-
铁可参与各种生物过程,如氧气运输、酶转化和DNA/RNA修复等[1-5]。但过量的铁会导致各种疾病,如细胞损伤、肝炎、器官功能障碍、阿尔茨海默病、帕金森病等。因此,定量检测铁对于监测细胞代谢和了解生物反应的机制具有重要意义[6-10],快速、灵敏、简便地测定铁离子(Fe3+)至关重要。
目前,针对Fe3+的检测方法主要有原子吸收光谱、荧光技术、电化学及比色等[11-18],所依据的检测原理主要包括氧化、填充、d-d跃迁等。其中,原子吸收光谱、荧光技术、电化学3种方法存在使用有毒溶剂、对设备要求较高、检测限高等缺点。相对其他检测方法而言,比色法具有易识别、灵敏度高、选择性好等优点,因此也是当下研究较多的领域。但是传统的比色法主要使用邻二氮菲试剂和硫氰化钾试剂来检测铁离子,存在使用稳定性差的缓冲溶液、用量不准、检测限高等缺点[19-20],因此,选择合适的比色试剂用于Fe3+的检测成为研究焦点。
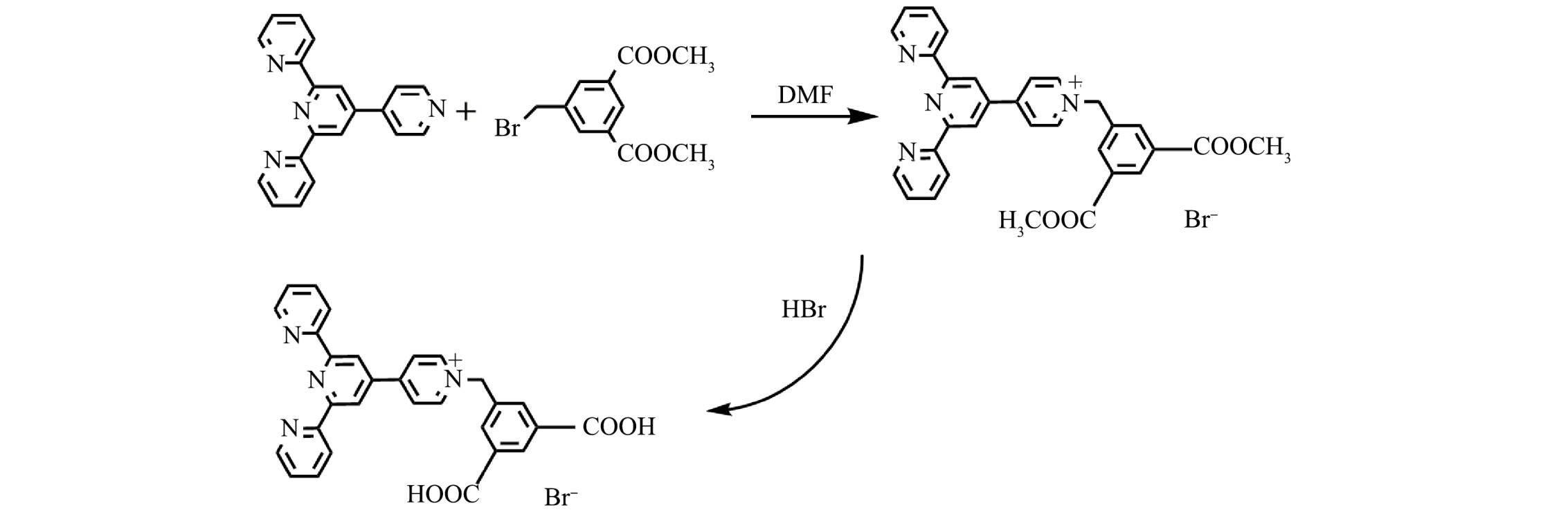
四联吡啶分子具有三齿吡啶氮原子的结构,能与金属离子进行配位[21-22]。羧基作为配体也易与金属离子进行配位[23-25]。本研究以四联吡啶和5-溴甲基-间苯二甲酸二甲酯为原料,以N,N-二甲基甲酰胺为溶剂,通过亲核取代和水解反应制备吡啶鎓盐分子,用于识别水中的Fe3+。
-
四联吡啶和5-溴甲基-间苯二甲酸二甲酯购于南京黄龙化工科技有限公司,均为分析纯。氢溴酸购于阿拉丁试剂有限公司,为分析纯。N,N-二甲基甲酰胺,乙醇购于北京化工厂,均为分析纯。各种金属离子溶解在蒸馏水中,室温保存备用。
-
一溴化4′-(N-(3,5-二甲酯基-苯亚甲基)-4-吡啶基)-2,2′:6′,2′'-三联吡啶分子(吡啶鎓盐分子D-COOCH3)的制备: 主要通过4'-(4-吡啶基)-2,2':6',2''-三联吡啶与5-溴甲基-间苯二甲酸二甲酯在N,N-二甲基甲酰胺溶剂中发生亲核取代而制备。具体操作如下:将4'-(4-吡啶基)-2,2':6',2''-三联吡啶(0.75 g,2.5 mmol)与5-溴甲基-间苯二甲酸二甲酯(1.15 g,4 mmol)溶于10 mL N,N-二甲基甲酰胺溶剂中,加热至90 °C,反应8 h,至产生大量白色沉淀,反应结束后,趁热过滤,用乙醇洗涤3次,放入70 ℃烘箱中烘干,得到白色固体,产率为75%。
一溴化4′-(N-(3,5-二羧基-苯亚甲基)-4-吡啶基)-2,2′:6′,2′'-三联吡啶分子(吡啶鎓盐分子D-COOH)的制备(图1): 主要通过酯基的水解反应得到。具体操作如下:将3 g吡啶鎓盐D-COOCH3分子和15 mL溴化氢水溶液放入反应釜中,搅拌30 min,放入90 ℃烘箱中;反应3 d,降温1 d,过滤,分别得到浅黄色晶体,产率为90%。
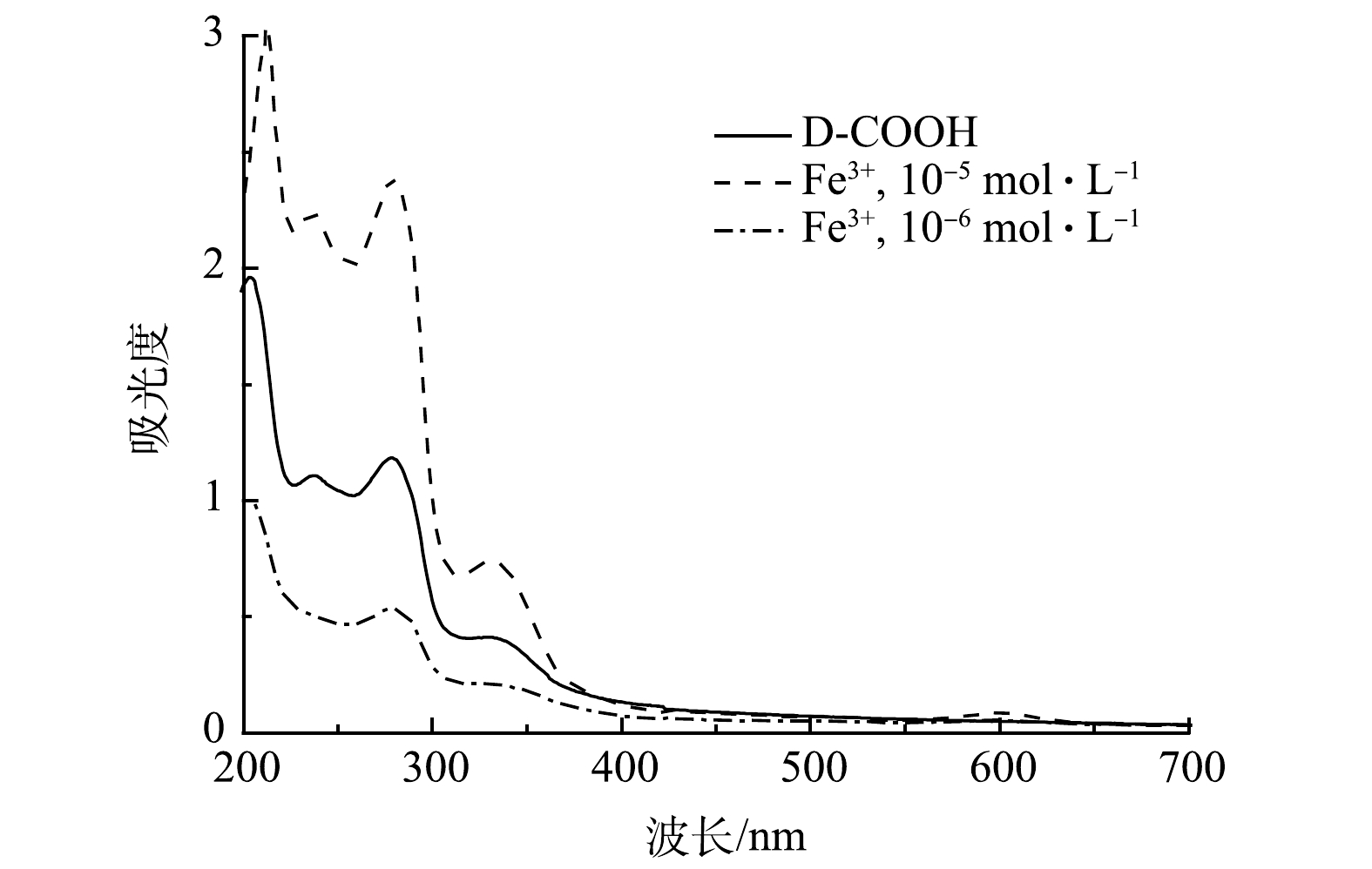
Fe3+的检测: 配制1.0×10−3 mol·L−1 Fe3+溶液,再逐步稀释成不同浓度( 1.0×10−5 、1.0×10−6 mol·L−1) 的Fe3+溶液,待用。取 1 μL D-COOH溶液加入到不同浓度的Fe3+溶液中,测定体系的吸收光谱。
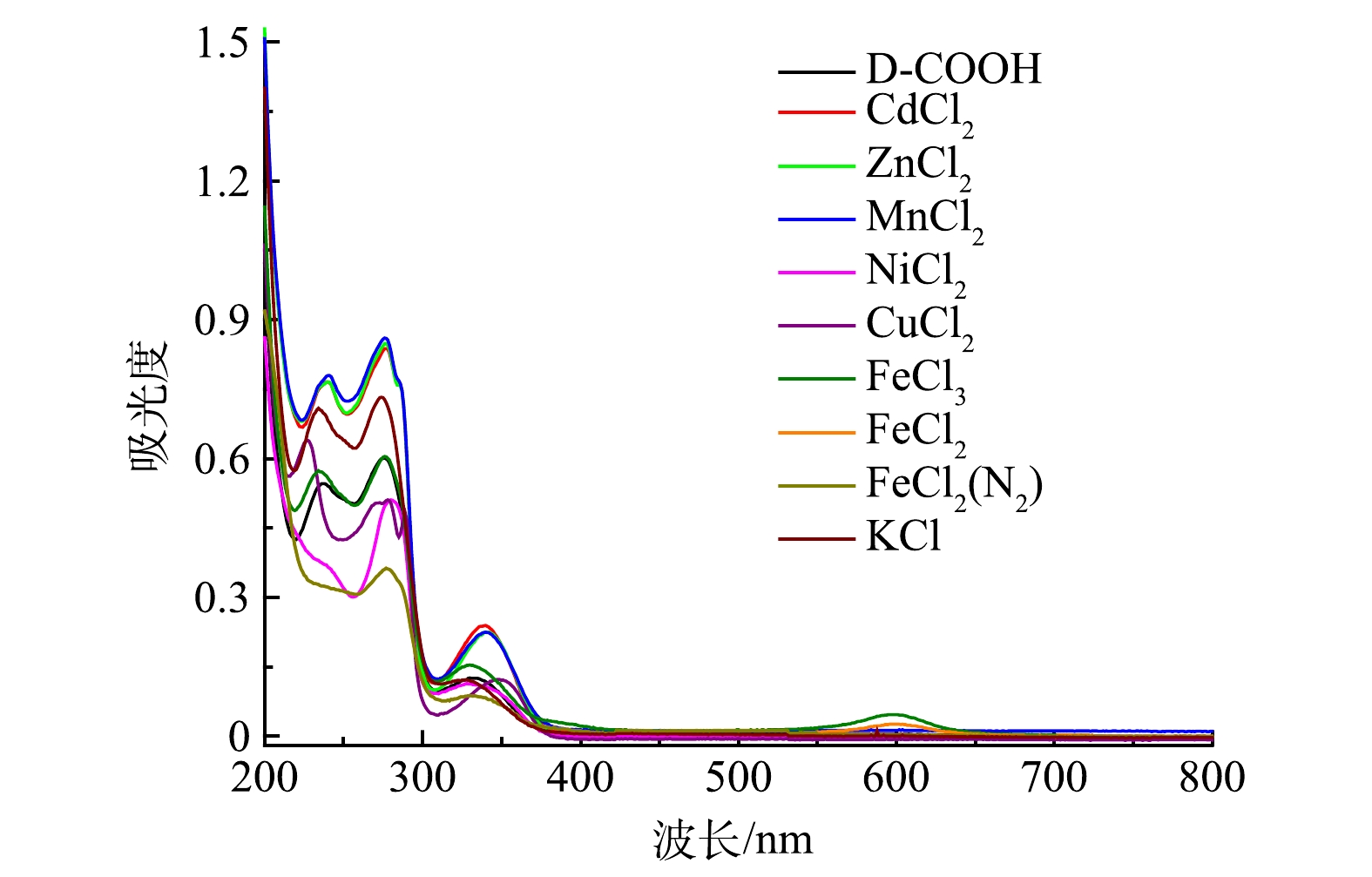
选择性实验: 取3 mL 1.0×10−5 mol·L−1不同的金属离子溶液(Cd2+、Zn2+、Mn2+、Ni2+、Cu2+、Fe3+、K+ ) 与 1 μL D-COOH溶液混合,测定其吸收光谱。
抗干扰实验: 分别取1.0×10−5 mol·L−1 Fe3+溶液和1.0×10−5 mol·L−1 其他离子(Cd2+、Zn2+、Mn2+、Ni2+、Cu2+、Fe2+、K+ ) 的溶液(比例为1∶5混合)后,取1 μL D-COOH溶液加入到上述混合溶液中,测定体系的吸收光谱。
-
红外光谱分析由傅里叶转换红外光谱(FT-IR)测试:采用KBr压片法,在NicoletiS10型红外光谱仪上测试,测试波数为400~4 000 cm−1。核磁共振氢谱在Bruker Ascend 400M仪器上进行测试。液体紫外在TU-1901型双光束紫外可见光分光光度计上测试,测量波长为190~600 nm。在一组比色管中,分别加入不同浓度的Fe3+标准溶液,按实验方法测量体系的吸光度,绘制校准曲线[26-27]。检测限(LOD) 按照式(1)计算。
式中:LLOD为检测限数值;s 为测定10个空白样品的标准偏差;k为斜率。
-
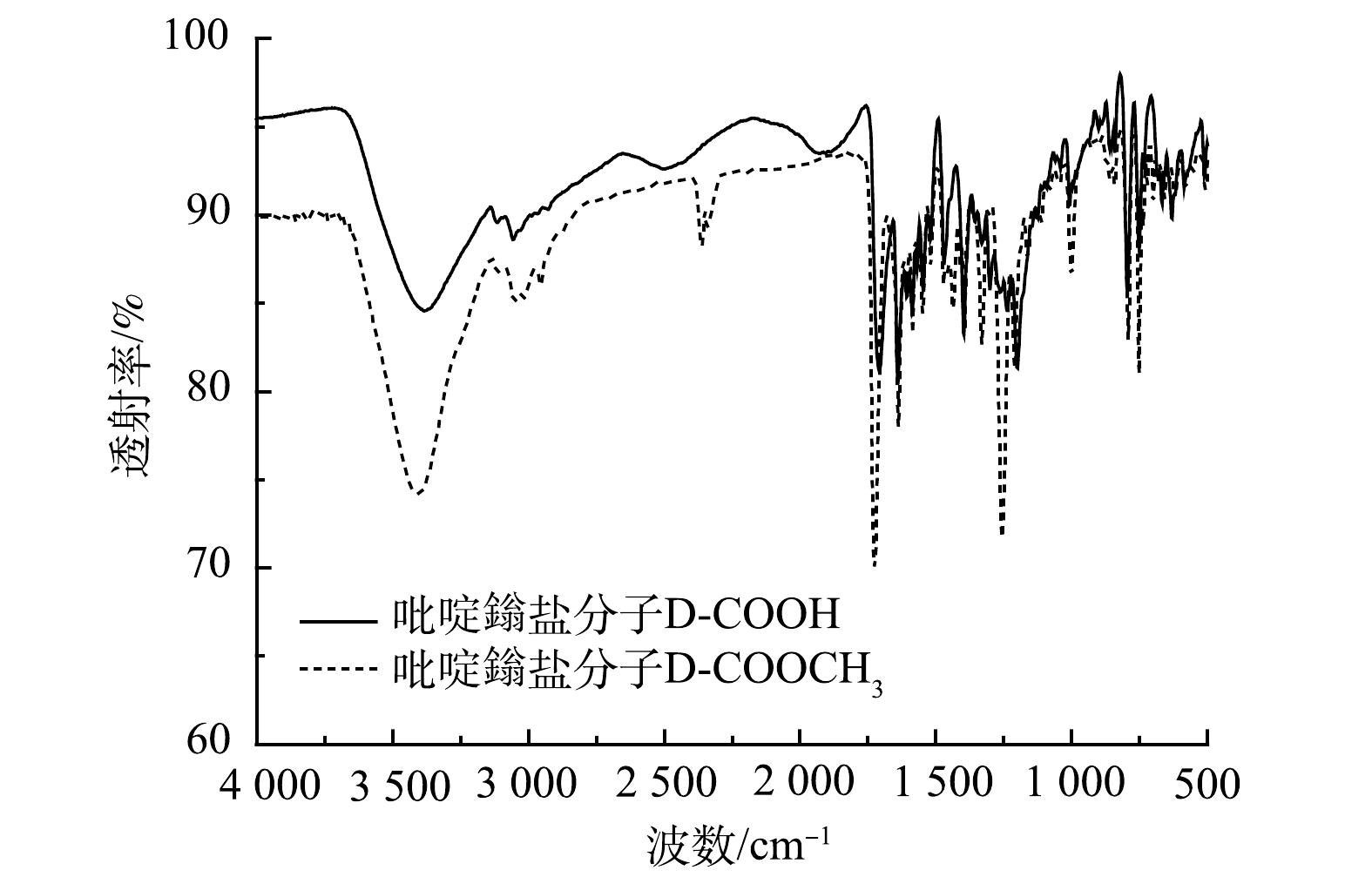
1)红外光谱分析。由图2可以看出:1 726 cm−1为酯基的C=O伸缩振动,1 641 cm−1为吡啶鎓盐的C=N的伸缩振动,1 583、1 548、1 444 cm−1为吡啶环与苯环的特征骨架伸缩振动;1 704 cm−1的吸收峰为羧基的C=O伸缩振动,1 640 cm−1为吡啶鎓盐的C=N的伸缩振动,1 583、1 545、1 470 cm−1为吡啶环与苯环的特征骨架伸缩振动;有机分子D-COOCH3和D-COOH在1 640 cm−1左右都有吡啶鎓盐特征的吸收峰,即C=N的伸缩振动吸收峰。在D-COOCH3、D-COOH这2个有机分子中,有机官能团从酯基变到羧基,极性增加,导致红外吸收带向低波数移动,因此酯基中羰基的吸收峰在1 726 cm−1,羧基中羰基的吸收峰在1 704 cm−1和1 709 cm−1。这说明该吡啶鎓盐分子结构中具有三齿吡啶氮和羧基。
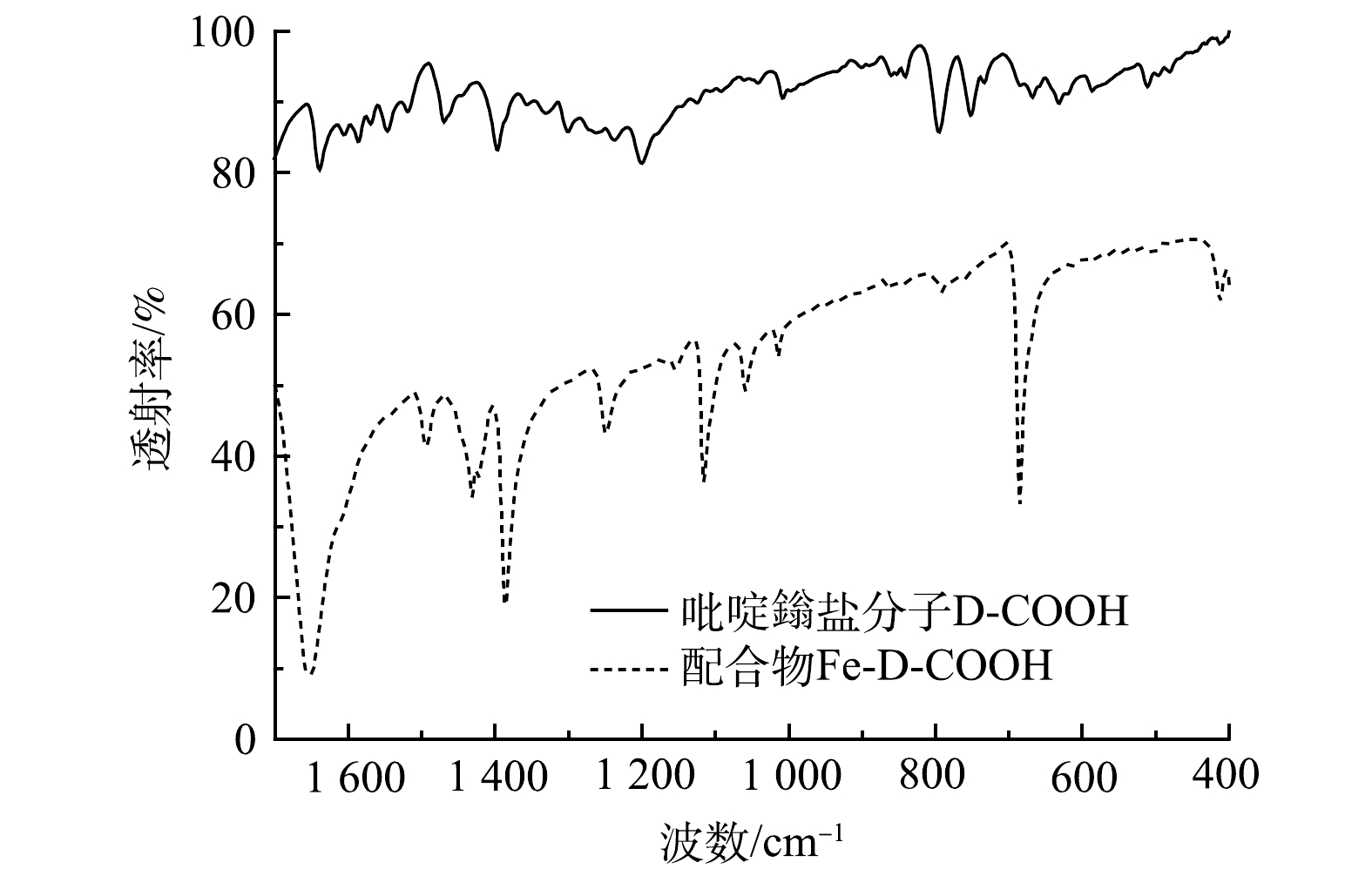
吡啶鎓盐分子D-COOH结构具有三齿吡啶氮和羧基,其与Fe3+之间具有很高的亲和力,可以与Fe3+配位形成金属络合物。由图3可以看出,417 cm−1和516 cm−1处的吸收峰分别归属于Fe—N、Fe—O[28]。说明Fe3+与D-COOH中的吡啶氮和羧基氧发生配位反应,形成三价铁的配合物Fe-D-COOH。
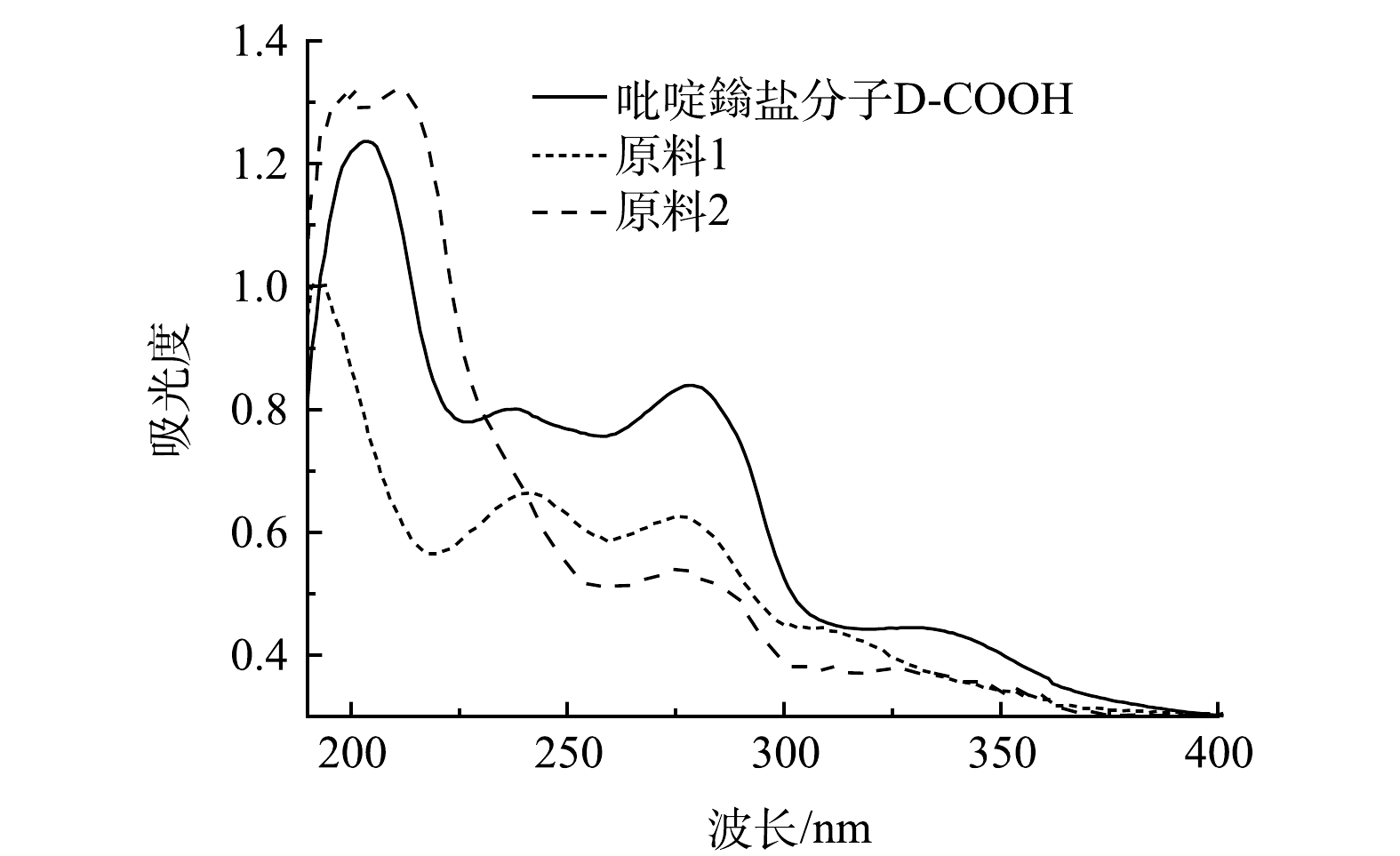
2)紫外光谱分析。对比原料与D-COOH的液体紫外吸收(图4),在液体紫外中D-COOH在280 nm、330 nm左右有吸收。280 nm处的吸收归属于吡啶环与吡啶鎓盐之间的π-π*跃迁,330 nm左右的吸收峰归属于吡啶环分子内的电荷转移[29-30]。
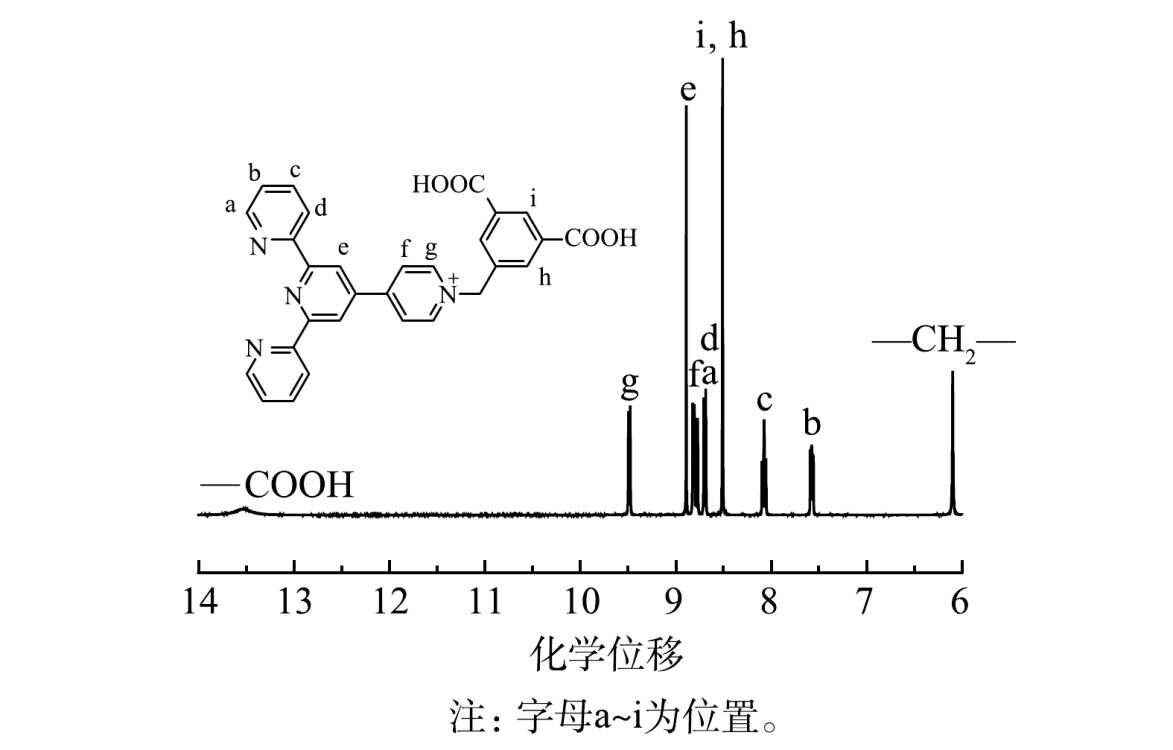
3)核磁共振氢谱分析。由图5可看出,化学位移13.5处出现2个H, 归属于—COOH中的H;化学位移9.48处出现2个H, 归属于图5中g位置的H;化学位移8.89处出现2个H, 归属于e位置的H;化学位移8.84 处出现2个H, 归属于f位置的H;化学位移8.78处出现2个H, 归属于a位置的H;化学位移8.68处出现2个H, 归属于d位置的H;化学位移8.51处出现3个H, 分别归属于i、h位置的H;化学位移8.12处出现2个H, 归属于c位置的H;化学位移7.61处出现2个H, 归属于b位置的H;化学位移6.07处出现2个H, 归属于—CH2—中的H。
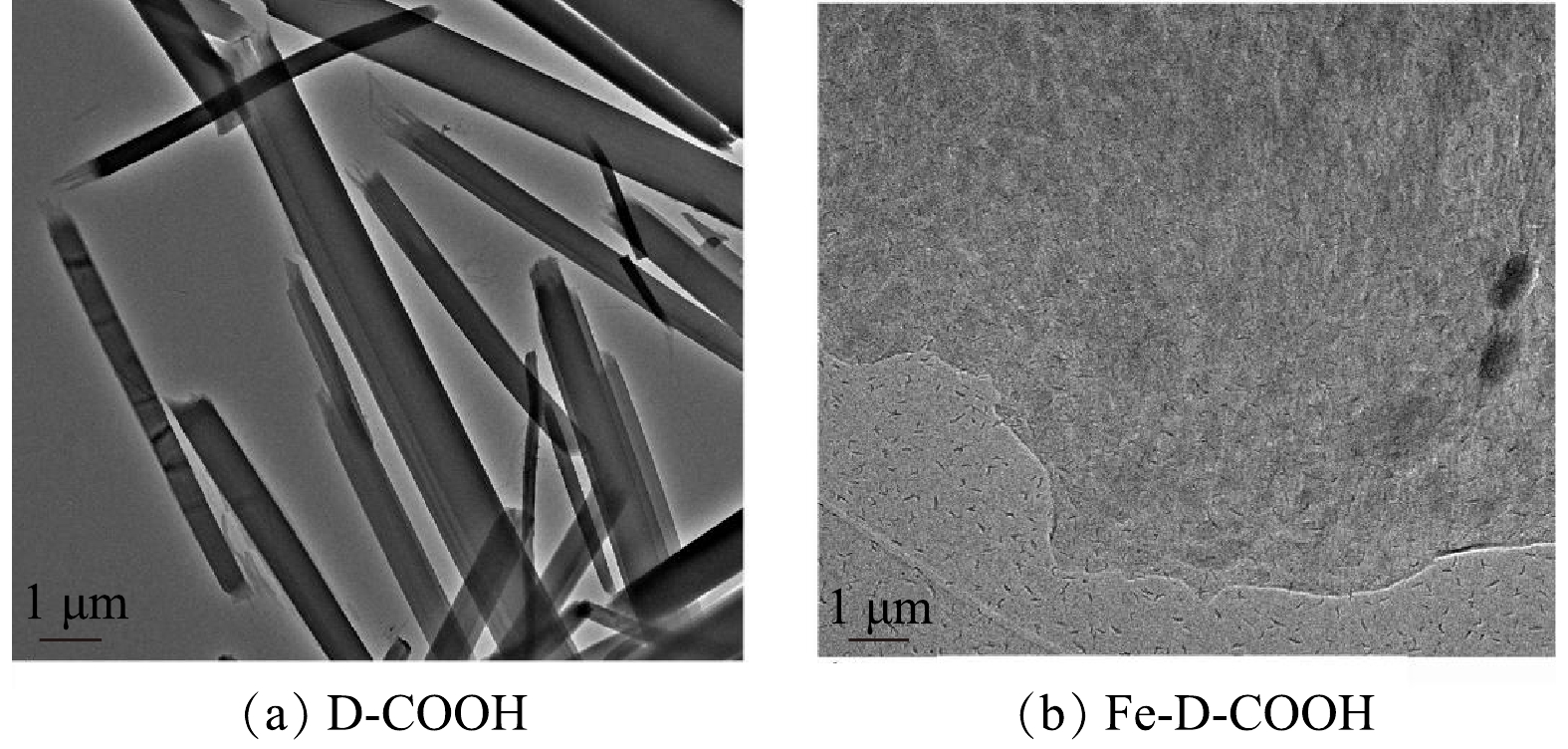
4) 电子透射分析。由图6可以看出,当粒径均为1 µm时,D-COOH与Fe3+形成配合物Fe-D-COOH,两者形态发生变化,由层状结构变为细小的条状,且分散良好。
-
1)识别与检测限实验。由于制备的吡啶鎓盐分子具有三齿吡啶氮和羧基,因而可实现对Fe3+的比色检测。如图7所示,当Fe3+ 的浓度为1.0×10−5 mol·L−1时,溶液立即由无色变为蓝色,说明吡啶鎓盐分子对Fe3+有良好的响应。对反应溶液进行液体紫外测试,发现在600 nm处有新的吸收峰,该吸收峰归属于三价铁离子与D-COOH络合形成的物质,其发生d-d跃迁使其显色。由上述红外谱图可以看出,417 cm−1和516 cm−1处的吸收峰分别归属于Fe—N、Fe—O[28]。这说明三价铁离子与D-COOH中的吡啶氮和羧基氧发生配位作用,形成三价铁的配合物。对不同浓度的Fe3+进行检测,发现在1.0×10−6 mol·L−1的时候仍能观察到颜色的变化(图7)。
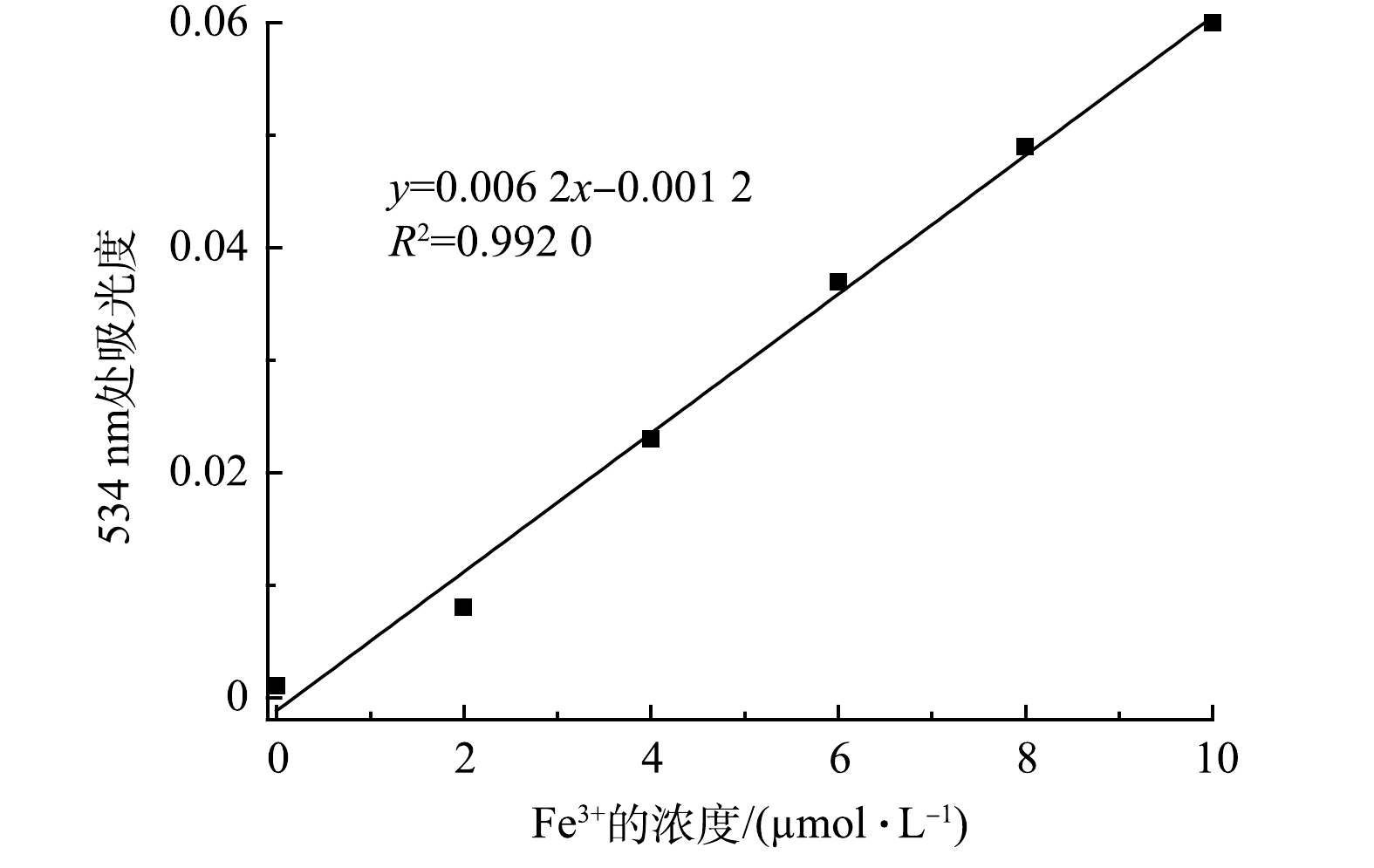
在一组比色管中,分别加入不同量的Fe3+标准溶液,按实验方法测量体系的吸光度,绘制校准曲线,如图8所示,线性浓度为0~10 μmol·L−1,可决系数R2= 0.992 0,其线性回归方程如式(2)所示。
式中:x为浓度;y为吸光度。
对试剂空白进行10次测定,求得标准偏差s =1×10−3,检测限(3s/k)为0.48 μmol·L−1(图8)。由表1可以看出:与以金属锌环化合物[31]、Ln-MOF[32]、磷腈[33]、MOF[34]、Eu-MOF-吡啶[35]为检测试剂的荧光法相比较,D-COOH显示出更低的检测限;而与以有机纳米片[36]为检测试剂的荧光法相比较,D-COOH虽然检测限更高,但其所用检测溶剂为水,有效避免了有毒溶剂的使用。同时,与以苯并咪唑[27]为检测试剂的比色法相比较,D-COOH具有检测线低、使用无污染的水为溶剂的优点。
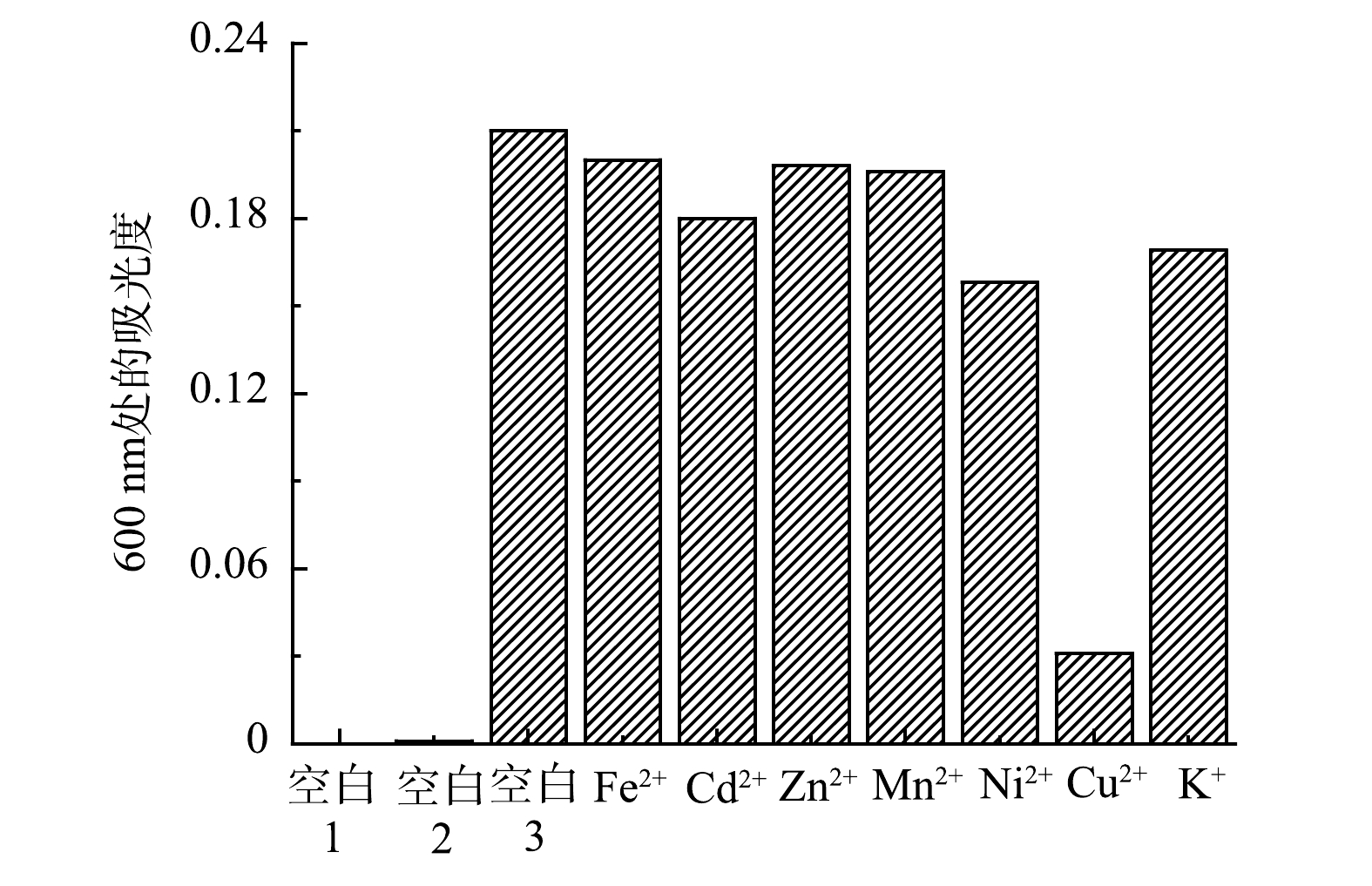
2)专一性实验。为了验证在其他金属离子存在的情况下吡啶鎓盐分子对Fe3+的检测具有专一性,分别检测了Cd2+、Zn2+、Mn2+、Ni2+、Cu2+、Fe2+和K+等多种干扰性金属离子对吡啶鎓盐分子识别信号的影响,各金属离子的浓度均为1.0×10−5 mol·L−1,结果如图9所示。可以看出,除 Fe3+、Fe2+遇吡啶鎓盐分子出现新的信号,其余离子对吡啶鎓盐分子都没有新增的信号。为排除Fe2+被氧化成Fe3+,使吡啶鎓盐分子在600 nm出现新的信号,在氮气氛围下进一步检测。实验结果表明,Fe2+对吡啶鎓盐分子检测Fe3+不存在干扰。这说明实验制备的吡啶鎓盐分子对 Fe3+有专一性响应。
3)抗干扰性实验。为了验证在多种金属离子存在下吡啶鎓盐分子对Fe3+的抗干扰性情况,以D-COOH(空白1)、Fe3+溶液(空白2)、单独Fe3+与吡啶鎓盐混合溶液(空白3)三者做为参比对象,检测了Fe2+、Cd2+、Zn2+、Mn2+、Ni2+、Cu2+和K+等多种干扰性金属离子与Fe3+混合后对吡啶鎓盐分子识别信号的影响,各金属离子的浓度均为1.0×10−5 mol·L−1,结果如图10所示。可以看出,即使其他金属离子浓度与Fe3+ 的比例增加到5∶1,吡啶鎓盐分子仍然显示出对Fe3+具有很好的识别性能,在600 nm处出现新的吸收峰。进一步观察图10,发现在Cu2+干扰组,溶液的吸光度明显降低,这表明Cu2+对吡啶鎓盐分子检测Fe3+产生了一定的干扰,但仍可较为灵敏的检测出Fe3+。上述实验结果说明实验制备的吡啶鎓盐分子对Fe3+检测具有较强的抗干扰能力。
-
1)以四联吡啶和5-溴甲基-间苯二甲酸二甲酯为原料,以N,N-二甲基甲酰胺为溶剂,通过亲核取代和水解反应制备了吡啶鎓盐分子,该分子结构具有三齿吡啶氮和羧基,与Fe3+之间具有很高的亲和力,可以与Fe3+配位形成金属络合物,使其显色。
2)吡啶鎓盐分子对Fe3+的络合显色实现了对Fe3+的专一性检测,其检测限为4.8×10−7 mol·L−1。吡啶鎓盐分子在Cd2+、Zn2+、Mn2+、Ni2+、Cu2+、Fe2+和K+ 等多种干扰离子的存在下,仍表现出较好的识别效果,具有较强的抗干扰性能。
3)使用吡啶鎓盐分子作为比色法检测试剂,相对于其他检测方法具有易识别、灵敏度高、绿色环保、选择性好等优点。
新型吡啶鎓盐分子的合成及其对Fe3+的检测
Syntheses of novel pyridinium salt and its performance on ferric ion detection
-
摘要: 针对水中铁离子(Fe3+)检测存在使用有毒溶剂、对设备要求较高、检测限高等缺点,以四联吡啶和5-溴甲基-间苯二甲酸二甲酯为原料,以N,N-二甲基甲酰胺为溶剂,通过亲核取代和水解反应制备出一种新型的铁离子检测探针——吡啶鎓盐分子。运用一系列的表征技术分析了吡啶鎓盐分子的性质,并通过实验探察了共存离子、Fe3+的浓度等对吡啶鎓盐分子识别Fe3+的影响。表征实验结果表明,吡啶鎓盐分子结构具有三齿吡啶氮和羧基,使其与Fe3+之间具有很高的亲和力,可以与Fe3+配位形成金属络合物。识别实验结果表明,吡啶鎓盐分子对Fe3+的络合显色实现了对Fe3+的专一性检测,其检测限为4.8 ×10−7 mol·L−1;在Cd2+、Zn2+、Mn2+、Ni2+、Cu2+、Fe2+和K+ 等多种干扰离子存在条件下,对Fe3+仍具有良好的识别效果,体现出较强的抗干扰性能。综上所述,新型吡啶鎓盐分子结构中具有三齿吡啶氮和羧基,使其对水中Fe3+具有易识别、灵敏度高、选择性好等优点。Abstract: To solve the problems existing in the process of Fe3+ detection, such as the used toxic solvents, high requirements for equipment and high detection limit, a novel Fe3+ detection probe-pyridinium salt molecule was prepared by nucleophilic substitution and hydrolysis reaction when tetrapyridine and dimethyl 5-bromomethyl-m-phthalate were taken as raw materials and N, N-dimethylformamide was taken as solvent. A series of characterization techniques were used to analyze the properties of pyridinium salt, and the effects of coexisting ions and Fe3+ concentration on Fe3+ recognition by pyridinium salt molecule were investigated. Characterizing experimental results showed that pyridinium salt molecular structure had tridentate pyridine nitrogen and carboxyl groups, which presented a high affinity with Fe3+ and could form metal complexes through coordination with Fe3+. The detection experimental results indicated that the specific detection of Fe3+ was achieved by complex chromogenic of pyridinium salt in the presence of Fe3+, and the detection limit was about 4.8×10−7 mol·L−1; No change occurred in the probe signal in the presence of multiple interfering ions, such as Cd2+, Zn2+, Mn2+, Ni2+, Cu2+, Fe2+, K+ and so on, showing good specific detection for Fe3+. In conclusion, the novel pyridinium salt has the advantages of easy recognition, high sensitivity and good selectivity to Fe3+ in water due to its tridentate pyridine nitrogen and carboxyl groups.
-
Key words:
- pyridinium salt /
- nucleophilic substitution /
- metal complexes /
- Fe3+ detection
-

-
表 1 D-COOH比色法与其他检测法的对比
Table 1. Comparison of D-COOH colorimetric method with other detection methods
检测试剂 溶剂 Fe3+检测限/
(mol·L−1)有机纳米片 TPE-BIMEG和994 µL HPES 0.1×10−9 金属锌环化合物 N,N-二甲基甲酰胺 2.5×10−6 Ln-MOF 乙醇 1.0×10−5 磷腈 四氢呋喃/水 4.8×10−6 MOF N,N-二甲基甲酰胺 1.7×10−4 Eu-MOF N,N-二甲基甲酰胺 3.3× 10−7 Eu-MOF-吡啶 水 5.0×10−4 苯并咪唑 三异丙基乙磺酰和N,N-二甲基甲酰胺 1.2×10−6 D-COOH 水 4.8×10−7 -
[1] ANDREWS N C N. Disorders of iron metabolism[J]. The New England Jouranl of Medicine, 1999, 341: 1986-1995. doi: 10.1056/NEJM199912233412607 [2] HAASJ D, BROWNLIE T J. Iron deficiency and reduced work capacity: A critical review of the research to determine a causal relationship[J]. Journal of Nutrition, 2001, 131: 676-690. doi: 10.1093/jn/131.2.676S [3] TESFALDETZ O, VAN STADENJ F, STEFAN R I. Sequential injection spectrophotometric determination of iron as Fe(Ⅱ) in multi-vitamin preparations using 1, 10-phenanthroline as complexing agent[J]. Talanta, 2004, 64(5): 1189-1195. doi: 10.1016/j.talanta.2004.02.044 [4] AKATSUKA K, MCLAREN J W, LAM J W, et al. Determination of iron and ten other trace elements in the Open Ocean Seawater reference material NASS-3 by inductively coupled plasma mass spectrometry[J]. Journal of Analytical Atomic Spectrometry, 1992, 7: 889-894. doi: 10.1039/ja9920700889 [5] ELROD V A, JOHNSON K S, COALE K H. Determination of subnanomolar levels of iron(II) and total dissolved iron in seawater by flow injection analysis with chemiluminescence detection[J]. Analytical Chemistry, 1991, 63(9): 893-898. doi: 10.1021/ac00009a011 [6] PERLMUTTER J S, TEMPEL L W, BLACK K J, et al. MPTP induces dystonia and parkinsonism. Clues to the pathophysiology of dystonia[J]. Neurology, 1997, 49(5): 1432-1436. doi: 10.1212/WNL.49.5.1432 [7] AISEN P, WESSLING-RESNICK M, LEIBOLD E A. Iron metabolism[J]. Current Opinion in Chemical Biology, 1999, 3(2): 200-206. doi: 10.1016/S1367-5931(99)80033-7 [8] MOON S Y, NA R C, KIM Y H, et al. New Hg2+-selective chromo- and fluoroionophore based upon 8-hydroxyquinoline[J]. The Journal of Organic Chemistry, 2004, 69: 181-183. doi: 10.1021/jo034713m [9] PRODI L, BARGOSSI C, MONTALTI M, et al. An effective fluorescent chemosensor for mercury ions[J]. Journal of the American Chemical Society, 2000, 122(28): 6769-6770. doi: 10.1021/ja0006292 [10] ZHOU G, WANG H, YANG M, et al. An NBD fluorophore-based colorimetric and fluorescent chemosensor for hydrogen sulfide and its application for bioimaging[J]. Tetrahedron, 2013, 69(2): 867-870. doi: 10.1016/j.tet.2012.10.106 [11] SEN S, SARKAR S, CHATTOPADHYAY B, et al. A ratiometric fluorescent chemosensor for iron: Discrimination of Fe2+ and Fe3+ and living cell application[J]. The Analyst, 2012, 137(14): 3335-3342. doi: 10.1039/c2an35258c [12] KUMAR M, KUMAR N, BHALLA V. FRET-induced nanomolar detection of Fe2+ based on cinnamaldehyde-rhodamine derivative[J]. Tetrahedron Letters, 2011, 52(33): 4333-4336. doi: 10.1016/j.tetlet.2011.06.044 [13] HONG Y, XIONG H, LAM J W, et al. Fluorescent bioprobes: structural matching in the docking processes of aggregation-induced emission fluorogens on DNA surfaces[J]. Chemistry-A European Journal, 2010, 16: 1232-1245. doi: 10.1002/chem.200900778 [14] LM A, YI A, SY A, et al. Recent advances and progress of fluorescent bio-/chemosensors based on aggregation-induced emission molecules[J]. Dyes and Pigments, 2019, 162: 611-623. doi: 10.1016/j.dyepig.2018.10.045 [15] ABBASPOUR A, KHAJEHZADEH A, GHAFFARINEJAD A. Development of a new method based on scanner electrochemistry: Applied for the speciation of iron(II) and iron(III)[J]. Analytical Methods, 2011, 3(10): 2268-2272. doi: 10.1039/c1ay05257h [16] KATO D, ODA A, TANAKA M, et al. Poly‐ε‐lysine modified nanocarbon film electrodes for LPS detection[J]. Electroanalysis, 2014, 26(3): 618-624. doi: 10.1002/elan.201300542 [17] ION A, BUDA M, MOUTET J, et al. Coordination of ferrocenyl ligands bearing bipy subunits: electrochemical, structural and spectroscopic studies[J]. European Journal of Inorganic Chemistry, 2002: 1357-1366. [18] KUMAR S A, THAKUR N, PARAB H J, et al. A visual strip sensor for determination of iron[J]. Analytica Chimica Acta, 2014, 851: 87-94. doi: 10.1016/j.aca.2014.08.047 [19] 廖滢滢. 邻二氮菲分光光度法测定水中铁离子含量实验设计[J]. 化工管理, 2008, 21(4): 72-73. [20] 陆燕海. 审视KSCN/K4[Fe(CN)6]溶液检验铁离子的实验[J]. 化学教学, 2011, 7(1): 52-53. [21] SAHA S, SANTRA S, GHOSH P. CuII-templated threading of a bis-amide-tris-amine macrocycle by substituted 2, 2′-bipyridyl derivatives assisted by strong π-π stacking and second-sphere H-bonding interactions[J]. European Journal of Inorganic Chemistry, 2014, 12: 2029-2037. [22] FU Z, CHEN Y, ZHANG J, et al. Correlation between the photoactive character and the structures of two novel metal organic frameworks[J]. Journal of Materials Chemistry, 2011, 21(22): 7895-7897. doi: 10.1039/c1jm10945f [23] SUN Y Q, ZHANG J, JU Z F, et al. Two-dimensional noninterpenetrating transition metal coordination polymers with large honeycomb-like hexagonal cavities constructed from a carboxybenzyl viologen ligand[J]. Crystal Growth & Design, 2005, 5(5): 1939-1943. [24] HIGUCHI M, TANAKA D, HORIKE S, et al. Porous coordination polymer with pyridinium cationic surface [Zn2(tpa)2(cpb)][J]. Journal of the American Chemical Society, 2009, 131(30): 10336-10337. doi: 10.1021/ja900373v [25] YAO Q X, JU Z F, JIN X H, et al. Novel polythreaded coordination polymer: From an armed-polyrotaxane sheet to a 3D polypseudorotaxane array, photo- and thermochromic behaviors[J]. Inorganic Chemistry, 2009, 48(4): 1266-1268. doi: 10.1021/ic8021672 [26] 周杉杉, 李人宇, 马文慧. Zn2+-邻菲啰啉-达旦黄体系光度法测定食盐中锌[J]. 中国调味品, 2012, 37(1): 69-73. doi: 10.3969/j.issn.1000-9973.2012.01.021 [27] KIM Y S, LEE J J, LEE S Y, et al. A highly sensitive benzimidazole-based chemosensor for the colorimetric detection of Fe(II) and Fe(III) and the fluorometric detection of Zn(II) in aqueous media[J]. RSC Advances, 2016, 6: 61505-61515. doi: 10.1039/C6RA10086D [28] NAKMOTO K Z. Infrared and Raman Spectra of Inorganic and Coordination Compounds[M]. Third Edition. John Wiler & Sons, 1978. [29] JIN X H, CHEN C, REN C X, et al. Bright white-light emission from a novel donor-acceptor organic molecule in the solid state via intermolecular charge transfer[J]. Chemical Communications, 2014, 50(98): 15878-15881. [30] CHEN C, JIN X H, ZHOU X J, et al. Photo-facilitated aggregation and correlated color temperature adjustment of single component organic solid state white-light emitting materials[J]. Journal of Materials Chemistry C, 2015, 3(17): 4563-4569. doi: 10.1039/C4TC02771J [31] KUMAR G, GUDA R, HUSAIN A, et al. A functional Zn(II) metallacycle formed from an N-heterocyclic carbene precursor: A molecular sensor for selective recognition of Fe3+ and IO4− ions[J]. Inorganic Chemistry, 2017, 56: 5017-5025. doi: 10.1021/acs.inorgchem.7b00098 [32] XU H, HU H C, CAO C S, et al. Lanthanide organic framework as a regenerable luminescent probe for Fe3+[J]. Inorganic Chemistry, 2015, 54: 4585-4587. doi: 10.1021/acs.inorgchem.5b00113 [33] KAGIT R, YILDIRIM M, OZAY O, et al. Phosphazene based multicentered naked-eye sluorescent sensor with high selectivity for Fe3+ ions[J]. Inorganic Chemistry, 2014, 53: 2144-2151. doi: 10.1021/ic402783x [34] WANG B, YANG Q, GUO C, et al. Stable Zr(IV)-based metal-organic frameworks with predesigned functionalized ligands for highly selective detection of Fe(III) Ions in water[J]. ACS Applied Materials & Interfaces, 2017, 9: 10286-10295. [35] ZHOU Z X, LI L, LI H H, et al. A flexible Eu(III)-based metal-organic framework: Turn-off luminescent sensor for the detection of Fe(III) and picric acid[J]. Dalton Transactions, 2013, 42: 12403-12409. doi: 10.1039/c3dt51081f [36] YANGY, WANG X, CUI Q, et al. Self-assembly of fluorescent organic nanoparticles for iron(III) sensing and cellular imaging[J]. ACS Applied Materials & Interfaces, 2016, 8: 7440-7448. -



 下载:
下载: