-
当前,我国许多污水处理厂面临进水碳氮比(本文以C/N表示)低,无法满足正常生物硝化后反硝化对碳源的需求。而当碳源不足时,容易导致反硝化无法进行到底,进而产生N2O;其次,当外加碳源耗尽后,反硝化菌将不得不利用内碳源进行反硝化,其反应的最终产物更容易是N2O[1]。作为一种强温室气体[2],N2O的全球增温潜势是CO2的265倍、CH4的28倍[3],且N2O的释放量每年正以0.3%的趋势增长[4]。N2O的持续排放将对人类生存环境及氮素平衡产生严重影响。此外,除了通过减少脱氮系统的N2O的释放率进行减量,SCHERSON等[5-6]利用CANDO(coupled aerobic-anoxic nitrous decomposition operation)工艺以聚羟基链烷酸酯(PHA)作为电子供体与NO2反应产生N2O气体并回收利用,获得了较高的N2O转化率和能源回收率,对于生物脱氮过程中产生大量N2O的工艺有重要的参考价值。因此,有机碳源的投加对于反硝化过程的N2O的产生有重要影响。
在反硝化过程中,
$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N浓度[7]、$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N浓度、C/N[8]等初始条件均可能影响脱氮效果和N2O的产生情况。以$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N为氮源的反硝化过程,需要先经由$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N再进行下一步反应。短程硝化反硝化作为污水处理中的新技术,在实现$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N积累后再进行亚硝酸盐反硝化,从而缩短反应时间,在一定C/N的条件下提高脱氮效率。常用的外加碳源为甲醇、乙醇和乙酸等低分子质量的有机化合物。甲醇过去作为一种常见碳源,由于近年来对危化品的管控,导致其运输困难,因此,在近年来的使用量日益减少[9]。而乙醇、乙酸运行成本较高,且乙酸会改变反应器内pH,影响其他菌种的生长[10],故乙醇和乙酸也不是理想的碳源。许多研究表明,葡萄糖的投加能够提高脱氮效果[11-12]。而关于碳源对反硝化过程中N2O释放量影响的相关研究已有一定的进展[13]。N2O的释放量因碳源的不同存在较大差别[14],而在不同C/N的条件下,N2O的释放特征也有所不同[15]。为提高出水水质并降低污水处理厂运行成本,需要探求各种廉价碳源进行生物反硝化。本研究以葡萄糖这类优质廉价的原料作为反硝化碳源,以$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N和$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N为电子受体,设计了不同的C/N进行实验,旨在研究葡萄糖碳源条件下的反硝化特征和N2O的释放规律,以减少N2O排放,或增加N2O释放以进行收集利用。
全文HTML
-
本研究采用批次实验的形式,污泥取自在SBR内驯化并适应了以葡萄糖为碳源的反硝化污泥。通过沉淀、离心去除上清液,加入蒸馏水后再次进行沉淀、离心、去除上清液,将以上步骤重复至少3次,以确保污泥中无化学物质残留。MLSS和MLVSS分别为2.61 g·L−1和2.42 g·L−1,温度维持在22 ℃,使用3 mol·L−1的HCl和3 mol·L−1的NaOH调节pH至6.5。此外,实验前进水用氮气吹脱以去除DO。污泥经淘洗后,加入不含
$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N和COD的原水混合均匀后,倒入500 mL的广口瓶中,随后将$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N、$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N和COD按实验要求分别配成浓缩液,在反应开始后,立即注射入广口瓶中,并将广口瓶置于磁力搅拌器上进行搅拌,转速为150 r·min−1。在
$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N或$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N分别为电子受体的条件下,分别探究C/N为1.5、3、6.5、10和20时对反硝化脱氮及N2O产生的影响。每隔10 min取水样,以检测反应器中$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N、$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N、溶解态N2O,持续100 min。每次取样结束后向广口瓶中通入适量氮气以补充瓶内气压。 -
实验通过投加NaNO3或者NaNO2分别调节
$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N和$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N浓度为100 mg∙L−1,投加不同量葡萄糖调节C/N为1.5、3、6.5、10、20。将$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N或$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N和葡萄糖按实验要求分别配成浓缩液,在反应开始后,立即注射入广口瓶中,实验具体运行条件如表1所示。 -
本实验中各污染物的检测指标均参考《水和废水监测分析方法》[16]的方法进行:
$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N采用N-(1-萘基)-乙二胺光度法;$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N采用紫外分光光度计法;COD采用重铬酸钾法;pH采用Five Go pH计进行测量;DO采用Multi 3620IDS溶解氧仪进行测量;MLSS和MLVSS采用重量法测定;溶解态N2O采用岛津气相色谱仪GC-2014通过顶空平衡法进行测量。
1.1. 实验装置和运行条件
1.2. 实验用水
1.3. 分析项目及方法
-
反硝化反应过程[17]如式(1)所示。
式中:Nar为硝酸盐还原酶(nitrate reductase);Nir为亚硝酸盐还原酶(nitrite reductase);Nor为一氧化氮还原酶(nitric oxide reductase);Nos为N2O还原酶(nitrous oxidereductase)。
由式(1)看出,反硝化过程需要大量电子供给,而这些电子供体通常来源于外部的有机碳源,内部碳源(poly-β-hydroxybutyrate,PHB)以及死亡的细胞组织等[18],反硝化的进行将受限于溶液中的电子供体数目,因此,C/N是衡量反硝化系统运行情况的重要指标。
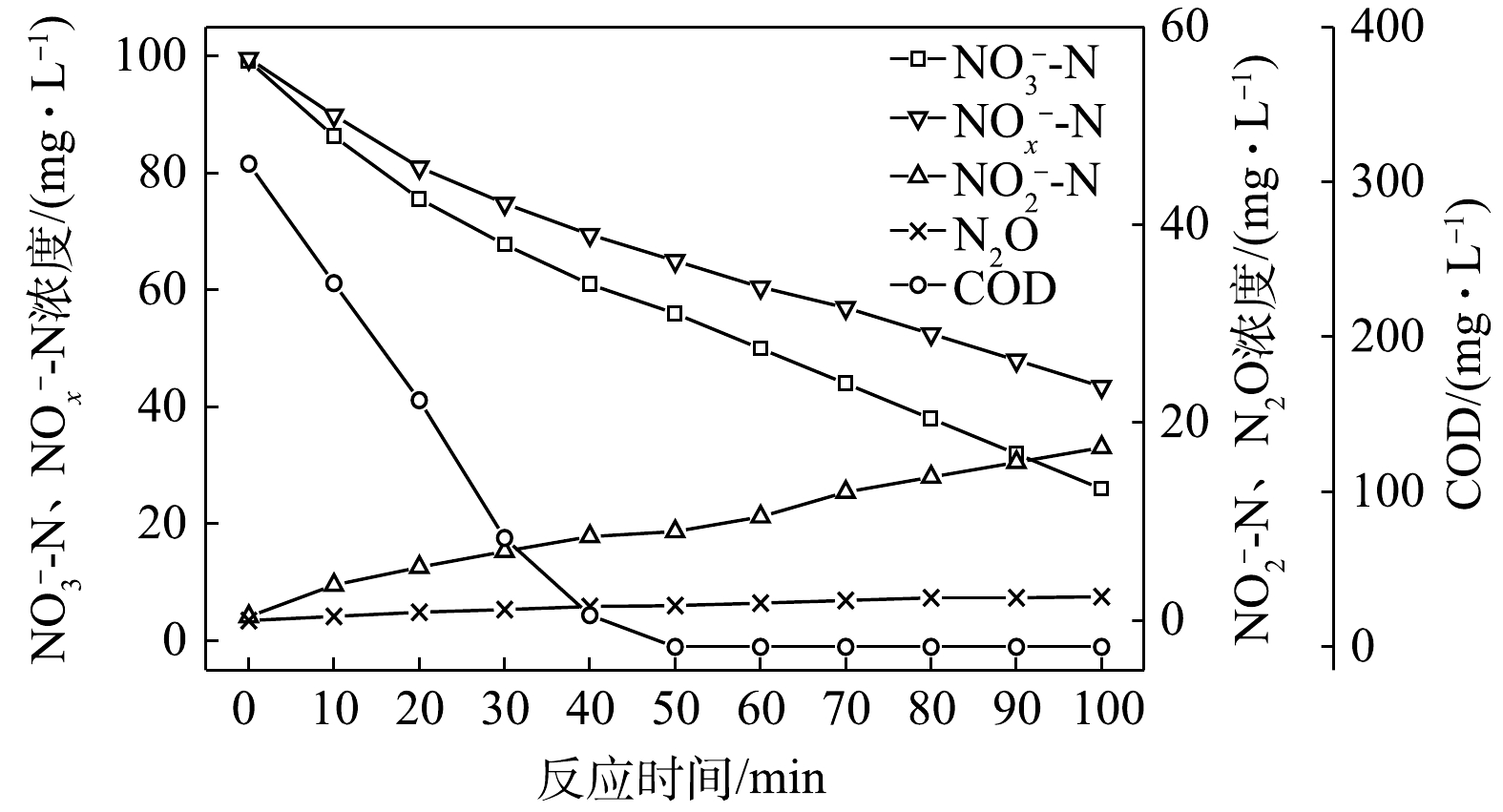
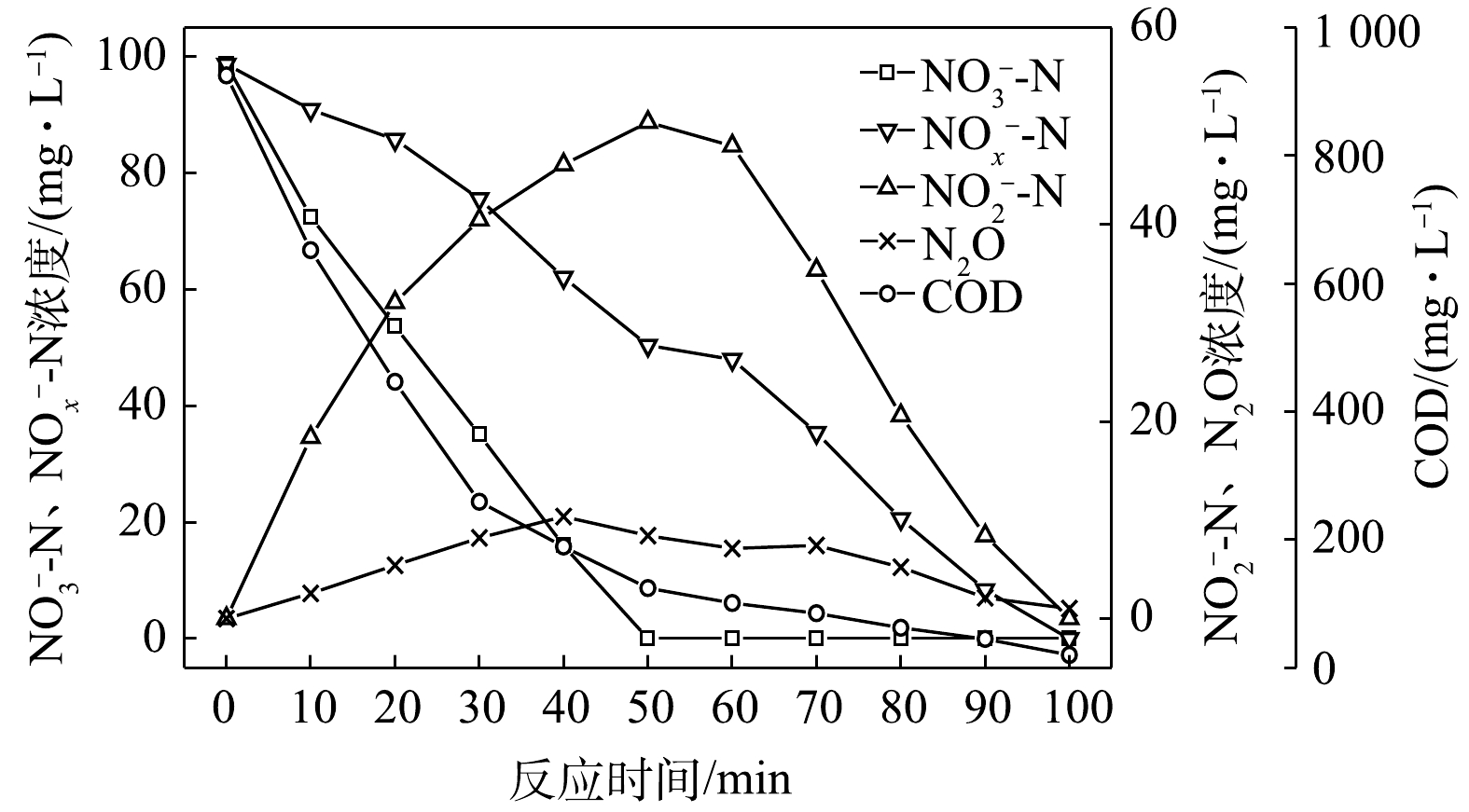
1) C/N=1.5时的反硝化的脱氮效果。当C/N为1.5时,以
$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N为电子受体时,反硝化过程中各氮素变化的情况如图1所示。以$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N为电子受体的条件下,在100 min内,$ {\rm{NO}}_x^{\rm{ - }}$ -N (=$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N+$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N)由100 mg∙L−1降至67 mg∙L−1,反硝化速率为8.81×10−3 g·(g·h)−1。其中$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N由99.8 mg∙L−1降至54 mg∙L−1,平均降解速率为1.14×10−2 g·(g·h)−1;$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N由0.2 mg∙L−1积累到13 mg∙L−1,平均积累速率为3.17×10−3 g·(g·h)−1。在反应过程中,N2O不断积累达到最大值为1.79 mg∙L−1,积累速率为0.45 mg·(g·h)−1,峰值转化率为3.43%。当C/N为1.5时,以
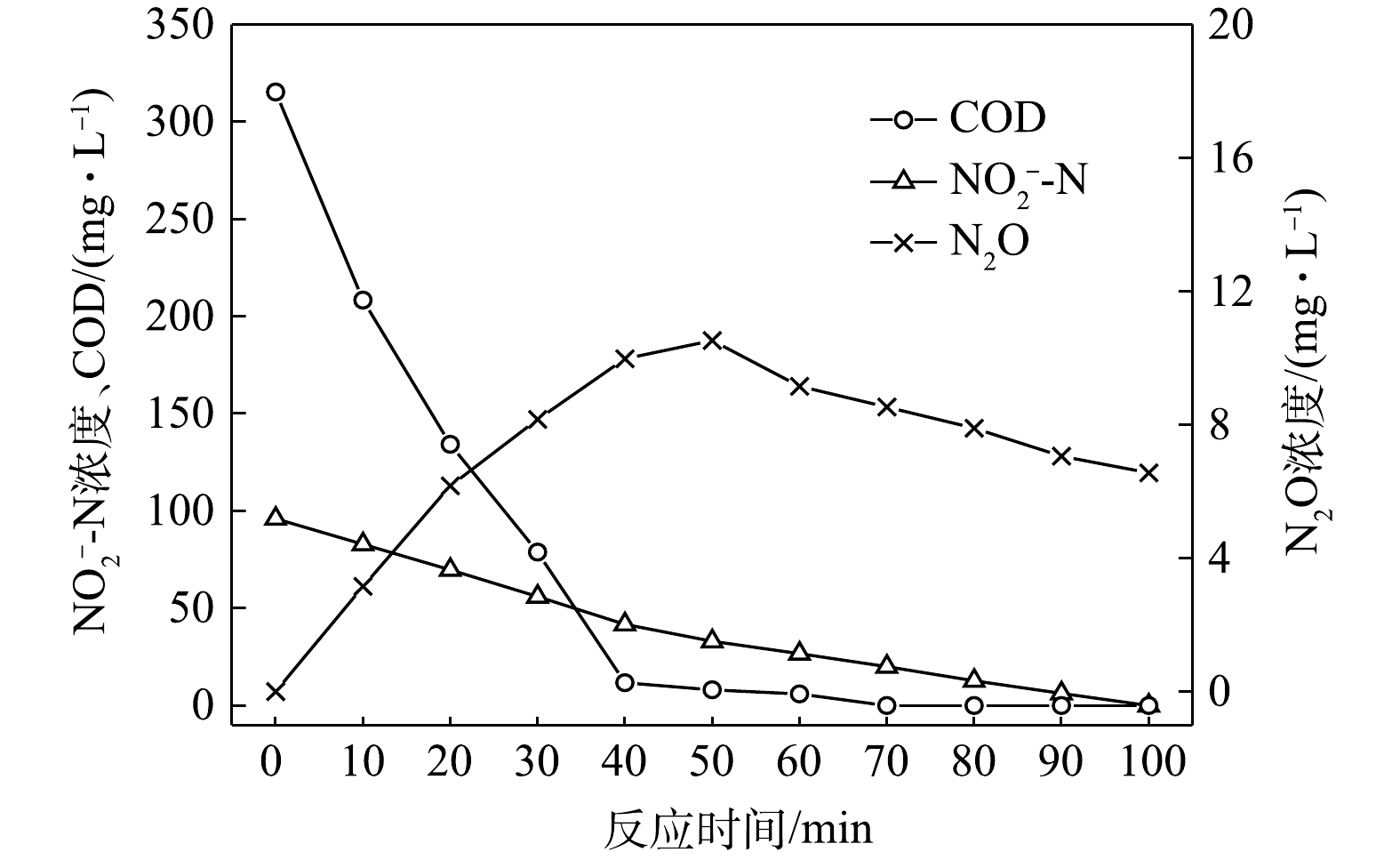
$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N为电子受体时,反硝化过程中各氮素的变化情况如图2所示。以$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N为电子受体的条件下,在100 min内,$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N由100 mg∙L−1降至35.8 mg∙L−1,反硝化速率为1.59×10−2 g·(g·h)−1。与以$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N为电子受体的工况比较,在相同的C/N条件下,以$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N为电子受体的反硝化速率是$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N的1.95倍。这是因为,$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N作为电子受体参与反硝化时必须转化为$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N才能继续进行,且$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N作为电子受体时的消耗反应速度要优于$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N作为电子受体时的速度[19]。N2O最大积累量为2.62 mg∙L−1,积累速率为1.63 mg·(g·h)−1,峰值转化率为4.08%,且N2O最大积累量、积累速率及峰值转化率均高于以$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N为电子受体的情况。这说明以$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N为电子受体进行反硝化反应时N2O的释放量高于以$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N做电子受体进行反硝化反应时的N2O释放量,主要是由于反硝化反应过程中浓度较高的$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N会抑制Nos酶的活性[20]。这与吴光学等[21]、翟晓峰等[22]的研究结果一致。2) C/N=3时的反硝化的脱氮效果。当C/N为3时,以
$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N为电子受体时,反硝化过程中各氮素变化的情况如图3所示。在以$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N为电子受体的条件下,在100 min内,$ {\rm{NO}}_x^{\rm{ - }}$ -N由99.6 mg∙L−1降至43.5 mg∙L−1,反硝化速率为1.39×10−2 g·(g·h)−1。其中$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N由99.2 mg∙L−1降至26 mg∙L−1,平均降解速率为1.81×10−2 g·(g·h)−1;$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N由0.4 mg∙L−1积累至17.5 mg∙L−1,平均积累速率为4.24×10−3 g·(g·h)−1。在反应过程中,N2O不断积累达到最大值,为2.42 mg∙L−1,较C/N为1.5时高,N2O积累速率为0.60 mg·(g·h)−1,N2O峰值转化率为4.32%。当C/N为3时,以
$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N为电子受体时,反硝化过程中各氮素变化的情况如图4所示。以$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N为电子受体的条件下,在100 min内,$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N由96 mg∙L−1降为0 mg∙L−1,反硝化速率为2.38×10−2 g·(g·h)−1,N2O最大积累量为10.53 mg∙L−1,积累速率为5.22 mg·(g·h)−1,峰值转化率为10.97%。虽然以$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N为电子受体时,N2O的最大积累量较高,但此时并非最终结果,随着反应进程的推进,N2O被还原被N2,TN(以$ {\rm{NO}}_x^{\rm{ - }}$ -N计)被完全去除,反硝化过程进行地较为充分。与C/N为1.5时所得的结果相似,以
$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N为电子受体的反硝化速率以及N2O的积累量、积累速率及峰值转化率均高于以$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N为电子受体时的情况。随着C/N的提高,此时各C/N下的反应速率均高于C/N为1.5时。这主要是由于碳源的增加提供了更多的电子受体,促进了反硝化过程的进行,而N2O积累量的增加除了与反硝化过程中各种酶之间的电子竞争[21]有关,也与葡萄糖[23]相对复杂的代谢过程有关。3) C/N=6.5时的反硝化的脱氮效果。当C/N为6.5时,以
$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N为电子受体时,反硝化过程中各氮素变化的情况如图5所示。在以$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N为电子受体的条件下,100 min内,$ {\rm{NO}}_x^{\rm{ - }}$ -N由101.2 mg∙L−1降至19.5 mg∙L−1,反硝化速率为2.03×10−2 g·(g·h)−1。$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N去除率达到100%,平均降解速率为3.09×10−2 g·(g·h)−1。$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N浓度先升高后降低,$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N的积累速率和降解速率分别为1.48×10−2 g·(g·h)−1和1.97×10−2 g·(g·h)−1,$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N浓度的最大积累值为43.3 mg∙L−1,截至反应进行到100 min时,$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N浓度降至19.5 mg∙L−1。N2O的最大积累量为5.01 mg∙L−1,积累速率为1.24 mg·(g·h)−1,峰值转化率为6.14%。当C/N为6.5时,以
$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N为电子受体时,反硝化过程中各氮素变化的情况如图6所示。在以$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N为电子受体的条件下,在反应进行的100 min内,$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N由98 mg∙L−1降为0 mg∙L−1,反硝化速率为4.05×10−2 g·(g·h)−1。N2O最大积累量为17.29 mg∙L−1,积累速率为14.29 mg·(g·h)−1,N2O峰值转化率为17.64%。与C/N为1.5和3时相似,以$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N为电子受体时的反硝化速率以及N2O的积累量、积累速率及峰值转化率均高于以$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N为电子受体时的情况。4) C/N=10时的反硝化的脱氮效果。当C/N为10时,以
$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N为电子受体时,反硝化过程中各氮素变化的情况如图7所示。以$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N为电子受体时,反硝化脱氮率达到100%,反硝化速率为2.45×10−2 g·(g·h)−1,$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N的平均降解速率为4.89×10−2 g·(g·h)−1。$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N浓度先升高后降低,前50 min内,$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N浓度上升至50.4 mg∙L−1,之后随着$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N浓度的耗尽;在后50 min,$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N浓度下降至0 mg∙L−1,$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N的积累速率为2.50×10−2 g·(g·h)−1。N2O积累量先增加后减少,在前40 min内,N2O浓度达到最大积累值10.36 mg∙L−1,之后N2O浓度开始下降;截至100 min时,溶液中N2O浓度减少为1.02 mg∙L−1。N2O积累速率为6.42 mg·(g·h)−1,N2O峰值转化率为10.49%。当C/N为10时,以
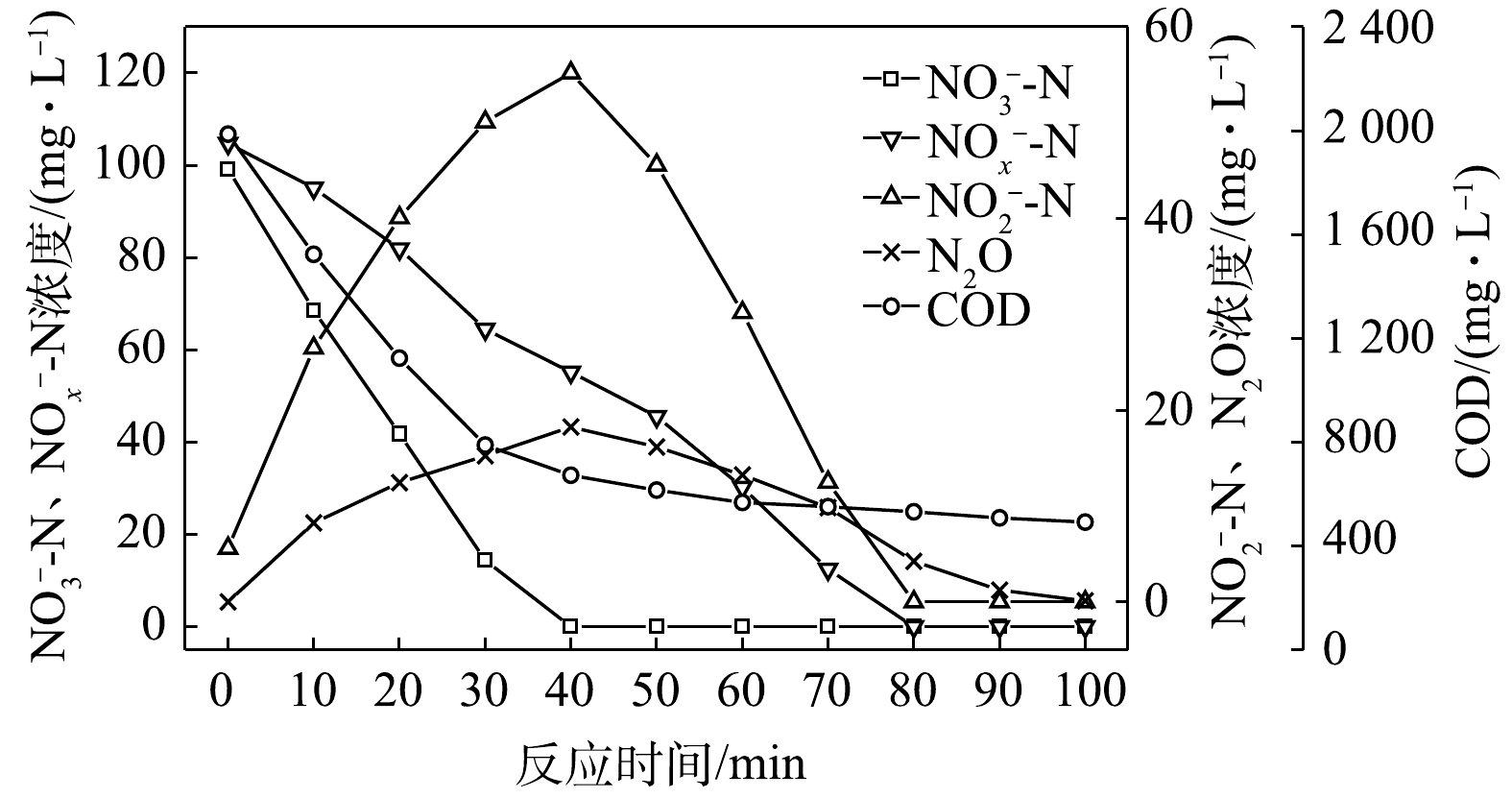
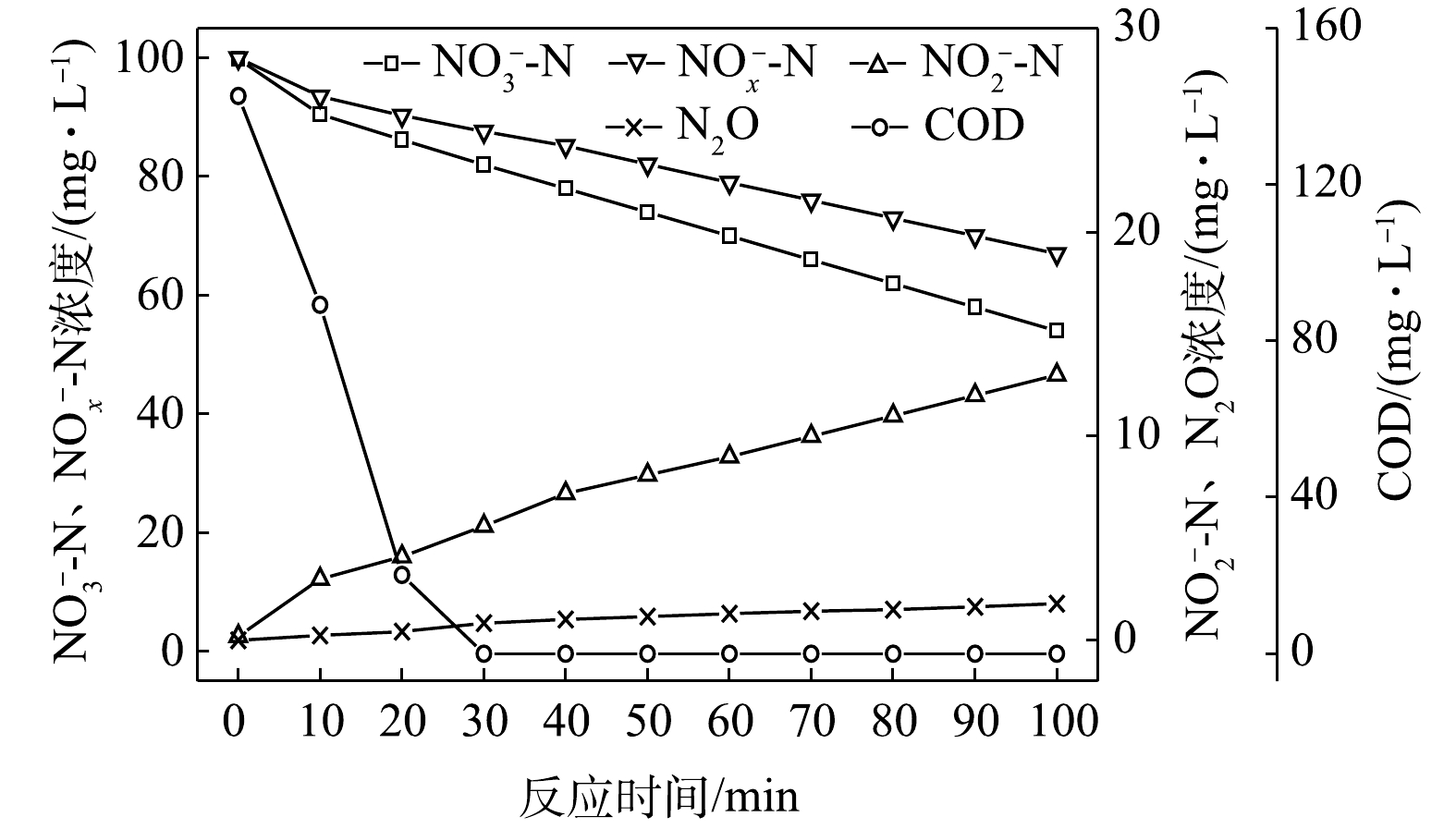
$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N为电子受体时,反硝化过程中各氮素变化的情况如图8所示。在以$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N为电子受体的条件下,$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N浓度由95.6 mg∙L−1迅速被完全降解,平均降解速率为5.92×10−2 g·(g·h)−1。溶液中N2O浓度先增加后减少,于20 min时达到最大积累值28.20 mg∙L−1,积累速率为34.96 mg·(g·h)−1,峰值转化率为29.49%。与C/N为1.5、3和6.5时相似,以$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N为电子受体时的反硝化速率以及N2O积累量、积累速率及峰值转化率均高于以$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N为电子受体的情况。5) C/N=20时的反硝化的脱氮效果。当C/N为20时,以
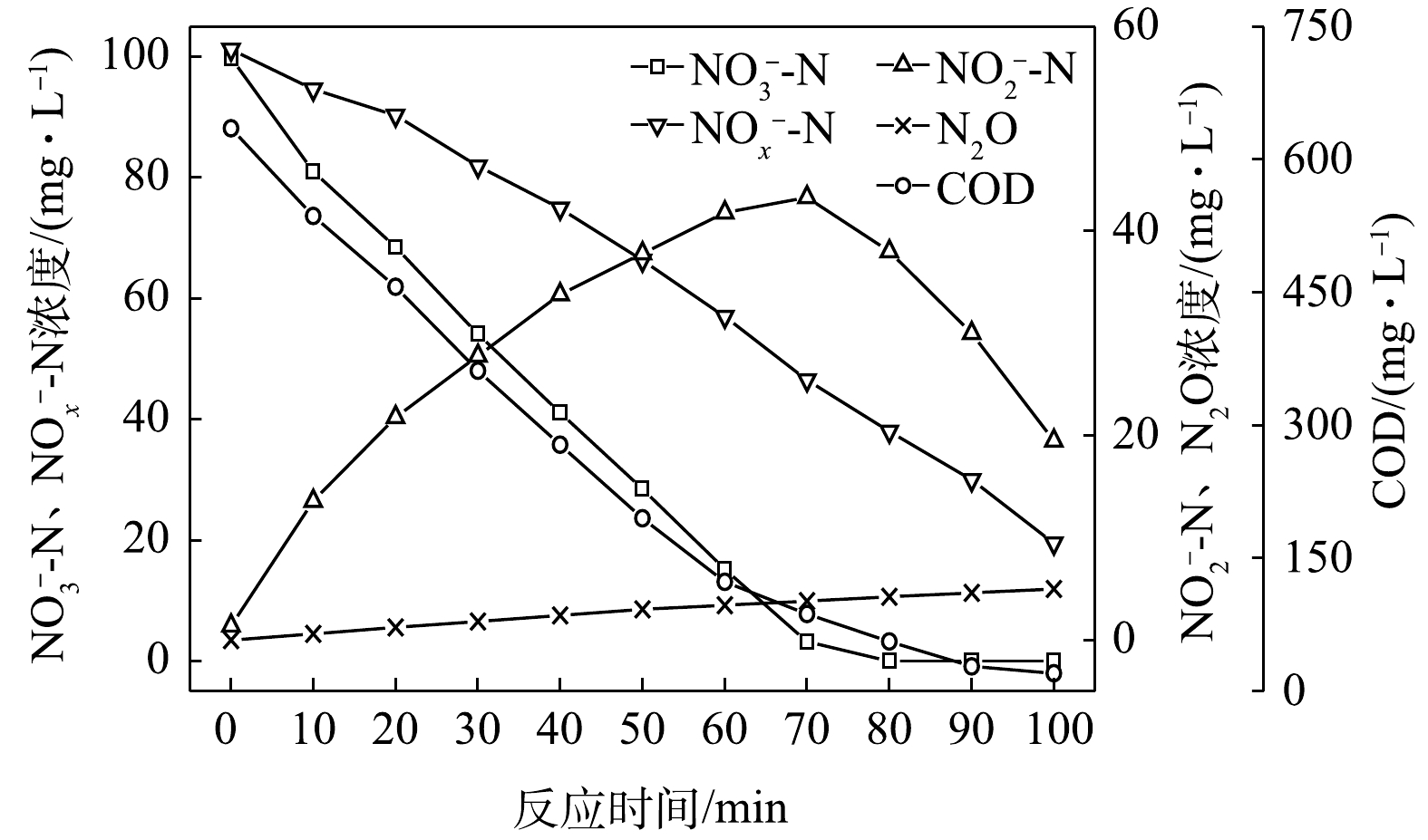
$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N为电子受体时,反硝化过程中各氮素变化的情况如图9所示。以$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N为电子受体时,反硝化脱氮率在反应进行的80 min内达到100%,反硝化速率为3.25×10−2 g·(g·h)−1,$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N在40 min内被降解完毕,平均降解速率达到6.15×10−2 g·(g·h)−1。$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N浓度先升高后降低,在前40 min内,$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N浓度上升至55.2 mg∙L−1,之后$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N浓度下降至0 mg∙L−1,反应在80 min内基本结束。$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N的积累速率为3.07×10−2 g·(g·h)−1。N2O浓度先增加后减少,在反应开始后的40 min时,N2O浓度达到最大积累值18.27 mg∙L−1,之后N2O浓度开始下降;截至100 min时,溶液中N2O浓度减少为0.12 mg∙L−1。此条件下,N2O积累速率为11.32 mg·(g·h)−1,N2O峰值转化率为17.43%。当C/N为20时,以
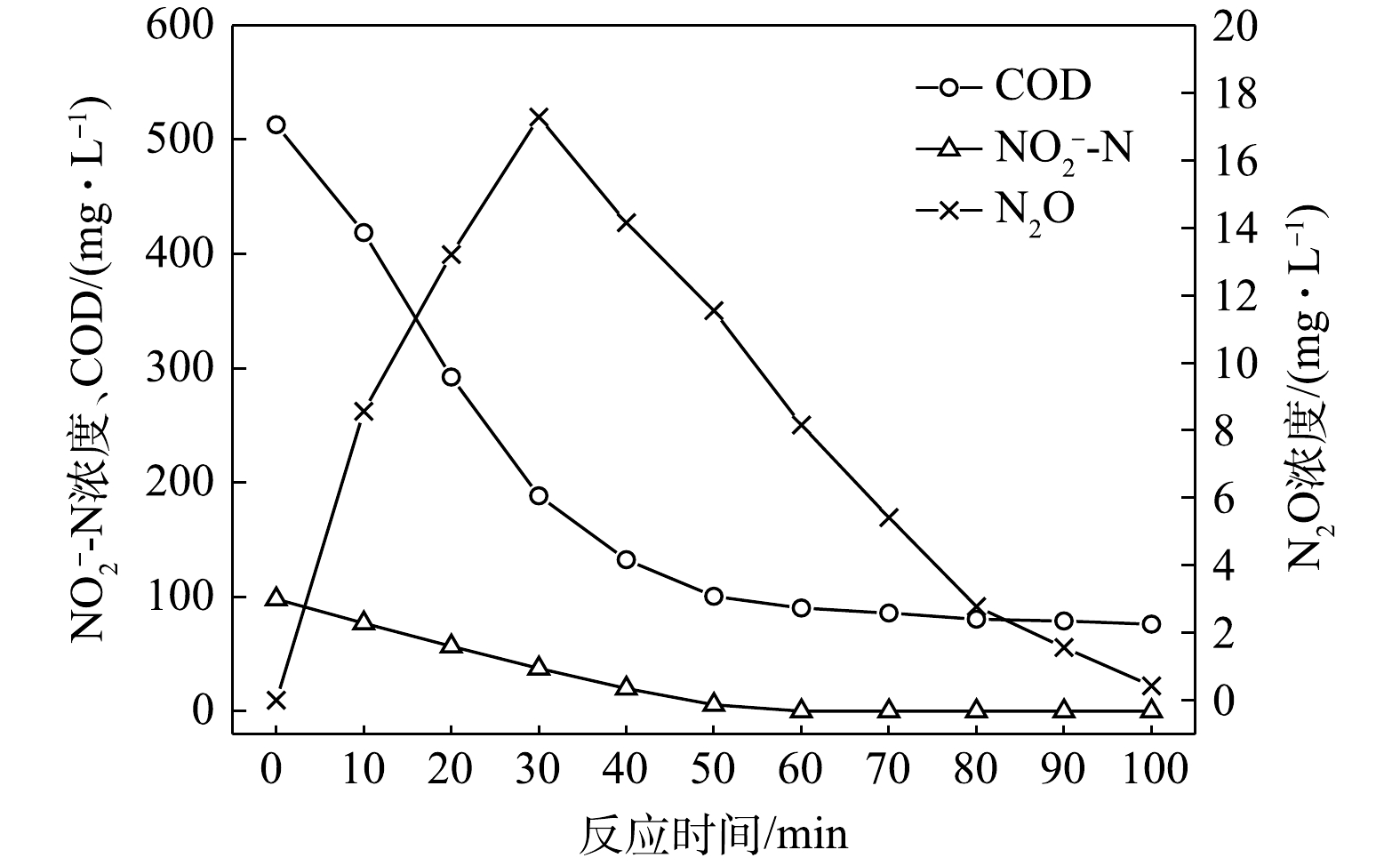
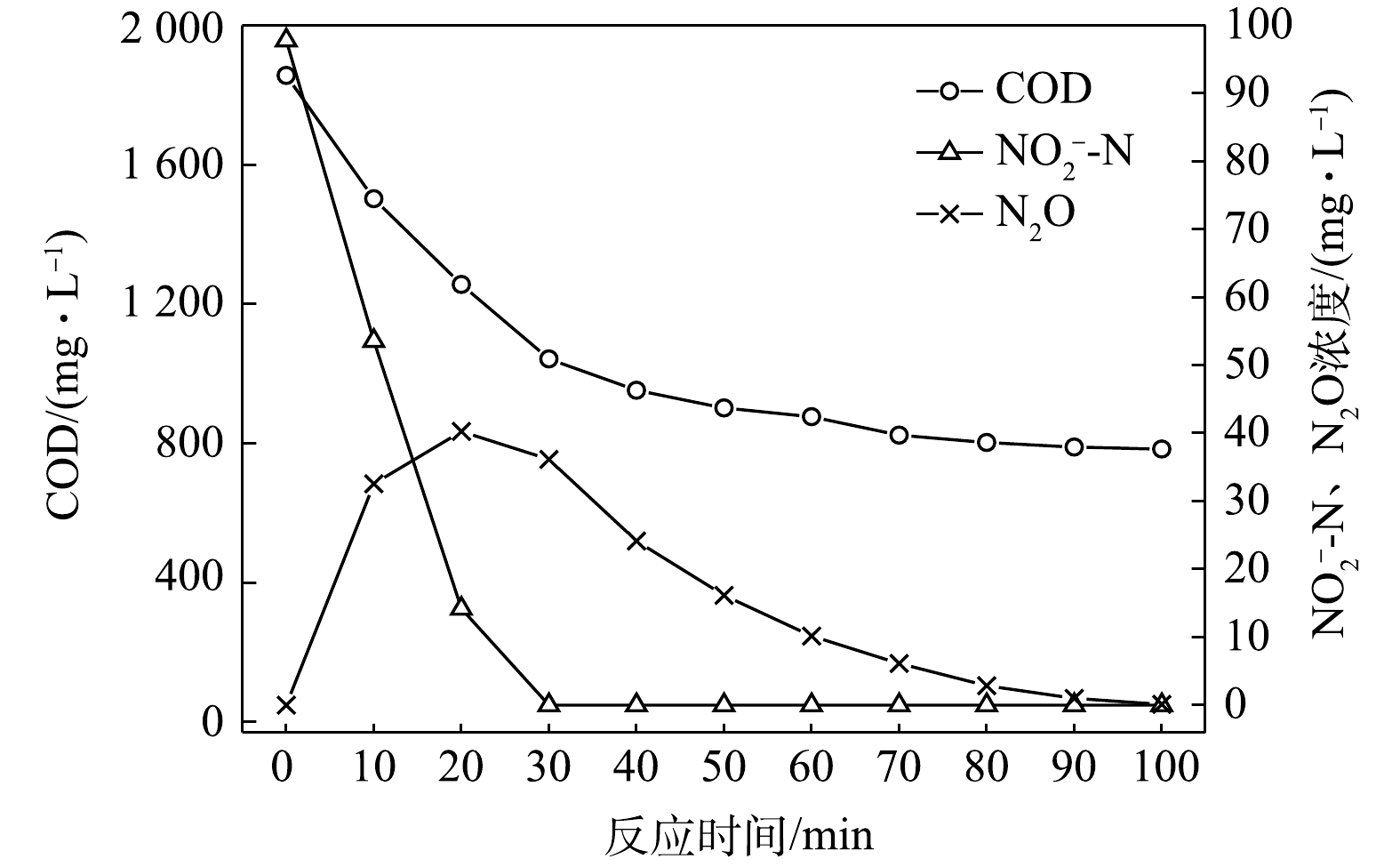
$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N为电子受体时,反硝化过程中各氮素变化的情况如图10所示。以$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N为电子受体的条件下,$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N浓度由97.8 mg∙L−1被迅速完全降解,平均降解速率为8.08×10−2 g·(g·h)−1。溶液中N2O浓度先增加后减少,于20 min时达到最大积累值40.27 mg∙L−1,积累速率为49.92 mg·(g·h)−1,峰值转化率为41.17%。随着C/N的增加,在不同电子受体情况下脱氮效率均有所升高,这说明低C/N条件限制了反硝化过程的进行。而且,以
$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N为电子受体的反硝化效果比以$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N为电子受体的反硝化效果差。当C/N为3、以$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N为电子受体时,产生的N2O在达到峰值后呈下降趋势,说明反硝化进程在N2O到达峰值后并未结束。但由于系统碳源不足,反硝化进行的不够充分,N2O在反应结束后并未完全降解。通过对比以$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N为电子受体,C/N为6.5、10、20时的N2O浓度,均呈现达到峰值后下降的趋势,并且产生峰值的时间随C/N的升高而缩短,N2O均反应完全。此趋势在以$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N为电子受体,C/N为10、20时也同样出现。主要是由于C/N的升高促进了系统的反硝化进程和N2O的降解。但是由于$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N作为电子受体参与反硝化时必须转化为$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N才能继续进行,导致以$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N为电子受体出现此现象滞后于$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N,碳源不足导致了其反应速率的滞后。而在相同实验条件下,以
$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N为电子受体的反硝化过程速率分别是以$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N为电子受体反硝化速率的2、1.7、2.05、2.46、2.53倍,与理论值1.67倍不符。这说明,$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N的还原速率在反硝化过程中受到了抑制,亦可以说以$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N为电子受体还原时受抑制的程度高于以$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N为电子受体时受抑制的程度。MA等[24]认为,在反硝化过程中会产生一定的FNA,而FNA对反硝化菌的活性有抑制作用[25]。在本研究中,在$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N为电子受体被还原的过程中,当C/N为1.5~20时,FNA最大值由0.01 mg·L−1上升至4.25×10−2 mg·L−1;在$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N为电子受体被还原的过程中,FNA最大值在0.074~0.077 mg·L−1内。有研究[26]表明,硝酸盐还原在FNA浓度为0.01~0.025 mg·L−1时,硝酸盐还原能力受抑制程度达到60%;同时,污泥亚硝酸盐还原能力也受到FNA的限制,当FNA浓度由0.01 mg·L−1上升至0.2 mg·L−1时,亚硝酸盐的还原能力下降80%。相对于Nir酶而言,Nar酶对C/N的变化更敏感。这也从另一个角度证明了:以$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N为电子受体时,随着C/N的增加,Nar酶因竞争电子的能力比Nir酶要强,从而导致$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N的浓度积累随着C/N的增加而升高。随着C/N的上升,加剧了FNA的积累以及各还原酶间的竞争[27],进而导致了反硝化过程被抑制以及N2O的释放。 -
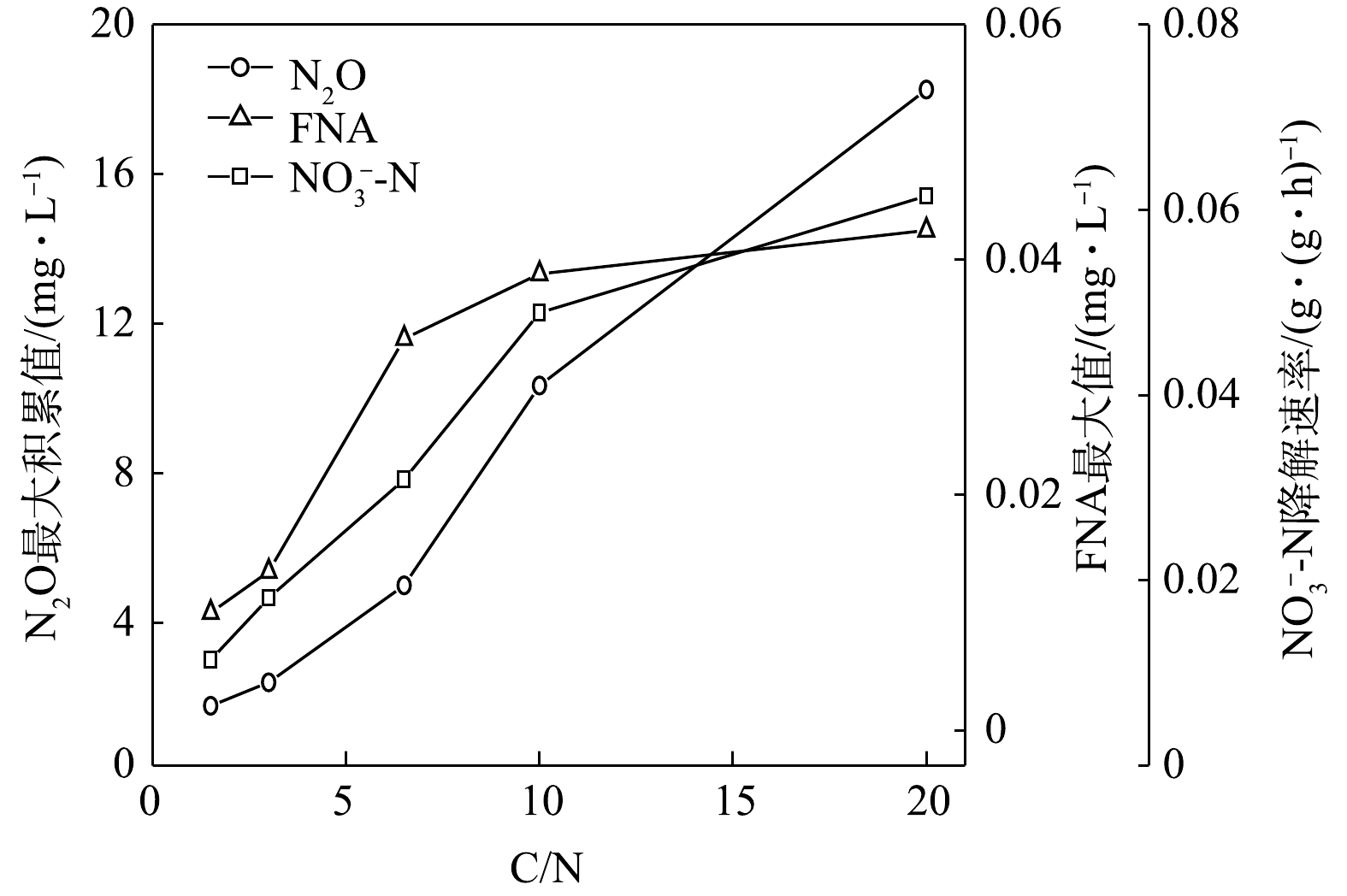
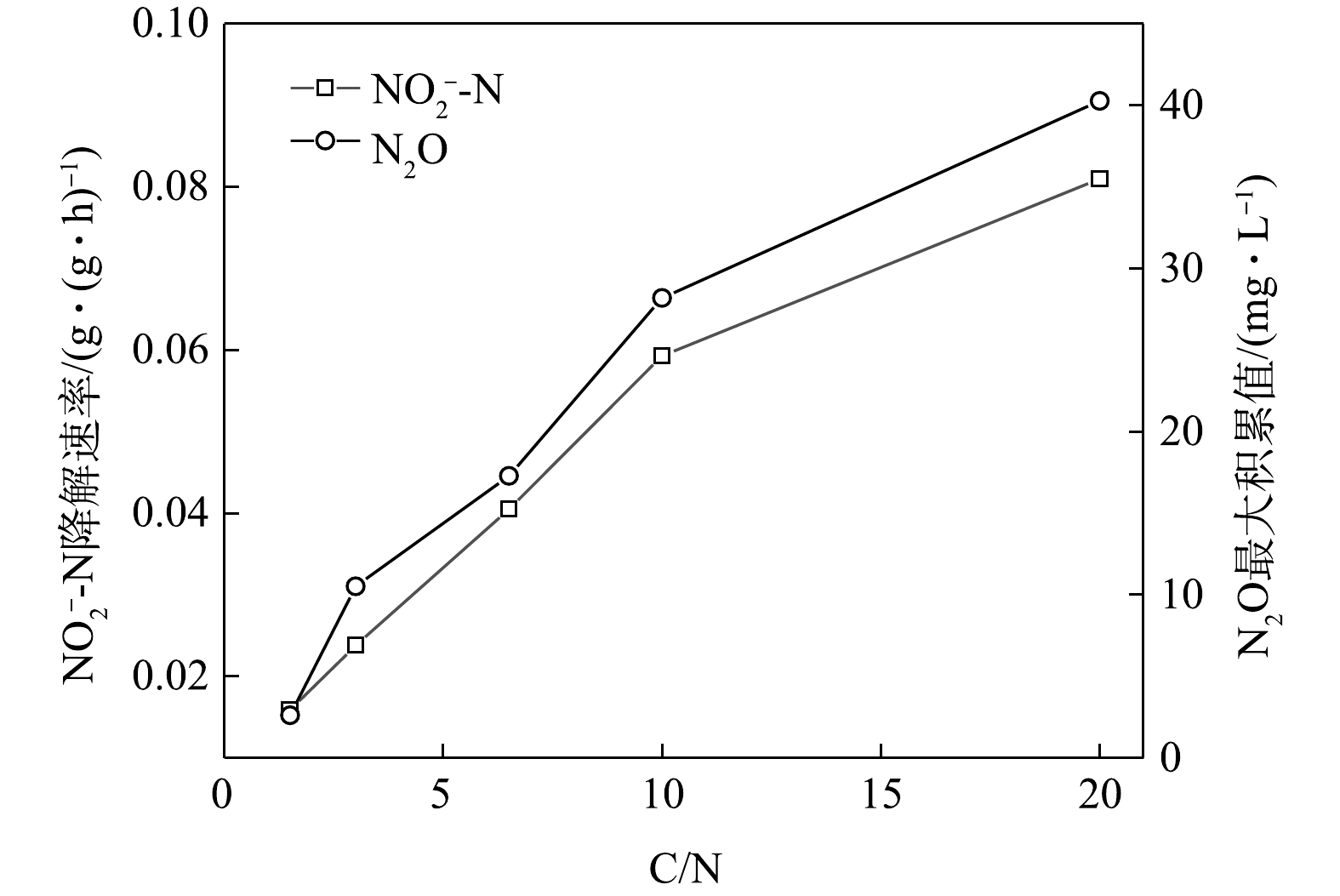
在不同C/N条件下,当以
$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N为电子受体时,反硝化过程的$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N的降解速率和N2O的最大积累值变化情况如图11所示。在不同C/N条件下,以$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N为电子受体时,反硝化过程中$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N的降解速率,N2O的最大积累值、FNA最大值变化情况如图12所示。由图11和图12可见,N2O的积累值均随着C/N的增加而增加。这可能是因为随着C/N的增加:一方面,各反硝化酶的电子消耗率差异拉大,使得更多的$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N被快速向$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N和N2O转化,增加了N2O的来源;另一方面,溶液中$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N积累值增加,使得FNA浓度升高,进而会抑制Nos酶活性[28-29],导致N2O得不到及时降解而造成积累[30-31]。王莎莎等[32]在探究不同电子受体在反硝化过程中N2O的产量时,发现在
$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N反硝化过程中N2O的含量是硝酸盐反硝化中的9.12倍。ALINSAFI等[33]认为,反硝化过程中亚硝酸盐的大量积累抑制了Nos酶的活性,导致大量N2O的产生。张兴兴等[27]指出,FNA对Nos酶的抑制浓度为0.001 5~0.002 5 mg·L−1。反应初期$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N的大量投加,FNA对Nos酶产生了抑制效果,反硝化以N2O为主要终产物;随着反应进行,FNA浓度逐渐降低,将N2O还原为N2。而随着C/N的升高,N2O的峰值亦逐渐上升。C/N的升高为反硝化提供了更多的电子,$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N的还原速率亦随之升高,导致N2O的积累量升高。而在相同的C/N条件下,以
$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N或$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N分别作为电子受体时,前者的反硝化脱氮速率和N2O产生量均比后者小。以C/N=1.5为例,分析其原因有2点:一方面,相对于$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N为电子受体时,以$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N为电子受体时溶液中FNA浓度变化更大,分别为0~0.010 0 mg∙L−1和0~0.073 8 mg∙L−1,本实验中药品在反应开始前一次性投加,而$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N的瞬时投加会导致其在系统中的积累,使$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N和水中的H+反应产生FNA,使反硝化过程中的Nos酶活性被抑制,从而促进了N2O积累量的上升;另一方面,相对于$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N为电子受体时,以$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N为电子受体时Nir酶和Nos酶电子排布落差更大。在反硝化过程中,碳源氧化与氮氧化物还原关联紧密,碳源氧化产生的电子被各氮氧化物还原酶利用,从而使各氮氧化物被还原。有研究[34-35]表明,在还原过程中,各氮氧化物还原酶同时存在着电子竞争的关系,被优先还原的氮氧化物竞争电子的能力优于后被还原的氮氧化物,反硝化过程中4种酶的竞争导致了N2O的积累。因此,可能的原因之一是:由于Nir酶竞争电子能力强于Nos酶,在没有$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N存在时,Nar酶不参与竞争电子,Nir酶能获得绝大部分电子;而$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N存在后,Nar酶优先参与竞争电子,使得流向Nir酶和Nos酶的电子总量很少[35],这大大削弱了Nir酶与Nos酶竞争电子之间存在的优势,故Nir酶和Nos酶之间电子排布的落差要小于$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N不存在的情况,因此,当$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N为电子受体时,更多N2O因没有足够电子而未能被Nos酶还原为N2。对于C/N对N2O释放量的影响,目前有不同的研究结论。有研究[36-37]表明,以乙酸钠为碳源时,随着C/N的增加,反硝化速率随之上升,而N2O的产量呈下降趋势。以葡萄糖为碳源时,N2O的产量随着C/N的增加而升高[38-39]。以葡萄糖为碳源时,无论电子受体是
$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N或$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N,系统中N2O的峰值产量均随C/N值的增加而增加。这主要是因为,相比于乙酸钠,葡萄糖作为较复杂的有机物,需要经过2个氧化过程才得以降解:首先反硝化细菌将其氧化得到丙酮酸和ATP;然后,丙酮酸进入三羧酸循环时被丙酮酸脱氢酶复合物转化为乙酰辅酶A[23],在无氧条件下,丙酮酸在乙酰辅酶A的作用下无法完全氧化,最终转化为乙醇,仍需进一步降解方能被异养菌吸收利用[40]。代谢过程越复杂,反硝化速率也就越慢,从而导致$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N的累积。同时,有机物质在反硝化过程中不仅要参与氧化,还要部分转化为细胞物质。不同物质转化为细胞物质的比例不尽相同。一般来说,单碳化合物的生长量比较低[9],这样对细胞生长造成阻碍,使其有更多的能量用来脱氮,葡萄糖作为高碳化合物,微生物生长量要高于醇类。此外,也有研究[41]表明,以葡萄糖驯化培养出的反硝化污泥中含有大量Comamonadaceae种属,其主要基因Brachymonas会促使反硝化过程产生大量的N2O。而以乙酸钠为碳源进行反硝化时,Thauera是系统中的主要反硝化基因[34],其所含的的NosZ基因能够将N2O还原为N2。为了达到污水脱氮过程中高效脱氮和N2O回收利用的目的,通过增加其产生量以回收利用,例如SCHERSON等[5-6]提出的CANDO(coupled aerobic-anoxic nitrous decomposition operation)工艺就获得了很高的N2O转化率和能源回收率。综上所述,以葡萄糖为碳源的反硝化系统中,尽管随着C/N的增加,反硝化速率有所增加,但是,会产生更多的峰值N2O。这一方面是由于反硝化菌对葡萄糖特殊的代谢过程导致的,另一方面是由于葡萄糖对反硝化菌群落结构的改变所导致的。系统中含有的大量与N2O生成相关的基因,FNA浓度的积累、反硝化酶之间的电子竞争可能是导致N2O大量产生的原因。当然,在碳源充足的情况下,这些N2O如果没有被释放到大气中,最终也将会被完全还原。因此,如果需要减少其排放,应该设法避免水中溶解的N2O吹脱到大气中;如果需要回收与利用N2O,需要朝相反的方向努力。
2.1. 不同C/N时反硝化的脱氮性能
2.2. 不同C/N时反硝化的N2O释放
-
1)在相同C/N条件下,相对于以
$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N为电子受体而言,以$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N为电子受体的反硝化系统中产生的N2O量更少。2)以
$ {\rm{NO}}_{\rm{3}}^{\rm{ - }}$ -N为电子受体时,随着C/N的增加,在5种C/N下峰值N2O转化率逐渐升高,分别为3.43%、4.32%、6.14%、10.49%和17.43%;反硝化速率由8.81×10−3 g·(g·h)−1升至3.25×10−2 g·(g·h)−1。3)以
$ {\rm{NO}}_{\rm{2}}^{\rm{ - }}$ -N为电子受体时,随着C/N的增加,在5种C/N下峰值N2O转化率逐渐增大,分别为4.08%、10.97%、17.64%、29.49%和41.17%;反硝化速率由1.59×10−2 g·(g·h)−1升至8.08×10−2 g·(g·h)−1。











 下载:
下载: