-
随着新型冠状病毒肺炎(COVID-19)在全球流行加剧,消毒剂在医院、医疗保健设施以及日常生活中广泛大量使用[1]. 消毒剂中最常见的活性成分之一就是苯扎氯铵(benzalkonium chlorides,BACs),它们是含有C8—C18烷基的各种烷基苄基二甲基氯化铵的混合物[2]. 目前环境中发现的BACs主要来自于医院废水和市政污水处理厂. 医院废水中的BACs浓度被检测高达2800 μg·L−1[3]. 在市政污水处理厂的检测中,其进水和出水中的BACs浓度分别为25—170 μg·L−1[4]和0.3—4.1 μg·L−1[5]. 然而,目前的污水处理工艺难以用于处理BACs,由于其化学结构中存在苄基而难以生物降解,导致BACs在处置时以生物固体形式存在或经过处理后作为微污染物释放到环境中[6]. 根据毒理学研究表明,BACs对无脊椎动物、鱼类、藻类、水蚤、轮虫和原生动物也表现出急性毒性,其半最大效应浓度EC50为5.1—2940 μg·L−1[5, 7 − 8]. 因此,我们需要关注其在环境中的排放和危害.
高级氧化工艺(AOPs)通过产生强氧化性的自由基将有机污染物降解甚至矿化为无毒的无机物,被视为处理难生物降解废水的有效方法. 关于使用AOPs降解BACs的研究较少,主要集中在紫外(Ultraviolet,UV)光催化降解BACs. UV可以直接光催化降解部分有机污染物. 通过投加一些化学氧化剂如过氧化氢(H2O2)、臭氧(O3)、过硫酸盐(PS)和氯(Cl)等来产生具有强氧化性的自由基(·OH、·
${\rm{SO}}_4^{2-} $ 、·Cl)也能实现大部分有机污染物的降解[9 − 12]. 与单独的UV光催化降解和氧化剂氧化降解相比,UV/PS[13]、UV/Cl[9]和UV/O3[6]对BACs的氧化降解有协同作用. 铁基催化剂由于其丰富和低成本以及无毒的性质也得到广泛的研究和应用. Hong等[14]通过使用Fe2+活化PS,在60 min的反应时间内去除了91.4%的BACs和52.5%的COD;Zhang等[15]通过使用Fe2+活化H2O2,在60 min的反应时间内BACs的去除率大于80%. 尽管Fe2+可有效活化H2O2和PS等氧化剂,但它们通过水被氧化消耗从而难以重复使用. 这种通过牺牲其他化学品来获得高效率并非是长久之计.开发具有光催化活性且稳定的固体铁基光催化材料值得去探索. 传统的半导体光催化材料如赤铁矿(Fe2O3)都存在明显的局限性,存在比表面积小、易光腐蚀和光生电子-空穴复合快等问题[16]. 铁基金属有机骨架MIL-88A由于具有高比表面积、晶格稳定和结构可调是个不错的选择. 更重要的是,大多数铁基MOF都是使用二甲基甲酰胺(DMF)合成的,一种国际癌症研究机构认定的致癌溶剂[17 − 18],而MIL-88A是唯一可以在没有有机溶剂参与的情况下用水去合成,没有造成二次污染. 因此,本研究将十二烷基二甲基苄基氯化铵(Dodecyl dimethyl benzyl ammonium chloride,DDBAC)作为目标污染物,BACs的最主要同系物,以水热法制备MIL-88A将其作为非均相光催化剂,实现了DDBAC在可见光下的降解,探究DDBAC在可见光下的降解性能、降解途径、降解机理、急性毒性以及材料的稳定性进行评估.
-
十二烷基二甲基苄基氯化铵(DDBAC,≥99%)、富马酸(C4H4O,≥99%)、乙酸铵(CH3COONH4,≥99%)购自阿拉丁生化科技股份有限公司;过氧化氢(H2O2,30%)、乙醇(EtOH,≥99%)、叔丁醇(tBuOH,≥99%)购自国药化学试剂有限公司. 六水合三氯化铁(Ⅲ)(FeCl3·6H2O)购自麦克林生化科技有限公司. 用于高效液相色谱(HPLC)流动相乙腈(CH3CN,99.9%)购自上海安谱实验科技有限公司. 所有溶液均使用Heal Force纯水机纯化的水制备.
-
根据先前报道的程序并进行优化[19, 20],MIL-88A进行典型的水热合成,即称取1.4616 g富马酸与3.4083 g FeCl3·6H2O溶解于63 ml去离子水. 使用磁力加热搅拌器以400 r·min−1和60 ℃下将溶液搅拌2 h,随后转移至100 mL聚四氟乙烯(PTFE)内衬之不锈钢高压釜中,并在烘箱中于85 ℃下加热2 h. 合成后的MIL-88A分别用无水乙醇和超纯水各洗涤五次,然后在高速离心机5000 r·min−1下离心10 min来回收沉淀. 最终在真空干燥箱中100 ℃下干燥10 h获得纯MIL-88A粉末.
-
合成的MIL-88A的形态通过扫描电子显微镜(SEM,日本日立SU5000)测得. 晶体结构通过X射线衍射(XRD,德国Bruker D8)进行鉴定. X射线光电子光谱(XPS,美国Thermo Fisher Scientific Escalab 250Xi)探究材料的化学性质,表明材料中的化学元素分布及组成. 官能团通过傅里叶变换红外光谱分析仪(FTIR,美国Thermo Fisher Scientific FT-IR6700)在4000 cm−1至450 cm−1范围内进行研究. 光催化活性通过紫外-可见漫反射光谱(UV-vis DRS,日本岛津SHIMADZU UV270)结果去表征. 材料的热稳定性采用热重分析仪(TGA,中国1000C)进行分析.
-
急性毒性测定按中国国家标准方法GB/T15441-1995进行,使用明亮发光杆菌T3(购自南京土壤研究所)对每个样品进行生物发光测定. 所有样品都使用牛过氧化氢酶来防止H2O2残留的影响. 使用酶标仪测量明亮发光杆菌的发光强度,急性毒性评价结果表示为发光抑制值[13](1−Lt/Lc,其中Lt是样品的发光强度,Lc是对照品在3% NaCl溶液中的发光强度),发光抑制值越高表明样品的急性毒性越大. 每项试验中都包含有HgCl2(Ⅱ)的阳性对照样品.
-
所有实验均在室温((25±1) ℃)下于体积为500 mL带有循环冷却装置的烧杯中进行. 使用光强度约为800 mW·cm−2的300 W氙灯(HF-GHX-XE-300,上海贺帆仪器)作为可见光源,光源与装有MIL-88A和DDBAC溶液的烧杯之间的距离固定为10 cm. 反应前在黑暗中磁力搅拌溶液30 min,以实现MIL-88A和DDBAC溶液之间的吸附-解吸平衡. 可见光照射后,每5 min抽取2 mL反应溶液,并通过0.22 μm水相针式过滤器过滤,加入20 μL异丙醇(IPA)以消除未反应的·OH自由基,送至高效液相色谱(HPLC)测试.
DDBAC的降解动力学通过DDBAC降解的反应速率常数来评估. 各实验体系均较好的遵循了准一阶速率动力学,因此通过准一阶模型来拟合实验数据[21],如以下方程式:
其中,Ct(mg·L−1)为处理时间t(min)时测得的DDBAC浓度,C0(mg·L−1)为初始DDBAC浓度,kobs为观察到的准一级反应速率常数(min−1). 通过该方程,kobs可计算为ln(Ct/C0)和t之间拟合线性回归的斜率.
-
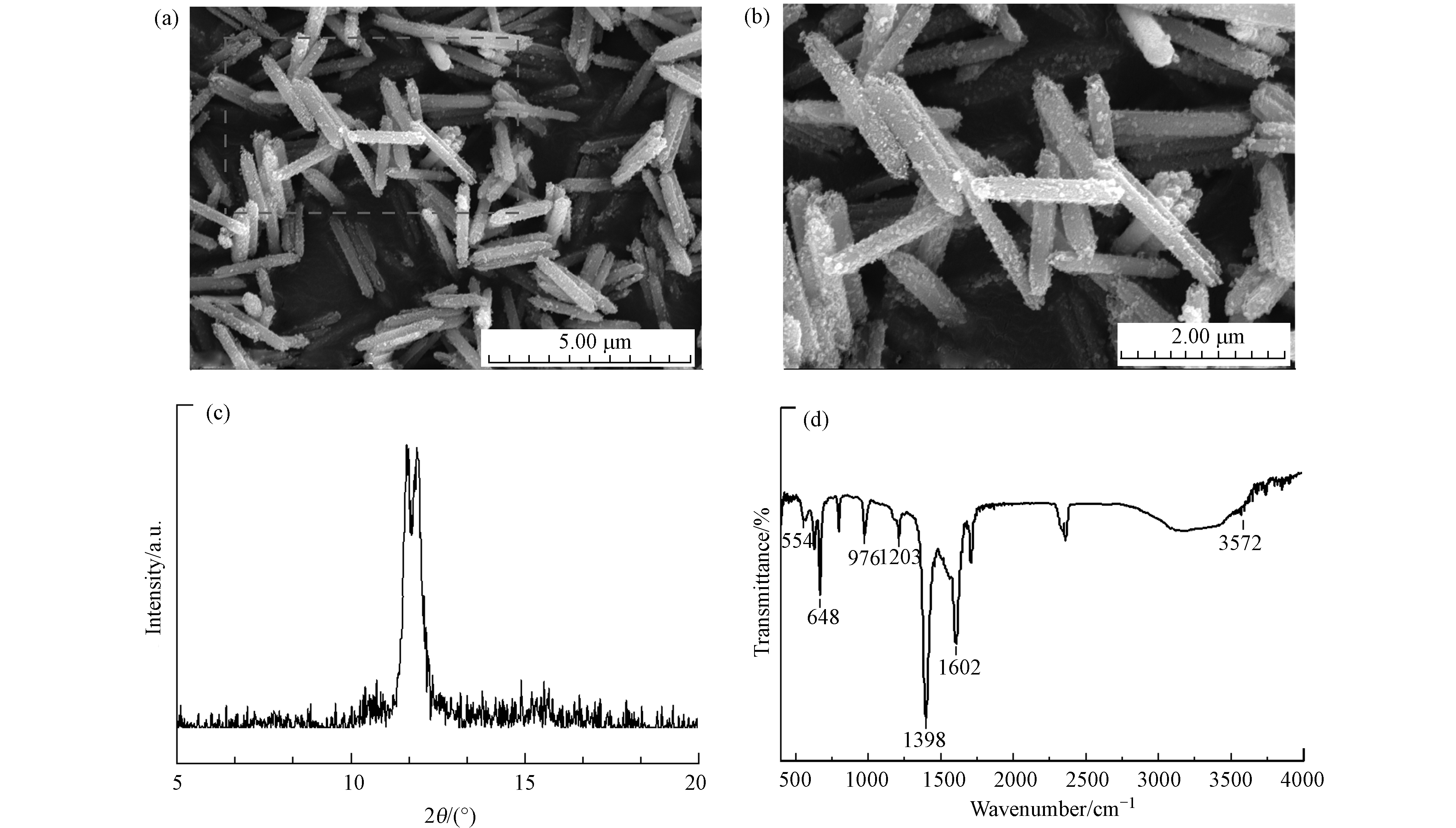
合成的MIL-88A的SEM图像如图1(a)、(b)所示,MIL-88A颗粒呈现六角棒状形态,大小尺寸分布相对均匀,这是文献报道中的典型形状[19, 22]. 根据研究发现,MIL-88A的类芬顿活性受纳米晶体形状的影响,形状不同导致光催化活性位点不同,进而影响光催化活性[23]. 六角棒状相对于MIL-88A的纺锤状和金刚石状的两种形态,它的光催化活性更好. 进一步分析MIL-88A的晶体结构,XRD图谱如图1(c)所示,在2θ位置11.6°和11.9°处显示出强峰,确保了MIL-88A的结晶性质. 有研究表明,XRD图谱和结晶度受合成参数影响[20],本研究中的MIL-88A是在85 ℃下晶化2 h的条件下合成,与类似条件下合成的MIL-88A的XRD图谱一致[24]. 此外,还评估了MIL-88A的FTIR光谱,由于合成MIL-88A的有机配体是富马酸,大部分主要特征峰都来自于它. 如图1(d)所示,1398 cm−1和1602 cm−1的峰分别来自富马酸中的羧基(—COOH)的对称和不对称振动模式[24],674 cm−1处的峰归因于羰基[25]. 1203、976、554 cm−1的峰分别属于富马酸中的C—O的拉伸振动、C—H的弯曲振动和Fe—O的拉伸振动[26]. 以上表征结果证实了MIL-88A材料的成功合成.
-
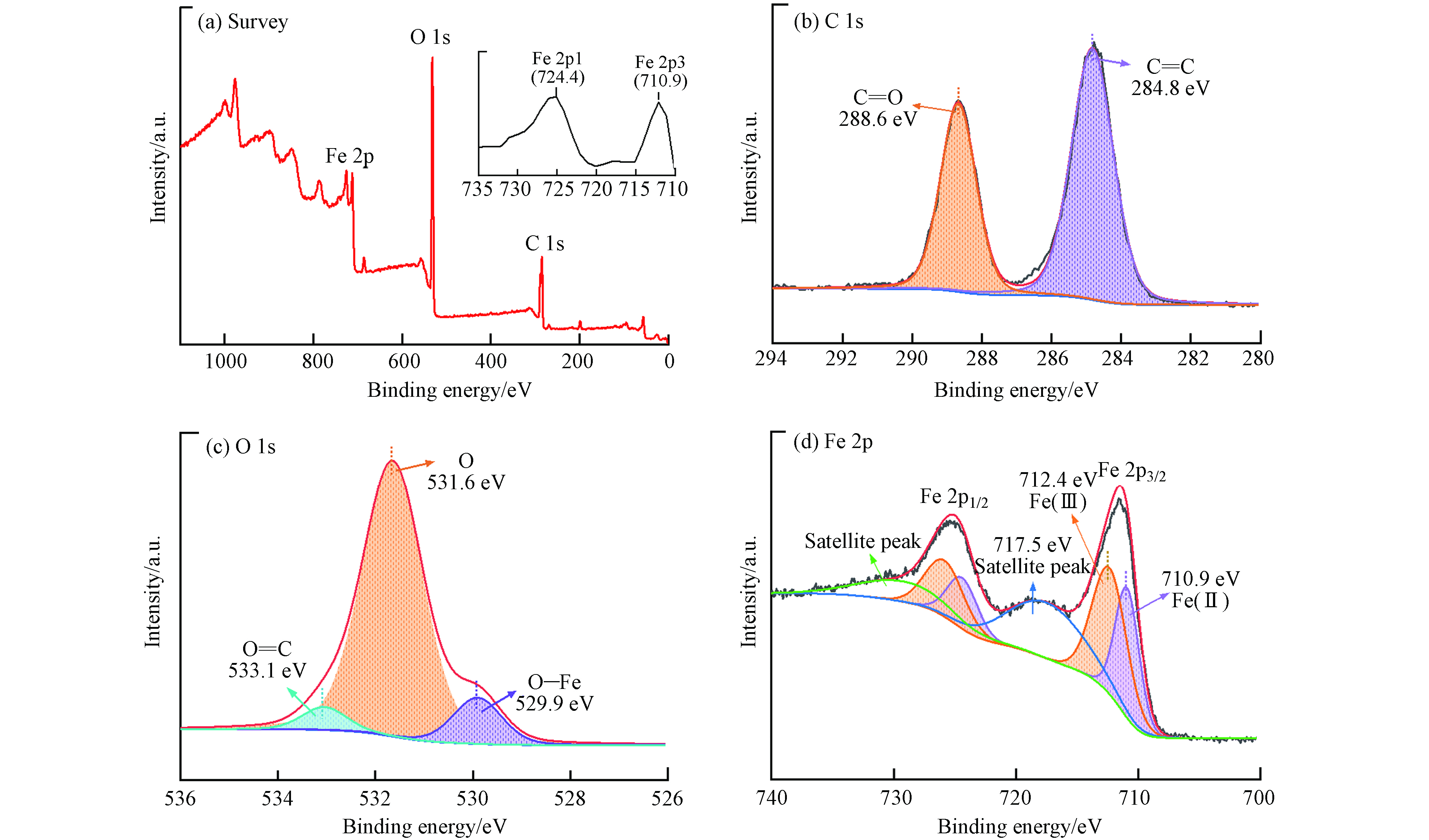
通过XPS测试确定MIL-88A的化学成分和键合. 如图2(a)所示,XPS光谱结果表明MIL-88A主要存在Fe、C和O元素. MIL-88A的C1s光谱如图2(b)所示,在288.6 eV和284.8 eV处出现的峰归因于羧基中的C—O和苯甲环中的C=C[22]. O1s的高分辨率光谱如图2(c)所示,在533.1 eV、531.6 eV和529.9 eV的3个峰分别归因与富马酸的O—C、O和O—Fe[27]. 图2(d)显示了Fe2p的高分辨率XPS光谱,710.9 eV和724.4 eV的峰分别归因于Fe2p3/2和Fe2p1/2,表明MIL-88A中存在Fe(Ⅱ)和Fe(Ⅲ)[28]. 在717.5 eV处的卫星峰证明MIL-88A中存在Fe(Ⅲ),因此XPS光谱的分析结果也进一步证明了MIL-88A的成功制备.
-
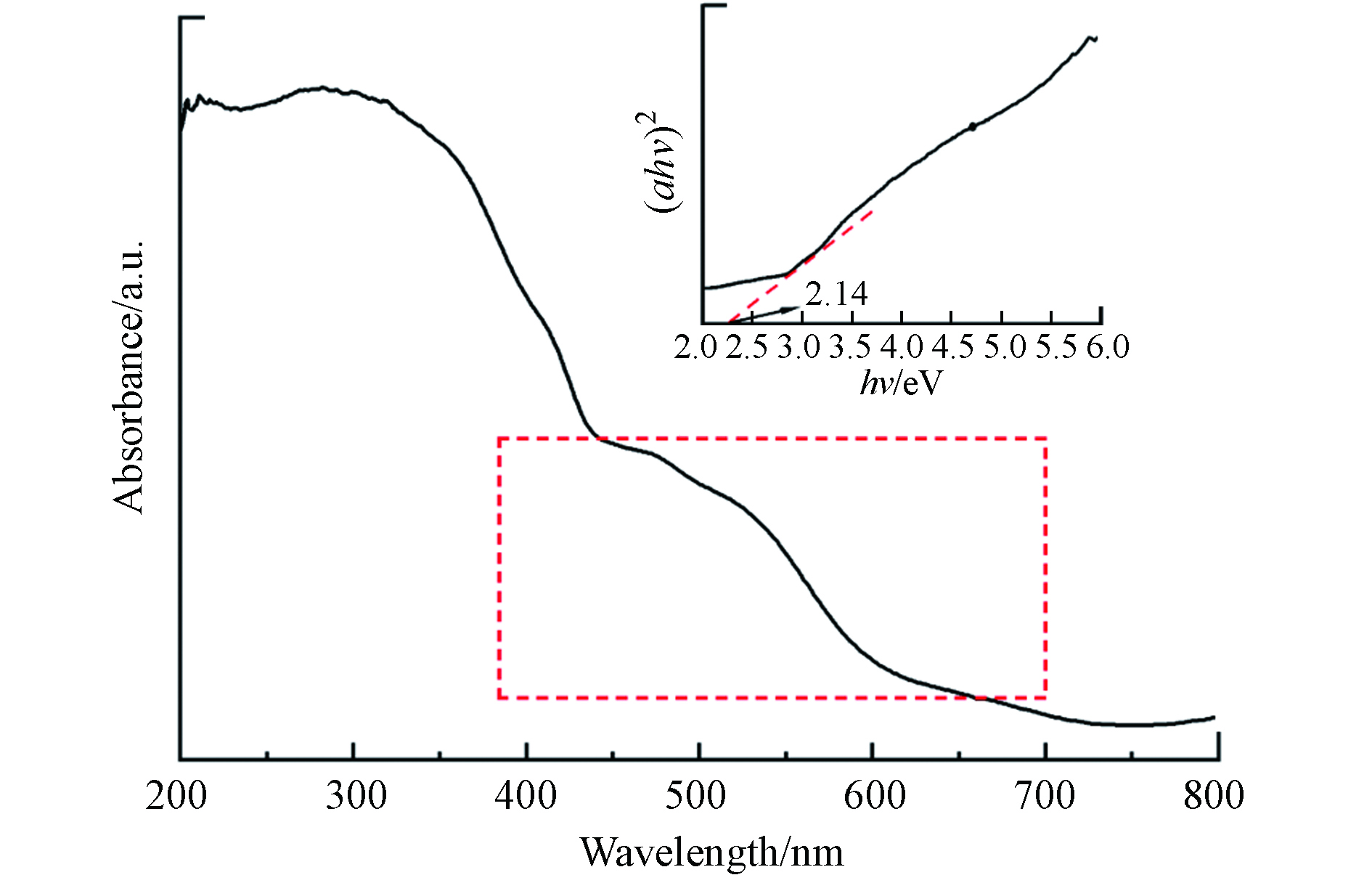
使用UV-vis DRS分析对MIL-88A的光学吸收特性进行了表征. MIL-88A的UV-vis DRS光谱图3显示了几个明显的吸收峰,其吸收边约为700 nm. MIL-88A的可见光吸收应该是由O2−到Fe3+的配体-金属电荷转移(LMCT)的光学跃迁引起的[29].
合成的MIL-88A的带隙能通过使用以下等式从UV-vis吸收光谱计算:
其中α是吸收系数,B是比例常数,h是普朗克常数,υ是光子的频率,Eg是吸收带隙[17]. 在一定条件下,带隙能越低,光照射下产生的光生电子和空穴浓度越高,光催化活性越高[30, 31]. 通过Tauc图(图3)估计MIL-88A带隙能为2.14 eV,表明其具有良好的光催化活性.
-
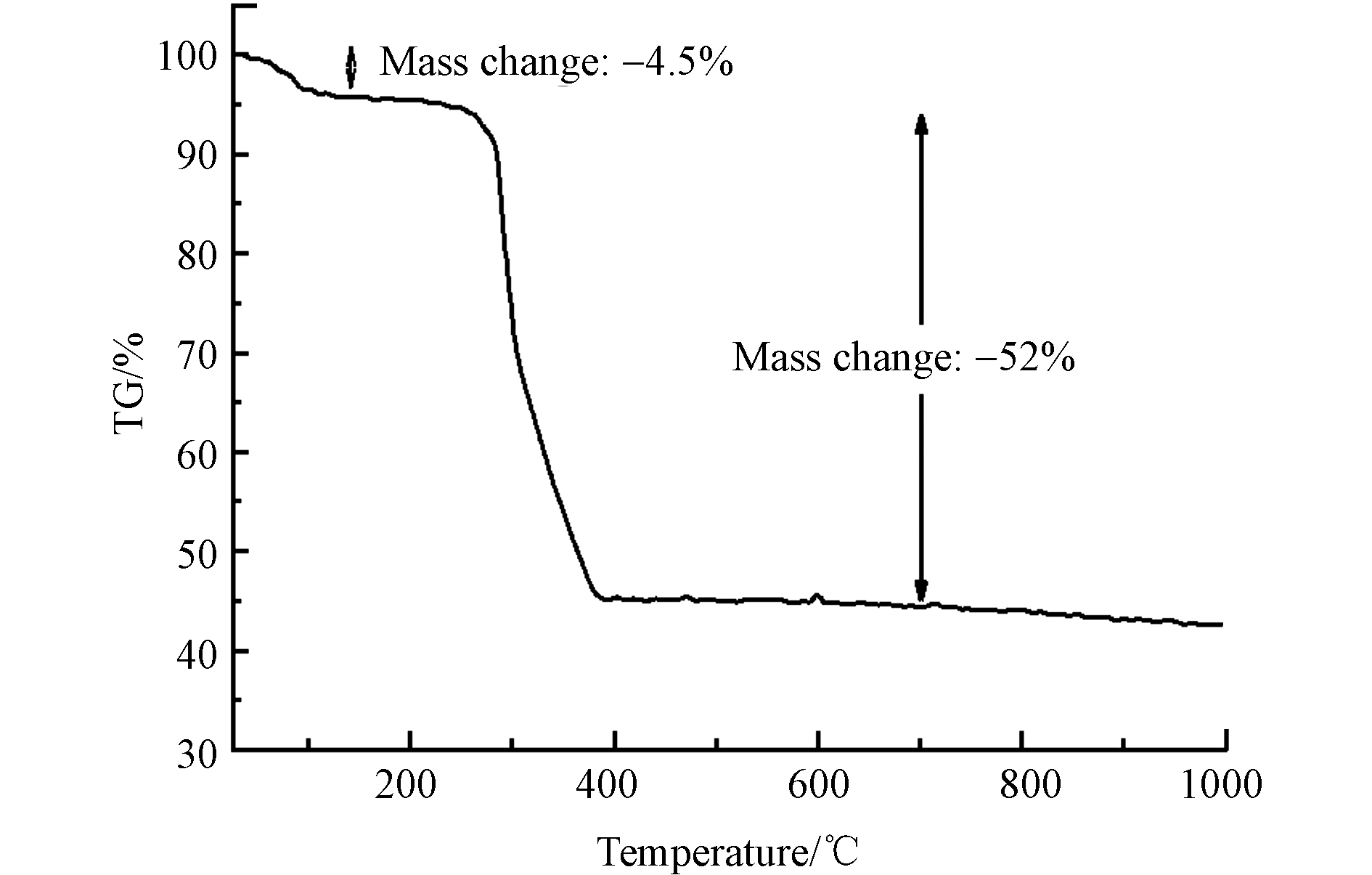
通过TGA评估了MIL-88A的热稳定性,结果显示了MIL-88A在不同温度下的重量损失. 图4的MIL-88A的TGA曲线表明,在110℃以下发生了4.5%的重量损失,这可能是由于MIL-88A吸附的水分和其他气体分子蒸发导致. 110—300℃下MIL-88A的重量保持相对稳定. 300—400 ℃,由于MIL-88A的配体富马酸开始分解,导致MIL-88A的重量大幅下降,发生了52%的重量损失. 在400 ℃之后MIL-88A的重量几乎没有变化. 因此,MIL-88A具有良好的热稳定性.
-
为了证实MIL-88A能够在可见光下有效地活化H2O2,进行了对照实验. 由于MIL-88A具有高比表面积,检查DDBAC的降解是否仅通过MIL-88A的吸附而产生至关重要. 如图5(a)所示,在黑暗条件下,将MIL-88A加入到DDBAC溶液中. 即使MIL-88A与DDBAC溶液混合70 min,溶液中DDBAC的浓度几乎没有下降,说明DDBAC不能简单地通过MIL-88A的吸附作用去除. 然而在黑暗条件下加入MIL-88A混合30 min后再投加H2O2时,DDBAC出现了轻微的降解,40 min内DDBAC的降解率为15%,这可能是由于MIL-88A表面的Fe-O团簇通过类Fenton反应产生·OH氧化所致,其反应描述为方程(4)和(5)[32]. 另外,在可见光照射的条件下,分别添加MIL-88A和H2O2对DDBAC的降解也是有限的,40 min内DDBAC的降解率仅为10%和21%. 当在可见光照射下同时加入MIL-88A和H2O2,DDBAC的降解明显增加,35 min内DDBAC的降解率为100%. DDBAC的降解数据符合拟一阶动力学模型. 图5(b)显示了DDBAC在Light/MIL-88A/H2O2体系中的kobs为0.067 min−1,比在其他体系中的kobs大了一个数量级. Light/MIL-88A/H2O2对DDBAC的协同氧化可能是由于加速了活性物质(例如·OH)的形成,这将在第2.4节中进一步讨论. 上述结果表明,MIL-88A作为一种非均相光Fenton催化剂在可见光下实现了DDBAC的高效降解.
-
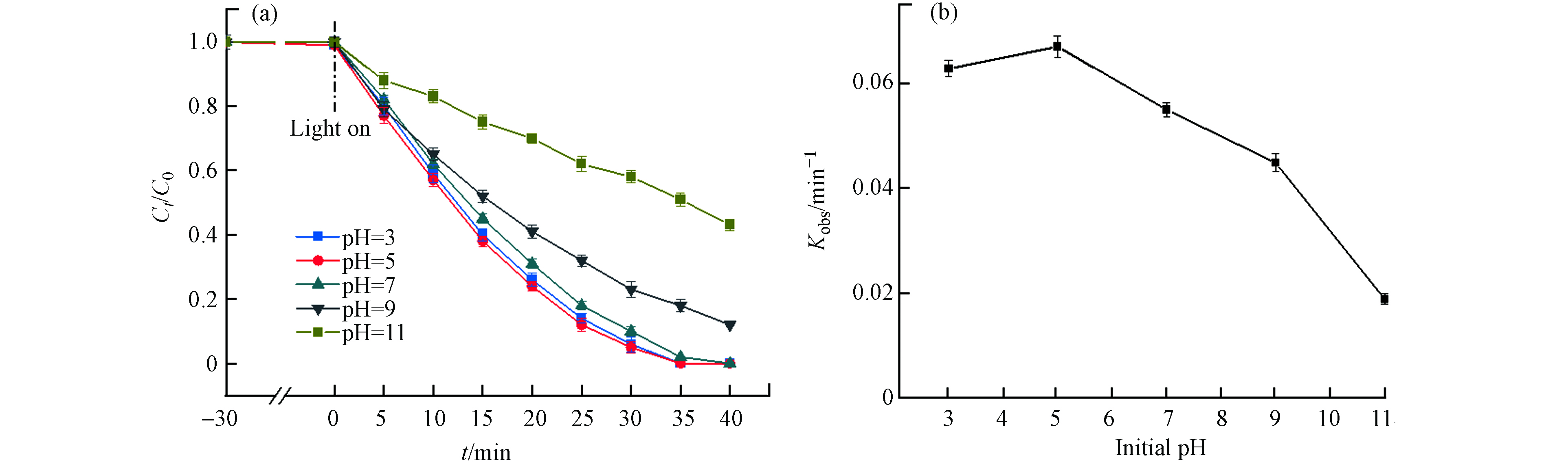
初始pH值对MIL-88A光Fenton氧化降解DDBAC的影响如图6(a)所示. 随着pH值从3增加到11,DDBAC在35 min内的降解率从100%降低到49%. 通过评估pH对降解速率常数(kobs)的影响,可以更清楚地观察到碱性条件对DDBAC降解的抑制作用,如图6(b)所示. 在酸性条件下,kobs始终保持在一个较高的水平. 这种现象最可能的原因是酸性条件下H+的存在能够阻止H2O2分解成HOO−,而HOO−是羟基自由基的捕获剂,对·OH有较强的清除作用. 随着pH值降低到3,kobs略有降低,可归因于H2O2是一种弱酸,在强酸条件下过于稳定,因此pH值过低反而会使H2O2的氧化能力降低. 随着pH值从5逐渐升高到11,kobs从0.067 min−1下降到0.018 min−1,kobs下降了73%. kobs随着pH值的升高下降幅度逐渐增大,这可能存在两方面原因. 一方面,当pH呈碱性时,H+被水体中的OH−消耗,促使H2O2水解为HOO−,H2O2大量被消耗导致·OH的浓度降低. 另一方面,降解产生的CO2在碱性溶液中易转化为
${\rm{CO}}_3^{2-} $ 和${\rm{HCO}}_3^{-} $ ,同样,·OH的形成在这里起着重要作用,${\rm{CO}}_3^{2-} $ 和${\rm{HCO}}_3^{-} $ 可与·OH的反应生成反应性较低的·${\rm{CO}}_3^{-} $ ,两种离子均为·OH的抑制剂(k(OH·,${\rm{CO}}_3^{2-} $ )=3.9×108 mol· L−1·s−1,k(OH·,${\rm{HCO}}_3^{-} $ )=8.5×106 mol· L−1·s−1)[33 − 34]. 因此,在偏酸性条件下Light/MIL-88A/H2O2氧化降解DDBAC的效率更高. -
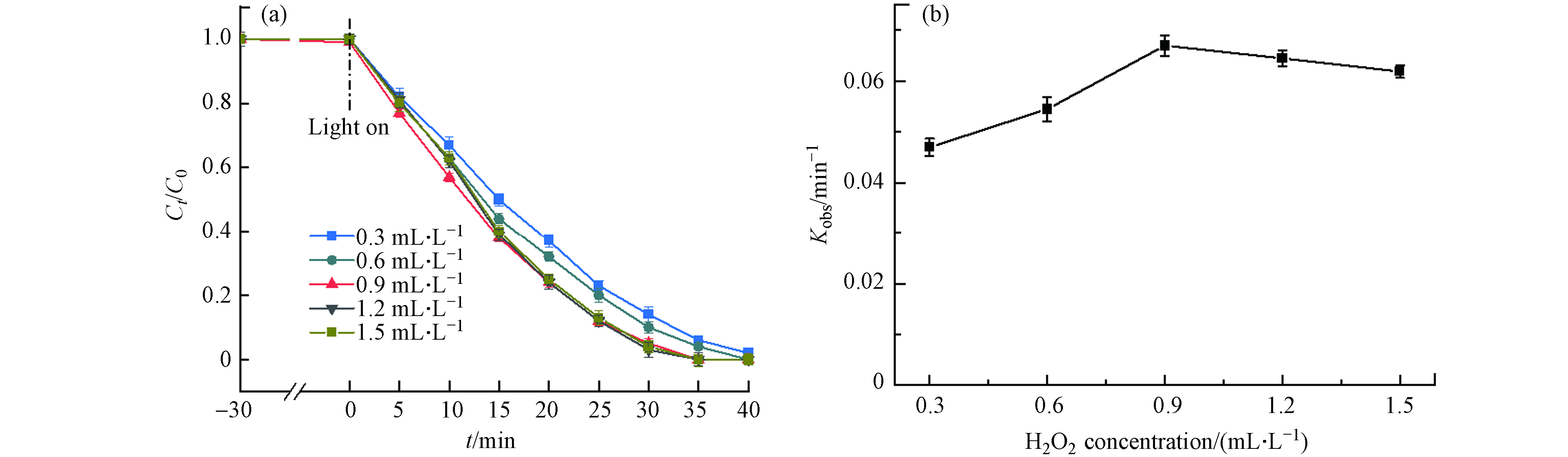
由于H2O2能够产生具有强氧化性的·OH,进一步研究了H2O2浓度对DDBAC降解的影响,如图7(a)所示. 当H2O2用量从0.3 mL·L−1增加到0.9 mL·L−1时,30 min内DDBAC的降解率从84%提高到95%. 而H2O2浓度进一步提高到1.5 mL·L−1,从图7(b)可以观察到kobs不仅没有升高反而还略微降低,这可能是水溶液中的H2O2量达到饱和甚至过量. H2O2在Light/MIL-88A体系下能够产生具有极强氧化性的·OH,当H2O2浓度未达到饱和前,·OH的浓度随着H2O2的浓度的增加而增加,DDBAC的降解率也随之增加. 但当溶液中存在过量的H2O2,H2O2既是·OH的发生剂又是·OH的清除剂,这与Zhang等[15]对BAC降解的观察结果一致. 因此,当H2O2的浓度为0.9 mL·L−1时,光Fenton氧化降解DDBAC的效率最高.
-
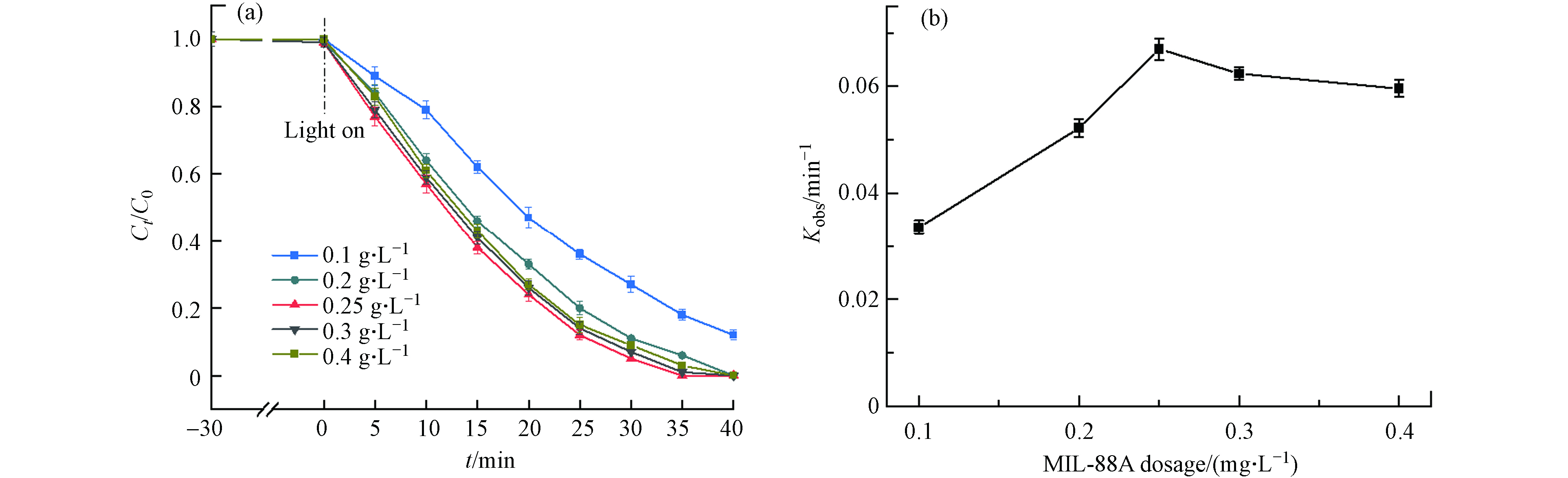
MIL-88A的剂量毫无疑问是光Fenton氧化降解DDBAC的另一重要因素. 一般而言,MI-88A的剂量越多,催化效率越高. 但在实际研究中,MIL-88A的剂量存在一个最佳剂量. 如图8(b)所示,MIL-88A的剂量在0.25 g·L−1时降解速率常数最大. 当MIL-88A的剂量从0.1 g·L−1增加到0.25 g·L−1时,如图8(a)所示DDBAC的降解率在30 min内从86%提高到95%,这归因于MIL-88A的剂量的增加将为H2O2的活化提供了更多的催化活性位点,从而产生更多的·OH. 当MIL-88A的剂量继续增加到0.4 g·L−1,kobs却从0.067 min−1下降到0.062 min−1,这可能是由于催化剂的聚集而导致. Le等[35]研究表明催化剂聚集不仅可能会减少活性位点的可用数量而且会增加光散射. 其次,大量的催化剂引起的竞争反应会减少有效自由基的量[36]. 因此,MIL-88A的剂量为0.25 g·L−1时,光Fenton氧化降解DDBAC的效率最高.
-
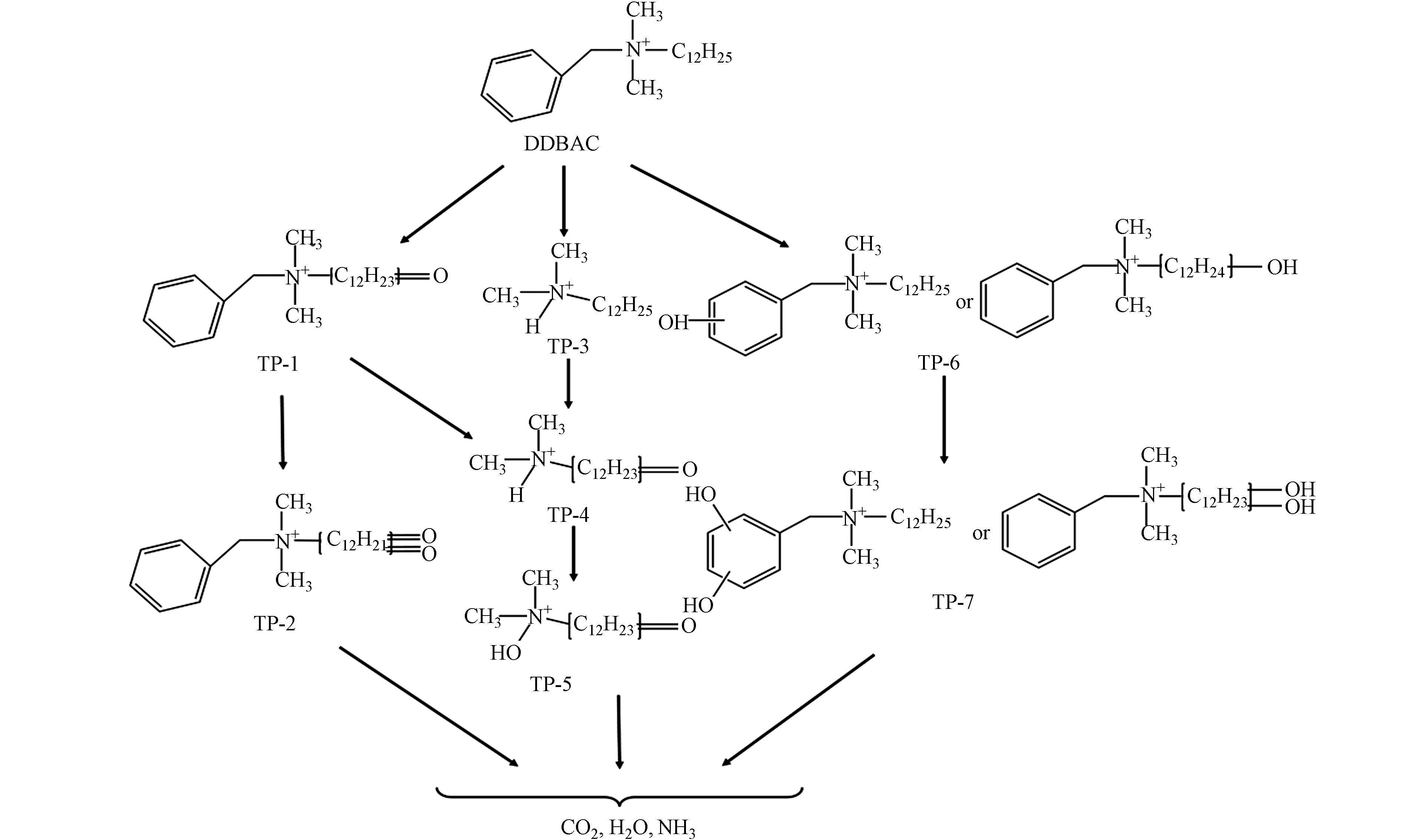
通过对UHPLC-Q-TOF的分析,在光Fenton氧化降解DDBAC过程中发现了7种主要的氧化产物(TP). 这些TPs的详细信息如色谱保留时间、质荷比(m/z)数据、和建议的结构等见表1. 在可见光下,Light/MIL-88A/H2O2降解DDBAC可能存在3种降解途径,如图9所示. 观察到了三种降解机制:烷基链的氢提取(TP-1);DDBAC(TP-3,叔铵盐)的苄基-氮键断裂;以及在芳香环或烷基链上添加羟基(TP-6,羟基化).
第一种可能的降解途径是氢提取过程. 通过羟基自由基对DDBAC的长烷基链进行抽氢,然后和溶液中的溶解氧反应生成羰基化合物TP-1(m/z 318.3,C21H36NO). TP-2(m/z 332.3,C21H34NO2)是对TP1的长烷基链进一步抽氢而形成的. 在O3和氯协同氧化DDBAC的过程中也观察到了相同的途径[3]. 第二种可能的降解途径通过苄基-氮键裂解而实现. 叔铵盐TP-3(m/z 214.3,C14H32N)是由DDBAC的苄基-氮键裂解而成. 在UV/过硫酸盐氧化DDBAC的过程中也检测到类似的氧化产物[13]. TP-4(m/z 228.3,C14H30NO)是由TP-1(m/z 318.3,C21H36NO)的苄基-氮键断裂形成. 此外,对TP-3通过羟基自由基抽氢,也可形成TP-4. 然后,TP-4的羟基化作用导致TP-5(m/z 244.3,C14H30NO2)的形成. 最后一种可能的降解途径通过羟基化作用而导致. TP-6(m/z 320.3,C21H38NO)是DDBAC的羟基化产物,根据羟基化的位置产生两种中间产物. 此外,TP-6的进一步羟基化作用形成TP-7(m/z 336.3,C21H38NO2)的形成. 最终,DDBAC及其中间体在包括羟基自由基在内的活性物质的攻击下被矿化为CO2、H2O和NH3.
-
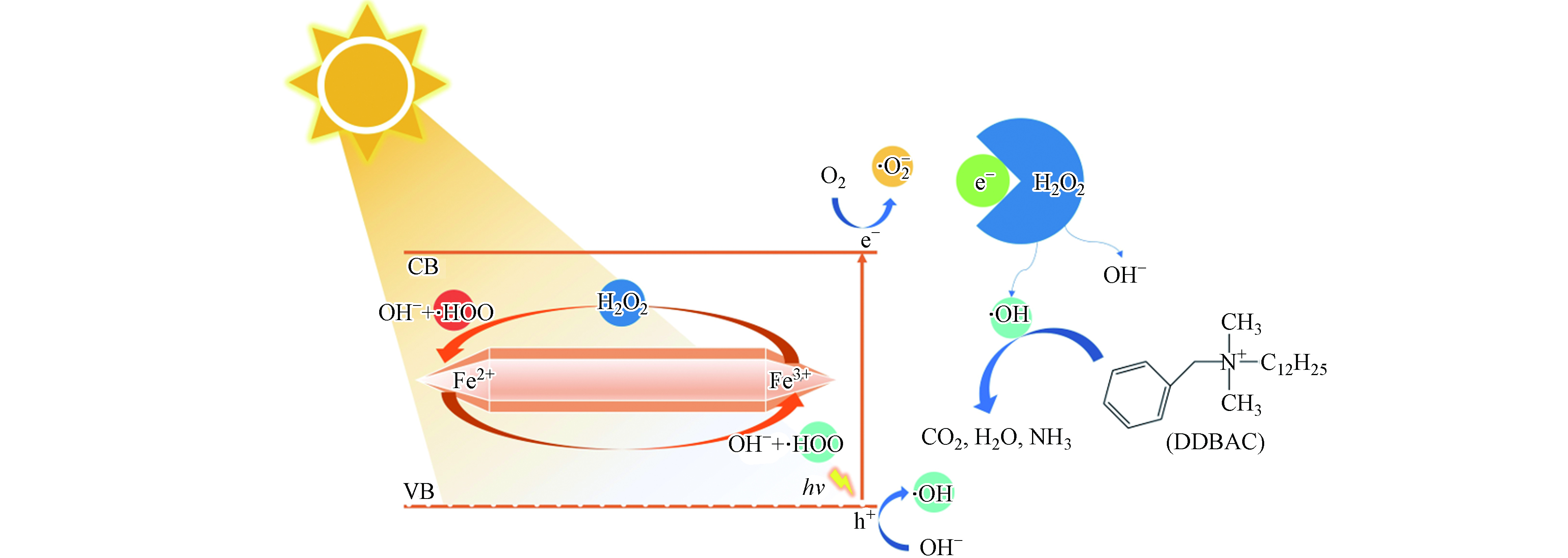
利用分子轨道理论对DDBAC的降解机理进行了探讨. 在可见光照射下,当MIL-88A被能量等于或大于其带隙的光子照射时,电子(e−)将从HOMO激发到LUMO,在HOMO上留下空穴(h+),其描述为方程(6)[37]. h+具有较强的氧化性,可以直接氧化吸附有机分子,还可以通过与氢氧根离子(OH−)反应生成具有强氧化能力的·OH(方程7))[37]. 同时,e−可以被溶解的O2捕获生成·
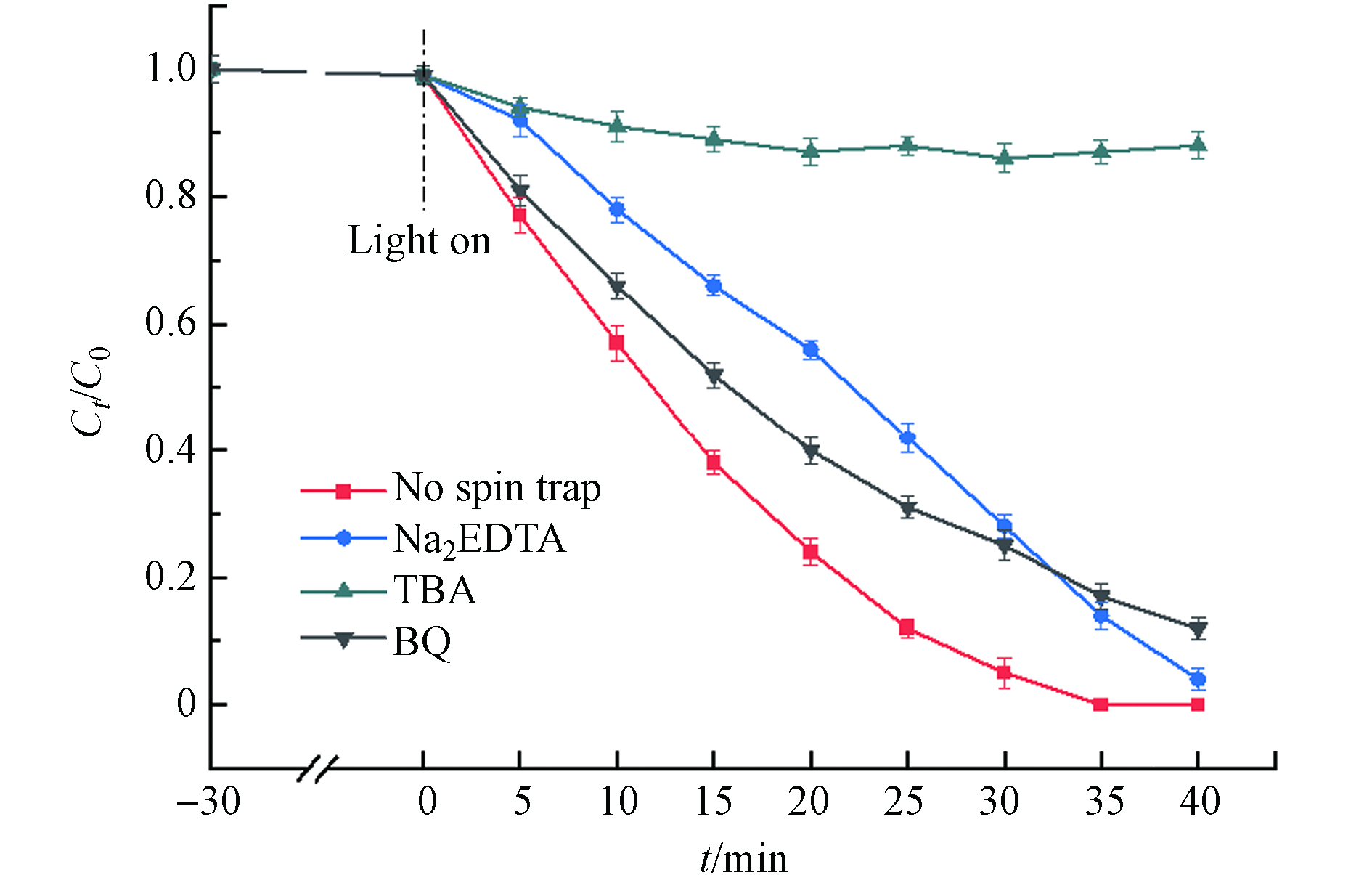
${\rm{O}}_2^- $ (方程(8))[38],由此产生的·${\rm{O}}_2^- $ 也具有较强的氧化能力. 然而,在第2.2.1节中可以了解到,单独的MIL-88A在可见光的照射下对DDBAC的降解受到限制,这可能归因于h+和e−的快速复合. 因此,引入外部电子受体(H2O2)有望可以抑制e−和h+的快速复合,从而提高光催化降解DDBAC的效率. 图5(a)显示,H2O2的加入显著提高了光降解效率,这归因于H2O2作为电子受体能够捕获e−而生成·OH(方程(9)). 综上所述,光Fenton是氧化降解DDBAC的主要机制,如图10所示.由于h+、·
${\rm{O}}_2^- $ 和·OH均具有较强的氧化能力,为了研究光Fenton降解DDBAC过程中起主要作用的活性物种,进行了自由基捕获实验. 用0.5 mmol·L−1 Na2EDTA,0.5 mmol·L−1对苯醌(BQ)和0.5 mmol·L−1 TBA分别捕获h+、·${\rm{O}}_2^- $ 和·OH. 如图11所示,TBA的加入明显抑制了DDBAC的降解效率,而Na2EDTA和BQ的加入使DDBAC的降解略有下降,说明h+和·${\rm{O}}_2^- $ 在光Fenton体系中起次要作用. 这些结果证明了在光Fenton氧化DDBAC过程中起主要作用的活性物种是·OH. -
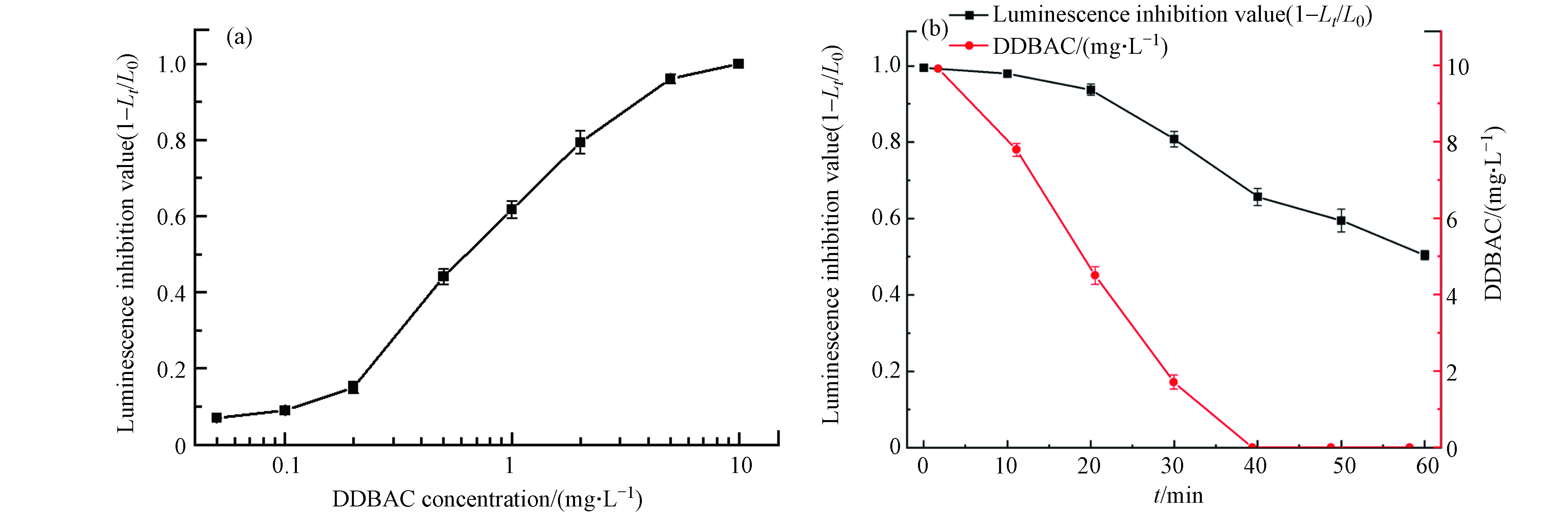
使用发光细菌(明亮发光杆菌T3)评估了DDBAC在MIL-88A光Fenton氧化处理过程中的急性毒性,将明亮发光杆菌的发光抑制值作为本研究中样品的急性毒性. DDBAC和明亮发光杆菌之间的浓度-反应关系如图12(a)所示,发光抑制值随着DDBAC浓度的增大而加速升高. 当DDBAC浓度为0.89 mg·L−1时,发光抑制值为0.58,这与Lee等[13]评估的发光抑制值接近. 当DDBAC浓度大于5 mg·L−1时,明亮发光杆菌的发光强度完全被抑制.
此外,图12(b)探究了MIL-88A光Fenton氧化工艺对DDBAC在不同反应时间下的解毒和降解效果. 该反应下的初始DDBAC浓度为10 mg·L−1. 当DDBAC在40 min内完全降解后,DDBAC溶液的发光抑制值下降到0.66. 在40 min内,发光抑制值与DDBAC浓度呈相似的下降趋势,这表明随着DDBAC从溶液中去除,急性毒性可以减弱. 然而,DDBAC完全去除后的溶液的残留毒性表明,在光Fenton过程中产生了有毒中间体. Yang等[39]和Huang等[9]的研究表明,在AOPs降解磺胺甲恶唑和DDBAC时也产生了毒性更强的中间体. 在40 min后,发光抑制值随着反应的进行而继续下降,60 min时发光抑制值为0.5. 毒性持续降低表明光Fenton氧化工艺能够进一步氧化有毒中间体,从而实现脱毒. 因此,Light/MIL-88A/H2O2是去除DDBAC和溶液脱毒的有效方法.
-
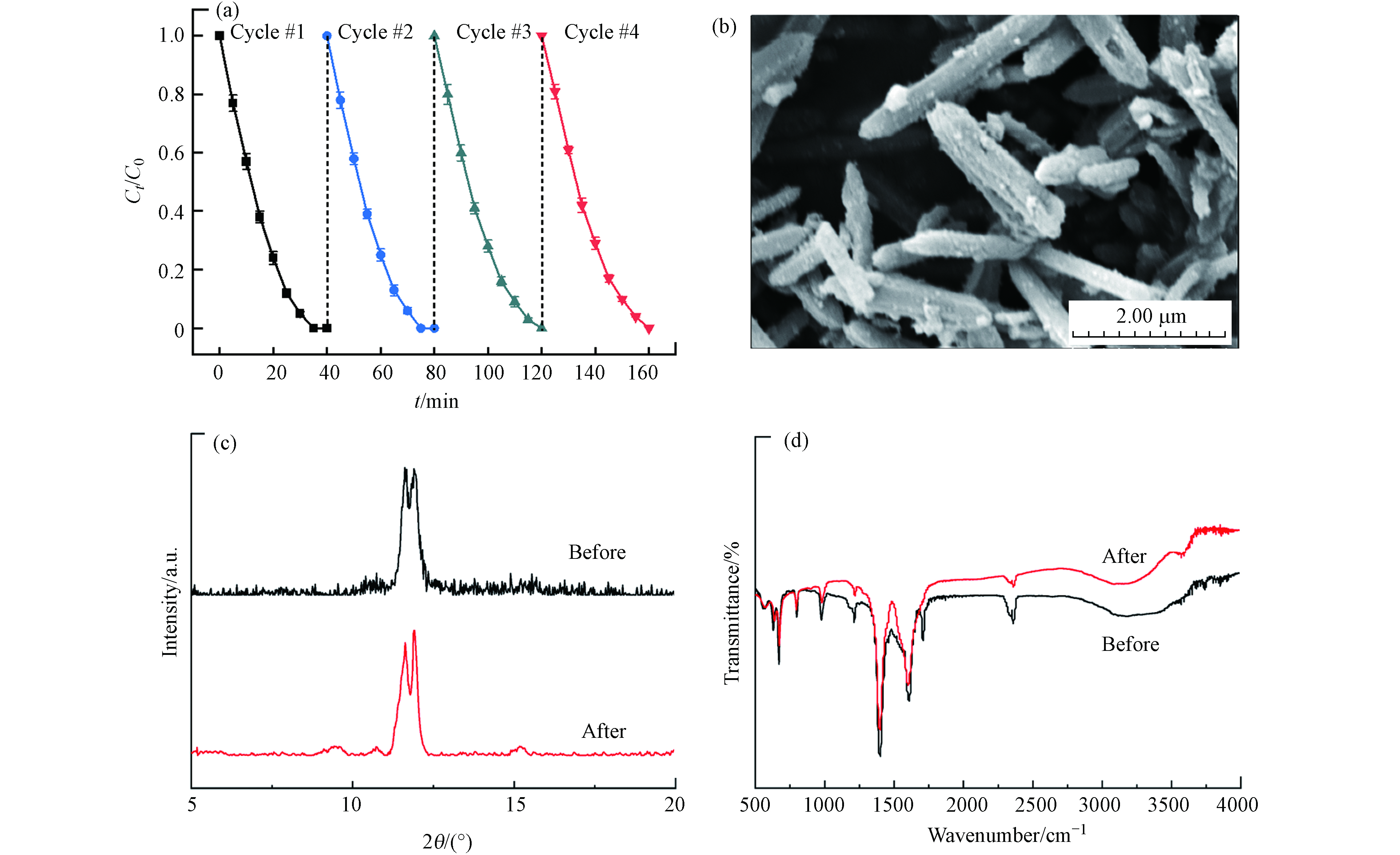
稳定性是评价多相光催化剂的重要参数. 为了考察MIL-88A的长期稳定性,进行了降解DDBAC的循环实验. 在每一次循环后,将MIL-88A进行回收并重新分散到DDBAC溶液中. 回收过程是通过离心分离和真空烘箱干燥完成的. 从图13(a)所示,经过4次循环后DDBAC的降解效率没有明显下降,表明MIL-88A具有稳定有效的光催化活性. 通过SEM、XRD和FT-IR进一步检查了经过4次循环前后的形态、晶体结构和化学状态. 图13(b)扫描电镜结果表明,反应后的MIL-88A仍保留了六角棒状形态,但尺寸相比于反应前变得更小,这可归因于MIL-88A的表面发生了氧化过程所导致. 此外,XRD和FT-IR的分析结果表明,反应后的MIL-88A的衍射图与最初合成的MIL-88A衍射图相似,仅在相应的峰强度(图13(c,d))上有所差异. 这些结果表明MIL-88A在光Fenton降解有机物方面具有良好的稳定性.
-
1)MIL-88A的引入成功实现了DDBAC在可见光下的降解. Light/MIL-88A/H2O2体系在pH=5,H2O2浓度为0.9 mL·L−1,MIL-88A剂量为0.25 g·L−1时,降解效果最佳,35 min内DDBAC降解效率达到100%.
2)DDBAC的降解机制:主要是由于MIL-88A作为光催化剂能够活化H2O2产生羟基自由基而产生的氧化作用.
3)急性毒性评估结果表明Light/MIL-88A/H2O2体系能够去除DDBAC,并对产生的有毒中间体进一步降解,实现溶液脱毒.
4)MIL-88A作为光催化剂不仅展现了优异的光催化性能,而且具有较强的稳定性. MIL-88A在可见光下光催化处理有机废水方面有很好的应用潜力.
MIL-88A作为非均相光芬顿催化剂在可见光下降解水中苯扎氯铵:性能、降解途径和急性毒性评估
MIL-88A as a heterogeneous photo-Fenton catalyst for the degradation of benzalkonium chloride under visible light: Performance, degradation pathway, and toxicity evaluation
-
摘要: 随着新型冠状病毒肺炎(COVID-19)在全球的流行,主要成分为苯扎氯铵的消毒剂大量使用对环境带来威胁. 本研究通过水热法成功制备了铁基金属有机骨架MIL-88A,将其作为光催化剂成功实现了苯扎氯铵在可见光下的高效降解. 通过SEM、XRD、XPS以及UV-vis DRS等表征方法研究了MIL-88A的形貌、结构以及光催化性能. 为了达到光催化降解苯扎氯铵的最佳效率,探究了MIL-88A在不同条件下的光催化降解性能. 结果表明,MIL-88A在pH=5,H2O2投加量为0.9 mL·L−1,MIL-88A剂量为0.25 g·L−1时,降解效果最好,35 min DDBAC降解效率达到100%. 采用UHPLC-Q-TOF-MS确定了降解中间产物,分析了苯扎氯铵可能的降解途径. 此外,基于分子轨道理论和自由基淬灭实验证明了氧化降解苯扎氯铵过程中羟基自由基是主要贡献者. 通过发光细菌法对DDBAC及其中间体的毒性进行了评估,结果表明,MIL-88A可见光光芬顿工艺能够实现溶液脱毒. 光催化降解循环实验以及对催化剂反应前后的表征证明了MIL-88A具有较高的稳定性.Abstract: With the increased global epidemic of novel coronavirus disease (COVID-19), the extensive use of disinfectants containing high levels of benzalkonium chlorides (BACs) poses a threat to the environment. In this study, iron-based organic framework MIL-88A was successfully prepared by hydrothermal method, which was used as a photocatalyst to achieve efficient degradation of BACs under visible light. The morphology, structure and photocatalytic properties of MIL-88A were studied by SEM, XRD, XPS and UV-vis DRS characterization. In order to achieve the best photocatalytic degradation efficiency of BACs, the photocatalytic degradation performance of MIL-88A was investigated under different conditions. The results showed the degradation efficiency of DDBAC was the best at pH=5, H2O2 concentration of 0.9 mL·L−1, and MIL-88A dosage of 0.25 g·L−1, and the degradation efficiency of DDBAC reached 100% after 35 minutes. The degradation intermediates were determined by UHPLC-Q-TOF-MS and the possible degradation pathway of BACs was analyzed. In addition, it was demonstrated that hydroxyl radicals are the main contributors to the oxidative degradation of benzalkonium chloride based on molecular orbital theory and free radical quenching experiments. The toxicity of DDBAC and its intermediates was assessed by the luminescent bacterial method, and the results showed that the MIL-88A visible light photo-Fenton process was able to achieve solution detoxification. The photocatalytic degradation cycle experiment and the characterization of the catalyst before and after the reaction proved that MIL-88A had high stability.
-
Key words:
- MIL-88A /
- DDBAC /
- visible light /
- degradation pathway /
- degradation mechanism /
- toxicity assessment.
-

-
图 5 (a)不同条件对DDBAC降解的影响;(b)不同条件下的DDBAC降解反应速率常数kobs实验条件:[DDBAC]0 = 40 mg·L−1;[H2O2]0 = 0.9 mL·L−1;[MIL-88A]0 = 0.25 g·L−1;pH = 5
Figure 5. (a) Effects of comparative experiments on the DDBAC photo-Fenton oxidation process;(b) degradation rate constants (kobs)as a function of the photo-Fenton oxidation process
图 12 (a)不同DDBAC浓度对发光细菌的急性毒性;(b)光Fenton氧化过程中DDBAC溶液的急性毒性和DDBAC浓度变化实验条件:[DDBAC]0 = 10 mg·L−1;[H2O2]0 = 30 μL·L−1;[MIL-88A]0 = 50 mg·L−1;pH = 7
Figure 12. (a) Acute toxicity of DDBAC of different concentrations to luminescent bacteria;(b) Acute toxicity and DDBAC concentration changes in DDBAC solutions during photo-Fenton oxidation
表 1 DDBAC氧化产物的经验公式和可能的结构
Table 1. The proposed empirical formulas and structures of oxidation products of DDBAC
化合物
Compound保留时间/min
Retention
Time
可能的化学式
Proposed empirical
formula质荷比
Mass (m/z)可能的结构
Proposed structure理论值
Theoretical实验值
ExperimentalDDBAC 1.64 C21H38N 304.3004 304.3026 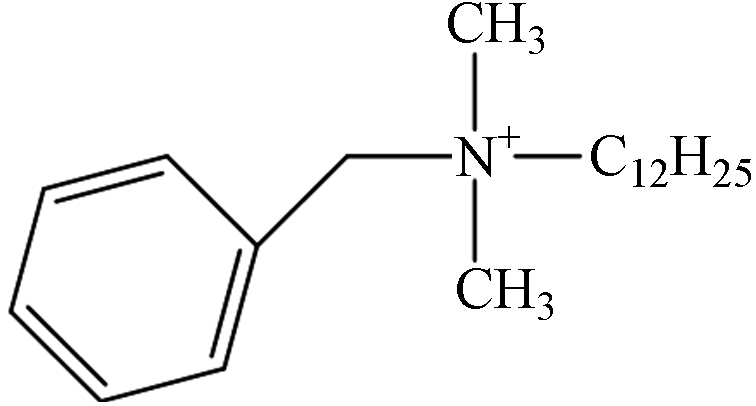
TP-1 1.76 C21H36NO 318.2797 318.3171 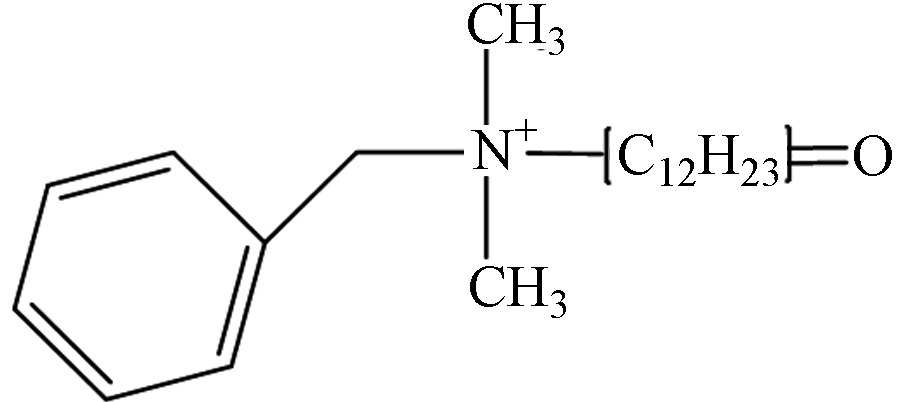
TP-2 2.09 C21H34NO2 332.2589 332.3345 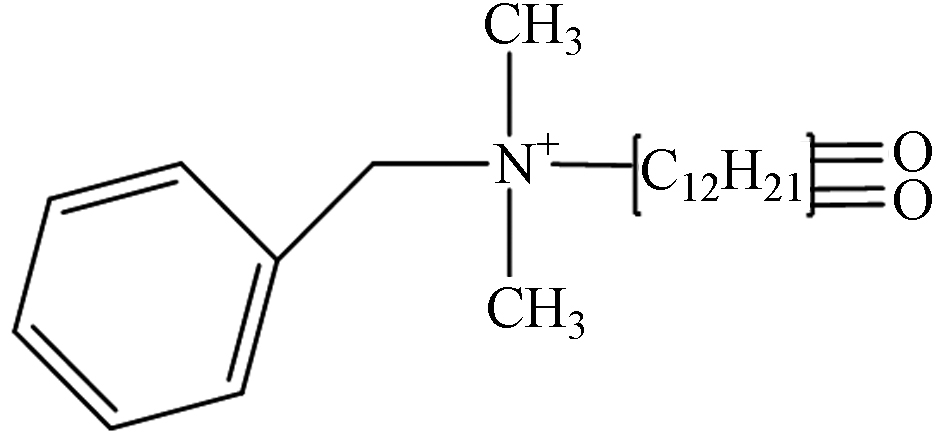
TP-3 1.23 C14H32N 214.2535 214.2551 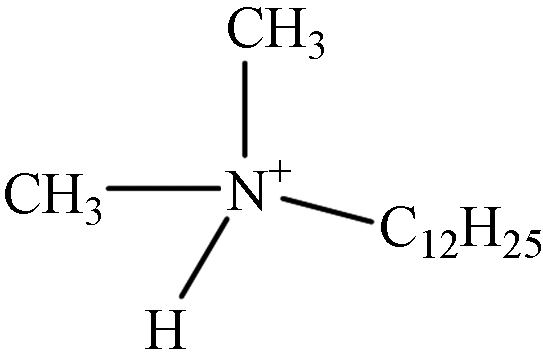
TP-4 1.34 C14H30NO 228.2327 228.2704 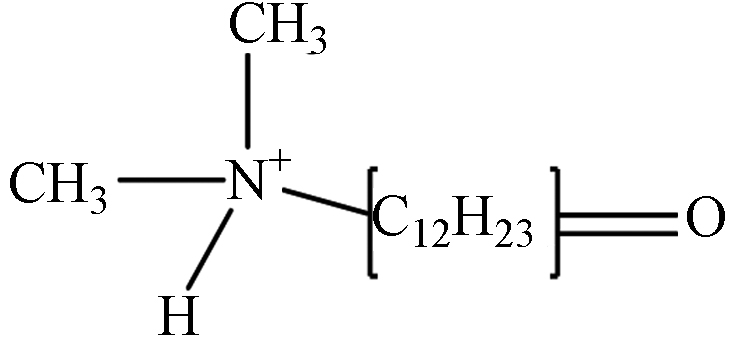
TP-5 1.41 C14H30NO2 244.2277 244.2656 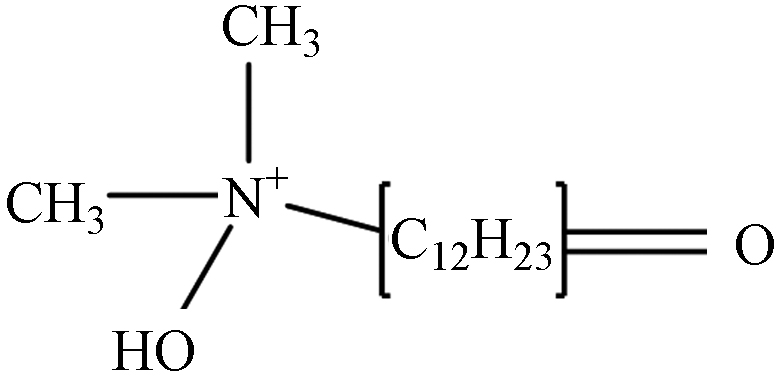
TP-6 1.16 C21H38NO 320.2953 320.2978 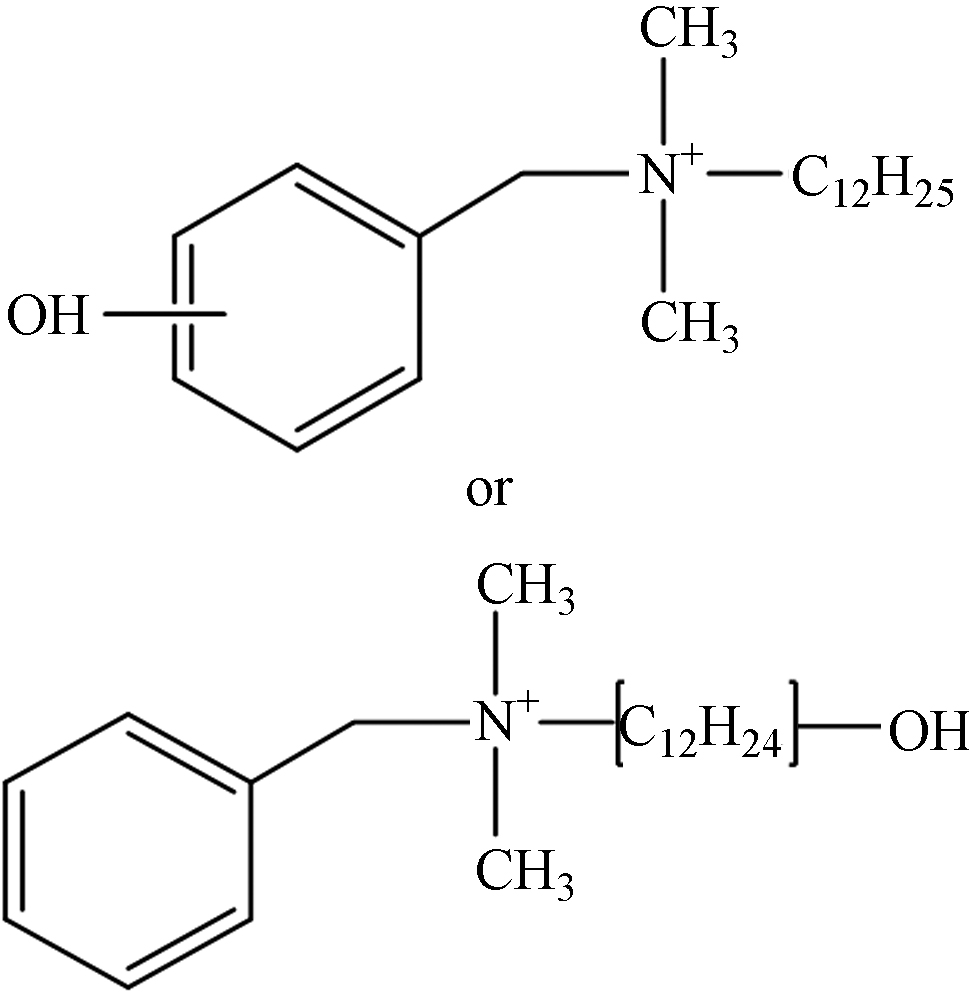
TP-7 1.27 C21H38NO2 336.2903 336.2939 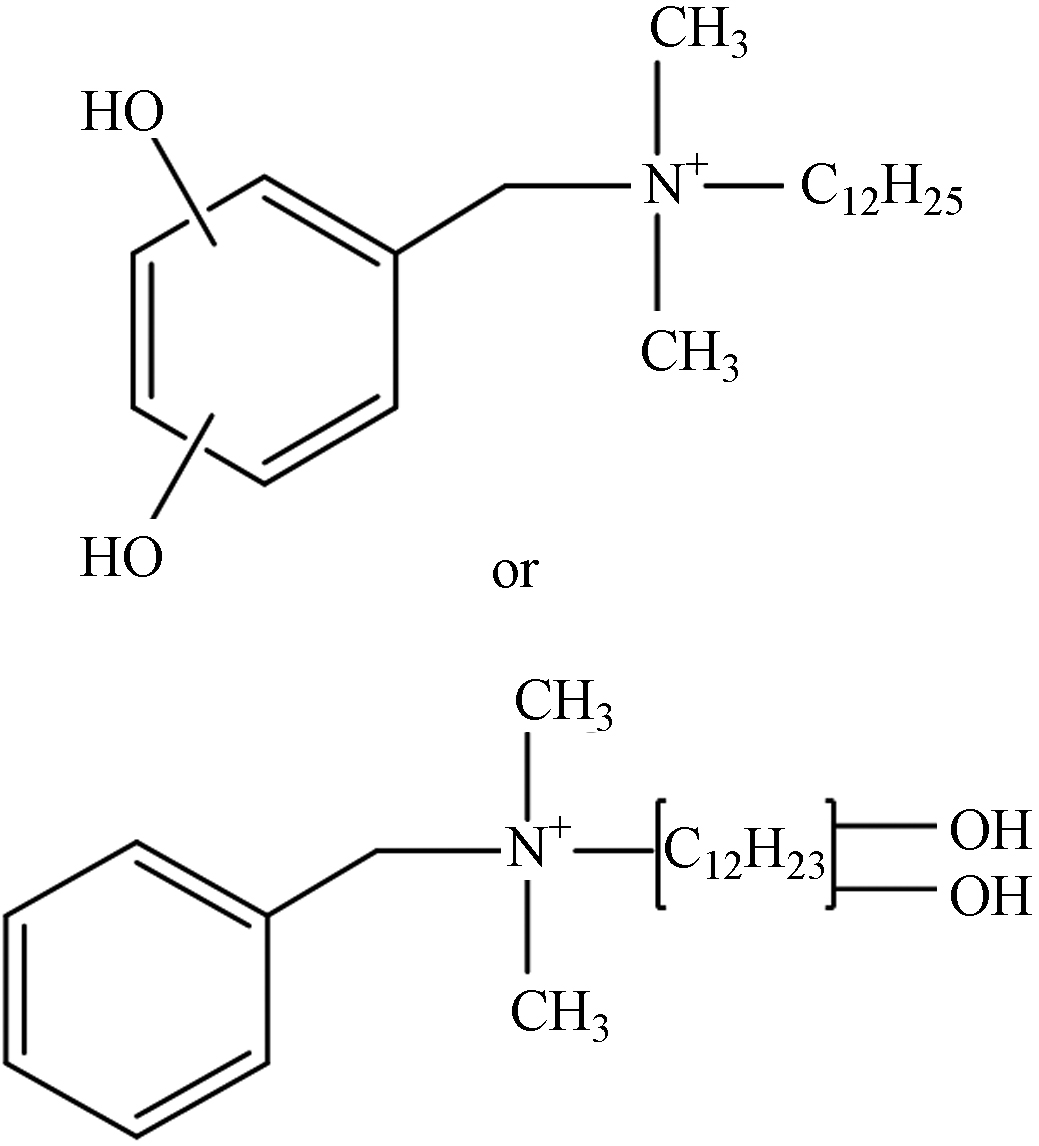
-
[1] BARBER O W, HARTMANN E M. Benzalkonium chloride: A systematic review of its environmental entry through wastewater treatment, potential impact, and mitigation strategies [J]. Critical Reviews in Environmental Science and Technology, 2022, 52(15): 2691-2719. doi: 10.1080/10643389.2021.1889284 [2] KAHRILAS G A, BLOTEVOGEL J, STEWART P S, et al. Biocides in hydraulic fracturing fluids: A critical review of their usage, mobility, degradation, and toxicity [J]. Environmental Science & Technology, 2015, 49(1): 16-32. [3] HUANG N, WANG W L, XU Z B, et al. A study of synergistic oxidation between ozone and chlorine on benzalkonium chloride degradation: Reactive species and degradation pathway [J]. Chemical Engineering Journal, 2020, 382: 122856. doi: 10.1016/j.cej.2019.122856 [4] CARBAJO J B, PETRE A L, ROSAL R, et al. Ozonation as pre-treatment of activated sludge process of a wastewater containing benzalkonium chloride and NiO nanoparticles [J]. Chemical Engineering Journal, 2016, 283: 740-749. doi: 10.1016/j.cej.2015.08.001 [5] LAVORGNA M, RUSSO C, D'ABROSCA B, et al. Toxicity and genotoxicity of the quaternary ammonium compound benzalkonium chloride (BAC) using Daphnia magna and Ceriodaphnia dubia as model systems [J]. Environmental Pollution, 2016, 210: 34-39. doi: 10.1016/j.envpol.2015.11.042 [6] YU X B, KAMALI M, VANAKEN P, et al. Advanced oxidation of benzalkonium chloride in aqueous media under ozone and ozone/UV systems - Degradation kinetics and toxicity evaluation [J]. Chemical Engineering Journal, 2021, 413: 127431. doi: 10.1016/j.cej.2020.127431 [7] MATARACI-KARA E, ATAMAN M, YILMAZ G, et al. Evaluation of antifungal and disinfectant-resistant Candida species isolated from hospital wastewater [J]. Archives of Microbiology, 2020, 202(9): 2543-2550. doi: 10.1007/s00203-020-01975-z [8] MOHAPATRA S, LIN Y, GOH S G, et al. Quaternary ammonium compounds of emerging concern: Classification, occurrence, fate, toxicity and antimicrobial resistance [J]. Journal of Hazardous Materials, 2023, 445: 130393. doi: 10.1016/j.jhazmat.2022.130393 [9] HUANG N, WANG T, WANG W L, et al. UV/chlorine as an advanced oxidation process for the degradation of benzalkonium chloride: Synergistic effect, transformation products and toxicity evaluation [J]. Water Research, 2017, 114: 246-253. doi: 10.1016/j.watres.2017.02.015 [10] MIKLOS D B, HARTL R, MICHEL P, et al. UV/H2O2 process stability and pilot-scale validation for trace organic chemical removal from wastewater treatment plant effluents [J]. Water Research, 2018, 136: 169-179. doi: 10.1016/j.watres.2018.02.044 [11] SRITHEP S, PHATTARAPATTAMAWONG S. Kinetic removal of haloacetonitrile precursors by photo-based advanced oxidation processes (UV/H2O2, UV/O3, and UV/H2O2/O3) [J]. Chemosphere, 2017, 176: 25-31. doi: 10.1016/j.chemosphere.2017.02.107 [12] WACŁAWEK S, LUTZE H V, GRÜBEL K, et al. Chemistry of persulfates in water and wastewater treatment: A review [J]. Chemical Engineering Journal, 2017, 330: 44-62. doi: 10.1016/j.cej.2017.07.132 [13] LEE M Y, WANG W L, WU Q Y, et al. Degradation of dodecyl dimethyl benzyl ammonium chloride (DDBAC) as a non-oxidizing biocide in reverse osmosis system using UV/persulfate: Kinetics, degradation pathways, and toxicity evaluation [J]. Chemical Engineering Journal, 2018, 352: 283-292. doi: 10.1016/j.cej.2018.04.174 [14] HONG J M, XIA Y F, ZHANG Q, et al. Oxidation of benzalkonium chloride in aqueous solution by S2O82−/Fe2+ process: Degradation pathway, and toxicity evaluation [J]. Journal of the Taiwan Institute of Chemical Engineers, 2017, 78: 230-239. doi: 10.1016/j.jtice.2017.06.005 [15] ZHANG Q, XIA Y F, HONG J M. Mechanism and toxicity research of benzalkonium chloride oxidation in aqueous solution by H2O2/Fe2+ process [J]. Environmental Science and Pollution Research, 2016, 23(17): 17822-17830. doi: 10.1007/s11356-016-6986-5 [16] XIA T L, LIN Y C, LI W Z, et al. Photocatalytic degradation of organic pollutants by MOFs based materials: A review [J]. Chinese Chemical Letters, 2021, 32(10): 2975-2984. doi: 10.1016/j.cclet.2021.02.058 [17] MAHMOODI N M, ABDI J, OVEISI M, et al. Metal-organic framework (MIL-100 (Fe)): Synthesis, detailed photocatalytic dye degradation ability in colored textile wastewater and recycling [J]. Materials Research Bulletin, 2018, 100: 357-366. doi: 10.1016/j.materresbull.2017.12.033 [18] YU J, XIONG W P, LI X, et al. Functionalized MIL-53(Fe) as efficient adsorbents for removal of tetracycline antibiotics from aqueous solution [J]. Microporous and Mesoporous Materials, 2019, 290: 109642. doi: 10.1016/j.micromeso.2019.109642 [19] EL ASMAR R, BAALBAKI A, ABOU KHALIL Z, et al. Iron-based metal organic framework MIL-88-A for the degradation of naproxen in water through persulfate activation [J]. Chemical Engineering Journal, 2021, 405: 126701. doi: 10.1016/j.cej.2020.126701 [20] WANG J M, WAN J Q, MA Y W, et al. Metal-organic frameworks MIL-88A with suitable synthesis conditions and optimal dosage for effective catalytic degradation of Orange G through persulfate activation [J]. RSC Advances, 2016, 6(113): 112502-112511. doi: 10.1039/C6RA24429G [21] DAOUD F, PELZER D, ZUEHLKE S, et al. Ozone pretreatment of process waste water generated in course of fluoroquinolone production [J]. Chemosphere, 2017, 185: 953-963. doi: 10.1016/j.chemosphere.2017.07.040 [22] VISWANATHAN V P, MATHEW S V, DUBAL D P, et al. Exploring the effect of morphologies of Fe(III) metal-organic framework MIL-88A(Fe) on the photocatalytic degradation of rhodamine B [J]. ChemistrySelect, 2020, 5(25): 7534-7542. doi: 10.1002/slct.202001670 [23] LIAO X Y, WANG F, WANG F, et al. Synthesis of (100) surface oriented MIL-88A-Fe with rod-like structure and its enhanced fenton-like performance for phenol removal [J]. Applied Catalysis B: Environmental, 2019, 259: 118064. doi: 10.1016/j.apcatb.2019.118064 [24] LIN K Y A, CHANG H A, HSU C J. Iron-based metal organic framework, MIL-88A, as a heterogeneous persulfate catalyst for decolorization of Rhodamine B in water [J]. RSC Advances, 2015, 5(41): 32520-32530. doi: 10.1039/C5RA01447F [25] LI Q W, LI L M, LONG X Y, et al. Rational design of MIL-88A(Fe)/Bi2WO6 heterojunctions as an efficient photocatalyst for organic pollutant degradation under visible light irradiation [J]. Optical Materials, 2021, 118: 111260. doi: 10.1016/j.optmat.2021.111260 [26] YU D Y, LI L B, WU M H, et al. Enhanced photocatalytic ozonation of organic pollutants using an iron-based metal-organic framework [J]. Applied Catalysis B: Environmental, 2019, 251: 66-75. doi: 10.1016/j.apcatb.2019.03.050 [27] XUE B, DU L, JIN J S, et al. In situ growth of MIL-88A into polyacrylate and its application in highly efficient photocatalytic degradation of organic pollutants in water [J]. Applied Surface Science, 2021, 564: 150404. doi: 10.1016/j.apsusc.2021.150404 [28] YI X H, JI H D, WANG C C, et al. Photocatalysis-activated SR-AOP over PDINH/MIL-88A(Fe) composites for boosted chloroquine phosphate degradation: Performance, mechanism, pathway and DFT calculations [J]. Applied Catalysis B: Environmental, 2021, 293: 120229. doi: 10.1016/j.apcatb.2021.120229 [29] XU W T, MA L, KE F, et al. Metal-organic frameworks MIL-88A hexagonal microrods as a new photocatalyst for efficient decolorization of methylene blue dye [J]. Dalton Transactions, 2014, 43(9): 3792-3798. doi: 10.1039/C3DT52574K [30] CHEN X J, DAI Y Z, WANG X Y, et al. Synthesis and characterization of Ag3PO4 immobilized with graphene oxide (GO) for enhanced photocatalytic activity and stability over 2, 4-dichlorophenol under visible light irradiation [J]. Journal of Hazardous Materials, 2015, 292: 9-18. doi: 10.1016/j.jhazmat.2015.01.032 [31] WANG D B, JIA F Y, WANG H, et al. Simultaneously efficient adsorption and photocatalytic degradation of tetracycline by Fe-based MOFs [J]. Journal of Colloid and Interface Science, 2018, 519: 273-284. doi: 10.1016/j.jcis.2018.02.067 [32] FU H, SONG X X, WU L, et al. Room-temperature preparation of MIL-88A as a heterogeneous photo-Fenton catalyst for degradation of rhodamine B and bisphenol a under visible light [J]. Materials Research Bulletin, 2020, 125: 110806. doi: 10.1016/j.materresbull.2020.110806 [33] BUXTON G V, GREENSTOCK C L, HELMAN W P, et al. Critical Review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals ( OH/ O–in Aqueous Solution [J]. Journal of Physical and Chemical Reference Data, 1988, 17(2): 513-886. doi: 10.1063/1.555805 [34] ZUO Z H, CAI Z L, KATSUMURA Y, et al. Reinvestigation of the acid–base equilibrium of the (bi)carbonate radical and pH dependence of its reactivity with inorganic reactants [J]. Radiation Physics and Chemistry, 1999, 55(1): 15-23. doi: 10.1016/S0969-806X(98)00308-9 [35] LE V T, TRAN V A, TRAN D L, et al. Fabrication of Fe3O4/CuO@C composite from MOF-based materials as an efficient and magnetically separable photocatalyst for degradation of ciprofloxacin antibiotic [J]. Chemosphere, 2021, 270: 129417. doi: 10.1016/j.chemosphere.2020.129417 [36] GAO Y X, YU G, LIU K, et al. Integrated adsorption and visible-light photodegradation of aqueous clofibric acid and carbamazepine by a Fe-based metal-organic framework [J]. Chemical Engineering Journal, 2017, 330: 157-165. doi: 10.1016/j.cej.2017.06.139 [37] WANG J Q, QIAN Q R, CHEN Q H, et al. Significant role of carbonate radicals in tetracycline hydrochloride degradation based on solar light-driven TiO2-seashell composites: Removal and transformation pathways [J]. Chinese Journal of Catalysis, 2020, 41(10): 1511-1521. doi: 10.1016/S1872-2067(19)63525-4 [38] XIE A T, CUI J Y, YANG J, et al. Graphene oxide/Fe(III)-based metal-organic framework membrane for enhanced water purification based on synergistic separation and photo-Fenton processes [J]. Applied Catalysis B: Environmental, 2020, 264: 118548. doi: 10.1016/j.apcatb.2019.118548 [39] YANG Y, LU X L, JIANG J, et al. Degradation of sulfamethoxazole by UV, UV/H2O2 and UV/persulfate (PDS): Formation of oxidation products and effect of bicarbonate [J]. Water Research, 2017, 118: 196-207. doi: 10.1016/j.watres.2017.03.054 -



 下载:
下载: