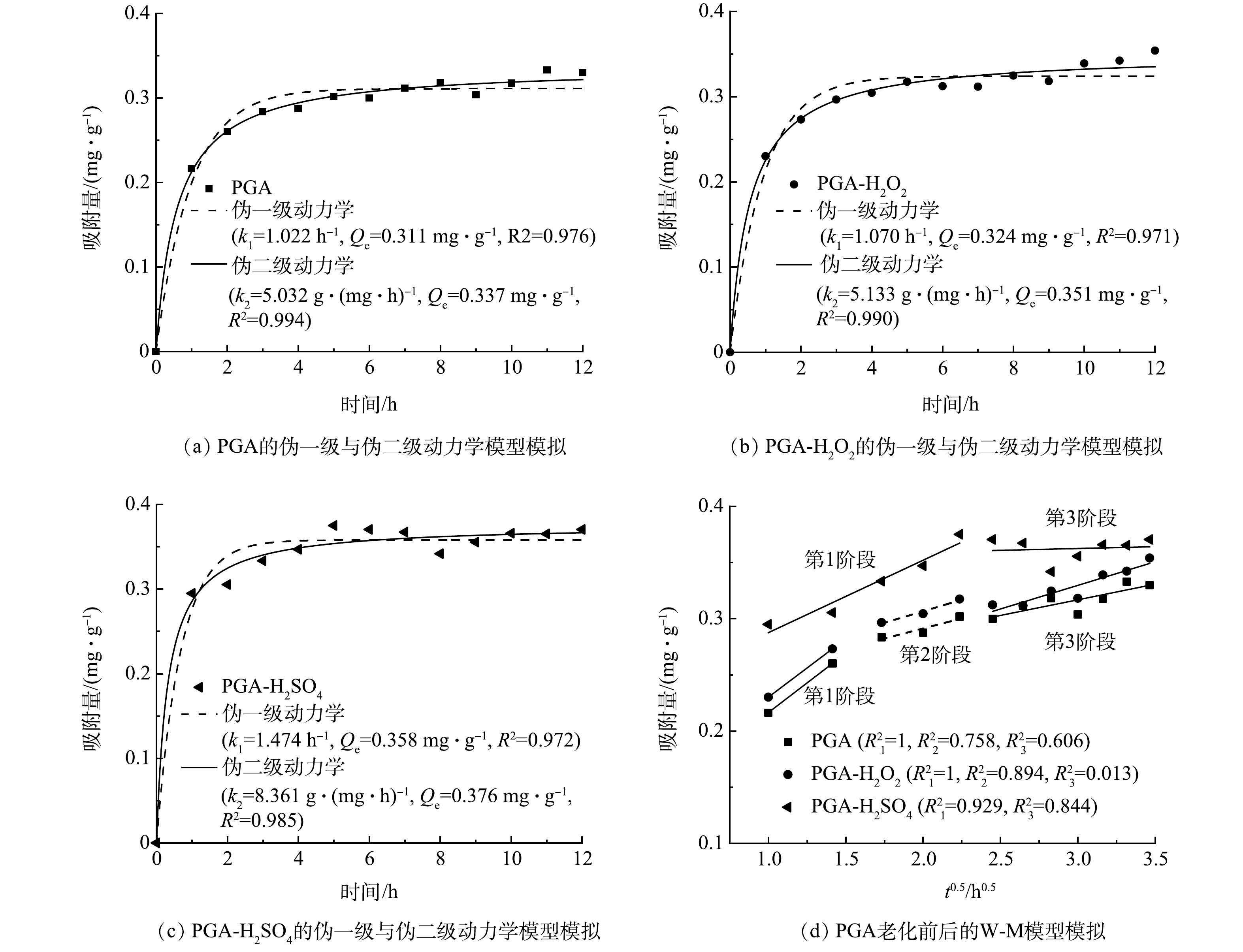
伪一级动力学和伪二级动力学吸附模型拟合结果如图6(a)~6(c)所示。可以看出,伪二级动力学模型(R2=0.985~0.994)较伪一级动力学模型(R2=0.971~0.976)可更好地描述老化前后PGA对TCH的吸附过程。这表明PGA对TCH的吸附过程可能存在物理吸附与化学吸附的共同作用,包含PGA颗粒周围的液膜扩散、颗粒内部的内扩散以及在颗粒表面吸附位点的物理或化学吸附等作用[24]。2种老化方式对TCH的平衡吸附量均有所增加,PGA-H2O2和PGA-H2SO4的Qe分别为0.351 mg·g−1与0.376 mg·g−1,较未老化的Qe(0.337 mg·g−1)分别提高了4.15%与11.57%。
W-M模型模拟结果如图6(d)所示。可以看出,PGA与PGA-H2O2呈现3个阶段的吸附过程,第1阶段为快速吸附阶段,TCH分子从溶液扩散到PGA表面,占据外部活化位点,扩散速率增大[25];第2阶段为内部扩散阶段,这一阶段在老化后PGA上有明显体现,因为老化前PGA表面较光滑,而老化后PGA比表面积增加,为TCH向PGA内部扩散增加位点。第3阶段为扩散平衡阶段,TCH在PGA上的吸附逐渐达到平衡。而PGA-H2SO4对TCH的吸附过程分为2个阶段,第1阶段和第2阶段间没有明显间隔,说明液膜扩散过程较短,拟合直线也不通过原点,仍表明TCH在PGA-H2SO4上的吸附过程仍以颗粒内扩散和表面吸附为主[26]。
-
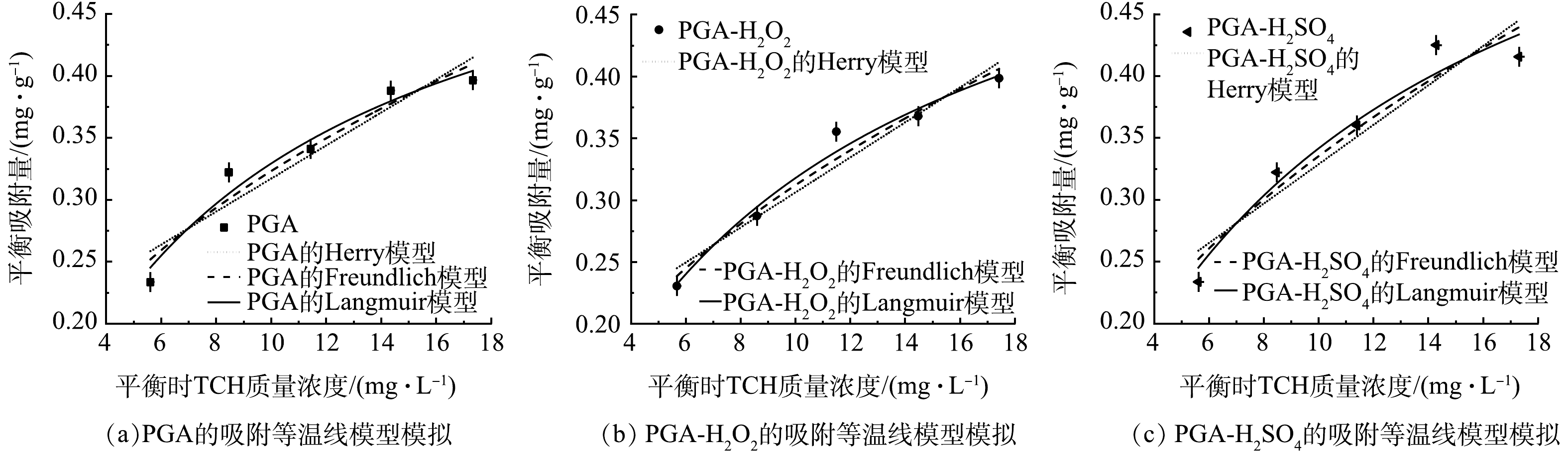
Herry、Freundlich和Langmuir等温吸附模型拟合结果见表2与图7。可以看出Langmuir模型(R2=0.941~0.974)较Herry模型(R2=0.807~0.848)和Freundlich模型(R2=0.905~0.954)能更好地描述老化前后PGA对TCH的吸附。这表明老化前后PGA对TCH的吸附是单分子层吸附,且均匀吸附于MPs的表面。从Langmuir等温吸附模型拟合结果来看,PGA-H2O2、PGA-H2SO4的最大吸附量Qmax分别是老化前PGA的1.05倍(0.617 mg·g−1)和1.17倍(0.686 mg·g−1),老化过程在一定程度上增强了PGA对TCH的吸附能力。
考虑到Herry模型与Freundlich模型的可决系数也较高,利用2个模型对吸附过程进行扩展分析。Herry模型中的Kh与Freundlich模型中的Kf均可以描述PGA对TCH的吸附能力,老化后的Kh与Kf均大于老化前,说明2种老化方式均增强了PGA对TCH的吸附能力,且吸附能力大小为PGA-H2SO4>PGA-H2O2>PGA。
-
一般来说,实际环境中MPs对污染物的吸附能力与塑料自身特性(粒径、结晶度、亲疏水性、老化方式等)与污染物性质密切相关[27]。有研究表明,纳米级聚苯乙烯(polystyrene,PS)的70 nm对多氯联苯的吸附量是微米级PE(10~180 μm)的1~2倍[28],粒径越小,吸附能力越强。MPs和有机污染物的亲疏水性对其吸附能力也有一定影响,表面具有亲水性的聚酰胺(polyamide,PA)相对于环丙沙星更容易吸附盐酸环丙沙星这种亲水性污染物(表3)。一定程度上结晶度越低的MPs对污染物表现出更强的吸附能力。老化会使MPs部分分子链发生氧化损伤,结晶度降低,无定形态区域增加,导致表面吸附活性增加[29]。
除了MPs与污染物自身性质之外,环境因素(pH、盐度、温度、共存物质等)也会对吸附产生影响。GUO等[30]研究pH对MPs吸附泰乐菌素的影响,发现pH的升高会导致MPs表面的电负性增加,与泰乐菌素之间的静电引力减小,导致MPs对泰乐菌素的吸附量随着pH的升高而减少。一般来说MPs对抗生素的吸附是自发吸热过程[27],温度升高会增加有机污染物的流动性和溶解度,使得MPs的吸附量也逐渐增加[33]。重金属的共存也会对MPs吸附有机污染物产生复杂的影响。刘迪等[31]发现Cu2+起到连接MPs和抗生素的作用,促进了MPs对CIP的吸附。但Cd2+因半径大而占据MPs表面吸附点位多,且有较弱的阳离子架桥作用,与CIP表现为竞争吸附,Cd2+抑制了MPs对CIP的吸附。
与现有MPs对抗生素类污染物吸附性能相比(表3),与聚氯乙烯(poly vinyl chloride,PVC)、 聚苯乙烯(poly styrene,PS)、聚丙烯(poly propylene,PP)、聚对苯二甲酸乙二醇酯(poly ethyleneglycol terephthalate,PET)等相比,PGA对TCH的吸附量与其他可降解与不可降解塑料相比普遍偏低。本研究中PGA的粒径为500 μm,较以往研究中MPs的粒径偏大,可能是相应吸附量较小的原因之一。一般来说,可生物降解塑料均具有亲水基团,在一定条件下对亲水性有机污染物的吸附能力大于疏水性有机污染物。在环境迁移过程中,可生物降解塑料因其良好的降解性能,故更容易在短时间内粒径变小,结晶度降低,对污染物的吸附能力增加。因此,需要进一步研究在真实环境条件下,PGA等可生物降解塑料对污染物的吸附和解吸,防范其环境风险。
-
1)经15 d H2O2和H2SO4老化后产生裂纹、凹坑,其中H2SO4老化后产生孔状结构,PGA经H2O2和H2SO4老化后比表面积从0.017 m2·g−1增加到0.327 m2·g−1和0.467 m2·g−1,表面含氧官能团分别增加了1.89%和3.49%,接触角从83.19°降至81.58°和50.07°,亲水性增强。这些变化会增强PGA对TCH的吸附能力。
2)伪二级动力学模型可以更好地描述老化前后PGA对TCH的吸附,说明在该吸附过程中可能存在物理吸附与化学吸附的共同作用。Langmuir等温吸附模型能更好地拟合PGA老化前后对TCH的吸附,说明PGA对TCH的吸附以单分子层吸附为主。
3)老化后PGA对TCH的吸附量均高于老化前。不同老化方式下TCH能力依次为:PGA-H2SO4(0.686 mg·g−1)>PGA-H2O2(0.617 mg·g−1)>PGA(0.585 mg·g−1),表明不同老化方式PGA对TCH的吸附量主要与其理化性质有关,如比表面积、表面官能团、接触角。
4)在复杂的实际环境下,除自身特性外,污染物的亲疏水性、水环境的pH、共存离子等因素都可能会对PGA的吸附能力产生影响,影响规律有待进一步研究。
可降解聚乙醇酸微塑料老化前后对盐酸四环素的吸附性能
Adsorption behavior of tetracycline hydrochloride on biodegradable poly glycolic acid microplastics before and after aging
-
摘要: 聚乙醇酸(poly glycolic acid,PGA)因其良好的降解性能会加快其老化过程,可能比传统塑料具有更大的环境风险,因此,评估PGA在环境迁移中对污染物的载体效应尤为重要。选用PGA颗粒微塑料(microplastics,MPs)为研究对象,盐酸四环素(tetracycline hydrochloride,TCH)为代表性污染物,探究老化过程对PGA吸附TCH行为的影响。结果表明:PGA在经过15 d H2O2和H2SO4老化后,表面均变得粗糙,比表面积由0.017 m2·g−1分别增至0.327 m2·g−1和0.467 m2·g−1,官能团含量分别增加了1.89%和3.49%,接触角由83.19°分别降至81.58°和50.07°。吸附动力学均符合伪二级动力学模型,吸附等温线均符合Langmuir等温吸附模型。老化后PGA对TCH的吸附量均高于老化前,PGA-H2O2和PGA-H2SO4最大表观吸附量分别为0.617 mg·g−1和0.686 mg·g−1,是PGA老化前的1.05倍和1.17倍。Abstract: The good degradation performance of Poly glycolic acid (PGA) can accelerate its aging process, which poses greater environmental risks than traditional plastics. Therefore, it is particularly important to evaluate the carrier effect of PGA on pollutants during its environmental migration. In this study, MP (Microplastic, MP ) of PGA particles was selected as the research object, and Tetracycline hydrochloride (TCH) was selected as the representative pollutant to explore the influence of aging process on the adsorption behavior of PGA. The results showed that the surface of PGA became rough after 15 d H2O2 or H2SO4 aging treatment, and the specific surface areas increased from 0.017 m2·g−1 to 0.327 with 0.467 m2·g−1, the function groups increased by 1.89% and 3.49%, the contact angles decreased from 83.19° to 81.58° and 50.07°, respectively. The adsorption kinetics all fitted the pseudo-second-order kinetic model, and the adsorption isotherms all fitted the Langmuir isotherm model. The adsorption capacity of PGA to TCH after aging was higher than before. The maximum apparent adsorption capacities of PGA-H2O2 and PGA-H2SO4 could reach 0.617 and 0.686 mg·g−1, which was 1.05 and 1.17 times that of PGA before aging, respectively.
-
Key words:
- biodegradable microplastics /
- polyglycolic acid /
- adsorption /
- tetracycline hydrochloride
-

-
表 1 PGA及TCH的理化性质
Table 1. The physico-chemical properties of PGA and TCH
化合物 简写 化学式 相对分子质量/Da 密度/(g·cm−3) 熔点/℃ 玻璃转化温度/℃ logP 水溶性 结晶度 聚乙醇酸 PGA (C2H4O3)n 1×104~100×104 1.30 220~240 35~40 — 82.79° 40~80 盐酸四环素 TCH C22H25N2O8Cl 480.9 1.13~1.35 220~223 — 1.288 50 g·L-1 — 表 2 PGA老化前后对TCH的吸附等温线参数
Table 2. Adsorption isotherm parameters of TCH on microplastics before and after aging
吸附剂 Herry模型 Freundlich模型 Langmuir模型 Kh/(L·g−1) R2 Kf/(L·mg−1) 1/n R2 K2/((mg·g−1)·(L·mg−1)1/n) Qmax/(mg·g−1) R2 PGA 0.026 0.807 0.108 0.431 0.916 0.128 0.585 0.952 PGA-H2O2 0.028 0.844 0.115 0.473 0.954 0.106 0.617 0.974 PGA-H2SO4 0.029 0.848 0.120 0.493 0.905 0.099 0.686 0.941 表 3 不同类型的微塑料对抗生素的吸附
Table 3. Adsorption of antibiotics by different types of microplastics
微塑料种类
有机污染物 微塑料粒径/
μmQmax/
(mg·g−1)吸附速率/
(g·(mg·h)−1)参考文献 聚氯乙烯(PVC)
(疏水)泰乐菌素(疏水) 74 3.33 0.010 [30] 土霉素(疏水) 450~1000 27.5 — [7] 环丙沙星(疏水) 135.9 1.86 — [31] 聚乙烯(PE)
(疏水)磺胺甲恶唑(疏水) 160.37 5.20 2.352 [11] 阿莫西林(亲水) 160.37 4.98 0.918 [11] 泰乐菌素(疏水) 74 1.67 0.010 [30] 头孢菌素C(亲水) 500~1000 0.72 1.000 [32] 聚丙烯(PP)
(疏水)盐酸环丙沙星(亲水) 74 5.46 1.720 [23] 泰乐菌素(疏水) 74 3.33 0.010 [30] 聚苯乙烯(PS)
(疏水)盐酸环丙沙星(亲水) 74 6.19 1.220 [23] 泰乐菌素(疏水) 74 3.33 0.010 [30] 头孢菌素C(亲水) 500-1000 0.71 0.100 [32] 聚对苯二甲酸乙二酯(PET)
(疏水)四环素(疏水) 100-150 3.62 0.437 [14] 聚酰胺(PA)
(亲水)盐酸环丙沙星(亲水) 74 7.46 1.350 [23] 环丙沙星(疏水) 164.7 1.85 — [31] 聚乳酸(PLA)
(疏水)四环素(疏水) 100-150 2.29 0.178 [14] 磺胺甲恶唑(疏水) 613.55 14.70 0.540 [11] 阿莫西林(亲水) 613.55 10.40 0.348 [11] 聚乙醇酸(PGA)
(亲水)盐酸四环素(亲水) 500 0.617 5.133 — 500 0.686 8.361 — -
[1] SHARMA M D, ELANJICKAL A I, MANKAR J S, et al. Assessment of cancer risk of microplastics enriched with polycyclic aromatic hydrocarbons[J]. Journal of Hazardous Materials, 2020, 398: 122994. doi: 10.1016/j.jhazmat.2020.122994 [2] 马思睿, 李舒行, 郭学涛, 等. 微塑料的老化特性、机制及其对污染物吸附影响的研究进展[J]. 中国环境科学, 2020, 40(9): 3992-4003. doi: 10.3969/j.issn.1000-6923.2020.09.032 [3] DONG Y, GAO M, SONG Z, et al. Adsorption mechanism of As(III) on polytetrafluoroethylene particles of different size[J]. Environmental Pollution, 2019, 254: 112950. doi: 10.1016/j.envpol.2019.07.118 [4] LIU S, HUANG J, ZHANG W, et al. Microplastics as a vehicle of heavy metals in aquatic environments: A review of adsorption factors, mechanisms, and biological effects[J]. Journal of Environmental Management, 2022, 302: 113995. [5] LI X, MEI Q, CHEN L, et al. Enhancement in adsorption potential of microplastics in sewage sludge for metal pollutants after the wastewater treatment process[J]. Water Research, 2019, 157: 228-237. doi: 10.1016/j.watres.2019.03.069 [6] WIJESEKARA H, BOLAN N S, BRADNEY L, et al. Trace element dynamics of biosolids-derived microbeads[J]. Chemosphere, 2018, 199: 331-339. doi: 10.1016/j.chemosphere.2018.01.166 [7] ZHANG H, WANG J, ZHOU B, et al. Enhanced adsorption of oxytetracycline to weathered microplastic polystyrene: Kinetics, isotherms and influencing factors[J]. Environmental Pollution, 2018, 243: 1550-1557. doi: 10.1016/j.envpol.2018.09.122 [8] 夏之学. 2021-2025年中国聚乙醇酸(PGA)发展前景浅析[J]. 中国化工贸易, 2020, 28: 2-3. doi: 10.3969/j.issn.1674-5167.2020.36.QKBJBD20202021062500011051 [9] 陈群, 许平, 崔爱军, 等. 煤基聚乙醇酸技术进展[J]. 化工进展, 2011, 30(1): 172-180. [10] 范秀磊, 甘容, 谢雅, 等. 老化前后聚乳酸和聚乙烯微塑料对抗生素的吸附解吸行为[J]. 环境科学研究, 2021, 34(7): 1747-1756. [11] JI Y, SHI Y, DONG W, et al. Thermo-activated persulfate oxidation system for tetracycline antibiotics degradation in aqueous solution[J]. Chemical Engineering Journal, 2016, 298: 225-233. doi: 10.1016/j.cej.2016.04.028 [12] RAZANAJATOVO R M, DING J, ZHANG S, et al. Sorption and desorption of selected pharmaceuticals by polyethylene microplastics[J]. Marine Pollution Bulletin, 2018, 136: 516-523. doi: 10.1016/j.marpolbul.2018.09.048 [13] 孔凡星, 许霞, 薛银刚, 等. 微塑料老化对四环素吸附行为的影响[J]. 环境科学研究, 2021, 34(9): 2182-2190. [14] 张瑞昌, 李泽林, 魏学锋, 等. 模拟环境老化对PE微塑料吸附Zn(Ⅱ)的影响[J]. 中国环境科学, 2020, 40(7): 3135-3142. [15] 唐四叶, 张瑞, 刘丰, 等. 一步合成的聚乙醇酸在不同介质中的熔融水解[J]. 河南大学学报:自然科学版, 2016, 44(2): 71-76. [16] 范秀磊, 常卓恒, 邹晔锋, 等. 可降解微塑料对铜和锌离子的吸附解吸特性[J]. 中国环境科学, 2021, 41(5): 2141-2150. [17] MAO R, LANG M, YU X, et al. Aging mechanism of microplastics with UV irradiation and its effects on the adsorption of heavy metals[J]. Journal Hazardous Materials, 2020, 393: 122515. doi: 10.1016/j.jhazmat.2020.122515 [18] KEDZIERSKI M, D'ALMEIDA M, MAGUERESSE A, et al. Threat of plastic ageing in marine environment. Adsorption/desorption of micropollutants[J]. Marine Pollution Bulletin, 2018, 127: 684-694. doi: 10.1016/j.marpolbul.2017.12.059 [19] WANG Q, ZHANG Y, WANGJIN X, et al. The adsorption behavior of metals in aqueous solution by microplastics effected by UV radiation[J]. Journal of Environmental Sciences, 2021, 101: 440-440. doi: 10.1016/j.jes.2020.11.001 [20] KACZMAREK H, KAMINâ SKA A, Sâ WIATEK M, et al. Photoinitiated degradation of polystyrene in the presence of low-molecular organic compounds[J]. European Polymer Journal, 2000, 36: 1167-1173. doi: 10.1016/S0014-3057(99)00175-5 [21] ZHOU Z, HUANG G, XIONG Y, et al. Unveiling the susceptibility of functional groups of poly(ether sulfone)/polyvinylpyrrolidone membranes to NaOCl: A Two-dimensional correlation spectroscopic study[J]. Environmental Science & Technology, 2017, 51(24): 14342-14351. [22] 吴迪, 陈亚芍. 微波辐射法合成聚乙醇酸及其性能研究[J]. 西北大学学报:自然科学版, 2010, 40(4): 641-645. [23] JIANG Z, HUANG L, FAN Y, et al. Contrasting effects of microplastic aging upon the adsorption of sulfonamides and its mechanism[J]. Chemical Engineering Journal, 2022, 430: 132939. doi: 10.1016/j.cej.2021.132939 [24] 王小玲, 马杰, 顾明华, 等. 砷和磷在不同污染类型土壤中的竞争吸附动力学[J]. 生态环境学报, 2015, 24(4): 694-699. [25] YOU H, HUANG B, CAO C, et al. Adsorption–desorption behavior of methylene blue onto aged polyethylene microplastics in aqueous environments[J]. Marine Pollution Bulletin, 2021, 167: 112287. doi: 10.1016/j.marpolbul.2021.112287 [26] 杨晨, 金舜铂, 李鹏飞, 等. 老化方式对聚苯乙烯与阿米替林吸附性能的影响[J]. 中南民族大学学报:自然科学版, 2022, 41(3): 305-311. [27] 刘沙沙, 陈诺, 杨晓茵, 等. 微塑料对有机污染物的吸附-解吸特性及其复合毒性效应研究进展[J]. 生态环境学报, 2022, 31(3): 610-620. [28] VELZEBOER I, KWADIJK C J, KOELMANS A A. Strong sorption of PCBs to nanoplastics, microplastics, carbon nanotubes, and fullerenes[J]. Environmental Science & Technology, 2014, 48(9): 69-76. [29] LIU J, MA Y, ZHU D, et al. Polystyrene nanoplastics-enhanced contaminant transport: Role of irreversible adsorption in glassy polymeric domain[J]. Environmental Science & Technology, 2018, 52(5): 2677-2685. [30] GUO X, PANG J, CHEN S, et al. Sorption properties of tylosin on four different microplastics[J]. Chemosphere, 2018, 209: 240-245. doi: 10.1016/j.chemosphere.2018.06.100 [31] 刘迪, 童非, 高岩, 等. 重金属存在下微塑料对环丙沙星的吸附特征及机制研究[J]. 农业环境科学学报. 2021, 40(5): 1017-1025. [32] XUAN G, WANG J, et al. Sorption of antibiotics onto aged microplastics in freshwater and seawater[J]. Marine Pollution Bulletin, 2019, 149: 110511. doi: 10.1016/j.marpolbul.2019.110511 [33] XU P, GE W, CHAI C, et al. Sorption of polybrominated diphenyl ethers by microplastics[J] Marine Pollution Bulletin, 2019, 145: 260-269. -



 下载:
下载: