-
随着科技及城市化与工业化水平的飞速发展,工业用水量大幅增加,约占总用水量的30%,工业用水中有80%用来冷却运行设备。为充分有效地利用水资源,须将冷却水循环使用,故称为循环冷却水[1]。而电厂中的循环冷却水用量几乎占全厂总用水量的97%以上[2]。目前,电厂中的冷却系统一般为开式循环系统,循环冷却水在与空气接触的条件下长期运行,水分不断蒸发,使水中存在的各种无机离子和有机物质富集浓缩。其中,浓度不断增加的硬度离子(主要是Ca2+和Mg2+)会导致系统结垢、腐蚀和微生物的大量滋生等[3],对冷却塔的热性能产生严重的负面影响。因此,硬度离子的浓度必须低于在传热表面结垢的最低浓度,这就需要运行过程中连续更换部分循环水,从而导致较高硬度的废水大量排放。通过去除或降低水中硬度离子的浓度,可以增加冷却水的循环次数,减少补充水量,有效减少冷却塔运行过程中产生的循环排污水量。因此,降低循环冷却水的硬度至关重要[4]。
去除水中硬度的主要方法包括膜分离法、反渗透法、离子交换法、加药软化法和电絮凝法等[5-8]。电絮凝法处理废水是利用阳极金属的溶出发生水解-聚合反应,产生具有强吸附能力的絮凝体,络合吸附水中污染物并聚集成团,通过沉降或气浮分离使污染物得到去除。电絮凝法具有去除污染物种类多(如大分子有机物、重金属离子、氟离子等)、效率高、适用pH范围广、装置紧凑、占地面积小等优势。电絮凝法可对多种水(如饮用水、工业过程水、反渗透水和海水等[8-11])进行软化,但将其应用于去除电厂冷却塔循环冷却水中结垢物质的研究较少。SCHULZ等[12]研究了电絮凝体系中铝和铁电极对电厂冷却塔排污水中的Ca2+和Mg2+的去除率,结果表明铝电极比铁电极对Ca2+和Mg2+有更好的去除率,电絮凝反应中生成2 mmol·L−1的铝可以去除20%~40%的Ca2+和Mg2+。HAFEZ等[13]研究了在电絮凝体系中使用铝、铁、锌电极对去除水中硬度离子和SiO2的影响,认为Al电极比Zn和Fe电极更有效地从电厂冷却塔排污水中去除硬度离子,对总硬度去除率可达到55.4%。如何进一步提高电絮凝过程对硬度的去除率,仍然有待进一步的研究。同时,不同水质对电絮凝的影响较大,因此,对不同来源的水进行电絮凝去除硬度效果的研究也具有重要意义。
本研究采用铝板电絮凝法去除某电厂冷却塔循环冷却水中的硬度,利用阳极电解产生铝离子,通过絮凝作用去除Ca2+和Mg2+等;为提高水中总硬度的去除率,对电絮凝过程中的影响因素包括电流密度、电解时间、溶液初始pH等进行研究;同时,对电絮凝过程中Ca2+和Mg2+等离子的去除机理也进行了探讨。
-
本研究用水为某电厂循环冷却水。此循环冷却水呈碱性,pH为7.86,所含重碳酸盐碱度和碳酸盐碱度分别为175.2、3.4 mg·L−1,电导率为1 492 µS·cm−1,水样中Ca2+、Mg2+、Sr4+、Na+、Al3+、Fe3+、F−、Cl−、
${\rm{NO}}_3^{-} $ 、${\rm{SO}}_4^{2-} $ 和硅元素含量分别为74.6、41.4、0.7、56、3.5、0.04、11.2、180.6、29.6、303.5和2.7 mg·L−1。实验试剂:无水碳酸钠(Na2CO3)、氢氧化钠(NaOH)、盐酸(HCl),均为分析纯,购于上海阿拉丁生化科技股份有限公司。
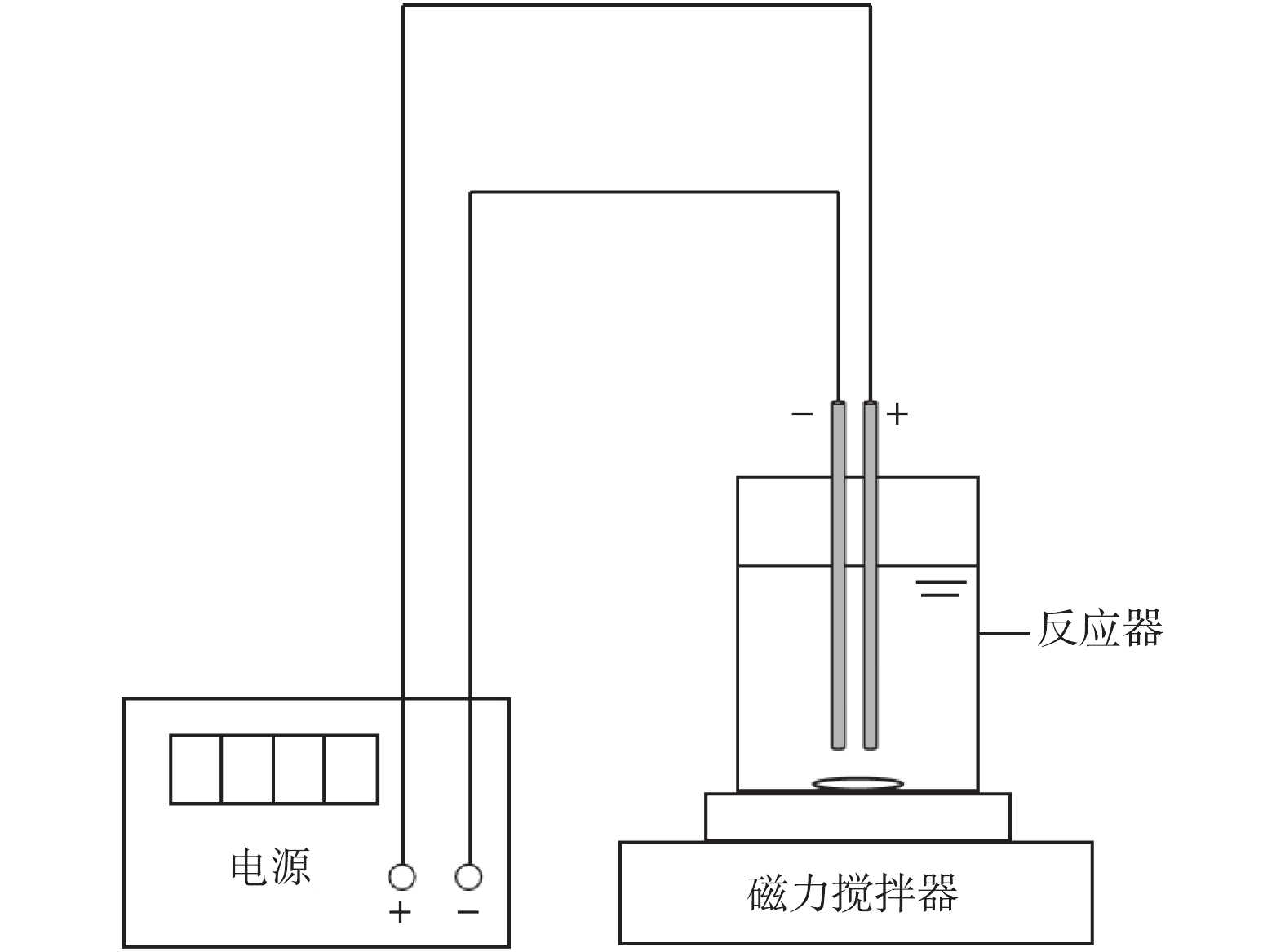
实验装置如图1所示,阳极(铝板)和阴极(不锈钢板)分别通过电极线与DH1765-1 型程控直流稳压稳流电源的相关输出端连接,使电絮凝体系在恒定电流下发生反应;MS-H-Pro型磁力搅拌器提供恒定搅拌速度,阳极和阴极的有效反应面积为30 cm2,极板间距为1 cm。电絮凝有效工作容积为500 mL。
-
在反应开始前,将所需极板在5%的盐酸溶液中浸泡30 min,然后用砂纸打磨,并用超纯水洗净,待用。
反应过程中,磁力搅拌器提供恒定搅拌速度400 r·min−1,直流电源提供稳定电流,反应时间90 min,每隔15 min取样5 mL,使用0.2 μm滤膜过滤;使用PHS-3E型pH计测定反应后水样的pH,使用9800 型电感耦合等离子光谱仪测定Ca2+和Mg2+浓度,水样总硬度(以CaCO3计)计算方法如式(1)所示。
式中:R为总硬度(以CaCO3计),mg·L−1;C1为Ca2+浓度,mg·L−1;C2为Mg2+浓度,mg·L−1。
-
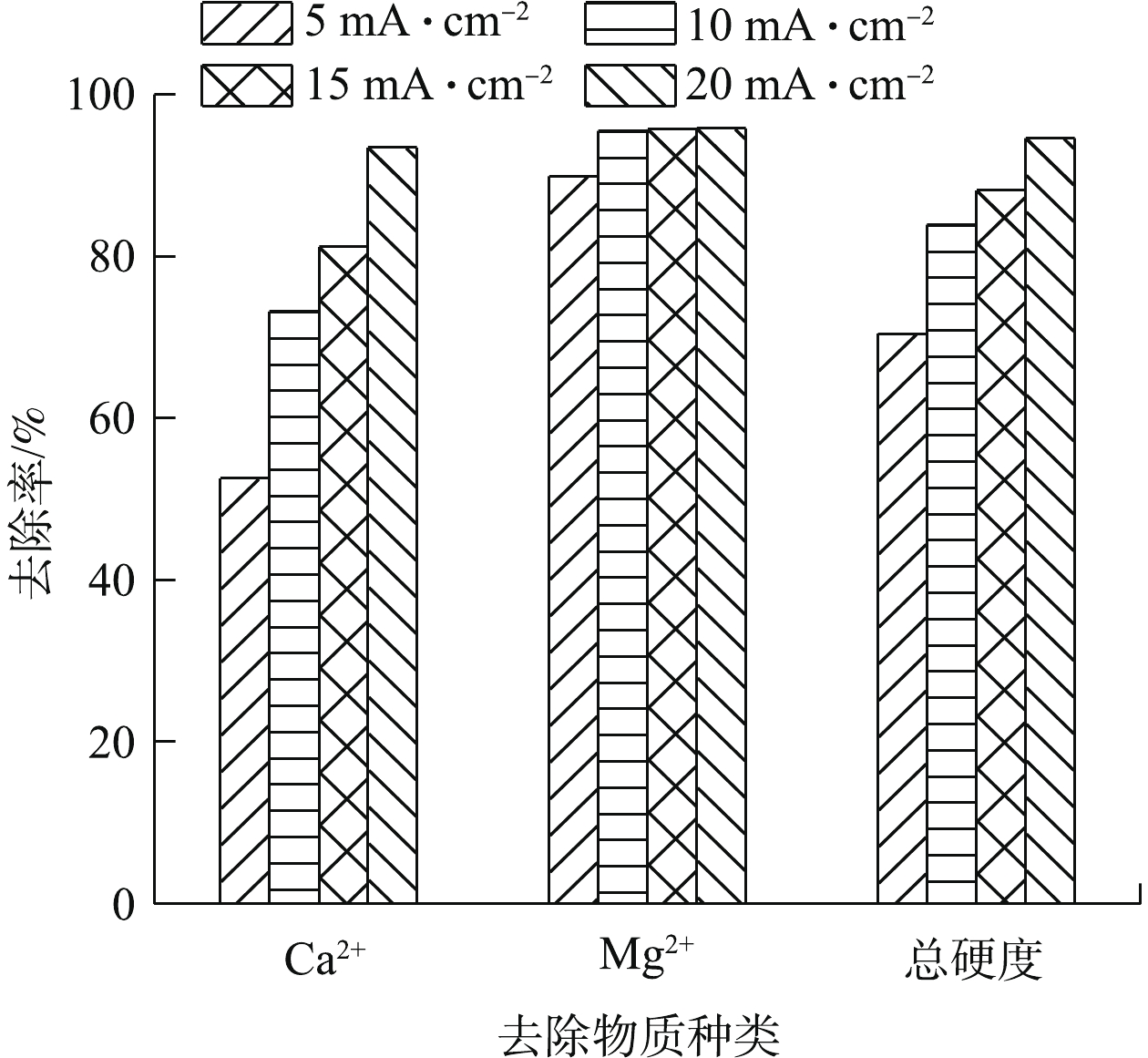
电流密度的大小会影响电絮凝反应中阳极的溶解速率、气泡的生成和体系pH的变化,从而影响絮凝剂的生成速率和絮凝效果。图2显示了不同电流密度对Ca2+、Mg2+及总硬度去除效果的影响。由图2可知,Ca2+的去除率随着电流密度的增加而不断提高,Mg2+的去除率在电流密度从5 mA·cm−2增加到10 mA·cm−2时有所提高,在电流密度为10~20 mA·cm−2时几乎不变。总硬度的去除率随着电流密度的增加也不断提高。在电流密度为20 mA·cm−2时,Ca2+、Mg2+以及总硬度的去除率可以达到93.5%、95.8%、94.6%,最终Ca2+、Mg2+的浓度以及总硬度为4.9、1.8、19.5 mg L−1。由于电流密度在20 mA·cm−2时,Ca2+、Mg2+以及总硬度的去除率很高(接近100%),不利于后续实验中研究其他条件对电絮凝过程中Ca2+、Mg2+以及总硬度去除率的影响,故后续的研究均在电流密度为10 mA·cm−2的条件下展开。
在电絮凝反应系统中主要发生的反应如式(2)~式(8)所示。阳极反应见式(2)和式(3),阴极反应见式(4)~式(6),沉淀反应见式(7)和式(8)。
随着电流密度的增加,阳极溶解速率增加,阴极析氢速率也不断提高,使得絮凝剂和OH−的量也相应增加。在室温下,Mg(OH)2、CaCO3、Ca(OH)2和MgCO3的溶度积分别为5.6×10−12、5.0×10−9、4.7×10−6、6.8×10−6。由此可推断,Ca2+和Mg2+去除的主要原因是:Mg2+消耗水中存在的OH−,生成大量的Mg(OH)2沉淀(式(5)),Ca2+消耗水样中的碳酸盐碱度和重碳酸盐碱度,生成CaCO3沉淀(式(6)~式(7))。由图3可知,随着电流密度的增加,反应结束时溶液的pH也随之增大,从而使得反应过程中OH−生成速率增加,导致Mg(OH)2沉淀生成速率提高,且Mg2+沉淀更加完全。同时,水中的碳酸盐碱度和重碳酸盐碱度很快消耗完全,过量的OH−可使Ca2+形成Ca(OH)2沉淀,使得Ca2+和Mg2+的去除率随电流密度的增大而升高,总硬度去除率升高。当电流密度为10 mA·cm−2时,体系中生成的OH−可使Mg2+去除率在反应90 min时达到95.4%,继续增加电流密度,Mg2+去除率几乎不变。这些在反应过程中逐渐生成的Mg(OH)2、CaCO3和Ca(OH)2沉淀,被电絮凝反应生成的絮凝剂通过压缩双电层、吸附架桥、集卷网捕等作用吸附并形成聚合物沉淀[14],或在阴、阳极产生的氢气、氧气作用下,发生气浮作用至液体表面,从而得到去除。
-
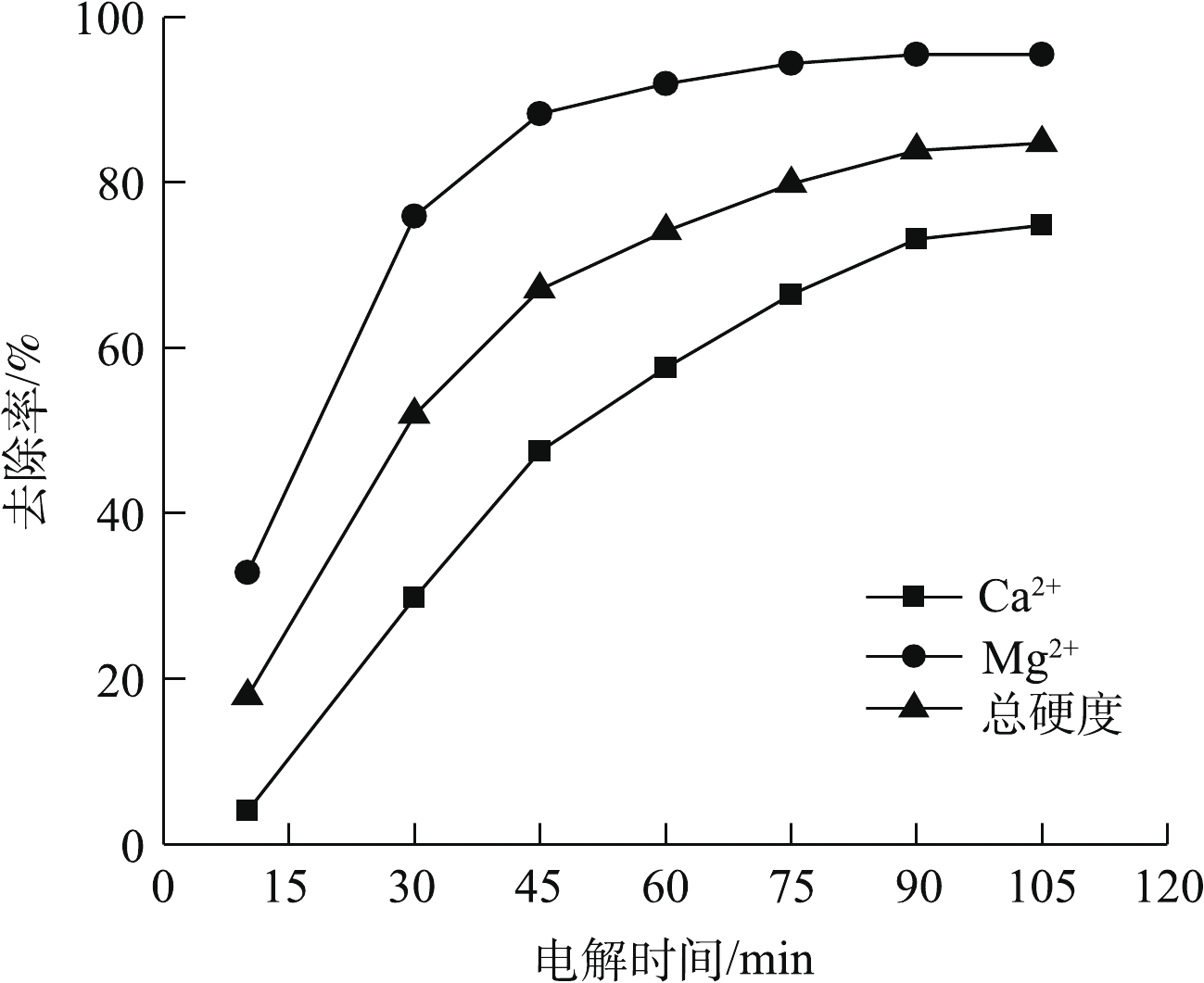
电解时间会影响电絮凝反应中阳极的溶解量和气泡的生成,从而影响产生絮凝剂的量和絮凝效果。图4为不同电解时间对Ca2+、Mg2+以及总硬度去除效果的影响结果。由图4可知,在105 min内,随着电解时间的延长,絮凝剂和OH−量增加[15],生成的Mg(OH)2、CaCO3和Ca(OH)2沉淀量也随之增加,被电絮凝反应生成的絮凝剂吸附并形成聚合物而得到去除[14],所以,Ca2+和Mg2+的去除率升高,使总硬度去除率随电解时间的延长而不断升高。但当电解时间从90 min延长到105 min时,Mg2+去除率可达到95.4%,几乎不再升高,Ca2+去除率升高,使总硬度去除率从83.9%升高到84.7%,考虑到当电解时间增加15 min而总硬度去除率升高较少为0.8%,所以采用90 min的电解时间较为经济。此时,Ca2+、Mg2+以及总硬度的去除率为73.2%、95.4%、83.9%;Ca2+、Mg2+的浓度以及总硬度降低至20、1.9、57.9 mg·L−1。
-
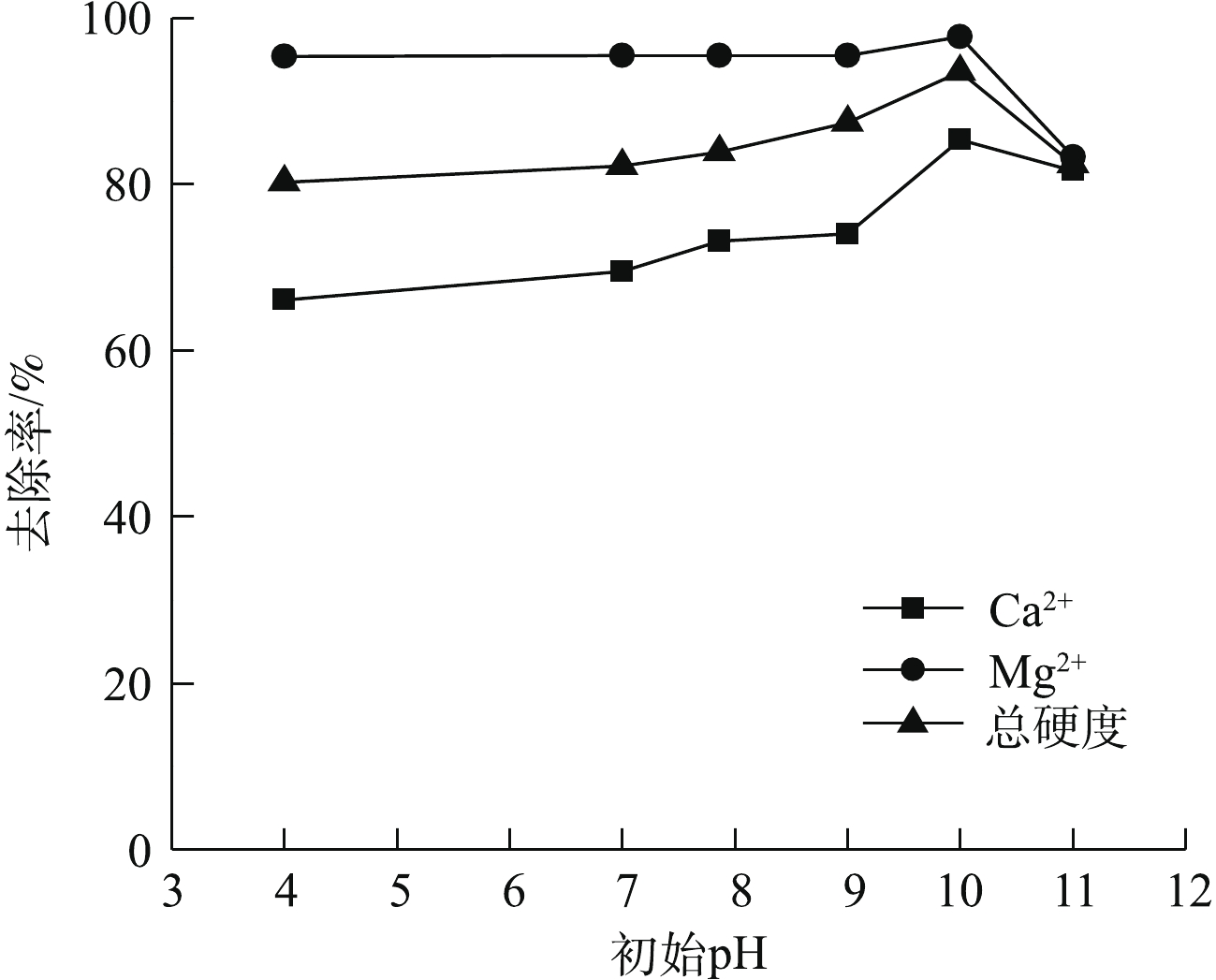
pH会改变体系中钙和镁的存在形式,对水体中铝的形态和溶解铝质量浓度影响也非常大[16]。图5为不同初始pH对Ca2+、Mg2+以及总硬度去除效果的影响。由图5可知,溶液初始pH=4时,钙和镁以Ca2+和Mg2+的形式存在,反应90 min后,Ca2+和Mg2+去除率较低,溶液初始pH为7、7.86、9、10时,钙和镁首先以Ca2+和Mg2+的形式存在,随着pH的增加,有一部分钙和镁以CaCO3和Mg(OH)2的形式存在。反应90 min后,随着pH的增加,Ca2+和Mg2+的去除率逐渐升高,总硬度去除率逐渐升高。在pH=10时,Ca2+、Mg2+以及总硬度的去除率达到最大,分别为85.4%、97.7%、93.5%,此时Ca2+和Mg2+的浓度以及总硬度降低到8.5、1.2、26.5 mg L−1。这是因为初始溶液为酸性时,反应体系中铝的存在形式主要是Al3+[17],且pH较低时,CaCO3、Mg(OH)2和Ca(OH)2沉淀的形成会受到抑制。在电絮凝反应过程中产生的OH−首先被H+消耗,随着反应时间的延长,pH进一步升高,CaCO3和Mg(OH)2沉淀形成,絮体生成,pH继续升高,Ca(OH)2沉淀形成,最终导致Ca2+和Mg2+的去除率升高。但pH=11时,Ca2+、Mg2+以及总硬度的去除率降低,这是因为pH过高,虽然有利于CaCO3、Mg(OH)2和Ca(OH)2沉淀的形成,但会导致絮体溶解或以
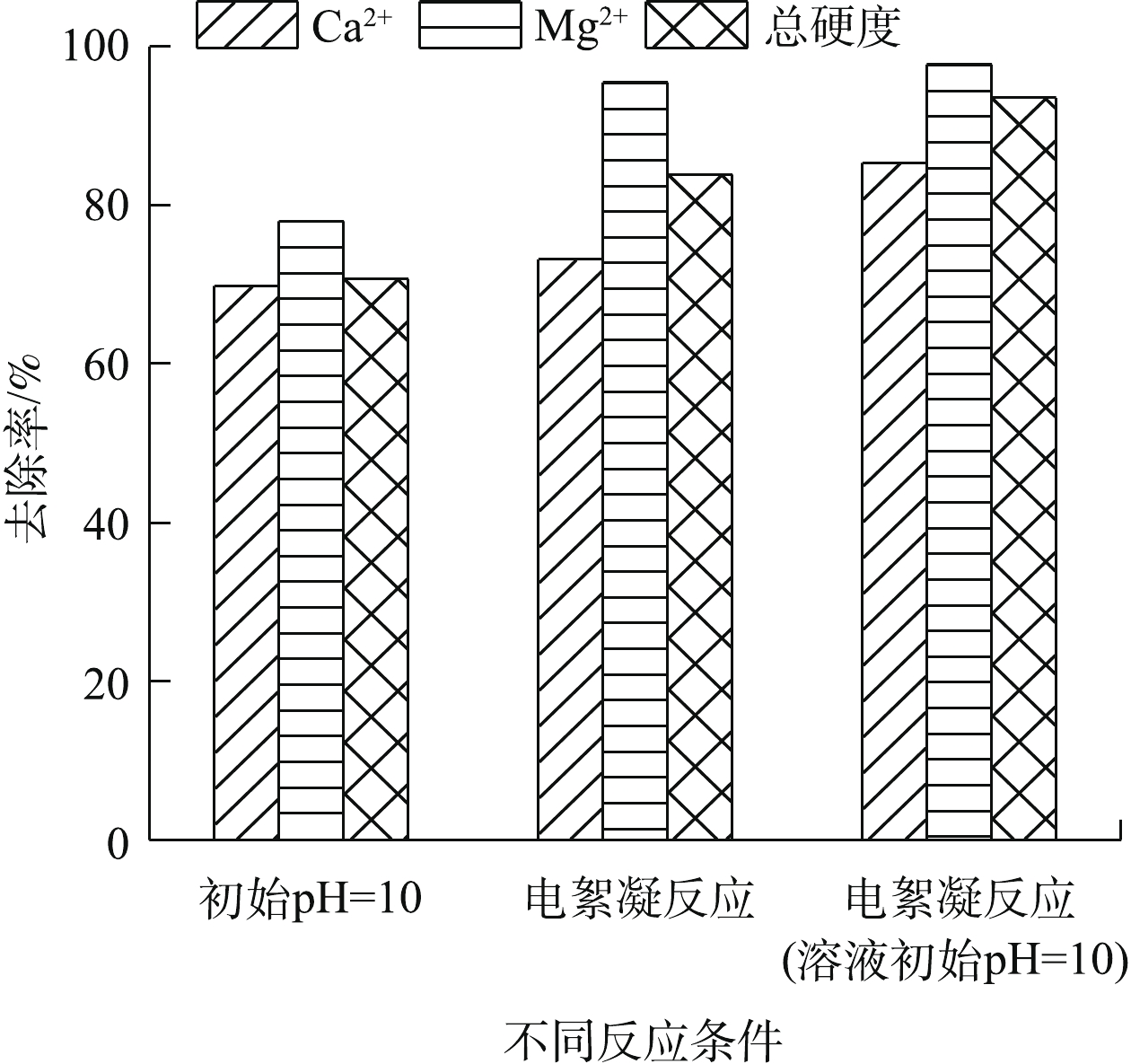
${\rm{Al}}\left( {{\rm{OH}}} \right)_4^ - $ 絮体的形式存在[14],使被絮体中和的Ca2+、Mg2+与被絮体吸附并形成聚合物沉淀的CaCO3和Mg(OH)2沉淀的量减少,总硬度去除率降低。溶液初始pH会影响体系中钙和镁的存在形式和沉淀去除效果。由图6可知,在未进行电絮凝反应的条件下,调节溶液初始pH为10,Ca2+、Mg2+以及总硬度的去除率可达69.8%、78.0%、70.7%。这一现象说明Ca2+和Mg2+可通过电絮凝过程中阴极产生的OH−生成Mg(OH)2、CaCO3和Ca(OH)2沉淀。但此时Ca2+、Mg2+以及总硬度去除率低于电絮凝反应下的去除率,且在电絮凝反应前,调节循环水初始pH为10,可进一步提高电絮凝过程中Ca2+、Mg2+以及总硬度的去除率。这进一步说明了在电絮凝反应中存在Mg(OH)2、CaCO3和Ca(OH)2沉淀被电絮凝反应生成的絮凝剂吸附并形成聚合物沉淀[14]的过程,这会不断促进Mg(OH)2、CaCO3和Ca(OH)2沉淀的生成,最终导致Ca2+、Mg2+以及总硬度去除率升高。
-
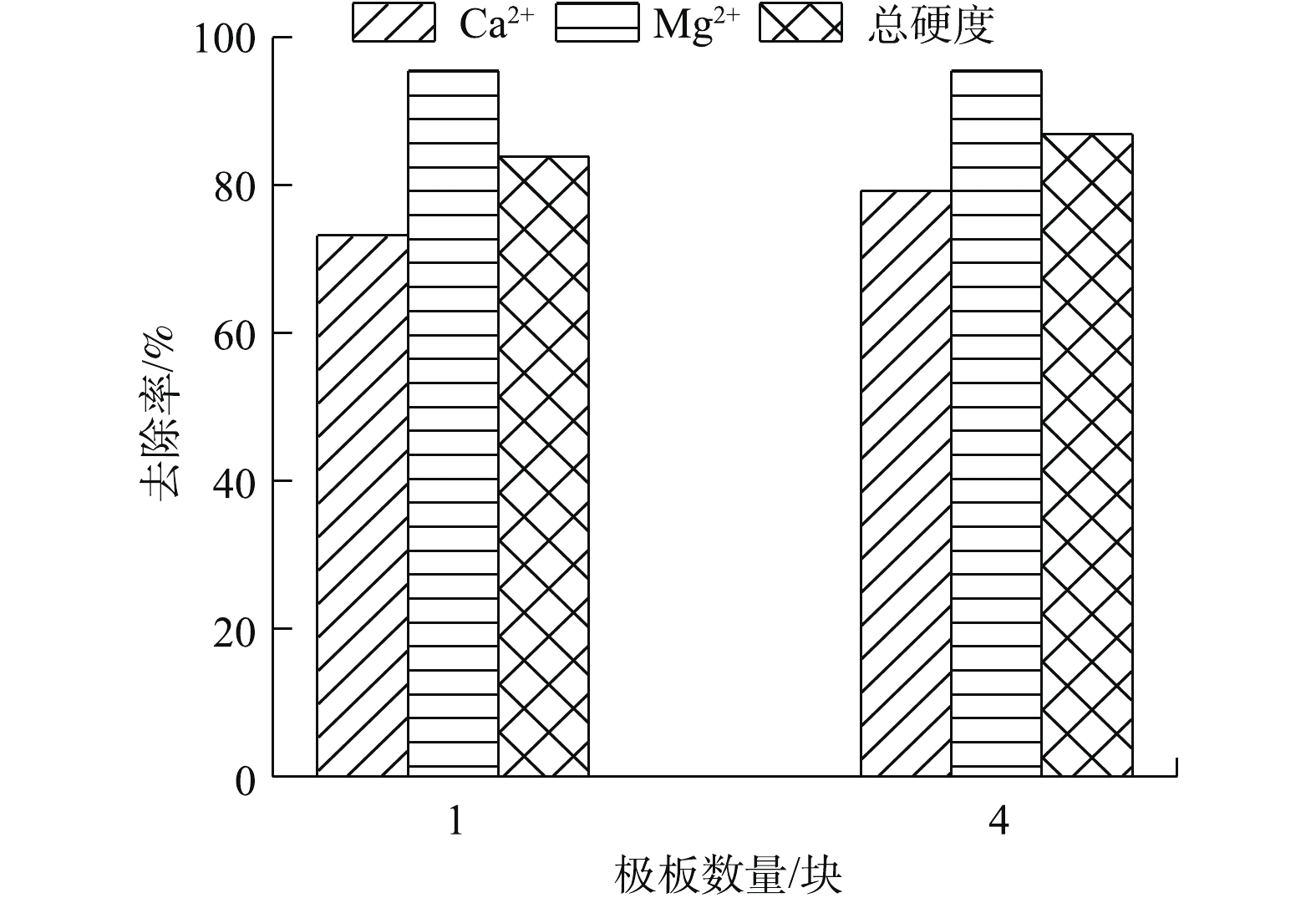
利用串联的方式增加极板数量会影响单位时间内阳极的溶解总量,进而影响絮凝物含量。图7为极板数量对Ca2+、Mg2+以及总硬度去除效果的影响。由图7可知,当阳极电极数量从1块增加到4块时,Ca2+去除率从73.2%升高到79.2%。这是因为阳极电极数量从1块增加到4块时,阳极金属溶出量增加,反应结束后,pH由7.84升高到8.91,OH−含量增加,有利于电絮凝反应中Ca2+的进一步去除;而Mg2+去除率已达到95.5%,浓度降低至1.6 mg L−1,去除率几乎不变;总硬度去除率可从83.9%升高到87.0%。
-
根据实验水质,水样中Ca2+和Mg2+浓度分别为74.6 mg·L−1和41.4 mg·L−1,总硬度为359.0 mg·L−1,假设Ca2+、Mg2+完全转化成CaCO3、MgCO3沉淀,则需
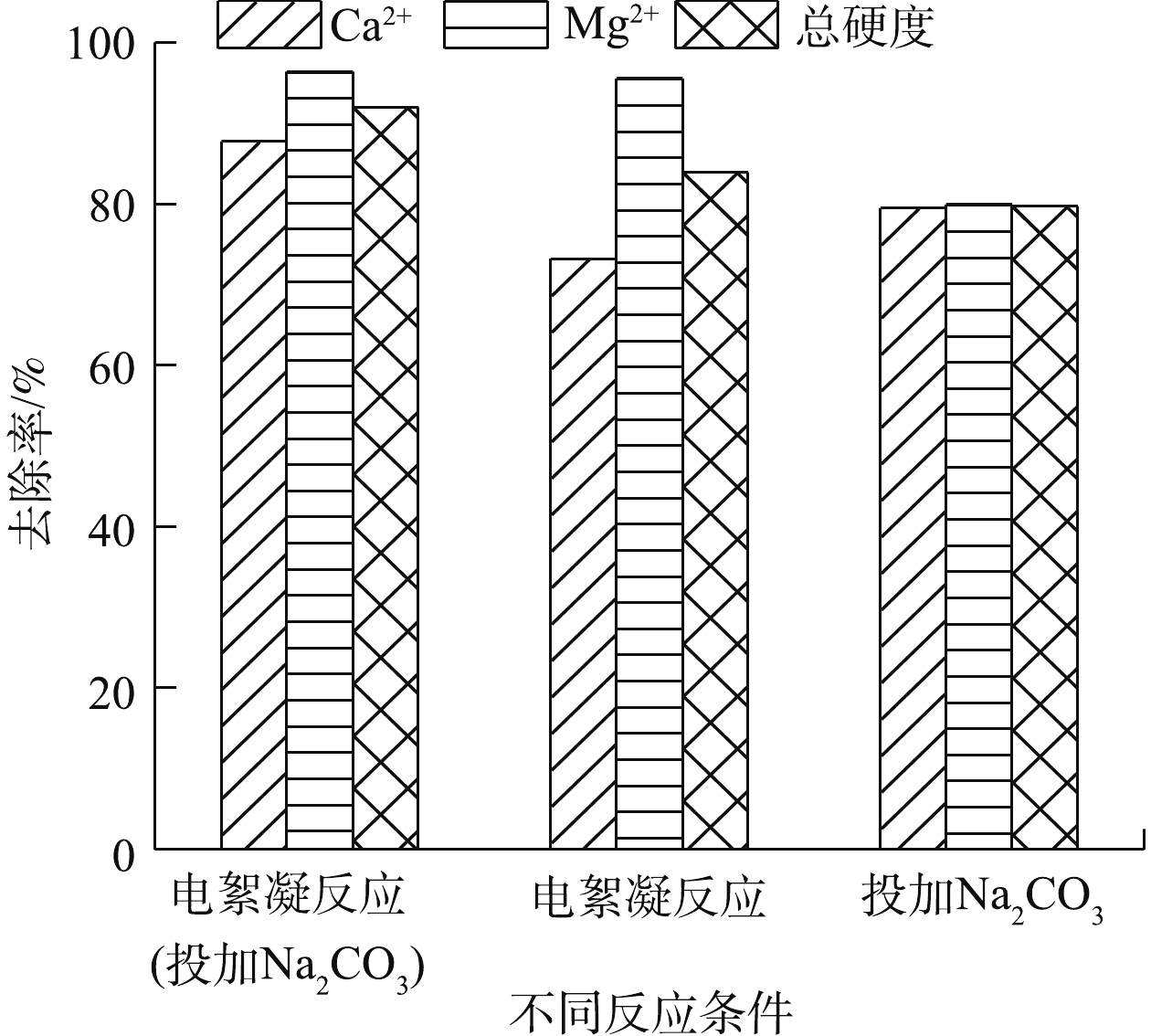
${\rm{CO}}_3^{2 - }$ 量为3.6 mmol·L−1(Na2CO3),本研究中取Na2CO3浓度为3 mmol·L−1。图8为投加3 mmol·L−1 Na2CO3对Ca2+、Mg2+、总硬度去除率的影响结果。由图8可知,Na2CO3的加入有利于进一步提高电絮凝反应中Ca2+的去除率,去除率从73.2%升高到87.7%,Mg2+去除率几乎不再升高,总硬度去除率从83.9%升高到91.9%。而单独投加3 mmol·L−1 Na2CO3,总硬度去除率较低,为79.7%,其原因可能为:一方面,反应中缺少电絮凝过程中的絮凝作用;另一方面,MgCO3溶度积大于Mg(OH)2溶度积。因此,单独投加Na2CO3时,Mg2+的去除率低于电混凝过程中Mg2+的去除率。 -
1)对于循环冷却水中的硬度离子,增加电流密度和延长电解时间均有利于Ca2+、Mg2+以及总硬度的去除。当电流密度为10 mA·cm−2,电解时间为90 min时,Ca2+和Mg2+的去除率分别可达到73.2%和95.4%,总硬度的去除率可达到83.9%。
2)相对于酸性条件和中性条件,碱性条件更有利于Ca2+、Mg2+以及总硬度的去除。但pH为11时,虽然pH过高有利于CaCO3、Mg(OH)2和Ca(OH)2沉淀的形成,但会导致絮体溶解或以
${\rm{Al}}\left( {{\rm{OH}}} \right)_4^ - $ 絮体的形式存在,使被絮体中和的Ca2+、Mg2+与被絮体吸附并形成聚合物沉淀的CaCO3和Mg(OH)2沉淀的量减少,最终使总硬度去除率反而降低。pH为10时,Ca2+、Mg2+以及总硬度去除率得到进一步提高,分别达到85.4%、97.7%、93.5%。pH过高,总硬度去除率会降低。3)增加阳极板数量、向溶液中投加Na2CO3,均可以进一步提高Ca2+去除率,进而进一步提高了总硬度去除率。
电絮凝对电厂循环冷却水中硬度的去除
Removal of hardness from circulating cooling water of power plant by electrocoagulation
-
摘要: 以某电厂冷却塔循环冷却水为处理对象,利用电絮凝法,以铝板为牺牲阳极去除水中的Ca2+和Mg2+,分别考察了电絮凝过程中不同电流密度、电解时间、溶液初始pH、阳极极板数量对总硬度去除率的影响。结果表明:增加电流密度、延长电解时间有利于Ca2+和Mg2+的去除;当电流密度为10 mA·cm−2,电解时间为90 min时,Ca2+和Mg2+去除率分别达到93.5%和95.8%,总硬度去除率为94.6%;相对于酸性和中性条件,碱性条件更有利于Ca2+和Mg2+的去除,当初始pH为10时,Ca2+和Mg2+去除率分别达到85.4%和97.7%,总硬度去除率为93.5%;随极板数量的增加,Ca2+和总硬度去除率均有所提高;投加Na2CO3有利于Ca2+和总硬度的去除。上述结果可为进一步提高电絮凝过程中总硬度的去除率提供参考。Abstract: The removal of Ca2+ and Mg2+ from circulating cooling water of power plant by electrocoagulation with the sacrificial aluminum anodes was studied in this work. The effects of current density, electrolysis time, initial pH and number of anodes on hardness removal in the electrocoagulation process were investigated. The results showed that the increase of current density and the extension of electrolysis time were benefit for the removal of Ca2+ and Mg2+. At the current density of 10 mA·cm−2 and the electrolysis time of 90 minutes, the removal efficiencies of Ca2+, Mg2+ and hardness were 93.5%, 95.8% and 94.6%, respectively. Compared with neutral and acidic conditions, alkaline condition was more benefit for the removal of Ca2+ and Mg2+. At the initial pH=10, the removal efficiencies of Ca2+, Mg2+ and hardness were 85.4%, 97.7% and 93.5%, respectively. With the increase of the number of anodes, the removal efficiencies of Ca2+ and hardness increased. The addition of sodium carbonate was also benefit for the removal of Ca2+ and hardness. The above results provide a reference and basis for further increasing the removal efficiency of hardness by electrocoagulation.
-
Key words:
- electrocoagulation /
- Ca2+ /
- Mg2+ /
- total hardness /
- circulating cooling water
-

-
-
[1] OTHMANI M, AISSA A, BACHOUA H, et al. Surface modification of calcium-copper hydroxyapatites using polyaspartic acid[J]. Applied Surface Science, 2013, 264: 886-891. doi: 10.1016/j.apsusc.2012.10.182 [2] 李培元. 火力发电厂水处理及水质控制[M]. 北京: 中国电力出版社, 1999. [3] NAYUNIGARI M K, MAITY A, AGARWAL S, et al. Curcumin-malic acid based green copolymers for control of scale and microbiological growth applications in industrial cooling water treatment[J]. Journal of Molecular Liquids, 2016, 214(95 Suppl): 400-410. [4] 李宏秀, 李文杰, 于宝缘. 电厂循环水排污水回用[J]. 华电技术, 2011, 33(6): 78-80. doi: 10.3969/j.issn.1674-1951.2011.06.030 [5] YAN W, LI W X, LIU F, et al. Removal of hardness from RO concentrate of paper mill effluents with NF membrane for water reuse[J]. Desalination and Water Treatment, 2017, 84: 59-68. doi: 10.5004/dwt.2017.21051 [6] CHOI J H, KIM B T. Hardness removal in membrane capacitive deionization with a selective cation exchange membrane[J]. Desalination and Water Treatment, 2017, 66: 97-102. doi: 10.5004/dwt.2017.20211 [7] 刘玉华, 丁海洋. 电厂循环冷却回用水的去除硬度工艺研究[J]. 炼油与化工, 2012, 23(2): 11-13. [8] MALAKOOTIAN M, MANSOORIAN H J, MOOSAZADEH M. Performance evaluation of electrocoagulation process using iron-rod electrodes for removing hardness from drinking water[J]. Desalination, 2010, 255(1/2/3): 67-71. [9] ZHAO S, HUANG G, CHENG G, et al. Hardness, COD and turbidity removals from produced water by electrocoagulation pretreatment prior to reverse osmosis membranes[J]. Desalination, 2014, 344: 454-462. doi: 10.1016/j.desal.2014.04.014 [10] CHEN Y, BAYGENTS J C, FARRELL J. Evaluating electrocoagulation and chemical coagulation for removing dissolved silica from high efficiency reverse osmosis (HERO) concentrate solutions[J]. Journal of Water Process Engineering, 2017, 16: 50-55. doi: 10.1016/j.jwpe.2016.12.008 [11] HAKIZIMANA J N, GOURICH B, VIAL C H, et al. Assessment of hardness, microorganism and organic matter removal from seawater by electrocoagulation as a pretreatment of desalination by reverse osmosis[J]. Desalination, 2016, 393: 90-101. doi: 10.1016/j.desal.2015.12.025 [12] SCHULZ M C, BAYGENTS J C, FARRELL J. Laboratory and pilot testing of electrocoagulation for removing scale-forming species from industrial process waters[J]. International Journal of Environmental Science and Technology, 2009, 6(4): 521-526. doi: 10.1007/BF03326091 [13] HAFEZ O M, SHOEIB M A, EL-KHATEEB M A, et al. Removal of scale forming species from cooling tower blowdown water by electrocoagulation using different electrodes[J]. Chemical Engineering Research and Design, 2018, 136: 347-357. doi: 10.1016/j.cherd.2018.05.043 [14] 周振, 姚吉伦, 庞治邦, 等. 电絮凝技术在水处理中的研究进展综述[J]. 净水技术, 2015, 34(5): 9-15. doi: 10.3969/j.issn.1009-0177.2015.05.002 [15] THAKUR L S, MONDAL P. Simultaneous arsenic and fluoride removal from synthetic and real groundwater by electrocoagulation process: parametric and cost evaluation[J]. Journal of Environmental Management, 2017, 190: 102-112. [16] 王文东, 杨宏伟, 蒋晶, 等. 水温和pH对饮用水中铝形态分布的影响[J]. 环境科学, 2009, 30(8): 2259-2262. doi: 10.3321/j.issn:0250-3301.2009.08.013 [17] 栾兆坤, 冯利, 汤鸿霄. 水解聚合铝溶液中形态分布的定量模拟研究[J]. 环境科学学报, 1995, 15(1): 39-47. -



 下载:
下载: