-
铬离子主要是通过铬盐生产行业及相关产业排放的废渣和废水流入环境中而引起污染. 其中的Cr(Ⅵ)具有强烈的毒性,可能造成遗传性基因缺陷,吸入可能致癌等,对环境危害极大并具有持久危险性[1]. 因此,Cr(Ⅵ)的污染治理已经引起研究者广泛关注. 对废水重金属铬污染的治理方法一般采用化学沉淀法[2]、氧化还原法[3]、离子交换法[4]和吸附法[5]等. 其中吸附法是使用较多的一种方法,而所选用的吸附剂的种类也很多,常用的有活性炭[1]、天然有机吸附剂[6]、无机吸附剂[7]和合成吸附剂[8]. 工业上最常用的吸附剂是活性氧化铝[9]、硅胶[10]、活性炭[11]和分子筛[12]. 在这种情况下,活性氧化铝因其对重金属离子的强亲和力而被认为是一种有前途的吸附剂. 一般来说,这些材料要么在其框架内提供大量官能团(如石墨烯氧化物和其他活性炭材料),要么晶格空位(如金属氧化物)可以有效去除废水中的污染物[13]. 废水中污染物富集的适宜材料应满足三个特点:(1)去除率快,对污染物的富集能力强;(2)环保、成本低;(3)结构稳定,可重复使用. 金属氧化物可以具备这些特性,各种金属氧化物由于其抗磨损的机械坚固性已被应用于废水中的污染物去除. Drisko等[14]发现,不同的大孔尺寸和形态的分层结构锆钛氧化物会极大地影响表面可进入性,从而影响扩散速率和U(Ⅵ)离子的空间容量. 为了提高材料的吸附速率和吸附容量,新的合成方法有望同时控制微/大孔特性(即孔体积和比表面积). 鉴于此,金属有机骨架(metal-organic frameworks, MOFs)合成金属氧化物为以简单、可控的方式合成定制功能材料提供了很大的可能性[15]. MOFs由与有机配体结合的金属离子簇或链组成[16],是一类具有超高比表面积和可调节孔径的新兴材料. MOFs经热煅烧后可生成孔隙均匀、比表面积高、结构有序的金属氧化物[17]. MOFs衍生的金属氧化物在电催化[18]和能量储存/转换[19]等方面都有很好的应用前景. 然而,目前废水中污染物的固定化应用还很少.
本研究针以MOFs为前驱体,在有氧条件下煅烧制备了多孔掺碳Al2O3材料,使用扫描电极(SEM)、X射线衍射仪(XRD)和孔隙度分析仪(BET)对该材料煅烧前后的表面形貌进行了表征分析,通过考察吸附剂投加量、初始浓度和共存阴离子等参数的影响分析其对水体中Cr(Ⅵ)的吸附能力,利用等温吸附模型和吸附动力学模型分析,揭示多孔掺碳Al2O3材料对水中Cr(Ⅵ)的去除提供新的途径.
-
九水合硝酸铝(Al(NO3)3•9H2O)、N,N-二甲基甲酰胺(C3H7NO,DMF)、盐酸(HCl)、重铬酸钾(K2Cr2O7)、氯化钠(NaCl)和氢氧化钠(NaOH)由成都科隆化工有限公司提供;迈瑞尔有限公司生产的氨基对苯二甲酸(C8H7NO4),所有的试剂均为分析纯且没有经过纯化处理.
-
a) 采用溶剂热法合成了NH2-MIL-53(Al)纳米晶体,将3.751 g九水合硝酸铝、1.81 g氨基对苯二甲酸和150 mL DMF加入到200 mL聚四氟乙烯内衬反应器中并进行搅拌,在150 ℃下反应24 h,冷却至室温,以10000 r·min−1高速离心分离得到固体产物. 将得到的固体产物在150 ℃下加入150 mL DMF活化12 h,再次高速离心分离得到黄色固体产物,用纯水反复洗涤3次,在60 ℃下真空干燥,得到黄色固体NH2-MIL-53(Al).
b) 将上述得到的NH2-MIL-53(Al)使用马弗炉在600 ℃下煅烧6 h,得到淡黄色多孔掺碳Al2O3粉末材料.
-
采用扫描电子显微镜(德国的 ZEISS Sigma 300)观察材料的表面形貌. 粉末X射线衍射图(PXRD)记录在Bruker AXS D8-ADVANCE衍射仪上,使用经过滤波的CuKα辐射源,工作在40 kV和30 mA,扫描速率为5 min−1. 使用美国的Micromeritics ASAP 2460全自动快速比表面与孔隙度分析仪对材料的孔径结构进行表征.
-
通过吸附平衡法测定多孔掺碳Al2O3对Cr(Ⅵ)的吸附等温线和动力学参数,取一定量的吸附剂加入到100 mL的不同浓度的重铬酸钾溶液中,振荡一定时间后过滤,通过二苯碳酰二肼分光光度法在波长540 nm处进行分光光度测定Cr(Ⅵ)的浓度. 根据式(1)计算吸附剂对Cr(Ⅵ)的吸附容量Qe(mg·g−1).
式中,C0 为Cr(Ⅵ)离子的初始浓度,mg·L−1;Ci 为吸附后剩余的Cr(Ⅵ)离子浓度,mg·L−1;V 为溶液体积L;m 为吸附剂的质量,g.
-
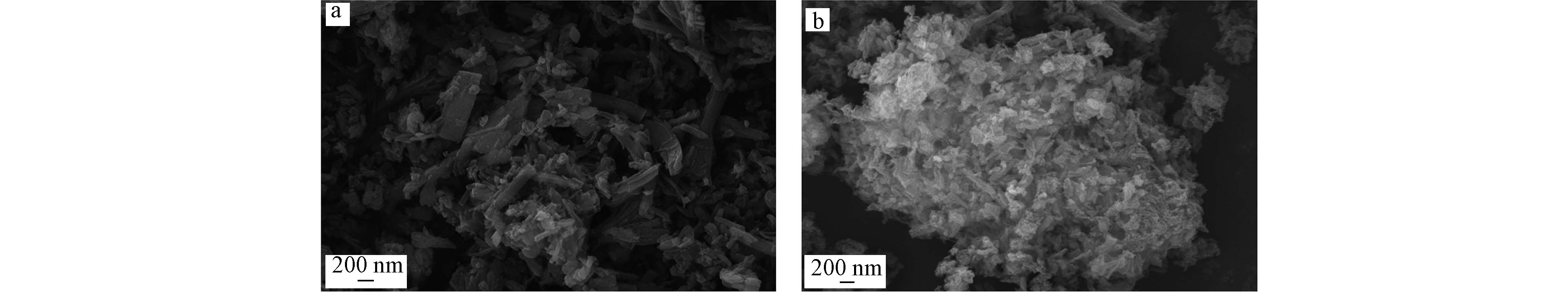
从图1a中可以观察到,NH2-MIL-53(Al)表面粗糙,同时在其表面上覆盖着不规则的长矩形片状结构,其由聚集的纳米晶体组成,层叠状堆聚,煅烧后的多孔掺碳Al2O3材料(图1b)总体结构与形貌与NH2-MIL-53(Al)类似,呈层叠状堆聚,但很明显看出其表面要较煅烧前的NH2-MIL-53(Al)材料更加粗糙,覆盖表面的片状结构变成絮状结构.
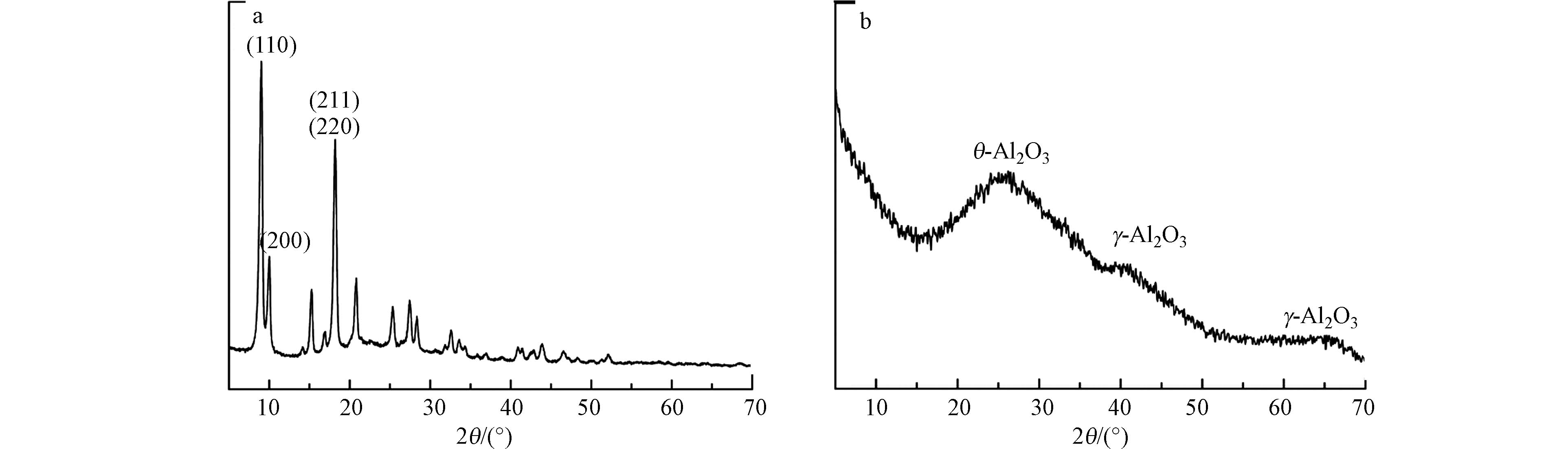
通过XRD测定了制备的NH2-MIL-53(Al)和多孔掺碳Al2O3材料的化学组成和晶体结构(图2). NH2-MIL-53(Al)的XRD谱图可以看出制备样品有明显衍射峰且特征峰形尖锐,表明结晶度良好. 在2θ=8.5°时,存在(110)峰,单峰宽度对应于(211)和(220)的反射,其峰值较高,说明其晶体尺寸大. 由图可以看出,NH2-MIL-53(Al)材料的衍射峰与Qin等 [20]研究结果相吻合.
在图2可以看出,煅烧后的样品没有明显的衍射峰,表明样品以非晶形式存在,在20°和36°附近没有峰,说明不存在对应的α-Al2O3[21]. 2θ=26.3处的峰与θ-Al2O3有关,2θ=41.1°和65.2°处的板状峰是γ-Al2O3的特征[22]. 根据这些峰的位置和形状,可以得出多孔掺碳Al2O3材料由非晶态氧化铝基体中的θ-Al2O3和γ-Al2O3晶粒混合组成.
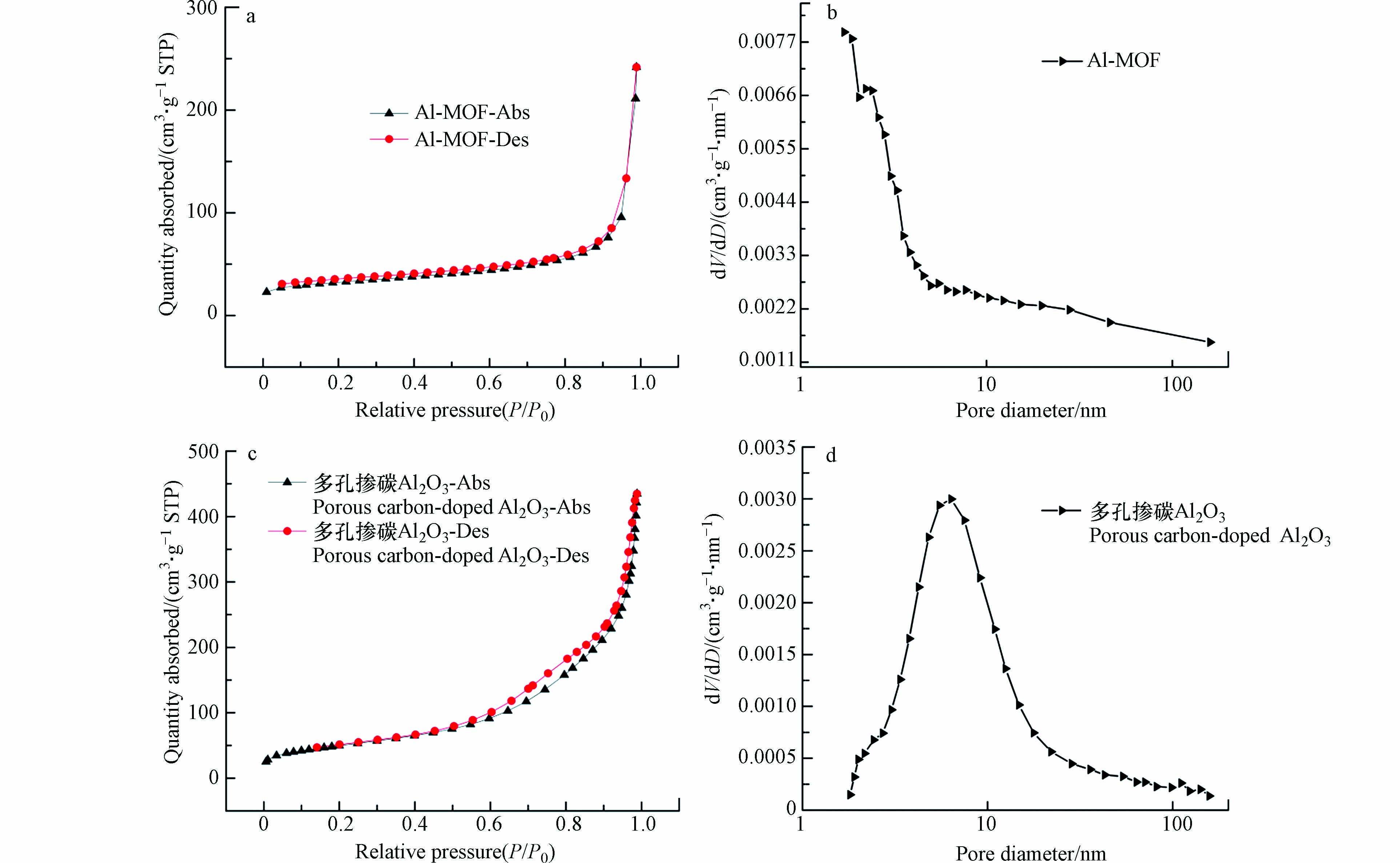
为了考察NH2-MIL-53(Al)和多孔掺碳Al2O3材料的孔道类型和孔径大小,进行了NH2-MIL-53(Al)和多孔掺碳Al2O3材料的氮气吸脱附测试,如图3a、c为NH2-MIL-53(Al)和多孔掺碳Al2O3材料的氮气吸脱附曲线,图3b、d为采用BEJ模型计算得到的材料孔径分布曲线. 图3a显示NH2-MIL-53(Al)为Ⅰ型吸附等温线,推测为微孔材料. 在图3可以看出,多孔掺碳Al2O3材料在P/P0为0.1—0.4的范围内没有二次吸收,吸附等温线可归为Ⅴ型,而在高压P/P0为0.7—0.9的范围内出现H4型迟滞回线,表明多孔掺碳Al2O3材料存在复合孔[23]. 通过孔径分布曲线可以更详细地验证. NH2-MIL-53(Al)的孔径主要分布在2 nm以前,大量微孔的存在进一步证明了其为微孔材料,而多孔掺碳Al2O3材料的孔径分布以6.36 nm为中心,主要以介孔为主. 用BET方程计算出NH2-MIL-53(Al)的比表面积(116.73 m²·g−1)要明显小于多孔掺碳Al2O3材料(180.24 m²·g−1),与预期结果一致. 基于上述结果,多孔掺碳Al2O3材料成功合成且其更高的比表面积使其成为污染物富集的高效材料之一.
-
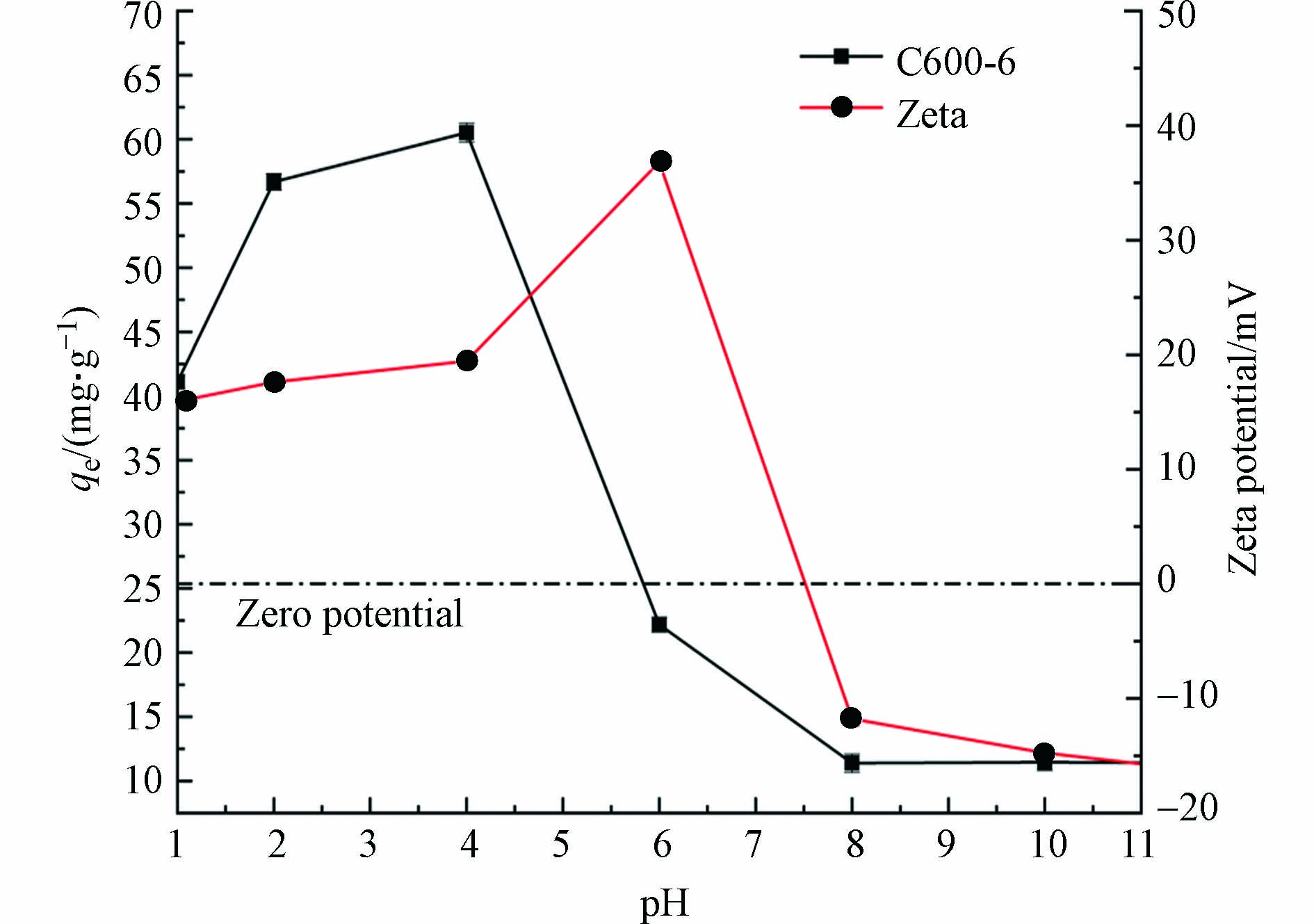
pH值是影响吸附剂的吸附效果的主要因素之一,因为pH通过影响吸附剂的表面电荷和溶液中Cr(Ⅵ)的离子形态来控制吸附剂表面的吸附能力[24],不同溶液pH条件下多孔掺碳Al2O3吸附剂对Cr(Ⅵ)的吸附效果的影响和材料在不同pH下的Zeta电位如图4所示. 数据分析表明,pH对BPA吸附容量有显著影响,材料在酸性条件比在碱性条件下对Cr(Ⅵ)的去除效果好,Cr(Ⅵ)在多孔掺碳Al2O3吸附剂上的最大吸附容量在pH=4左右出现,最大吸附容量为60.71 mg·g−1. pH较低时,Cr(Ⅵ)主要以HCrO4−存在[25],材料在这个范围内的Zeta电位显示其时带正电荷,促进了吸附材料与HCrO4−的静电吸引作用. 溶液pH为碱性时,主要以CrO42−形式存在[26],此时吸附材料开始去质子化,表面带负电,与CrO42−存在静电排斥作用,且OH−会与CrO42−竞争吸附剂上的吸附位点[8],因此在碱性环境下吸附剂对Cr(Ⅵ)的吸附能力大幅下降.
-
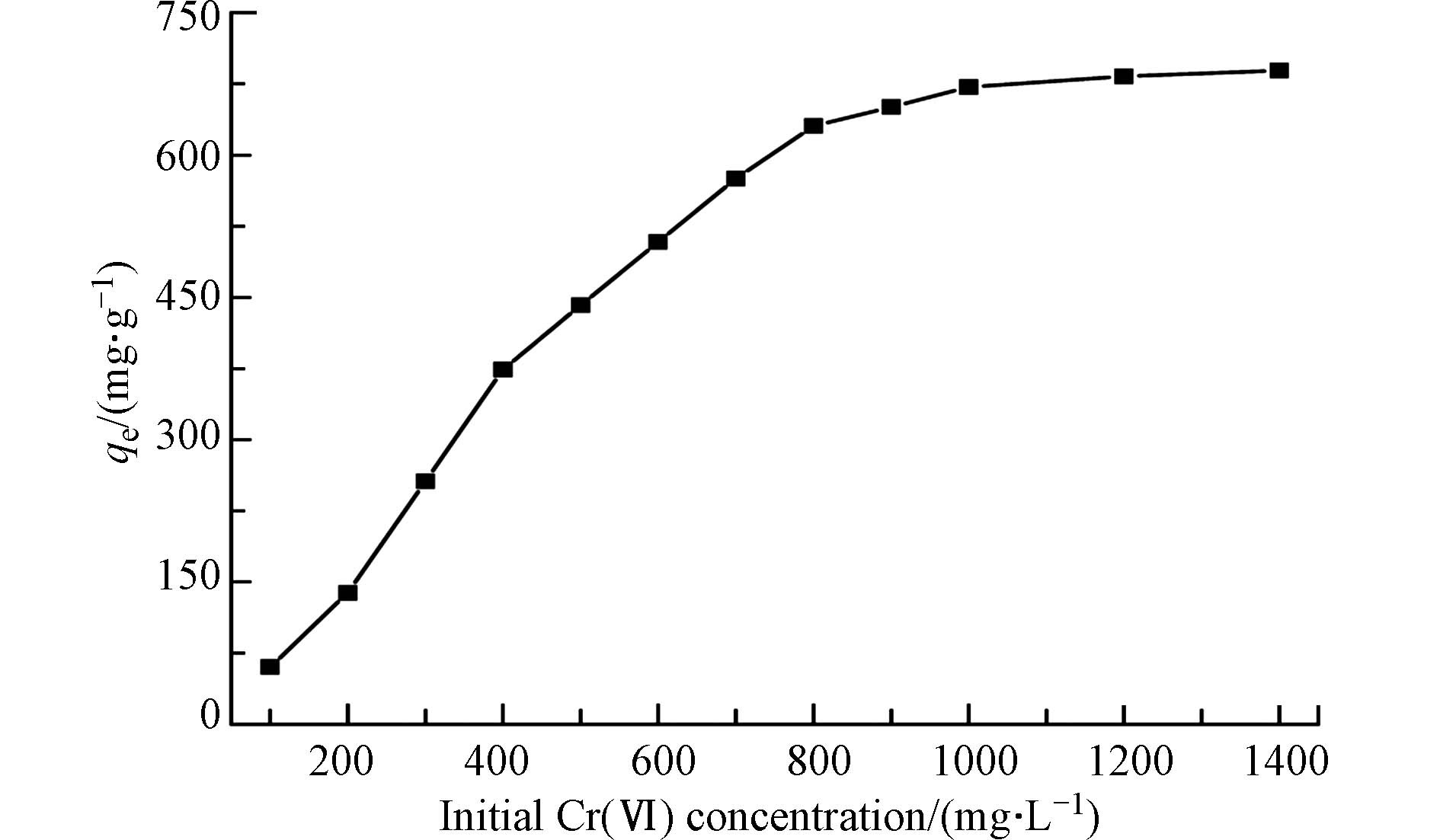
图5显示了Cr(Ⅵ)初始浓度对多孔掺碳Al2O3吸附剂的影响. 由图5可见,随着Cr(Ⅵ)初始浓度从100 mg·L−1增加到1400 mg·L−1,Cr(Ⅵ)在多孔掺碳Al2O3吸附剂上的吸附容量也越来越高,低浓度时,Cr(Ⅵ)初始浓度的增加显著提高了平衡吸附容量(qe),这是由于与活性吸附位点接触的Cr(Ⅵ)增加所致. 当Cr(Ⅵ)初始浓度超过800 mg·L−1时,qe值仍然可以缓慢增加. 这是因为高浓度可以提供更强的驱动力,克服了传质阻力,促进了吸附剂对Cr(Ⅵ)的吸附[27]. 当Cr(Ⅵ)初始浓度大于1000 mg·L−1时,由于吸附位点饱和,吸附容量保持不变[28]. 从图得到的Cr(Ⅵ)的qe值为671.56 mg·g−1.
-
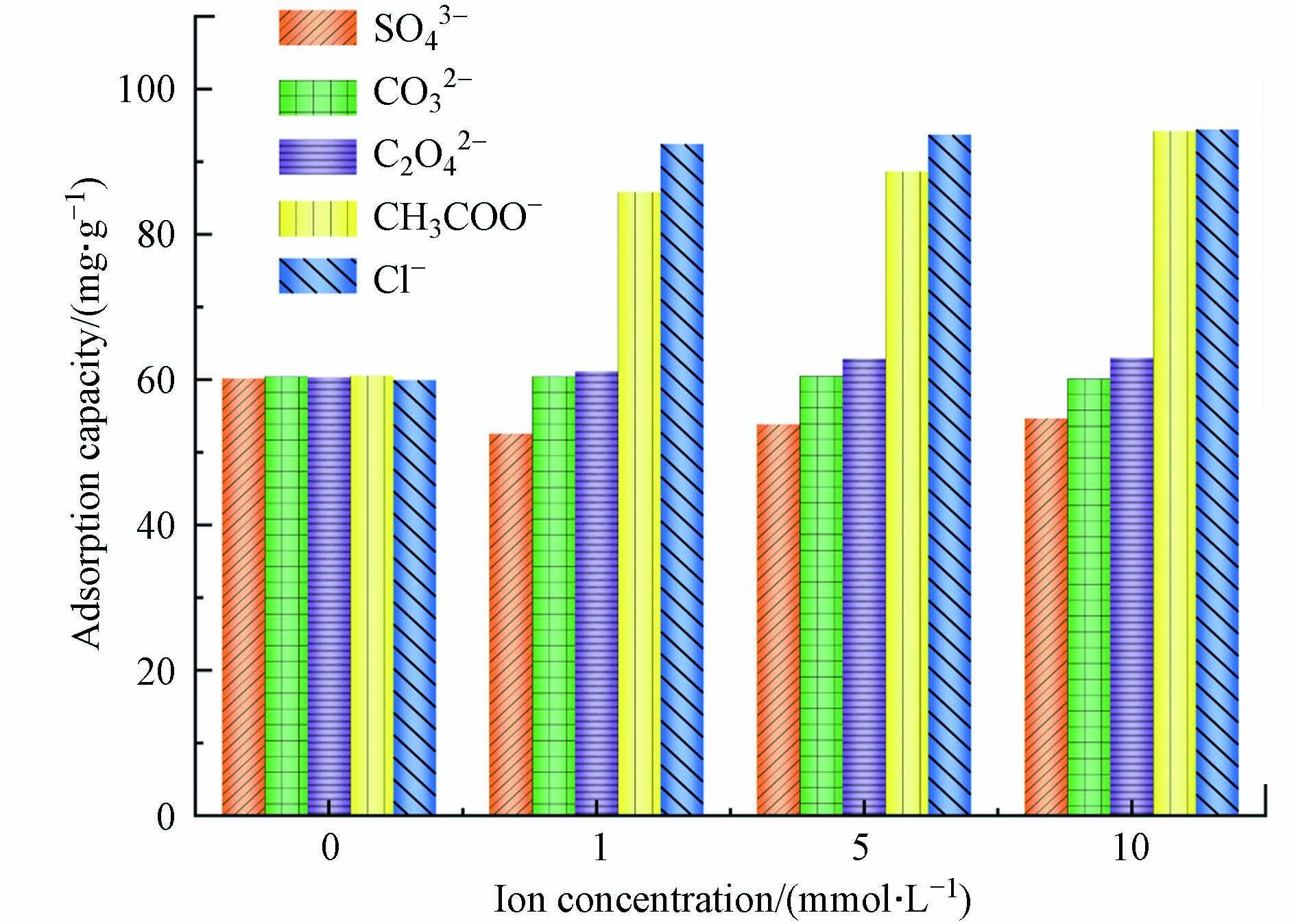
在实际生产中,工业废水的成分非常复杂. 因此,实验还应考虑不同离子类型对吸附的影响. 在本实验中制备了0、1、5、10 mmol·L−1 SO42−、CO32−、C2O42−、CH3COO−、Cl−和Cr(Ⅵ)的混合溶液. 研究多孔掺碳Al2O3吸附剂在混合溶液中对Cr(Ⅵ)的吸附效果. 实验数据如图6所示. 在CH3COO−和Cl−介质中,Cr(Ⅵ)的吸附量增加. 当SO42−存在时,多孔掺碳Al2O3吸附剂对Cr(Ⅵ)的吸附能力降低. 这可能是由于SO42−和Cr(Ⅵ)氧阴离子之间具有相似的化学性质,从而导致它们的竞争吸附,降低吸附容量[29],且SO42−与吸附剂竞争溶液中的H+,生成HSO4−,从而降低吸附剂表面的正电荷.
-
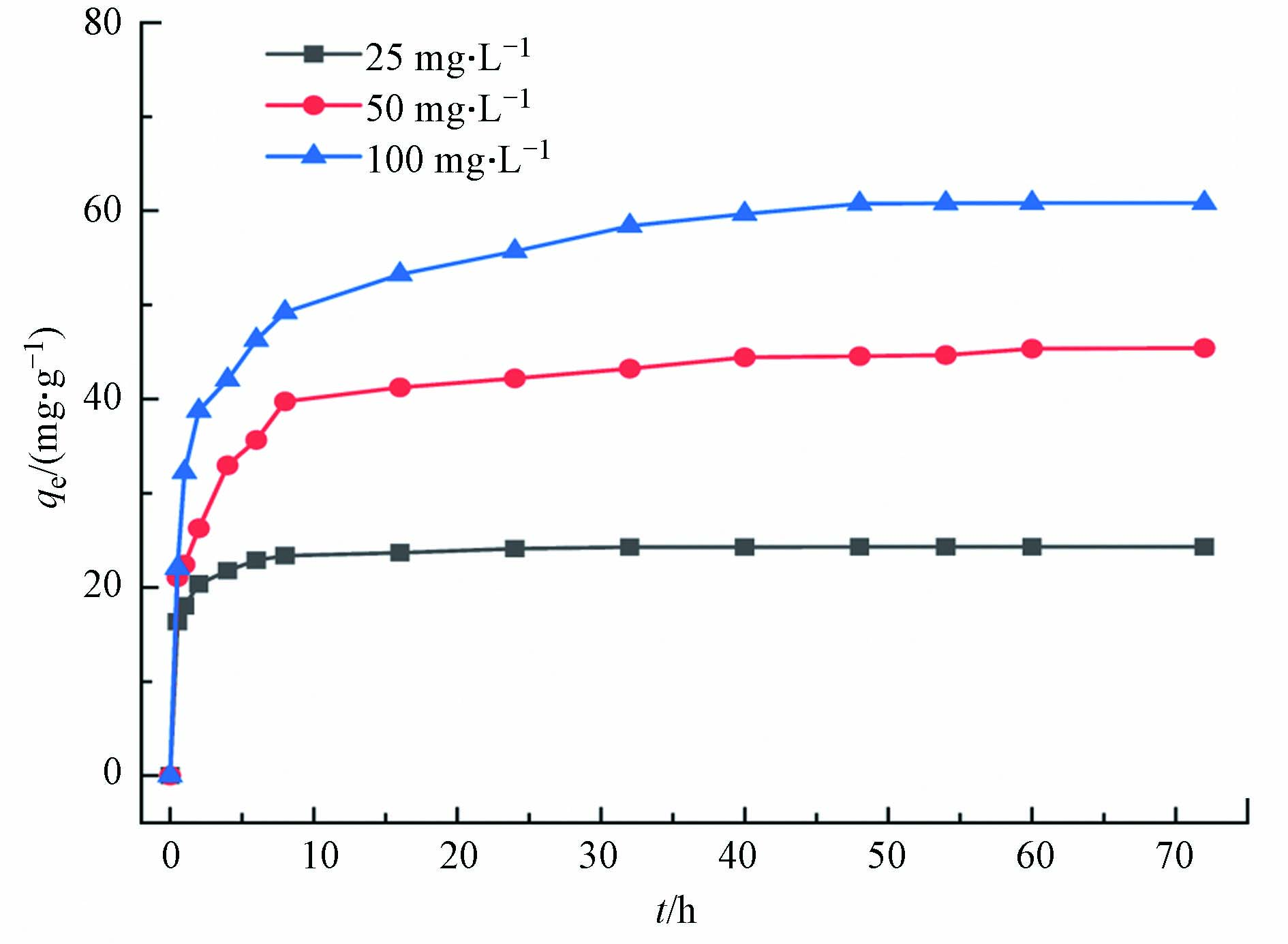
图7为多孔掺碳Al2O3吸附剂对Cr(VI)的吸附容量随时间的变化,可以看出初始浓度为25、50、100 mg·L−1的Cr(Ⅵ)随时间的增加,吸附容量的变化过程都是相似的,均随时间的增加而增加,在前10 h内吸附速率很快,然后逐渐减慢,在24 h达到了吸附平衡,最大吸附容量为60.75 mg·g−1. 反应前期,多孔掺碳Al2O3吸附剂材料表面的吸附位点较多并且溶液中的Cr(Ⅵ)此时浓度最高,吸附驱动力大,因此吸附速率快. 然而随着时间的推移,多孔掺碳Al2O3吸附剂表面的吸附位点逐渐被占据并且Cr(Ⅵ)浓度逐渐降低,因此吸附驱动力减弱.
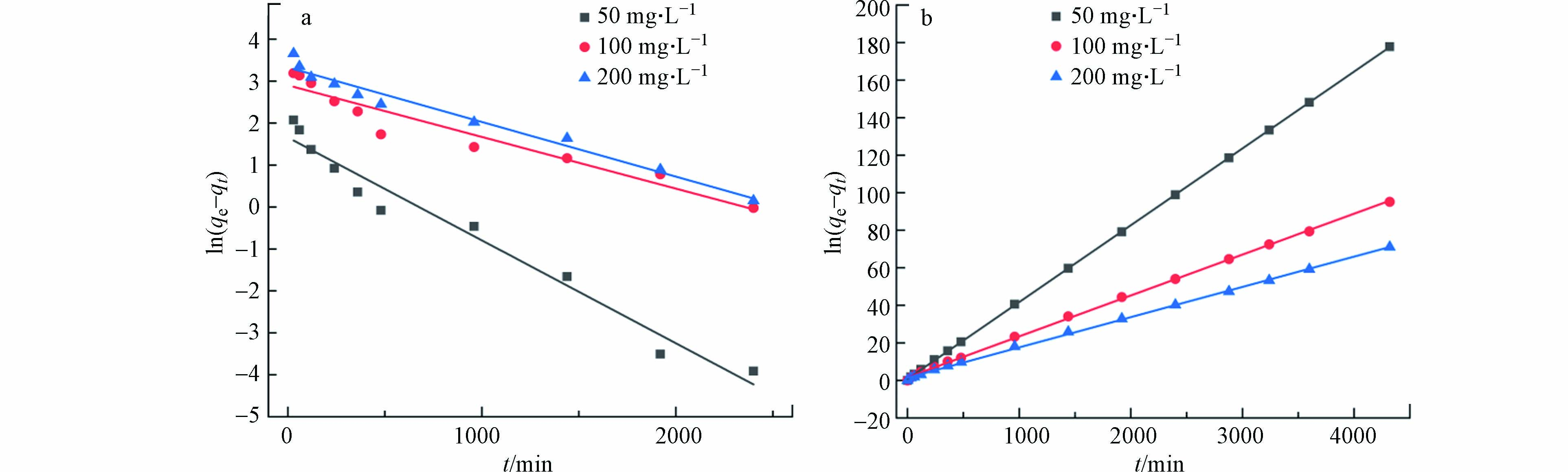
将实验数据拟合在伪一级动力学模型、伪二级动力学模型中,方程见(2)及(3).
其中,K1为伪一阶动力学模型吸附速率常数,min−1;K2为伪二阶动力学模型吸附速率常数,g·(mg·min)−1;qe为平衡时的吸附量,mg·g−1;qt为t时刻的吸附量,mg·g−1.
拟合的图像及相关参数如图8和表1所示. 由表1可知,伪二阶动力学模型的回归系数(0.9999、0.9991、0.9997)均高于伪一阶动力学模型的回归系数(0.9685、0.9282、0.9733),且在100 mg·L−1 Cr(Ⅵ)下,伪二级动力学模型的吸附容量(60.75 mg·g−1)更接近实验吸附容量值,表明伪二阶动力学模型更适合多孔掺碳Al2O3吸附剂对Cr(Ⅵ)的吸附过程,同时也说明了该吸附过程是化学吸附[30],且化学键取代过程可能是限制该吸附过程的主要机理.
-
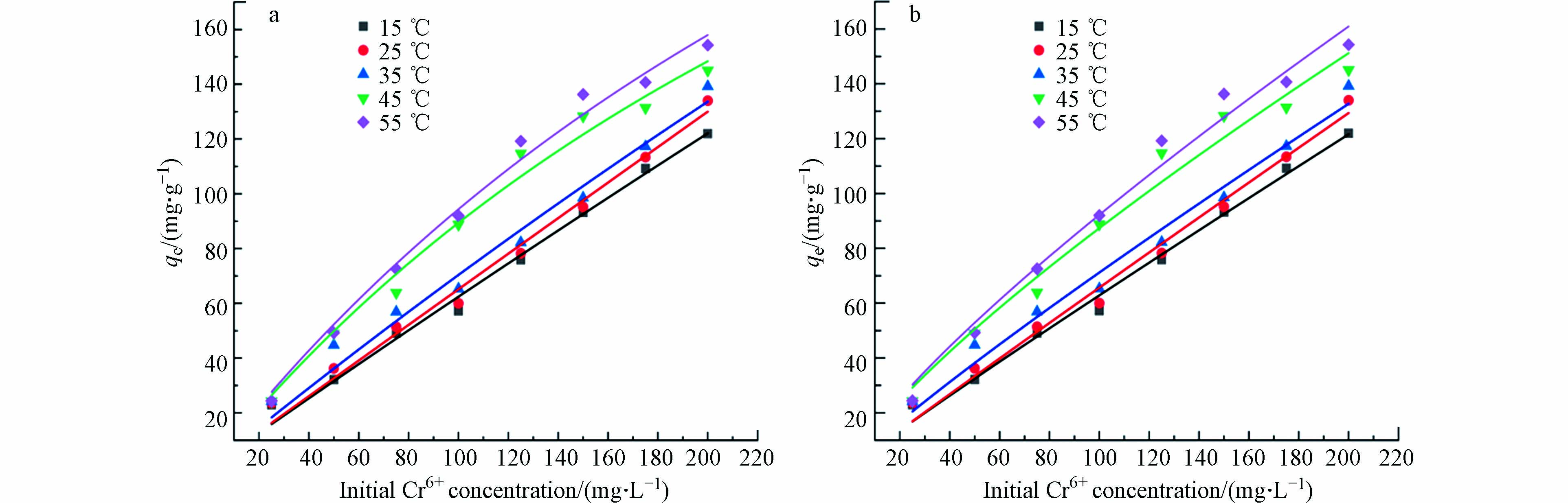
Langmuir和Freundlich吸附等温线是经典的等温线模型,用于解释固体中疏水化合物吸附的非线性性质,因此在本研究中使用Langmuir和Freundlich吸附模型分析多孔掺碳Al2O3吸附剂对Cr(Ⅵ)的吸附等温线.
Langmuir吸附等温线表明,吸附剂表面会形成均匀的单分子吸附层,能量均匀,吸附分子之间在不同位置没有相互作用[31]. Langmuir方程的非线性形式用(4)方程表示. 而Freundlich等温线是已知最早描述吸附平衡的关系式,用来描述非均相能量吸附剂表面对多层吸附的非理想可逆吸附过程,非线性形式的Freundlich等温方程如(5)所示.
式中,qm为最大吸附量,mg·g−1;b为Langmuir吸附平衡常数,L·mg−1;Kf为Freundlich吸附容量常数,mg·g−1;n为Freundlich亲和常数.
不同等温吸附模型的拟合参数如表2和图9所示. 由表2可知,使用Langmuir等温吸附模型拟合R2(0.9963—0.9901)值大于Freundlich等温吸附模型的R2(0.9912—0.9830)值,说明Langmuir等温吸附模型更适合描述多孔掺碳Al2O3吸附剂对Cr(Ⅵ)的吸附过程,说明该吸附是单层吸附[32]. 由Langmuir等温吸附模型拟合得到的理论最大吸附量与实际测得的吸附量接近,说明其具有可信度. 此外,Freundlich等温吸附模型拟合得到的n值可以很好的反应多孔掺碳Al2O3吸附剂对Cr(Ⅵ)的吸附能力,n<1,说明吸附较难;n>1,说明吸附能力较强. 本研究中的Freundlich等温吸附模型中的n(1.0503—1.2450)值均大于1,说明多孔掺碳Al2O3吸附剂对Cr(Ⅵ)的吸附能力较强.
-
在250 mL的锥形瓶中配制100 mL水溶液,加入1 g·L−1的多孔掺碳Al2O3吸附剂和纳米氧化铝吸附剂,并用1 mol·L−1的HCl或者NaOH溶液调节pH为1和12,溶液振荡反应24 h后,使用0.22 μm滤膜分离剩下的吸附剂,取25 mL分离得到的液体于50 mL比色管中,利用铬天青s检测溶液中Al3+的析出量,其反应变化如图10所示,水样1为纯水样,水样2、4分别为多孔掺碳Al2O3在pH为1和12条件下Al3+的析出量,水样3、5分别为纳米氧化铝在pH为1和12条件下Al3+的析出量. 在酸性条件下Al3+和铬天青s反应生成蓝绿色的四元胶束,碱性条件下生成紫红色. 从图10可以明显看出,多孔掺碳Al2O3在酸碱条件下产生的絮状物要明显少于纳米氧化铝,说明多孔掺碳Al2O3在酸碱条件下的稳定性要明显优于纳米氧化铝.
-
吸附剂的再生对吸附剂的实用性和可行性至关重要. 通过连续5次吸附脱吸实验,评价多孔掺碳Al2O3吸附剂的重复使用性. 将100 mg 多孔掺碳Al2O3吸附剂与100 mL Cr(Ⅵ) (100 mg·L−1)结合,振荡24 h进行吸附实验,固液分离后测定Cr(Ⅵ)浓度. 脱附液100 mL与吸附剂混合,振荡24 h得到脱附上清. 脱附液由10%的硫脲和2%的盐酸组成. 图11a为5次循环中Cr(Ⅵ)的吸附速率. 各吸附率分别为98.4%、97.6%、96.5%、95.1%和94.5%. 多孔掺碳Al2O3吸附剂在5次循环后仍具有较强的吸附势. 值得注意的是,吸附率的轻微下降可能是由于未洗Cr(Ⅵ)的积累或实验过程中不可避免的活性位点的损失. 为了确定是否将污染物完全脱附以及脱附后是否有改变吸附剂的结构,使用傅立叶变换红外光谱仪对多孔掺碳Al2O3吸附剂脱附前后的光谱进行分析,如图11b所示,脱附再生后的多孔掺碳Al2O3与原多孔掺碳Al2O3相似. 从图中可以观察到,877 cm−1的峰值代表了吸附Cr(Ⅵ)之后多孔掺碳Al2O3上的C—H伸缩振动在脱附后的多孔掺碳Al2O3上消失了,说明脱附液洗涤吸附的Cr(Ⅵ)被成功清除. 结果验证了多孔掺碳Al2O3的稳定性和可重复使用性.
-
吸附Cr(Ⅵ)后的多孔掺碳Al2O3红外光谱如图11b所示,3453 cm−1处为OH 的伸缩振动,羟基与Cr(Ⅵ)结合后,νOH相对强度减弱,并发生约12 cm−1的位移,表明结合Cr(Ⅵ)后羟基的振动峰强减弱. 同样的位于615 cm−1左右处的Al—O键晶格振动的相对强度减弱,并发生约15 cm−1的位移. 红外分析结果表明,多孔掺碳Al2O3具有较高的吸附能力是由于大量羟基的存在,这些羟基能有效地与阳离子结合并形成表面复合物.
-
(1)本研究将水热法合成的NH2-MIL-53(Al)作为原材料进行碳化,利用碳化开发制备出一种新型制备多孔掺碳Al2O3吸附材料,并将其用于吸附水中的Cr(Ⅵ)污染物. 根据经过XRD、SEM、BET的测试方法分析,XRD、SEM结果表明多孔掺碳Al2O3成功合成,呈低石墨化状态,晶体结构稳定;SEM则表明多孔掺碳Al2O3材料表面呈絮状结构,但其结构没有受到破坏;通过BET测试表明,多孔掺碳Al2O3的比表面积为180.24 m²·g−1,其比表面积要大于煅烧前,其孔径主要为介孔.
(2)探究了吸附过程中各因素对多孔掺碳Al2O3吸附剂吸附Cr(Ⅵ)的影响,结果表明,多孔掺碳Al2O3吸附剂对Cr(Ⅵ)的吸附平衡时间为48 h,平衡吸附量最大可达为671.56 mg·g−1;pH值为4时,多孔掺碳Al2O3吸附剂对Cr(Ⅵ)的吸附容量达到最大;同时,SO42−和Cr(Ⅵ)氧阴离子之间具有相似的化学性质,因此增加SO42−会导致它们的竞争吸附,降低吸附容量在吸附剂表面竞争活性位点,抑制吸附过程.
(3)通过吸附模型结果表明,多孔掺碳Al2O3吸附剂对Cr(VI)的吸附过程与Langmuir等温线模型和伪二阶动力学模型拟合更好,说明吸附是单层的化学吸附.
以NH2-MIL-53(Al)为前驱体制备多孔掺碳Al2O3吸附剂及其对水中Cr(Ⅵ)的吸附性能
Porous carbon-doped Al2O3 derived from metal organic frameworks and its adsorption performance for Cr(Ⅵ) from water
-
摘要: 铬是污染性金属元素,铬含量是水质污染控制的一项重要指标,其中Cr(Ⅵ)的毒性最大,且易被人体吸收. 本研究以水中的Cr(Ⅵ)吸附传质分离为目标,利用以铝为金属源水热法合成的铝基MOFs为前驱体,600 ℃煅烧后制备了多孔掺碳Al2O3吸附材料,利用现代分析技术对其进行微观结构表征,探究了其吸附作用能力与机制. 研究结果表明,XRD、SEM、BET等表征手段证明了NH2-MIL-53(Al)与多孔掺碳Al2O3结构的成功合成. 前驱体NH2-MIL-53(Al)和煅烧后的衍生物多孔掺碳Al2O3,在形貌上相似,且多孔掺碳Al2O3材料(180.24 m2·g−1)的比表面积要大于NH2-MIL-53(Al)(116.73 m2·g−1). 多孔掺碳Al2O3材料对Cr(Ⅵ)的平衡吸附量最大可达到671.56 mg·g−1. 吸附动力学模型拟合结果显示,多孔掺碳Al2O3材料对Cr(Ⅵ)的吸附行为与Langmuir等温线模型和伪二阶动力学模型更加拟合. 研究显示,多孔掺碳Al2O3材料可以作为除Cr材料实现对Cr(Ⅵ)的高效去除.Abstract: Chromium is a pollutant metal element, and its content is an important index of water pollution control, among which Cr(Ⅵ) is the most toxic and easily absorbed by the human body. In this study, the adsorption and mass transfer separation of Cr(Ⅵ) in water was taken as the goal, and the porous carbon-doped Al2O3 adsorption material was prepared by calcination at 600℃ using aluminum-based MOFs synthesized by hydrothermal method with aluminum as metal source. The microstructure was characterized by modern analytical technology, and the adsorption capacity and mechanism were explored. The results showed that XRD, SEM, BET and other characterization methods proved the successful synthesis of NH2-MIL-53(Al) and porous carbon-doped Al2O3 structure. The precursor NH2-MIL-53 (Al) and the calcined derivative porous carbon-doped Al2O3 are similar in morphology, and the specific surface area of porous carbon-doped Al2O3 material (180.24 m2·g−1) is larger than that of NH2-MIL-53 (Al) (116.73 m2·g−1). The maximum equilibrium adsorption capacity of Cr(Ⅵ) on porous carbon-doped Al2O3 material is 671.56 mg·g−1. The fitting results of the adsorption kinetics model show that the adsorption behavior of porous carbon doped Al2O3 material for Cr(Ⅵ) is more consistent with the Langmuir isotherm model and pseudo-second-order kinetics model. The study shows that porous carbon-doped Al2O3 material can be used as a Cr removal material to achieve effective removal of Cr(Ⅵ).
-
Key words:
- MOFs derivatives /
- adsorption /
- metal Cr(Ⅵ) /
- adsorption kinetics.
-

-
图 3 NH2-MIL-53(Al)的氮气吸附脱附等温线图(a),孔径分布图(b);多孔掺碳Al2O3的的氮气吸附脱附等温线图(c),孔径分布图(d)
Figure 3. Nitrogen adsorption and desorption isotherm of NH2-MIL-53(Al) (a), pore size distribution of NH2-MIL-53(Al) (b); Nitrogen adsorption and desorption isotherm of Porous carbon-doped Al2O3 (c), pore size distribution of Porous carbon-doped Al2O3 (d)
表 1 多孔掺碳Al2O3吸附剂吸附Cr(VI)的动力学模型参数
Table 1. Kinetic model parameters of Cr(VI) adsorption on Porous carbon-doped Al2O3
C0/(mg·L−1) 伪一阶动力学模型
Pseudo-first-order model伪二阶动力学模型
Pseudo-second-order modelQe/(mg·g−1) R2 k1/(min−1) Qe/(mg·g−1) R2 k2/ (g·(mg·min)−1) 25 24.28 0.9685 2.45×10−3 24.32 0.9999 4.09×10−2 50 44.46 0.9282 1.23×10−3 45.10 0.9991 2.18×10−2 100 59.60 0.9733 1.30×10−2 60.75 0.9997 1.61×10−2 表 2 多孔掺碳Al2O3吸附剂吸附Cr(Ⅵ)的等温吸附模型参数
Table 2. Parameters of the isotherm adsorption model for Cr(Ⅵ) adsorption on Porous carbon-doped Al2O3
温度/℃ Langmuir 模型 Freundlich 模型 Qm/(mg·g−1) R2 b/(L·mg−1) R2 Kf/(mg·g−1) n 15 122.10 0.9963 2.31×10−4 0.9912 0.7836 1.0503 25 130.02 0.9883 2.17×10−5 0.9876 0.7254 1.0221 35 133.65 0.9889 2.31×10−4 0.9830 1.1342 1.1125 45 148.39 0.9853 2.58×10−3 0.9782 2.2831 1.2636 55 157.98 0.9901 2.42×10−3 0.9830 2.2828 1.2450 -
[1] 赵曼淑, 刘涛, 鹿文慧, 等. 基于CTAC改性活性炭的信封式膜包用于水溶液中六价铬去除 [J]. 环境化学, 2020, 39(9): 2593-2601. doi: 10.7524/j.issn.0254-6108.2019062401 ZHAO M S, LIU T, LU W H, et al. Envelope membrane packed with CTAC modified active carbon for Cr(Ⅵ) removal from aqueous solution [J]. Environmental Chemistry, 2020, 39(9): 2593-2601(in Chinese). doi: 10.7524/j.issn.0254-6108.2019062401
[2] 张诚, 陈远洪, 王庆森, 等. 连续电镀锡钝化六价铬废水的处理 [J]. 电镀与涂饰, 2020, 39(13): 875-878. ZHANG C, CHEN Y H, WANG Q S, et al. Treatment of hexavalent chromium-containing wastewater discharged from passivation phase in continuous tin electroplating process [J]. Electroplating & Finishing, 2020, 39(13): 875-878(in Chinese).
[3] 高卫国, 钱林波, 韩璐, 等. 锰铁氧体吸附及催化柠檬酸还原六价铬的过程及机理 [J]. 环境化学, 2018, 37(7): 1525-1533. doi: 10.7524/j.issn.0254-6108.2017101302 GAO W G, QIAN L B, HAN L, et al. Iron manganese minerals catalyzed Cr(Ⅵ) reduction by citric acid and its mechanism [J]. Environmental Chemistry, 2018, 37(7): 1525-1533(in Chinese). doi: 10.7524/j.issn.0254-6108.2017101302
[4] 范俊英. 回收电镀废水中六价铬的离子交换法应用分析 [J]. 资源节约与环保, 2015(8): 56. FAN J Y. Application analysis of ion exchange method for recovering hexavalent chromium from electroplating wastewater [J]. Resources Economization & Environmental Protection, 2015(8): 56(in Chinese).
[5] 张铁军, 李博, 韩剑宏, 等. 磁性改性玉米秸秆材料吸附铬的性能及机理研究 [J]. 工业水处理, 2020, 40(12): 100-105. ZHANG T J, LI B, HAN J H, et al. Study on adsorption performance and mechanism of chromium on magnetic modified corn stalk [J]. Industrial Water Treatment, 2020, 40(12): 100-105(in Chinese).
[6] 彭鑫, 王静蕾, 常金明, 等. 基于壳聚糖的吸附材料在六价铬吸附中的应用 [J]. 高分子材料科学与工程, 2021, 37(6): 181-190. PENG X, WANG J L, CHANG J M, et al. Removal of hexavalent chromium by chitosan-based adsorbents [J]. Polymer Materials Science & Engineering, 2021, 37(6): 181-190(in Chinese).
[7] 周晓倩, 郭华明, 赵凯. 改性天然菱铁矿去除水中六价铬 [J]. 环境工程学报, 2015, 9(9): 4171-4177. ZHOU X Q, GUO H M, ZHAO K. Removal of hexavalent chromium from water solution by modified natural siderite [J]. Chinese Journal of Environmental Engineering, 2015, 9(9): 4171-4177(in Chinese).
[8] DIM P E, MUSTAPHA L S, TERMTANUN M, et al. Adsorption of chromium (Ⅵ) and iron (Ⅲ) ions onto acid-modified kaolinite: Isotherm, kinetics and thermodynamics studies [J]. Arabian Journal of Chemistry, 2021, 14(4): 103064. doi: 10.1016/j.arabjc.2021.103064 [9] 聂兰玉, 陈海, 白智勇, 等. 羟基氧化铝吸附去除六价铬 [J]. 环境工程学报, 2015, 9(8): 3847-3853. doi: 10.12030/j.cjee.20150842 NIE L Y, CHEN H, BAI Z Y, et al. Adsorption of chromium(Ⅵ) by aluminum oxyhydroxide [J]. Chinese Journal of Environmental Engineering, 2015, 9(8): 3847-3853(in Chinese). doi: 10.12030/j.cjee.20150842
[10] 李雯, 张光华, 刘林涛. 硅胶负载微波交联壳聚糖对制革废水中Cr(Ⅵ)的吸附研究 [J]. 西部皮革, 2010, 32(1): 33-35,39. LI W, ZHANG G H, LIU L T. Silica gel based cross-linked chitosan prepared under microwave irradiation as Cr(Ⅵ) adsorbent [J]. West Leather, 2010, 32(1): 33-35,39(in Chinese).
[11] BOUSTILA H, BOUTILLARA Y, VELASCO L F, et al. Tailoring activated carbon properties for Pb(II) and Cr(VI) removal from water in continuous mode [J]. Chemical Engineering & Technology, 2022, 45(2): 258-265. [12] 贺龙强, 胡鹏, 付克明. 利用粉煤灰制备分子筛及对水体中六价铬的吸附研究 [J]. 硅酸盐通报, 2017, 36(10): 3493-3497,3503. doi: 10.16552/j.cnki.issn1001-1625.2017.10.045 HE L Q, HU P, FU K M. Adsorption of hexavalent chromium by zeolite synthesized of fly ash [J]. Bulletin of the Chinese Ceramic Society, 2017, 36(10): 3493-3497,3503(in Chinese). doi: 10.16552/j.cnki.issn1001-1625.2017.10.045
[13] HUANG S Y, PANG H W, LI L, et al. Unexpected ultrafast and high adsorption of U(Ⅵ) and Eu(Ⅲ) from solution using porous Al2O3 microspheres derived from MIL-53 [J]. Chemical Engineering Journal, 2018, 353: 157-166. doi: 10.1016/j.cej.2018.07.129 [14] DRISKO G L, CHEE KIMLING M, SCALES N, et al. One-pot preparation and uranyl adsorption properties of hierarchically porous zirconium titanium oxide beads using phase separation processes to vary macropore morphology [J]. Langmuir:the ACS Journal of Surfaces and Colloids, 2010, 26(22): 17581-17588. doi: 10.1021/la103177h [15] NI Y Y, YANG J H, SUN L X, et al. La/LaF3 co-modified MIL-53(Cr) as an efficient adsorbent for the removal of tetracycline [J]. Journal of Hazardous Materials, 2022, 426: 128112. doi: 10.1016/j.jhazmat.2021.128112 [16] OUYANG B W, CHEN Q, YUAN H H, et al. Reversible environmental impacts of iron-based metal-organic framework MIL-53(Fe) on nitrogen-fixing bacterium Azotobacter vinelandii [J]. Journal of Environmental Chemical Engineering, 2022, 10(3): 107794. doi: 10.1016/j.jece.2022.107794 [17] LI X, LIU Y, ZHANG C L, et al. Porous Fe2O3 microcubes derived from metal organic frameworks for efficient elimination of organic pollutants and heavy metal ions [J]. Chemical Engineering Journal, 2018, 336: 241-252. doi: 10.1016/j.cej.2017.11.188 [18] HUANG H L, LI J R, WANG K K, et al. An in situ self-assembly template strategy for the preparation of hierarchical-pore metal-organic frameworks [J]. Nature Communications, 2015, 6: 8847. doi: 10.1038/ncomms9847 [19] ZHANG L, WU HB, et al. Porous Fe2O3 nanocubes derived from MOFs for highly reversible lithium storage[J]. CrystEngComm. 2013(15): 9332-9335. [20] QIN Y Y, WANG Q Y, GE J L, et al. Microwave ultrasound-assisted synthesis of NH2-MIL-53(Al) for fluorescence detection of organosulfur compounds in model fuel [J]. Inorganic Chemistry Communications, 2021, 132: 108828. doi: 10.1016/j.inoche.2021.108828 [21] 邹文兵, 沈军, 邹丽萍, 等. La2O3掺杂氧化铝气凝胶的制备与耐温性能[J]. 稀有金属材料与工程, 2018, 47(S2): 99-103. ZOU W B, SHEN J, ZOU L P, et al. Fabrication and thermal stability of La2O3 doped alumina aerogel[J]. Rare Metal Materials and Engineering, 2018, 47(Sup 2): 99-103(in Chinese).
[22] 矫宝庆, 唐克, 洪新, 等. 活性氧化铝吸附脱除模拟油中吡啶的研究 [J]. 石油炼制与化工, 2022, 53(3): 91-98. JIAO B Q, TANG K, HONG X, et al. Study on adsorption removal of pyridine from model fuels by three kinds of activated alumina [J]. Petroleum Processing and Petrochemicals, 2022, 53(3): 91-98(in Chinese).
[23] LI G, ZHAO H F, GUO P T, et al. Effective removal of tinidazole by MIL-53(Al)-NDC metal-organic framework from aqueous solution [J]. Journal of Solid State Chemistry, 2022, 310: 123066. doi: 10.1016/j.jssc.2022.123066 [24] JIN X Y, LIU Y, TAN J, et al. Removal of Cr(VI) from aqueous solutions via reduction and absorption by green synthesized iron nanoparticles [J]. Journal of Cleaner Production, 2018, 176: 929-936. doi: 10.1016/j.jclepro.2017.12.026 [25] 谢发之, 李海斌, 李国莲, 等. 富里酸对针铁矿吸附Cr(Ⅵ)的影响机理 [J]. 环境科学研究, 2016, 29(10): 1506-1512. doi: 10.13198/j.issn.1001-6929.2016.10.14 XIE F Z, LI H B, LI G L, et al. Effects of fulvic acid on the adsorption of chromium(Ⅵ) to goethite [J]. Research of Environmental Sciences, 2016, 29(10): 1506-1512(in Chinese). doi: 10.13198/j.issn.1001-6929.2016.10.14
[26] DING J, PU L T, WANG Y F, et al. Adsorption and reduction of Cr(Ⅵ) together with Cr(Ⅲ) sequestration by polyaniline confined in pores of polystyrene beads [J]. Environmental Science & Technology, 2018, 52(21): 12602-12611. [27] LI Y X, HAN Y C, WANG C C. Fabrication strategies and Cr(Ⅵ) elimination activities of the MOF-derivatives and their composites [J]. Chemical Engineering Journal, 2021, 405: 126648. doi: 10.1016/j.cej.2020.126648 [28] WANG C, XIONG C, HE Y L, et al. Facile preparation of magnetic Zr-MOF for adsorption of Pb(Ⅱ) and Cr(VI) from water: Adsorption characteristics and mechanisms [J]. Chemical Engineering Journal, 2021, 415: 128923. doi: 10.1016/j.cej.2021.128923 [29] YI Y, WANG X Y, MA J, et al. Fe(Ⅲ) modified Egeria najas driven-biochar for highly improved reduction and adsorption performance of Cr(Ⅵ) [J]. Powder Technology, 2021, 388: 485-495. doi: 10.1016/j.powtec.2021.04.066 [30] LIM A, CHEW J J, NGU L H, et al. Synthesis, characterization, adsorption isotherm, and kinetic study of oil palm trunk-derived activated carbon for tannin removal from aqueous solution [J]. ACS Omega, 2020, 5(44): 28673-28683. doi: 10.1021/acsomega.0c03811 [31] FOO K Y, HAMEED B H. Insights into the modeling of adsorption isotherm systems [J]. Chemical Engineering Journal, 2010, 156(1): 2-10. doi: 10.1016/j.cej.2009.09.013 [32] KHAMWICHIT A, DECHAPANYA W, DECHAPANYA W. Adsorption kinetics and isotherms of binary metal ion aqueous solution using untreated Venus shell [J]. Heliyon, 2022, 8(6): e09610. doi: 10.1016/j.heliyon.2022.e09610 -



 下载:
下载: