-
莠去津(atrazine, 2-氯-4-乙胺基-6-异丙胺基-1,3,5-三嗪)是一类被广泛应用在玉米、甘蔗和高粱等作物中的三嗪类除草剂,据估计,2001年的使用量为3.5×107 t[1]。莠去津长时间施用会造成接茬种植的蔬菜、大豆等死苗[2],进入水体会危害人类及水生生物等生长[3]。地下水和饮用水中经常检测到该农药,已被美国环保署确定为潜在致癌物、内分泌干扰物和持久毒性化合物[4-5]。我国化学合成农药的产量较低,平均产量不到40%,其他原料、中间体或副产品均以“三废”的形式排放,导致莠去津生产废水中不仅具有高浓度合成原料(三聚氯氰、异丙胺、甲苯)和合成中间体(乙胺等),还具有高含盐量和强碱性的特点[6]。这些特点不仅对处理装置、管路的运行造成负担,更对后续传统生化处理造成极大困难。
目前,此类实际生产废水处理技术的详细报道很少见,研究对象主要是实验室模拟废水。ARELLANO等[7]用光芬顿和光催化技术降解初始浓度为35 mg·L−1的纯莠去津溶液,得到72%的矿化率。而实际生产废水呈强碱性,使用芬顿法须加入大量化学试剂进行中和,增加系统负荷的同时还可能导致二次污染;有研究考虑筛选可降解莠去津的菌株,如ZHU等[8]将筛选得到的菌株Arthrobacter sp. strain HB-5引入0.3%含盐废水样品中,初步实现了莠去津的半衰期低于7 d的降解效果;也有研究[9]筛选出耐盐的(3%~14%含盐量)莠去津降解菌。然而实际生产废水中的含盐量均在5%~20%,再加之废水可生化性差,毒性大,对各种微生物处理方法都具有很大挑战。
光催化氧化法作为一种处理高浓度难降解有机废水的方法,近40年来被广泛研究和应用。该方法产生的羟基自由基(·OH)是一种强氧化物质,具有2.8 V标准电极电势,可对污染物迅速降解[10-14]。但·OH的选择性低,这就意味着有机物和其他共存物之间存在竞争反应[10, 15]。莠去津生产废水含盐量高,水中的氯离子会与有机物竞争·OH,使得降解效率受到影响,这种竞争作用在其他高级氧化体系(如UV/O3、钴/过氧硫酸盐和UV/TiO2等)中也有体现[16-18]。针对这些问题,本研究制备了活性炭负载三价铁催化剂(AC-Fe3+),并将其与光解臭氧法(UV/O3)结合,开发了能够有效降解莠去津生产废水的方法,对催化剂投加量、紫外功率、曝气强度3个因素进行了优化,并从理论上探索负载型光催化氧化(UV/O3/AC-Fe3+)体系对莠去津废水的降解机理及吸附降解动力学。
全文HTML
-
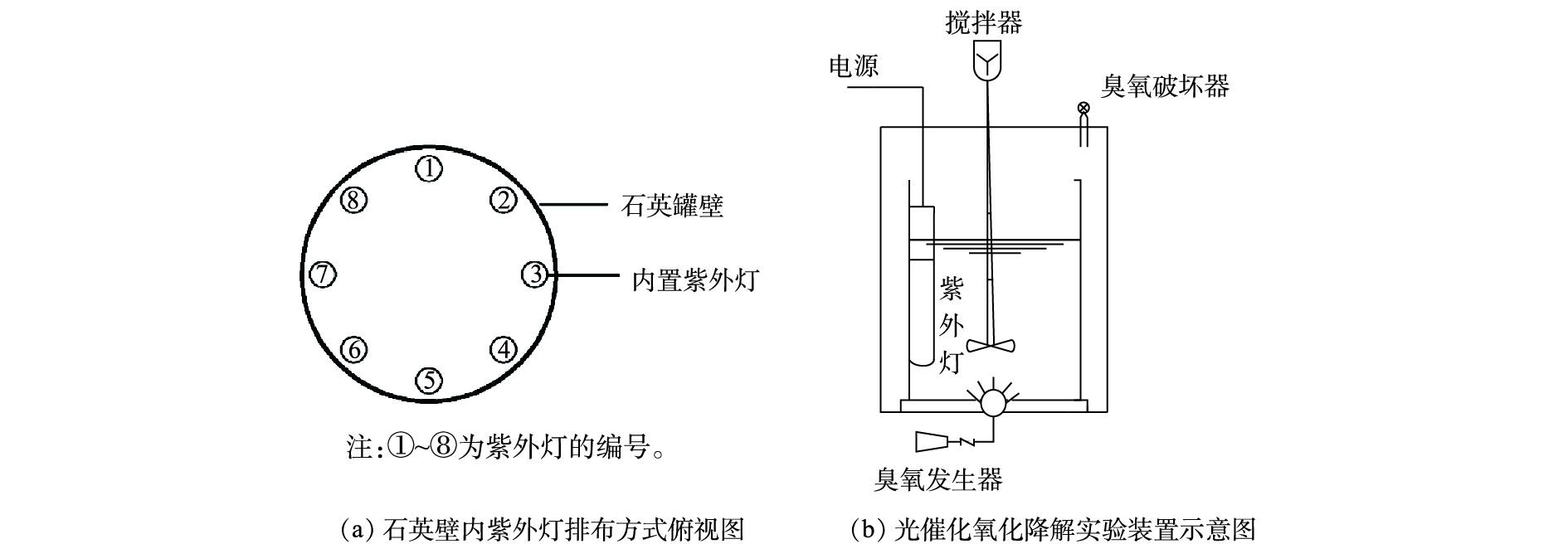
实验中的光催化降解装置(见图1)主体为4 L圆柱形石英反应器,内径为20 cm,壁厚为4 mm,壁高为25 cm,置于不锈钢罐中,石英反应器配有紫外灯(UVC-7中山市利贞电器有限公司)、搅拌器、臭氧装置(FL-815Y深圳市飞立电器科技有限公司),实验中与水样接触的部件均采用聚四氟乙烯等性质稳定材料,避免引入其他物质造成误差。实验所用莠去津废水取自农药化工厂,莠去津废水是一类具有高浓度难降解有机物的高盐碱性废水,部分水质参数如下:化学需氧量(COD)为10 000~15 000 mg·L−1;五日生化需氧量(BOD5)为2 000~3 850 mg·L−1;氯化物为15 000~197 500 mg·L−1;氨氮为40~60 mg·L−1;pH为13~14,污水主要表现为高盐度、高碱性和低可生化性。
催化剂的制备和改进参考ZAZO等[19]的方法,采用颗粒活性炭(市售)为载体,活性炭粒度为4~8目,强度为90%。活性组分是浓度为0.5 mol·L−1的硝酸铁(Fe(NO3)3·9H2O,北京化工厂)。将活性炭颗粒用纯水洗涤后,放入鼓风干燥箱(DHG-9245A 上海一恒科学仪器有限公司),105 ℃烘干12 h,在硝酸铁溶液中振荡浸渍6 h,然后在干燥箱60 ℃烘干12 h,在马弗炉(SX-4-10 北京KSW公司)中500 ℃焙烧4 h,使其自然冷却,用筛子除去粉末颗粒,制得负载型催化剂(AC-Fe3+)。
-
在光催化实验中,取2 L水样置于石英反应罐中,投入一定量的负载型催化剂,打开搅拌装置,并同时打开紫外光和臭氧装置,并开始计时。分别在0、20、40、60、80、100、120、140、160、180 min时经蠕动泵(YZ1515X 保定创锐泵业有限公司)取样,取样时留取蠕动泵3 s后的水样,以消除管内残留水样对实验结果的影响。COD的测定方法参考国家环保总局《水和废水监测分析方法》中的重铬酸钾法[20],NH3-N的测定方法选用HJ 536-2009中的水杨酸分光光度法[21]。
1.1. 实验仪器与材料
1.2. 实验与方法
-
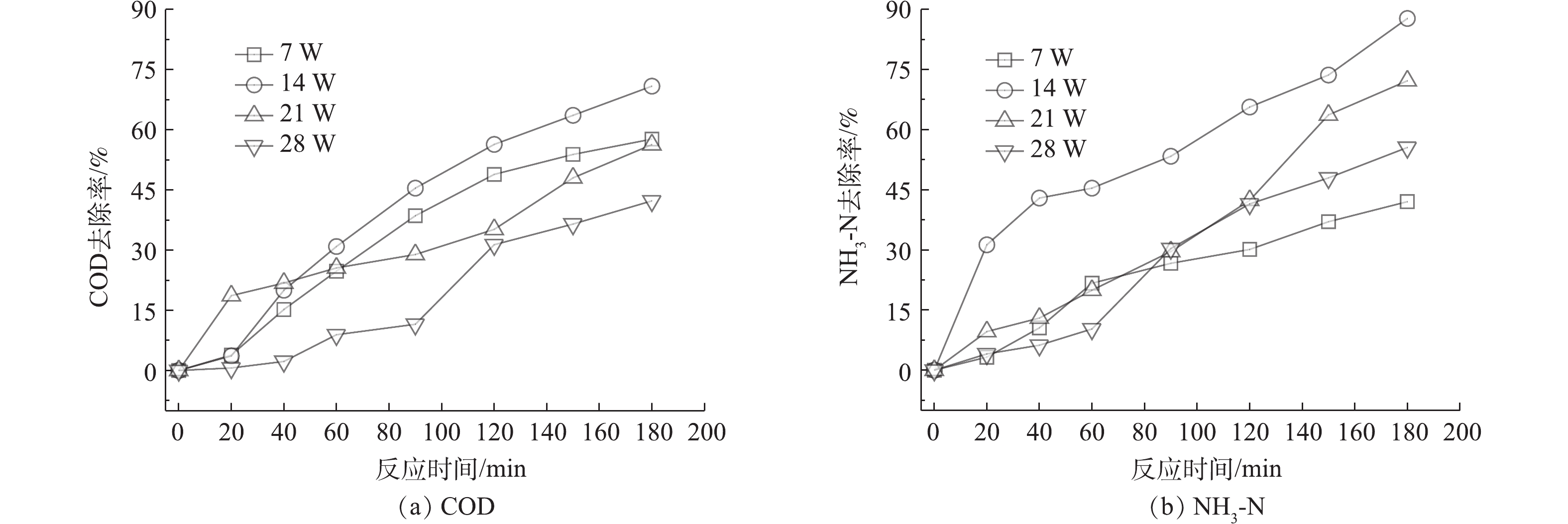
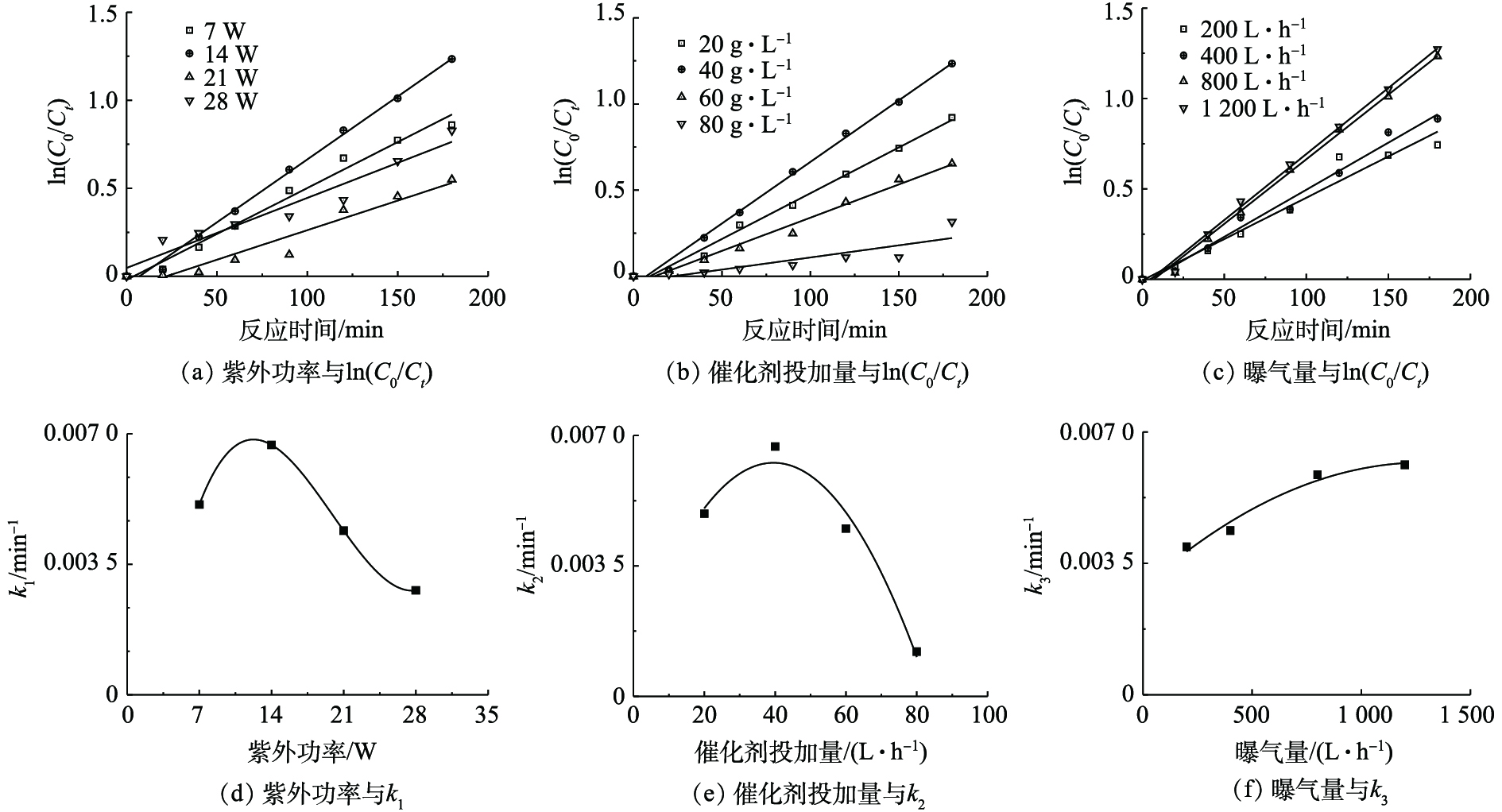
根据前期研究结果[22-23],实验中调整紫外灯的数量,考察了催化体系下紫外功率分别为7、14、21和28 W的处理效果,以上实验均在室温条件下进行,并同时进行空白样和平行样的实验控制,结果如图2所示。UV/O3/AC-Fe3+体系下降解莠去津废水,随着紫外功率的提高,COD去除率随之增加,当紫外灯功率为14 W时,废水COD和NH3-N的去除率最佳。在紫外功率继续增大时,去除率降低,在紫外灯功率为21 W和28 W时,COD的去除率比7 W时更低,这可能是由于过高的光强对降解产生了某种抑制作用或者发生了加成反应,抑制了降解效率。
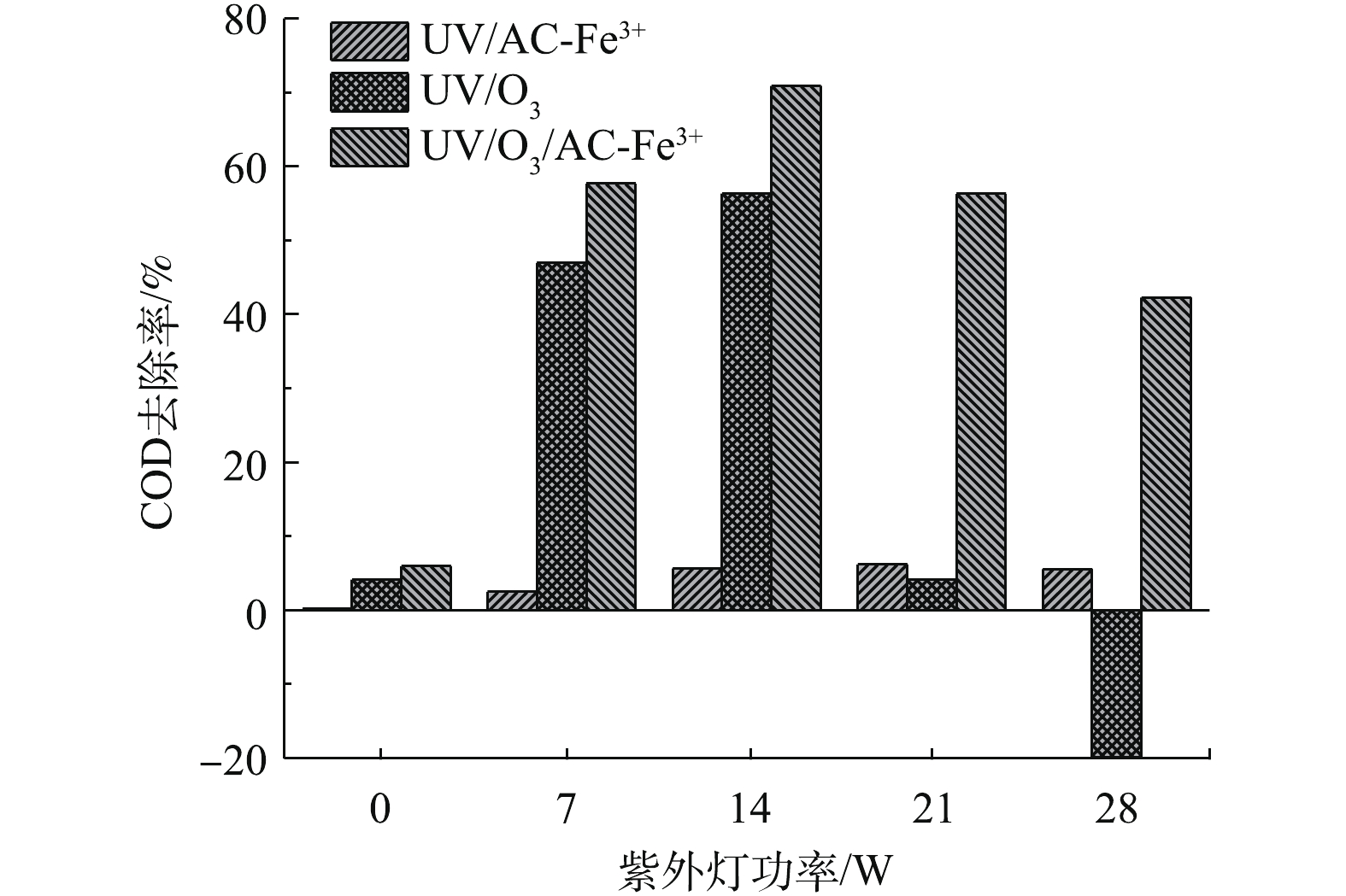
实验进一步研究了光解臭氧(UV/O3)体系和单纯光催化体系(UV/AC-Fe3+),反应进行180 min时紫外功率对废水中COD去除率的影响,并与UV/O3/AC-Fe3+体系的实验结果进行了比较,结果如图3所示。当没有AC-Fe3+存在时,随着紫外功率的增加,UV/O3体系的抑制作用更明显,在紫外功率为28 W时,出现了COD去除率为负值的情况,即反应中产生了某种物质使COD值增加,这说明抑制作用和加成作用可能同时存在。单纯光催化对莠去津废水的降解能力较弱,在紫外功率分别为7、14、21和28 W时,COD去除率分别仅有2.52%、5.68%、6.21%和5.56%,废水COD去除率在21 W时仅为6.21%。3种降解体系相比而言,UV/O3/AC-Fe3+体系对COD的去除效果最佳。
单纯UV/AC-Fe3+降解莠去津废水,莠去津发生脱氯作用产生三嗪类中间产物(如HIET和CAIT),这些中间产物十分稳定,难以直接光解。莠去津及大量三嗪类物质没有得到矿化,因此,废水中COD去除率不高,这与BIANCHI等[24]和JAIN等[25]的研究具有一致性。
在UV/O3/AC-Fe3+体系下,紫外光解臭氧产生具有强氧化能力的羟基自由基,莠去津废水中大量的三嗪类物质在254 nm波长下发生羟基自由基夺氢反应,导致COD降低,增大紫外功率后,COD不降甚至升高,可能有2方面原因:一方面是氯离子的抑制作用,废水中存在大量的氯离子,与有机物竞争·OH,有研究证明氯离子与·OH的反应速率(k·OH-Cl− = 4.3×109 L·(mol·s)−1)要高于目标污染物莠去津(k·OH-Atrazine =2.4×109 L·(mol·s)−1),从而抑制了莠去津的降解反应;另一方面是加成作用,光强过高时,容易促使三嗪类物质直接光解,生成联三嗪类物质,废水中的氯离子也会与有机物争夺氧气,生成次氯酸,在强光辐射下生成氯自由基,进而与有机物发生加成反应,使COD升高,光强越高,加成反应越显著。氯离子对高级氧化的抑制在其他研究中也常有报道[16, 26-27],有研究[28-30]发现,在羟基自由基氧化染料体系中,在低pH条件下,氯离子加速降解过程,在高pH条件下,氯离子显著抑制降解过程,这种现象与本研究结果具有一致性。从本研究结果来看,UV/O3/AC-Fe3+体系可针对性地吸附降解有机物,相比于单一光催化或光解臭氧体系更有效,可削弱因高浓度的氯离子和强碱废水环境对降解产生的抑制作用,更适合莠去津废水。
-
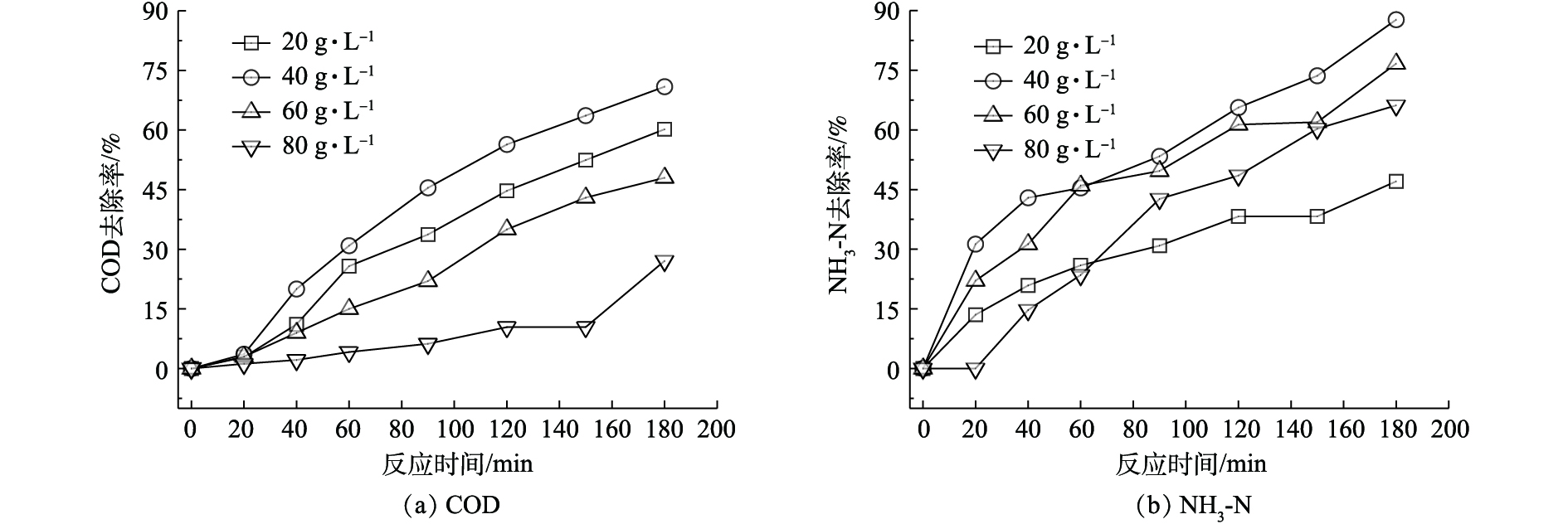
AC-Fe3+的投加有助于吸附水中有机物,单独催化剂吸附莠去津废水,催化剂投加量为20、40、60和80 g·L−1时,COD和NH3-N在180 min时的最高去除率分别仅为5.32%和12.16%。因此,考察了催化剂投加量对光催化氧化(UV/O3/AC-Fe3+)体系降解效果的影响,结果如图4所示。实验前提条件是在相同的光照强度和臭氧流量下,UV/O3体系在180 min内对莠去津废水中的COD最大去除率为56.4%,随着AC-Fe3+的投加,废水中COD去除率有所升高。如图4所示,当AC-Fe3+投加量为40 g·L−1时,莠去津废水中COD的去除效果最好,180 min后废水中COD去除率为70.9%,增加催化剂投加量,没有对去除率有所提高反而呈降低趋势。NH3-N去除率和COD去除率变化趋势类似,当催化剂投加量为20、40、60和80 g·L−1时,NH3-N降解效率分别为47.1%、87.7%、76.7%和66.2%,呈先升高后降低的趋势。COD和NH3-N去除率变化趋势均表明,本研究中催化剂最佳投加量为40 g·L−1。
光催化氧化技术由于产生了光生空穴和氧化基团从而提高了反应效率,但废水中高浓度的氯离子会影响氧化基团的去除率。一方面AC-Fe3+作为吸附剂能够吸附分离废水中的有机物,使得羟基自由基等氧化基团与难降解有机物反应,避免氯离子或者氯自由基与有机物争夺氧化基团,实现选择性降解;另一方面,活性炭负载的Fe3+可以作为催化剂,通过不同价态的转换提高反应效率。推测可能发生的反应步骤如下:废水中的Cl−可与O3反应生成ClO−[31](式(1));然后通过湿式氧化产生高价铁,即Fe3+在碱性条件下被溶液中的ClO−氧化,形成电极电势仅次于羟基自由基·OH(2.4 V)的高铁酸根
${\rm{Fe}}{{\rm{O}}_4^{2 - }}$ (2.2 V)[32-33](式(2));高铁酸根极不稳定,与废水中的有机物进行快速的氧化反应,自身被还原为中间价态铁(式(3));还原产物Fe(Ⅴ)和Fe(Ⅳ)仍具有很强的氧化活性,能对有机物进行进一步的氧化降解[34-35](式(4)),高铁酸根的最终还原产物为三价铁(式(5)),继续参与反应,活性组分三价铁在反应中完成了一个完整循环[36]。溶液中存在的氧化基团、高铁酸根${\rm{Fe}}{{\rm{O}}_4^{2 - }}$ 以及中间的强氧化价态铁共同作用,可加强对废水中有机物的氧化。但AC-Fe3+用量增加没有提高降解效率,可能由于:一方面催化剂颗粒影响了紫外光在溶液中的传播能力;另一方面,AC-Fe3+投量增加使得${\rm{Fe}}{{\rm{O}}_4^{2 - }}$ 浓度升高,高浓度的${\rm{Fe}}{{\rm{O}}_4^{2 - }}$ 会加速其自身分解过程。 -
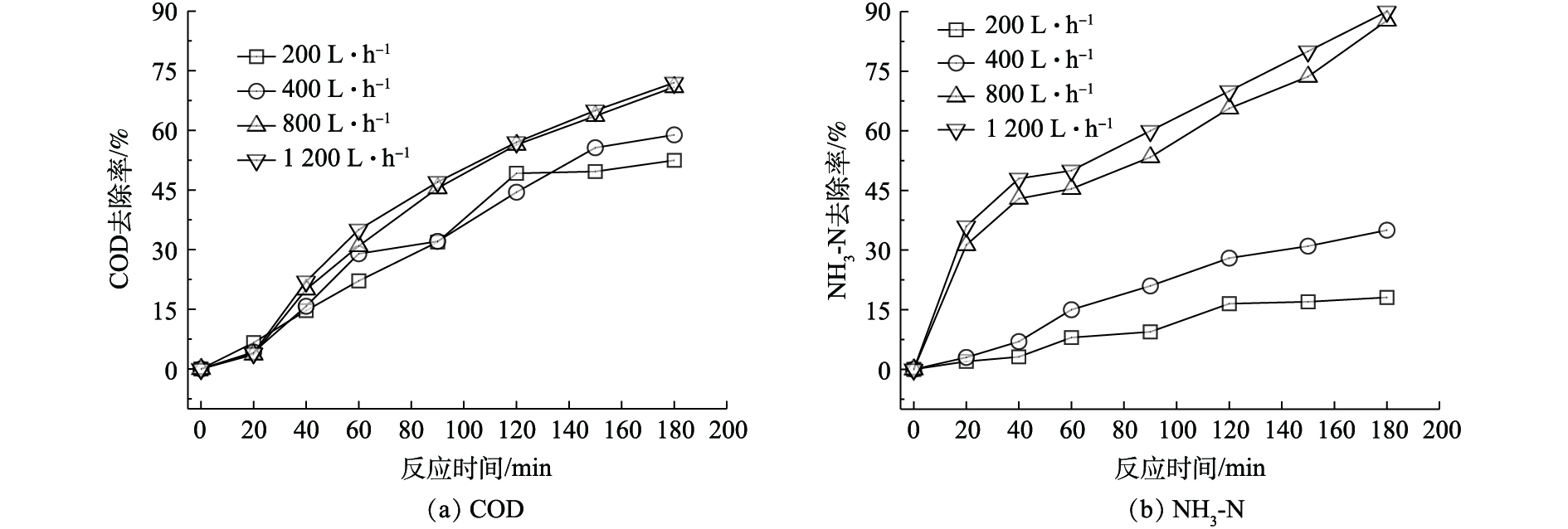
研究考察了臭氧产量为15 g·h−1,空气曝气量分别为200、400、800和1 200 L·h−1时对废水中COD和NH3-N去除率的影响,结果如图5所示。随着曝气量的增加,莠去津废水COD和NH3-N去除率都得到了明显提高。反应进行180 min后,曝气量为200 L·h−1时的COD和NH3-N的去除率分别仅为52.5%和18.1%,当曝气量为800 L·h−1时,COD和NH3-N的去除率可分别增至70.9%和87.7%,去除率分别提高了18.4%和69.6%,特别是废水中NH3-N的催化氧化效果得到明显提高,继续提高曝气量,降解效果提高不明显,考虑到能耗问题,本研究将最佳的空气曝气量定为800 L·h−1。
废水中COD去除率随空气曝气量提高而增加,可能的原因是由于空气中的氧起到了贡献作用。一方面,O2阻止了氯自由基的加成反应(式(6)),使得自由基加成反应链得以终止,比如甲基自由基与氧气反应,生成了反应活性很差的的过氧甲基自由基;另一方面,O2促进了羟基自由基与降解中间体的反应,从而促进了有机物的矿化反应(式(7)),双重作用下,光催化氧化过程的效率得以提高。研究表明,O2在均相体系和非均相体系矿化污染物中都具有重要作用,在非均相体系中,O2清除导带上电子,从而防止电子和空穴复合,增加羟基自由基的产量的同时,还促进中间产物的降解。研究发现,一旦O2被消耗,中间体的降解就可能停止[37-39];在均相体系中,O2直接参与环状污染物的开环,有助于初级中间体以及副产物的矿化[39]。
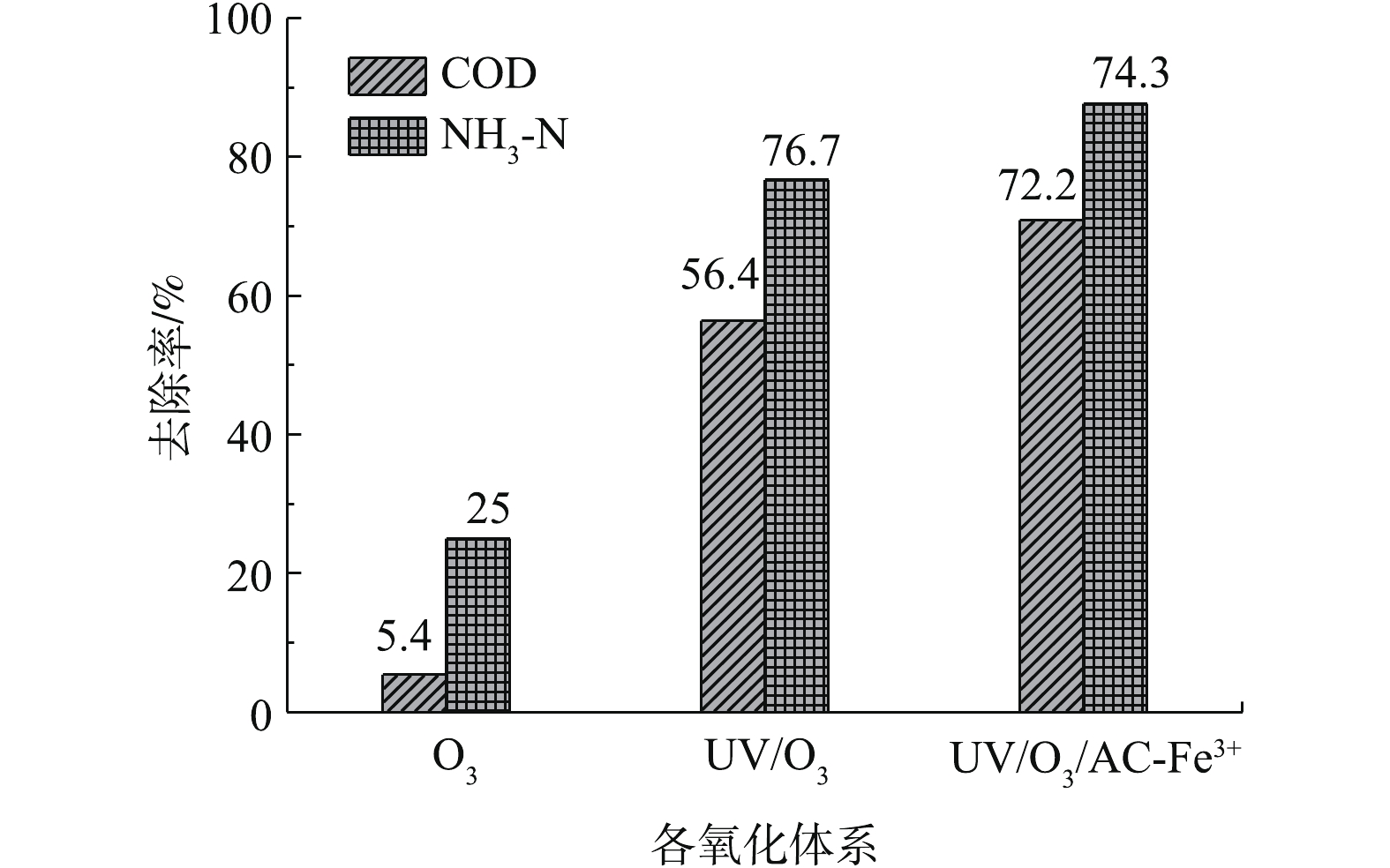
研究对比分析了800 L·h−1曝气量下不同氧化体系的去除率(图6),单独O3氧化对废水COD去除率最低,仅为5.4%;O3与UV结合后,光解臭氧产生的羟基自由基可对废水中有机物快速降解,COD去除率提高了51%;在UV/O3体系投加催化剂AC-Fe3+后,光催化臭氧化作用对废水COD去除率最高,比单独O3提高了66.8%。可以发现,单独O3氧化能力较弱,UV和AC-Fe3+的加入为莠去津废水降解起到主要贡献作用。事实上,单独O3法很难将污染物完全降解,须与其他技术(如紫外、超声、活性炭等)结合来达到处理要求[40]。
-
将不同紫外功率、催化剂投加量以及曝气量的莠去津废水COD光催化降解实验数据进行积分处理,求出不同影响因素在各个水平下的反应速率常数k (表1),并绘制ln(C0/Ct)与反应时间的曲线(图7)。可以看出,ln(C0/Ct)与t基本呈线性关系,即表明莠去津废水中的COD光催化降解过程可用一级反应动力学方程来描述。当紫外功率从7 W上升到28 W时,k先从0.005 1上升到0.006 7,然后降到0.002 8,同理,COD催化剂投量和空气曝气量的反应速率常数也有先升后降的规律。
利用Origin 8.5®进行非线性回归,得到每个影响因素与废水中COD一级反应速率常数k之间的拟合曲线(见图7),不同紫外功率(P)、不同催化剂投加量(M)和不同空气曝气量(Q)与一级反应速率常数k1、k2和k3之间的拟合关系见式(8)~式(10),莠去津废水COD的Langmuir降解速率常数k= k1k2k3。
2.1. 紫外功率对莠去津废水降解的影响
2.2. 催化剂投加量对莠去津废水降解的影响
2.3. 曝气强度对莠去津废水降解的影响
2.4. 负载型活性炭光催化臭氧氧化降解莠去津废水中COD的动力学
-
1) 研究针对高盐度莠去津生产废水开发了活性炭负载三价铁催化剂(AC-Fe3+)光催化臭氧氧化方法,实验证明,AC-Fe3+的存在与否对废水中COD的去除率影响显著。当无AC-Fe3+存在时,降解过程受到水中氯离子的影响,提高光强反而抑制了降解过程,AC-Fe3+可以有效延缓高盐水质的不利影响。
2) 通过对各影响因素进行优化实验,进一步确定了最佳反应条件:催化剂投加量为40 g·L−1,紫外线功率为14 W,曝气强度为800 L·h−1时,废水中COD和NH3-N去除率分别可以达到70.9%和87.7%。
3) 对AC-Fe3+光催化臭氧氧化降解废水中COD的动力学进行研究,发现废水中COD的降解过程符合一级动力学。分别拟合了催化剂投加量、紫外线功率和曝气强度与废水中COD反应速率常数k之间的关系,计算值与实验值具有很好的相关性。



 下载:
下载: