-
Pb2+是环境中主要的重金属污染物之一,其无法被生物降解且能够生物积累,即使低剂量暴露也会危害人体健康和生态安全[1-2]。水体重金属污染处理方法包括化学沉淀、吸附、膜分离、离子交换和生物絮凝法等[3-6]。其中,由于吸附法具有操作简便、吸附材料来源广泛、适用范围广等优点近年来备受关注,开发高效、廉价的新型吸附材料是国内外水体重金属修复领域的研究热点之一。生物炭(biochar)一般指生物质在低氧或缺氧的环境中,高温缓慢热解得到的一种含碳量高、富有孔隙结构的碳化物质,具有较大的比表面积和孔隙度,表面官能团丰富,可以高效吸附重金属,是一种具有良好环境效益的吸附材料。
2019年我国市政污泥年均产量超6 000×104 t[7],市政污泥是水处理的副产品,含有多种有害污染物(如重金属、病菌等),处理处置不当将引起严重的二次污染。现有的污泥处理方式主要有焚烧、填埋、堆肥、厌氧消化等。上述方式可能导致土壤、水体污染且存在处理不完全、成本较高等问题[8-10]。考虑到污泥中富含有机质,有研究发现可依托高温热解工艺处理污泥并获得污泥基生物炭,该产物可用于吸附水体中的重金属[11-13]。郑凯琪等[14]研究了不同热解温度下污泥生物炭对重金属的吸附效果,结果表明吸附效率随热解温度增加而增加。
如何将吸附剂从水体中分离,是当前吸附处理的工艺难题之一。有研究[15-16]表明,通过磁改性生物炭,既可以提高生物炭的吸附能力,产物又可通过磁力回收以解决吸附剂难回收的技术难题。目前,磁性生物炭的制备大多采用湿法,即将生物炭原料浸泡于含铁离子的溶液中,然后超声分散、干燥后再进行煅烧,进而获得磁性生物炭[15-17]。赵冰等利用FeCl2和FeCl3复配溶液进行了湿法负载,经改性后获得的磁性生物炭最大Cu2+吸附容量较未改性生物炭增加了60.08%[18]。但实际操作中,湿法改性会出现热解前需消耗大量能源脱水干化、浸渍后颗粒团聚现象严重、固液分离困难等环境不友好的问题[15-17, 19-21]。
为解决上述湿法制备磁性生物炭的不足,本研究以市政污泥为基质,基于无溶剂法制备了磁性污泥基生物炭,并利用扫描电子显微镜(SEM)、傅立叶变换红外吸收光谱(FTIR)、X射线光电子能谱(XPS)、磁滞回线(VMS)、拉曼光谱(Raman)等技术对材料进行表征;选取Pb2+作为代表性重金属,分析了pH、温度、背景离子强度及吸附剂投加量对污泥基磁性生物炭吸附效能的影响,结合吸附动力学、吸附等温线、吸附热力学及吸附前后表征结果,系统揭示了无溶剂法制备的污泥基磁性生物炭的Pb2+吸附机理,以期为开发环境友好型重金属吸附材料提供参考。
-
本研究所用三水合硝酸铅为分析纯、硝酸(65%~68%)为优级纯,购买自国药集团化学试剂有限公司;硝酸钠与氢氧化钠为分析纯、无水乙醇纯度99.5%、纳米四氧化三铁纯度99.5%,购买自上海麦克林生化科技有限公司。
-
本研究所用脱水市政污泥取自昆明市第二水质净化厂,含水率(80.20±0.07)%(收到基),VS为(43.99±0.05)%(干基),C/N为6.48。经生物-物理干化后含水率降至约30%,风干后含水率降至5%以下,研磨过60目筛后待用。称取5 g风干后的市政污泥与一定质量纳米Fe3O4混合,通过机械研磨使其混合均匀。将上述混合物在管式炉中以5 ℃·min−1(N2气氛下)加热至800 ℃,热解2 h,待冷却至室温后取出样品并研磨过60目筛,储存在棕色密封瓶中并置于干燥皿中待用,标记为MSBC-x(其中,x代表Fe元素质量分数)。纳米Fe3O4用量由目标产物所设计铁元素质量分数确定。本研究中,Fe元素质量分数依次被设置为0.07%、0.15%、0.36%、0.58%、0.72%、1%、2%及5%。预实验结果表明,当Fe质量分数达到2%后,Pb2+吸附容量基本不再发生变化,故研究对象设置为Fe的质量分数为2%的磁性污泥基生物炭(MSBC-2)。
-
本研究采用ZEISS Gemini 300扫描电子显微镜(SEM;蔡司,英国)观察样品的微观形貌;采用赛默飞世尔6700型傅里叶变换红外光谱仪(FTIR;Thermo Fisher,美国)表征吸附前后样品表面官能团分布;采用Thermo ESCALAB 250Xi型X射线光电子能谱仪(XPS;Thermo Fisher,美国)表征吸附前后样品表面元素组成及化学状态;采用7404型振动样品磁强计(VMS;LakeShore,美国)进行磁滞回线测定;利用Renishaw Micro-Raman System 2000型拉曼光谱仪(Raman;Renishaw,英国)进行样品拉曼光谱测定。
-
磁性污泥基生物炭对Pb2+的吸附行为受多种因素影响,本实验探究了pH、温度、背景离子强度、吸附剂投加量对Pb2+去除率的影响。其中,生物炭对Pb2+的吸附容量及去除率根据式(1)和式(2)进行计算。
式中:Qe为生物炭对Pb2+的吸附容量,mg·g−1;C0、Ce分别为初始和吸附平衡时Pb2+的质量浓度,mg·g−1;V0为加入的Pb2+溶液体积,L;m为生物炭的投加量,g;E为Pb2+去除率,%。
称取一定质量的MSBC-2加入到40 mL一定质量浓度的Pb2+溶液中,用0.1 mol·L−1 HNO3溶液和0.1 mol·L−1 NaOH溶液调节反应pH,放置于恒温箱中,经摇床振荡(350 r·min−1)后取样,过0.45 μm PES针式过滤器,采用ICP-OES(Prodigy7,LeemanLabs,美国)测定Pb2+质量浓度,测定波长为220.353 nm。在MSBC-2投加量为1 g·L−1、初始Pb2+质量浓度为100 mg·L−1的条件下,分别考察不同pH(2、3、4、5、6、7、8),反应温度(25、35、45 ℃)及背景离子强度(0.005、0.01、0.05、0.1、0.5 mol·L−1)对吸附效果的影响。将MSBC-2投加量分别设置为0.1、0.5、1、2、4 g·L−1,在初始Pb2+质量浓度为100 mg·L−1、pH = 6、反应温度为25 ℃的条件下,研究磁性生物炭投加量对Pb2+去除效果的影响。以上实验均振荡24 h,并设置3组平行样。
-
选取0.04 g MSBC-2,100 mg·L−1的Pb2+溶液,反应体系pH为6,培养箱温度为25 ℃,取样时间分别为1、3、5、10、20、30、60、90、120、180、240、360、720、1 440 min。以上实验均设置3组平行。将上述实验结果分别用准一级动力学模型(式(3))、准二级动力学模型(式(4))、Elovich模型(式(5))和颗粒内扩散模型(式(6))拟合。
式中:Qt为t时刻生物炭对Pb2+的吸附容量,mg·L−1;Qe为平衡吸附容量,mg·g−1;k1为准一级动力学方程的反应速率常数,min−1;k2为准二级动力学方程的反应速率常数,g·(mg·min)−1,a和b分别代表与初始吸附速率和活化能相关的常数,单位分别为mg·(g·min)−1和g·mg−1;Kid为内扩散速率常数,mg·(g·min1/2)−1;C反映边界层效应。
选取0.04 g MSBC-2,100 mg·L−1的Pb2+溶液,反应体系pH为6,培养箱温度分别为25、35、45 ℃,振荡24 h。以上实验均设置3组平行。分别用Langmuir和Freundlich模型对上述实验结果进行拟合,如式(7)和式(8)所示。
式中:Ce为Pb2+吸附平衡质量浓度,mg·L−1;Qe为平衡吸附容量,mg·g−1;Qm为理论最大吸附容量,mg·g−1;KL为Langmuir方程平衡常数;KF为Freundlich常数;n为经验常数;RL为分离因子。
用Langmuir方程拟合得到的KL进行热力学拟合,分别求得ΔH°和ΔS°(式(10)~式(12))。
式中:ΔS0为吸附熵变,J·(mol·K)−1;ΔH0为吸附焓变,J·mol−1;R为理想气体常数,8.314 J·(mol·K)−1;T为热力学温度,K。
-
在环境因素对MSBC-2的Pb2+去除效果影响研究中,对所获得的Pb2+去除率与吸附容量数据使用One-way ANOVA进行分析,采用post-hoc Turkey检验来确定不同处理之间的差异,显著性水平P设置为0.05(SPSS 23.0,IBM,美国)。
-
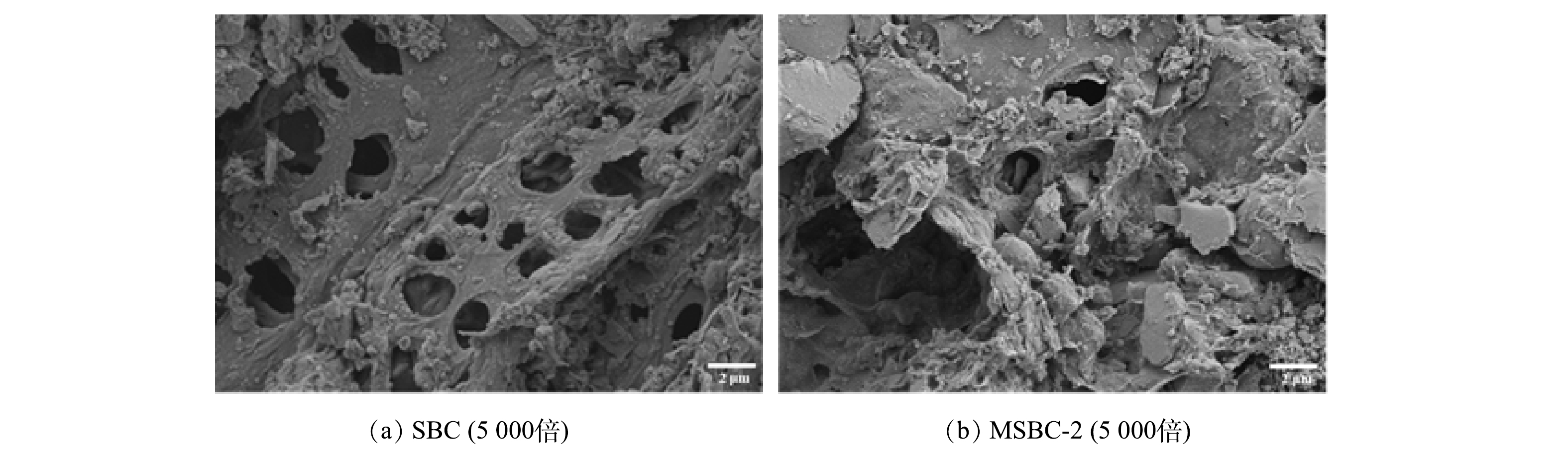
1)比表面积及SEM分析。磁性污泥基生物炭的比表面积、孔结构特征如表1所示,SEM结果如图1所示。未改性污泥基生物炭(SBC)比表面积为59.38 m2·g−1,纤维素、脂肪、蛋白质和其他沉积物成分通过化学活化和热解过程降解,形成具有粗糙表面外观和孔隙结构的生物炭基质(图1(a)),可为纳米Fe3O4颗粒的负载提供足够的空间[18]。经过改性后,磁性污泥基生物炭(MSBC-2)比表面积增大为63.68 m2·g−1,负载于生物炭表面的纳米铁氧化物比表面积相对较大,且磁改性有一定扩孔作用,导致磁性生物炭的比表面积增加。由图1(b)可见,磁改性后所得生物炭表面较为粗糙,表面大孔数量变少,主要是一些中微孔结构,其表面附着较多颗粒,这更有利于吸附反应的发生。
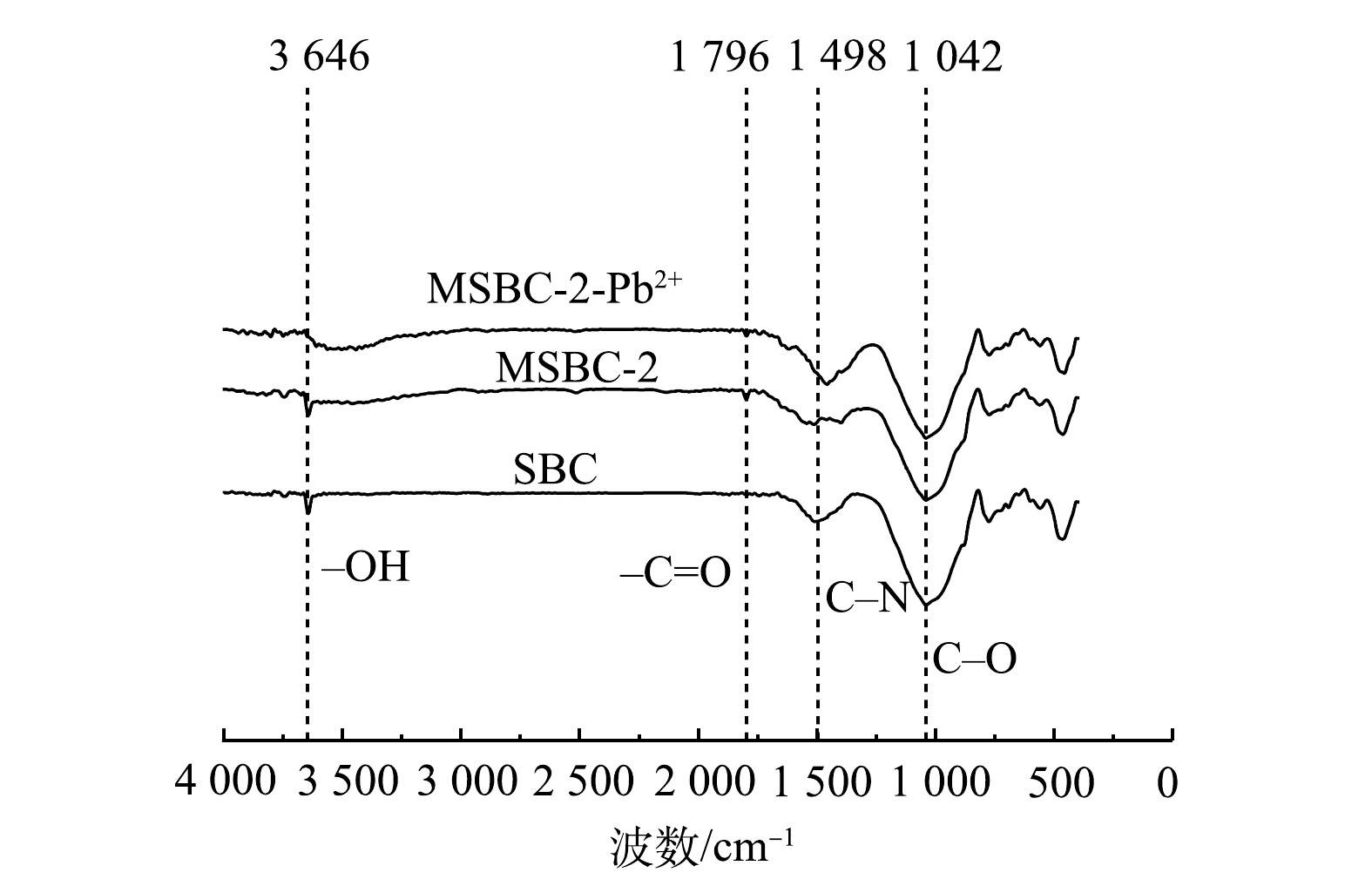
2)表面官能团。磁改性会改变生物炭表面官能团分布,采用FTIR对改性前后污泥基生物炭官能团进行分析。如图2所示,改性前后生物炭的红外特征峰位置基本不变,而峰的强度稍有不同。在3 646、1 796、1 498、1 042和760 cm−1处的吸收峰分别对应—OH(羟基)、C=O、C—N、C—O、Si—O—Si基团。在1 042 cm−1处的宽峰为C–O伸缩振动峰[22];3 646cm−1处为–OH基团的伸缩振动峰[23];1 796 cm−1处吸收峰为芳香环C=C和—COOH振动峰[24],改性后峰值增强,说明改性增加了生物炭的芳香性;1 498 cm−1处为C—N的伸缩振动峰[25];760 cm−1对应Si—O—Si伸缩振动峰,说明热解过程SiO2被保留在生物炭基质中[26]。
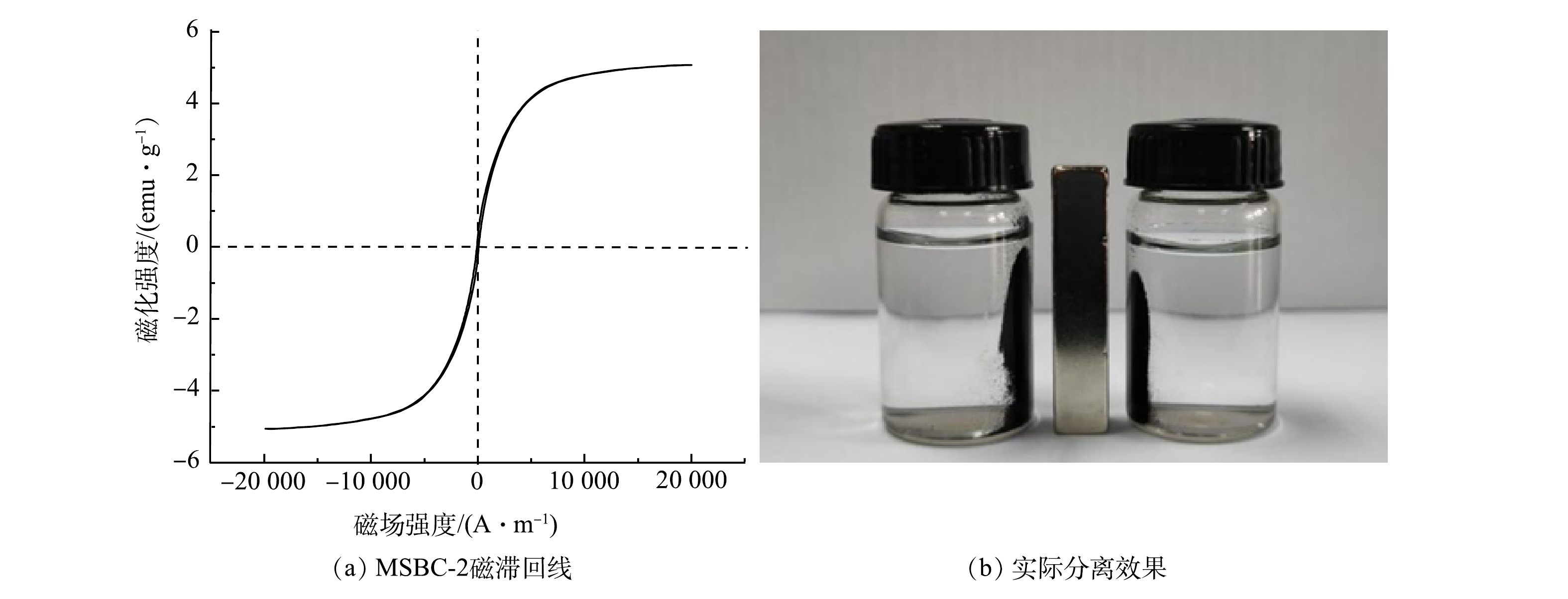
3)磁滞回线分析。MSBC-2磁滞回线呈S型(图3(a)),具有良好的磁性,饱和磁化强度为5.07 emu·g−1,可以通过外加磁场回收利用。由图3(b)可见,水中MSBC-2在吸附Pb2+后可被磁铁吸引至一侧,说明MSBC-2的磁性较好。
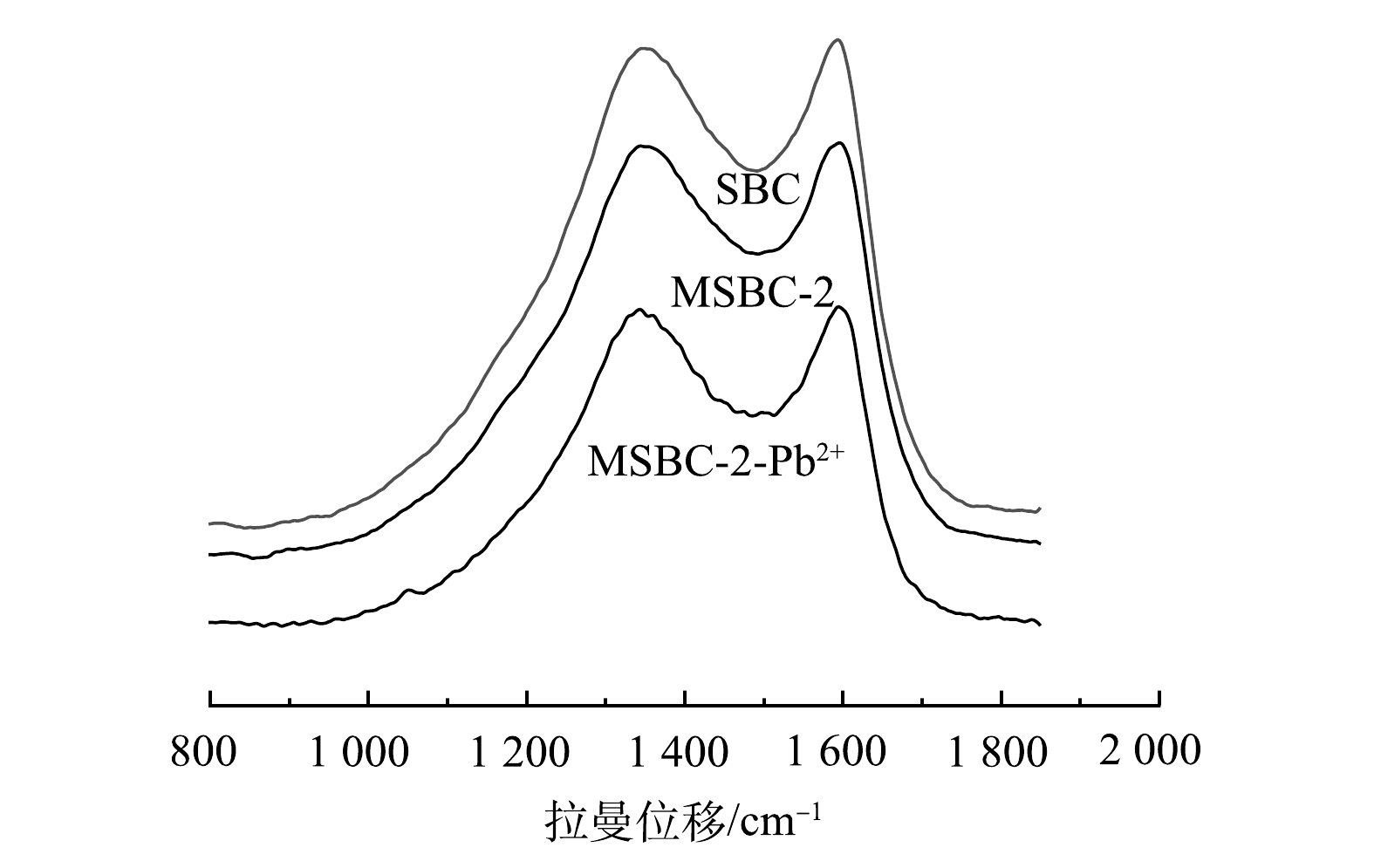
4) Raman分析。为进一步分析MSBC-2结构,对其进行Raman光谱表征,并对D峰和G峰进行分峰处理,结果如图4和表2所示。位于1 360~1 370 cm−1处的D带峰强越大,代表生物炭无序度越高;位于1 585~1 595 cm−1处的G带峰强越大,代表生物炭结晶度越高。可采用D带和G带的强度比(ID/IG)代表碳材料的石墨化程度[27]。由此可见,MSBC-2较SBC结晶度较高。
-
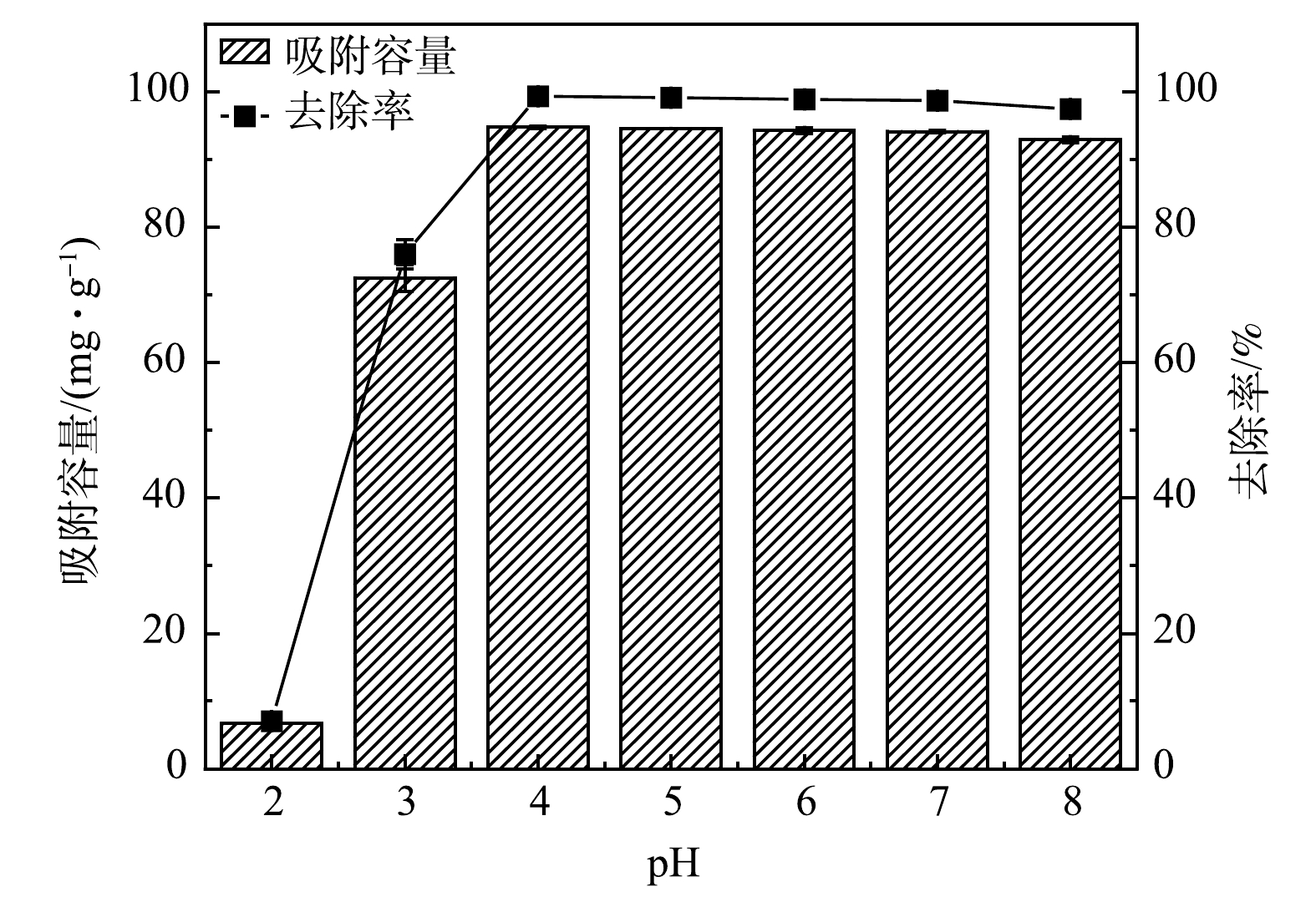
1) pH对Pb2+吸附的影响。如图5所示,当pH为2时,MSBC-2对Pb2+的去除率仅为7.5%。这是由于在酸性条件下,溶液中H+浓度较高,H+可以与Pb2+竞争吸附点位;此外,MSBC-2表面官能团会发生质子化,质子化的官能团可以与Pb2+产生静电排斥[28]。当pH为2~4时,随着pH增大,MSBC-2的Pb2+去除率由7.5%升高为99.3%,吸附容量由6.7 mg·g−1增大为94.8 mg·g−1。这是因为吸附剂表面含有更多带负电的羧基基团及游离羟基等,可为Pb2+提供吸附位点[26]。当pH≥4时,带负电的吸附位点继续增加,Pb2+被完全吸附,故MSBC-2的Pb2+去除率与吸附容量基本不再发生变化。综上所述,磁性污泥基生物炭MSBC-2在pH为3~8时均可实现较好的Pb2+去除效果。
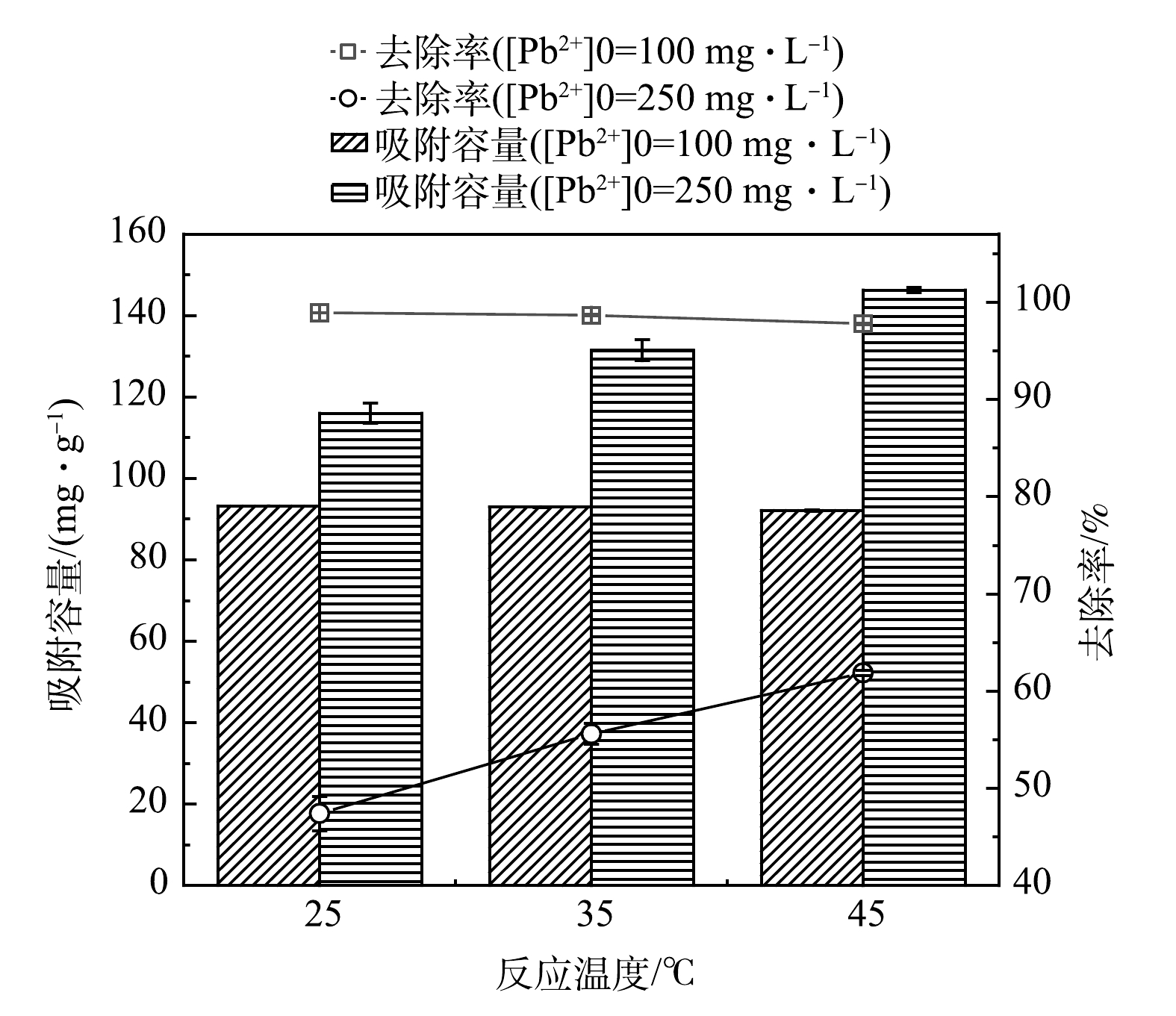
2)反应温度。图6反映了MSBC-2在不同温度下对Pb2+的去除效果。当初始Pb2+质量浓度为100 mg·L−1时,MSBC-2去除率基本不受温度影响;当初始Pb2+质量浓度提高到250 mg·L−1时,去除率随温度升高而显著增大(P<0.05),去除率由25 ℃的47.4%增加到45 ℃的61.9%。由此推断,MSBC-2吸附Pb2+的过程可能属于吸热反应,升温使化学反应活化能增加,活化分子百分数增大,进而导致吸附容量升高[29]。
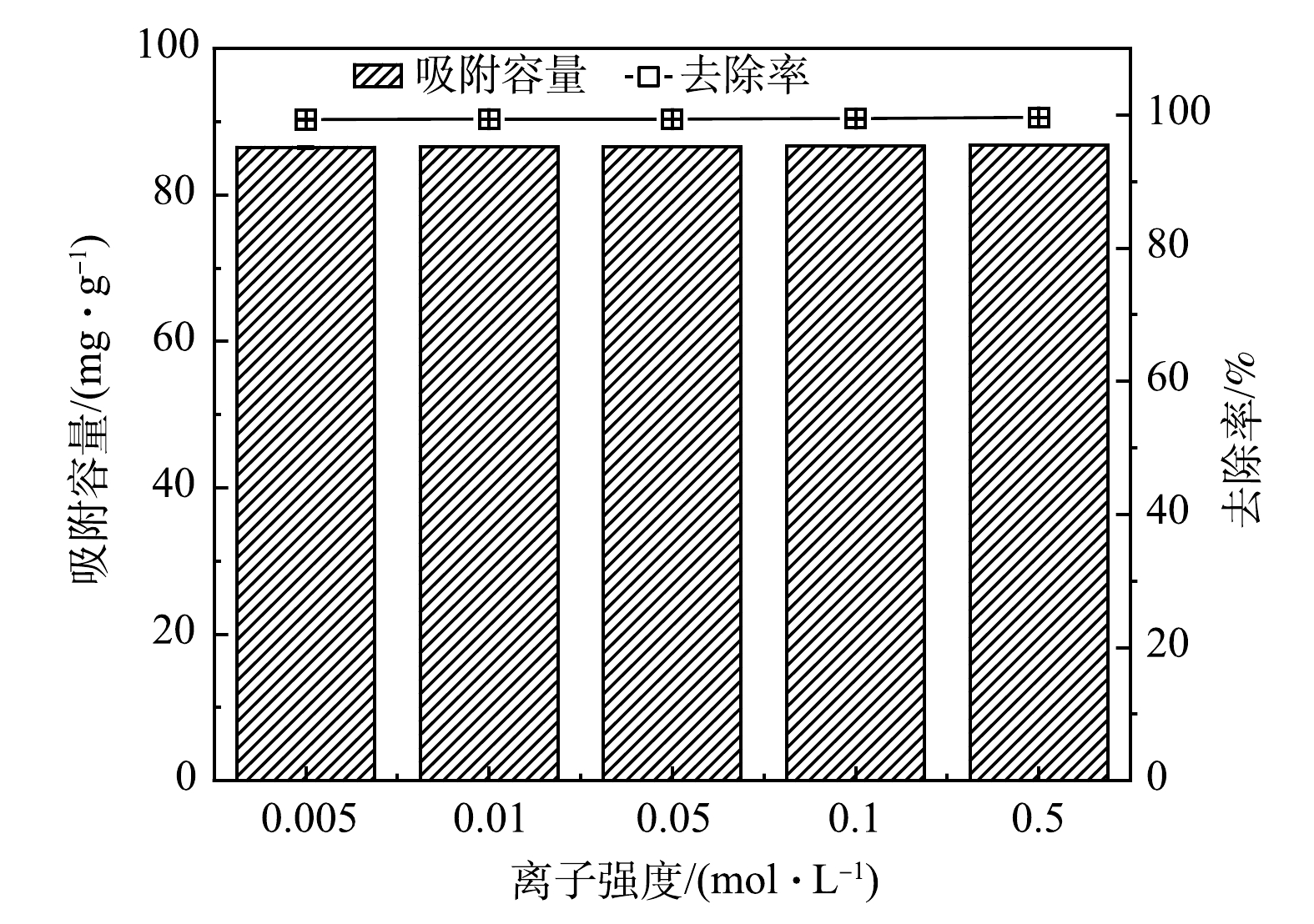
3)背景离子强度。图7反映了在不同背景离子强度下(0.005~0.5 mol·L−1NaNO3)下MSBC-2对Pb2+的去除效果。在该背景离子强度范围内,离子强度对Pb2+去除效果影响不大,说明MSBC-2吸附机制可能为内层络合,且MSBC-2吸附点位较多[30]。
4)吸附剂投加量。吸附剂投加量是影响吸附反应的重要因素,投加量不足水体中重金属将无法得到有效去除,投加量过大则会造成资源浪费并在实际应用中增加成本。图8为MSBC-2在不同投加量(0.1~4 g·L−1)下的Pb2+去除效果。Pb2+去除率随MSBC-2投加量增加而增大,由25.4% (0.1 g·L−1)增大至98.6% (1 g·L−1),当投加量大于1 g·L−1时,去除率基本不再发生变化。吸附容量随MSBC-2投加量增加而减小,这可能与单位磁性生物炭的位点和活性基团的数量有关。一定量的生物炭表面位点和活性基团可促进Pb2+的吸附,但过量的位点和活性基团则会引起溶液中Pb2+与之发生竞争吸附,进而导致单位质量吸附剂的Pb2+吸附容量降低。当MSBC-2投加量为4 g·L−1时,Pb2+去除率略有下降。这可能是由于部分吸附剂聚集导致[31]。
-
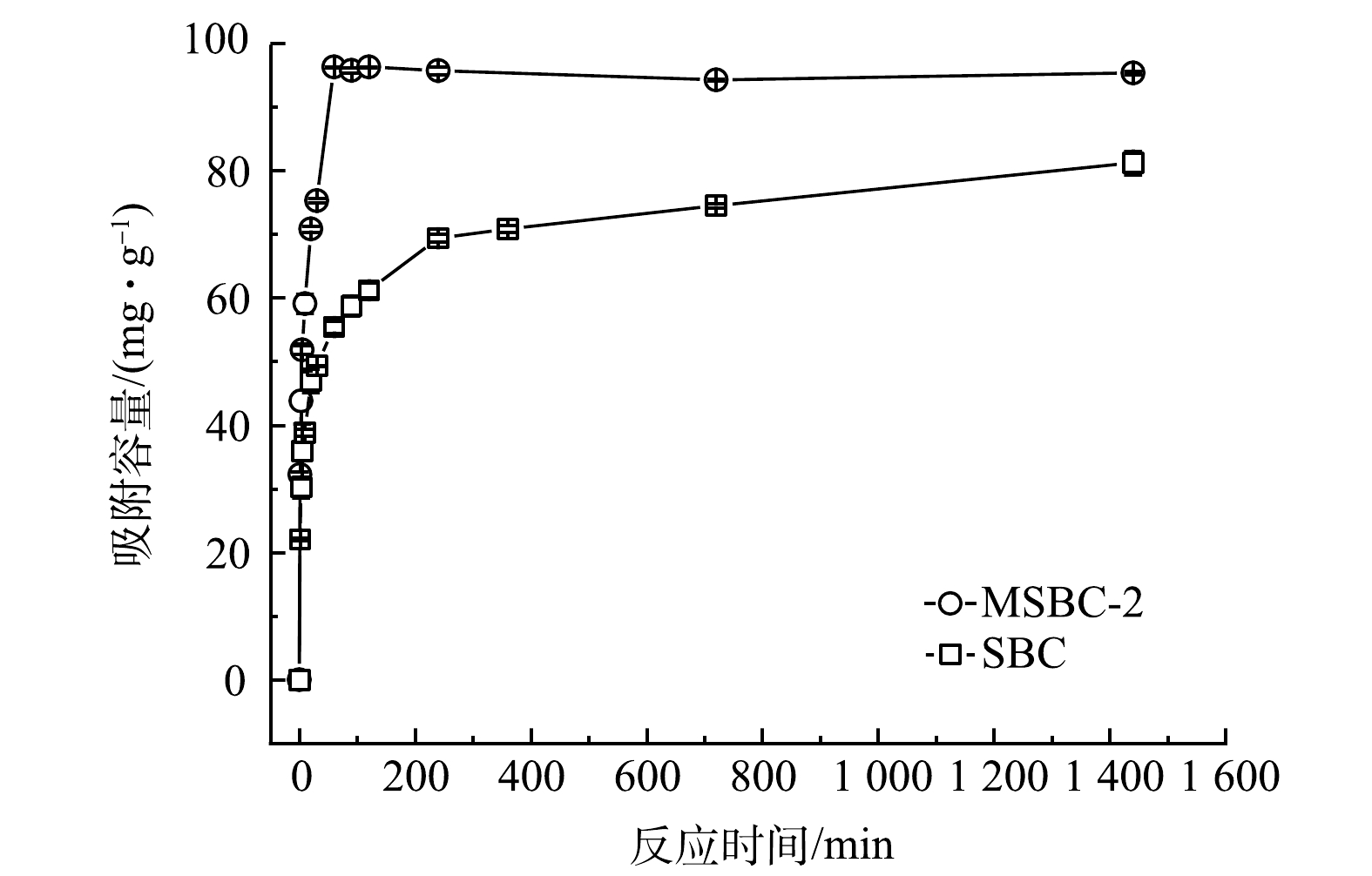
图9为磁性污泥基生物炭(MSBC-2)与未改性污泥基生物炭(SBC)对Pb2+的吸附容量随时间的变化。MSBC-2对Pb2+的吸附可分为快速吸附、慢速吸附和吸附平衡3个阶段[32]。在初始阶段(0~60 min),MSBC-2对Pb2+的吸附容量和去除率迅速增大,这是由于溶液重金属离子浓度高和MSBC-2表面可以提供大量吸附位点;60 min之后,吸附过程进入慢速阶段,这是由于随着吸附时间的增加,MSBC-2的吸附位点逐渐被占据,Pb2+吸附容量增加变缓最后达到平衡(240 min)。
为了进一步探究MSBC-2对Pb2+的吸附过程,采用准一级动力学模型[33]、准二级动力学模型[33]、Elovich模型和颗粒内膜扩散模型[34]对Pb2+吸附过程进行拟合分析,结果见表3。可见,准二级动力学模型的可决系数高于准一级动力学模型、Elovich模型和颗粒内膜扩散模型,表明准二级动力学模型能更好地描述MSBC-2对Pb2+的吸附过程(R2=0.999 7, P<0.000 1)。MSBC-2和SBC对Pb2+的吸附过程中限速步骤是化学反应,吸附过程主要受化学吸附控制[35]。颗粒内膜扩散模型常用来判断颗粒内扩散过程是否为吸附速率控制步骤,其可决系数R2仅为0.5252,说明MSBC-2的Pb2+吸附过程不是简单的物理扩散过程,在速率控制阶段粒子内的扩散作用不是吸附反应的唯一的影响因素[36]。Elovich模型用于描述由反应速率和扩散因子综合调控的非均相扩散过程。对于MSBC-2,其可决系数R2仅为0.889 1,说明该模型不能很好地描述其Pb2+吸附过程;但对于SBC,其可决系数R2=0.995 6,说明SBC在整个吸附过程中具有均匀分布的表面吸附能,Pb2+吸附主要为非均质表面吸附[37]。反应过程中,MSBC-2对Pb2+的吸附容量一直高于SBC,反应结束时MSBC-2吸附容量为95.75 mg·g−1,而SBC吸附容量为81.24 mg·g−1;MSBC-2的反应速率常数k2(3.90×10−3 g·(mg·min)−1),是SBC(k2=9.56×10−4 g·(mg·min)−1)的4.1倍,这说明磁改性后污泥基生物炭的吸附性能得到了一定程度的提高。
-
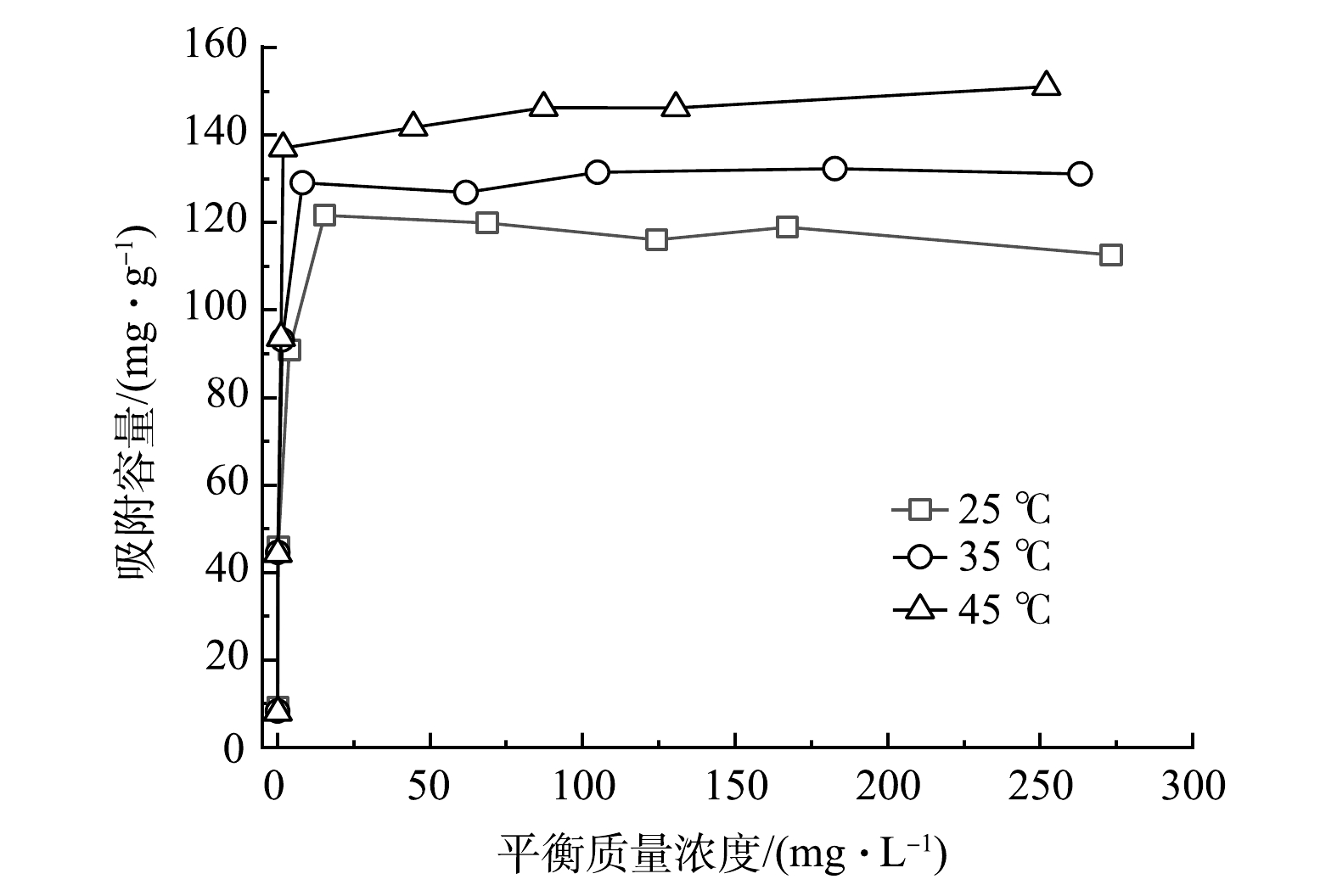
图10为MSBC-2在25、35、45 ℃下对Pb2+的吸附等温线。可见,随着初始Pb2+质量浓度增大,MSBC-2对Pb2+的吸附容量先逐渐增加后趋于平衡。当Pb2+初始质量浓度超过150 mg·L−1时,温度越高,吸附容量越大。这说明升高温度有利于吸附反应的进行。
为进一步解释MSBC-2的吸附机理,采用Langmuir模型和Freundlich模型对实验数据进行拟合(表4)。Freundlich模型假设吸附是发生在非均相表面上的多层吸附,并且不考虑吸附剂的饱和度[38]。Langmuir模型常用于模拟发生在均匀表面上的单层化学吸附,且吸附分子之间没有相互作用[39]。当反应温度从25 ℃上升至45 ℃时,MSBC-2的最大Pb2+吸附容量Qm由113.64 mg·g−1上升至151.52 mg·g−1,这说明吸附过程为吸热反应。由表4可见,Langmuir模型(R2=0.999 7~0.999 9, P<0.000 1)较Freundlich模型(R2=0.603 6~0.752 7, P<0.05)可决系数更高,可更好地描述MSBC-2对Pb2+的吸附行为。这表明Pb2+的吸附主要为单分子层吸附[36-39]。基于Langmuir模型计算得出的分离因子RL(式(9))可用于评价吸附剂吸附污染物的能力,当RL=0时,吸附过程为不可逆吸附;0<RL<1时,有利吸附过程的发生;RL=1时,吸附过程为线性吸附;RL>1时,吸附过程则为不利吸附[40]。在25、35、45 ℃时,RL分别为0.001 1~0.045 8、0.002 0~0.076 8、0.004 2~0.150 1,表明MSBC-2对Pb2+的吸附易于发生。在Freundlich模型中,KF是吸附容量指标,KF越大,表明其吸附容量越大;1/n表示吸附强度,1/n数值越小,表明吸附强度越强[41],且当0.1<1/n<1时,表明吸附反应易于进行。在25、35、45 ℃时,MSBC-2的KF值分别为39.958、44.228、49.511 mg·g−1·(L·mg−1)1/n,1/n的值分别为0.249、0.251、0.260。这说明温度越高MSBC-2吸附性能越强,有利于MSBC-2吸附Pb2+。
-
为了更好的揭示温度对磁性污泥基生物炭吸附Pb2+的影响,用Langmuir方程拟合得到的KL来进行热力学模拟(式(10)~(12))[42],拟合结果如表5所示。吸附过程ΔG>0,表明该反应为非自发反应。但是随着温度的增加,ΔG°逐渐降低,吸附自发性逐渐增加,温度增加更有利于吸附的发生。ΔH>0,为91.413 9 kJ·mol−1,表明该吸附过程是吸热过程,与吸附等温实验结果相对应。通常当吸附焓ΔH<40 kJ·mol−1时,吸附主要作用力是范德华力,属物理吸附;50 kJ·mol−1<ΔH<200 kJ·mol−1时,吸附作用力是化学键,属化学吸附[43]。上述结果说明MSBC-2吸附Pb2+主要依靠化学吸附,吸附过程有化学键的形成,故可以推测得出MSBC-2主要是依靠其表面的有机官能团或者无机灰分与Pb2+相互作用,这与吸附动力学、吸附等温线拟合结果吻合。ΔS仅为0.321 8 kJ·(mol·K)−1,说明吸附中固液界面无序性基本不变[44]。综上所述,MSBC-2的吸附反应是以化学吸附为主的非自发吸热过程。
-
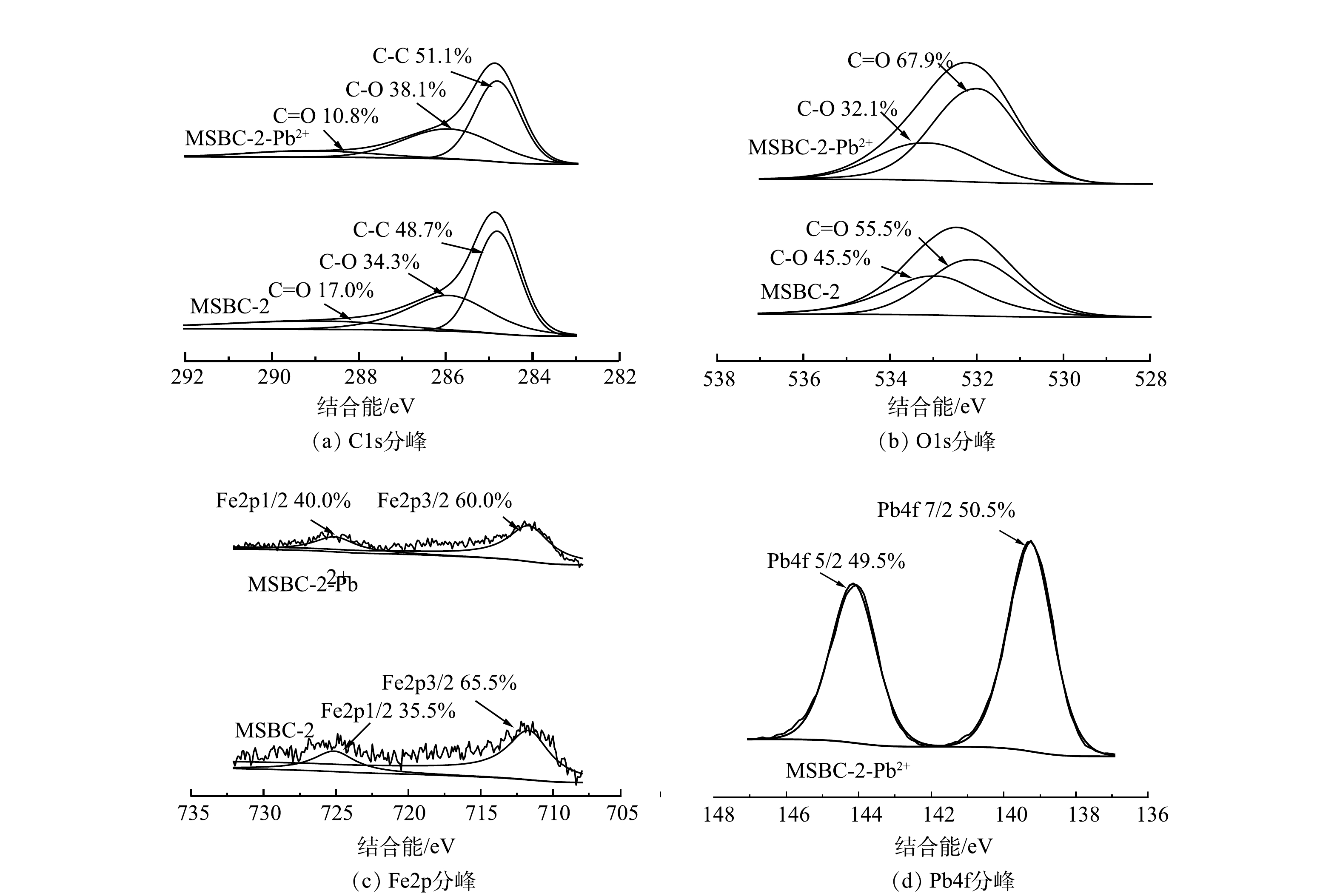
磁性生物炭的吸附机理通常为物理吸附和化学吸附。BET和SEM分析结果表明,磁改性过程具有扩孔作用,使MSBC-2的比表面积增大,有利于对Pb2+的吸附。为进一步探究MSBC-2的吸附机制,本研究在吸附动力学、吸附等温线、吸附热力学研究的基础上,结合FTIR、XPS及拉曼分析,进一步从微观层面揭示了MSBC-2的化学吸附机理。由图11可见,吸附前MSBC-2样品中的284.80、285.92、288.71 eV分别对应C—C、C—O、C=O的吸收峰,相应官能团含量占比分别为48.7%、34.3%、17.0%;在吸附Pb2+后,C—C、C—O含量占比升高,C=O含量占比降低至10.8%,这可能是由于Pb2+与羧基发生络合反应,使C=O含量占比降低[45]。吸附前后O元素XPS分析如图11(b)所示。吸附前531.75eV和532.52 eV分别对应C=O和C—O吸收峰,吸附后结合能分别为531.83 eV和532.58 eV;结合能升高可能是C=O及C—O与Pb2+形成配合物所致[45-46]。MSBC-2吸附后Pb2+的XPS表征结果如图11(d)所示。在139.28 eV和144.15 eV附近的2个吸收峰分别对应Pb4f7/2和Pb4f5/2[37],说明Pb2+与磁性生物炭中的官能团形成了配位键[46]。由图2可见,MSBC-2吸附Pb2+后—OH伸缩振动峰峰强降低,且振动峰移至3 576 cm−1处,结合XPS结果中在139 eV出现的Pb–O吸收峰与2.2节中pH对MSBC-2吸附的影响推测,羟基可能参与了表面络合,或者通过提供H+的方式与Pb2+发生离子交换反应[46-47]。图4表明吸附Pb2+后的MSBC-2图谱发生蓝移,MSBC-2、MSBC-2-Pb中的D峰与G峰的强度比分别为5.32、3.32,可进一步说明表面官能团与金属离子络合。综上所述,MSBC-2对Pb2+的吸附机理主要包括表面络合、离子交换与物理吸附。
-
为进一步评价无溶剂法制备磁性污泥基生物炭的Pb2+去除能力与应用潜力,表6总结了近年由湿法制备的磁性生物炭反应速率常数k2与最大理论吸附容量Qm。由表6可见,湿法制备磁性生物炭的反应速率常数k2为6.33×10−4~4.00×10−2 g·(mg·min)−1,最大Pb2+理论吸附容量Qm为17.7~103.10 mg·g−1。本研究中,由无溶剂法制备的磁性生物炭k2=3.90×10−3 g·(mg·min)−1,此数值在湿法制备磁性生物炭的范围内;最大Pb2+理论吸附容量Qm为113.6 mg·g−1,高于绝大多数由湿法制备的磁性生物炭。这证实了由无溶剂法制备的磁性生物炭具备高吸附性。此外,湿法制备磁性生物炭会出现热解前需消耗大量能源脱水干化、固液分离困难等多种环境不友好的问题,本研究所使用的无溶剂制备法全程不需要溶剂浸泡,不产生废液,制备能耗低,制备更加方便快捷,因此更具实际应用潜力。
-
1)相较于未改性生物炭,无溶剂法制备的磁性污泥基生物炭比表面积增大,结晶度提高,表面官能团基本不变;本研究制备的磁性污泥基生物炭具有良好的磁性,饱和磁化强度为5.07 emu·g−1,可以通过外加磁场回收利用。
2) MSBC-2对Pb2+的去除率随pH增大而增大,pH>4后其去除率基本不变(>99%);MSBC-2对Pb2+的去除率随温度提高而升高,离子强度对其去除率基本无影响;MSBC-2最佳投加量为1 g·L−1。
3)准二级动力学模型及Langmuir模型能更好地描述MSBC-2对Pb2+的吸附过程,表明该吸附反应的限速步骤是化学反应,Pb2+的吸附主要为单分子层吸附;吸附过程为非自发、吸热且熵增的过程。
4) MSBC-2对Pb2+吸附机理主要包括表面络合、离子交换与物理吸附。
5) 25 ℃时MSBC-2最大理论吸附容量Qm为113.64 mg·g−1,高于大多数由湿法制备的磁性生物炭。
磁性污泥基生物炭对Pb2+的吸附性能
Adsorption performance of magnetic sludge-derived biochar towards Pb2+
-
摘要: 为克服湿法制备磁性生物炭颗粒时团聚严重、固液分离困难、热解前需消耗大量能源脱水干化的问题,本研究以市政污泥为原料,通过无溶剂法热解制备了磁性污泥基生物炭(MSBC-2),并利用SEM、FTIR、XPS、VMS和Raman等方法对产物的表面结构与特征进行了表征。基于序批实验,分析了pH、温度、背景离子强度、生物炭投加量对该吸附材料的Pb2+吸附性能的影响,并进行了吸附动力学、吸附等温线及吸附热力学研究。结果表明:MSBC-2的Pb2+去除率随pH及温度的升高而升高,pH>4后去除率基本不变,离子强度对Pb2+去除率基本无影响。MSBC-2对Pb2+的吸附行为符合准二级动力学模型及Langmuir模型,表明吸附过程的限速步骤为化学反应,吸附为单分子层吸附;MSBC-2的反应速率常数k2是未改性生物炭的4.1倍,25 ℃时最大理论吸附容量为113.36 mg·g−1,高于大多数湿法制备的磁性生物炭;该吸附过程非自发、吸热且熵增过程;MSBC-2对Pb2+的吸附机理主要包括表面络合、离子交换和物理吸附。Abstract: In order to overcome severe agglomeration, solid-liquid separation problems for magnetic biochar particles in wet preparation process, and high energy consumption for dewatering and drying before pyrolysis, a type of magnetic sludge-derived biochar (MSBC-2) was prepared by solvent-free pyrolysis method with municipal sludge as the raw material. The SEM, FTIR, XPS, VMS, and Raman techniques were applied to characterize MSBC-2. Based on the batch experiment, the effects of pH, temperature, background ionic strength, and adsorbent dosage on the Pb2+ adsorption performance were investigated, together with adsorption kinetics, isotherms, and thermodynamics. The results revealed that Pb2+ removal efficiency by MSBC-2 increased as pH and temperature rose, but remained stable when pH was above 4. The removal efficiency was almost unaffected by background ionic strength. The Pb2+ adsorption process onto MSBC-2 followed the pseudo-second-order kinetic model and Langmuir model, implying that chemisorption was the rate-limiting step and the adsorption process was monolayer adsorption. The reaction rate constant k2 of MSBC-2 was 3.1 times greater than that of the sludge-derived biochar without modification. The maximum theorical adsorption capacity of MSBC-2 was 113.36 mg·g−1 at 25℃. The adsorption was non-spontaneous, endothermic, and entropy-increasing process. The Pb2+ adsorption mechanism onto MSBC-2 included surface complexation, ion-exchange reactions and physical absorption.
-
Key words:
- solvent-free method /
- sludge /
- magnetic biochar /
- Pb2+ /
- adsorption performance
-

-
表 1 磁性污泥基生物炭比表面积、孔结构特征
Table 1. Specific surface area and pore structure characteristics of MSBC-2 and SBC
样品名称 BET比表面积
/(m2·g-1)总孔体积
/(m3·g-1)平均孔径
/nmMSBC-2 63.68 0.089 5.96 SBC 59.38 0.073 4.56 表 2 拉曼曲线D带和G带分析
Table 2. D-band and G-band analysis from Raman spectrum
样品名称拉曼位移/cm−1 面积 D G D G SBC 1 376.5 1 594.6 823 962 151 072 MSBC-2 1 377.0 1 597.1 691 440 129 894 MSBC-2-Pb2+ 1 363.5 1 592.9 420 701 126 569 表 3 不同吸附动力学模型拟合参数
Table 3. Adsorption kinetics parameters for various adsorption kinetics models
样品名称 Qe,exp/
(mg·g−1)准一级动力学模型 准二级动力学模型 Qe,cal/
(mg·g−1)k1/
(min−1)R2 P Qe,cal/
(mg·g−1)k2/
(g·(mg·min)−1)R2 P MSBC-2 95.75 44.35 4.79×10−3 0.878 8 0.000 6 94.97 3.90×10−3 0.999 7 <0.000 1 SBC 81.24 40.61 3.08×10−3 0.766 5 <0.000 1 75.02 9.56×10−4 0.998 3 <0.000 1 样品名称 Qe,exp/
(mg·g−1)Elovich模型 颗粒内扩散模型 a/
(mg·(g·min)−1)b/
(g·mg−1)R2 P Kid/
(mg·(g·min0.5)−1)C/
(mg·g−1)R2 P MSBC-2 95.75 3.16×102 1.48 0.889 1 <0.000 1 2.211 56.43 0.525 2 <0.000 1 SBC 81.24 1.12×102 1.44 0.995 6 <0.000 1 1.921 33.90 0.818 3 <0.000 1 表 4 不同温度下MSBC-2吸附Pb2+的Langmuir模型和Freundlich模型拟合结果
Table 4. Adsorption isotherms parameters of Pb2+ onto MSBC-2 at different temperatures for Langmuir and Freundlich models
温度
/℃Langmuir模型 Freundlich模型 Qm/(mg·g−1) KL/(L·mg−1) R2 P RL KF/(mg·g−1·(L·mg−1)1/n ) n R2 P 25 113.64 2.270 0.999 7 <0.000 1 0.001 1~0.045 8 39.958 4.024 0.752 7 0.005 2 35 131.58 1.310 0.999 9 <0.000 1 0.002 0~0.076 8 44.228 3.986 0.670 9 0.012 9 45 151.52 0.617 0.999 7 <0.000 1 0.004 2~0.150 1 49.511 3.849 0.603 6 0.023 3 表 5 MSBC-2吸附Pb2+的吸附热力学参数
Table 5. Thermodynamic parameters of Pb2+ onto MSBC-2
温度/K ΔG0/
(kJ·mol−1)ΔS0/
(kJ·(mol·K)−1)ΔH0/
(kJ·mol−1)R2 P 298.15 2.199 0 0.321 8 91.413 9 0.999 9 0.006 4 308.15 1.785 1 318.15 1.355 3 表 6 湿法制备与无溶剂法制备磁性生物炭反应速率常数和最大Pb2+理论吸附容量的对比
Table 6. Comparison of k2 and Qm of Pb2+ onto magnetic biochar prepared by wet process and this study
-
[1] JIANG W, CHEN X, PAN B, et al. Spherical polystyrene- supported chitosan thin film of fast kinetics and high capacity for copper removal[J]. Journal of Hazardous Materials, 2014, 276: 295-301. doi: 10.1016/j.jhazmat.2014.05.032 [2] 万顺利, 薛瑶, 马钊钊, 等. 茶叶基水合氧化铁吸附水中Pb(Ⅱ)的性能[J]. 环境科学, 2014, 35(10): 3782-3788. [3] ZHOU Z, LIU Y, LIU S, et al. Sorption performance and mechanisms of arsenic(V) removal by magnetic gelatin- modified biochar[J]. Chemical Engineering Journal, 2017, 314: 223-231. doi: 10.1016/j.cej.2016.12.113 [4] ZHE X, ZHANG Q, LI X, et al. A critical review on chemical analysis of heavy metal complexes in water/wastewater and the mechanism of treatment methods[J]. Chemical Engineering Journal, 2022: 429131688. [5] CHAI W, CHEUN J, KUMAR PS, et al. A review on conventional and novel materials towards heavy metal adsorption in wastewater treatment application[J]. Journal of Cleaner Production, 2021: 296126589. [6] ANKITA A, UPADHYAY U, SREEDHAR I, et al. A review on valorization of biomass in heavy metal removal from wastewater[J]. Journal of Water Process Engineering, 2020: 38101602. [7] 戴晓虎. 我国污泥处理处置现状及发展趋势[J]. 科学, 2020, 72(6): 30-34. [8] 薛重华, 孔祥娟, 王胜, 等. 我国城镇污泥处理处置产业化现状、发展及激励政策需求[J]. 净水技术, 2018, 37(12): 33-39. [9] 刘莹. 城市污水处理厂污泥处理处置现状与技术研究[J]. 节能与环保, 2019(1): 78-79. doi: 10.3969/j.issn.1009-539X.2019.01.031 [10] 谢昆, 尹静, 陈星. 中国城市污水处理工程污泥处置技术研究进展[J]. 工业水处理, 2020, 40(7): 18-23. [11] 丁文川, 杜勇, 曾晓岚, 等. 富磷污泥生物炭去除水中Pb (Ⅱ)的特性研究[J]. 环境化学, 2012, 31(9): 1375-1380. [12] 范世锁, 李雪, 胡凯, 等. 污泥基生物炭吸附重金属Cd的动力学和热力学[J]. 环境工程学报, 2016, 10(10): 5971-5977. doi: 10.12030/j.cjee.201505069 [13] IFTHIKAR J, WANG J, WANG Q, et al. Highly efficient lead distribution by magnetic sewage sludge biochar: Sorption mechanisms and bench applications[J]. Bioresource Technology, 2017, 238: 399-406. doi: 10.1016/j.biortech.2017.03.133 [14] 郑凯琪, 王俊超, 刘姝彤, 等. 不同热解温度污泥生物炭对Pb2+、Cd2+的吸附特性[J]. 环境工程学报, 2016, 10(12): 7277-7282. doi: 10.12030/j.cjee.201507083 [15] YI Y, HUANG Z, LU B, et al. Magnetic biochar for environmental remediation: A review[J]. Bioresource Technology, 2020: 298122468. [16] LI X, WANG C, ZHANG J, et al. Preparation and application of magnetic biochar in water treatment: A critical review[J]. Science of the Total Environment, 2020: 711134847. [17] 魏太庆, 王博, 艾丹, 等. 磁性生物炭的制备及其在环境修复中的研究进展[J]. 功能材料, 2021, 52(10): 10039-10047. doi: 10.3969/j.issn.1001-9731.2021.10.006 [18] 赵冰, 张冉, 徐新阳. 污泥基磁性生物炭及其对水体中铜离子的吸附性能[J]. 东北大学学报(自然科学版). 2021, 42(7): 1012-1018. [19] LI Y, ZIMMERMAN A, HE F, et al. Solvent-free synthesis of magnetic biochar and activated carbon through ball-mill extrusion with Fe3O4 nanoparticles for enhancing adsorption of methylene blue[J]. Science of the Total Environment, 2020: 722137972. [20] JOSHUA J, HUGGINS T, HUANG Y, et al. Production of magnetic biochar from waste-derived fungal biomass for phosphorus removal and recovery[J]. Journal of Cleaner Production, 2019, 224: 100-106. doi: 10.1016/j.jclepro.2019.03.120 [21] 张连科, 王洋, 王维大, 等. 磁性羟基磷灰石/生物炭复合材料的制备及对Pb2+的吸附性能[J]. 环境科学学报, 2018, 38(11): 4360-4370. [22] VAUGHN S, KENAR J, TISSERAT B, et al. Chemical and physical properties of Paulownia elongata biochar modified with oxidants for horticultural applications[J]. Industrial Crops and Products, 2017, 97: 260-267. doi: 10.1016/j.indcrop.2016.12.017 [23] 李蕊宁, 王兆炜, 郭家磊, 等. 酸碱改性生物炭对水中磺胺噻唑的吸附性能研究[J]. 环境科学学报, 2017, 37(11): 4119-4128. [24] 周振扬, 杨驰浩, 尹微琴, 等. 氧化松木生物炭高效去除水中Pb及定量吸附机理[J]. 工业水处理, 2021, 41(7): 88-93. [25] 张伟, 杨柳, 蒋海燕, 等. 污泥活性炭的表征及其对Cr(Ⅵ)的吸附特性[J]. 环境工程学报, 2014, 8(4): 1439-1446. [26] 陈婷婷. 稻壳灰及改性稻壳灰吸附性能研究[D]. 南京: 南京理工大学, 2013. [27] 赵佳明. 生物质基多孔活性炭制备及其吸附Cr(VI)离子性能研究[D]. 哈尔滨: 黑龙江大学, 2021. [28] 于长江, 董心雨, 王苗, 等. 海藻酸钙/生物炭复合材料的制备及其对Pb(Ⅱ)的吸附性能和机制[J]. 环境科学, 2018, 39(8): 3719-3728. [29] 王鑫宇, 张曦, 孟海波, 等. 温度对生物炭吸附重金属特性的影响研究[J]. 中国农业科技导报, 2021, 23(2): 150-158. [30] 赵天赐. 负载铁基复合氧化物的生物炭对Pb(Ⅱ)的吸附作用[D]. 石家庄: 河北师范大学, 2020. [31] ZHOU Y, NIE H, BRANFORD-WHITE C. Removal of Cu(II) from aqueous solution by chitosan-coated magnetic nanoparticles modified with α-ketoglutaric acid[J]. Journal of Colloid and Interfaced Science. 2009, 330(1): 29-37. [32] 胡学玉, 陈窈君, 张沙沙, 等. 磁性玉米秸秆生物炭对水体中Cd的去除作用及回收利用[J]. 农业工程学报, 2018, 34(19): 208-218. doi: 10.11975/j.issn.1002-6819.2018.19.027 [33] ZIMMERMANN A, MECABÔ A, FAGUNDS T, et al. Adsorption of Cr(VI) using Fe-crosslinked chitosan complex (Ch-Fe)[J]. Journal of Hazardous Materials, 2010, 179(1): 192-196. [34] ZHU S, KHAN M, WANG F, et al. Rapid removal of toxic metals Cu2+ and Pb2+ by amino trimethylene phosphonic acid intercalated layered double hydroxide: A combined experimental and DFT study[J]. Chemical Engineering Journal, 2019, 392: 123711. [35] ÇELEKLI A, İLGÜN G, BOZKURT H. Sorption equilibrium, kinetic, thermodynamic, and desorption studies of Reactive Red 120 on Chara contraria[J]. Chemical Engineering Journal, 2012, 191: 228-235. doi: 10.1016/j.cej.2012.03.007 [36] ZHOU X, LIU Y, ZHOU J, et al. Efficient removal of lead from aqueous solution by urea-functionalized magnetic biochar: Preparation, characterization and mechanism study[J]. Journal of the Taiwan Institute of Chemical Engineers, 2018: 91457-467. [37] PRIYA AK, YOGESHWARAN V, RAJENDRAN S, et al. Investigation of mechanism of heavy metals (Cr6+, Pb2+& Zn2+) adsorption from aqueous medium using rice husk ash: kinetic and thermodynamic approach[J]. Chemosphere, 2022: 286131796. [38] LI T, LIU Y, PENG Q, et al. Removal of lead(II) from aqueous solution with ethylenediamine-modified yeast biomass coated with magnetic chitosan microparticles: kinetic and equilibrium modeling[J]. Chemical Engineering Journal, 2013, 214(1-13): 189-197. [39] WANG P, CAO M, WANG C, et al. Kinetics and thermodynamics of adsorption of methylene blue by a magnetic graphene-carbon nanotube composite[J]. Applied Surface Science, 2014, 290: 116-124. doi: 10.1016/j.apsusc.2013.11.010 [40] ZHAO Y, ZHANG B, ZHANG X, et al. Preparation of highly ordered cubic NaA zeolite from halloysite mineral for adsorption of ammonium ions[J]. Journal of Hazardous Materials, 2010, 178(1-3): 658-664. doi: 10.1016/j.jhazmat.2010.01.136 [41] 曹玮. 磁性生物炭去除废水中Pb2+、Cd2+的效果及机制初探[D]. 长沙: 中南林业科技大学, 2016. [42] SUN J, LIAN F, LIU Z, et al. Biochars derived from various crop straws: Characterization and Cd(II) removal potential[J]. Ecotoxicology and Environmental Safety, 2014, 106: 226-231. doi: 10.1016/j.ecoenv.2014.04.042 [43] 周守勇, 赵宜江, 薛爱莲, 等. 盐泥吸附剂对直接染料的平衡吸附行为和热力学性质研究[J]. 环境工程学报, 2009, 3(8): 1414-1418. [44] HO S, CHEN Y, YANG Z, et al. High-efficiency removal of lead from wastewater by biochar derived from anaerobic digestion sludge[J]. Bioresource Technology, 2017, 246: 142-149. doi: 10.1016/j.biortech.2017.08.025 [45] DONG J, SHEN L, SHAN S, et al. Optimizing magnetic functionalization conditions for efficient preparation of magnetic biochar and adsorption of Pb(II) from aqueous solution[J]. Science of the Total Environment, 2022: 806151442. [46] 刘国成. 生物炭对水体和土壤环境中重金属铅的固持[D]. 青岛: 中国海洋大学, 2014. [47] 王棋, 王斌伟, 谈广才, 等. 生物炭对Cu(II)、Pb(II)、Ni(II)和Cd(II)的单一及竞争吸附研究[J]. 北京大学学报(自然科学版), 2017, 53(6): 1122-1132. [48] ZAHEDIFAR M, SEYEDI N, SHAFIEI S, et al. Surface-modified magnetic biochar: Highly efficient adsorbents for removal of Pb(II) and Cd(II)[J]. Materials Chemistry and Physics, 2021, 271: 124860. doi: 10.1016/j.matchemphys.2021.124860 [49] MOHAN D, SINGH P, SARSWAT A, et al. Lead sorptive removal using magnetic and nonmagnetic fast pyrolysis energy cane biochars[J]. Journal of Colloid and Interface Science, 2015, 448: 238-250. doi: 10.1016/j.jcis.2014.12.030 [50] KARUNANAYAKE A, TODD O, CROWLEY M, et al. Lead and cadmium remediation using magnetized and nonmagnetized biochar from Douglas fir[J]. Chemical Engineering Journal, 2018, 331: 480-491. doi: 10.1016/j.cej.2017.08.124 [51] WANG S, GUO W, GAO F, et al. Lead and uranium sorptive removal from aqueous solution using magnetic and nonmagnetic fast pyrolysis rice husk biochars[J]. RSC advances, 2018, 8(24): 13205-13217. doi: 10.1039/C7RA13540H [52] HAN Z, SANI B, MROZIK W, et al. Magnetite impregnation effects on the sorbent properties of activated carbons and biochars[J]. Water Research, 2015, 70: 394-403. doi: 10.1016/j.watres.2014.12.016 [53] JIA Y, ZHANG Y, FU J, et al. A novel magnetic biochar/MgFe-layered double hydroxides composite removing Pb2+ from aqueous solution: Isotherms, kinetics and thermodynamics[J]. Colloids and Surfaces A:Physicochemical and Engineering Aspects, 2019, 567: 278-287. [54] SUN C, CHEN T, HUANG Q, et al. Enhanced adsorption for Pb(II) and Cd(II) of magnetic rice husk biochar by KMnO4 modification[J]. Environmental Science and Pollution Research, 2019, 26(9): 8902-8913. doi: 10.1007/s11356-019-04321-z [55] CHEN T, QUAN X, JI Z, et al. Synthesis and characterization of a novel magnetic calcium-rich nanocomposite and its remediation behaviour for As(III) and Pb(II) co-contamination in aqueous systems[J]. Science of the Total Environment, 2020, 706: 135122. doi: 10.1016/j.scitotenv.2019.135122 [56] 曹玮, 周航, 邓贵友, 等. 改性谷壳生物炭负载磁性Fe去除废水中Pb2+的效果及机制[J]. 环境工程学报, 2017, 11(3): 1437-1444. doi: 10.12030/j.cjee.201511081 -



 下载:
下载: