-
目前,矿井废水的利用率不断提高,处置后的矿井废水多用于工业用水等,只有少部分能作为居民饮用水回用,其中最主要的制约因素之一就是氟离子含量超标[1]。氟是一种持久性和不可降解的有毒物质,饮用含有过量氟的饮用水,会对人体健康造成威胁[1]。长期饮用高氟水而导致的氟斑牙、氟骨症等蓄积性氟中毒是全球影响最广的地方病之一,氟过量摄入对儿童智商也有明显影响[2-3]。然而,最近的研究[1]表明,人体软组织也会受到氟离子影响,这种类型的氟中毒被称为非骨骼性氟中毒。已经发布的地下水质量标准(GB/T 14848-2017)和污水排放标准(GB 8978-1996)中均明确规定了氟离子和氟化物的排放质量浓度限值,即1.0 mg·L−1和10 mg·L−1。同时,地下水中也发现了各种不同浓度的金属和非金属离子,如铁离子、镁离子、钙离子、钠离子、氯离子、碳酸根离子等,相比于其他离子,氟离子过量被认为具有更大危害[4]。此外,氟甚至可以引起神经系统[5]、内分泌腺[6]、生殖系统、肾脏、肝脏[7]等器官的代谢、结构和功能损伤。氟中毒的数量惊人[1, 8],全球约有25个国家受到氟中毒的影响。氟化物污染在世界不同地区的地下水中被广泛报道,其中包括非洲、拉丁美洲和亚洲的潮湿热带地区[9]。鉴于氟化物对人体健康的毒性影响,迫切需要找到一种有效且稳健的技术来去除水环境中过量的氟化物[10]。
去除矿井废水中氟离子的主要方法有混凝沉淀法[11-12],离子交换法[13]、吸附法[14]、膜分离法[15]等。混凝沉淀法因具有效率高、操作简单、投资少等优点而受到普遍认可。混凝沉淀法是向水中投放具有凝聚能力的物质,形成大量胶体,通过沉淀将氟离子从水中去除的过程[16]。常规混凝剂有硫酸铝[17]、氯化铝[18]、硫酸亚铁[19]、硫酸钙[20]等。相对于其他混凝剂,聚合氯化铝(polyaluminum chloride,PAC)具有水解速度快、吸附能力强、巩花大、质密、沉淀快等优点而备受关注[21]。
响应曲面法(response surface methodology,RSM)是一种能够优化工艺参数、减少实验次数以及评估各种影响因素水平及交互作用的有效方法[22-26]。与传统的单因素法和正交实验法相比,RSM优势是,在实验条件优化过程中,可以连续地对实验各个水平进行分析,通过建立影响因素和响应值之间的多元二次回归方程来拟合二者之间的函数关系,克服了单因素法和正交实验法无法解释各因素之间的相互作用,以及无法给出因素和响应值之间的明确回归模型的缺陷[23-24]。
本研究依据RSM中的Box-Behnken Design(BBD)实验设计原理,对铝氟摩尔比(r)、pH和凝聚时间3个影响因素对氟离子的水平影响及其之间的交互作用进行探究,明确去除氟离子的最佳条件参数,旨在为含氟矿井水的处理提供新的技术方案和科学依据。
-
实验试剂:氟化钠(NaF)、硝酸钠(NaNO3)购于国药集团化学试剂有限公司;无水柠檬酸钠(C6H5Na3O7)、聚合氯化铝(PAC)均为分析纯并购于上海阿拉丁生化科技股份有限公司;实验水样来自某煤矿矿井处理工艺出水。
实验仪器:搅拌机(84-1A6S);氟离子计(PXSJ-216F);pH计(PB-10);分光光度计(DR6000);分析天平(AL104);Milli-Q超纯水系统。
-
除氟实验方法:将1 L的含氟废水放置在六联搅拌机上,将铝氟摩尔比r调整为10~20,将凝聚时间设置为1~3 min,将废水的pH调节为6.0~7.0,用氟离子选择电极测试上清液中氟离子质量浓度。
氟离子分析方法:氟离子质量浓度分析采用《水质 氟化物的测定 离子选择电极法》(GB 7484-1987)中的方法。采用氟离子计测量质量浓度,测量区间0.05~1 900 mg·L−1。将待测溶液置于塑料烧杯内,插入电极并放入转子,慢速搅拌,待数值稳定后,记录示数。
-
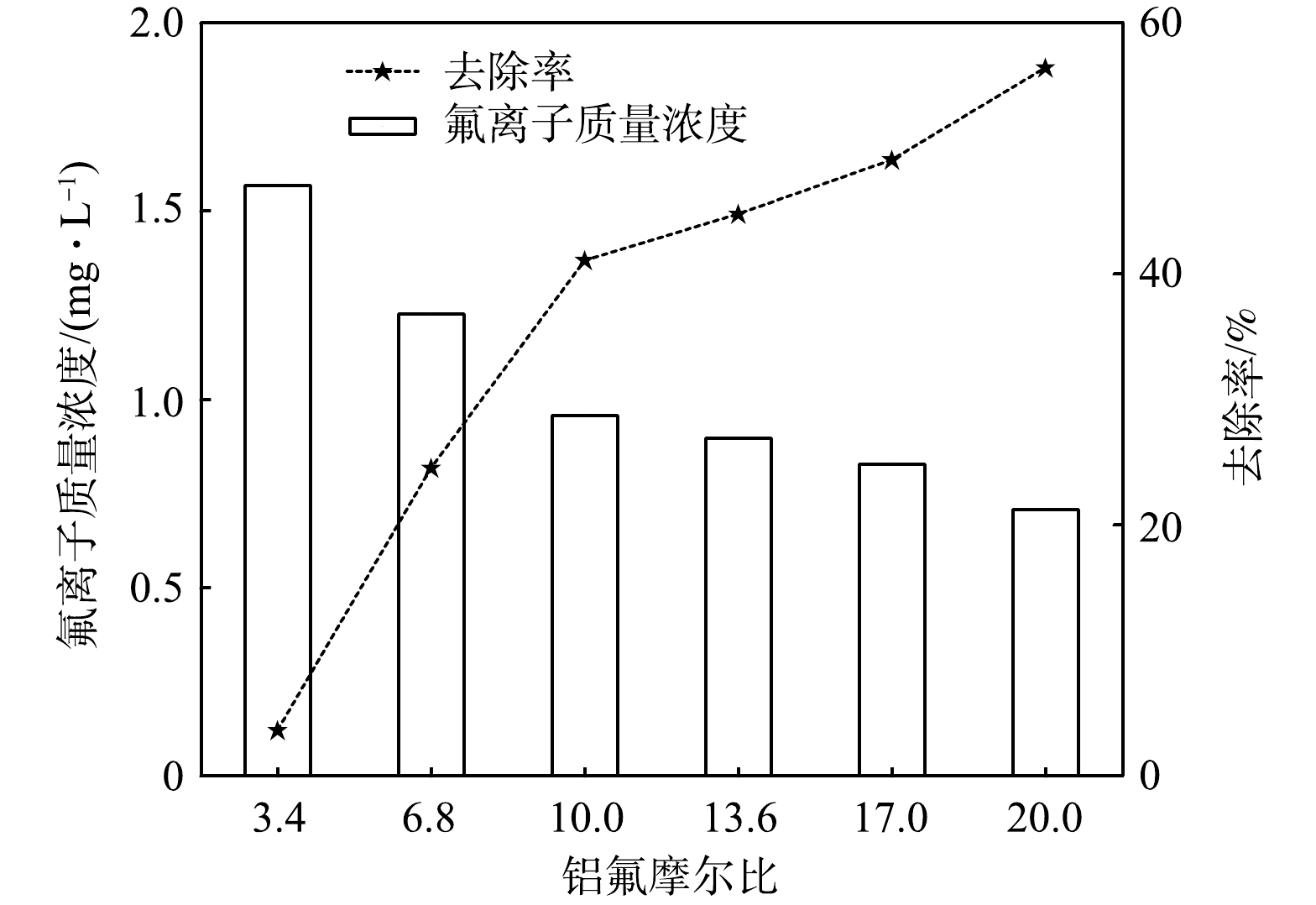
向1 L氟离子质量浓度为1.63 mg·L−1矿井排放废水中分别投加r为3.4、6.8、10、13.6、17、20的5% PAC,混凝反应结束后,记录氟离子电位,根据氟离子标准曲线计算氟离子质量浓度。实验水样pH维持在6.5左右,实验结果见图1。
由图1可知,随着r的逐渐增加,矿井排放废水中氟离子质量浓度逐渐下降,氟离子的去除率逐渐上升。r由3.4增至20时,矿井排放废水中氟离子质量浓度逐渐减小;r由3.4增至10时,矿井排放废水中氟离子质量浓度低于1 mg·L−1,达到排放标准。然而r不能一直增加,一方面由于投加浓度过高时,沉淀矾花较大且容易造成上翻、余浊高等负面作用;另一方面,投加浓度过高也会造成经济损失和资源浪费[27-29]。因此,响应曲面法中r应为10、15、20。
-
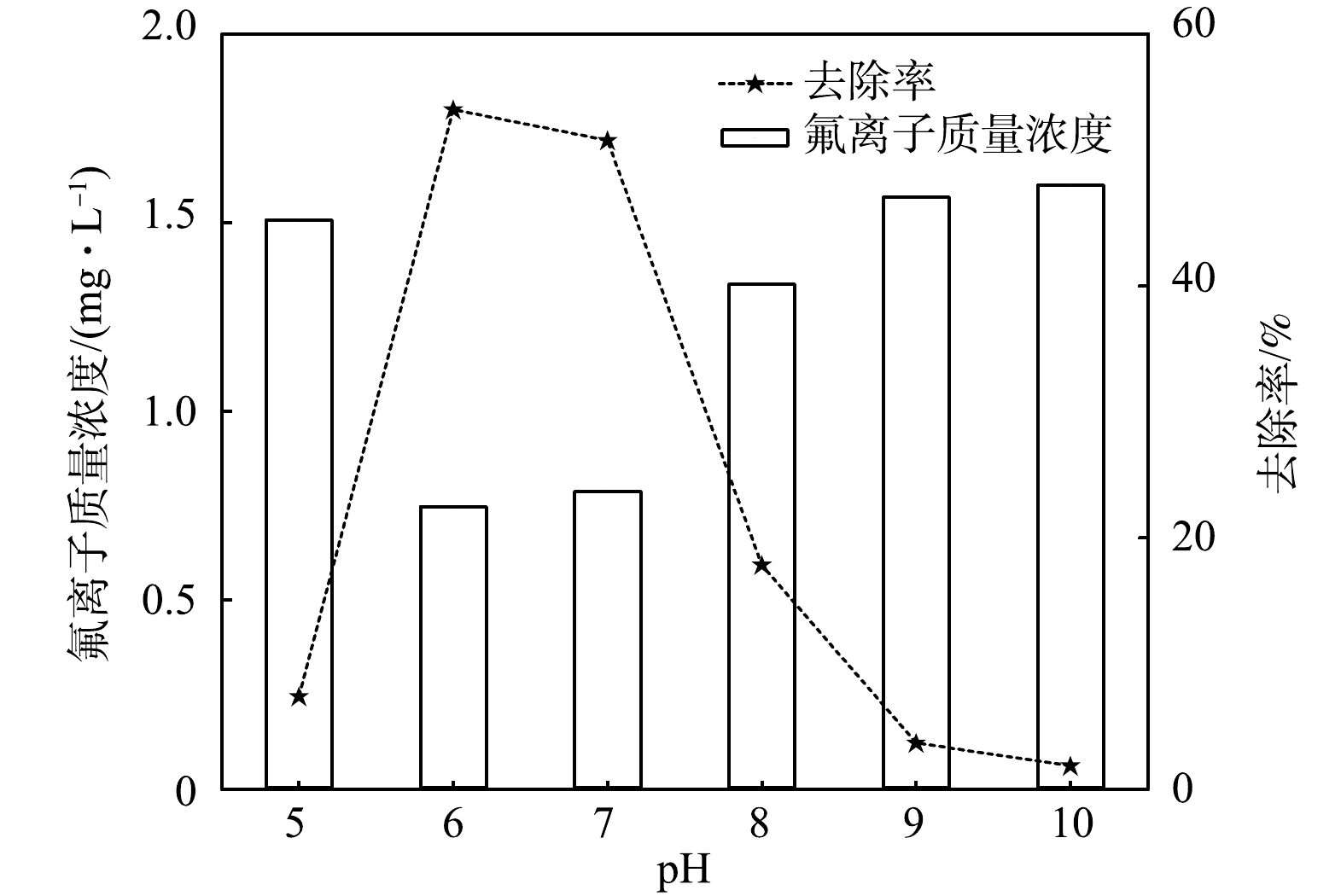
pH对混凝效果有较大影响,pH会影响混凝剂的水解速度、水解产物的存在形态以及性能。实验采用矿井排放出口废水,r为20,调节pH为5.0~10.0,沉淀完成之后,记录氟离子电位,根据氟离子标准曲线测定氟离子质量浓度并计算其去除率。实验结果见图2。
由图2可知,PAC去除矿井排放废水中氟离子的pH最佳区间为6.0~7.0,PAC水解效果较好,水解产物对实际水样中氟离子的吸附较充分。pH为6.0~7.0时,氟离子去除率呈现出先升高,后下降的趋势,pH在此区间平均去除率达到最高。溶液的pH会影响PAC的水解程度、水解产物形态,当pH低于4.0时,Al3+不能水解成Al(OH)3,主要以Al3+离子形式存在,混凝效果较差;pH为6.0~7.0时,Al3+水解聚合成聚合程度较大Al(OH)3中性胶体,混凝效果较好;pH大于8.0时,Al3+水解成AlO2−,混凝效果下降,因此,控制混凝过程pH十分必要。
-
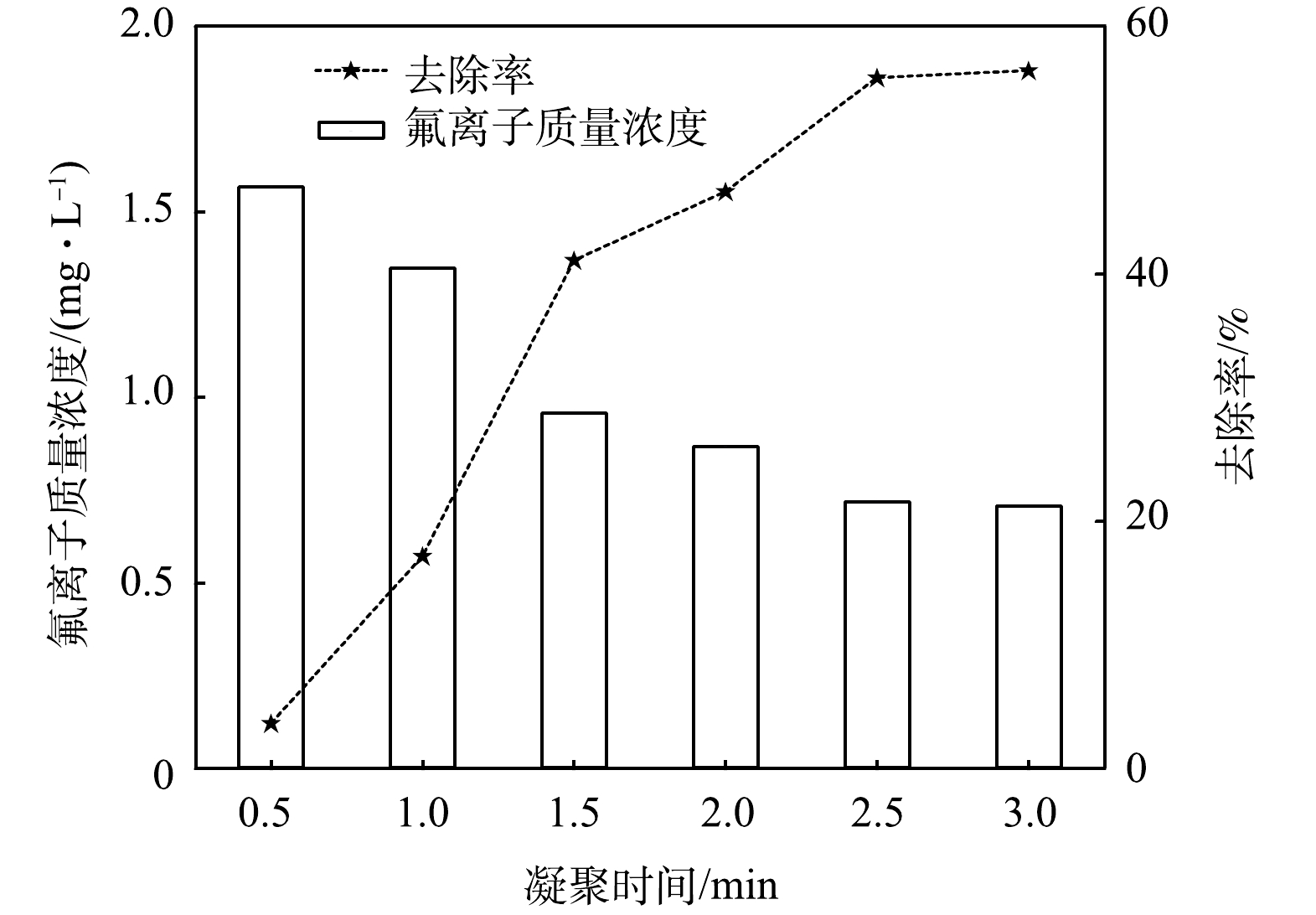
凝聚时间对混凝效果有一定影响,已有研究[30]表明,正常的凝聚时间持续2 min左右,并且伴随着水体逐渐浑浊。实验从投加PAC后开始计时,每隔10 s观察烧杯实验现象。调节凝聚时间分别为0.5、1、1.5、2、2.5和3 min,沉淀完成之后,记录氟离子电位,根据氟离子标准曲线测定氟离子质量浓度并计算其去除率。实验结果见图3。
由图3可以看出,在凝聚1 min后,观察到烧杯中出现微细矾花;在凝聚2 min时,矾花达到最密集,氟离子去除率均在40%以上;在凝聚2.5 min后,氟离子去除率基本维持水平;在凝聚3 min后,开始进行絮凝阶段。为节省时间,响应曲面法中凝聚时间应为1、2和3 min。
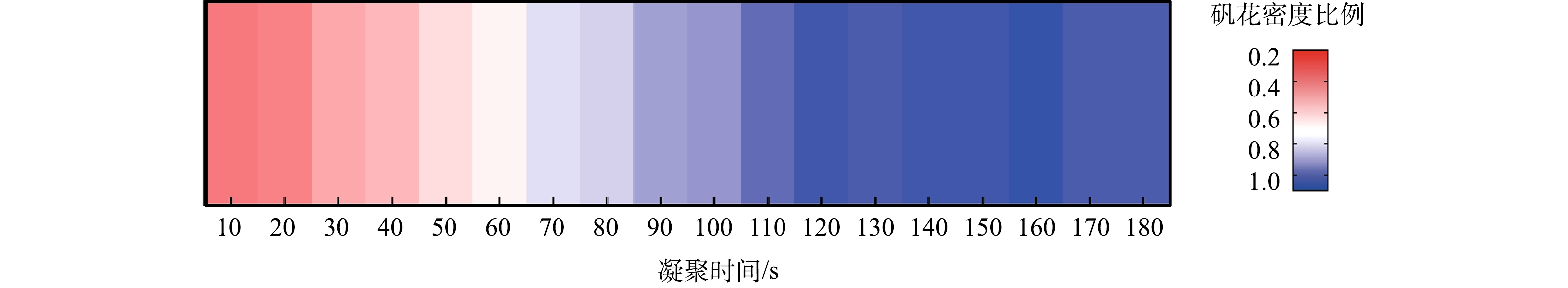
在凝聚过程中,实验从投加PAC后开始计时,每隔10 s观察烧杯中矾花密集程度。凝聚时间达到1 min,观察到烧杯中出现微细矾花,2 min矾花达到最密集,3 min后开始进行絮凝阶段(图4)。
-
根据Box-Behnken设计的原理,选择r(A)、pH(B)、凝聚时间(C) 3个因素作为响应曲面模型设计的考察变量,以氟离子去除率(Y)为指标进行3因素3水平响应曲面分析(表1)。
-
将各因素及水平值一次输入系统,则生成实验方案表。按照该方案进行实验,记录每组因素组合的实验结果,并将实验结果填入对应表格。采用Design-Expert软件对实验结果进行分析,首先点击Analysis按钮,对线性函数,2FI模型、二阶模型、三阶模型进行显著性检验,并对模型显著性、失拟项、相关性的数据进行对比,依据对比结果,推荐适合的模型[31]。根据实验结果,推荐采用二阶模型。然后对选择的模型进行方差分析以及显著性检验。使用方差分析对影响二次方程模型的常数项、一次项、二次项、平方项的显著性进行检验。
根据表2的实验结果,使用Design-Expert软件进行模拟分析,得到矿井排放废水氟离子质量浓度与影响因子有关的二次响应回归方程(式(1))。
该模型可以预测自变量与响应变量(Y)之间的关系,并采用Fisher统计检验进行方差分析(ANOVA),以获得变量与响应之间的交互作用,评估“拟合优度”质量。拟合模型的质量用决定系数R2和调整后的R2表示,在同一程序中采用Fisher的F检验统计显著性[24]。模拟项的选择或拒绝是基于P值(概率)的95%置信水平。对方程进行方差分析和信度分析结果,见表3。
二次响应面回归模型可信度分析结果表明,决定系数为0.994 3,调整后的决定系数为0.984 0,预测决定系数为0.940 8,信噪比为34.043 4,标准差为1.40,变异系数为3.46%。二阶回归方程模型F=96.42(>1),P<0.000 1,信噪比为34.043 4(>4),表明模型拟合准确。失拟项P值表示模型与实际实验差异程度,本模型失拟项P值为0.549 7(>0.05),失拟项差异性不显著[28]。该方程的决定系数R2=0.994 3,变异系数为3.46%(<10%),表明模拟与实际拟合较好,拟合的可信度和精密度较高。
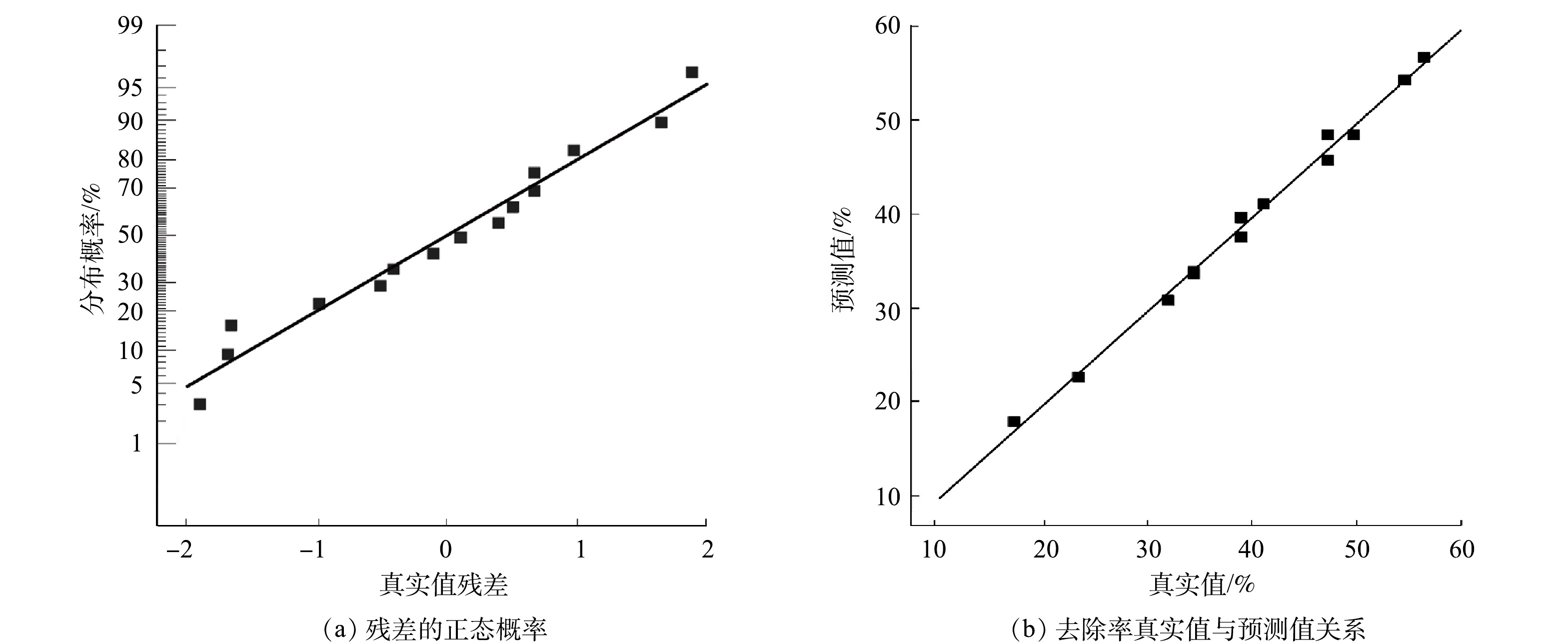
由图5可以看出,预测值与真实值的偏差较小,得到的实验数据服从正态分布,真实值的残差总体上较小。因此,二次响应回归模型具有较好的拟合度[29]。氟离子质量浓度的真实值基本分布在直线上或靠近两侧,说明真实值与预测值有较高的拟合度。证明了拟合二次响应回归模型的可靠性[29]。
-
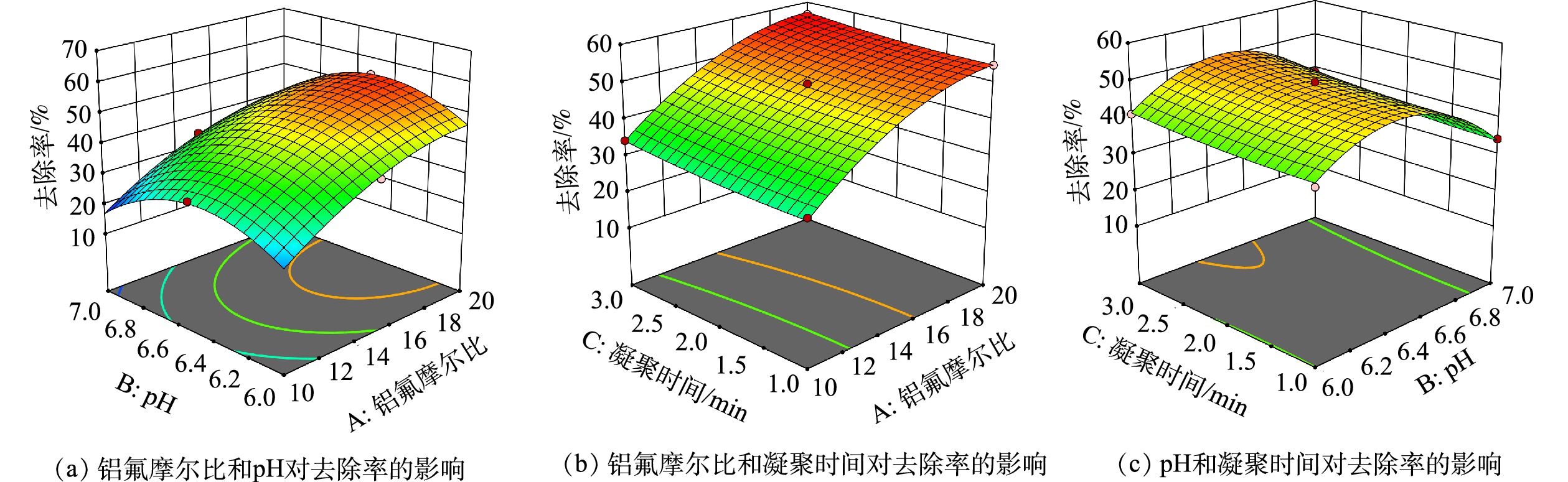
响应曲面法克服了正交实验不能给出直观图形的缺陷[27, 32]。根据二次方程模型,分别做出实验因素间交互作用的三维立体响应曲面图,考察某个因素固定在中心值不变的情况下,其他2个因素的交互作用对氟离子去除率的影响,结果见图6。由图6(a)可看出,r和pH的交互作用较明显。pH为6.0~7.0时,氟离子去除率呈现出先升高,后下降的趋势,pH在6.5附近去除率达到最高。由图6(b)可以看出,r和凝聚时间的交互作用不明显,整体呈较平缓状态,凝聚时间为1~3 min时,对氟离子去除率影响较小。随着r的增加,氟离子去除率逐渐增加。由图6(c)可以看出,pH和凝聚时间的交互作用不明显。同样,pH为6.0~7.0时,氟离子去除率呈先升高后下降的趋势,pH在6.5左右去除率达到最高。凝聚时间为1~3 min时,曲面平缓,说明凝聚时间对氟离子去除率影响较小。
-
模型预测的最佳实验条件:r=19.04,pH为6.5,凝聚时间为2.90 min。根据模型预测进行验证实验,结果见表4。
在模型预测的最佳实验条件下,模拟实验平均去除率为56.44%,而理论去除率预测值为56.46%。由此可知,实验值与预测值的误差为0.02%,表明采用此二次响应曲面预测优化得到的最佳实验条件可靠。
-
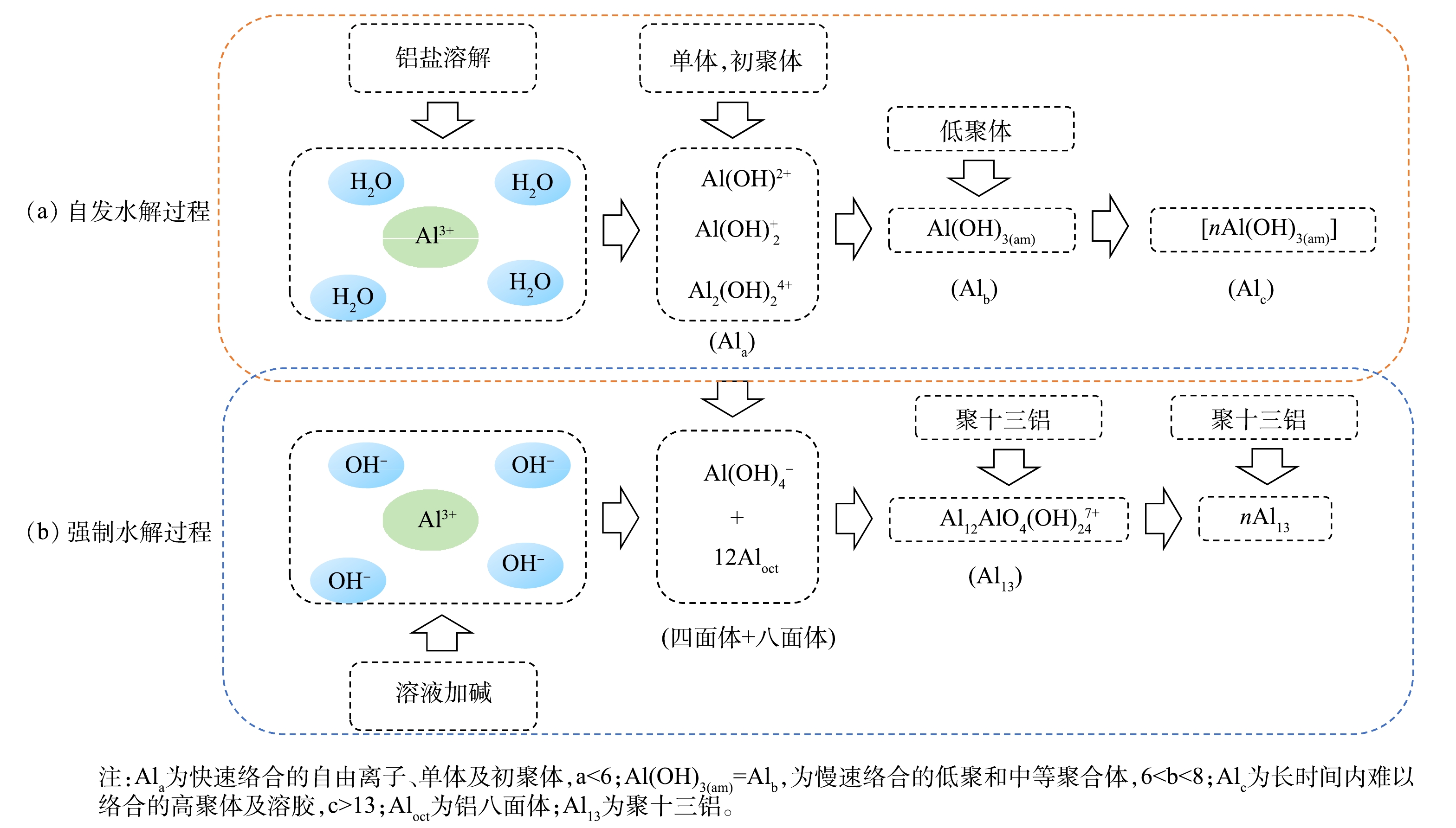
PAC作为最常用的无机高分子絮凝剂,很多研究者[33-35]对其混凝机理做了探究。通常认为,PAC是一种含有羟基的聚合物,有很强的界面吸附能力,是库仑力、分子间范德华力、憎水斥力以及羟基与表面的键合力等多种因素综合作用的结果。静电库仑力、分子间范德华力、憎水斥力随着聚合物电荷及分子质量的增大而增大,使水中PAC最大限度接近颗粒表面,大大提高了混凝效率。Al3+在溶液中水解,产生羟基多核络合物,水解过程见式(2)。
Al3+的各种水解状态就是铝盐的水解-聚合-沉淀的动力学过程。其形态和结构特征主要取决于水解反应中的2种转化过程:一种是铝盐在水中的自然溶解及水解过程,即自发水解;另一种则是铝盐溶液中加入强碱导致pH升高而强烈水解的过程,即强制水解。2种转化路径[33]见图7。
在PAC絮凝沉淀去除氟离子过程中,PAC投加到水中,利用Al3+与氟离子的络合以及铝盐水解中间产物和最后生成的Al(OH)3(am)矾花,对氟离子进行离子吸附、离子交换、络合作用,从而达到去除水中的氟离子的目的。在离子吸附过程中,一方面,含氟絮体吸附了带负电荷的氟离子,正电荷被部分中和;另一方面,当水中阴离子的浓度较高时,使絮凝过程中形成的Al(OH)3(am)矾花对氟离子的吸附容量显著减少,原因在于,在PAC混凝去除氟离子过程中,生成了具有很大比表面积的Al(OH)3(am)絮体,对氟离子产生氢键吸附,氟离子半径小,电负性强,使得这一吸附方式容易发生,由此说明PAC去除氟离子过程中离子吸附是一重要的作用方式。氟离子与氢氧根离子半径和电荷都较为接近,PAC投加到水中生成的Al12AlO4(OH)247+以及Al(OH)3(am)絮体的氢氧根离子均可以与氟离子发生交换,反应过程见方程式(3)和式(4)。
由此可见,这一过程会使体系的pH升高,说明离子交换也是铝盐除氟的一种重要作用方式。在PAC去除氟离子过程中,络合沉淀也起到一定作用,氟离子能与Al3+形成AlF2+、AlF2+、AlF3、AlF4−、AlF52−、AlF63−6种络合物。在PAC混凝去除氟离子体系中,这些铝氟络合离子在絮凝过程中会形成铝氟络合物或夹杂在新形成的Al(OH)3(am)絮体中沉降下来。
-
1) BBD优化法模型预测与实际处理效果基本一致(R2=0.99)。根据模型预测结果,处理含氟离子矿井废水最优处理工艺参数:r为19.04,pH为6.5,凝聚时间为2.90 min,氟离子出水达到排放标准。
2) PAC去除矿井废水中氟离子的影响因素显著性大小顺序为r > pH > 凝聚时间,其中r和pH是主要控制因素。
3)在实际矿井废水中,弱酸条件有利于PAC通过氟离子絮凝、离子交换和络合沉降等多种方式的综合作用去除氟离子。
基于响应曲面法的聚合氯化铝对矿井废水中氟离子混凝去除工艺优化
Optimization of coagulation removal of fluoride ions from mine wastewater by polyaluminum chloride based on response surface methodology
-
摘要: 为有效去除矿井废水中氟离子,利用聚合氯化铝(PAC)对某矿井含氟废水进行混凝效果研究,设计单因素实验,研究了铝氟摩尔比(r)、pH和凝聚时间等因素对PAC混凝去除氟离子的影响,依据响应曲面法的Box-Behnken Design(BBD)实验设计原理,探究了r、pH和凝聚时间对混凝效果的影响,并优化工艺参数。结果表明:各因素对混凝效果的影响顺序为r > pH > 凝聚时间;在r=19.04、pH=6.5、凝聚时间为2.9 min的最佳条件下,氟离子的去除率为56.4%,与预测值(56.46%)基本吻合;去除氟离子的机理包括PAC对氟离子絮凝沉淀、离子交换和络合沉降等;pH影响PAC在溶液中的存在状态,凝聚时间则影响矾花在溶液中形成的速度以及密集程度,进而影响混凝沉淀效果。由此可以看出,BBD优化模型预测与实际处理效果基本一致,铝氟摩尔比和pH是去除氟离子的主要控制因素。本研究使用的实验方法具有处理工艺简单、效果稳定、成本低等优点,可为实际矿井废水中氟离子的去除提供参考。
-
关键词:
- 聚合氯化铝(PAC) /
- 混凝 /
- 矿井废水 /
- 氟离子
Abstract: In order to effectively remove fluoride ions from mine wastewater, the coagulation effect of polymerized aluminum chloride (PAC) on a mine fluoride wastewater was studied, a single-factor experiment was designed to investigate the effects of aluminum-fluoride molar ratio (r), pH and coagulation time on the removal of fluoride ions by PAC coagulation. Based on the Box-Behnken Design (BBD) experimental design principle of response surface method, the effects of r, pH and coagulation time on the coagulation effect were investigated, and the process parameters were optimized. The results showed that the coagulation effect was orderly influenced by r>pH>coagulation time. Under the optimal conditions of r=19.04, pH=6.5 and coagulation time of 2.9 min, the removal rate of fluoride ions was 56.4%, which was consistent with the predicted value (56.46%). The mechanism of fluoride ions removal included flocculation and precipitation of fluoride ions by PAC, ion exchange and complexation precipitation, etc.. pH affected the speciation of PAC in the solution, and the coagulation time affected the formation rate and compactness of alum flocs in the solution, which further affected the coagulation and precipitation effect. It can be seen that the prediction of BBD optimization model was basically consistent with the actual treatment effect, and the molar ratio of aluminum to fluorine and pH were the main controlling factors for fluoride ions removal. The experimental method used in this study has the advantages of simple treatment process, stable effect and low cost, which can provide a reference for the removal of fluoride ions in actual mine wastewater.-
Key words:
- polymeric aluminum chloride(PAC) /
- coagulation /
- mine wastewater /
- fluoride
-

-
表 1 响应面实验设计
Table 1. Response surface test design
水平 因素 (A)铝氟摩尔比 (B)pH (C)凝聚时间/min −1 10 6 1 0 15 6.5 2 +1 20 7 3 表 2 响应面实验设计和结果
Table 2. Response surface test design and results
实验号 (A)铝氟摩尔比 (B)pH (C)凝聚时间 去除率/% 1 15 6.0 3 41.10 2 20 7.0 2 41.10 3 20 6.5 3 56.44 4 15 7.0 3 38.95 5 15 6.5 2 49.70 6 15 7.0 1 34.36 7 10 7.0 2 17.18 8 20 6.0 2 47.24 9 15 6.0 1 38.95 9
1015
206.0
6.51
138.95
54.6011 15 6.5 2 47.24 12 10 6.5 3 34.36 13 15 6.5 2 49.70 14 10 6.0 2 23.31 15 10 6.5 1 31.90 表 3 回归方程的方差分析与回归模型的可信度分析表
Table 3. Analysis of variance of regression equations and credibility of regression model
方差来源 平方和 自由度 均方 F值 P值 模型 1 697.33 9 188.59 96.42 <0.000 1 (A)铝氟摩尔比 1 072.54 1 1 072.54 548.37 <0.000 1 (B)pH 45.17 1 45.17 23.10 0.004 9 (C)凝聚时间 15.24 1 15.24 7.79 0.038 4 AB 0.000 0 1 0.000 0 0.000 0 0.997 3 AC 0.096 1 1 0.096 1 0.049 1 0.833 3 BC 1.49 1 1.49 0.761 0 0.422 9 A2 105.44 1 105.44 53.91 0.000 7 B2 473.87 1 473.87 242.28 <0.000 1 C2 2.30 1 2.30 1.17 0.328 0 残差 9.78 5 1.96 失拟项 5.74 3 1.91 0.949 3 0.549 7 误差 4.03 2 2.02 总离差 1 707.11 14 表 4 二次响应面回归模型预测值验证
Table 4. Verification of the predicted value of the quadratic response surface regression model
铝氟摩尔比(r) pH 凝聚时间/min 实际去除率/% 19.04 6.50 2.90 56.44 19.04 6.50 2.90 56.44 19.04 6.50 2.90 56.44 -
[1] HU C Y, LO S L, KUAN W H, et al. Removal of fluoride from semiconductor wastewater by electrocoagulation-flotation[J]. Water Research, 2005, 39(5): 895-901. doi: 10.1016/j.watres.2004.11.034 [2] WAN K, HUANG L, YAN J, et al. Removal of fluoride from industrial wastewater by using different adsorbents: A review[J]. Science of the Total Environment, 2021, 773: 145535. doi: 10.1016/j.scitotenv.2021.145535 [3] CASTAÑEDA L F, RODRÍGUEZ J F, NAVA J L. Electrocoagulation as an affordable technology for decontamination of drinking water containing fluoride: A critical review[J]. Chemical Engineering Journal, 2021, 413: 127529. doi: 10.1016/j.cej.2020.127529 [4] DAS D, NANDI B K. Simultaneous removal of fluoride and Fe(II) ions from drinking water by electrocoagulation[J]. Journal of Environmental Chemical Engineering, 2020, 8(1): 103643. doi: 10.1016/j.jece.2019.103643 [5] VALDEZ-JIMÉNEZ L, SORIA FREGOZO C, MIRANDA BELTRÁN M L, et al. Efectos del flúor sobre el sistema nervioso central[J]. Neurología, 2011, 26(5): 297-300. [6] GARCÍA-MONTALVO E A, REYES-PÉREZ H, DEL RAZO L M. Fluoride exposure impairs glucose tolerance via decreased insulin expression and oxidative stress[J]. Toxicology, 2009, 263(2): 75-83. [7] CHATTOPADHYAY A, PODDER S, AGARWAL S, et al. Fluoride-induced histopathology and synthesis of stress protein in liver and kidney of mice[J]. Archives of Toxicology, 2011, 85(4): 327-335. doi: 10.1007/s00204-010-0588-7 [8] HUANG L, LUO Z, HUANG X, et al. Applications of biomass-based materials to remove fluoride from wastewater: A review[J]. Chemosphere, 2022, 301: 134679. doi: 10.1016/j.chemosphere.2022.134679 [9] VITHANAGE M, BHATTACHARYA P. Fluoride in the environment: sources, distribution and defluoridation[J]. Environmental Chemistry Letters, 2015, 13(2): 131-147. doi: 10.1007/s10311-015-0496-4 [10] HE J, YANG Y, WU Z, et al. Review of fluoride removal from water environment by adsorption[J]. Journal of Environmental Chemical Engineering, 2020, 8(6). [11] LIN J Y, MAHASTI N N N, HUANG Y H. Recent advances in adsorption and coagulation for boron removal from wastewater: A comprehensive review[J]. Journal of Hazardous Materials, 2021, 407: 124401. doi: 10.1016/j.jhazmat.2020.124401 [12] 王兵, 王佩洁, 祝伟, 等. 混凝-吸附联用预处理页岩气压裂返排液[J]. 环境工程学报, 2019, 13(10): 2475-2481. [13] KOILRAJ P, KANNAN S. Aqueous fluoride removal using ZnCr layered double hydroxides and their polymeric composites: Batch and column studies[J]. Chemical Engineering Journal, 2013, 234: 406-415. doi: 10.1016/j.cej.2013.08.101 [14] 刘敏. 羟基磷灰石去除煤矿矿井水氟化物工艺研究及参数优化[J]. 煤化工, 2021, 49(1): 80-85. [15] 刘锐平. 饮用水氟污染控制原理与技术[J]. 应用生态学报, 2019, 30(1): 30-36. [16] 马宏涛, 孙水裕, 许明鑫. 臭氧联合混凝沉淀法去除浮选废水中有机磷[J]. 环境工程学报, 2017, 11(1): 285-290. [17] LEE W, WESTERHOFF P. Dissolved organic nitrogen removal during water treatment by aluminum sulfate and cationic polymer coagulation[J]. Water Research, 2006, 40(20): 3767-3774. doi: 10.1016/j.watres.2006.08.008 [18] LIU Z, ZHOU L, LIU F, et al. Impact of Al-based coagulants on the formation of aerobic granules: Comparison between poly aluminum chloride (PAC) and aluminum sulfate (AS)[J]. Science of the Total Environment, 2019, 685: 74-84. doi: 10.1016/j.scitotenv.2019.05.306 [19] GEORGIOU D, AIVAZIDIS A, HATIRAS J, et al. Treatment of cotton textile wastewater using lime and ferrous sulfate[J]. Water Research, 2003, 37(9): 2248-2250. doi: 10.1016/S0043-1354(02)00481-5 [20] ZHANG J, JIN J, GUO B, et al. Effect of mixed basalt fiber and calcium sulfate whisker on chloride permeability of concrete[J]. Journal of Building Engineering, 2023, 64: 105633. doi: 10.1016/j.jobe.2022.105633 [21] 刘皓月, 王磊, 吕永涛, 等. 微生物絮凝剂与聚合氯化铝复配处理污水厂二级出水[J]. 环境工程学报, 2017, 11(1): 111-115. [22] 彭赵旭, 牛宁琪, 王炬, 等. 响应面法优化石灰处理高氟废水的研究[J]. 河南师范大学学报(自然科学版), 2022, 50(1): 108-114. [23] LEE E Y, WONG S Y, PHANG S J, et al. Additively manufactured photoreactor with immobilized thermoset acrylic-graphitic carbon nitride nanosheets for water remediation: Response surface methods and adsorption modelling studies[J]. Chemical Engineering Journal, 2022, 455: 140633. [24] TOOR U A, DUONG T T, KO S Y, et al. Optimization of Fenton process for removing TOC and color from swine wastewater using response surface method (RSM)[J]. Journal of Environmental Management, 2021, 279: 111625. doi: 10.1016/j.jenvman.2020.111625 [25] 严博文, 叶长文, 龚锐, 等. 响应曲面分析优化改性粉煤灰漂珠对水中氟的吸附性能及机理研究[J]. 环境科学研究, 2019, 32(4): 709-717. [26] ZHU X, TU X, MEI D, et al. Investigation of hybrid plasma-catalytic removal of acetone over CuO/γ-Al2O3 catalysts using response surface method[J]. Chemosphere, 2016, 155: 9-17. doi: 10.1016/j.chemosphere.2016.03.114 [27] KONG Y, ZHANG Z, PENG Y. Multi-objective optimization of ultrasonic algae removal technology by using response surface method and non-dominated sorting genetic algorithm-II[J]. Ecotoxicology and Environmental Safety, 2022, 230: 113151. doi: 10.1016/j.ecoenv.2021.113151 [28] MAO J, GUANHUA N, YUHANG X, et al. Modeling and optimization of mechanical properties of drilling sealing materials based on response surface method[J]. Journal of Cleaner Production, 2022, 377: 134452. doi: 10.1016/j.jclepro.2022.134452 [29] OFGEA N M, TURA A M, FANTA G M. Activated carbon from H3PO4 activated Moringa Stenopetale Seed Husk for removal of methylene blue: Optimization using the response surface method (RSM)[J]. Environmental and Sustainability Indicators, 2022, 16: 100214. doi: 10.1016/j.indic.2022.100214 [30] 周鑫, 孙海龙, 张泽乾. 响应面法在污水处理工艺优化中的应用[J]. 化学研究与应用, 2017, 29(6): 753-760. [31] LUO Y, GUO P, GAO J, et al. Application of design-expert response surface methodology for the prediction of rejuvenated asphalt fatigue life[J]. Journal of Cleaner Production, 2022, 379: 134427. doi: 10.1016/j.jclepro.2022.134427 [32] LIU Y, WANG X J, ZHOU S, et al. Enhancing public building energy efficiency using the response surface method: An optimal design approach[J]. Environmental Impact Assessment Review, 2021, 87: 106548. doi: 10.1016/j.eiar.2020.106548 [33] 汤鸿霄. 羟基聚合氯化铝的絮凝形态学[J]. 环境科学学报, 1998, 18(1): 3-12. [34] 唐婉莹, 翟宇峰, 王连军, 等. 聚合氯化铝絮凝机理探讨[J]. 南京理工大学学报, 1997, 21(4): 41-44. [35] 张忠国, 栾兆坤, 赵颖, 等. 聚合氯化铝(PACl)混凝絮体的破碎与恢复[J]. 环境科学, 2007, 28(2): 346-351. -



 下载:
下载: