-
化石燃料的燃烧已造成更多的CO2排入大气[1]。1800—2020年,大气中CO2的体积分数由280 cm3·m−3升至410 cm3·m−3,造成全球表面气温上升约1.2 ℃[2-3]。为应对全球气候变暖,各国纷纷出台了CO2减排政策,中国亦明确了“碳中和”目标[4]。CO2捕集技术能从工业排放点源处减少CO2排放,还可吸收大气中已有的CO2,因而对水泥和钢铁等重工业的深度脱碳、低碳氢能的规模化生产、低碳电力供给、实现“碳中和”等具有重要意义[5-7]。
CO2捕集技术包括化学吸收、固体吸附和膜分离等[8-10]。综合考虑对设备腐蚀性、能耗、分离效率和适用范围等问题,固体吸附法(包括钙基吸附剂、多孔物理吸附剂和固态胺吸附剂)被认为是很有前途的CO2分离技术[11-12]。其中,固态胺吸附剂具有以下优势:CO2选择性高、吸附能力强、吸附速率快和能耗低;适用范围广,可用于低浓度CO2捕集;且操作温度低,通常运行温度为50~180 ℃,可广泛应用于工业烟气CO2捕集[9]。固态胺吸附剂通常是将有机胺如聚乙烯亚胺(Polyethyleneimine,PEI)、四乙烯五胺(Tetraethylenepentamine,TEPA)、五乙烯六胺(Pentaethylenehexamine,PEHA)等浸渍或嫁接到多孔载体上制备而成[13]。多孔载体的结构和性质很大程度上决定了固态胺吸附剂的CO2吸附性能。包括多孔沸石、介孔二氧化硅、有机框架材料、多孔活性炭、多孔树脂等在内的多种载体材料陆续被研究人员开发出。宋春山等[14]采用模板剂法合成出具有倒锥形孔道的介孔二氧化硅并负载PEI制备固态胺吸附剂,其CO2吸附能力达到214 mg·g−1。IRANI等[15]采用TEPA浸渍碳纳米管,制备得到的固态胺吸附剂具有220 mg·g−1以上的CO2吸附能力。然而,过于复杂的制备过程及多种昂贵试剂的使用导致吸附剂的成本居高不下。另外,李凯敏等[11]发现,在使用CO2作为解吸气升温解吸固态胺吸附剂过程中,该吸附剂往往会在几个循环后因生成尿素链而快速失活。而只有使用CO2作为解吸气方能获得高纯CO2用于后续利用或封存[16]。因此,亟需开发在CO2解吸气氛下,廉价且具备高稳定性的固态胺吸附剂。
多孔Al2O3具有较高的比表面,且热稳定性好,在催化剂、催化剂载体、吸附剂载体等领域应用广泛。Al2O3载体作为两性物质含有较多的路易斯酸性位点,使得制备得到的材料往往具有较特殊的性能[17]。然而,多孔Al2O3的孔体积往往小于介孔二氧化硅、活性炭等载体,使得难以负载高含量的活性胺,进而导致铝基固态胺吸附剂的CO2吸附性能不尽如人意[18]。
本研究以铝酸钠和硫酸铝2种从铝土矿中提取的工业副产物为原料,采用共沸蒸馏的方式进行扩孔合成大孔体积Al2O3,并以此负载PEI制备铝基固态胺吸附剂,进一步优化多孔Al2O3的孔隙结构。然后,重点研究铝基固态胺吸附剂的吸附性能和循环稳定性,并结合分析表征,揭示铝基固态胺吸附剂的循环稳定性机制,以期为进一步开发低成本、高稳定性固态胺吸附剂提供参考。
-
铝酸钠(纯度99%,阿拉丁),十八水硫酸铝(纯度99%,阿拉丁),正丁醇(纯度99.5%,麦克林),聚乙烯亚胺(纯度99%,阿法埃莎;PEI,平均分子量为1200 Da),甲醇(纯度99.5%,麦克林),商业SiO2(德国赢创,孔体积1.32 cm3·g−1)。
-
1)多孔Al2O3载体制备步骤。分别称取4.92 g和6.66 g铝酸钠和十八水硫酸铝于2个烧杯中,各加入100 mL去离子水于25 ℃搅拌溶解;然后快速混合,并在25 ℃下持续搅拌30 min;将混合物转移至恒温水浴锅于80 ℃老化0~6 h,取出混合物洗涤过滤;将沉淀物与正丁醇按质量比1∶10进行混合打浆,使滤饼充分分散;将混合物转移至共沸蒸馏装置中,在93 ℃共沸0.5 h,之后升温至117 ℃共沸1 h;待冷却后取出离心,离心所得沉淀在80 ℃烘箱中干燥12 h;最后将干燥后的样品置于500 ℃马弗炉中焙烧4 h,制备得到多孔Al2O3。
2)吸附剂的制备步骤。称取2.05 g PEI于烧杯中,加入25 mL甲醇充分搅拌0.5 h;然后加入1 g新合成的多孔Al2O3或1 g商业SiO2;同时再加入5 mL甲醇,在25 ℃下持续搅拌;待甲醇基本蒸发完,转移至60 ℃真空干燥箱继续干燥4 h。所制备的固态胺吸附剂分别命名为60%PEI@Al2O3-未老化、60%PEI@Al2O3-4 h、60%PEI@商业SiO2(4 h表示多孔Al2O3制备过程中的老化时间)。
-
多孔Al2O3样品的N2吸脱附曲线采用ASAP 2460物理吸附分析仪(Micromeritics,USA)进行测定。样品的比表面积和孔径分布分别由Brunauer-Emmett-Teller和BJH模型进行计算。用蔡司-Sigma 300型扫描电子显微镜(SEM)来观察载体和吸附剂的表面形貌。吸附剂的热稳定性由热重分析仪(TGA,Setsys EVO Easy 1750)进行测定。在Ar气氛下,以10 ℃·min−1的升温速率升温至800 ℃,得到材料的热稳定性曲线。
采用热重分析仪进行吸附剂的CO2吸附性能和循环稳定性测试。对于吸附剂单次吸附实验,每次称取15~20 mg吸附剂样品于氧化铝坩埚中,在120 ℃ Ar气氛下预脱气0.5 h,然后降温至60~105 ℃,将气流转换为CO2,进行60 min的吸附,吸附完成后将气氛切换为Ar,并升温至120 ℃解吸30 min。对于在惰性解吸气氛下的循环吸附实验,吸附剂首先在120 ℃ Ar气氛下脱气0.5 h,并降温至90 ℃;第1次循环开始,先将气流切换为CO2,进行0.5 h的吸附实验,接着将CO2气氛切换为Ar气氛,并升温至120 ℃解吸15 min,随后降温至90 ℃,第1次循环结束;进入下一个循环,完全重复第1次循环的步骤,整个吸附实验的循环次数为10次。对于在CO2解吸气氛下的循环吸附实验,吸附剂首先在120 ℃ Ar气氛下脱气0.5 h,并降温至90 ℃;第1次循环开始,先将气流切换为CO2,进行0.5 h的吸附实验,接着无需切换气氛,直接在CO2气氛下升温至165 ℃解吸15 min,随后降温至90 ℃,第1次循环结束;进入下一个循环,完全重复第1次循环的步骤,整个吸附实验的循环次数为10次。
-
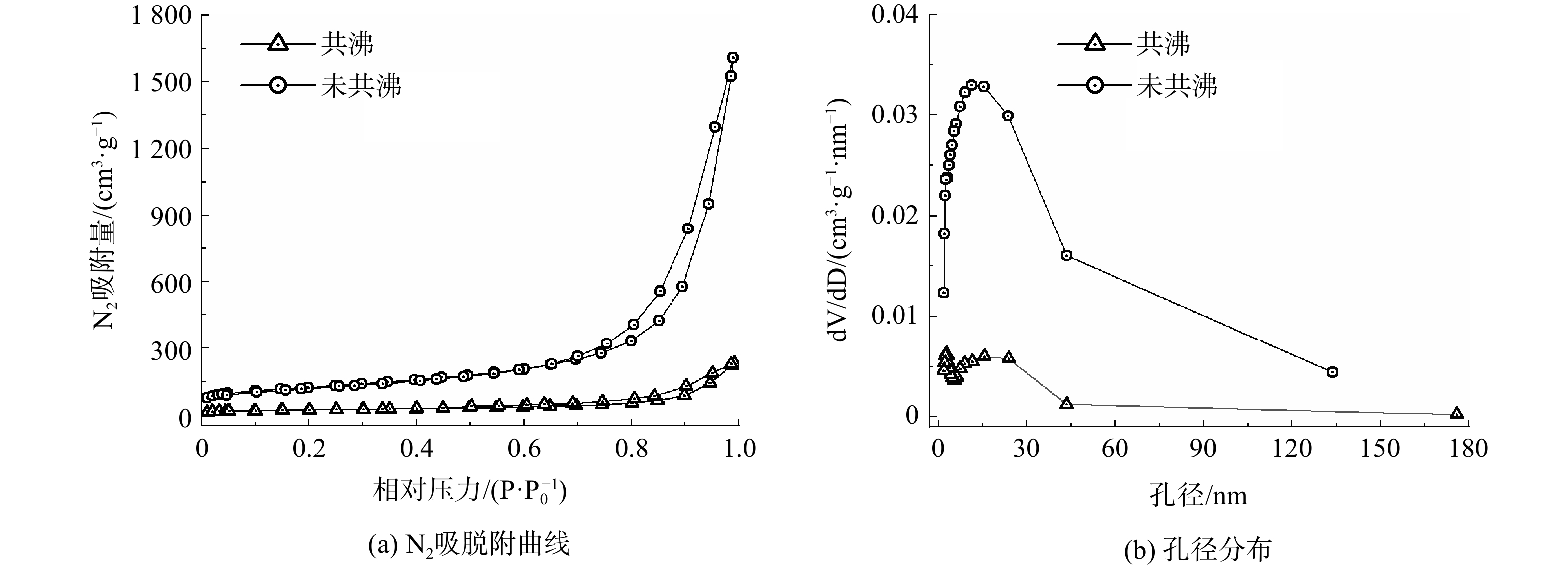
共沸过程对多孔Al2O3结构的影响如图1所示。无论共沸与否,多孔Al2O3的氮气吸脱附等温线均为Ⅳ型,并存在明显的H3型回滞环。这表明样品具有一定的中大孔结构,且这种孔结构并不规整[19]。与不共沸样品相比,共沸多孔Al2O3的饱和氮气吸附体积远大于不共沸样品,并且其氮气吸附增量主要存在于相对压力为0.8~1.0时。这表明共沸过程大大增加了中大孔的数量。2种样品均具有较宽的孔径分布,为5~40 nm,其孔结构特性见表1。与未共沸样品相比,共沸多孔Al2O3的比表面、孔体积和平均孔径均明显增大,分别达到449.4 cm2·g−1、2.59 cm3·g−1和21.3 nm。共沸扩孔Al2O3的机制可归纳如下[20-21]:新生成的拟薄水铝石表面含有大量的吸附水,在随后的干燥和焙烧过程中,由于水分子的表面张力较大,在水分子的快速迁移与去除中,拟薄水铝石晶粒会彼此靠近形成团聚物,并导致多孔Al2O3孔体积较小;加入的丁醇与新生成拟薄水铝石中的水一起组成二元共沸体系,在低于水沸点的93 ℃共沸点下进行共沸,从而将拟薄水铝石中的水分子带走并替代它存在凝胶中;由于丁醇的表面张力小,在随后的干燥、焙烧过程中,样品的孔结构最大限度地被保留下来。
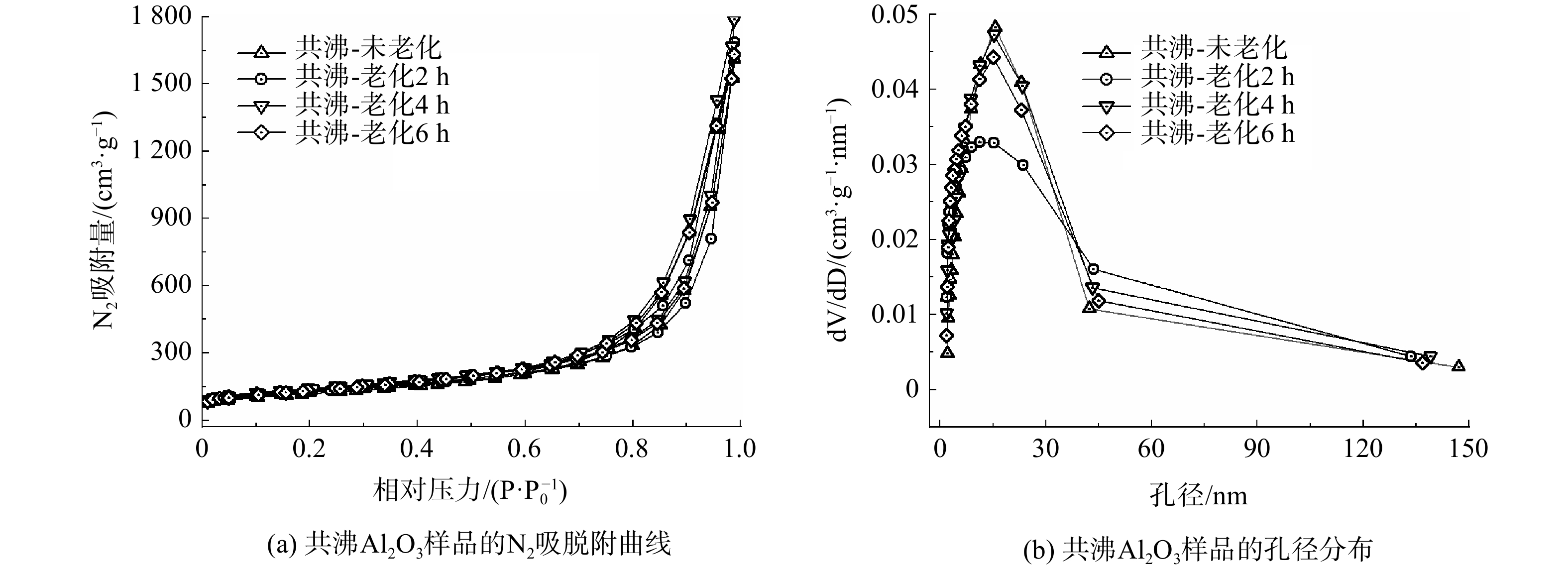
进一步探索了老化过程对Al2O3孔结构的影响,不同老化时间的共沸多孔Al2O3的氮气吸附等温曲线和孔径分布如图2所示,其比表面、孔体积和平均孔径列于表1中。数据表明:老化时间对Al2O3的孔结构影响并不大;随着老化时间增加,Al2O3的比表面和孔体积均先增大后减小,老化时间为4 h时达到最佳,比表面和孔体积分别达到495.7 cm2·g−1、2.75 cm3·g−1。
图3为未老化和老化4 h时的共沸多孔Al2O3的微观结构。与比表面分析结果不同的是,老化过程对Al2O3的微观结构有显著影响。尽管2种样品均有大量丰富的孔隙结构,然而其孔隙结构截然不同。未老化Al2O3样品微观结构呈现出大量细小的蝌蚪状晶粒,且以无规则方式堆积,晶粒之间充满大量细小孔隙;而老化4 h的Al2O3样品微观结构中,细小晶粒消失,出现花瓣状的团簇结构,团簇中充满丰富的褶皱构成孔隙。2种多孔Al2O3均含有丰富的孔隙结构,这有利于负载高含量的活性胺以制备高CO2吸附量的固态胺吸附剂。
-
分别以未老化和老化4 h的共沸多孔Al2O3为载体负载60%的PEI制备吸附剂,并以商业SiO2负载相同含量的PEI作为对照组。采用热重分析仪分析了3种吸附剂的热稳定性,结果见图4。首先对纯PEI进行分析,发现纯PEI存在2个DTG分解峰:第1阶段是200 ℃前,主要是少量水蒸气和极少量的小分子PEI的分解;第2阶段是在200~500 ℃出现的主峰,是由PEI分解造成的。计算这段温度区间的热失重达97.6%,表明PEI的分解温度就在这一区间,因此,可用此温度区间的热失重来计算吸附剂的PEI负载量。由此计算得到60%PEI@商业SiO2、60%PEI@Al2O3-未老化和60%PEI@Al2O3-4 h的PEI负载量分别为56.5%、62.1%和62.4%。3种吸附剂的PEI分解峰的起始温度分别达到了234 ℃、248 ℃和245 ℃。这表明3种吸附剂均具有较高的热稳定性,能在较宽的温度范围内进行CO2捕集。
图5为3种载体负载60%PEI后的微观形貌。通常,商业SiO2的微观形貌是无定型纳米颗粒。当负载60%PEI时,颗粒表面基本被PEI包裹,并相互粘结成大块,看不到颗粒物,且几乎没有留下孔隙。而对于60%PEI@Al2O3-未老化和60%PEI@Al2O3-4 h,尽管载体原本丰富多孔的形貌消失了,但表面并没有出现被PEI包裹的光滑形貌,尤其是60%PEI@Al2O3-4 h吸附剂还残留较多的孔隙,可为CO2由外界扩散到吸附剂内部提供必要扩散通道,有助于提高CO2的吸附能力和吸附速率。
-
在温度为90 ℃时考察了不同吸附剂的CO2吸附能力,结果见图6(a)。3种吸附剂的吸附过程均可分为快速吸附阶段(0~10 min)和慢速吸附剂阶段(10~60 min)。60%PEI@商业SiO2、60%PEI@Al2O3-未老化和60%PEI@Al2O3-4 h 3种吸附剂的饱和CO2吸附量分别为165、172和194 mg·g−1。其中,在前10 min的吸附量分别达到了138、150和174 mg·g−1,快速吸附阶段在整个吸附过程中贡献了主要的CO2吸附量。60%PEI@Al2O3-4 h吸附剂不仅拥有最高的CO2吸附能力,同时也拥有较高的吸附效率,在前10 min的CO2吸附量即可达到饱和吸附量的90%。当固态胺吸附剂的CO2吸附量超过4 mmol·g−1(176 mg·g−1)时,吸附剂的解吸能耗可以显著降低,因此,建议优秀固态胺吸附剂的CO2吸附量标准定为176 mg·g−1 [22]。基于该研究结果,本研究所制备的60%PEI@Al2O3-4 h吸附剂已达到优秀吸附剂的标准。这主要得益于多孔Al2O3载体的发达孔隙结构,有利于CO2扩散到活性胺吸附位点发生吸附反应。同时,也可看出老化4 h的多孔Al2O3更适合作为载体负载PEI用于CO2捕获。此外,3种吸附剂均拥有优异的解吸性能,在惰性气氛下解吸15 min即可彻底解吸。
图6(b)反映了不同吸附温度对60%PEI@Al2O3-4 h吸附剂CO2吸附性能的影响。升高温度有利于提高吸附剂的CO2吸附量,然而超过90 ℃时,吸附量却开始下降。温度对CO2吸附的影响可归纳为2方面[23]:1)降低效应,即CO2和活性胺的反应是一种放热过程,其吸附能力随温度升高而降低;2)促进效应,即升高温度可降低活性胺的粘度,从而使得CO2的扩散阻力减小,有利于CO2扩散吸附。因此,90 ℃是60%PEI@Al2O3-4 h吸附剂的最佳吸附温度。
-
循环稳定性是固态胺吸附剂的重要指标,直接关系到实际应用过程中材料使用寿命和CO2捕集成本。尽管目前针对固态胺吸附剂的循环稳定性已开展了大量研究,但大多数是用惰性解吸气氛进行研究,故得不到高纯CO2,难以用于后续利用或封存。分别测试了3种吸附剂在惰性气氛和CO2气氛下解吸的循环稳定性(见图7(a)),在惰性气氛下解吸,3种吸附剂均拥有超高的循环稳定性。经过10次循环,60%PEI@商业SiO2、60%PEI@Al2O3-未老化和60%PEI@Al2O3-4 h这3种吸附剂的保有吸附量分别为157、171和186 mg·g−1,衰减率均低于1.0%。这是由于吸附剂具有良好的热稳定性,在120 ℃的惰性解吸气氛下难以挥发。图7(b)为3种吸附剂在CO2解吸气氛下的循环稳定性。随着循环进行,60%PEI@商业SiO2吸附剂经历了快速失活过程,吸附量从初始的146 mg·g−1快速下降到19 mg·g−1,衰减率高达87.0%。而60%PEI@Al2O3-未老化和60%PEI@Al2O3-4 h却依然保持着良好的循环稳定性,经过10次循环依然保有吸附量136、148 mg·g−1,衰减率分别仅为15.9%、15.2%。
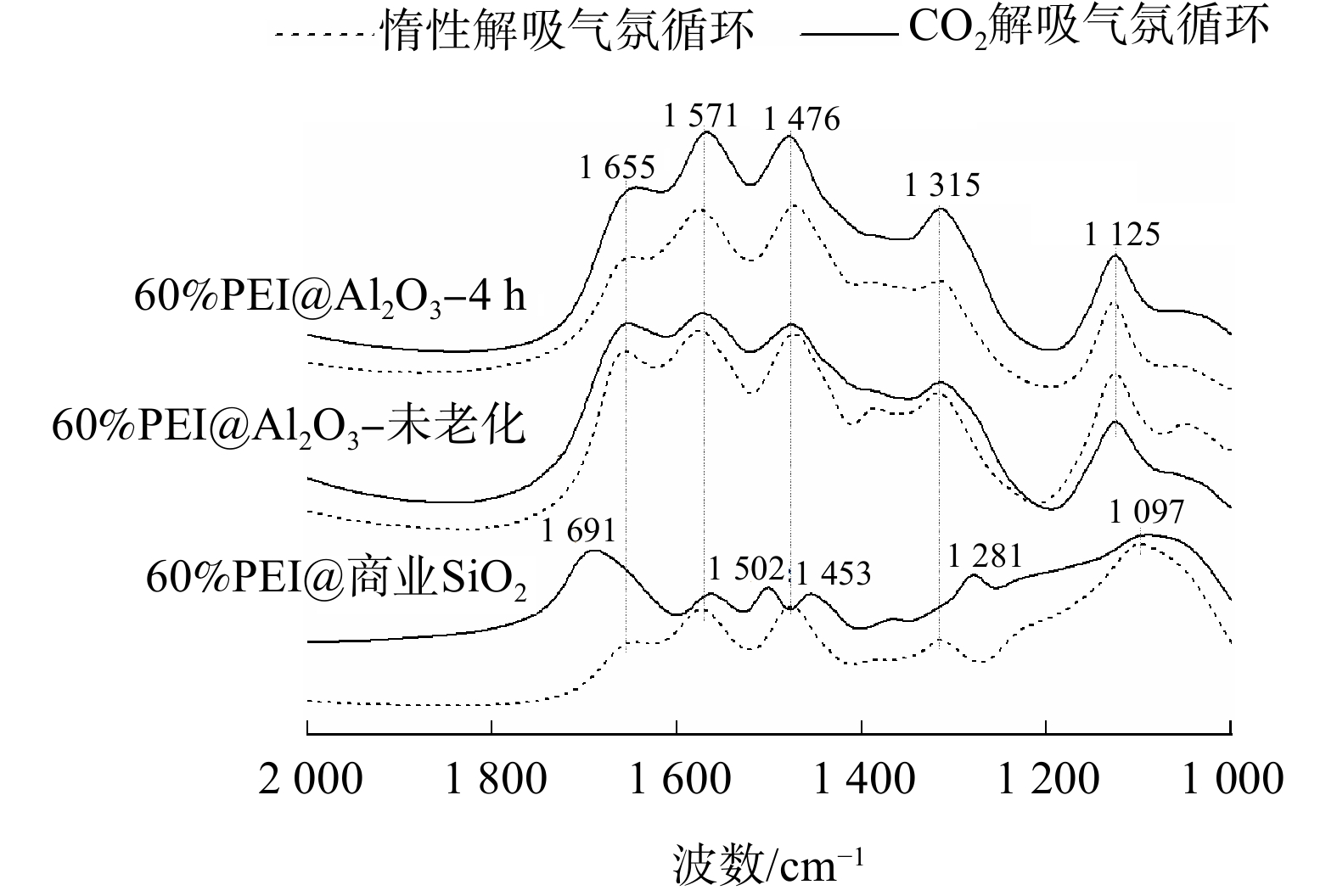
进一步测试了3种吸附剂循环10次后的红外光谱,以探究其失活机制,结果见图8。在惰性解吸气氛循环10次后,3种吸附剂在1 200~1 800 cm−1 均出现相同的峰。这是PEI分子上的特征峰:1 315 cm−1对应C—N弯曲振动;1 476 cm−1对应—CH2—伸缩振动;1 571 cm−1和1 655 cm−1对应N—H弯曲振动[24];而出现在1 125 cm−1的峰对应C—N伸缩振动;1 097 cm−1对应SiO2的特征峰Si—O—Si键[25]。当在CO2解吸气氛下循环10次后,60%PEI@Al2O3-未老化和60%PEI@Al2O3-4 h 的红外图谱基本和惰性解吸气氛下的图谱一致。然而,60%PEI@商业SiO2吸附剂却在1 281、1 453、1 502和1 691 cm−1处出现4个新峰,这是典型的尿素化合物的特征峰[26]。红外图谱结果表明,硅基固态胺吸附剂在CO2气氛下解吸极易形成尿素化合物而快速失活,而铝基固态胺吸附剂却具有抵抗尿素化合物形成的优异性能,故能在CO2解吸气氛下维持良好的循环稳定性。
对于实际工业应用而言,铝基固态胺吸附剂在CO2解吸气氛下的循环性能仍不够稳定,造成衰减的原因可能源于过高的解吸温度。固态胺吸附剂在CO2气氛下解吸时由于缺少浓度梯度驱动力,需要在165 ℃下方能接近完全解吸。而由此可能造成吸附剂来自2方面的衰减:1)PEI通常含有一些分子量低于PEI平均分子量的PEI小分子,165 ℃的解吸温度对于这些小分子而言可能过高,导致这些小分子容易挥发出去,造成吸附剂物理性衰减;2)对于传统硅基固态胺吸附剂,在解吸温度高于120 ℃时,尿素化合物就会大量生成,而165 ℃的高温对于尿素化合物的生成极为有利[26]。尽管铝基固态胺吸附剂的红外图谱未检测出尿素化合物的生成,但依然存在尿素化合物生成的可能性,或者由于生成量少而未被检测到,从而造成吸附剂的化学性衰减。因此,在CO2解吸气氛下,如何在不造成吸附剂解吸效率大幅度下降的前提下尽可能地降低解吸温度是进一步提高铝基固态胺吸附剂循环稳定性的关键所在。
-
1)通过铝酸钠溶液与硫酸铝溶液进行简单混合,经过老化和共沸,可制备得到具有大孔体积的多孔Al2O3;在最佳工艺参数下,所制备的多孔Al2O3的比表面、孔体积和孔径分别达到495.7 cm2·g−1、2.75 cm3·g−1和20.3 nm。
2)采用简单的浸渍法将PEI负载到合成的多孔Al2O3上,制备得到的优等级铝基固态胺吸附剂(60%PEI@Al2O3-4 h)的CO2吸附量达到194 mg·g−1,达到了优秀吸附剂的标准(176 mg·g−1以上)。
3)该吸附剂具有稳定的循环性能,且具备优异的抗尿素化合物形成性能;在惰性解吸气氛和CO2解吸气氛下进行10次循环,CO2吸附量依然还保留186 mg·g−1和148 mg·g−1,分别衰减不到1%和15.2%。
高性能铝基固态胺吸附剂的制备及其对CO2的吸附
Preparation and CO2 adsorption of high-performance aluminum-based solid amine adsorbent
-
摘要: 以铝酸钠和硫酸铝为原料,通过共沸蒸馏合成大孔体积Al2O3,并以此作为载体浸渍聚乙烯亚胺(PEI)制备铝基固态胺吸附剂,再系统研究了其对CO2的吸附性能及循环稳定性。通过老化、共沸蒸馏等工艺,在最佳工艺参数下制得孔体积为2.75 cm3·g−1的多孔Al2O3载体,进而制备得到优等级吸附剂(60%PEI@Al2O3-4 h),其对CO2的饱和吸附能力可达到194 mg·g−1。该吸附剂在惰性解吸气氛和CO2解吸气氛下均具有稳定的循环性能,10次循环后其对CO2的吸附量依然为186 mg·g−1和148 mg·g−1,分别衰减不到1%和15.2%。本研究结果可为开发低成本、高稳定性固态胺吸附剂提供参考。Abstract: The porous Al2O3 with large-pore volume was synthesized by azeotropic distillation with sodium aluminate and aluminum sulfate as raw materials, which was used as a support to impregnate polyethyleneimine (PEI) for preparing aluminum-based solid amine adsorbent. The CO2 adsorption performance and cyclic stability of the adsorbent were systematically investigated subsequently. The porous Al2O3 supports with apore volume of 2.75 cm3·g−1 were prepared by aging and azeotropic distillation processes under the optimal technological parameters, and then the superior grade adsorbent (60%PEI@Al2O3-4 h) with a saturation CO2 adsorption capacity of 194 mg·g−1 was prepared. In addition, the adsorbent had stable cyclic performance in inert desorption atmosphere and CO2 desorption atmosphere. After 10 cycles, the CO2 adsorption capacity of 60%PEI@Al2O3-4 h still retained 186 mg·g−1 and 148 mg·g−1, with attenuation of less than 1% and 15.2%, respectively. This study can provide reference for developing low-cost and high-stability solid amine adsorbents.
-

-
表 1 不同合成条件制备得到的多孔Al2O3孔结构参数
Table 1. Pore structure parameters of the porous Al2O3 under different synthesis conditions
样品 SBET/(cm2·g−1) V/( cm3·g−1) D/nm 未共沸-未老化 77.1 0.36 17.6 共沸-未老化 433.1 2.49 20.2 共沸-老化2 h 449.4 2.59 21.3 共沸-老化4 h 495.7 2.75 20.3 共沸-老化6 h 474.2 2.51 19.0 注:SBET表示比表面积;V表示孔体积;D表示BJH吸附平均
孔径。 -
[1] 李函珂, 党成雄, 杨光星, 等. 面向二氧化碳捕集的过程强化技术进展[J]. 化工进展, 2020, 39(12): 4919-4939. [2] JAHANDAR LASHAKI M, KHIAVI S, SAYAYI A. Stability of amine-functionalized CO2 adsorbents: a multifaceted puzzle[J]. Chemical Society Reviews, 2019, 48(12): 3320-3405. doi: 10.1039/C8CS00877A [3] 王君雅, 羊莹, 宁平. 碱金属硝酸盐对促进LDH基材料吸附CO2 性能的影响[J]. 环境工程学报, 2018, 12(12): 3379-3388. doi: 10.12030/j.cjee.201808095 [4] 张亚朋, 崔龙鹏, 刘艳芳, 等. 3种典型工业固废的CO2矿化封存性能[J]. 环境工程学报, 2021, 15(7): 2344-2355. doi: 10.12030/j.cjee.202101003 [5] TIAN S C, YAN F, ZHANG Z T, et al. Calcium-looping reforming of methane realizes in situ CO2 utilization with improved energy efficiency[J]. Science advances, 2019, 5: eaav5077. doi: 10.1126/sciadv.aav5077 [6] 米剑锋, 马晓芳. 中国CCUS 技术发展趋势分析[J]. 中国电机工程学报, 2019, 39(9): 2537-2543. [7] FANG M X, YI N T, DI W T, et al. Emission and control of flue gas pollutants in CO2 chemical absorption system - a review[J]. International Journal of Greenhouse Gas Control, 2020, 93: 102904. doi: 10.1016/j.ijggc.2019.102904 [8] 陈旭, 杜涛, 李刚, 等. 吸附工艺在碳捕集中的应用现状[J]. 中国电机工程学报, 2019, 39(S1): 155-163. [9] 秦红艳, 张晓云, 张鹏志, 等. 氨基改性CO2固体吸附材料的研究进展[J]. 材料导报, 2013, 27(19): 39-42. [10] YANG Z Q, HE C Q, SUI H, et al. Recent advances of CO2-responsive materials in separations[J]. Journal of CO2 Utilization, 2019, 30: 79-99. doi: 10.1016/j.jcou.2019.01.004 [11] LI K M, JIANG J G, TIAN S C, et al. Polyethyleneimine–nano silica composites: a low-cost and promising adsorbent for CO2 capture[J]. Journal of Materials Chemistry A, 2015, 3(5): 2166-2175. doi: 10.1039/C4TA04275A [12] 马双忱, 韩剑, 方文武, 等. 燃煤烟气中CO2脱除方法的分析与探讨[J]. 电力科技与环保, 2011, 27(2): 4-8. doi: 10.3969/j.issn.1674-8069.2011.02.002 [13] 仇雪霞, 刘新民. 以废塑料为基材的大孔型离子交换树脂的制备, 表征和吸附性能[J]. 环境工程学报, 2020, 14(1): 224-235. doi: 10.12030/j.cjee.201903198 [14] LOU F J, ZHANG A F, ZHANG G H, et al. Enhanced kinetics for CO2 sorption in amine-functionalized mesoporous silica nanosphere with inverted cone-shaped pore structure[J]. Applied Energy, 2020, 264: 114637. doi: 10.1016/j.apenergy.2020.114637 [15] IRANI M, JACOBSON A T, GASEM K A M, et al. Modified carbon nanotubes/tetraethylenepentamine for CO2 capture[J]. Fuel, 2017, 206: 10-18. doi: 10.1016/j.fuel.2017.05.087 [16] JEON S, JUNG H, KIM S H, et al. Double-layer structured CO2 adsorbent functionalized with modified polyethyleneimine for high physical and chemical stability[J]. ACS Applied Materials Interfaces, 2018, 10: 21213-21223. doi: 10.1021/acsami.8b01749 [17] 蔡卫权, 余小锋. 高比表面大中孔拟薄水铝石和γ-Al2O3的制备研究[J]. 化学进展, 2007, 19(9): 1323-1330. [18] BHOWMIK K, CHAKRAVARTY A, BYSAKH S, et al. γ-Alumina nanorod/reduced graphene oxide as support for poly(ethylenimine) to capture carbon dioxide from flue gas[J]. Energy Technology, 2016, 4(11): 1409-1419. doi: 10.1002/ente.201600186 [19] SHEN X H, YAN F, LI C Y, et al. Biogas upgrading via cyclic CO2 adsorption: Application of highly regenerable PEI@nano-Al2O3 adsorbents with anti-urea properties[J]. Environmental Science Technology, 2021, 55(8): 5236-5247. doi: 10.1021/acs.est.0c07973 [20] CAI W Q, LI H Q, ZHANG Y. Azeotropic distillation-assisted preparation of macro-mesostructured γ-Al2O3 nanofibres of crumpled sheet-like morphology[J]. Materials Chemistry and Physics, 2006, 96(1): 136-139. doi: 10.1016/j.matchemphys.2005.06.053 [21] SHEN X H, YAN F, LI C Y, et al. Amine-functionalized nano-Al2O3 adsorbent for CO2 separation from biogas: Efficient CO2 uptake and high anti-urea stability[J]. Journal of Cleaner Production, 2022, 332: 130078. doi: 10.1016/j.jclepro.2021.130078 [22] SIEGELMAN R L, MILNER P J, KIM E J, et al. Challenges and opportunities for adsorption-based CO2 capture from natural gas combined cycle emissions[J]. Energy & Environmental Science, 2019, 12(7): 2161-2173. [23] 赵蓓蓓, 王际童, 王梅, 等. 树脂基固态胺吸附剂室温下对低浓度CO2的吸附性能研究[J]. 环境科学学报, 2014, 34(11): 2892-2898. [24] WANG W J, MOTUZAS J, ZHAO X S, et al. 2D/3D assemblies of amine-functionalized graphene silica (templated) aerogel for enhanced CO2 sorption[J]. ACS Applied Materials Interfaces, 2019, 11(33): 30391-30400. doi: 10.1021/acsami.9b07192 [25] LI C Y, YAN F, SHEN X H, et al. Highly efficient and stable PEI@Al2O3 adsorbents derived from coal fly ash for biogas upgrading[J]. Chemical Engineering Journal, 2021, 409: 128117. doi: 10.1016/j.cej.2020.128117 [26] LI K M, JIANG J G, CHEN X J, et al. Research on urea linkages formation of amine functional adsorbents during CO2 capture process: Two key factors analysis, temperature and moisture[J]. Journal of Physical Chemistry C, 2016, 120(45): 25892-25902. doi: 10.1021/acs.jpcc.6b08788 -



 下载:
下载: