-
近年来,随着人类工农业的发展,氮肥的过度使用以及化石燃料燃烧和工业污水排放,水体中硝酸盐污染日益严重[1-3]。水中高浓度硝酸盐会导致水体富营养化,破坏水生态环境,并危害人类健康[4-5]。目前已有物理、化学、生物等方法用于去除水体中的硝酸盐[6-7],但大多存在效率低、浓水需再处置、受外部环境影响大、产生有机残留物等问题。电化学还原法是一种新型高浓度硝酸盐有效去除方法[8-9],不仅可以去除水中的有机污染物,还可以将水中高浓度的硝酸盐转化为氮气或更具经济价值的氨[10-11]。此外,电还原所需的电能可以来自风能和太阳能等清洁能源,可以潜在地减少能源使用和碳足迹,并实现资源的循环利用[12-13]。
然而,硝酸盐电还原是一个复杂的多电子转移过程,涉及氮价态为+5价至-3价的多种含氮物质,其反应效率与选择性受到8电子转移过程和竞争性析氢反应(hydrogen evolution reaction, HER)的阻碍[14-15]。因此,迫切需要开发高效且高选择性的电极材料以及电场、流场相协同的电化学反应器[16-17],以提高传质效率、电荷效率和产物转化率。铜作为一种低成本的过渡金属,其电子结构可调,具有灵活的电化学活性,相比许多贵金属,铜有更好的催化硝酸盐转氨性能。因此,铜基电极在硝酸盐电还原方面得到了广泛研究[18-21]。然而,纯铜催化剂容易因为还原过程中中间产物的强吸附而快速失活。有研究[22-24]表明,将铜与贵金属或其他过渡金属合金化,可调节质子和电子的转移以及吸附在铜中心上的部分还原中间体的吸附强度,从而能减缓这些限制。
本研究通过构建电场与流场相协同的穿透式电化学反应器,以溶液穿流通过电极的方式提高电极和电解质溶液的接触面积,构造高度凝缩的传质和反应空间,强化电子传递;同时通过电化学还原制备穿透式镍铜二元金属纳米线电极,提高催化位点密度,调节中间产物吸附,提升催化效率。最后,对该体系硝酸盐电化学还原效率和体系的运行稳定性进行了评价。
-
泡沫铜(99.9%)、质子交换膜(杜邦Nafion 117)、丙酮(C3H6O)、盐酸(HCl)、过硫酸铵((NH4)2S2O8,≥98%)、硝酸钾(KNO3,99.9%)、六水合氯化镍(NiCl2·6H2O,99.99%)、氢氧化钾(KOH,75%)、氨基磺酸、磷酸、4-氨基苯磺酰胺、N-(1-萘基)-乙二胺二盐酸盐、纳氏试剂、酒石酸钾钠(99%)、5,5-二甲基-1-吡咯啉-N-氧化物(DMPO),以上试剂均为分析纯。
-
首先,依次用丙酮、3 mol·L−1 HCl、超纯水清洗直径为2 cm厚度为3 mm的泡沫Cu以去除表面的油脂和氧化层。接下来,将洗涤过的泡沫Cu在25 mL 1 mol·L−1 NaOH和25 mL (NH4)2S2O8中浸泡1 h,将铜转化为氢氧化铜纳米线(Cu(OH)2 NW),然后用超纯水清洗Cu(OH)2 NW,干燥后在10 mmol·L−1 NiCl2溶液中浸泡12 h进行阳离子交换。此后,产物在烘箱中70 ℃干燥1 h,然后在200 ℃的流动Ar气氛中退火2 h。最后进行原位电化学预还原,使用计时电位法在100 mA·cm−2条件下处理12 h,最终得到Ni-Cu NW电极。采用相同的方法合成未负载Ni元素的Cu NW作为对照。
-
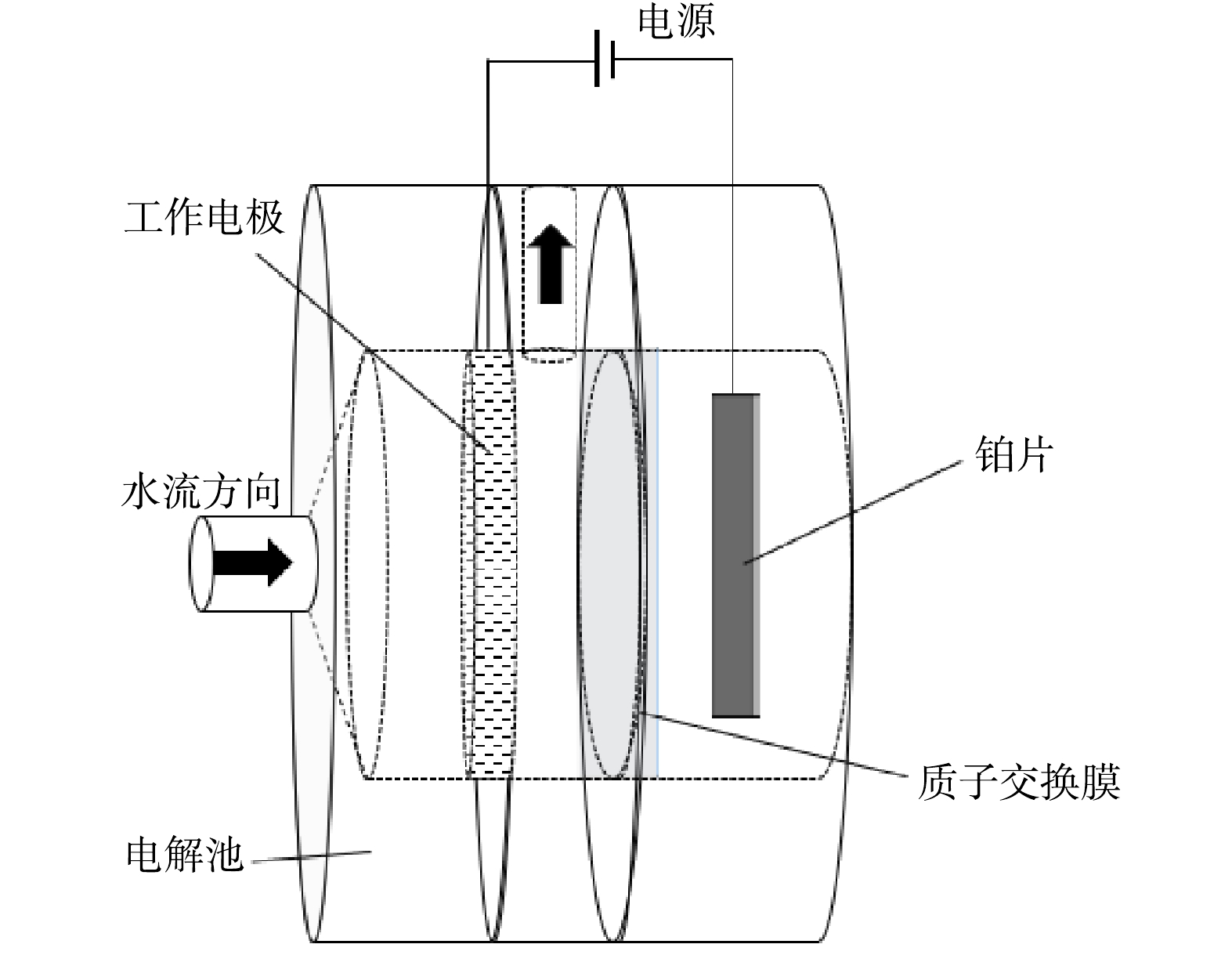
所有电化学还原NO3−-N实验均在定制的可穿透式电解池(图1)中进行,并使用质子交换膜(杜邦Nafion 117)将阴极室和阳极室分隔。在阳极室和阴极室分别加入25 mL的电解质溶液,电解质溶液包含支持电解质(1 mol·L−1 KOH)和反应物(1 400 mg·L−1 NO3−-N)。电化学测试在三电极体系中进行,以铂片(2 cm×2 cm)和填充饱和KCl的Ag/AgCl电极作为对电极和参比电极(ERHE=EAg/AgCl+0.059 1pH+0.19) ,分别用Cu、Cu NW、Ni-Cu NW电极作为工作电极。
-
通过紫外-可见分光光度计(U-3900,日立公司)检测水中的氮素的浓度变化。硝酸盐氮含量采用标准紫外-可见分光光度法测量,收集电解质溶液稀释至可检测范围,加入1 mol·L−1 HCl和0.8%氨基磺酸溶液,在室温下静置20 min后,记录220 nm和275 nm的吸光度。根据A=A220nm-2A275nm计算最终的吸光度,根据吸光度和标准曲线计算硝酸盐氮的浓度。亚硝酸盐氮含量采用标准紫外-可见分光光度法测量,以磷酸、4-氨基苯磺酰胺、N-(1-萘基)-乙二胺二盐酸盐混合溶液作为显色剂,收集电解质溶液稀释至可检测范围,加入显色剂,在室温静置20 min后,记录540 nm的吸光度,根据吸光度和标准曲线计算亚硝酸盐氮浓度。氨氮含量采用纳氏试剂分光光度法测量,加入酒石酸钾钠溶液后,再加入纳氏试剂,在室温静置10 min后,记录420 nm的吸光度,根据吸光度和标准曲线计算氨氮的浓度。
分别利用高分辨蔡司场发射扫描显微镜(Scanning electron microscope, SEM)和X射线能谱分析(Energy dispersive spectrometer, EDS)观察电极表面形貌,表征表面元素比例。采用D/Max 2500H X射线衍射仪(X-ray diffraction, XRD)使用Cu-Kα辐射,在40 kV和40 mA的操作条件下对电极表面的晶形结构进行分析。采用赛默氏公司ESCALAB XI能谱仪进行X射线光电子能谱(X-ray photoelectron spectroscopy, XPS)测试,分析不同电极表面元素组成。以5,5-二甲基-1-吡咯啉-N-氧化物(DMPO)作为原子氢(Hads)的捕获剂,并利用Bruker E500 X波段电子自旋共振(electron spin resonance, ESR)谱仪进行自由基检测。
-
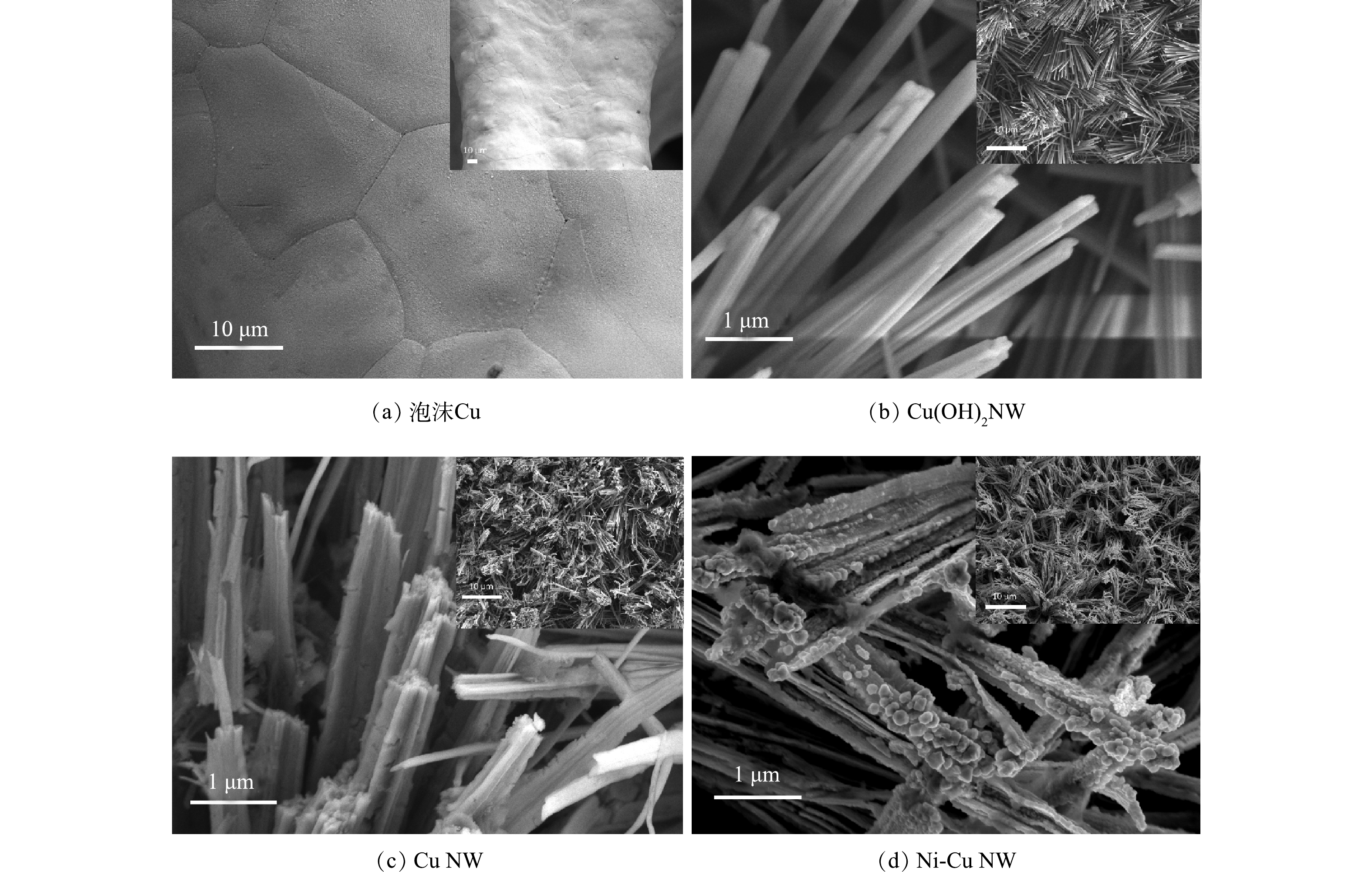
通过阳离子交换方法在泡沫Cu基体上合成了Ni-Cu NW电极,电极的SEM图像如图2所示。原始的泡沫Cu表面非常光滑(图2(a)),经过化学氧化后,泡沫Cu表面均匀地覆盖了大量Cu(OH)2 NW(图2(b)),其直径约为200 nm。在200 ℃的流动Ar气氛中退火2 h,进行原位电化学预还原,生成铜纳米线(Cu NW)(图2(c)),此时其直径增长至约为500 nm,表面变得更加粗糙。当掺杂Ni后,纳米线尖端出现颗粒(图2(d)),由EDS表征结果可知,Ni掺杂在催化剂Cu NW基质中,Ni:Cu原子比为1:9。Ni-Cu NW具有尖端颗粒沉积的纳米异质结构,有效地扩大了电极的比表面积,从而提升了反应活性位点的数量。
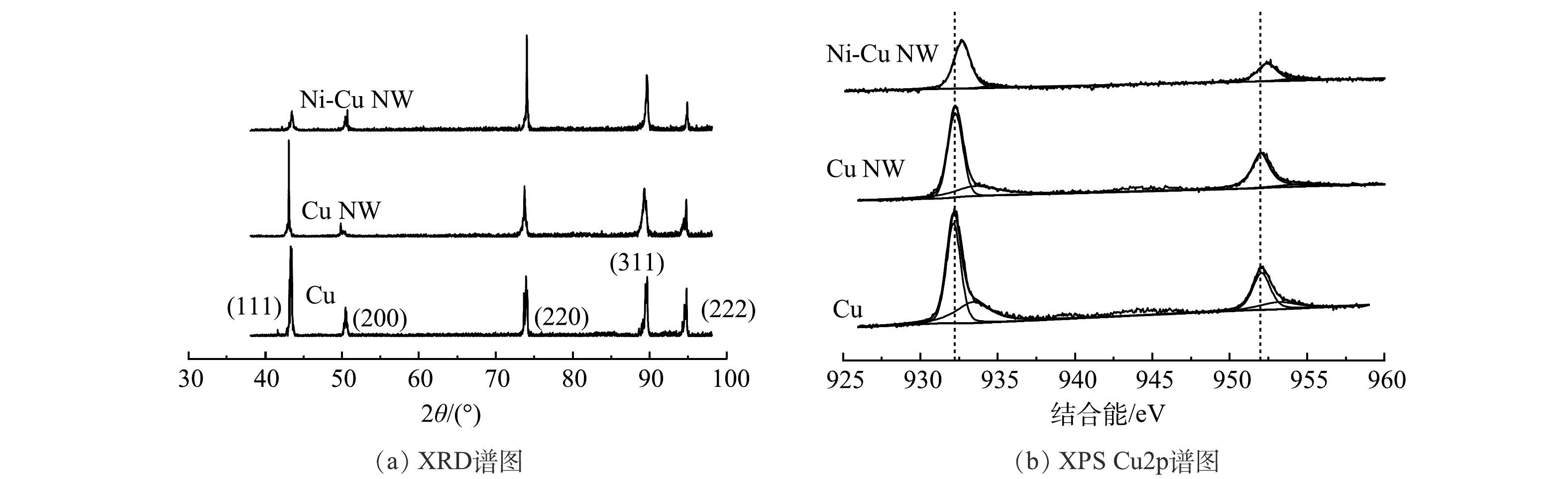
根据X射线衍射(XRD)表征结果(图3(a)),对比标准卡片(PDF#01-1241)可知,(111)、(200)、(220)、(311)、(222)为催化剂的主要晶面。其中Cu和Cu NW的最强衍射峰为(111),Ni掺杂后Ni-Cu NW的最强衍射峰转变为(220)。其原因在于晶格Cu原子在合成的过程中向外扩散并与Ni离子发生交换[11]。在电化学预还原过程中,表面氧化物被还原为金属,晶格结构重新排列。X射线光电子能谱(XPS)(图3(b))结果表明,Ni-Cu NW催化剂Cu2p结合能显著增加,结合能偏移为~0.5 eV,说明Cu NW的Ni掺杂使得Cu最外层电子再分布,结合能升高。以上结果说明Ni成功掺杂入Cu NW基体中,并形成了稳定的Ni-Cu合金相。
-
采用三电极体系对电极进行电化学表征,Cu、Cu NW、Ni-Cu NW电极分别作为工作电极,填充饱和KCl的Ag/AgCl和Pt片(2 cm×2 cm)作为参比电极和对电极,NO3、NO2、NH3通过紫外-可见分光光度法和标准曲线进行定量。
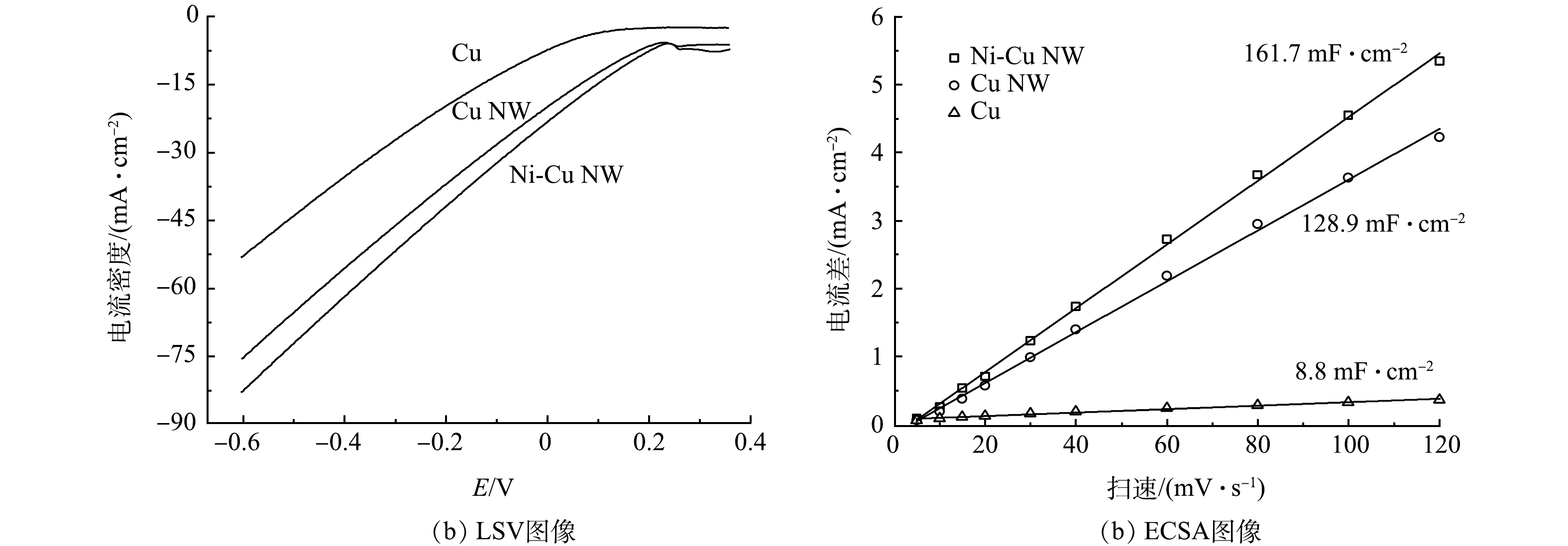
在1 mol·L−1 KOH和100 mmol·L−1 KNO3电解质中,以10 mV·s−1的速度在0.3~-0.6V进行线性扫描伏安法(LSV)测量(图4(a))。与原始Cu相比,Cu NW和Ni-Cu NW电极的电流密度存在明显的提升,在−0.6 V(vs. RHE)电位下,Ni-Cu NW的电流密度(82.8 mA·cm−2)是Cu的电流密度(53.05 mA·cm−2)的1.56倍。电化学活性表面积(ECSA)(图4(b))可以反映出实际参与电化学反应的活性面积,为进一步比较催化剂的活性,进行了ECSA测试。结果表明,Cu NW(128.9 μF·cm−2)和Ni-Cu NW(161.7 μF·cm−2)比Cu(8.8 μF·cm−2)有更大的活性比表面积,拥有更多的硝酸盐还原活性位点,可以更高效地还原硝酸盐。
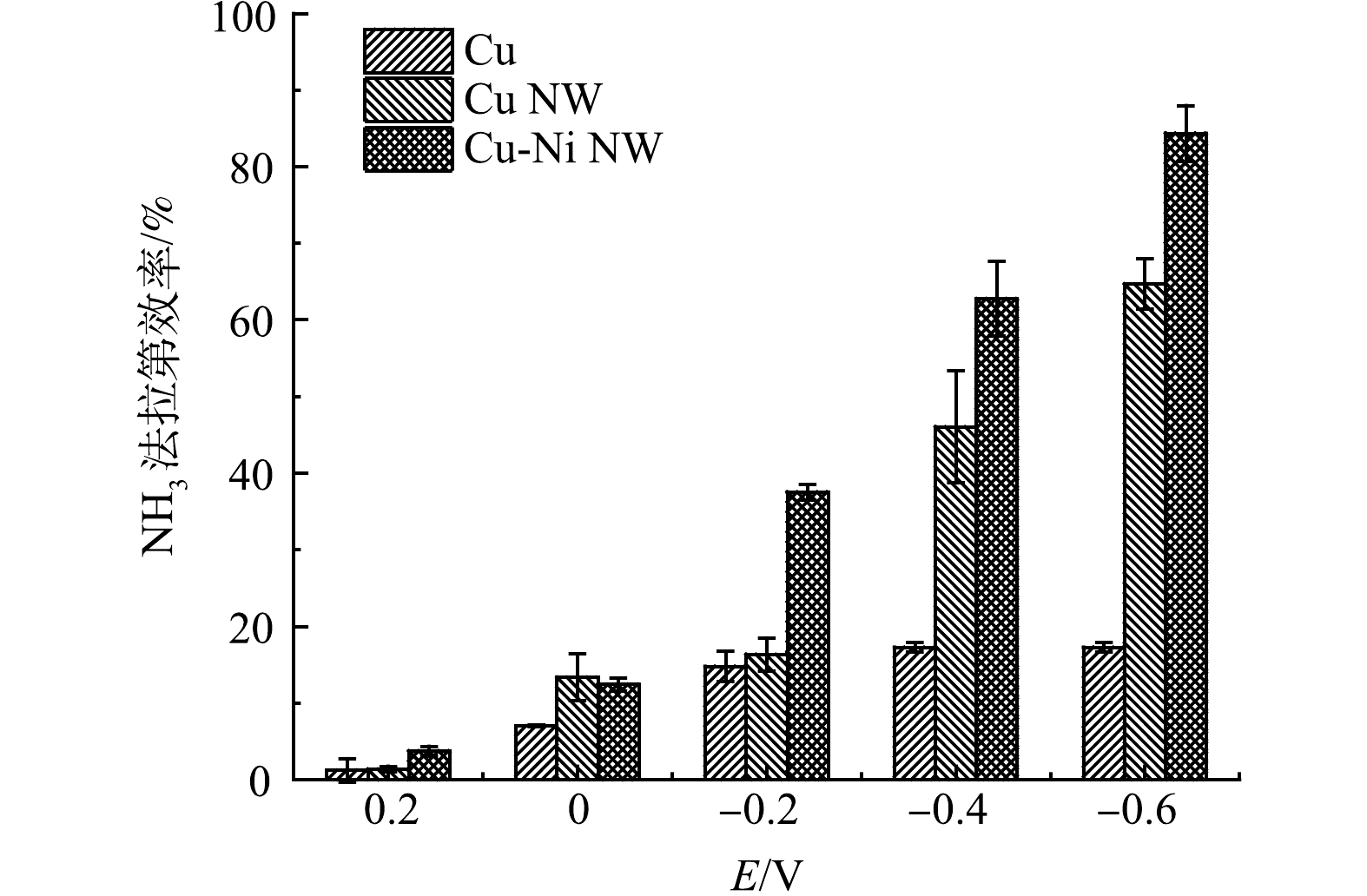
电位是影响电极电催化活性的重要因素,在定制的穿透式电化学反应器中,通过施加不同的电位,比较Cu、Cu NW和Ni-Cu NW 3种电极的法拉第效率(FE)(图5(c))。在−0.2 V下,Ni-Cu NW的FE(37.5%)显著高于Cu(14.7%)和Cu NW(16.3%),说明Ni-Cu NW能在更正的电位下拥有更高的氨选择性。当施加更负的电位时,如在−0.6 V下,Ni-Cu NW的FE达到84.35%,远高于Cu NW(64.6%)和Cu(17.2%)。在−0.6 V下,Ni-Cu NW的FE达到最高,能量的利用效率保持在较高的水平。因此,后续实验选择在−0.6 V下进行。
-
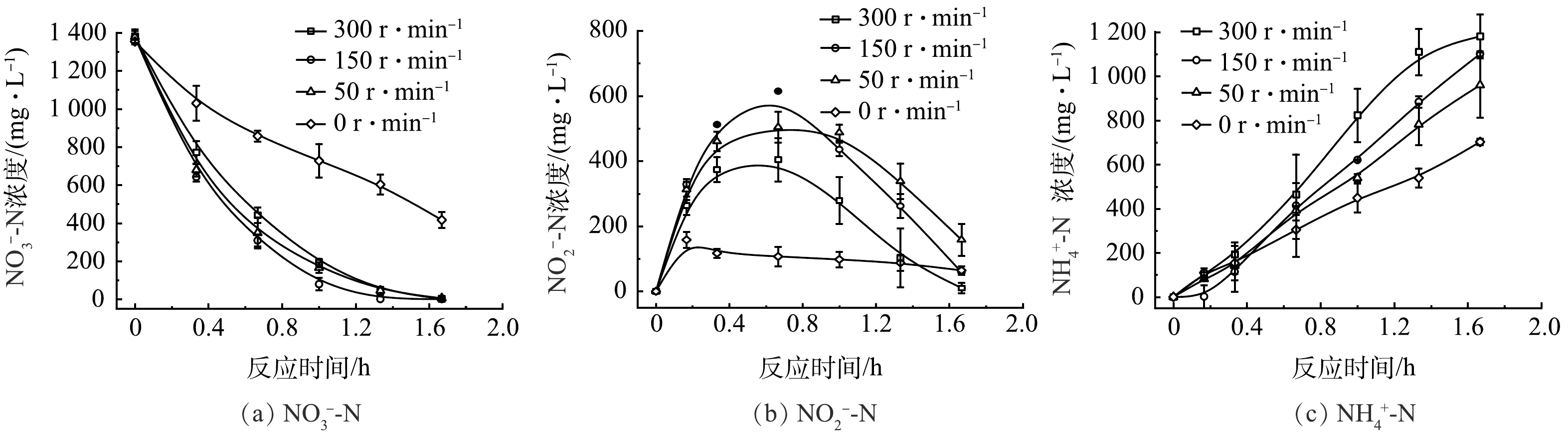
为了进一步探究流场环境对硝酸盐的影响,在定制的穿透式反应器中,通过使用不同的流速,探究不同流场环境对硝酸盐还原的影响。如图6(a)所示,通过蠕动泵施加300、150、50和0 r·min−1的转速,NO3−-N去除率分别为99.75%、100%、99.58%和69.23%。增加流速使传质加快,硝酸盐的转化率明显提升,接近100%。如图6(b)所示,在转速分别为300、150、50 r·min−1时,均在40 min左右出现了NO2−-N累积的情况,说明在NO3−向NH3还原的8电子过程中,在Ni-Cu NW表面NO3−向*NO2的转变速率比*NO2向NH3的转变速率更快,导致NO2−从电极表面解吸到水中。当水中NO2−增加到一定浓度,解吸反应被抑制到较低水平,从而促进NO3−向NH3的8电子转移过程。如图6(c)所示,随着转速的增大,氨产量也逐步增加,说明增加流速可提升NO3−向NH3整体反应的效率。
-
硝酸盐还原是一个复杂的多电子转移过程,涉及氮价态为+5价至-3价的多种含氮物质,其中亚硝酸盐是硝酸盐完全还原为氨或氮气之前主要的稳定中间体。硝酸盐直接电还原的过程包括2种途径[15],一是通过阴极的直接电子还原,将吸附在阴极表面的NO3−以及中间产物通过电子转移直接还原(式(1)~式(9))。二是通过活性氢原子还原,电子首先将吸附在阴极表面的H2O还原为Hads,通过逐步脱氧加氢将NO3−还原为NH4+后,硝酸盐还原终止[25]。
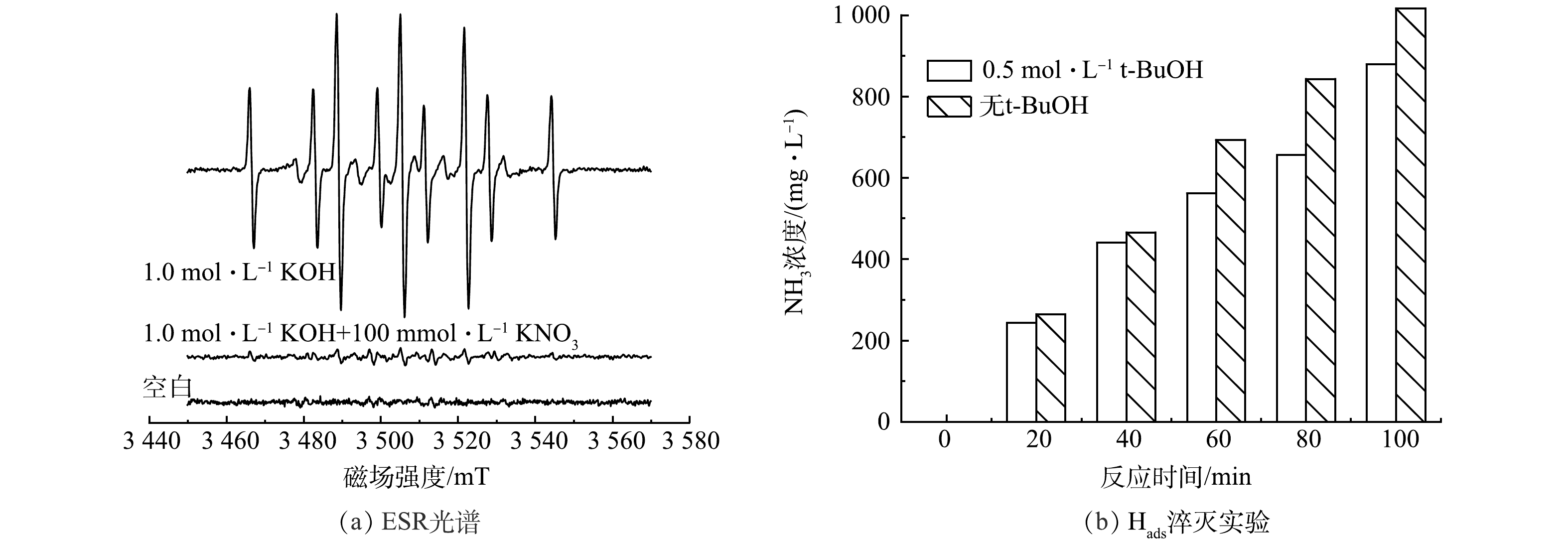
为了鉴定还原过程中生成的自由基,以5,5-二甲基-1-吡咯啉-N-氧化物(DMPO)为捕获剂,通过ESR对活性物种进行了鉴定。在1 mol·L−1 KOH溶液中观察到9个典型的信号峰(图7(a)),强度比接近1:1:2:1:2:1:2:1:1,这表示产生了DMPO-H,即确认了Hads的产生[26]。再加入100 mmol·L−1硝酸盐后DMPO-H的信号强度降低,这证实了在硝酸根还原的过程中,产生的Hads被消耗。在电解质中加入0.5 mol·L−1叔丁醇(t-BuOH)以淬灭Hads,未加入叔丁醇的实验组中氨产量均高于加入叔丁醇淬灭自由基的实验组(图7(b))。以上结果表明,硝酸根的还原除了有直接电极还原外,体系中可产生Hads,帮助进一步还原硝酸根。
-
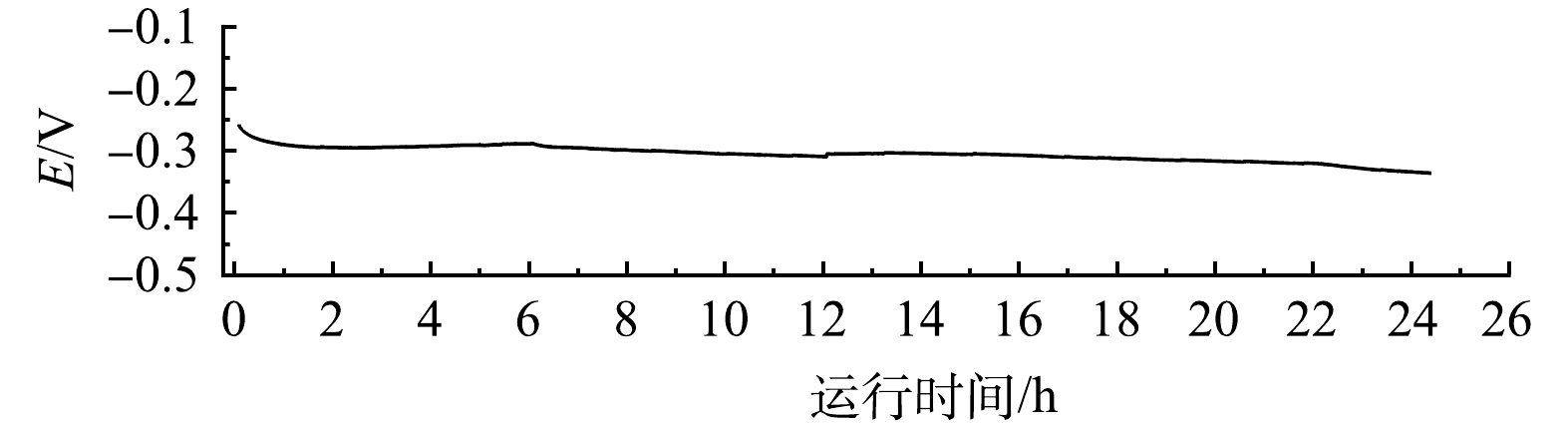
为了验证电极的电还原硝酸盐氮的稳定性,以电流密度为50 mA·cm−2恒电流模式,对1 L含有1 mol·L−1 KOH、1 400 mg·L−1 NO3−-N的电解质溶液进行长期运行实验(图8),电极的电位在24 h内无明显的变化,表明电极在24 h内保持稳定。
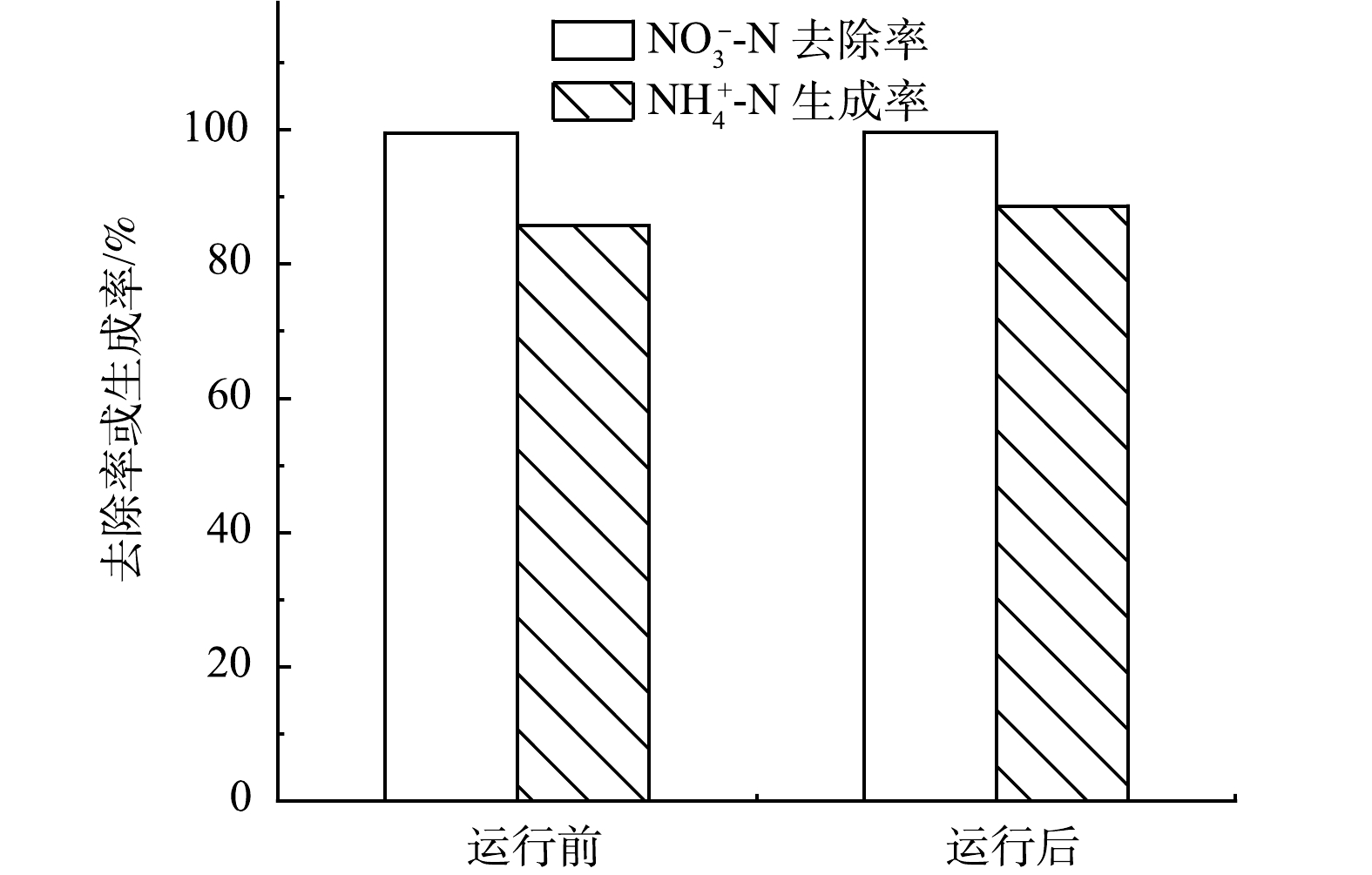
长期运行实验前后分别在-0.6 V恒电位条件下进行硝酸盐氮电还原实验。如图9所示,NO3−-N转化效率分别为99.58%和99.71%,氨生成率分别85.72%和88.62%,运行前后硝酸盐电还原性能未发生明显变化。
-
1)采用离子交换电化学预还原的方式制备了具有良好的催化活性Ni-Cu NW电极,硝酸盐电化学还原的法拉第效率为(84.35±3.63)%,100 min内硝酸盐去除率接近100%,其中有85.4%的硝酸盐还原为有价值的氨。
2)以恒电流方式运行24 h,Ni-Cu NW电极保持稳定,且对硝酸盐电还原效果保持稳定。
3)通过ESR确认了Hads的产生,通过Hads淬灭实验证明,硝酸根的还原除了有直接电极还原外,体系中存在Hads吸附介导的电化学硝酸盐还原路径。
铜镍纳米结构修饰多孔电极的电化学硝酸根还原
Electrochemical nitrate reduction of porous electrodes modified with Ni-Cu nanostructures
-
摘要: 利用电化学将硝酸根转化为有价值的氨是硝酸盐处置绿色、低碳的途径。本研究制备了铜镍纳米结构修饰多孔电极(Ni-Cu NW),同时构建了电场与流场相协同的穿透式电化学反应器体系。在施加-0.6 V (vs. RHE)电位条件下,该体系氨法拉第效率可达到(84.35±3.63)%,而泡沫Cu电极的法拉第效率仅为(17.2±0.63)%。在定制的穿透式反应器中,Ni-Cu NW电极在−0.6 V、300 r·min−1转速条件下对1 400 mg·L−1 NO3--N 溶液电解,100 min后硝酸盐的转化率接近100%,氨选择性为85.5%。电子自旋共振能谱(ESR)和Hads淬灭实验证明除了电极直接还原硝酸盐,体系中的Hads可帮助进一步还原硝酸盐。以50 mA·cm−2恒电流密度运行24 h,运行前后电极的硝酸盐还原性能未产生明显变化。Abstract: Electrochemical conversion of nitrate ions into valuable ammonia is a green and low carbon approach for nitrate disposal. A porous electrode modified with Ni-Cu nanostructure was prepared and the according flow-through electrochemical reactor system was constructed with the synergy of electric field and flow field. At −0.6V(vs. RHE), the Faraday efficiency of ammonia reached (84.35±3.63)%, whereas that of foam Cu electrode was only (17.2±0.63)%. By using the flow-through reactor, the Ni-Cu NW electrode electrolyzed 1 400 mg·L−1 NO3--N at the potential of −0.6 V, a stirring rate of 300 r·min−1, After 100 min treatment, the results exhibited that the conversion rate of nitrate and the selectivity of ammonia was 100% and 85.5%, respectively. Electron spin resonance spectroscopy (ESR) and Hads quenching experiments showed that in addition to direct reduction of nitrate by electrode, the produced Hads could further facilitate nitrate reduction. With a current of 50 mA·cm−2 for 24 hours, no obvious change in nitrate reduction performance could occur before and after running.
-
Key words:
- electric reduction /
- nitrate /
- Cu foam /
- copper-nickel alloy
-

-
-
[1] HAN D, CURRELL M J, CAO G. Deep challenges for China's war on water pollution[J]. Environmental Pollution, 2016, 218: 1222-1233. doi: 10.1016/j.envpol.2016.08.078 [2] 张鑫, 张妍, 毕直磊, 等. 中国地表水硝酸盐分布及其来源分析[J]. 环境科学, 2020, 41(4): 1594-1606. doi: 10.13227/j.hjkx.201909078 [3] GRUBER N, GALLOWAY J N. An Earth-system perspective of the global nitrogen cycle[J]. Nature, 2008, 451: 293-296. doi: 10.1038/nature06592 [4] SCHULLEHNER J, HANSEN B, THYGESEN M, et al. Nitrate in drinking water and colorectal cancer risk: A nationwide population‐based cohort study[J]. International Journal of Cancer, 2018, 143: 73-79. doi: 10.1002/ijc.31306 [5] EBDRUP N H, SCHULLEHNER J, KNUDSEN U B, et al. Drinking water nitrate and risk of pregnancy loss: A nationwide cohort study[J]. Environmental Health, 2022, 21(1): 87. doi: 10.1186/s12940-022-00897-1 [6] YOU Q G, WANG J H, QI G X, et al. Anammox and partial denitrification coupling: A review[J]. RSC Advances, 2020, 10: 12554-12572. doi: 10.1039/D0RA00001A [7] BARRABéS N, Sá J. Catalytic nitrate removal from water, past, present and future perspectives[J]. Applied Catalysis B:Environmental, 2011, 104: 1-5. doi: 10.1016/j.apcatb.2011.03.011 [8] CHAUHAN R, SRIVASTAVA V C. Electrochemical denitrification of highly contaminated actual nitrate wastewater by Ti/RuO2 anode and iron cathode[J]. Chemical Engineering Journal, 2020, 386: 122065. doi: 10.1016/j.cej.2019.122065 [9] DUCA M, KOPER M T M. Powering denitrification: The perspectives of electrocatalytic nitrate reduction[J]. Energy & Environmental Science, 2012, 5: 9726-9742. [10] GAO J, SHI N, LI Y, et al. Electrocatalytic upcycling of nitrate wastewater into an ammonia fertilizer via an electrified membrane[J]. Environmental Science & Technology, 2022, 56: 11602-11613. [11] CHEN F Y, WU Z Y, GUPTA S, et al. Efficient conversion of low-concentration nitrate sources into ammonia on a Ru-dispersed Cu nanowire electrocatalyst[J]. Nature Nanotechnology, 2022, 17: 759-767. doi: 10.1038/s41565-022-01121-4 [12] GHIMIRE U, SARPONG G, GUDE V G. Transitioning wastewater treatment plants toward circular economy and energy sustainability[J]. ACS Omega, 2021, 6: 11794-11803. doi: 10.1021/acsomega.0c05827 [13] CHEN J G, CROOKS R M, SEEFELDT L C, et al. Beyond fossil fuel–driven nitrogen transformations[J]. Science, 2018, 360: 1-7. [14] CHEN G F, YUAN Y, JIANG H, et al. Electrochemical reduction of nitrate to ammonia via direct eight-electron transfer using a copper–molecular solid catalyst[J]. Nature Energy, 2020, 5: 605-613. doi: 10.1038/s41560-020-0654-1 [15] GARCIA-SEGURA S, LANZARINI-LOPES M, HRISTOVSKI K, et al. Electrocatalytic reduction of nitrate: Fundamentals to full-scale water treatment applications[J]. Applied Catalysis B:Environmental, 2018, 236: 546-568. doi: 10.1016/j.apcatb.2018.05.041 [16] PERRY S C, PONCE DE LEóN C, WALSH F C. Review: The design, performance and continuing development of electrochemical reactors forclean electrosynthesis[J]. Journal of the Electrochemical Society, 2020, 167: 155525. doi: 10.1149/1945-7111/abc58e [17] MOUSSET E, DIONYSIOU D D. Photoelectrochemical reactors for treatment of water and wastewater: A review[J]. Environmental Chemistry Letters, 2020, 18: 1301-1318. doi: 10.1007/s10311-020-01014-9 [18] WANG X, ZHU M, ZENG G, et al. A three-dimensional Cu nanobelt cathode for highly efficient electrocatalytic nitrate reduction[J]. Nanoscale, 2020, 12: 9385-9391. doi: 10.1039/C9NR10743F [19] WU K, SUN C, WANG Z, et al. Surface reconstruction on uniform Cu nanodisks boosted electrochemical nitrate reduction to ammonia[J]. ACS Materials Letters, 2022, 4: 650-656. doi: 10.1021/acsmaterialslett.2c00149 [20] XUE Y, YU Q, MA Q, et al. Electrocatalytic hydrogenation boosts reduction of nitrate to ammonia over single-atom Cu with Cu(I)-N3C1 sites[J]. Environmental Science & Technology, 2022, 56: 14797-14807. [21] WANG Y, ZHOU W, JIA R, et al. Unveiling the activity origin of a copper-based electrocatalyst for selective nitrate reduction to ammonia[J]. Angewandte Chemie International Edition, 2020, 59: 5350-5354. doi: 10.1002/anie.201915992 [22] WANG Y, XU A, WANG Z, et al. Enhanced nitrate-to-ammonia activity on copper-nickel alloys via tuning of intermediate adsorption[J]. Journal of the American Chemical Society, 2020, 142: 5702-5708. doi: 10.1021/jacs.9b13347 [23] MATTAROZZI L, CATTARIN S, COMISSO N, et al. Electrochemical reduction of nitrate and nitrite in alkaline media at CuNi alloy electrodes[J]. Electrochimica Acta, 2013, 89: 488-496. doi: 10.1016/j.electacta.2012.11.074 [24] MATTAROZZI L, CATTARIN S, COMISSO N, et al. Hydrogen evolution assisted electrodeposition of porous Cu-Ni alloy electrodes and their use for nitrate reduction in alkali[J]. Electrochimica Acta, 2014, 337-344. [25] ROSCA V, DUCA M, DE GROOT M T, et al. Nitrogen cycle electrocatalysis[J]. Chemical Reviews, 2009, 109: 2209-2244. doi: 10.1021/cr8003696 [26] FAN K, XIE W, LI J, et al. Active hydrogen boosts electrochemical nitrate reduction to ammonia[J]. Nature Communications, 2022, 13: 7958. doi: 10.1038/s41467-022-35664-w -



 下载:
下载: