-
扬子鳄(Alligator sinensis)是中国特有物种。20世纪80年代以来,扬子鳄野生种群数量急剧下降[1-2],国际自然保护联盟(IUCN)已将扬子鳄列为极度濒危物种。然而,扬子鳄的致濒机制尚不十分清楚。研究认为,栖息地的减少及碎片化、全球环境变化及环境污染可能是扬子鳄野生种群数量锐减的主要原因[3-4]。
滴滴涕(DDT)及其代谢产物二氯二苯二氯乙烯(DDE)和二氯二苯二氯乙烷(DDD)等化合物是环境中广泛存在的内分泌干扰物。我国于20世纪50年代开始生产DDT,随后于1983年被禁用。然而,某些农药(如三氯杀螨醇)和防锈漆中仍然含有DDT成分,这些产品中的DDT还将持续释放到环境。这些污染物具有生物富集效应并可以沿食物链产生生物放大效应,从而对生物的内分泌系统和繁殖系统产生毒害作用[5]。鳄类处于食物链的顶端,很容易蓄积这类污染物。研究证实,DDT类污染物暴露可能使鳄类在性别分化过程中产生性别反转,并可导致新生鳄和幼鳄存活率降低[6-7]。此外,这些污染物可以导致成鳄繁殖力下降、血清性激素水平改变和第二性征异形等不利后果[8-12],最终影响野生种群数量[8-9]。
圈养是保护极度濒危物种-扬子鳄的重要手段。我国主要的扬子鳄圈养地(安徽宣城和浙江长兴)位于长江中下游著名的鱼米之乡,历史上该地区DDT曾作为杀虫剂大量使用。我们的初步研究发现,圈养扬子鳄及卵中DDT及其降解产物的含量处于非点源污染地鳄类这些污染物含量报道值的高端,卵中DDE含量高值已接近可能产生性别反转的DDE含量限值[13]。然而,圈养扬子鳄栖息环境中DDT类化合物污染现状尚不清楚。
本研究测试了我国最大的扬子鳄圈养基地水塘沉积物中DDT、DDE、DDD、2,2-二(氯苯基)1-氯乙烯(DDMU)和二氯二苯甲烷(DDM)等DDT相关污染物的含量和组成,评估了这些污染物潜在的生态风险。
-
沉积物样品分别于2017年3月(春季)、6月(夏季)和9月(秋季)采集于安徽省扬子鳄繁育研究中心扬子鳄栖息水塘(繁殖塘)。该中心位于安徽省宣城市,是我国最大的扬子鳄圈养基地。利用Van veen抓斗式采泥器采集表层沉积物样品,挑取树叶等杂物后置聚乙烯样品袋,采样当天运回实验室。
沉积物样品的前处理及净化流程参照文献[14]。样品经真空冷冻干燥后,研磨过75目(0.2 mm)不锈钢筛。称取约3 g样品,加入回收率指示物(13C-PCB 141、PCB 65和PCB 204)和铜片(去除元素硫)后,用丙酮/正己烷混合溶剂(1:1,V/V)索氏抽提48 h。抽提液浓缩至1 mL左右,过多层硅胶氧化铝色谱柱净化,用二氯甲烷/正己烷混合溶剂(1:1,V/V)洗脱。洗脱液浓缩至1 mL左右,转换溶剂为正己烷,氮吹定容至100 μL。加入60 ng内标化合物(13C-PCB 208、PCB 24、PCB 82 和PCB 198),上气相色谱/质谱(GC/MS)测试。
-
使用GC-MS-EI(Agilent 6890 GC-5975B Series MS)测定DDT及其降解产物的含量。选择离子检测(SIM)模式,无分流进样,进样量为1 μL。采用DB-5 MS毛细色谱柱(60 m × 250 μm i.d. × 0.25 μm;J&W Scientific)进行目标化合物的分离。仪器测试条件参见文献[15]。
-
质量控制和保证措施包括程序空白、加标空白以及样品重复样分析。样品中回收率指示物13C-PCB 141、PCB 65和PCB 204的回收率范围分别为86.8%±5.6%、82.9%±5.8%和90.8% ±5.8%。程序空白(n = 4)中检出p,p'-DDE,但其含量远低于样品中该化合物含量(< 5%)。加标空白(n = 5)中8种化合物(p,p'-DDT、p,p'-DDE、p,p'-DDD、o,p'-DDT、o,p'-DDT、o,p'-DDT、p,p'-DDMU和p,p'-DDM)的回收率范围为92.6%±5.8%。样品中目标化合物最终浓度未经回收率校正和空白校正。样品重复样中目标化合物含量的相对标注偏差(RSD)小于15%。
p,p'-DDE的检测限(LOD)按方法空白中的含量加3倍标准偏差计算,其他目标化合物的LOD按5倍信噪比(S/N)计算。沉积物样品中DDT及其降解产物的检测限为0.01—0.11 ng·g−1干重。
-
实验数据采用Excel2019和Origin9.0软件进行统计检验、分析。沉积物中p,p'-DDMU与p,p'-DDE和p,p'-DDD之间的含量相关性采用Pearson相关分析,利用方差分析(ANOVA)检验不同采样季节∑DDXs含量差异的显著性。
-
塘沉积物中共检出p,p'-DDT、p,p'-DDE、p,p'-DDD、o,p'-DDT、o,p'-DDT、o,p'-DDT、p,p'-DDMU和p,p'-DDM等8种DDT类化合物,除o,p'-DDT外,其余化合物的检出率均为100%。这些污染物及∑DDXs(DDT及其降解产物含量之和)的含量见表1。水塘沉积物中∑DDXs的平均含量(7.97 ng·g−1 干重)高于法国洛林地区池塘沉积物中∑DDXs含量(均值:1.22 ng·g−1)[16],与波兰华沙地区池塘沉积物中∑DDXs含量(均值 6.70 ng·g−1)相当[17],但低于我国广东顺德地区鱼塘(均值 30.0 ng·g−1)[18]和巢湖流域池塘(均值 81.9 ng·g−1)[19]及印度阿萨姆邦两个池塘(分别为366 ng·g−1和449 ng·g−1)[20]沉积物中∑DDXs含量的1—3个数量级。
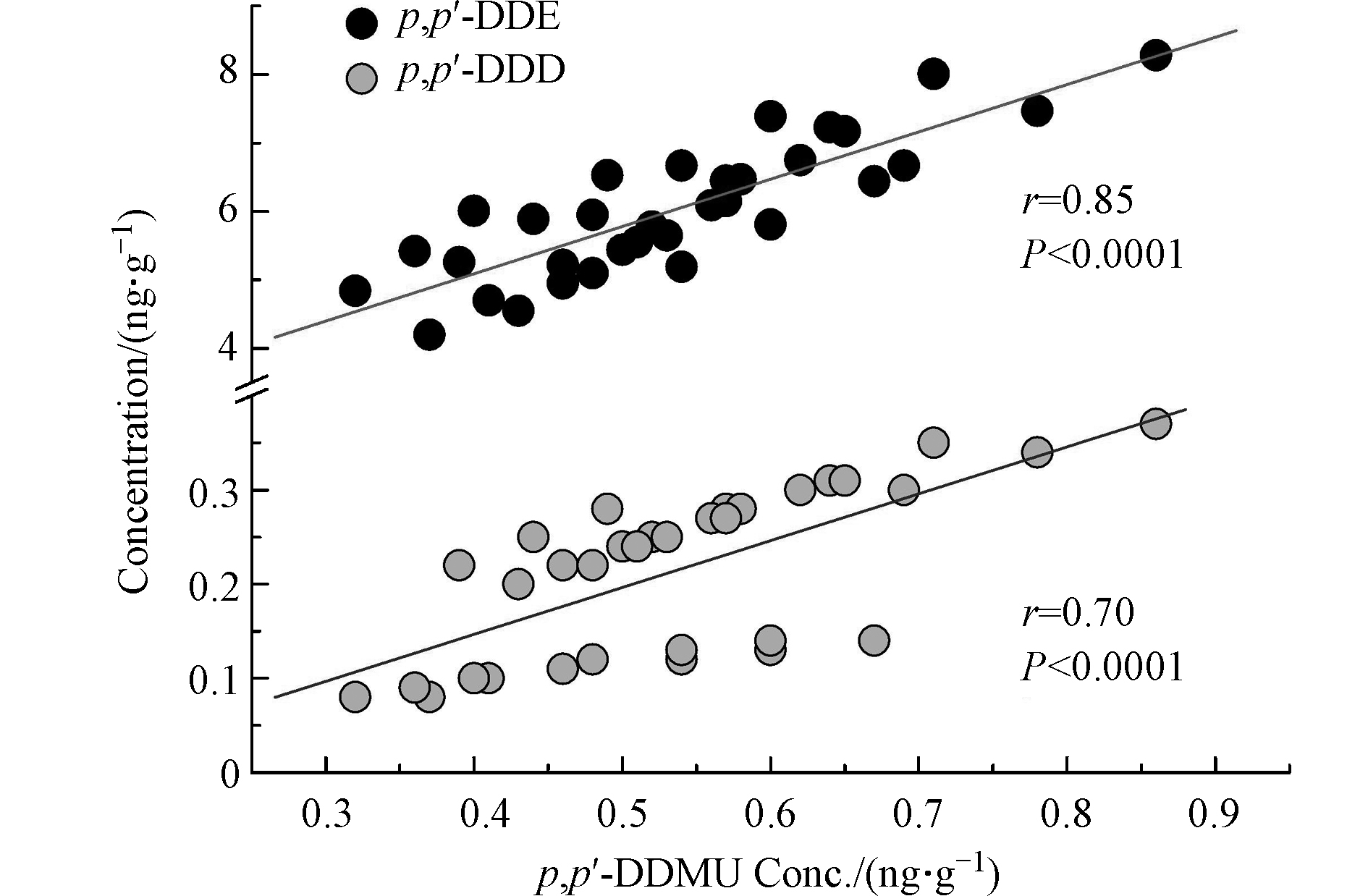
DDT的两种高阶降解产物p,p'-DDMU和p,p'-DDM也在沉积物中有检出,其含量均值分别为0.53 ng·g−1和0.04 ng·g−1干重(表1)。沉积物中DDT新型降解产物含量报道较少(表2)。本研究的沉积物中p,p'-DDMU的平均含量比广州东莞和顺德农村地区两个鱼塘中的p,p'-DDMU含量高出1个数量级[21],与渤海湾沉积物中p,p'-DDMU含量相当[22],但低于长江、海河以及波兰维斯图拉河沉积物中p,p'-DDMU含量[23-25]。美国的帕洛斯弗迪斯大陆架以及旧金山湾某运河沉积物中检测到了极高浓度的p,p'-DDMU,其含量高出当前报道值2—3个数量级[26-27]。沉积物p,p'-DDM的报道值极少。本研究沉积物中p,p'-DDM的含量低于长江(3.56 ng·g−1)[23]以及美国的帕洛斯弗迪斯大陆架沉积物(12 ng·g−1)[26]中p,p'-DDM含量2—3个数量级。DDMU可能是DDE或DDD的降解产物 [22] 。本研究沉积物中 p,p'-DDMU含量与 p,p'-DDE( r = 0.85, P < 0.0001 )和
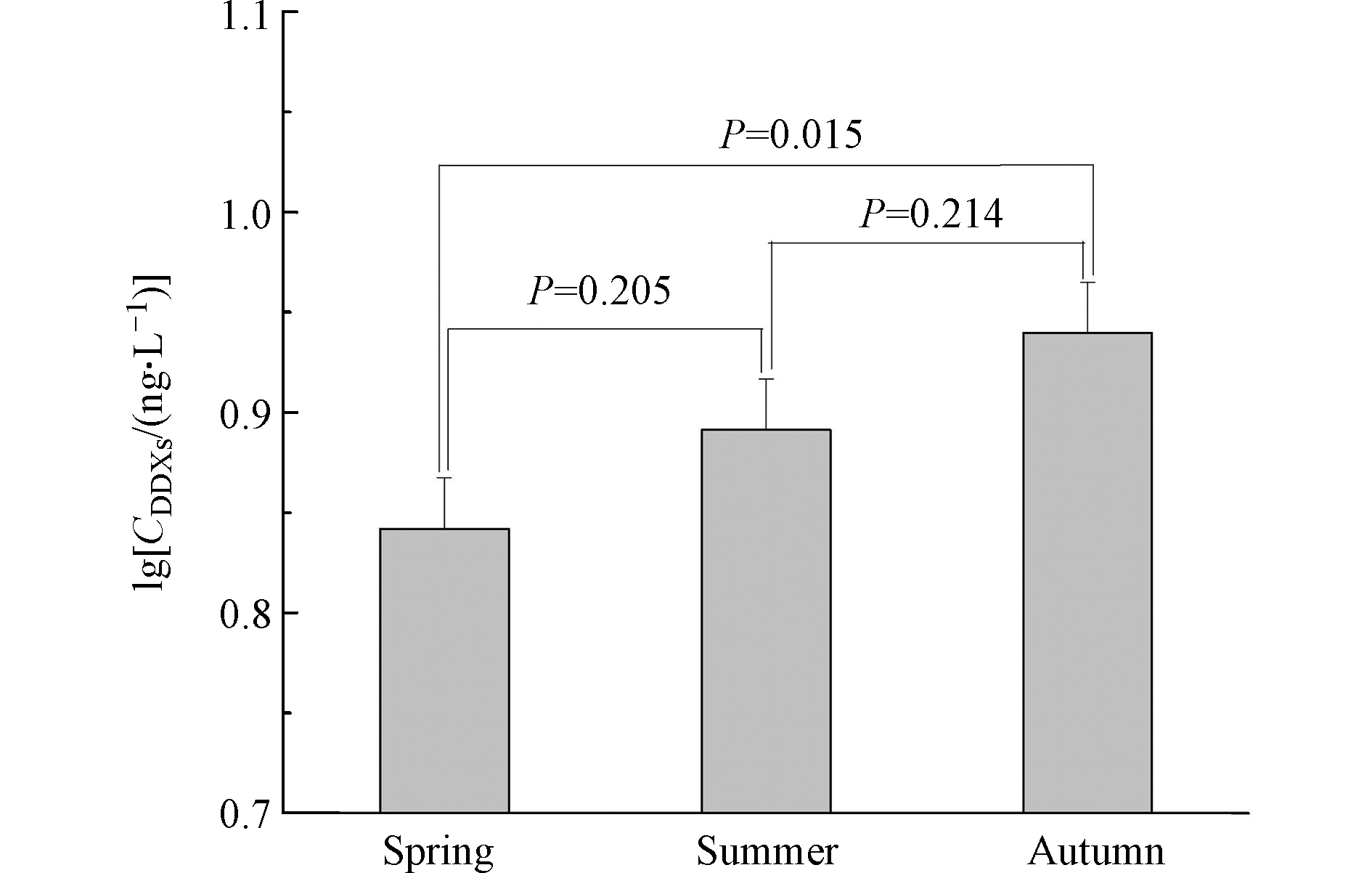
p,p'-DDD( r = 0.70, P < 0.0001 )的含量均具有显著相关性(图1),表明DDMU可能主要来自于沉积物中DDE和DDD的降解,或DDMU与其母体化合物具有相似的环境化学行为。p,p'-DDMU是一种雌激素类物质,对生物体的内分泌功能及生殖能力可能产生毒害作用[28]。圈养扬子鳄栖息水塘沉积物中p,p'-DDMU的高检出频率应引起关注。 对比不同采样季节沉积物中的∑DDXs含量发现,秋季沉积物中∑DDXs含量显著高于春季(P = 0.02;图2),而春季和夏季的∑DDXs含量无显著性差异(P = 0.2;图2)。这可能与夏季和初秋相对较高的温度有关。较高的温度有利于DDXs从沉积物中重新释放到水中,随后沉入表层沉积物中。此外,较高的温度也会加速土壤中DDXs的挥发,而后随地表径流等作用最终累积在表层沉积物中。扬子鳄幼鳄在初秋孵出并进入水塘,而幼鳄对环境污染物(包括DDT及其代谢产物)较为敏感[9]。秋季水塘沉积物中较高的∑DDXs含量对幼鳄是否具有毒害作用应进一步研究。
-
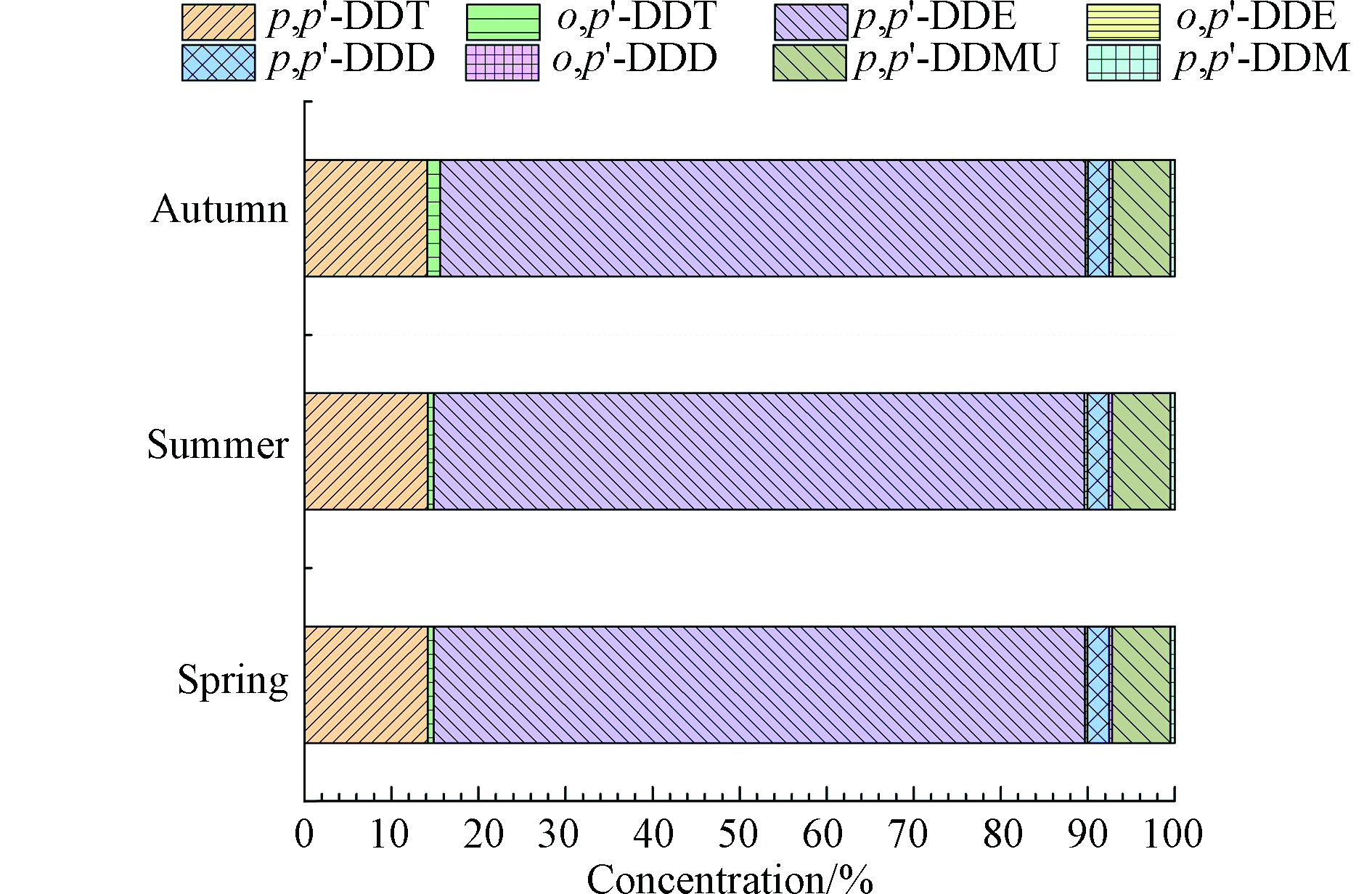
所有沉积物样品中,p,p'-DDE为最主要的DDT类污染物(占∑DDXs含量的73%),其次为p,p'-DDT(占∑DDXs含量的15%)(图3)。沉积物样品中DDT新型降解产物p,p'-DDMU和p,p'-DDM的比例分别为6.5%和0.5%,与前人报道的沉积物中这些新型降解产物的相对含量相符[21]。
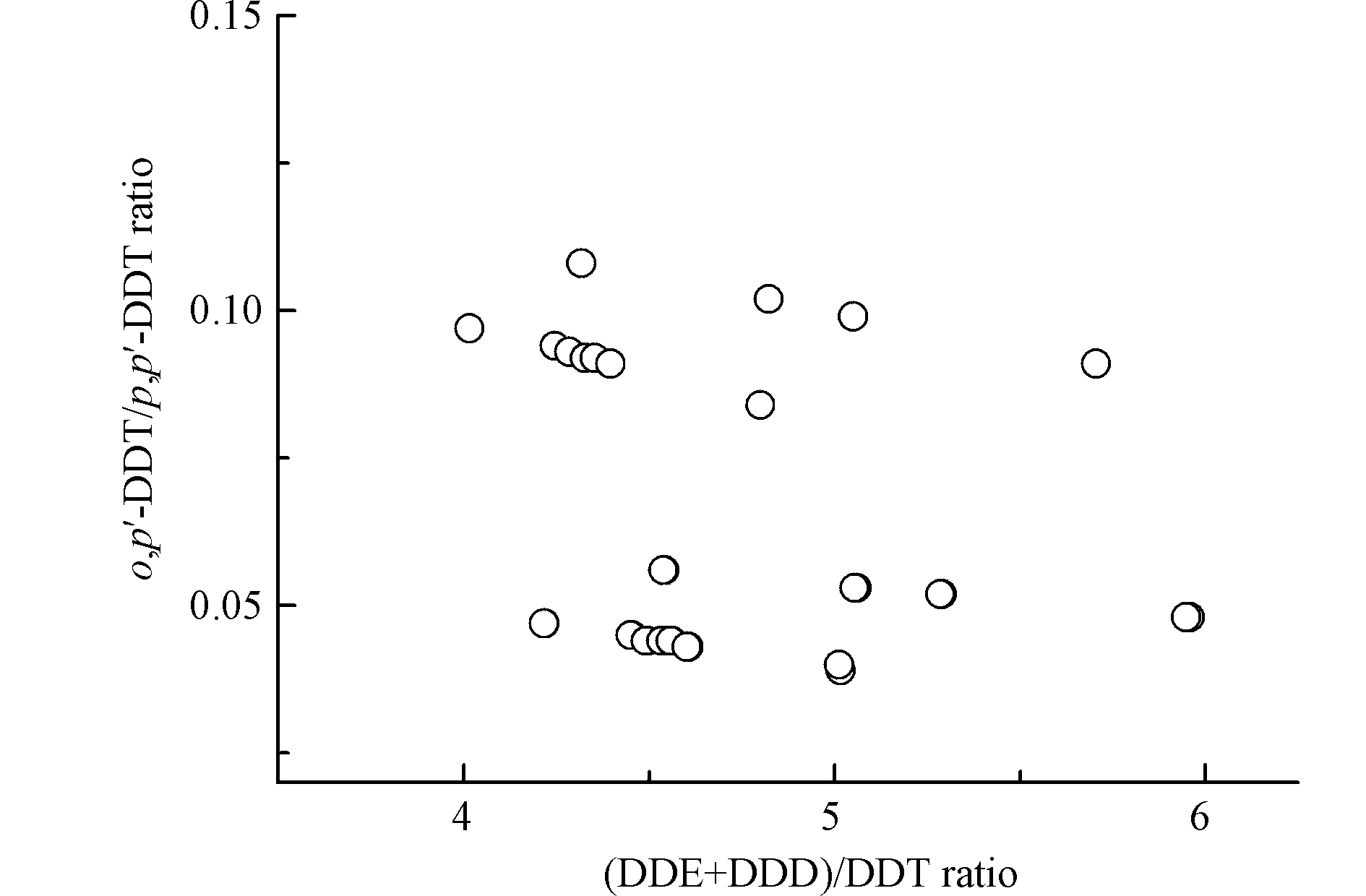
DDT在环境中可以降解为DDD和DDE等,可用DDT降解产物的比例指示环境中DDT类农药的输入历史:当(DDE+DDD)/DDT含量比值 > 1时,表明环境中DDT类污染物主要为历史残留[29]。此外,农药三氯杀螨醇是DDT新源输入的主要来源之一[30]。三氯杀螨醇中DDT主要为o,p'-DDT(o,p'-DDT/p,p'-DDT = 1.3—9.3),而DDT工业品中DDT主要为p,p'-DDT(o,p'-DDT/p,p'-DDT = 0.2—0.3)[31]。本研究沉积物中(DDE+DDD)/DDT含量比值均 > 4,且o,p'-DDT/p,p'-DDT含量比值均 < 0.15(图4),表明圈养扬子鳄栖息环境中DDT主要来源于历史残留。本研究圈养扬子鳄繁殖中心地处长江中下游鱼米之乡,历史上DDT作为农药曾大量使用[31]。
-
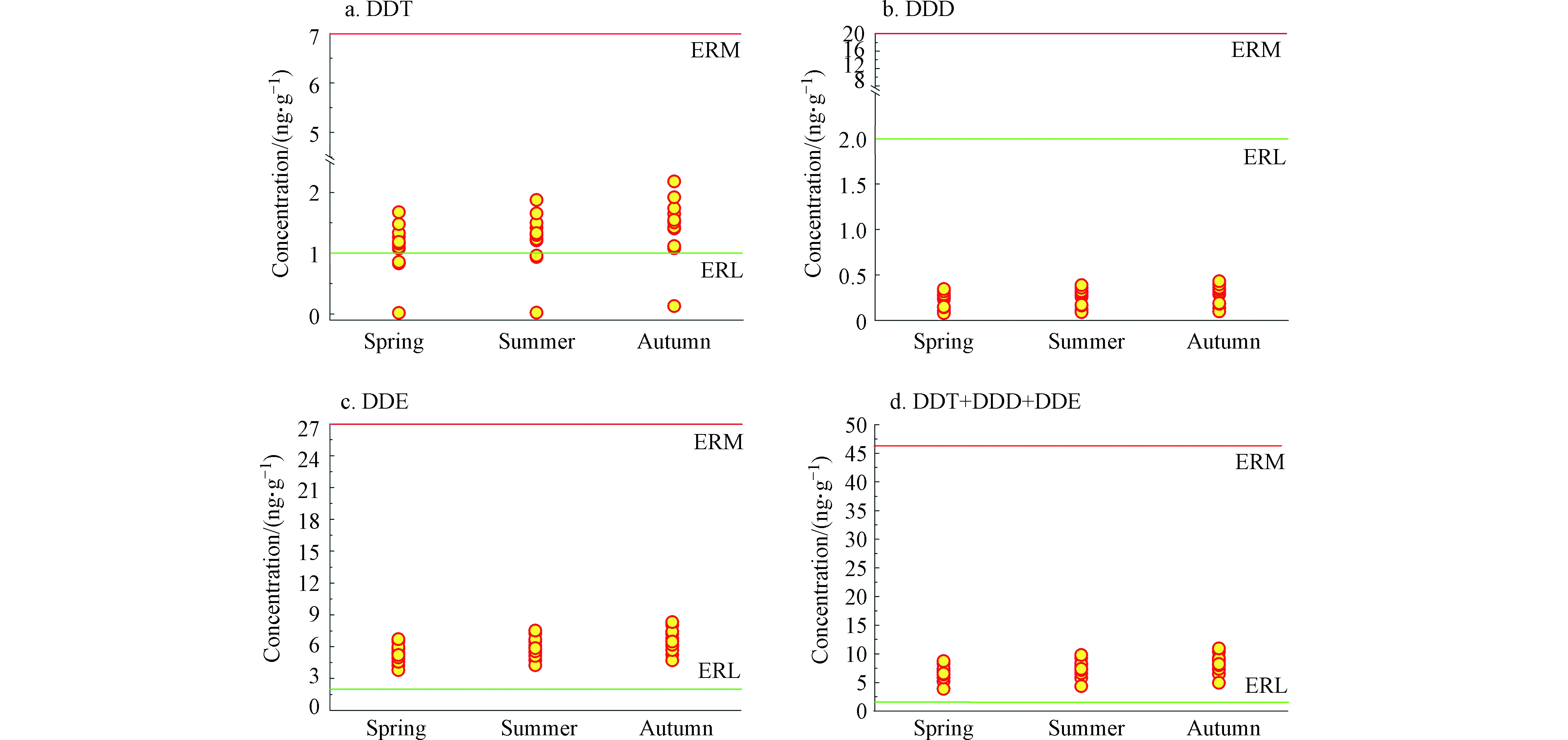
目前尚无沉积物中DDXs对鳄类的生态风险评估标准。Long等用风险评价低值(ERL)和风险评价中值(ERM)评估沉积物中DDT及其降解产物的生态风险[32]。该评价方法是基于沉积物中DDT及其降解产物对水生生物产生毒害效应的概率进行评价,即沉积物中DDT及其降解产物的含量达到ERL或ERM值时,这些污染物对水生生物产生毒害的几率分别为 < 10%和 > 50%[32]。ERL和ERM可评估沉积物中污染物的生态风险:当沉积物中DDT及其降解产物的含量≤ERL时,这些污染物的生态风险较低;当这些污染物的含量介于ERL和ERM之间时,表明其具有中等程度生态风险;当污染物含量≥ERM,这些化合物对水生生物具有较高的生态风险。
所有沉积物样品中DDT、DDE和∑DDTs(DDT、DDE和DDD含量之和)的含量均介于ERL和ERM值之间(DDT、DDE和∑DDTs的ERL分别为1、2、1.58,ERM分别为7、15、46.1)(图5a、图5c和图5d),表明圈养扬子鳄栖息水塘沉积物中这些污染物对水生生物具有中等程度的生态风险。沉积物中DDD的含量<ERL(2)(图5b),圈养扬子鳄栖息水塘中DDD随水生生物的生态风险较低。
-
(1) 圈养扬子鳄栖息水塘沉积物中∑DDXs的含量范围为4.30—11.9 ng·g−1干重,秋季∑DDXs含量显著高于春季。
(2) p,p'-DDE是沉积物中最主要的DDT类化合物,其含量占∑DDXs的73%。DDT的两种高阶降解产物p,p'-DDMU和p,p'-DDM在沉积物中广泛检出,其含量分别占∑DDXs 的6.5%和0.5%。DDT类污染物的组成分析显示,圈养扬子鳄栖息水塘无DDT新源输入。
(3) 生态风险评价结果表明,沉积物中DDE和DDT对水生生物具有中等程度的生态风险。这些污染物对圈养扬子鳄可能的毒害作用应进一步研究。
圈养扬子鳄栖息水塘沉积物中滴滴涕(DDT)及其降解产物残留
Residues of DDT and its metabolites in the sediments from a captive breeding center for Chinese alligator
-
摘要: 圈养是保护极度濒危物种-扬子鳄的重要手段,但圈养环境中滴滴涕(DDT)等污染物可能对扬子鳄产生毒害作用。本研究测试了我国最大的扬子鳄圈养基地水塘沉积物中DDT及其降解产物的含量和组成,并评估了这些污染物的潜在生态风险。沉积物中∑DDXs(DDT及其降解产物含量之和)的含量范围为4.30—11.9 ng·g−1干重;秋季含量显著高于春季。p,p'-DDE是最主要的DDT类污染物,其含量占∑DDXs的73%。DDT的两种高阶降解产物2-氯-1,1-双(4-氯苯基)乙烯(p,p'-DDMU)和1-氯-4-[(4-氯苯基)甲基]苯(p,p'-DDM)也在沉积物中广泛检出,其含量分别占∑DDXs的6.5%和0.5%。DDT类污染物的组成分析结果显示,圈养扬子鳄栖息地没有DDT的新源输入。沉积物中DDE和DDT的含量超过了这些污染物的阈值效应水平,表明圈养扬子鳄栖息水塘中这些化学品存在潜在的生态风险。Abstract: Captive breeding of the Chinese alligator serves as the last resort for ex situ conservation of this critically endangered species. However, this effort may be compromised by the environmental chemicals such as DDT-related compounds which are proposed stressors contributing to the declines in some crocodilian populations. Here, we reported the presence of DDT and its metabolites in the sediments from the largest captive breeding center for the Chinese alligator in China. The concentrations of ∑DDXs (sum of the concentrations of DDT and its metabolites) in the sediments ranged from 4.30 to 11.9 ng·g−1 dry weight, with significantly higher levels in autumn than spring. The most abundant DDT compound in the sediments was p,p'−DDE, comprising approximately 73% of the ∑DDXs. In particular, two high-order metabolites of DDT, p,p'−DDMU and -DDM, were also consistently detected in the sediments, with contributions of 6.5% and 0.5% to the ∑DDXs, respectively. The profiles of DDT isomers and its metabolites indicated that there is no DDT fresh input in the captive breeding center. The concentrations of DDE and DDT in the sediment samples clearly exceeded the threshold effects level set in sediment quality guidelines, suggesting potential ecological risks of these chemicals.
-
Key words:
- DDT /
- DDMU /
- Sediment /
- Alligator sinensis /
- Ecological risks
-

-
表 1 圈养扬子鳄栖息水塘沉积物中DDT及其代谢产物的含量(ng·g−1干重)
Table 1. Concentrations (ng·g−1 dry weight) of DDT and its metabolites in the pond sediments collected from a captive breeding center for Chinese alligator
化合物
Compounds春季(Spring)(n = 12) 夏季(Summer)(n = 12) 秋季(Autumn)(n = 12) 均值
Average标准差
Standard error范围
Range均值
Average标准差
Standard error范围
Range均值
Average标准差a
Standard error范围
Rangep,p'-DDT 1.05 0.11 0.02—1.60 1.17 0.13 0.02—1.79 1.30 0.14 0.02—1.99 o,p'-DDT 0.05 0.01 0.01—0.07 0.05 0.01 0.00—0.08 0.13 0.01 0.09—0.19 p,p'-DDE 5.26 0.25 3.79—6.67 5.89 0.28 4.24—7.47 6.53 0.31 4.70—8.28 o,p'-DDE 0.02 0.003 0.01—0.04 0.03 0.003 0.01—0.05 0.03 0.003 0.01–0.05 p,p'-DDD 0.18 0.02 0.07—0.30 0.20 0.03 0.08—0.34 0.23 0.03 0.09—0.37 o,p'-DDD 0.03 0.003 0.01—0.04 0.03 0.003 0.01—0.05 0.04 0.003 0.01—0.06 p,p'-DDMU 0.47 0.03 0.32—0.69 0.53 0.03 0.36—0.78 0.59 0.04 0.40—0.86 p,p'-DDM 0.03 0.002 0.03—0.05 0.04 0.002 0.03—0.05 0.04 0.002 0.03—0.06 ∑DDXs 7.09 0.39 4.30—9.47 7.94 0.44 4.82—10.6 8.88 0.49 5.45—11.9 ∑DDXs,DDT及其代谢产物的含量之和.
∑DDXs, Sum concentrations of DDT and its metabolites examined.表 2 文献报道的沉积物中p,p'-DDMU含量(干重)
Table 2. The concentrations of p,p'-DDMU (dry wt) in the sediments reported in the literature
采样地点
Sampling sites采样时间
Sampling year浓度范围/(ng·g−1)
Concentration range均值/(ng·g−1)
Average concentration参考文献Reference 圈养扬子鳄栖息水塘 2017 0.32—0.86 0.53 本研究 广东省东莞某池塘 2006—2007 ND—0.22 0.04 [20] 广东省顺德某池塘 2006—2007 ND—0.12 0.01 [20] 渤海湾 2002 NCR 0.2 [21] 长江 2010 0.03—3.76 1.16 [22] 海河 2002 0.48—5.8 1.64 [23] 波兰维斯图拉河 1991—1995 NCR 1.9 [24] 美国帕洛斯弗迪斯大陆架 1992 510—956 712 [25] 美国旧金山湾 1993 1.1—190 39.89 [26] ND,未检出;NCR,无明确范围.
ND, not detected; NCR, have not reported the ranges. -
[1] MAQSOOD I, RONG K. Existing status and resurgence strategies for Chinese Alligator (Alligator sinensis) [J]. Pakistan Journal of Zoology, 2019, 51(3): 1169-1177. [2] 周永康, 余本付, 吴孝兵, 等. 我国扬子鳄种群及栖息地保护现状 [J]. 动物学杂志, 2012, 47(1): 133-136. [3] THORBJARNARSON J, WANG X M, MING S, et al. Wild populations of the Chinese alligator approach extinction [J]. Biological Conservation, 2002, 103(1): 93-102. doi: 10.1016/S0006-3207(01)00128-8 [4] 吴孝兵, 顾长明, 朱家龙, 等. 安徽扬子鳄国家级自然保护区综合研究[M]. 合肥: 合肥工业大学出版社, 2008. WU X B, GU C M, ZHU J L, et al. Comprehensive study on Anhui National Nature Reserve for Chinese Alligator [M]. Hefei: Hefei University of Technology Press, 2008 (in Chinese).
[5] JOHNSTON S. Endocrine disrupting chemicals: Occurrence, exposures and health risks [M]. New York: Nova Publishers, 2016. [6] CRAIN D A, GUILLETTE L J JR, ROONEY A A, et al. Alterations in steroidogenesis in alligators (Alligator mississippiensis) exposed naturally and experimentally to environmental contaminants [J]. Environmental Health Perspectives, 1997, 105(5): 528-533. doi: 10.1289/ehp.97105528 [7] MILNES M R, BRYAN T A, MEDINA J G, et al. Developmental alterations as a result of in ovo exposure to the pesticide metabolite p, p’-DDE in Alligator mississippiensis [J]. General and Comparative Endocrinology, 2005, 144(3): 257-263. doi: 10.1016/j.ygcen.2005.06.013 [8] SEMENZA J C, TOLBERT P E, RUBIN C H, et al. Reproductive toxins and alligator abnormalities at Lake Apopka, Florida [J]. Enviornmental Health Perspectives, 1997, 105(10): 1030-1032. doi: 10.1289/ehp.971051030 [9] GUILLETTE L J JR, CRAIN D A, GUNDERSON M P, et al. Alligators and endocrine disrupting contaminant: A current perspective [J]. American Zoologist, 2000, 40(3): 438-452. [10] GUILLETTE LJ JR, GROSS T S, MASSON G R, et al. Developmental abnormalities of the gonad and abnormal sex hormone concentrations in juvenile alligators from contaminated and control lakes in Florida [J]. Environmental Health Perspectives, 1994, 102(8): 680-688. doi: 10.1289/ehp.94102680 [11] GUILLETTE L J JR, PICKFORD D B, CRAIN D A, et al. Reduction in penis size and plasma testosterone concentrations in juvenile alligators living in a contaminated environment [J]. General and Comparative Endocrinology, 1996, 101(1): 32-42. doi: 10.1006/gcen.1996.0005 [12] STOKERE C, ZAYAS M A, FERREIRA M A, et al. The eggshell features and clutch viability of the broad-snouted caiman (Caiman latirostris) are associated with the egg burden of organochlorine compounds [J]. Ecotoxicology and Environmental Safety, 2013, 98: 191-195. doi: 10.1016/j.ecoenv.2013.08.022 [13] WU T, HONG B, WU X B, et al. Persistent halogenated compounds in captive Chinese alligators (Alligator sinensis) from China [J]. Chemosphere, 2014, 110: 23-30. doi: 10.1016/j.chemosphere.2014.03.015 [14] ZHANG Y, WU J P, LUO X J, et al. Biota-sediment accumulation factors for dechlorane plus in bottom fish from an electronic waste recycling site, South China [J]. Environment International, 2011, 37(8): 1357-1361. doi: 10.1016/j.envint.2011.06.005 [15] WU J P, ZHANG Y, LUO X J, et al. DDTs in frogs (Rana limnocharis) from an agricultural site, South China: Tissue distribution, biomagnification, and potential toxic effects assessment [J]. Environmental Toxicology and Chemistry, 2012, 31(4): 705-711. doi: 10.1002/etc.1717 [16] THOMAS M, LAZARTIGUES A, BANAS D, et al. Organochlorine pesticides and polychlorinated biphenyls in sediments and fish from freshwater cultured fish ponds in different agricultural contexts in north-eastern France [J]. Ecotoxicology and Environmental Safety, 2012, 77: 35-44. doi: 10.1016/j.ecoenv.2011.10.018 [17] BOJAKOWSKA I, TOMASSI-MORAWIEC H, MARKOWSKI W. PAHs and DDTs in soil and sediment of inland water bodies of Warsaw city and its surroundings [J]. Journal of Geochemical Exploration, 2018, 187: 57-71. doi: 10.1016/j.gexplo.2017.07.013 [18] LI H S, LING W F, LIN C X. Fishpond sediment-borne DDTs and HCHs in the Pearl River Delta: Characteristics, environmental risk and fate following the use of the sediment as plant growth media [J]. Journal of Hazardous Materials, 2011, 186 (2–3) 1474–1480. [19] ZHANG, H, SHAN B Q. Historical distribution of DDT residues in pond sediments in an intensive agricultural watershed in the Yangtze-Huaihe region, China [J]. Journal of Soils and Sediments, 2014, 14(5): 980-990. doi: 10.1007/s11368-013-0839-3 [20] MISHRA K, SHARMA R C, KUMAR S. Contamination profile of DDT and HCH in surface sediments and their spatial distribution from North-East India [J]. Ecotoxicology and Environmental Safety, 2013, 95: 113-122. doi: 10.1016/j.ecoenv.2013.05.029 [21] ZHANG B Z, YU H Y, YOU J, et al. Input pathways of organochlorine pesticides to typical freshwater cultured fish ponds of South China: Hints for pollution control [J]. Environmental Toxicology and Chemistry, 2011, 30(6): 1272-1277. doi: 10.1002/etc.503 [22] HU J Y, WAN Y, SHAO B, et al. Occurrence of trace organic contaminants in Bohai Bay and its adjacent Nanpaiwu River, North China[J]. Marine Chemistry, 2005, 95(1–2): 1–13. [23] TANG Z W, HUANG Q F, YANG Y F, et al. Organochlorine pesticides in the lower reaches of Yangtze River: Occurrence, ecological risk and temporal trends [J]. Ecotoxicology and Environmental Safety, 2013, 87: 89-97. doi: 10.1016/j.ecoenv.2012.10.001 [24] WAN Y, HU J Y, LIU J L, et al. Fate of DDT-related compounds in Bohai Bay and its adjacent Haihe Basin, North China [J]. Marine Pollution Bulletin, 2005, 50(4): 439-445. doi: 10.1016/j.marpolbul.2004.11.037 [25] FALANDYSZ J, STRANDBERG B, STRANDBERG L, et al. Tris(4-chlorophenyl)methane and tris(4-chlorophenyl)methanol in sediment and food webs from the Baltic South Coast [J]. Environmental Science & Technology, 1999, 33(4): 517-521. [26] KUCHER S, SCHWARZBAUER J. DDT-related compounds as non-extractable residues in submarine sediments of the Palos Verdes Shelf, California, USA [J]. Chemosphere, 2017, 185: 529-538. doi: 10.1016/j.chemosphere.2017.07.041 [27] PEREIRA W E, HOSTETTLER F D, RAPP J B. Distributions and fate of chlorinated pesticides, biomarkers and polycyclic aromatic hydrocarbons in sediments along a contamination gradient from a point-source in San Francisco Bay, California [J]. Marine Environmental Research, 1996, 41(3): 299-314. doi: 10.1016/0141-1136(95)00021-6 [28] WETTERAUER B, RICKING M, OTTE J C et al. Toxicity, dioxin-like activities, and endocrine effects of DDT metabolites—DDA, DDMU, DDMS, and DDCN [J]. Environmental Science and Pollution Research, 2012, 19(2): 403-415. doi: 10.1007/s11356-011-0570-9 [29] 王元, 刘桂建, 刘荣琼. 巢湖湖区及入湖河流表层水体、沉积物中有机氯农药分布及风险评价 [J]. 环境化学, 2019, 38(3): 669-678. doi: 10.7524/j.issn.0254-6108.2018051603 WANG Y, LIU G J, LIU R Q. Distribution and ecological risk assessment of organochlorine pesticides in surface water and sediment samples of Lake Chaohu and its inflow rivers [J]. Environmental Chemistry, 2019, 38(3): 669-678(in Chinese). doi: 10.7524/j.issn.0254-6108.2018051603
[30] QIU X, ZHU T, YAO B, et al. Contribution of dicofol to the current DDT pollution in China [J]. Environmental Science & Technology, 2005, 39(12): 4385-4390. [31] LAI W Y. Pesticide use and health outcomes: Evidence from agricultural water pollution in China [J]. Journal of Environmental Economics and Management, 2017, 86: 93-120. doi: 10.1016/j.jeem.2017.05.006 [32] LONG E, MACDONALD D, SMITH S, et al. Incidence of adverse biological effects within ranges of chemical concentrations in marine and estuarine sediments [J]. Environmental Management, 1995, 19(1): 81-97. doi: 10.1007/BF02472006 -



 下载:
下载: