-
过氧化氢(hydrogen peroxide),即H2O2,是世界上100种最重要的化学品之一[1],在全pH 范围内具有高氧化电位(pH 为 0 时 E0=1.763 V,pH 为 14 时 E0 =0.878 V)[2]. 在合适活化条件下(如臭氧、紫外线、Fe2+等),可有效产生羟基自由基(•OH),在短时间内氧化有机污染物,将其转化成易生物降解的中间产物,因此可广泛应用于处理市政饮用水、工业废水和生活污水[2-3]. H2O2的反应产物为氧气和水,安全无毒易处理,所以H2O2也被称为最清洁的氧化剂[4] . 目前,H2O2的工业价值接近每年40亿美元,年需求量约550万吨[5-6],也正因如此,探寻高效廉价制取H2O2的方法具有重要的现实意义.
蒽醌法是目前国内外生产H2O2的主要方法,世界上大约 95% 的H2O2都是通过蒽醌法生产的[7]. 然而,该方法无法原位生产H2O2,且合成的H2O2浓度较高,在运输过程中存在爆炸风险,储存过程中也易发生分解,运输和储存成本较高.
由于现行方法的不足,如何绿色高效地合成H2O2成为越来越多学者研究的课题. 在各种方法当中,电催化two-electron oxygen reduction reaction(2e− ORR)法合成H2O2最具潜力. 该方法可在常温、常压下实现H2O2的合成,相比传统的蒽醌法具有进行原位水处理、绿色安全、工艺简单、能耗较低、无污染物排放等优点,更符合当今绿色化学的发展理念.
-
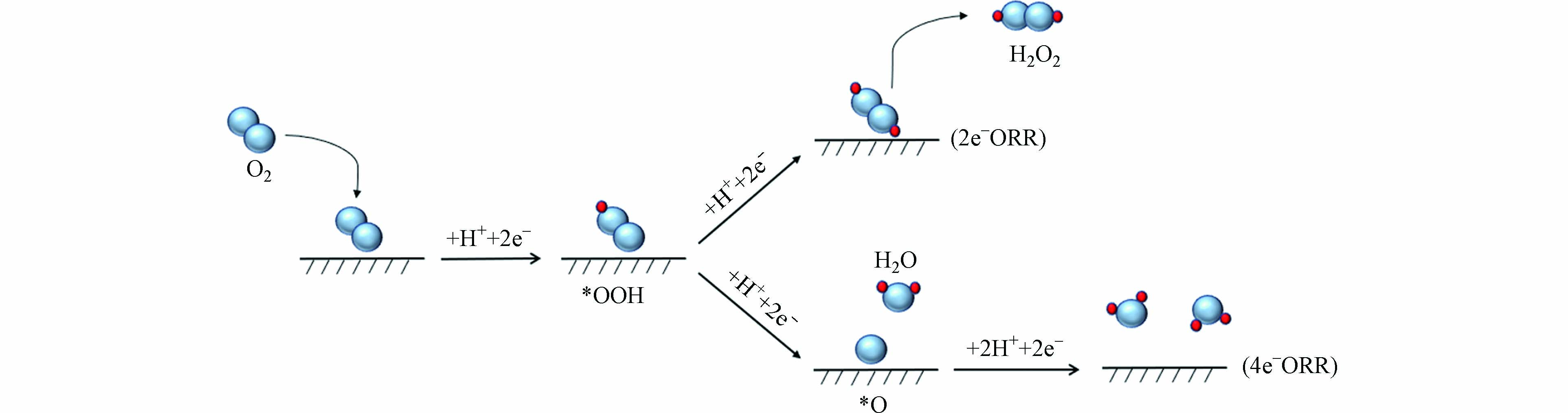
电化学氧还原反应是在一定电压下,将通入阴极的O2直接与电解液中的质子结合,实现H2O2的产生 [8]. ORR是一个多步骤,多质子耦合的电子转移过程,包括多个基元步骤和反应中间产物[9-10]. 一般认为,ORR反应包括两条途径,即吸附于催化剂阴极表面的O2分子通过2e− ORR途径被还原为H2O2或4e− ORR途径被还原为H2O [11-12](图1).
在2e− ORR中,O2分子吸附于阴极催化剂表面,吸附的O2分子首先得到1个电子,同时从溶液中得到一个质子形成反应中间体*OOH(*表示催化剂表面未被占据的活性位点),由于*OOH的弱吸附,在继续得到一个电子和质子后,会从催化剂表面脱附形成H2O2. 酸性条件下2e− ORR的化学式如(1)所示:
在4e− ORR中,第一步同样是被阴极催化剂表面吸附的O2分子得到1个电子,再从溶液中得到一个质子形成*OOH,与2e− ORR有所不同的是,*OOH的强吸附阻止了其从催化剂表面解吸,并最终分解成了*O和*OH,继续得到电子和质子后形成H2O. 酸性条件下4e− ORR的化学式如(2)所示:
通过对比可以发现,O2分子和反应中间体*OOH在催化剂阴极表面活性位点的吸附性能是发生2e− ORR的关键. *OOH吸附过强阻碍了其在催化剂表面的解吸,导致生成副产物H2O,加剧竞争反应;*OOH吸附过弱则会降低反应活性,需要额外的过电位[13]. ORR反应体系中H2O2的生成是发生2e− ORR的标志,从化学式可以看出,2e− ORR是合成H2O2最高效的途径. 因此,越来越多的研究者致力于合成兼具高2e− ORR活性和选择性的稳定催化剂,它们具有更强O2吸附性能和合适的*OOH结合性能,可以在最大程度上实现*OOH的保留,进而有利于H2O2的生成.
-
目前,贵金属基催化剂,如Pd[14-16]、Pt[17-18]及其合金[19-20]等,它们对O2电还原具有较小的过电位和高2e− ORR选择性. FORTUNATO[14]等利用氯化物-低金属Pd负载的催化剂通过ORR反应制备H2O2,发现在几何效应和电子效应的相互作用下H2O2的选择性接近100%,具有极高的质量活性[21],但是贵金属数量稀少、成本过高、易脱落造成污染的缺点限制了其规模化应用.
碳材料是常见的阴极材料,很早以前便有研究者将碳材料用于催化O2还原制备H2O2. 然而,原始碳材料的表面积较小且难以提高[22],O2分子需要先溶解于溶液中再扩散到电极表面. 此外,未改性的碳材料的表面活性位点也较少,这些都限制了它的催化活性. Da POZZO[23]、SALARI[24]、ÖZCAN[25]、WANG[26]等曾将原始碳材料(石墨、石墨毡、碳毡、活性炭纤维)作为催化剂用于合成H2O2,可是效果并不理想,远没有达到规模化生产的要求. 因此,对碳材料催化剂阴极进行改性成为提升其电化学性能的首选方法.
根据ORR的过程机理,催化剂改性原理主要分为两点[14,27]:
①几何效应:适当增加催化剂中活性位点之间的距离,对活性位点进行修饰,通过改变催化剂空间结构,包括催化剂活性位点分布和空间结构等,提高催化剂的催化性能.
②电子效应:根据Sabatier的中间化合物理论,催化过程的中间产物(在本反应中为*OOH)与催化剂之间的吸附能应适中,寻找活性位点具有合适电子密度的催化剂,使其对*OOH具有合适的吸附能.
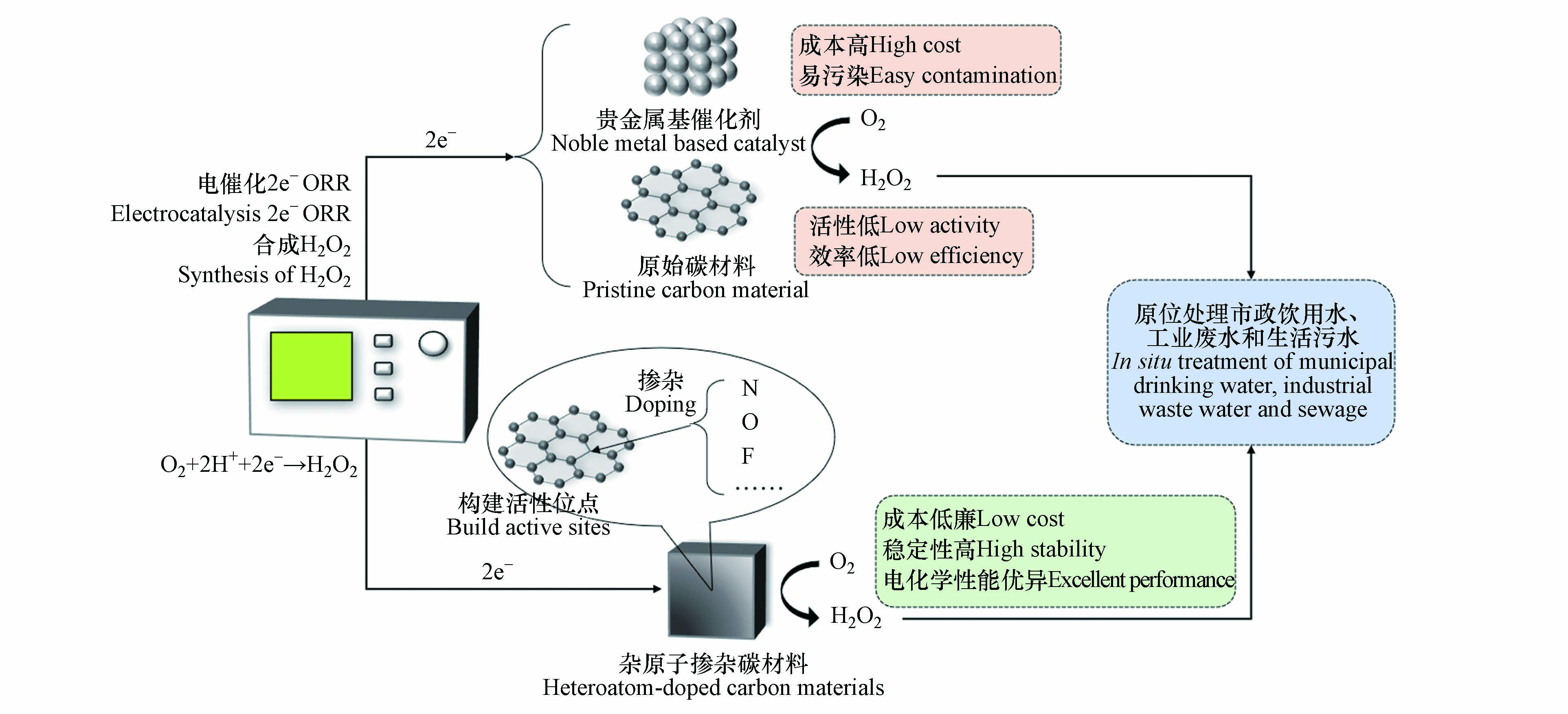
当前,有关电催化2e− ORR 合成H2O2过程催化剂的改性重点集中在向碳基基底引入其他碳材料(炭黑[28]、乙炔黑[29]、石墨烯[30]等)、杂原子(N[31-34]、O[35-37]、F[38-39]等)掺杂、材料结构的调控、反应条件的优化等方法提高O2分子在还原过程中与催化剂表面结合的活性、选择性以及催化剂的稳定性. 由于掺入的杂原子具有不同的电负性,碳基体的局部电荷和自旋密度发生了显著改变,促进了对O2的吸附,降低了反应能垒,增强了碳材料电催化ORR的活性[40-41]. 杂原子掺杂后的碳材料与贵金属基催化剂和原始碳材料相比具有可操作性好、电化学性能优异、成本低廉等优点,可用于原位处理市政饮用水、工业废水和生活污水,拥有良好的发展前景(图2). 表1展示了部分杂原子掺杂碳材料催化剂电合成H2O2的性能.
-
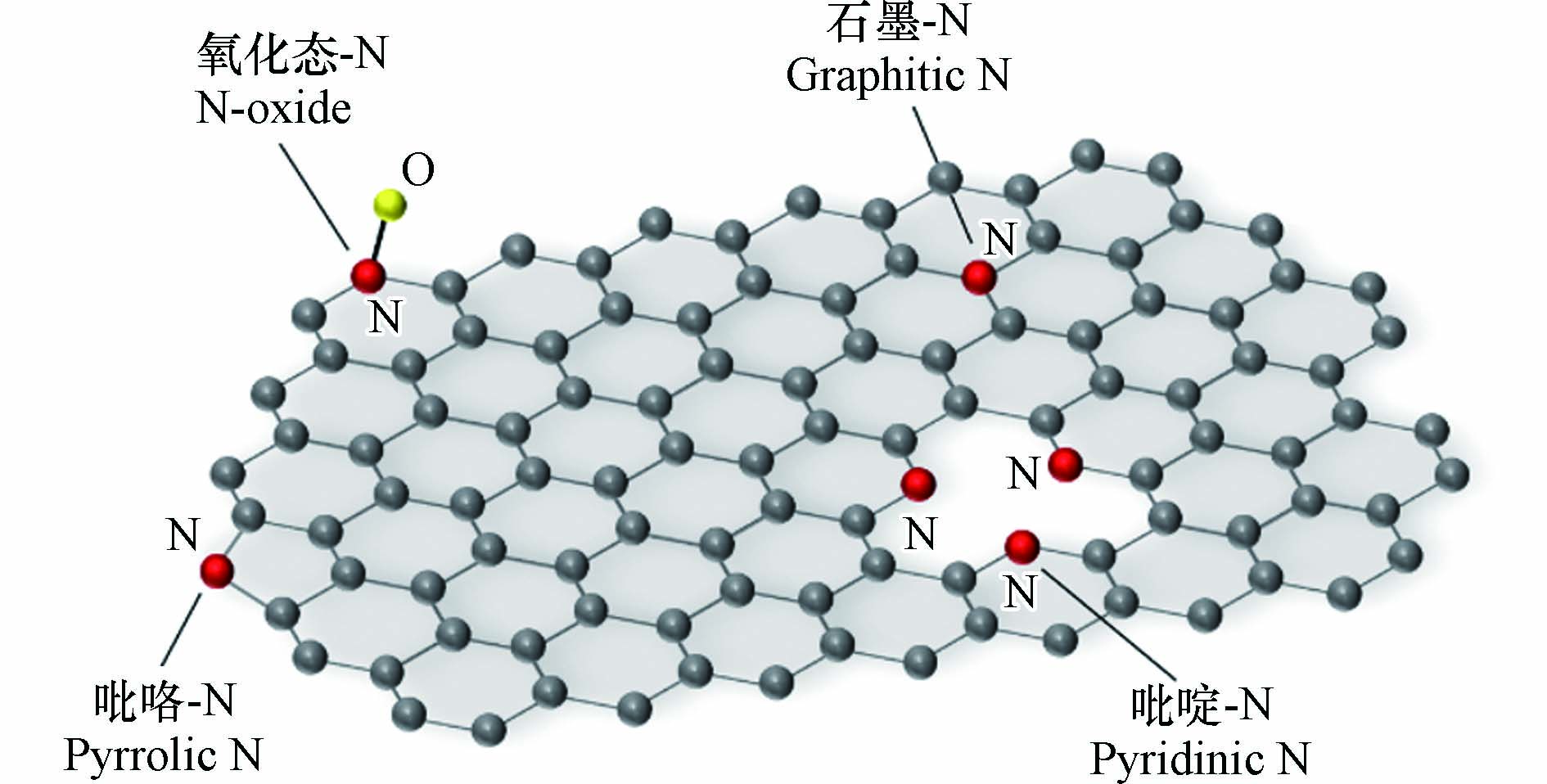
N掺杂碳材料是指将N原子通过化学键连接或结合到碳骨架中产生的新型材料. N原子和C原子尺寸相接近,在N原子取代C原子的过程当中,碳材料骨架所收到的破坏程度较小,具有较高的稳定性和耐久性. 此外, N可以改变相邻C原子上的电荷分布,从而产生更多的活性位点,促进ORR的发生 [52]. N原子掺杂碳材料中的N主要有4种类型:石墨-N、吡啶-N、吡咯-N和氧化态-N(图3),由于自身电子结构差异,不同N物种具有不同的结构性能. 例如,由于吡啶-N物种具有未杂化的孤对电子,有较强的给电子能力,可以有效提供 Lewis 碱位点,所以富含吡啶-N物种的N掺杂碳材料催化剂在ORR中具有更优异的催化性能[53].
目前的N掺杂方法尚不能建立有效的结构性能关系,所以明确不同键合类型的N掺杂效果,识别N掺杂碳材料的活性位点具有重要意义. 2012年,FELLINGER等[31]采用离子液体N-丁基-3-甲基吡啶二氰胺(BMP-dca)作为前体合成N掺杂介孔碳,催化剂产生1 g H2O2的实际比能量为0.241 W·(gcat·h)−1,法拉第电流效率达65.15%. N掺杂介孔碳催化剂有着较高的2e− ORR选择性,这是因为掺杂到碳材料中具有较高电负性的N原子可以通过破坏π共轭系统完整性并诱导电荷再分布来激活π电子,从而改变碳材料的吸附性能,有利于H2O2的生成.
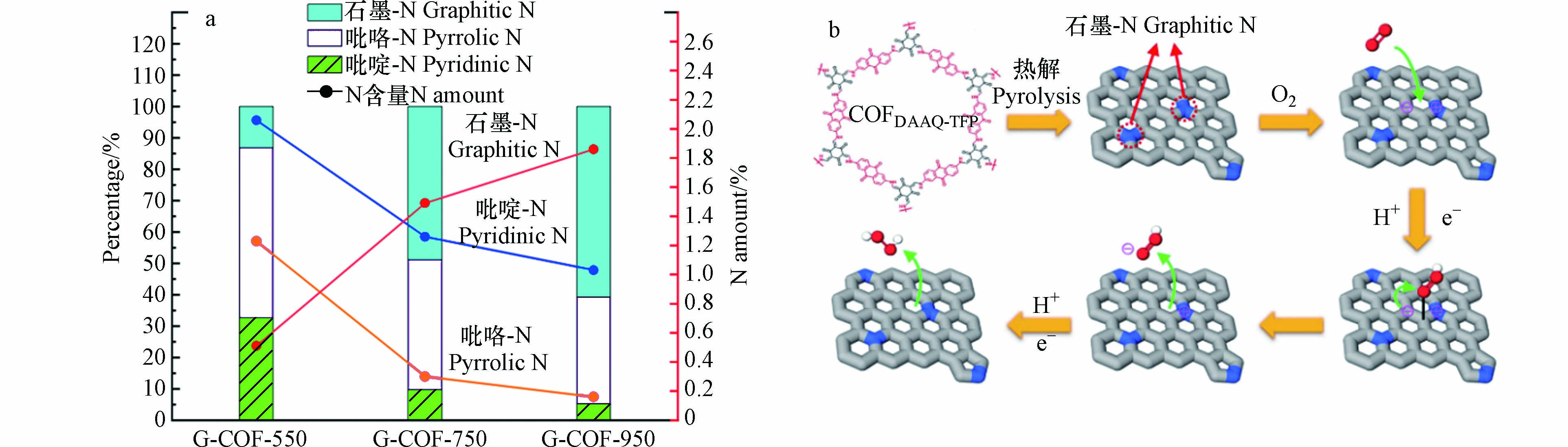
2020年,ZHANG等[42]采用共价有机框架(COF)作为前体,在Ar气氛下热解,加热至恒温合成了N掺杂催化剂 G-COF-T(G-COF-550、G-COF-750、G-COF-950). X射线光电子能谱(XPS)分析发现催化剂中总氮含量随热解温度的升高而降低:吡啶-N和吡咯-N的含量减少,石墨-N的含量增加. 其中,950 ℃下处理的G-COF中石墨N的含量从0.51%增加到1.86%,占总氮的比从13.5%增加到60.7%(图4a). 在0.1 mol·L−1的KOH溶液中,该催化剂H2O2生成速率为1286.9 mmol· (gcat·h)−1,法拉第效率达69.8%. 进一步推断认为,石墨-N可以通过给p-π共轭体系提供一个单电子来诱导电荷再分配,从而增强氧气在相邻的C原子上的吸附,将其转变为中间体HOO*后,石墨-N的不稳定结构趋于电荷平衡,促使*OOH解吸(图4b). 因此,高密度、高活性的石墨-N有助于N掺杂的碳材料高效催化2e− ORR的发生.
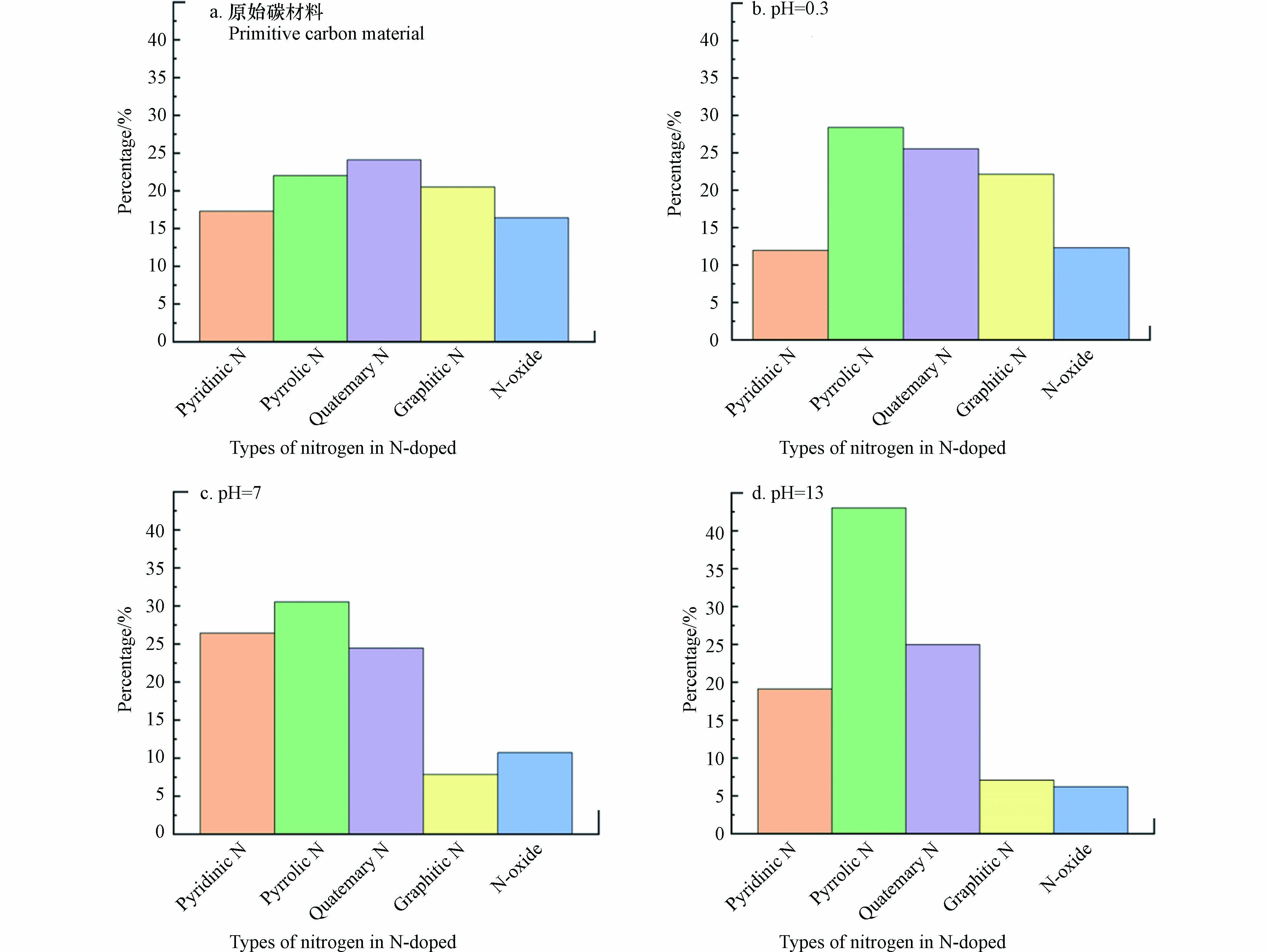
此外, SUN等[43] 将有序介孔碳(CMK-3)与聚乙烯亚胺(PEI)直接热解制备得到N掺杂多孔碳材料PEI50CMK3-800T. 分析发现,在0.05 mol·L−1 H2SO4溶液,电位为0.35 V (vs. RHE) 的条件下,H2O2的选择性从未改性时的77.2%大幅提高到98.5%,;在中性条件下,H2O2的生成速率达到570.1 mmol· (gcat·h)−1. XPS结果表明,在不同pH条件下,不同类型的N掺杂效果不同. 酸性条件下,吡啶- N在催化过程中起关键作用,而在中性和碱性条件下,石墨- N基团的催化活性更高(图5). 最近有研究表明[54-56],ORR过程中吸附的*OOH中间体可以改变催化剂的催化活性位点或结构基序的直接化学环境. 在酸性条件下,—OH与相邻吡啶-N的碳原子共价结合,形成一个羟基吡啶-N结构,该结构中N的1s结合能由初始的398.8 eV上升到400.2 eV,与吡咯-N的结合能非常接近. 与之类似的是,—OH与相邻石墨N的碳原子也会共价结合,使N的1s结合能降低至400 eV左右,同样与吡咯-N的结合能接近. 吡咯-N含量的增加证实了其作为有效活性位点结构促进了2e− ORR的发生.
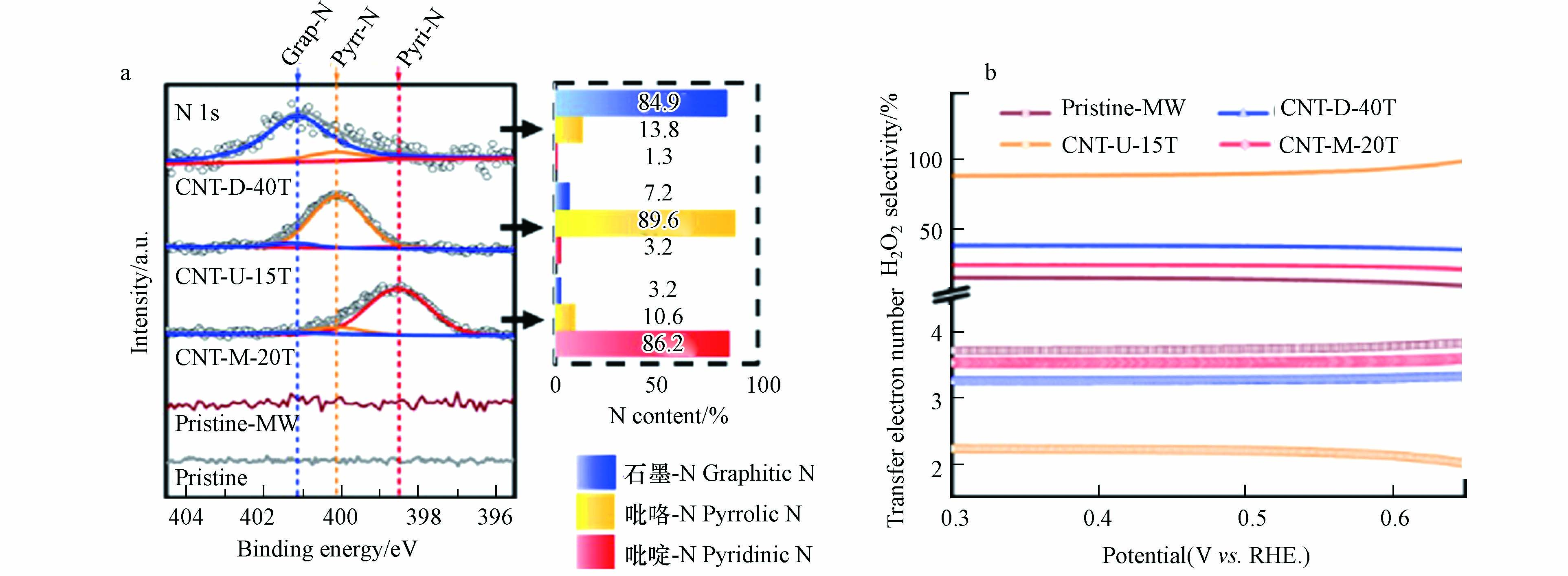
2022年,WAN等[44] 通过微波辅助脉冲加热(MAPH)方法,成功制备了具有多种键合类型(石墨-N,吡咯-N和吡啶-N)的N掺杂单壁碳纳米管(CNT-D-40T、CNT-U-15T、CNT-M-20T)(图6a). N掺杂可以通过扰乱π共轭体系的完整性来激活C的π电子的ORR活性. π电子的活性与其电子亲和力密切相关,在吡啶-N构型中的N原子会与两个碳原子结合,为π体系贡献一个p电子,而吡咯-N可以为构型中的π共轭体系贡献两个p电子. 除此以外,计算电子转移数发现,吡咯-N掺杂的CNT-U-15T在很宽的电位范围内(0.30—0.65 V,vs. RHE)具有最小的电子转移数值,与2最为接近,小于石墨-N掺杂的CNT-D-40T和吡啶-N掺杂的CNT-M-20T(图6b). 这些都无疑证明了吡咯-N掺杂的优异2e− ORR催化性能.
近期,HU等[45]将聚多巴胺碳球碳化,并在Ar气氛下进行进一步高温处理得到了具有丰富微孔的空心N掺杂碳球, 其在0.1 mol·L−1 KOH溶液,0.7 V(vs. RHE)的条件下,相比于原始碳材料(77.0%和61.8%),具有更高的2e− ORR选择性[91.9%,0.7 V(vs. RHE)]和法拉第效率[85.1%,0.7 V(vs. RHE)],H2O2生成速率达618.5 mmol· (gcat·h)−1. 研究发现,N掺杂碳球中更多的微孔体积通常有利于活性位点的暴露,此外,微孔隙率还可以减少H2O2的停留产物,从而防止进一步还原成为H2O,这也证明了空心结构的杂原子掺杂碳材料极大地有利于2e− ORR合成H2O2.
-
O掺杂可以将各种含氧官能团(OG)引入碳材料表面,例如羧基(―COOH)、醛基(―CHO)、羰基(—C=O)、醚键(C—O—C)等. O掺杂的碳材料具有合成简单、成本低廉的特点,引入的OG会显著地改变其表面性质,如反应性和亲水性. 与N掺杂类似,O掺杂也可以显著改变相邻C原子上的电荷分布,从而调整*OOH在碳材料表面的吸附特性,促进2e− ORR的发生. 因此,氧化碳纳米管(O-CNT)[35]、氧化石墨烯[46]、活性炭黑[57]等O掺杂碳材料作为2e− ORR催化剂的研究已经取得一定进展.
2010年,MATSUBARA等[47]就利用120 °C的2 mol·L−1硫酸和1:1浓硝酸处理多壁碳纳米管(MWCNTs),发现了在表面形成的OG可以使反应过电位降低. 2013年,Zhou等[36]通过阳极氧化石墨毡发现,酸性的OG可以提高碳材料表面的亲水性能,更易于获得水中的溶解氧,促进电极和电解质之间的电子传输. 2014年,MIAO等[37]在硫酸中电化学氧化石墨毡电极,将某些OG经过修饰后引入石墨毡电极表面,发现处理后的石墨毡电极阴极上2e− ORR反应得到增强,H2O2合成效率提高到了原来的6倍,进一步证明了OG对ORR反应的促进作用.
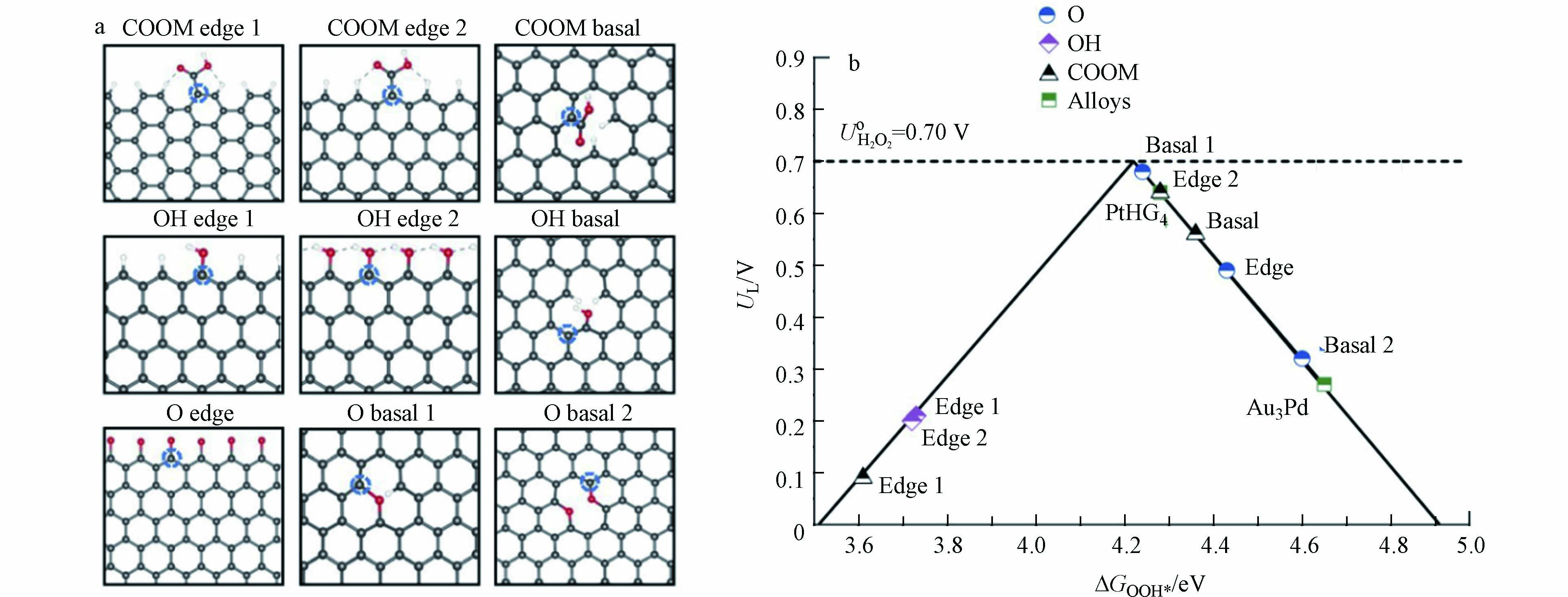
关于OG提高碳材料催化活性的机理,由于不同实验室采用不同的碳材料和氧化过程,给出的解释也不尽相同. 2018年,LU等[35]通过浓硝酸氧化的方式成功制备了O-CNT. 与标准的CNT相比,O-CNT在0.1 mol·L−1 KOH溶液,0.2 mA的电流下的过电位降低了约130 mV,将2e− ORR反应选择性从60%提高到了90%. 与此同时,LU等发现O-CNT的活性和选择性都与其氧含量呈正相关,不同结构的催化活性由反应中间体与催化剂活性中心的结合能决定(图7a). LU等通过进一步密度泛函理论(DFT)计算,根据*OOH吸附能的变化,绘制了不同OG通过2e− ORR合成H2O2的火山图(图7b),结果表明,在石墨烯基面和边缘上的醚键具有极高的2e− ORR反应活性,它们的过电位分别为0.02 V和0.06 V,这与贵金属催化剂Pt-Hg和Pt-Au相当[20].
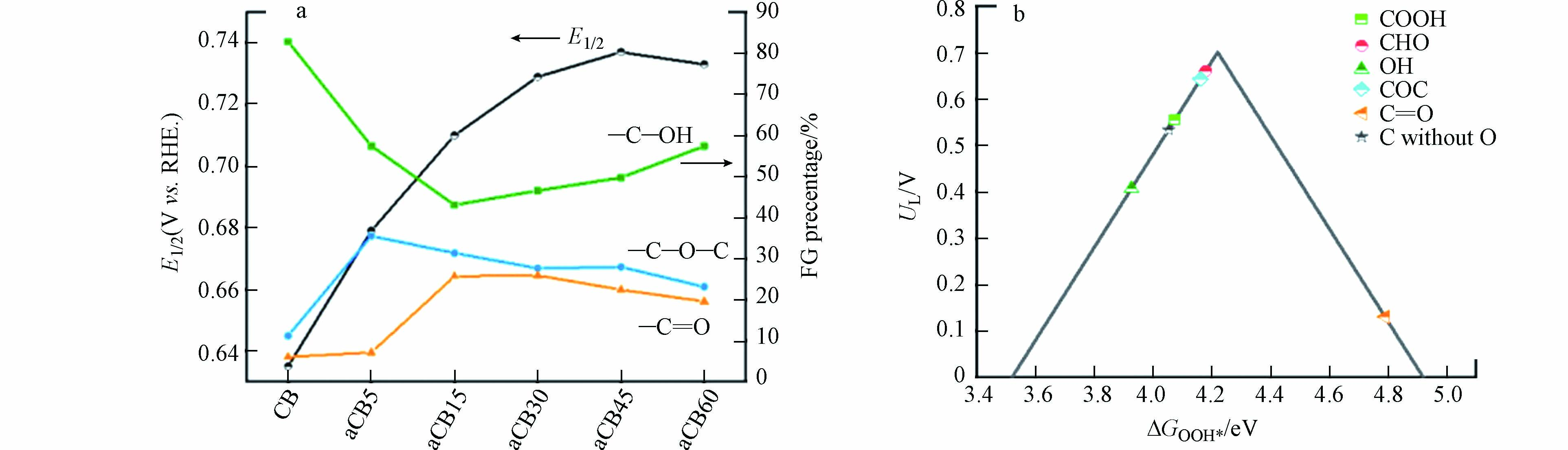
LU等在利用浓硝酸氧化制备O-CNT的实验中,未提到是否会引入N. 对此,一些研究者提供了其他的氧化方法. Kim等[46]利用氧化石墨烯材料,通过热还原富集氧化石墨烯中的醚键和环氧树脂,使用碳纸成功合成了多层mrGO催化剂(F-mrGO(600)),该催化剂过电位小于10 mV且具有极高的2e− ORR活性和稳定性,选择性更是接近100%. 此外,WANG[57]等通过在500 ℃空气气氛中退火来提高活性炭黑(aCB)中的C—O键和O—H键浓度,有效将过电位降到了5 mV以下,在0.75V(vs. RHE)下H2O2的最大生成速率达770 mmol· (gcat·h)−1. 这种简单的活化方法可以在不改变含氧量的情况下操纵含氧基团的比例,从而准确地将催化活性与含氧基团相关联(图8a). 根据实验结果和DFT计算,绘制出火山图,得出添加醚键和醛基的碳材料催化剂表面活性位点对*OOH的吸附能最低,过电位较小,是生成H2O2的活性中心(图8b).
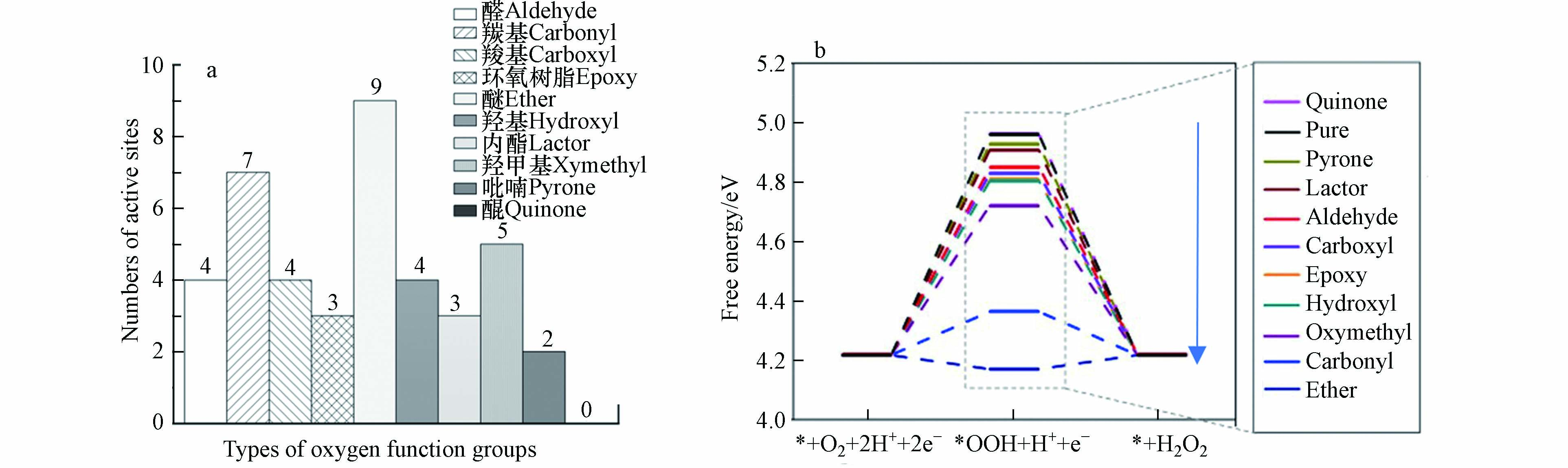
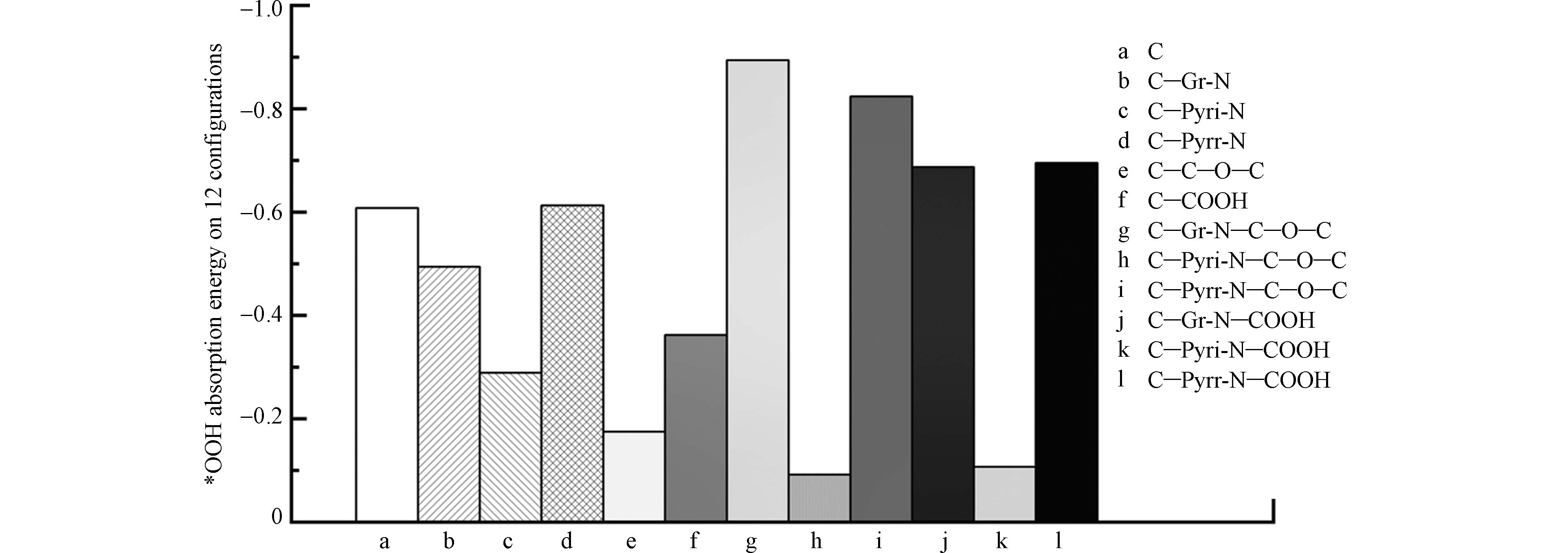
先前研究表明,电催化剂上各位点吸附反应物的能力与其活性直接相关[58]. 杂原子掺杂后电荷密度和自旋密度的再分布会影响碳材料中活性位点的分布,在掺杂剂附近具有高电荷密度或高自旋密度的碳原子可能成为活性位点 [59]. 2022年,XIE等[60] 基于DFT计算,引入福井函数,用以识别活性位点并系统地研究O掺杂碳材料(OCM)的活性. 结果表明,醚键和羰基可能是2e− ORR的高活性来源. 通过研究不同OG共掺杂的碳材料的2e− ORR活性,XIE等发现不同OG之间具有显著的协同效应. OCM中所有碳原子*OOH吸附游离能的计算表明,除醌外的所有OG的引入都能增加碳材料中2e− ORR活性位点的数量(图9a). 醚键和羰基分别可以引入9个和7个活性位点,相较于其余OG可以更大程度地提高OCM的催化活性. XIE等还绘制的自由能变化图总结了掺杂各种OG对碳材料催化活性的影响,当掺杂羰基或醚键时,自由能变化相对平稳,过电位分别为0.05 V或0.15 V,而当掺杂其他OG时,碳材料的自由能图非常陡峭,过电势至少为0.5 V(图9b),这意味着低过电位的材料会具有更多的活性位点. 最终得到不同OG的催化活性大小如下:醚键>羰基>>羟甲基>羟基≈环氧≈羧基≈醛>内酯≈吡喃>碳≈醌. 这也与Chen[61]等得出的结论:羰基>羧基≈羟基一致.
-
F作为第二周期卤族元素,具有极高的电负性,可以通过诱导相邻的碳极化以产生活性位点,从而增强*OOH在催化剂表面的特异性吸附.2011年,Sheng等[62] 以乙炔黑粉(ABP)为催化剂,聚四氟乙烯(PTFE)为粘结剂,制备了一种新型的乙炔黑-聚四氟乙烯电极,在pH=3,电流密度为 20 mA·cm−2的条件下,H2O2的平均生成速率达到58.9 mg·L−1·h−1·cm−2,电流效率效率高达92.7%,展示出了含F碳材料良好的ORR反应特性.
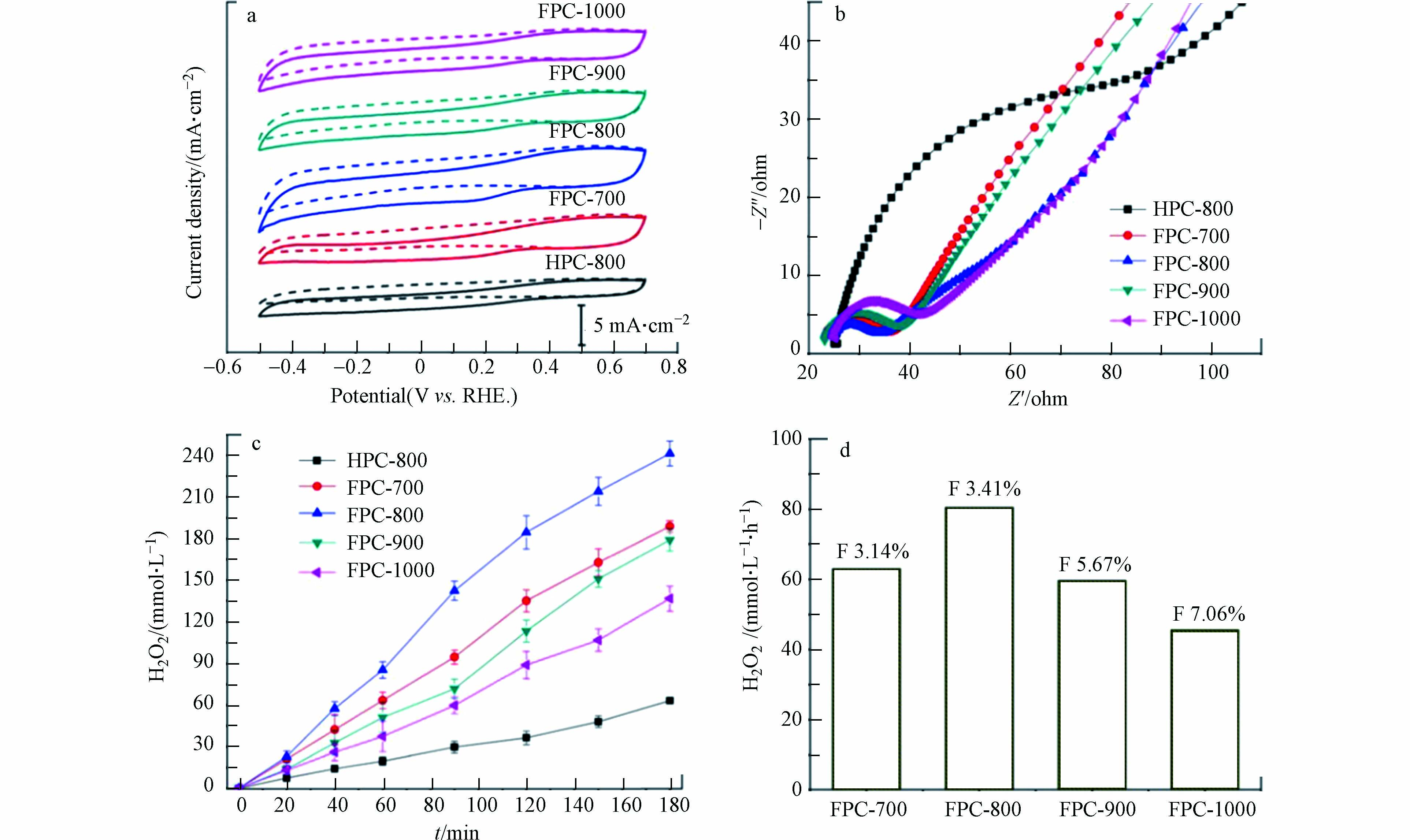
先前有研究表明,F掺杂碳材料主要通过抑制4e− ORR反应来提高2e− ORR合成 H2O2的活性和选择性[48]. 为了进一步讨论不同密度和分布的F掺杂的碳材料对H2O2电合成的活性和选择性的影响,ZHAO等[39]采用铝基MOF(MIL-53(Al))作为前体在不同碳化温度下合成了F掺杂的分级多孔碳(FPC-700、FPC-800、FPC-900、FPC-1000). 将FPC与未掺杂F的碳材料(HPCs)进行比较,发现 FPC在ORR反应中具有更高的电流密度,更高的负起始电位,和更低的电荷转移电阻(图10a、b),FPC-800 270 mV的过电位,也低于HPC-800的311 mV. 这些观察结果表明,将F原子掺入碳框架中可以最大限度地降低电荷转移阻力,提高催化剂活性,加速ORR反应(图10c). FPC-800在pH=1,外加电位为0.2V条件下的H2O2生成速率达到714.1 mmol· (gcat·h)−1,电流效率达82.1%,表现出出色的2e− ORR催化性能.
随着热解温度在700—1000°C范围内变化,F的掺杂含量从3.10%增加到7.06%(图10d). 一般认为,在碳材料中掺杂F原子会破坏碳的电荷均匀性,并为H2O2的产生提供活性位点. 然而,当材料中F原子含量过高时,材料的石墨化程度会随着F原子含量的提高而降低,从而导致电子电导率降低,产生 H2O2的活性降低.
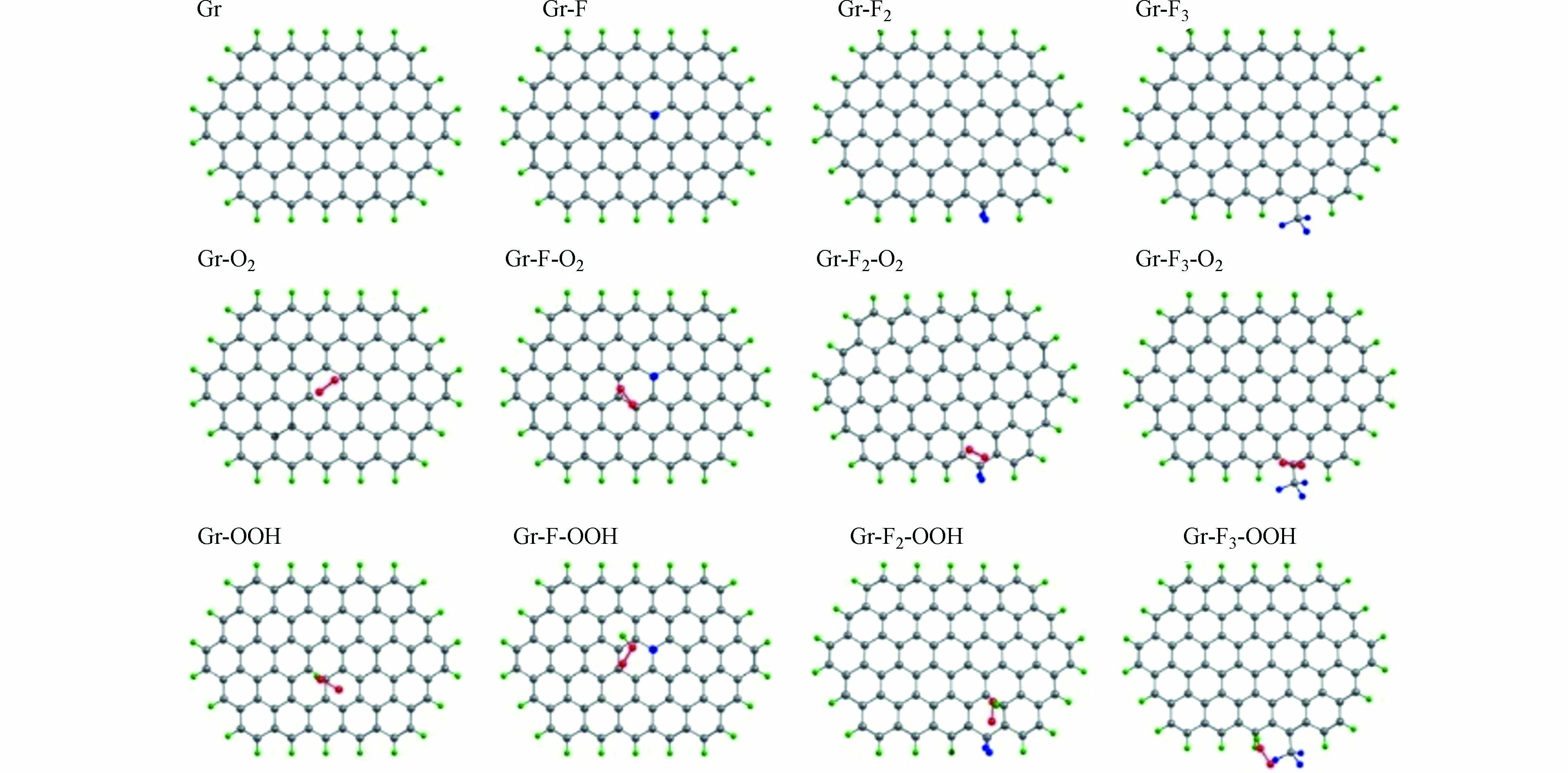
对此,ZHAO等构建了3种不同的F掺杂碳材料(Gr-F、Gr-F2和Gr-F3 )以及未进行F掺杂的纯石墨碳(Gr)模型(图11),确定了反应中间体在不同类型F掺杂碳材料和纯石墨碳表面的吸附能差异. 对比发现,Gr-F、Gr-F2和Gr-F3相比Gr对O2分子具有较弱的吸附能,这有利于O2分子在催化剂表面的活化;Gr-F2具有较弱的OOH结合能,可以促进H2O2的生成. 因此,Gr-F2被认为是最有利于2e− ORR的F掺杂类型.
WANG[63]等采用F改性CNT,以F掺杂碳纳米管(F-CNT)制备的气体扩散电极(GDE)作为阴极生产H2O2. 实验发现,用0.6 mol·L−1 HF溶液制备的F-CNT(CNT-F-0.6)具有更高的电产H2O2浓度和电流效率,分别达到47.6 mg·L−1和89.4%,高于未改性时的的27.6 mg·L−1和70.1%. 与Zhao等的结论一致:WANG等也认为F原子掺杂可以破坏碳的电荷均匀性,促进O2的吸附和*OOH的形成,而CF2与*OOH之间较弱的吸附能又有利于H2O2的形成.
-
由于不同掺杂原子之间存在的协同效应,双杂原子或多杂原子掺杂往往会更大程度地增加催化剂表面的活性位点和2e− ORR选择性,从而起到更好的改性效果. 基于同族元素化学性质相似的特点,近年来出现了许多不同杂原子组合掺杂改性碳材料以合成H2O2的实验,例如B/N[49,64]、N/O[50]、N/S[65-66]、O/F[67]等,它们具有优秀的2e− ORR选择性和较高的H2O2产率,进一步验证了杂原子掺杂改性碳材料具有广阔的应用前景.
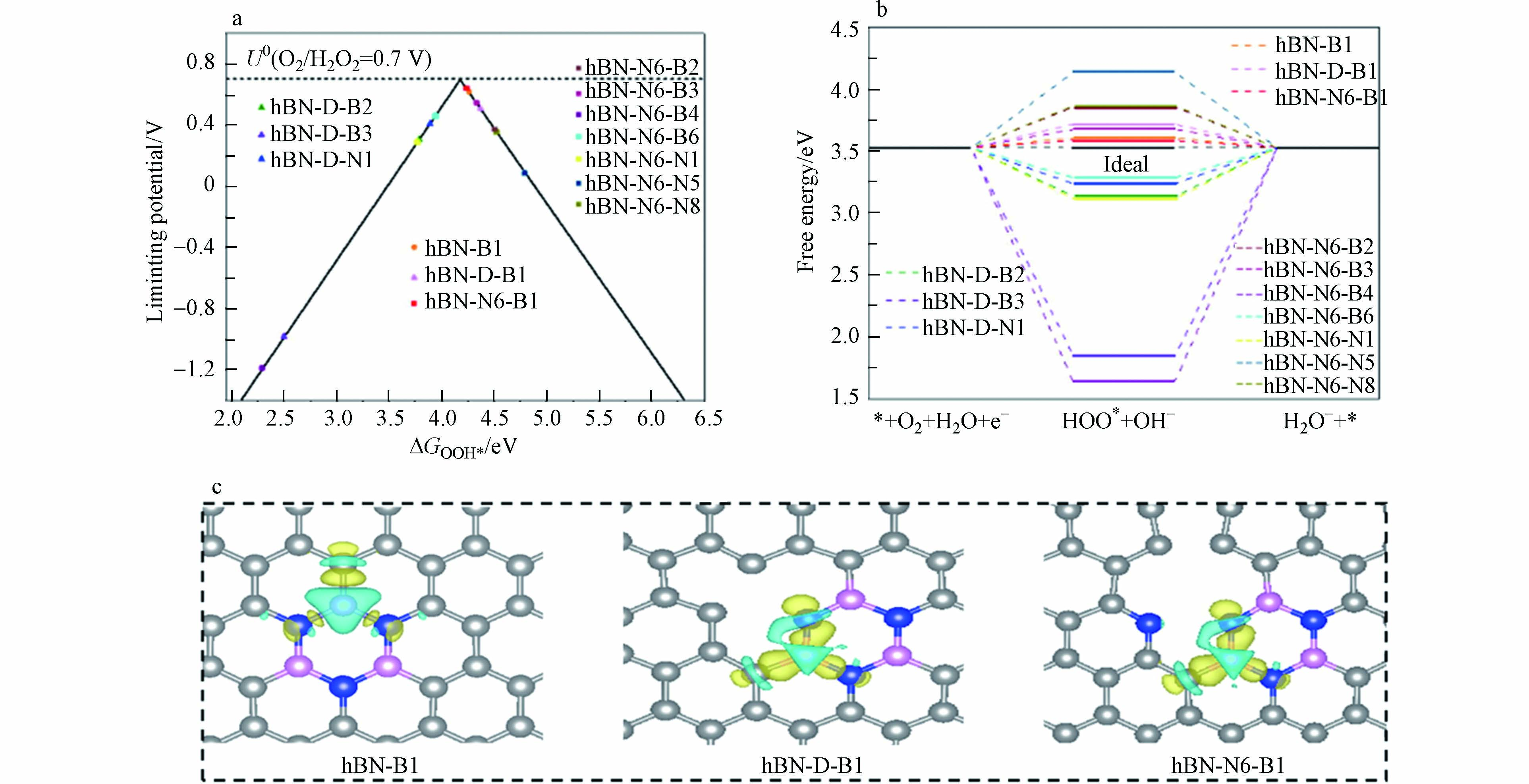
2021年,LI等[49]通过将聚乙烯醇-石墨烯、硼砂和聚苯胺凝胶化后进行热解,成功制备具有可调B、N构型的B/N共掺杂多孔碳气凝胶. H2O2的合成选择性在碱性溶液,在外加正电位(0.6 V vs. RHE)的条件下达到了94.16%,表现出优异的电催化性能. 由于B(2.0)与C(2.5)、N(3.0)原子电负性的差异,使B原子上的电子逐渐向C、N原子积累,这有利于B原子产生空轨道以接受来自HOO*中间体的电子. DFT研究发现,hBN-N6-B1位点具有最低的过电位和最小的自由能势垒(图12a、b),hBN与邻近的吡咯-N位点相结合是进行2e− ORR合成H2O2最活跃的位点(图12c). 与LI等[49]的想法类似,LIU等[64]通过水热和热解工艺设计合成了BCN催化剂,B原子能有效结合N原子,使两种原子进入石墨晶格,取代C原子形成BCN,其在较宽电位范围内的2e− ORR选择性超过70%,可稳定工作12 h.
鉴于N、O原子单独掺杂时均取得了较为良好的效果,QIN等[50]利用己胺(HMT)、聚环氧乙-b-聚环氧丙-b-聚氧化乙二醇三嵌段共聚物(Pluronic F127)和间苯二酚通过高压水热和碳化处理成功制备了N/O共掺杂有序介孔碳材料,其2e− ORR选择性高达95%,在pH=3,外加电位(-0.2 V vs. RHE)的条件下,H2O2合成速率达47.40 mg·L−1·h−1. 研究发现,吡啶N与C—O—C/COOH的结合相比于其他结构大大降低了OOH*的吸附能(图13),从而促进了2e− ORR反应.
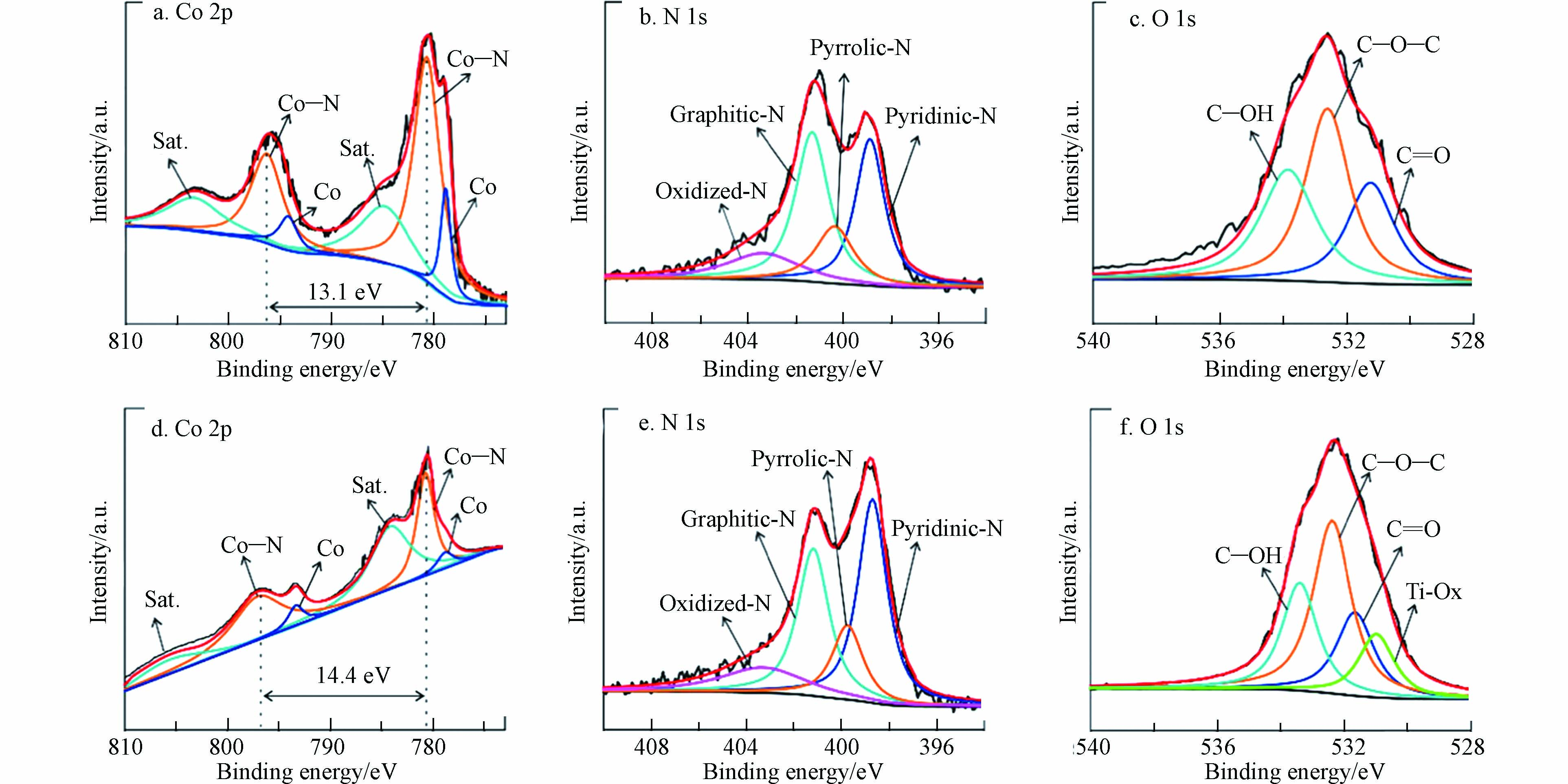
HUANG[68]等以Co—N—C催化剂(ZIF-67)和Ti3C2Tx为前体物,合成了过渡金属碳氮共掺杂碳材料催化剂,在酸性条件下H2O2合成效率达94.1 mmol·L−1·h−1. 光谱分析发现,与ZIF-67相比(13.32%),掺杂Ti的催化剂中Co的含量有所降低(4.78%)(图14a、d),这是由于Ti3C2Tx的层状结构,有利于Co—N—C的均匀分布和去除暴露的Co纳米颗粒[69]. 此外,Co—N—C/Ti3C2Tx的自旋轨道分裂值(ΔCo = 14.4 eV)比Co—N—C(ΔCo = 13.1 eV)大,这表明Co原子的电荷密度状态较低,使其具有合适的*OOH结合能,以促进H2O2的形成. 此外,在Co—N—C/Ti3C2Tx中,吡啶-N占总配位N的主导地位(39.56%),高于Co—N—C(31.86%)(图14b、e), C—O—C比例(8.30%)也高于Co—N—C(1.02%)(图14c、f). C—O—C具有优异的2e− ORR催化活性,XPS结果表明,Ti3C2Tx上的氧官能团可以有效地微调Co原子的电子结构,使其处于较低的电荷密度状态,这将使*OOH具有合适的结合能,从而实现高效电合成H2O2.
除此以外,其他不同类型的杂原子掺杂组合也展现出了良好的应用潜力. 2016年PERAZZOLO等[66]利用二氧化硅作为无机模板,将其和有机前体物(1,10-菲罗啉、吩噻嗪、二苯并噻吩和)溶解与丙酮中,加热后冷却制成了N/S共掺杂介孔碳. 其在氧化还原反应中显示出高达80%的2e− ORR选择性,作为电解池阴极时可更快更有效地降解甲基橙,矿化了53%的TOC. 后来,ZHANG等[70]采用5-氨基-2-巯基-1,3,4-噻二唑(AMT)作为N和S掺杂剂,通过简单的热退火工艺制备了无金属的“石墨烯合金”(NS-rGO). 电化学阻抗谱(EIS)和循环伏安法(CV)结果显示,N和S共掺杂产生的协同效应引入了不成对电子,提供的活性位点增强了H2O2的吸附能力,并将电荷转移电阻从原始的360.1 Ω降低到了17.7 Ω,增强了碳基质的导电性,进一步提高了电子转移效率. 2019年,JASPER等[70]将氮基三乙酸锰前驱体水热合成后热解和酸洗,合成了Mn—N—C纳米棒. 使用旋转环盘电极评估其2e− ORR活性和H2O2选择性发现, Mn—N—C纳米棒不仅在酸性介质中表现出高活性,而且在外加电位(0.5 V vs. RHE)时表现出的H2O2选择性高达98%. 随后使用Mn—N—C纳米棒催化降解亚甲基蓝(MB),将工作电极浸入电解质中以排除吸附影响,通电后观察到MB浓度逐渐明显下降,证实了电催化亚甲基蓝分解的发生. 近期,GU等[67]将MWCNTs分散在H2SO4与HF的混合溶液中,干燥退火后浸泡于聚四氟乙烯合成了新型O/F共掺杂石墨毡气体扩散阴极(GF-GDC) ,在pH=7,20 mA·cm−2 的电流密度下1 h的H2O2累计浓度高达232.2 mg·L−1,电流效率高达83%,且15个周期内电流效率仅降低10%,具有良好的稳定性.
-
相比于国内外目前广泛采用的蒽醌法,电催化2e− ORR是一种有效、绿色、安全制备H2O2的方法,具有良好的应用前景. 不同类型的杂原子掺杂的碳材料可以不同程度地提高碳材料催化剂的活性、2e− ORR选择性、循环稳定性等,是催化ORR合成H2O2的最佳方法之一. 杂原子掺杂碳材料催化能力的提高与掺杂原子类型和形成的掺杂结构密切相关,在众多科研工作者的努力下,不同杂原子的作用机理已经逐步明晰. 其中, N掺杂中形成的吡咯-N、O掺杂中形成的醚键和F掺杂中形成的CF2具有优秀的催化活性,以及多原子掺杂中不同原子之间形成的协同效应,均可以改善催化剂表面活性位点的分布,极大提高材料对O2分子的吸附能力以及对ORR反应中间体*OOH的脱附能力,发挥最佳的电化学催化性能.
与此同时,杂原子掺杂碳材料催化剂还不可避免地存在着一些问题,①不同实验中制备的碳材料催化剂结构复杂,重复性差,理论与实际难以形成一致的结论,增加了探明催化位点对O2作用机理的难度;②催化剂的性能需要进一步提高以满足实际应用;③催化剂的制备方法还需进一步简化,以满足工业领域的大规模生产. ④多杂原子掺杂时对ORR选择性的提升方向具有明显的差异,需进一步加以鉴别. 随着科学技术的不断进步,先进的实验方法和计算手段的加入,杂原子掺杂碳材料催化ORR的反应机理会变得更加明晰, 2e− ORR的催化活性会得到进一步提高,合成后的H2O2可应用于分散式水处理场景,极大地提高污水处理效率. 随着技术和工艺的不断升级,H2O2的处理能力会不断增强,应用领域将不断扩大,这也势必会推动水处理技术的革新与进步.
杂原子掺杂碳材料用于电合成过氧化氢的研究进展
Progress of heteroatom-doped carbon materials for the electro-synthesis of hydrogen peroxide
-
摘要: 过氧化氢(H2O2)可广泛应用于市政饮用水、工业废水和城市污水的处理. 然而,过氧化氢在储存和运输方面的风险(如腐蚀和爆炸)限制了其在分散式水处理中的应用. 以二电子氧还原反应(2e− ORR)原位合成H2O2用于分散式水处理具有良好的应用前景. 然而,未被催化的2e− ORR动力学缓慢、选择性差,不能满足大规模生产的要求. 相比之下,杂原子掺杂碳材料具有良好的2e− ORR活性、选择性和稳定性. 本文回顾了2e− ORR的反应机理和催化剂的改性原理,进一步描述了杂原子N、O、F掺杂碳材料的作用机理,并总结了几种具有优秀2e− ORR催化活性和选择性的掺杂结构. 最后,对杂原子掺杂碳材料合成H2O2应用于水处理的未来发展进行了展望.Abstract: Hydrogen peroxide (H2O2) is widely used in the treatment of municipal drinking water, industrial wastewater and municipal wastewater. However, the application of H2O2 in decentralized water treatment is limited by the risks (e.g., corrosion and explosion) associated with the transport and storage of H2O2 stocks. On-site production of H2O2 from 2-electron oxygen reduction (2e− ORR) is a promising way to supply H2O2 for decentralized water treatment. However, uncatalyzed 2e−ORR has slow kinetics and poor selectivity, and thus cannot meet the requirements of large-scale production. In contrast, heteroatom-doped carbon materials have good 2e− ORR activity, selectivity and stability. This paper reviews the mechanism of 2e− ORR reaction and the modification principle of the catalyst. Further, the mechanism of heteroatom nitrogen, oxygen and fluorine-doped carbon materials is described, and several doped structures with excellent 2e− ORR catalytic activity and selectivity are summarized. Finally, the future development of heteroatom-doped carbon material synthesis H2O2 and its application in water treatment is prospected.
-

-
图 4 (a)G-COF在不同温度处理后不同N掺杂类型的比例及含量;(b)N原子掺杂碳材料的可能机理[42]
Figure 4. (a) Percentages and amount of nitrogen functionality present in catalysts ; (b) Proposed mechanism of electrochemical 2e− ORR on COF-derived N-doped carbon catalysts
图 7 (a)不同官能团的ORR活性,用蓝色圆圈表示的碳原子为活性中心[44];(b)不同OG根据*OOH吸附能合成H2O2的活性火山图[44],包括了Pt–Hg和Pd–Au合金的活性[20].
Figure 7. (a) Different oxygen functional group type configurations examined in this study. The carbon atoms denoted by a blue circle are the active sites under investigation [44]; (b) Calculated two-electron (solid black) ORR-related volcano plot for the electro-reduction of oxygen to H2O2 displayed with the limiting potential plotted as a function of∆ GOOH*[44], including the activity of Pt -- Hg and Pd -- Au alloys[20].
图 8 (a)不同退火时间的催化剂中不同OG的催化活性和比率;(b)不同OG根据*OOH吸附能合成H2O2的活性火山图[57].
Figure 8. (a) Catalytic activity and ratio of the different oxygen-containing functional groups as a function of the series catalyst annealed with different activation time; (b) ORR activity volcano plot of the different oxygen-containing functional groups modified carbon for the synthesis of H2O2 according to the adsorption energy of HOO*[57].
图 10 (a)FPCs和HPC-800在(虚线-Ar)和(实线-O2)中的循环伏安曲线( 扫描速度10 mV·s−1 ,0.05 mol·L−1 H2SO4);(b)FPCs和HPC-800在0.05 mol·L−1H2SO4中的EIS数据(交流电压为5 mV,频率范围在100 kHz—0.1 Hz);(c)FPCs和HPC-800 的H2O2产量随时间的变化;(d)FPCs在不同F掺杂含量下H2O2的生成速率[39].
Figure 10. (a) CV curves of FPCs and HPC-800 in (Ar- dash line) or (O2-saturated solid line) 0.05 mol·L−1 H2SO4 solution with a scan rate of 10 mV·s−1; (b) EIS data of FPCs and HPC-800 in O2-saturated 0.05 mol·L−1 H2SO4 solution with an AC of 5 mV and a frequency range between 100 kHz and 0.1 Hz; (c) The concentration of H2O2 produced by FPCs and HPC-800 obtained at different carbonization temperature; (d) H2O2 production ration with F content [39]
图 12 (a) 不同B/N构型根据*OOH吸附能的2e− ORR活性火山图;(b)标准电位下O2还原成H2O2自由能变化图;(c) hBN-B1、hBN-D-B1 和 hBN-N6-B1 配置的电荷密度差,黄色和青色区域分别代表电子积累和耗尽[49].
Figure 12. (a) The calculated oxygen reduction volcano plot for the two-electron reduction of O2, with the limiting potential plotted as a function of ΔGHOO*; (b) The free energy diagram of O2 reduction to H2O2 at the standard potential (U = 0 V); (c) Charge density difference of hBN-B1, hBN-D-B1, and hBN-N6-B1 configurations. Yellow and cyan regions represent electron accumulation and depletion, respectively[49].
图 14 (a, d) Co—N—C 和 Co—N—C /Ti3C2Tx 的 Co 2p 光谱;(b, e)Co—N—C 和 Co—N—C / Ti3C2Tx的 N 1s 光谱;(c,f)Co—N—C 和 Co—N—C /Ti 3C2Tx的 O 1s 光谱[68].
Figure 14. (a, d) Co 2p spectra of Co—N—C and Co—N—C /Ti3C2Tx. (b, e) N 1s spectra of Co—N—C and Co—N—C /Ti3C2Tx; (c, f) O 1s spectra of Co—N—C and Co—N—C /Ti3C2Tx[68].
表 1 部分杂原子掺杂碳材料催化剂电合成H2O2的性能
Table 1. Performance of partially heteroatom-doped carbon material catalysts for the electro-synthesis of H2O2
掺杂类型
Doping type催化剂
Catalyst电解液
Electrolyte电位/V
Potential(vs. RHE)合成速率/ mmol·(gcat·h)−1
Synthesis rate选择性/%
Selectivity参考文献
ReferenceN掺杂 G-COF-950 0.1 mol·L−1KOH 0.1 1286.9 69.8(FE) [42] PEI50CMK3-800T 0.5 mol·L−1 H2SO4 0.35 570.1① 98.5 [43] CNT-M-15T 0.1 mol·L−1 KOH 0.6 — 93.5 [44] rGO-U-25T 0.1 mol·L−1 KOH 0.6 — 98.35 [44] 空心N掺杂碳球 0.1 mol·L−1 KOH 0.7 618.5 91.9,85.1(FE) [45] O掺杂 F-mrGO(600) 0.1 mol·L−1 KOH 0.75 770 接近100 [46] O-CNT 0.1 mol·L−1 KOH 0.65 — 约90 [35] 石墨毡 0.05 mol·L−1 Na2SO4 0.4 比未改性提高6倍 — [47] F掺杂 FPC-800 0.05 mol·L−1 H2SO4 0.2 714.1 82.1(CE) [39] CNT-F-0.6 0.6 mol·L−1 HF -0.323 — 89.5(CE) [48] B/N共掺杂 B/N-C-2 1 mol·L−1 KOH 0.82 — 94.16 [49] N/O共掺杂 O/N-OMC-700 0.05 mol·L−1 H2SO4 0.4 47.40 mg·L−1·h−1② 95 [50] Mn/N共掺杂 Mn-N-C纳米棒 0.1 mol·L−1 HClO4 0.5 — 98 [51] 注:①是在中性条件下取得的;②是在−0.2 V(vs. RHE)下取得的;FE表示法拉第效率;CE表示电流效率.
Note: ① is obtained under neutral conditions; ② is obtained at −0.2 V (vs. RHE); FE denotes Faraday efficiency; CE denotes current efficiency -
[1] MYERS R L. The 100 most important chemical compounds a reference guide[M]. Westport, Conn. : Greenwood Press, 2007 [2] CAMPOS-MARTIN J M, BLANCO-BRIEVA G, FIERRO J L G. Hydrogen peroxide synthesis: An outlook beyond the anthraquinone process [J]. Angewandte Chemie International Edition, 2006, 45(42): 6962-6984. doi: 10.1002/anie.200503779 [3] 刘淼, 钱美荣, 苏荣梅, 等. 羟基自由基氧化处理垃圾渗滤液中高浓度氨氮 [J]. 水处理技术, 2007, 33(11): 79-81,88. doi: 10.16796/j.cnki.1000-3770.2007.11.022 LIU M, QIAN M R, SU R M, et al. High strength nh3-n removal in landfill leachate by hydroxy radical oxidation [J]. Technology of Water Treatment, 2007, 33(11): 79-81,88(in Chinese). doi: 10.16796/j.cnki.1000-3770.2007.11.022
[4] DOLL M, MORGAN D J, ANDERSON D, et al. Touchless technologies for decontamination in the hospital: A review of hydrogen peroxide and UV devices [J]. Current Infectious Disease Reports, 2015, 17(9): 498. [5] 潘智勇, 邢定峰. 过氧化氢市场现状和技术发展趋势 [J]. 现代化工, 2021, 41(4): 11-16. doi: 10.16606/j.cnki.issn0253-4320.2021.04.003 PAN Z Y, XING D F. Market status and technology development trend of hydrogen peroxide [J]. Modern Chemical Industry, 2021, 41(4): 11-16(in Chinese). doi: 10.16606/j.cnki.issn0253-4320.2021.04.003
[6] ZHANG Z H, MENG H S, WANG Y J, et al. Fabrication of graphene@graphite-based gas diffusion electrode for improving H2O2 generation in Electro-Fenton process [J]. Electrochimica Acta, 2018, 260: 112-120. doi: 10.1016/j.electacta.2017.11.048 [7] 陈君伟, 孙绍晖. 工业制过氧化氢的研究进展 [J]. 河南化工, 2017, 34(12): 12-17. doi: 10.14173/j.cnki.hnhg.2017.12.004 CHEN J W, SUN S H. Research progress of industrial hydrogen peroxide [J]. Henan Chemical Industry, 2017, 34(12): 12-17(in Chinese). doi: 10.14173/j.cnki.hnhg.2017.12.004
[8] 何峰, 张静静, 陈奕君, 等. 电化学氧还原反应合成H2O2碳基催化剂研究进展 [J]. 储能科学与技术, 2021, 10(6): 1963-1976. HE F, ZHANG J J, CHEN Y J, et al. Recent progress on carbon-based catalysts for electrochemical synthesis of H2O2 via oxygen reduction reaction [J]. Energy Storage Science and Technology, 2021, 10(6): 1963-1976(in Chinese).
[9] CHAI G L, HOU Z F, IKEDA T, et al. Two-electron oxygen reduction on carbon materials catalysts: Mechanisms and active sites [J]. The Journal of Physical Chemistry C, 2017, 121(27): 14524-14533. doi: 10.1021/acs.jpcc.7b04959 [10] 闫啸, 石文武, 王新中. 碳基电催化材料选择性合成过氧化氢研究进展 [J]. 新型炭材料, 2022, 37(1): 223-236. doi: 10.1016/S1872-5805(22)60582-1 YAN X, SHI W W, WANG X Z. Carbon based electrocatalysts for selective hydrogen peroxide conversion [J]. New Carbon Materials, 2022, 37(1): 223-236(in Chinese). doi: 10.1016/S1872-5805(22)60582-1
[11] ZHOU Y, CHEN G, ZHANG J J. A review of advanced metal-free carbon catalysts for oxygen reduction reactions towards the selective generation of hydrogen peroxide [J]. Journal of Materials Chemistry A, 2020, 8(40): 20849-20869. doi: 10.1039/D0TA07900F [12] BU Y F, WANG Y B, HAN G F, et al. Carbon-based electrocatalysts for efficient hydrogen peroxide production [J]. Advanced Materials, 2021, 33(49): e2103266. doi: 10.1002/adma.202103266 [13] VERDAGUER-CASADEVALL A, DEIANA D, KARAMAD M, et al. Trends in the electrochemical synthesis of H2O2: Enhancing activity and selectivity by electrocatalytic site engineering [J]. Nano Letters, 2014, 14(3): 1603-1608. doi: 10.1021/nl500037x [14] FORTUNATO G V, PIZZUTILO E, MINGERS A M, et al. Impact of palladium loading and interparticle distance on the selectivity for the oxygen reduction reaction toward hydrogen peroxide [J]. The Journal of Physical Chemistry C, 2018, 122(28): 15878-15885. doi: 10.1021/acs.jpcc.8b04262 [15] LEDENDECKER M, PIZZUTILO E, MALTA G, et al. Isolated Pd sites as selective catalysts for electrochemical and direct hydrogen peroxide synthesis [J]. ACS Catalysis, 2020, 10(10): 5928-5938. doi: 10.1021/acscatal.0c01305 [16] LEE S, CHUNG Y M. Direct synthesis of H2O2 over acid-treated Pd/C catalyst derived from a Pd-Co core-shell structure [J]. Catalysis Today, 2020, 352: 270-278. doi: 10.1016/j.cattod.2019.09.038 [17] CHOI C H, KWON H C, YOOK S, et al. Hydrogen peroxide synthesis via enhanced two-electron oxygen reduction pathway on carbon-coated Pt surface [J]. The Journal of Physical Chemistry C, 2014, 118(51): 30063-30070. doi: 10.1021/jp5113894 [18] CHOI C H, KIM M, KWON H C, et al. Tuning selectivity of electrochemical reactions by atomically dispersed platinum catalyst [J]. Nature Communications, 2016, 7: 10922. doi: 10.1038/ncomms10922 [19] PIZZUTILO E, KASIAN O, CHOI C H, et al. Electrocatalytic synthesis of hydrogen peroxide on Au-Pd nanoparticles: From fundamentals to continuous production [J]. Chemical Physics Letters, 2017, 683: 436-442. doi: 10.1016/j.cplett.2017.01.071 [20] SIAHROSTAMI S, VERDAGUER-CASADEVALL A, KARAMAD M, et al. Enabling direct H2O2 production through rational electrocatalyst design [J]. Nature Materials, 2013, 12(12): 1137-1143. doi: 10.1038/nmat3795 [21] 周伟. H2O2的电催化合成及·OH产用效能提升[D]. 哈尔滨: 哈尔滨工业大学, 2019. ZHOU W. H2O2 electrocatalytic synthesis and the enhancement of generation and utilization efficiency of ·OH[D]. Harbin: Harbin Institute of Technology, 2019(in Chinese).
[22] LIU H, CHENG S A, LOGAN B E. Power generation in fed-batch microbial fuel cells as a function of ionic strength, temperature, and reactor configuration [J]. Environmental Science & Technology, 2005, 39(14): 5488-5493. [23] da POZZO A, PALMA L D, MERLI C, et al. An experimental comparison of a graphite electrode and a gas diffusion electrode for the cathodic production of hydrogen peroxide [J]. Journal of Applied Electrochemistry, 2005, 35(4): 413-419. doi: 10.1007/s10800-005-0800-2 [24] SALARI D, NIAEI A, KHATAEE A, et al. Electrochemical treatment of dye solution containing C. I. Basic Yellow 2 by the peroxi-coagulation method and modeling of experimental results by artificial neural networks [J]. Journal of Electroanalytical Chemistry, 2009, 629(1/2): 117-125. [25] ÖZCAN A, ŞAHIN Y, SAVAŞ KOPARAL A, et al. Carbon sponge as a new cathode material for the electro-Fenton process: Comparison with carbon felt cathode and application to degradation of synthetic dye basic blue 3 in aqueous medium [J]. Journal of Electroanalytical Chemistry, 2008, 616(1/2): 71-78. [26] WANG A M, QU J H, RU J, et al. Mineralization of an azo dye Acid Red 14 by electro-Fenton's reagent using an activated carbon fiber cathode [J]. Dyes and Pigments, 2005, 65(3): 227-233. doi: 10.1016/j.dyepig.2004.07.019 [27] SANG Z Y, HOU F, WANG S H, et al. Research progress on carbon-based non-metallic nanomaterials as catalysts for the two-electron oxygen reduction for hydrogen peroxide production [J]. New Carbon Materials, 2022, 37(1): 136-151. doi: 10.1016/S1872-5805(22)60583-3 [28] YU F K, ZHOU M H, YU X M. Cost-effective electro-Fenton using modified graphite felt that dramatically enhanced on H2O2 electro-generation without external aeration [J]. Electrochimica Acta, 2015, 163: 182-189. doi: 10.1016/j.electacta.2015.02.166 [29] SHENG Y P, ZHAO Y, WANG X L, et al. Electrogeneration of H2O2 on a composite acetylene black-PTFE cathode consisting of a sheet active core and a dampproof coating [J]. Electrochimica Acta, 2014, 133: 414-421. doi: 10.1016/j.electacta.2014.04.071 [30] YANG W L, ZHOU M H, CAI J, et al. Ultrahigh yield of hydrogen peroxide on graphite felt cathode modified with electrochemically exfoliated graphene [J]. Journal of Materials Chemistry A, 2017, 5(17): 8070-8080. doi: 10.1039/C7TA01534H [31] FELLINGER T P, HASCHÉ F, STRASSER P, et al. Mesoporous nitrogen-doped carbon for the electrocatalytic synthesis of hydrogen peroxide [J]. Journal of the American Chemical Society, 2012, 134(9): 4072-4075. doi: 10.1021/ja300038p [32] LEE Y H, LI F, CHANG K H, et al. Novel synthesis of N-doped porous carbons from collagen for electrocatalytic production of H2O2 [J]. Applied Catalysis B:Environmental, 2012, 126: 208-214. doi: 10.1016/j.apcatb.2012.06.031 [33] ZHONG R S, QIN Y H, NIU D F, et al. Effect of carbon nanofiber surface functional groups on oxygen reduction in alkaline solution [J]. Journal of Power Sources, 2013, 225: 192-199. doi: 10.1016/j.jpowsour.2012.10.043 [34] HASCHÉ F, OEZASLAN M, STRASSER P, et al. Electrocatalytic hydrogen peroxide formation on mesoporous non-metal nitrogen-doped carbon catalyst [J]. Journal of Energy Chemistry, 2016, 25(2): 251-257. doi: 10.1016/j.jechem.2016.01.024 [35] LU Z Y, CHEN G X, SIAHROSTAMI S, et al. High-efficiency oxygen reduction to hydrogen peroxide catalysed by oxidized carbon materials [J]. Nature Catalysis, 2018, 1(2): 156-162. doi: 10.1038/s41929-017-0017-x [36] ZHOU L, ZHOU M H, ZHANG C, et al. Electro-Fenton degradation of p-nitrophenol using the anodized graphite felts [J]. Chemical Engineering Journal, 2013, 233: 185-192. doi: 10.1016/j.cej.2013.08.044 [37] MIAO J, ZHU H, TANG Y, et al. Graphite felt electrochemically modified in H2SO4 solution used as a cathode to produce H2O2 for pre-oxidation of drinking water [J]. Chemical Engineering Journal, 2014, 250: 312-318. doi: 10.1016/j.cej.2014.03.043 [38] ZHAO K, QUAN X, CHEN S, et al. Enhanced electro-Fenton performance by fluorine-doped porous carbon for removal of organic pollutants in wastewater [J]. Chemical Engineering Journal, 2018, 354: 606-615. doi: 10.1016/j.cej.2018.08.051 [39] ZHAO K, SU Y, QUAN X, et al. Enhanced H2O2 production by selective electrochemical reduction of O2 on fluorine-doped hierarchically porous carbon [J]. Journal of Catalysis, 2018, 357: 118-126. doi: 10.1016/j.jcat.2017.11.008 [40] LIU L, ZHANG J, MA W J, et al. Co/N co-doped graphene-like nanocarbon for highly efficient oxygen reduction electrocatalyst [J]. Science China Materials, 2019, 62(3): 359-367. doi: 10.1007/s40843-018-9322-y [41] ZHANG W M, CHU J J, LI S F, et al. CoNxC active sites-rich three-dimensional porous carbon nanofibers network derived from bacterial cellulose and bimetal-ZIFs as efficient multifunctional electrocatalyst for rechargeable Zn-air batteries [J]. Journal of Energy Chemistry, 2020, 51: 323-332. doi: 10.1016/j.jechem.2020.04.067 [42] ZHANG J Y, ZHANG G, JIN S Y, et al. Graphitic N in nitrogen-Doped carbon promotes hydrogen peroxide synthesis from electrocatalytic oxygen reduction [J]. Carbon, 2020, 163: 154-161. doi: 10.1016/j.carbon.2020.02.084 [43] SUN Y Y, LI S, JOVANOV Z P, et al. Structure, activity, and faradaic efficiency of nitrogen-doped porous carbon catalysts for direct electrochemical hydrogen peroxide production [J]. ChemSusChem, 2018, 11(19): 3388-3395. doi: 10.1002/cssc.201801583 [44] WAN J, ZHANG G Q, JIN H R, et al. Microwave-assisted synthesis of well-defined nitrogen doping configuration with high centrality in carbon to identify the active sites for electrochemical hydrogen peroxide production [J]. Carbon, 2022, 191: 340-349. doi: 10.1016/j.carbon.2022.01.061 [45] HU Y Z, ZHANG J J, SHEN T, et al. Efficient electrochemical production of H2O2 on hollow N-doped carbon nanospheres with abundant micropores [J]. ACS Applied Materials & Interfaces, 2021, 13(25): 29551-29557. [46] KIM H W, ROSS M B, KORNIENKO N, et al. Efficient hydrogen peroxide generation using reduced graphene oxide-based oxygen reduction electrocatalysts [J]. Nature Catalysis, 2018, 1(4): 282-290. doi: 10.1038/s41929-018-0044-2 [47] MATSUBARA K, WAKI K. The effect of O-functionalities for the electrochemical reduction of oxygen on MWCNTs in acid media [J]. Electrochemical and Solid-State Letters, 2010, 13(8): F7. doi: 10.1149/1.3428472 [48] SUN X J, SONG P, ZHANG Y W, et al. A class of high performance metal-free oxygen reduction electrocatalysts based on cheap carbon blacks [J]. Scientific Reports, 2013, 3: 2505. doi: 10.1038/srep02505 [49] LI X Y, WANG X P, XIAO G Z, et al. Identifying active sites of boron, nitrogen co-doped carbon materials for the oxygen reduction reaction to hydrogen peroxide [J]. Journal of Colloid and Interface Science, 2021, 602: 799-809. doi: 10.1016/j.jcis.2021.06.068 [50] QIN M C, FAN S Y, WANG L, et al. Oxygen and nitrogen co-doped ordered mesoporous carbon materials enhanced the electrochemical selectivity of O2 reduction to H2O2 [J]. Journal of Colloid and Interface Science, 2020, 562: 540-549. doi: 10.1016/j.jcis.2019.11.080 [51] BIEMOLT J, van der VEEN K, GEELS N J, et al. Efficient oxygen reduction to H2O2 in highly porous Manganese and nitrogen co-doped carbon nanorods enabling electro-degradation of bulk organics [J]. Carbon, 2019, 155: 643-649. doi: 10.1016/j.carbon.2019.09.034 [52] 魏家崴, 李平, 强富强, 等. 杂原子掺杂碳材料用于氧还原反应催化剂的研究 [J]. 功能材料, 2021, 52(2): 2098-2108. WEI J W, LI P, QIANG F Q, et al. Review of heteroatom-doped carbon materials as catalysts for oxygen reduction reaction [J]. Journal of Functional Materials, 2021, 52(2): 2098-2108(in Chinese).
[53] 李宇明, 刘梓烨, 张启扬, 等. 氮掺杂碳材料的制备及其在催化领域中的应用 [J]. 化工学报, 2021, 72(8): 3919-3932. LI Y M, LIU Z Y, ZHANG Q Y, et al. Preparation of nitrogen-doped carbon materials and their applications in catalysis [J]. CIESC Journal, 2021, 72(8): 3919-3932(in Chinese).
[54] GUO D H, SHIBUYA R, AKIBA C, et al. Active sites of nitrogen-doped carbon materials for oxygen reduction reaction clarified using model catalysts [J]. Science, 2016, 351(6271): 361-365. doi: 10.1126/science.aad0832 [55] XING T, ZHENG Y, LI L H, et al. Observation of active sites for oxygen reduction reaction on nitrogen-doped multilayer graphene [J]. ACS Nano, 2014, 8(7): 6856-6862. doi: 10.1021/nn501506p [56] SCARDAMAGLIA M, SUSI T, STRUZZI C, et al. Spectroscopic observation of oxygen dissociation on nitrogen-doped graphene [J]. Scientific Reports, 2017, 7: 7960. doi: 10.1038/s41598-017-08651-1 [57] WANG W, HU Y C, LIU Y C, et al. Self-powered and highly efficient production of H2O2 through a Zn-air battery with oxygenated carbon electrocatalyst [J]. ACS Applied Materials & Interfaces, 2018, 10(38): 31855-31859. [58] WANG Y W, QIU W J, SONG E H, et al. Adsorption-energy-based activity descriptors for electrocatalysts in energy storage applications [J]. National Science Review, 2017, 5(3): 327-341. [59] GONG K P, DU F, XIA Z H, et al. Nitrogen-doped carbon nanotube arrays with high electrocatalytic activity for oxygen reduction [J]. Science, 2009, 323(5915): 760-764. doi: 10.1126/science.1168049 [60] XIE L, ZHOU W, QU Z B, et al. Understanding the activity origin of oxygen-doped carbon materials in catalyzing the two-electron oxygen reduction reaction towards hydrogen peroxide generation [J]. Journal of Colloid and Interface Science, 2022, 610: 934-943. doi: 10.1016/j.jcis.2021.11.144 [61] CHEN S Y, LUO T, CHEN K J, et al. Chemical identification of catalytically active sites on oxygen-doped carbon nanosheet to decipher the high activity for electro-synthesis hydrogen peroxide [J]. Angewandte Chemie, 2021, 60(30): 16607-16614. doi: 10.1002/anie.202104480 [62] SHENG Y P, SONG S L, WANG X L, et al. Electrogeneration of hydrogen peroxide on a novel highly effective acetylene black-PTFE cathode with PTFE film [J]. Electrochimica Acta, 2011, 56(24): 8651-8656. doi: 10.1016/j.electacta.2011.07.069 [63] WANG W, LU X Y, SU P, et al. Enhancement of hydrogen peroxide production by electrochemical reduction of oxygen on carbon nanotubes modified with fluorine [J]. Chemosphere, 2020, 259: 127423. doi: 10.1016/j.chemosphere.2020.127423 [64] LIU Z M, GAO D Z, HU L N, et al. Metal-free boron-rich borocarbonitride catalysts for high-efficient oxygen reduction to produce hydrogen peroxide [J]. ChemistrySelect, 2022, 7(5): e202104203. [65] PARSE H, PATIL I M, SWAMI A S, et al. TiO2-decorated titanium carbide MXene co-doped with nitrogen and sulfur for oxygen electroreduction [J]. ACS Applied Nano Materials, 2021, 4(2): 1094-1103. doi: 10.1021/acsanm.0c02695 [66] PERAZZOLO V, DURANTE C, GENNARO A. Nitrogen and sulfur doped mesoporous carbon cathodes for water treatment [J]. Journal of Electroanalytical Chemistry, 2016, 782: 264-269. doi: 10.1016/j.jelechem.2016.10.037 [67] GU Y Y, FU H J, HUANG Z W, et al. O/F co-doped CNTs promoted graphite felt gas diffusion cathode for highly efficient and durable H2O2 evolution without aeration [J]. Journal of Cleaner Production, 2022, 341: 130799. doi: 10.1016/j.jclepro.2022.130799 [68] HUANG X, LIU W, ZHANG J J, et al. Coupling Co-N-C with MXenes yields highly efficient catalysts for H 2 O2 production in acidic media [J]. ACS Applied Materials & Interfaces, 2022, 14(9): 11350-11358. [69] CHEN J N, YUAN X L, LYU F L, et al. Integrating MXene nanosheets with cobalt-tipped carbon nanotubes for an efficient oxygen reduction reaction [J]. Journal of Materials Chemistry A, 2019, 7(3): 1281-1286. doi: 10.1039/C8TA10574J [70] ZHANG T T, LI C, GU Y, et al. Fabrication of novel metal-free “graphene alloy” for the highly efficient electrocatalytic reduction of H2O2 [J]. Talanta, 2017, 165: 143-151. doi: 10.1016/j.talanta.2016.12.018 -



 下载:
下载: