-
溴代阻燃剂(BFRs)由于具有优良的阻燃性能而被广泛应用[1-3]。传统BFRs如六溴环十二烷和部分多溴二苯醚(PBDEs)因具有持久性有机污染物的性质而被禁用[4-7]。新型溴代阻燃剂(NBFRs)是传统BFRs的替代品,在其生产、使用和处置过程中,会不可避免地进入环境,危害生态系统和人类健康[6, 8-10]。NBFRs已在海水[11]、湖水[12]、沉积物[13]和水生生物体[14]等水环境介质中被广泛检出。一些NBFRs对生物具内分泌干扰[15]和基因毒性[16]等不利影响。因此,有必要研究NBFRs的水环境转化行为,以评价其生态风险。
光化学转化是污染物在水环境中重要的转化途径之一,并受广泛存在于水中的溶解性有机质(DOM)的影响[17]。DOM吸光后能生成光生活性中间体(PPRIs),如羟基自由基(·OH)、单线态氧(1O2)和激发三线态溶解性有机质(3DOM*),促进污染物的光化学转化[18-21]。3DOM*是一类重要的PPRI,其与污染物的反应机理包括能量转移(如与山梨酸、山梨醇和百菌清等的反应[22-24])、电子转移和质子转移(如与酚、胺和苯胺类化合物的反应[25-29])。然而3DOM*与其他化学结构的反应机理尚不清楚。
前人对NBFRs或PBDEs的环境光化学研究,多聚焦1O2和·OH参与的反应[30]、以及DOM的光屏蔽抑制效应[31],仅有少量研究涉及3DOM*的反应。已有研究表明,3DOM*能促进PBDEs的光降解[32];类似于3DOM*的激发态丙酮酸能够引发PBDEs的光致脱溴反应[33];Zhang等[7]发现,3DOM*可与一种重要的NBFR,2,3-二溴丙基-2,4,6-三溴苯基醚(DPTE)发生反应,但具体反应机理并不明确。3DOM*与NBFRs反应机理研究的缺乏,限制了对NBFRs环境光化学行为的理解。
稳态光解实验、子结构模型化合物(SSMCs)实验以及密度泛函理论(DFT)计算,是探究3DOM*与污染物反应机理的重要途径。DOM分子结构复杂,难以直接表征,常用DOM模型化合物(如4-羧基苯甲酮、苯乙酮、3-甲氧基苯乙酮、蒽醌-2-磺酸等)探究微观反应机理[26]。SSMCs指具有目标物部分特征结构且不含其他活性基团,能代表目标物部分特征性质的化合物。Li等[26]通过研究SSMCs与DOM模型化合物的反应性,确定了胺基氮为磺胺嘧啶与3DOM*的反应位点,并使用DFT计算阐释机理。
本研究选择苯乙酮作为DOM模型化合物,以DPTE为代表性NBFRs,探究3DOM*与NBFRs的反应活性、反应位点和反应途径。为鉴别DPTE与3DOM*的反应位点,选择2,4,6-三溴苯甲醚(TBA)、3-苯氧基溴丙烷(3BPE)和丙氧基苯(PB)作为DPTE的SSMCs考察反应活性,并通过DFT计算阐释反应机理。
-
2,3-二溴丙基-2,4,6-三溴苯基醚(DPTE,纯度99%)购于AccuStandard公司;2,4,6-三溴苯甲醚(TBA,纯度98%)购于上海TCI有限公司;2,4,6-三甲基苯酚(TMP,纯度99%)、苯乙酮(AP,纯度98%)、3-甲氧基苯乙酮(3MAP,纯度99%)和3-苯氧基溴丙烷(3BPE,纯度95%)购于北京百灵威科技(J&K)有限公司;丙氧基苯(PB,纯度97%)购于Fluorochem公司;异丙醇(IPA,纯度99.5%)购于Tedia公司;山梨醇(SA,纯度97%)和乙腈(色谱纯)购于Sigma-Aldrich公司。超纯水(18.25 MΩ·cm)由上海涞科仪器有限公司的OKP超纯水机制备。
-
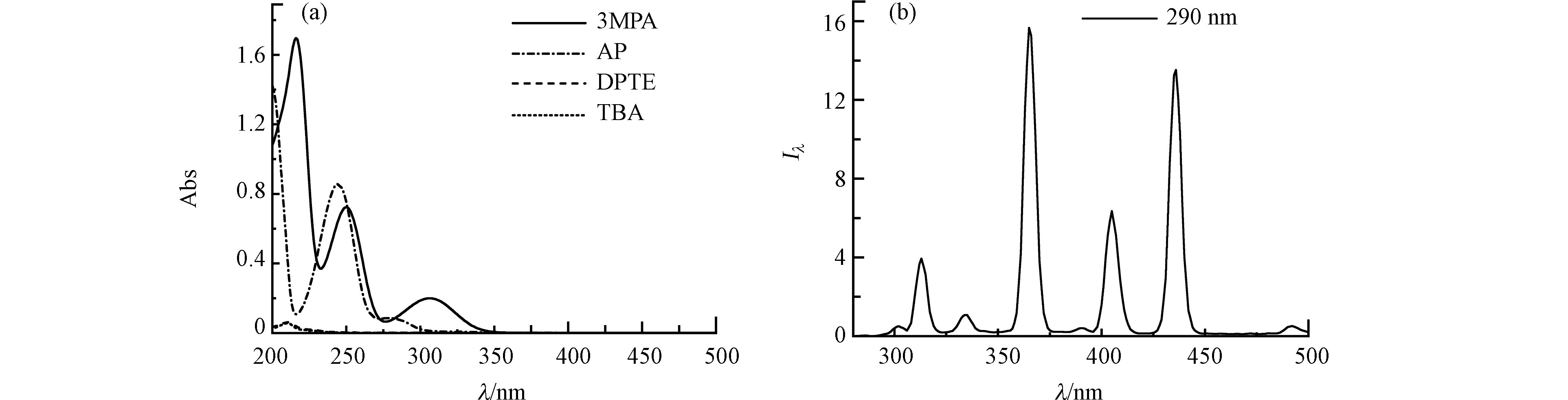
光化学实验在外连冷却循环装置的XPA-1型旋转式光化学反应仪(南京胥江机电厂)中进行,实验光源为辅以290 nm截止滤光片的500 W汞灯。光解液置于石英试管中,温度控制为(25 ± 1) ℃。DPTE、TBA和DOM模型化合物的紫外-可见吸收光谱,使用紫外-可见分光光度计(Hitachi U2900, 日本)测定(图1a),化合物浓度与光解实验初始浓度一致,使用20%乙腈含量的水溶液作为溶剂和参比。光源发射光谱使用TriOS水下光谱仪(RAMSES-ACC-UV, 德国)测定(图1b)。
化合物的储备液使用乙腈配制。实验前量取储备液于容量瓶中,使用N2吹至近干,并配置光解溶液。光解实验中,DPTE和TBA的初始浓度为1 μmol·L−1,3BPE和PB的初始浓度为5 μmol·L−1。苯乙酮和3MAP的浓度根据天然水体中常见DOM的浓度范围(2.2—17.8 mg·L−1C[34])设定,为5 mg·L−1C。使用SA (1 mmol·L−1)和IPA (20 mmol·L−1)分别淬灭光照过程中产生的激发三线态分子和·OH。通入高纯N2 (20 min),去除溶解氧以消除1O2等由O2生成的PPRIs。每组淬灭实验重复3次。由于DPTE, SSMCs和DOM模型化合物分子结构中无可离解基团,光化学行为受溶液pH值的影响较小。为减少溶液离子强度对反应体系的干扰,未额外调整反应溶液的pH值,初始pH值介于6.4和6.8。
采用配备二极管阵列检测器的Agilent 1260高效液相色谱进行定量分析。使用Zorbax XDB-C18色谱柱(3.0 mm× 150 mm, 3.5 μm)进行分离,进样量为40 μL。具体分析条件列于表1。
-
根据图1a所示,苯乙酮或3MAP与DPTE的紫外吸收光谱部分重叠,因此存在竞争吸光,需考虑光屏蔽效应。通过式(1)和(2)计算光屏蔽因子(
$\sum {{S_\lambda }} $ ):其中,
${\alpha _\lambda }$ 是苯乙酮或3MAP的光吸收系数(cm−1);${\varepsilon _\lambda }$ 是DPTE的摩尔吸光系数(L·cm−1·mol−1);l是光程,根据Wenk等[35]提出的方法,计算为1.65 cm;[DPTE]是DPTE的初始浓度(mol·L−1);${I_\lambda }$ 是光源在波长为λ nm处的光强(W·m−2·nm−1)。通过式(3)计算得到苯乙酮或3MAP存在条件下,DPTE的直接光解速率常数(kd′):其中,kd为无苯乙酮或3MAP存在条件下,DPTE的直接光解速率常数(h−1)。
以TMP (0.5 mmol·L−1)为激发三线态探针,测定苯乙酮存在下TMP的转化速率,根据式(4)计算3AP*的生成速率(
${R_{{}^3{\rm{AP*}}}}$ ),并根据式(5)获得3AP*的稳态浓度([3AP*]):其中,
${R_{{}^3{\rm{AP*}}}}$ 和${R_{{\rm{TMP}}}}$ 是3AP*的生成速率和TMP的降解速率(mol·L−1·s−1),${{k_{\rm{S}}'}} $ 是3DOM*的淬灭速率常数(5 × 105 s−1[36]),${k_{{\rm{TMP,}}{}^{\rm{3}}{\rm{AP*}}}}$ 是TMP与3AP*的二级反应速率常数(使用TMP与3DOM*的二级反应速率常数代替,1.8 × 109 L·mol−1·s−1[37]),[TMP]是TMP的初始浓度(mol·L−1)。根据式(6)计算3AP*与DPTE的二级反应速率常数(${k_{{}^{\rm{3}}{\rm{AP*,DPTE}}}}$ ):其中,
${k_{^{\rm{3}}{\rm{AP*}}}}$ 为苯乙酮存在条件下,DPTE的表观光解速率常数(kobs)与加入SA后DPTE的表观光解速率常数之差(s−1)。苯乙酮在光照条件下产生的各活性物种对DPTE降解的贡献通过式(7)计算:其中,Pi表示某种PPRI的贡献率,i代表3DOM*, 1O2等PPRIs;kq,i为加入某种PPRI淬灭剂后,DPTE的表观光解速率常数(s−1)。
-
应用Gaussian 09[38]进行DFT计算,几何构型优化和频率分析的泛函和基组为B3LYP/6-31++G(d,p),计算单点能的泛函和基组为B3LYP/6-311++G(3df,2p)。溶剂效应通过基于自洽反应场(SCRF)的极化连续模型的积分方程形式(IEFPCM)进行模拟。初始反应构型通过势能面柔性扫描确定,使用的泛函和基组为B3LYP/6-31g(d,p)。通过频率分析,选择有且仅有一个虚频的几何构型为反应过渡态(TS);通过内禀反应坐标分析,将TS连接到对应的反应前络合物和产物。基于含时密度泛函理论(TDDFT),计算化合物激发单线态和三线态的垂直激发能(ES1和ET1)。
以垂直电子亲和势(VEA)和垂直电离能(VIE),分别表征分子的得电子能力(EAA)和供电子能力(EDA),以评价光致电子转移反应的热力学[39-41]。VEA和VIE的值越小,表明EAA和EDA越大。基态VEA和VIE(VEAS0 and VIES0)、激发单(三)线态VEA和VIE(VEAS1(T1) and VIES1(T1))的计算方式如式(8) – (11)所示:
式中,E(electron acceptor)和E(electron donor)为电子受体和电子供体的单点能;E(electron acceptor + e)和E(electron donor - e)为电子受体(供体)得到(失去)1个电子后的单点能。
-
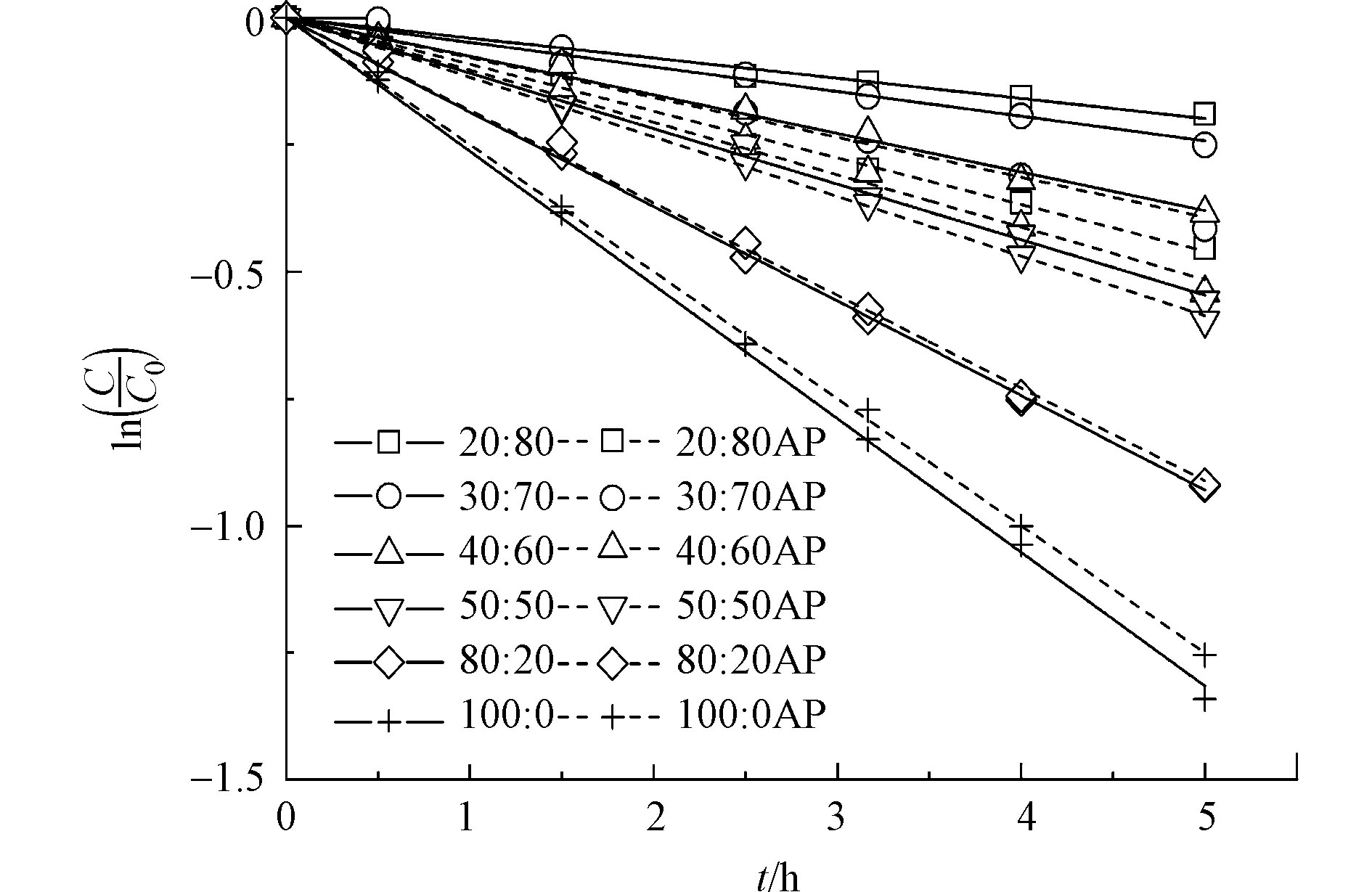
由于DPTE在纯水中的溶解度无法达到仪器定量限,本研究使用乙腈/水混合液增加其溶解度。图2显示不同乙腈/水比例溶液中,DPTE的光解动力学。当乙腈含量从20%增加至100%,DPTE的直接光解加快,直接光解速率常数kd从4.1 × 10−2 h−1升高至2.6 × 10−1 h−1。Xie等[42]发现,在不同溶剂中,PBDEs的kd与其第一激发单线态垂直激发能(Eex)和平均溴原子形式电荷(qBr+)相关。Eex越低、qBr+越高,PBDEs越容易发生直接光解。本研究计算DPTE在水和乙腈中的Eex分别为107.74 kcal·mol−1和107.73 kcal·mol−1,qBr+分别为0.045和0.046。DPTE在乙腈中的Eex略低于水中,qBr+略高于水中,导致DPTE在乙腈中的直接光解比在水中快,与Xie等[42]的结论一致。
如图2所示,苯乙酮的加入影响DPTE光降解动力学,且这种影响与乙腈的含量相关。随着乙腈含量的增加,苯乙酮对DPTE光降解的促进作用减弱。当乙腈含量高于80%时,加入苯乙酮,DPTE的表观光降解速率不再增加。这是由于乙腈作为一种极性有机溶剂,能够淬灭激发三线态分子[42-43]。随着乙腈含量的增加,激发三线态苯乙酮(3AP*)的稳态浓度降低,导致DPTE的间接光降解被减缓。同时,苯乙酮也会通过光屏蔽效应抑制DPTE的直接光解。因此,乙腈含量高的情况下,苯乙酮的存在会抑制DPTE的表观光降解。
使用20%乙腈含量的水溶液作为溶剂的暗对照实验中,7 h后DPTE及其SSMCs浓度变化低于1%。鉴于乙腈对3AP*稳态浓度的影响,以及DPTE及SSMCs的暗对照损耗情况,在后续的光化学实验中,使用20%乙腈含量的目标物水溶液,既减少对3AP*稳态浓度的影响,又能保证目标物一定的溶解度。
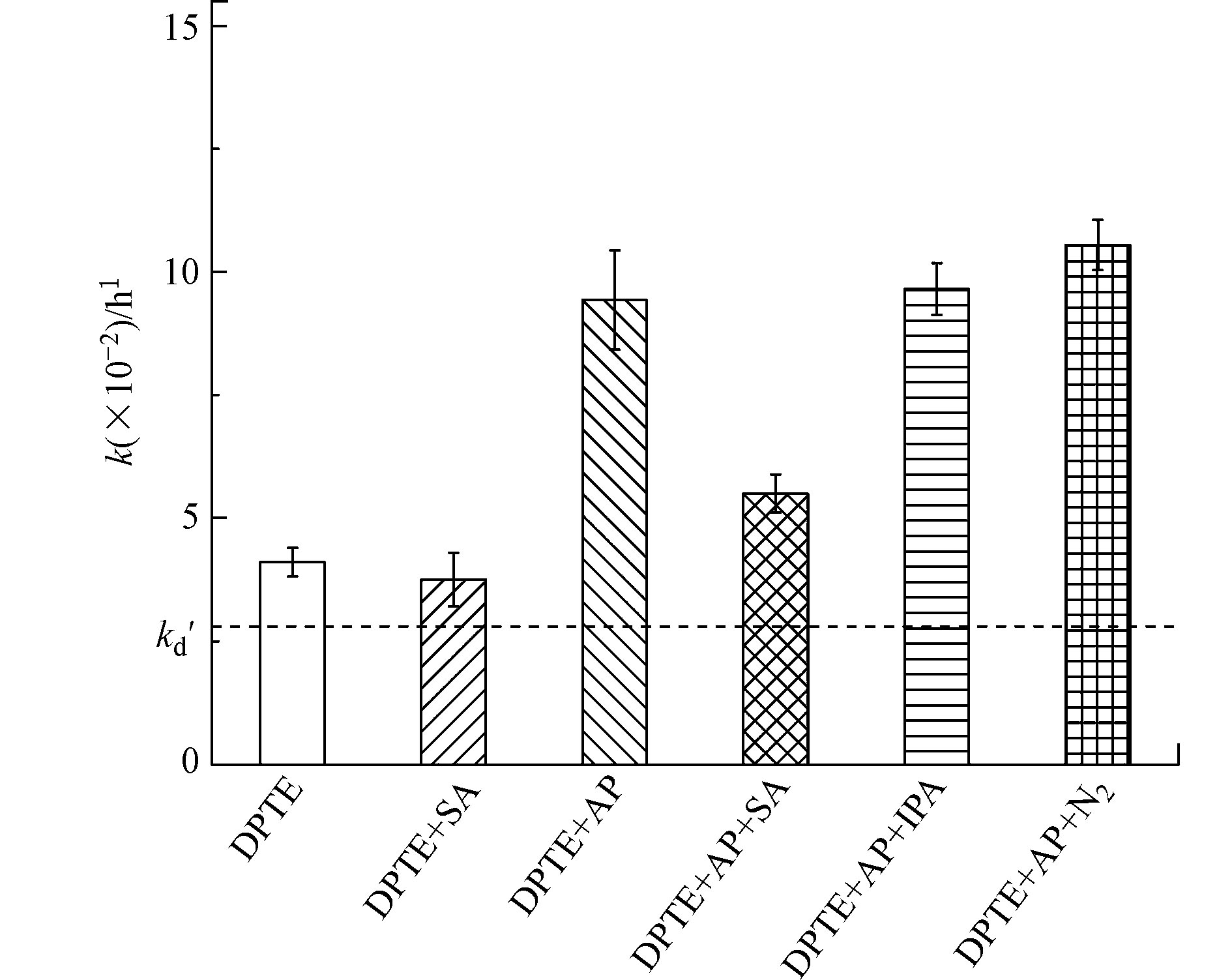
如图3所示,·OH淬灭剂IPA的加入,对DPTE的光降解无影响,表明本研究体系中·OH对DPTE光降解的贡献可以忽略。这说明3AP*并非通过生成·OH引发DPTE的光降解。激发三线态淬灭剂SA的加入,对DPTE直接光解影响较小,表明DPTE的直接光解并非由激发三线态(3DPTE*)主导。SA的加入,显著抑制了苯乙酮存在下DPTE的表观光降解,说明3AP*在DPTE的光降解中起到重要作用。根据式(7)计算,3AP*对DPTE光降解的贡献为41.7% ± 2.2%。
溶解氧是一种天然激发三线态淬灭剂,能够淬灭3DOM*并生成1O2。为证明3AP*能与DPTE直接反应,向反应液中通入N2清除溶解氧以增加3AP*稳态浓度[26]。通入N2还能排除其他由O2衍生的PPRIs干扰,如O2·-和H2O2[44]。如图3所示,通入N2后,DPTE的表观光降解速率常数增加至(10.5 ± 0.5) × 10−2 h−1,说明3AP*能够直接与DPTE发生反应且主导DPTE的间接光降解。
使用TMP测得3AP*稳态浓度为7.31 × 10−14 mol·L−1,通过式(6)计算得到
${k_{{}^{\rm{3}}{\rm{AP*,DPTE}}}}$ 为(1.49 ± 0.24) × 108 L·mol−1·s−1。虽然${k_{{}^{\rm{3}}{\rm{AP*,DPTE}}}}$ 比DPTE与·OH的二级反应速率常数(2.4 × 109 L·mol−1·s−1[7])低1个数量级,但天然水体中3DOM*的稳态浓度(10−14—10−12 mol·L−1[45])远高于·OH的稳态浓度(10−18—10−16 mol·L−1[46])。因此天然水体中3DOM*与DPTE的表观反应速率可能高于·OH与DPTE的表观反应速率。由此可知,3DOM*对于天然水中DPTE的光化学转化,可能起到不可忽视的作用。根据式(1)—(3)计算得到苯乙酮对DPTE直接光解的∑Sλ为0.69,kd′ 为(2.8 ± 0.2) × 10−2 h−1。苯乙酮存在时,DPTE的kobs为(9.4 ± 1.0) × 10−2 h−1,扣除kd′后,间接光降解的总贡献应为70.1% ± 1.4%。淬灭实验表明,3AP*对DPTE光降解的贡献为41.7% ± 2.2%,而其他PPRIs对DPTE光降解的贡献不显著。这说明DPTE表观光降解中,尚有28.4% ± 1.5%的未知光降解途径有待明确。综合考虑光解溶液中可能存在的物种,推测这部分光降解可能由基态苯乙酮引发。
-
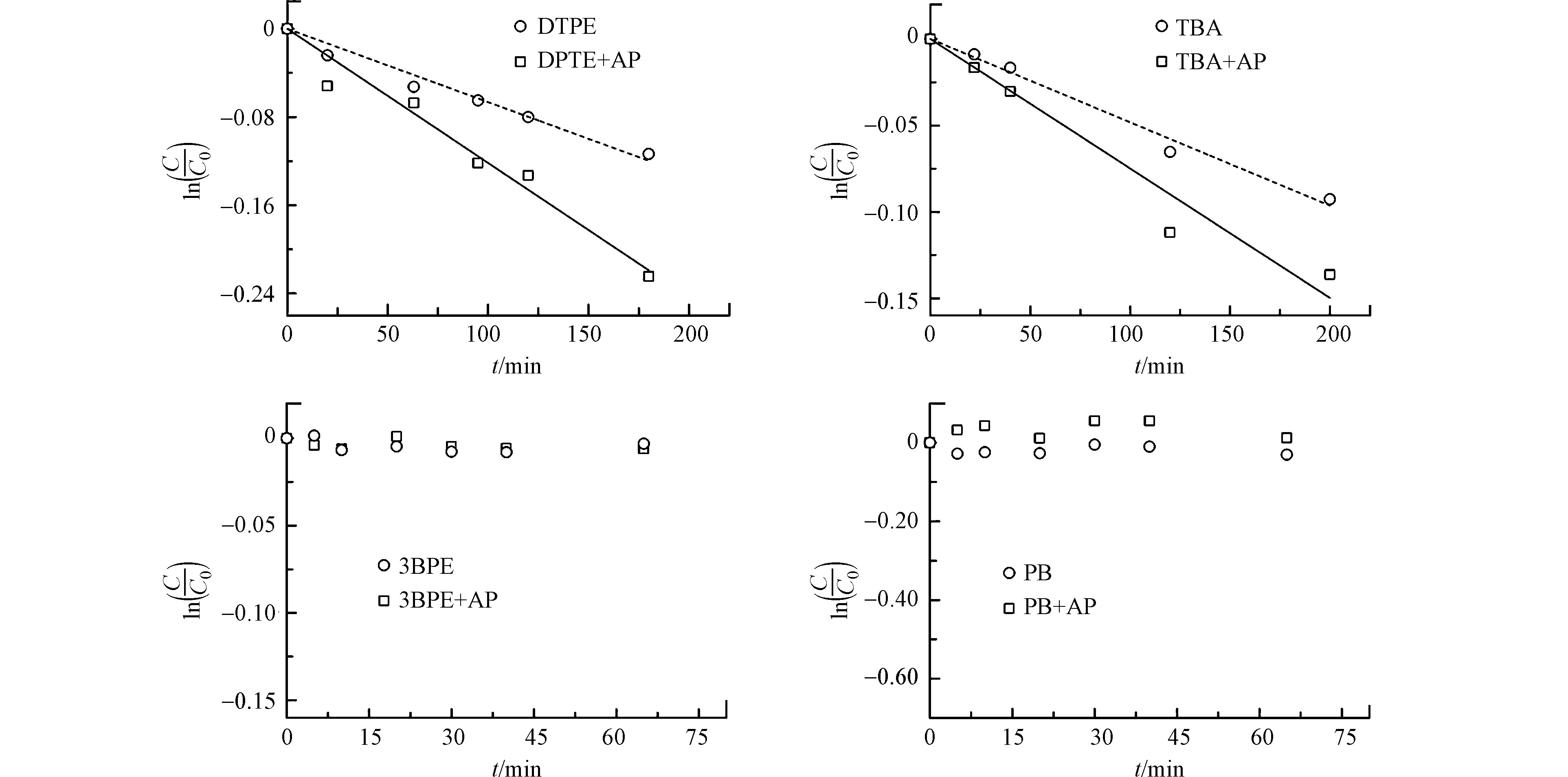
图4显示了3种SSMCs的光解动力学结果。3BPE和PB几乎不发生直接光解,而TBA可直接光解,kd为3.0 × 10−2 h−1。苯乙酮不能促进3BPE和PB的光降解,但可显著促进TBA的光降解(4.2 × 10−2 h−1)。根据分子结构(表2),3种SSMCs分别代表DPTE的溴代苯(TBA)、溴代烷烃(3BPE)和芳香醚(PB)子结构。因此,DPTE苯基上的溴为3AP*的主要反应位点。
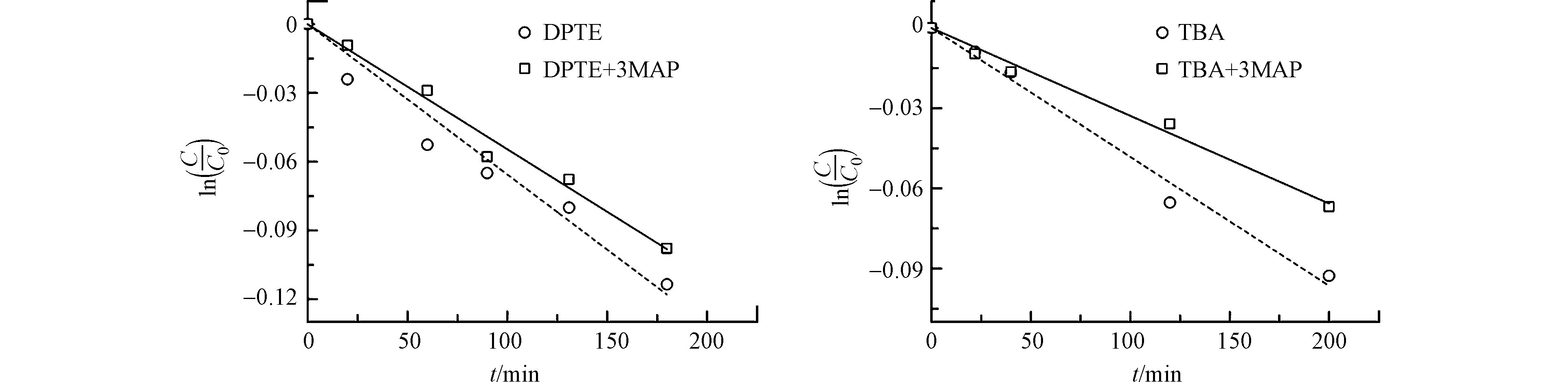
为深入探究3DOM*与DPTE的反应同DOM性质的关系,选用另一种DOM模型化合物3MAP进行SSMCs实验。如图5所示,3MAP抑制了DPTE和TBA的表观光降解。计算3MAP对DPTE和TBA直接光解的∑Sλ分别为0.64和0.62。光屏蔽矫正后,3MAP存在时,DPTE和TBA的kd'分别为2.6 × 10−2 h−1和1.9 × 10−2 h−1,低于表观光解速率常数(DPTE: 4.2 × 10−2 h−1; TBA: 2.1 × 10−2 h−1)。这表明33MAP*能引发DPTE和TBA的间接光降解但促进程度低于3AP*。
3MAP的三线态垂直激发能(303 kJ·mol−1[45])和三线态氧化还原电势(1.64 V[47])较苯乙酮(308 kJ·mol−1和1.77 V[45])低,意味3MAP和苯乙酮对DPTE及SSMCs的影响差异可能是两种DOM模型化合物反应活性不同所致。
-
能量转移和电子转移相关参数计算结果如表3所示,DPTE的ET1和ES1均高于苯乙酮的ET1。因此,从热力学角度分析,3AP*向DPTE的能量转移不可行。相反,激发态DPTE (DPTE*)则可能被基态苯乙酮淬灭。表4列出了DPTE与3AP*能量转移和电子转移反应式及相应的Gibbs自由能变(ΔG)。3AP*与DPTE之间电子转移反应(反应2,3)的ΔG > 0,为热力学非自发反应;3AP*与DPTE* (包括3DPTE*和1DPTE*)之间的电子转移反应(反应4—7)的ΔG < 0, 为热力学自发反应。
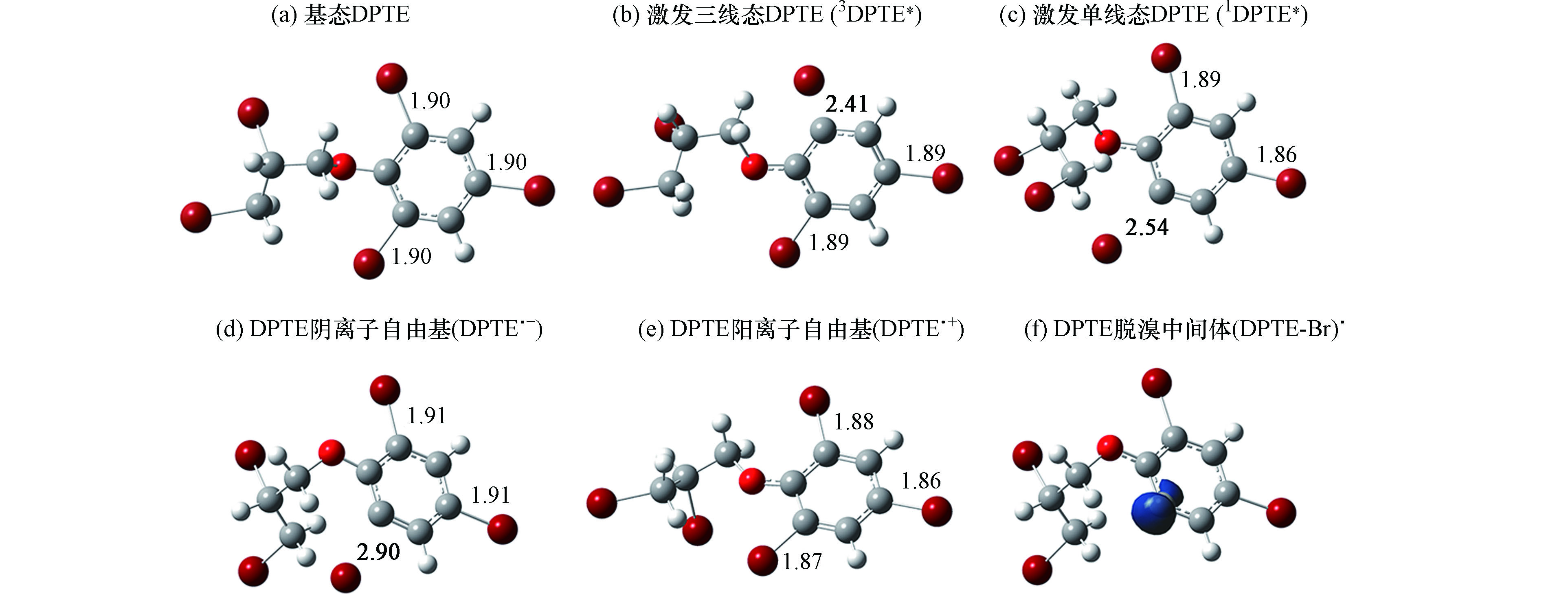
DPTE与3AP*发生电子转移反应后,可生成阴离子自由基(DPTE·-, 图6d)或阳离子自由基(DPTE·+, 图6e)。DPTE·+与DPTE相比无显著的结构变化,而DPTE·-的C—Br键键长增至0.290 nm并发生断裂,生成脱溴中间体(DPTE-Br)·。1DPTE*和3DPTE*同3AP*的电子转移反应均为热力学自发过程,因此仅能得到电子转移通过DPTE*发生,而DPTE参与反应的激发态仍需在后续研究中通过开发新淬灭剂及探针确定.
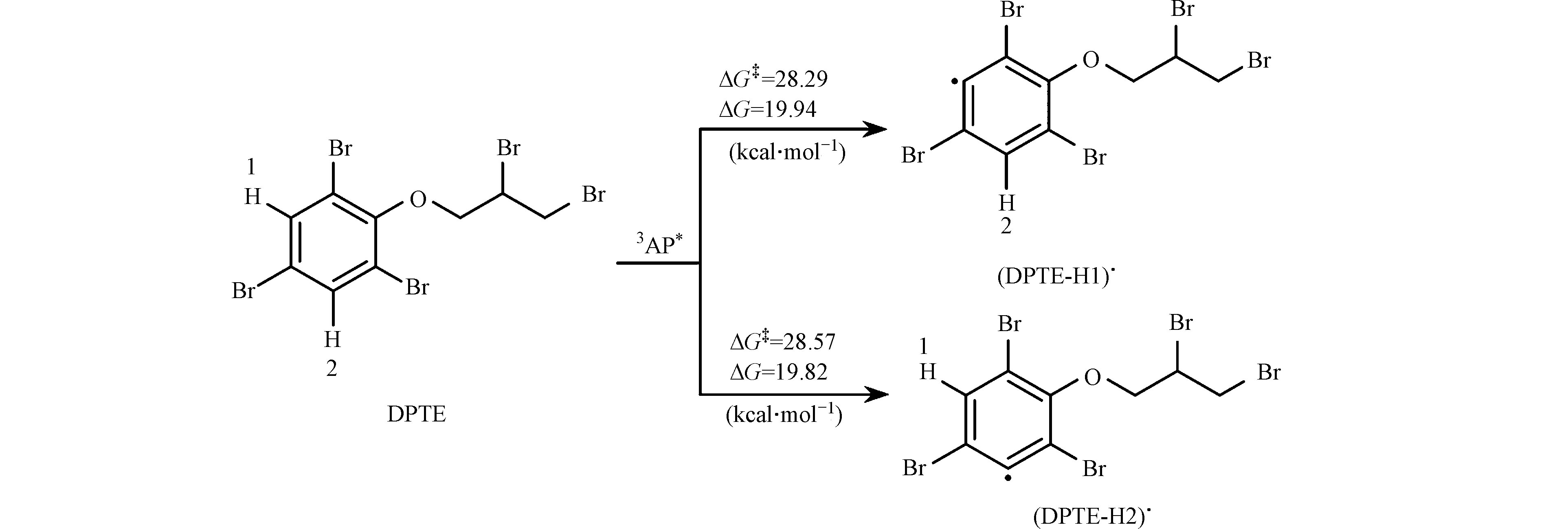
质子转移是3DOM*与污染物反应的另一种主要途径。本研究考察了DPTE苯基上氢原子向3AP*的转移过程。如图7所示,苯基上两个氢原子的转移反应均具有较大的能垒(ΔG‡),分别为28.29 kcal·mol−1 (H1)和28.57 kcal·mol−1 (H2),且反应ΔG分别为19.94 kcal·mol−1 (H1)和19.82 kcal·mol−1 (H2),为热力学非自发过程,反应较难发生。
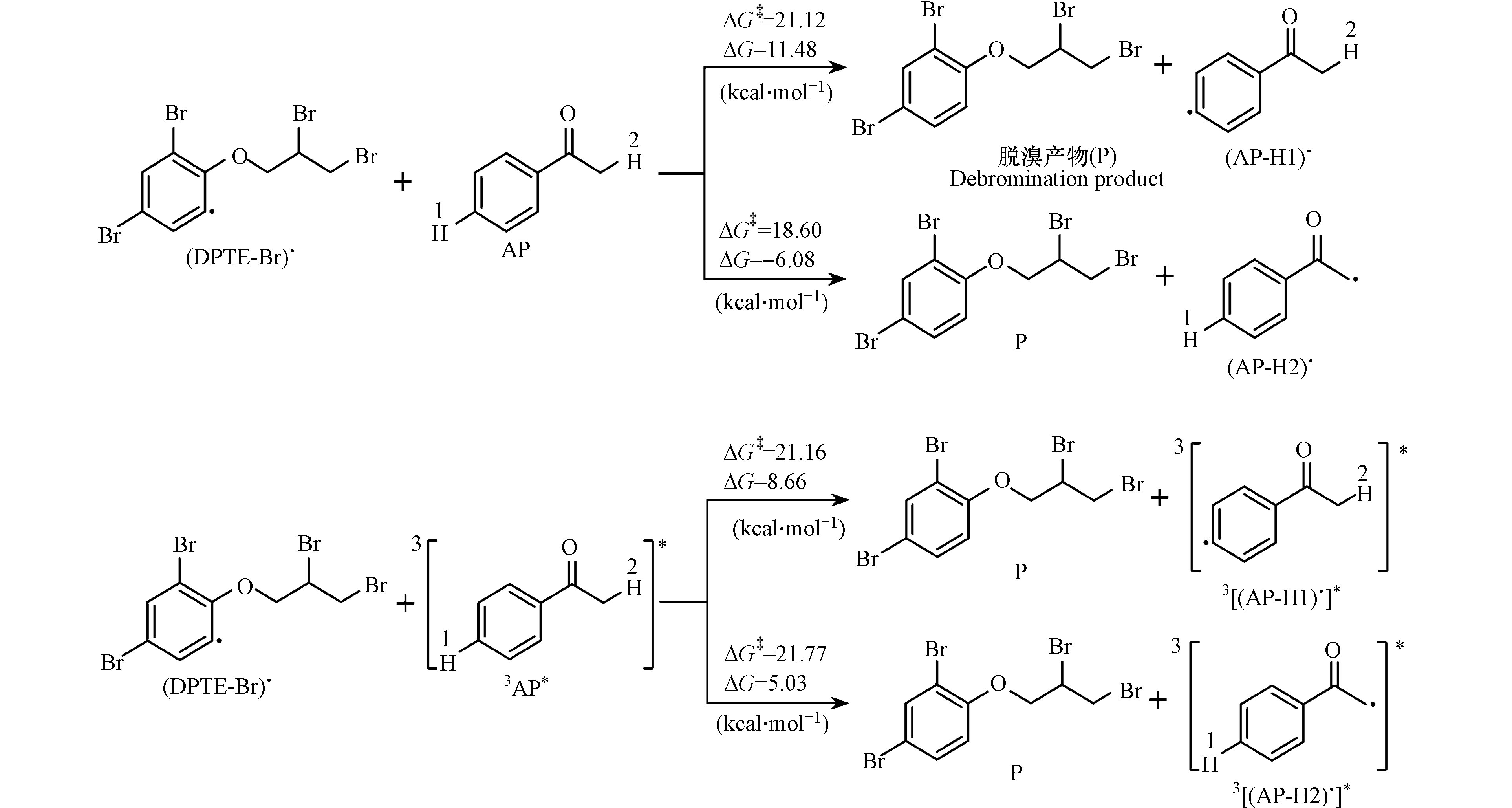
前面实验表明DPTE表观光降解中28.4%的贡献可能由基态苯乙酮引发。DFT计算表明,(DPTE-Br)· (图6 f)是DPTE光解过程中的重要中间体,可能通过质子转移,与浓度较高的基态苯乙酮或3AP*反应生成加氢产物,进而促进DPTE的转化。
苯乙酮分子结构中含有两类氢原子,分别为苯基氢和烷基氢。分别考察了基态苯乙酮和3AP*的两类氢原子向(DPTE-Br)·的转移过程。根据DPTE·-的几何构型(图6 d),脱溴位点为苯基上醚键邻位取代溴,因此选择该位点的(DPTE-Br)·进行后续计算。结果如图8所示,基态苯乙酮烷基氢转移的ΔG‡最小(18.60 kcal·mol−1),反应性最强。并且仅有此位点反应的ΔG < 0,为热力学可自发反应。实验中,苯乙酮的浓度为5.20 × 10−5 mol·L−1,3AP*的稳态浓度为7.31 × 10−14 mol·L−1,因此基态苯乙酮比3AP*更易与(DPTE-Br)·发生质子转移反应,生成稳定的脱溴加氢产物。
-
DFT计算表明,3AP*向1DPTE*或3DPTE*的电子转移,是促进DPTE降解的主要原因。一直以来,3DOM*被认为与基态污染物分子发生反应[27, 47-50],而非激发态,主要因为两个激发态分子相遇的概率较低。然而,综合考虑本研究体系中可能存在的物种,激发态 – 激发态之间的电子转移反应,是3AP*促进DPTE光降解最可能的原因。
除表4中所列出的激发态之间的电子转移反应外,水合电子(eaq−)及DOM阴离子自由基(DOM·-)也是由DOM生成的可能有供电子能力的活性物种。Sharpless等[44]总结了前人关于DOM的研究,提出eaq−来自DOM成分中酚类及其衍生物、芳香羧酸及芳香氨基酸的激发单线态失电子过程;DOM·-则可通过DOM中不同组分(如芳香酮和酚类化合物)之间的光致电子转移反应生成。Zhang等[19, 29]发现,电子转移反应是由激发态电子供体和电子受体相互作用引发。而DPTE及苯乙酮分子结构中不含有富电子基团,因此理论上光照过程中不易生成eaq−或苯乙酮阴离子自由基。
未加入苯乙酮时,(DPTE-Br)·的摘氢反应可能与H2O或H3O+有关。(DPTE-Br)·夺取H2O中的氢原子,可生成·OH。然而,淬灭实验表明,体系中无·OH存在。根据质子转移柔性扫描的DFT计算,(DPTE-Br)·夺取H3O+中氢原子是无能垒的放热过程。因此,未加入苯乙酮的情况下,(DPTE-Br)·主要从H3O+夺取氢原子,生成稳定的光解产物。
基于上述结果,提出苯乙酮存在的情况下,DPTE的光化学转化路径(图9)。苯乙酮促进DPTE的光化学转化体现在两方面:(1) 3AP*向DPTE*的电子转移引发C—Br键断裂生成(DPTE-Br)·;(2) 基态苯乙酮向(DPTE-Br)·转移氢原子促进加氢产物生成。此外,脱溴产物(2,3-二溴丙基-2,4-二溴苯基醚)仍可吸光发生深度脱溴,该过程也可能以相似的机理被苯乙酮促进。
-
本研究以苯乙酮模拟DOM,通过光化学实验与DFT计算结合的方法,对DOM与DPTE的反应过程进行了探究。发现DOM能够通过两种方式参与DPTE的光降解,包括:(1) 吸光生成3DOM*向DPTE*转移电子,引发C—Br键断裂生成(DPTE-Br)·,反应位点为DPTE苯基上的溴;(2) 向(DPTE-Br)·转移氢原子促进加氢产物生成。苯乙酮可促进DPTE的光转化,但未改变DPTE的转化产物。意味在天然水体中,3DOM*引发的NBFRs间接光降解,只有反应路径不同于直接光解而降解产物不变。所揭示的DPTE光转化机理,有助于评价和预测水体中NBFRs及其他具有溴代苯基醚结构的污染物的光降解产物和动力学。
溶解性有机质引发2,3-二溴丙基-2,4,6-三溴苯基醚光转化机理
Phototransformation mechanism of 2,3-dibromopropyl-2,4,6-tribromophenyl ether induced by dissolved organic matter
-
摘要: 光转化是水体中有机污染物的重要转化途径,决定污染物的环境暴露和风险。激发三线态溶解性有机质(3DOM*)能够引发酚类、胺类和共轭二烯类污染物的间接光降解,反应机理涉及电子转移、质子转移和能量转移。然而,3DOM*与新型溴代阻燃剂(NBFRs)及具有类似结构的污染物的反应机理尚不清楚。本研究选取2,3-二溴丙基-2,4,6-三溴苯基醚(DPTE)为NBFRs模型化合物,以苯乙酮模拟DOM,通过光化学实验和密度泛函理论(DFT)计算,探究3DOM*与NBFRs的反应活性、反应位点和反应途径。结果表明,激发三线态苯乙酮(3AP*)对DPTE表观光降解的贡献为41.7% ± 2.2%,二者的二级反应速率常数为(1.49 ± 0.24) × 108 L·mol−1·s−1。DPTE的子结构模型化合物与苯乙酮的光化学实验表明,3AP*与DPTE的反应位点为DPTE苯基上的溴。DFT计算表明,3AP*通过向激发态DPTE转移电子使DPTE生成脱溴中间体,脱溴中间体与基态苯乙酮发生质子转移反应生成脱溴产物。所揭示的3AP*引发 DPTE 光转化机理,有助于评价和预测水体中其他有溴代苯基醚结构的污染物光转化产物和动力学。Abstract: Phototransformation is an important transformation pathway determining environmental exposure and risks of organic pollutants in natural waters. Excited triplet state of dissolved organic matter (3DOM*) can react with phenolics, amines and conjugated dienes through electron transfer, proton transfer and energy transfer. However, reaction mechanism of 3DOM* and novel brominated flame retardants (NBFRs) or other pollutants with similar structures remains unclear. In this study, reactivity, reaction sites and reaction pathways of 3DOM* and NBFRs were investigated through simulating photochemical experiments and density functional theory (DFT) calculation, adopting 2,3-dibromopropyl-2,4,6-tribromophenyl ether (DPTE) as a representative of NBFRs and acetophenone (AP) as a model of DOM. Results show that contribution of excited triplet state of acetophenone (3AP*) to apparent photodegradation of DPTE is 41.7% ± 2.2%. The second-order reaction rate constant between 3AP* and DPTE was determined to be (1.49 ± 0.24) × 108 L·mol−1·s−1. Photochemical experiments on sub-structural moieties of DPTE and acetophenone indicates that the reaction site of DPTE and 3AP* is the bromine on the phenyl. According to the DFT calculation, electron transfer from 3AP* to excited state of DPTE leads to the generation of debromination intermediates, which further react with ground state of acetophenone via proton transfer reaction and generate debromination product. The phototransformation mechanism of DPTE induced by 3AP* unveiled in this study contributes to evaluating and predicting phototransfomation products and kinetics of pollutants with bromophenyl ether structures in water body.
-

-
图 1 (a) 2,3-二溴丙基-2,4,6-三溴苯基醚(DPTE)、2,4,6-三溴苯甲醚(TBA)、苯乙酮(AP)和3-甲氧基苯乙酮(3MAP)的UV-vis吸收光谱;(b) 配备290 nm滤光片的500 W汞灯的发射光谱(Iλ, W·m−2·nm−1)
Figure 1. (a) UV-vis absorption spectra of 2,3-dibromopropyl-2,4,6-tribromophenyl ether (DPTE), 2,4,6-tribromoanisole (TBA), acetophenone (AP) and 3-methoxyacetophenone (3MAP); (b) Emission spectrum of 500 W Hg lamp filtered by 290 nm filters (Iλ, W·m−2·nm−1).
图 3 淬灭实验中DPTE的光降解速率常数[kd′ = (2.8 ± 0.2) × 10−2 h−1,为对苯乙酮(AP)光屏蔽校正后,DPTE的直接光解速率常数(误差线代表95%置信区间,n= 3)]
Figure 3. Photodegradation rate constants of DPTE observed in quenching experiments [kd′ = (2.8 ± 0.2) × 10−2 h−1, representing direct photolysis rate constant corrected by light screening factors of acetophenone (AP). The error bars represent the 95% confidence interval, n = 3)]
表 1 光化学实验中涉及化合物的高效液相色谱检测方法
Table 1. HPLC detection methods for compounds involved in the photochemical experiments
化合物
Compounds流动相/% Mobile phase 温度/℃
Temperature检测波长/nm
Wavelength保留时间/min
Retention time乙腈 Acetonitrile 水 Water 2,3-二溴丙基-2,4,6-三溴苯基醚
(DPTE)100 0 25 210 4.7 2,4,6-三溴苯甲醚(TBA) 100 0 25 220 4.4 3-苯氧基溴丙烷(3BPE) 80 20 25 220 4.7 丙氧基苯(PB) 90 10 25 220 3.7 2,4,6-三甲基苯酚(TMP) 60 40 30 220 5.2 表 2 DPTE及子结构模型化合物
Table 2. DPTE and sub-structural model compounds
化合物
Compounds结构图
Structure diagramCAS号
CAS number分子量
Molecular weight2,3-二溴丙基-2,4,6-三溴苯基醚
2,3-Dibromopropyl-2,4,6-tribromophenyl ether (DPTE)
35109-60-5 530.67 2,4,6-三溴苯甲醚
2,4,6-Tribromoanisole (TBA)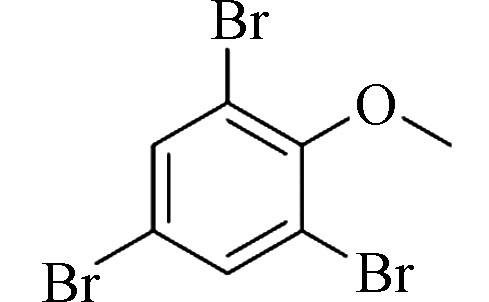
607-99-8 370.86 3-苯氧基溴丙烷
3-Bromopropyl phenyl ether (3BPE)
588-63-6 215.09 丙氧基苯
Propoxybenzene (PB)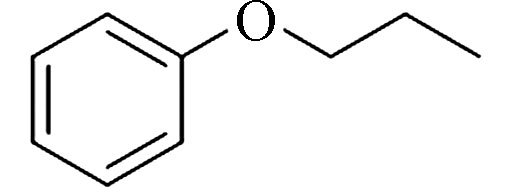
622-85-5 136.19 表 3 DPTE与苯乙酮的激发单线态(三线态)垂直激发能(ES1和ET1),垂直电子亲和势(VEA)和垂直电离能(VIE) (kcal·mol-1)
Table 3. Vertical excitation energies of the excited singlet (triplet) state (ES1 and ET1), vertical electron affinities (VEA) and vertical ionization energies (VIE) of DPTE and acetophenone (kcal·mol-1).
物质Substances ET1 ES1 VEAS0 VEAS1 VEAT1 VIES0 VIES1 VIET1 DPTE 79.17 107.74 − 38.35 − 146.09 − 117.53 159.77 52.03 80.59 苯乙酮 76.76 — − 51.10 — − 127.31 168.06 — 91.85 表 4 DPTE与3AP*可能发生的反应和热力学评价标准
Table 4. Possible reactions of DPTE and 3AP* and thermodynamic assessment criteria.
No. 反应式
Reaction equations热力学自发反应的评价标准
Criteria of thermodynamic spontaneityΔG/(kcal·mol− 1) 1 DPTE + 3AP* → 3DPTE* + AP ET1,DPTE < ET1,AP — 2 DPTE + 3AP* → DPTE·+ + AP·− ΔG1 = VIEDPTE,S0 + VEAAP,T1 < 0 32.46 3 DPTE + 3AP* → DPTE·− + AP·+ ΔG2 = VEADPTE,S0 + VIEAP,T1 < 0 53.50 4 3DPTE* + 3AP* → DPTE·+ + AP·− ΔG3 = VIEDPTE,T1 + VEAAP,T1 < 0 − 46.71 5 3DPTE* + 3AP* → DPTE·− + AP·+ ΔG4 = VEADPTE,T1 + VIEAP,T1 < 0 − 25.68 6 1DPTE* + 3AP* → DPTE·+ + AP·− ΔG5 = VIEDPTE,S1 + VEAAP,T1 < 0 − 75.28 7 1DPTE* + 3AP* → DPTE·− + AP·+ ΔG6 = VEADPTE,S1 + VIEAP,T1 < 0 − 54.24 -
[1] XIONG P, YAN X T, ZHU Q Q, et al. A review of environmental occurrence, fate, and toxicity of novel brominated flame retardants [J]. Environmental Science & Technology, 2019, 53(23): 13551-13569. [2] KALACHOVA K, HRADKOVA P, LANKOVA D, et al. Occurrence of brominated flame retardants in household and car dust from the Czech Republic [J]. Science of the Total Environment, 2012, 441: 182-193. doi: 10.1016/j.scitotenv.2012.09.061 [3] BESIS A, CHRISTIA C, POMA G, et al. Legacy and novel brominated flame retardants in interior car dust - Implications for human exposure [J]. Environmental Pollution, 2017, 230: 871-881. doi: 10.1016/j.envpol.2017.07.032 [4] COVACI A, HARRAD S, ABDALLAH M A E, et al. Novel brominated flame retardants: A review of their analysis, environmental fate and behaviour [J]. Environment International, 2011, 37(2): 532-556. doi: 10.1016/j.envint.2010.11.007 [5] XIE Z Y, MÖLLER A, AHRENS L, et al. Brominated flame retardants in seawater and atmosphere of the Atlantic and the Southern Ocean [J]. Environmental Science & Technology, 2011, 45(5): 1820-1826. [6] NAKARI T, HUHTALA S. In vivo and in vitro toxicity of decabromodiphenyl ethane, a flame retardant [J]. Environmental Toxicology, 2010, 25(4): 333-338. [7] ZHANG Y N, WANG J Q, CHEN J W, et al. Phototransformation of 2,3-dibromopropyl-2,4,6-tribromophenyl ether (DPTE) in natural waters: Important roles of dissolved organic matter and chloride ion [J]. Environmental Science & Technology, 2018, 52(18): 10490-10499. [8] LIU L Y, SALAMOVA A, VENIER M, et al. Trends in the levels of halogenated flame retardants in the Great Lakes atmosphere over the period 2005-2013 [J]. Environment International, 2016, 92-93: 442-449. doi: 10.1016/j.envint.2016.04.025 [9] VENKATESAN A K, HALDEN R U. Brominated flame retardants in US biosolids from the EPA national sewage sludge survey and chemical persistence in outdoor soil mesocosms [J]. Water Research, 2014, 55: 133-142. doi: 10.1016/j.watres.2014.02.021 [10] SMYTHE T A, BUTT C M, STAPLETON H M, et al. Impacts of unregulated novel brominated flame retardants on human liver thyroid deiodination and sulfotransferation [J]. Environmental Science & Technology, 2017, 51(12): 7245-7253. [11] MÖLLER A, XIE Z Y, CAI M H, et al. Polybrominated diphenyl ethers vs alternate brominated flame retardants and dechloranes from East Asia to the Arctic [J]. Environmental Science & Technology, 2011, 45(16): 6793-6799. [12] RUAN T, WANG Y W, WANG C, et al. Identification and evaluation of a novel heterocyclic brominated flame retardant tris(2,3-dibromopropyl) isocyanurate in environmental matrices near a manufacturing plant in Southern China [J]. Environmental Science & Technology, 2009, 43(9): 3080-3086. [13] LIU H H, HU Y J, LUO P, et al. Occurrence of halogenated flame retardants in sediment off an urbanized coastal zone: Association with urbanization and industrialization [J]. Environmental Science & Technology, 2014, 48(15): 8465-8473. [14] VÉNISSEAU A, BICHON E, BROSSEAUD A, et al. Occurrence of legacy and novel brominated flame retardants in food and feed in France for the period 2014 to 2016 [J]. Chemosphere, 2018, 207: 497-506. [15] SKLEDAR D G, TOMAŠIČ T, CARINO A, et al. New brominated flame retardants and their metabolites as activators of the pregnane X receptor [J]. Toxicology Letters, 2016, 259: 116-123. doi: 10.1016/j.toxlet.2016.08.005 [16] BEARR J S, STAPLETON H M, MITCHELMORE C L. Accumulation and DNA damage in fathead minnows (Pimephales promelas) exposed to 2 brominated flame-retardant mixtures, Firemaster® 550 and Firemaster® BZ-54 [J]. Environmental Toxicology and Chemistry, 2010, 29(3): 722-729. doi: 10.1002/etc.94 [17] GE L K, CHEN J W, WEI X X, et al. Aquatic photochemistry of fluoroquinolone antibiotics: Kinetics, pathways, and multivariate effects of main water constituents [J]. Environmental Science & Technology, 2010, 44(7): 2400-2405. [18] JANSSEN E M L, ERICKSON P R, MCNEILL K. Dual roles of dissolved organic matter as sensitizer and quencher in the photooxidation of tryptophan [J]. Environmental Science & Technology, 2014, 48(9): 4916-4924. [19] ZHANG Y, VECCHIO R D, BLOUGH N V. Investigating the mechanism of hydrogen peroxide photoproduction by humic substances [J]. Environmental Science & Technology, 2012, 46(21): 11836-11843. [20] 孙国新, 王杰琼, 周成智, 等. 四溴双酚A在近岸海水中的光降解动力学研究 [J]. 环境化学, 2018, 37(8): 1683-1690. doi: 10.7524/j.issn.0254-6108.2018010602 SUN G X, WANG J Q, ZHOU C Z, et al. Photodegradation kinetics of tetrabromobisphenol A in coastal water [J]. Environmental Chemistry, 2018, 37(8): 1683-1690(in Chinese). doi: 10.7524/j.issn.0254-6108.2018010602
[21] GE L K, CHEN J W, QIAO X L, et al. Light-source-dependent effects of main water constituents on photodegradation of phenicol antibiotics: Mechanism and kinetics [J]. Environmental Science & Technology, 2009, 43(9): 3101-3107. [22] ZHOU H X, YAN S W, MA J Z, et al. Development of novel chemical probes for examining triplet natural organic matter under solar illumination [J]. Environmental Science & Technology, 2017, 51(19): 11066-11074. [23] ROSARIO-ORTIZ F L, CANONICA S. Probe compounds to assess the photochemical activity of dissolved organic matter [J]. Environmental Science & Technology, 2016, 50(23): 12532-12547. [24] PORRAS J, FERNÁNDEZ J J, TORRES-PALMA R A, et al. Humic substances enhance chlorothalonil phototransformation via photoreduction and energy transfer [J]. Environmental Science & Technology, 2014, 48(4): 2218-2225. [25] GUERARD J J, CHIN Y P, MASH H, et al. Photochemical fate of sulfadimethoxine in aquaculture waters [J]. Environmental Science & Technology, 2009, 43(22): 8587-8592. [26] LI Y J, WEI X X, CHEN J W, et al. Photodegradation mechanism of sulfonamides with excited triplet state dissolved organic matter: A case of sulfadiazine with 4-carboxybenzophenone as a proxy [J]. Journal of Hazardous Materials, 2015, 290: 9-15. doi: 10.1016/j.jhazmat.2015.02.040 [27] CANONICA S, HELLRUNG B, MÜLLER P, et al. Aqueous oxidation of phenylurea herbicides by triplet aromatic ketones [J]. Environmental Science & Technology, 2006, 40(21): 6636-6641. [28] CHEN Y, LI H, WANG Z P, et al. Photodegradation of selected β-blockers in aqueous fulvic acid solutions: Kinetics, mechanism, and product analysis [J]. Water Research, 2012, 46(9): 2965-2972. doi: 10.1016/j.watres.2012.03.025 [29] ZHANG Y, SIMON K A, ANDREW A A, et al. Enhanced photoproduction of hydrogen peroxide by humic substances in the presence of phenol electron donors [J]. Environmental Science & Technology, 2014, 48(21): 12679-12688. [30] HAN S K, SIK R H, MOTTEN A G, et al. Photosensitized oxidation of tetrabromobisphenol A by humic acid in aqueous solution [J]. Photochemistry and Photobiology, 2009, 85(6): 1299-1305. doi: 10.1111/j.1751-1097.2009.00608.x [31] LEAL J F, ESTEVES V I, SANTOS E B H. BDE-209: Kinetic studies and effect of humic substances on photodegradation in water [J]. Environmental Science & Technology, 2013, 47(24): 14010-14017. [32] WANG H L, WANG M, WANG H, et al. Aqueous photochemical degradation of BDE-153 in solutions with natural dissolved organic matter [J]. Chemosphere, 2016, 155: 367-374. doi: 10.1016/j.chemosphere.2016.04.071 [33] JIANG Z W, LINGHU W S, LI Y M, et al. Photoreductive debromination of decabromodiphenyl ether by pyruvate [J]. Catalysis Today, 2014, 224: 89-93. doi: 10.1016/j.cattod.2014.01.002 [34] COOPER W J, ZIKA R G. Photochemical formation of hydrogen-peroxide in surface and ground waters exposed to sunlight [J]. Science, 1983, 220(4598): 711-712. doi: 10.1126/science.220.4598.711 [35] WENK J, von GUNTEN U, CANONICA S. Effect of dissolved organic matter on the transformation of contaminants induced by excited triplet states and the hydroxyl radical [J]. Environmental Science & Technology, 2011, 45(4): 1334-1340. [36] CANONICA S, FREIBURGHAUS M. Electron-rich phenols for probing the photochemical reactivity of freshwaters [J]. Environmental Science & Technology, 2001, 35(4): 690-695. [37] MCCABE A J, ARNOLD W A. Reactivity of triplet excited states of dissolved natural organic matter in stormflow from mixed-use watersheds [J]. Environmental Science & Technology, 2017, 51(17): 9718-9728. [38] FRISCH M J, TRUCKS G W, SCHLEGEL H B, et al. Gaussian 09[Z]. Revision A. 02. ed ed. Wallingford CT: Gaussian, Inc, 2009. [39] ZHANG S Y, CHEN J W, QIAO X L, et al. Quantum chemical investigation and experimental verification on the aquatic photochemistry of the sunscreen 2-phenylbenzimidazole-5-sulfonic acid [J]. Environmental Science & Technology, 2010, 44(19): 7484-7490. [40] JIANG L, QIU Y L, LI Y. Effects analysis of substituent characteristics and solvents on the photodegradation of polybrominated diphenyl ethers [J]. Chemosphere, 2017, 185: 737-745. doi: 10.1016/j.chemosphere.2017.07.063 [41] KAVARNOS G J, TURRO N J. Photosensitization by reversible electron transfer: Theories, experimental evidence, and examples [J]. Chemical Reviews, 1986, 86(2): 401-449. doi: 10.1021/cr00072a005 [42] XIE Q, CHEN J W, SHAO J P, et al. Important role of reaction field in photodegradation of deca-bromodiphenyl ether: Theoretical and experimental investigations of solvent effects [J]. Chemosphere, 2009, 76(11): 1486-1490. doi: 10.1016/j.chemosphere.2009.06.054 [43] ZHANG Y N, CHEN J W, XIE Q, et al. Photochemical transformation of five novel brominated flame retardants: Kinetics and photoproducts [J]. Chemosphere, 2016, 150: 453-460. doi: 10.1016/j.chemosphere.2015.12.125 [44] SHARPLESS C M, BLOUGH N V. The importance of charge-transfer interactions in determining chromophoric dissolved organic matter (CDOM) optical and photochemical properties [J]. Environmental Science: Processes & Impacts, 2014, 16(4): 654-671. [45] MCNEILL K, CANONICA S. Triplet state dissolved organic matter in aquatic photochemistry: Reaction mechanisms, substrate scope, and photophysical properties [J]. Environmental Science: Processes & Impacts, 2016, 18(11): 1381-1399. [46] VIONE D, MINELLA M, MAURINO V, et al. Indirect photochemistry in sunlit surface waters: Photoinduced production of reactive transient species [J]. Chemistry - A European Journal, 2014, 20(34): 10590-10606. doi: 10.1002/chem.201400413 [47] WENK J, EUSTIS S N, MCNEILL K, et al. Quenching of excited triplet states by dissolved natural organic matter [J]. Environmental Science & Technology, 2013, 47(22): 12802-12810. [48] PFLUG N C, SCHMITT M, MCNEILL K. Development of N-cyclopropylanilines to probe the oxidative properties of triplet-state photosensitizers [J]. Environmental Science & Technology, 2019, 53(9): 4813-4822. [49] CHEN Y, ZHANG X, FENG S X. Contribution of the excited triplet state of humic acid and superoxide radical anion to generation and elimination of phenoxyl radical [J]. Environmental Science & Technology, 2018, 52(15): 8283-8291. [50] WENK J, CANONICA S. Phenolic antioxidants inhibit the triplet-induced transformation of anilines and sulfonamide antibiotics in aqueous solution [J]. Environmental Science & Technology, 2012, 46(10): 5455-5462. -



 下载:
下载: