-
人工湿地作为一种生态型水处理技术,具有污染物去除率高、易于维护、运行成本低等优点,已被广泛用于处理不同类型的污水,如污水厂尾水、村镇生活污水等[1-3]。潮汐流人工湿地(tidal flow constructed wetland,TFCW)通过对湿地进行周期性的浸没和排空,使得湿地内部不断形成好氧-厌氧环境,可在去除有机污染物的同时实现脱氮[4]。TFCW具有较好的复氧效果,常用于强化硝化过程,通过增加淹水期时长,使得湿地内部某一阶段处于缺氧或厌氧状态,形成适于反硝化微生物活动的缺氧/厌氧微环境,保证TFCW达到强化脱氮的目的。在人工湿地中,微生物反硝化作用是脱氮的主要途径,通过此途径去除的氮量可达湿地脱氮总量的60%~70%[5],而通过湿地植物吸收的氮量占比较小(约10%)[6]。温度、pH、溶解氧(dissolved oxygen, DO)以及碳源等均是影响反硝化作用的主要因素,其中关键因素是碳源。碳源可为反硝化细菌提供电子供体,促进硝酸盐(NO3−-N)还原为气态氮化物和氮气(N2),实现脱氮。
采用人工湿地深度处理污水厂尾水时,由于反硝化过程中缺少电子供体,脱氮效率普遍偏低。为此,国内外学者围绕湿地外加碳源强化反硝化作用开展了大量研究。乙酸钠、甲醇、葡萄糖等传统化学碳源都可作为人工湿地的外加碳源[7-9],显著提高人工湿地的脱氮效率,但存在成本较高且投加不当易造成二次污染的缺点[10]。工业有机废水中有机物种类丰富、浓度较高,也可作为碳源强化人工湿地脱氮,但其成分复杂,需考虑应用过程中是否会引进新的污染物质以及是否对微生物产生不良影响[9,11]。固态碳源如稻草秸秆、玉米芯、芦苇杆等具有成本低、可为微生物提供附着条件等优点,但存在碳源释放不稳定的问题[9,12-13]。
餐厨垃圾具有有机质含量高、易降解的特点,被认为是生产碳源的适宜基质[14]。2020年,我国产生城市固体废物约2.35×108 t,其中餐厨垃圾约占50%~60%。目前,餐厨垃圾处理技术中厌氧发酵在我国使用最为广泛。截至2015年,118座50 t∙d−1以上处理规模的餐厨垃圾处理厂中近70%采用厌氧发酵技术[15]。餐厨垃圾厌氧发酵是生产碳源的常用方法。有研究[16-17]表明,餐厨垃圾发酵液(food waste fermentation liquid, FWFL)含有大量可作为反硝化碳源的碳水化合物、挥发性脂肪酸(volatile fatty acids, VFAs)等。
目前将FWFL作为碳源的研究主要集中在FWFL的有效组分以及反硝化性能方面,将FWFL应用于人工湿地反硝化脱氮的研究报道较少。FWFL成分较为复杂,应用于污水厂尾水脱氮时其中的无效干扰组分可能会引起二次污染和出水水质波动,因此,验证FWFL投加时人工湿地的综合除污效能十分关键。本实验通过向污水处理厂尾水湿地投加FWFL调节其碳氮比(carbon/nitrogen, C/N),研究了不同C/N对TFCW脱氮和综合除污效果、湿地填料生物膜硝化速率、反硝化速率、厌氧氨氧化速率及相关酶活性、电子传递系统活性(electron transport system activity, ETSA)、微生物群落结构的影响,解析了FWFL强化人工湿地反硝化脱氮的机理,以期为FWFL强化人工湿地脱氮应用于实际工程提供技术支撑。
-
构建了12个TFCW实验装置,分为3组。每组尺寸的长×宽×高分别为80 cm×30 cm×40 cm、80 cm×30 cm×70 cm、80 cm×30 cm×130 cm,编号为L、M、H。每组包含4个装置,分别编号为1、2、3、4,这些编号对应的C/N为4、6、7、8。填料为粒径1~4 cm的鹅卵石,填料高度(同湿地高度)分别为40、70、130 cm,淹水高度(出水口距离湿地顶端10 cm)低于填料高度10 cm。填料的空隙率约为49%。湿地表面种植蓝花鸢尾(Iris tectorum,12株∙m−2)。人工湿地以潮汐流方式运行,每3 d运行1个周期,每个周期分别由进水期(30 min)、淹水期、出水期(30 min)和排空闲置期(60 min)组成。TFCW-H的淹水期为24、12 h,TFCW-M、L的淹水期为36 h。实验过程中TFCW-H、M、L的水力负荷分别为1.2、0.6、0.3 m3∙ (m2∙d)−1。
ZHENG等对安徽省合肥市某污水厂尾水进行了连续10 d的监测,结果表明C/N比在2.0~5.5[18]。本实验选择C/N=4作为对照组(未添加FWFL),通过添加FWFL将进水C/N分别调至6、7、8。实验于2021年10月27日至2022年1月13日进行,期间水温为4.9~20 ℃,平均水温12.8 ℃。水质指标测定频率为3~4 d一次。在实验末期从人工湿地中获取填料测定填料生物膜的相关脱氮速率、反硝化酶活性、硝化酶活性、ETSA和微生物群落结构。
-
本实验通过将8.35 g富里酸、17.33 g硝酸钾、1.53 g氯化铵、0.39 g磷酸二氢钾溶于200 L自来水中配置污水处理厂尾水[19]。人工湿地进水水质如表1所示。
-
实验所使用的餐厨垃圾(food waste, FW)来自某大学学生食堂。首先对FW进行预处理,去除其中的纸巾、骨头等杂质,然后粉碎,用自来水将混合物的总固体含量控制在12%左右,并在4 ℃环境下保存。取安徽省合肥市某污水处理厂曝气池的活性污泥(activated sludge, AS),将预处理后的FW与AS以5:1的比例混合均匀放入发酵罐中。通入N2使其保持无氧状态,接着放入30 ℃的摇床中(35 r∙min−1)厌氧发酵3 d。发酵结束后,经纱布过滤得到FWFL[16-17]。
-
样品的COD、NH4+-N、NO3−-N、NO2−-N、TN、TP分别采用快速消解-分光光度法(HJ/T399-2007)、纳氏试剂分光光度法(HJ/T535-2009)、紫外分光光度法(HJ/T346-2007)、分光光度法(GB 7493-87)、碱性过硫酸钾消解-紫外分光光度法(HJ636-2012)、钼酸铵分光光度法(GB 11893-89)测定。样品的pH、氧化还原电位(oxidation-reduction potential, ORP)、DO分别采用玻璃电极法、电化学探头法测定。人工湿地的脱氮性能通过反硝化速率、厌氧氨氧化速率、硝化酶活性、反硝化酶活性以及ETSA进行衡量,此5项指标参照文献[20-22]测定。测定微生物群落结构时,先进行DNA的提取,再开展文库构建,最后进行测序。提取DNA时使用高度特异性引物富集目标序列,引物对为341F(CCTACGGGNGGCWGCAG)和805R(GACTACHVGGGTATCTAATCC)。PCR产物通过检测试剂盒定量。通过16S rRNA基因测序,对微生物种类进行研究。本实验中的基因测序委托生工生物工程(上海)股份有限公司测定。
-
采用Excel软件进行单因素方差分析(one-way ANOVA),判断实验数据的显著性差异(P<0.05);数据统计图采用Origin 2019绘制。
-
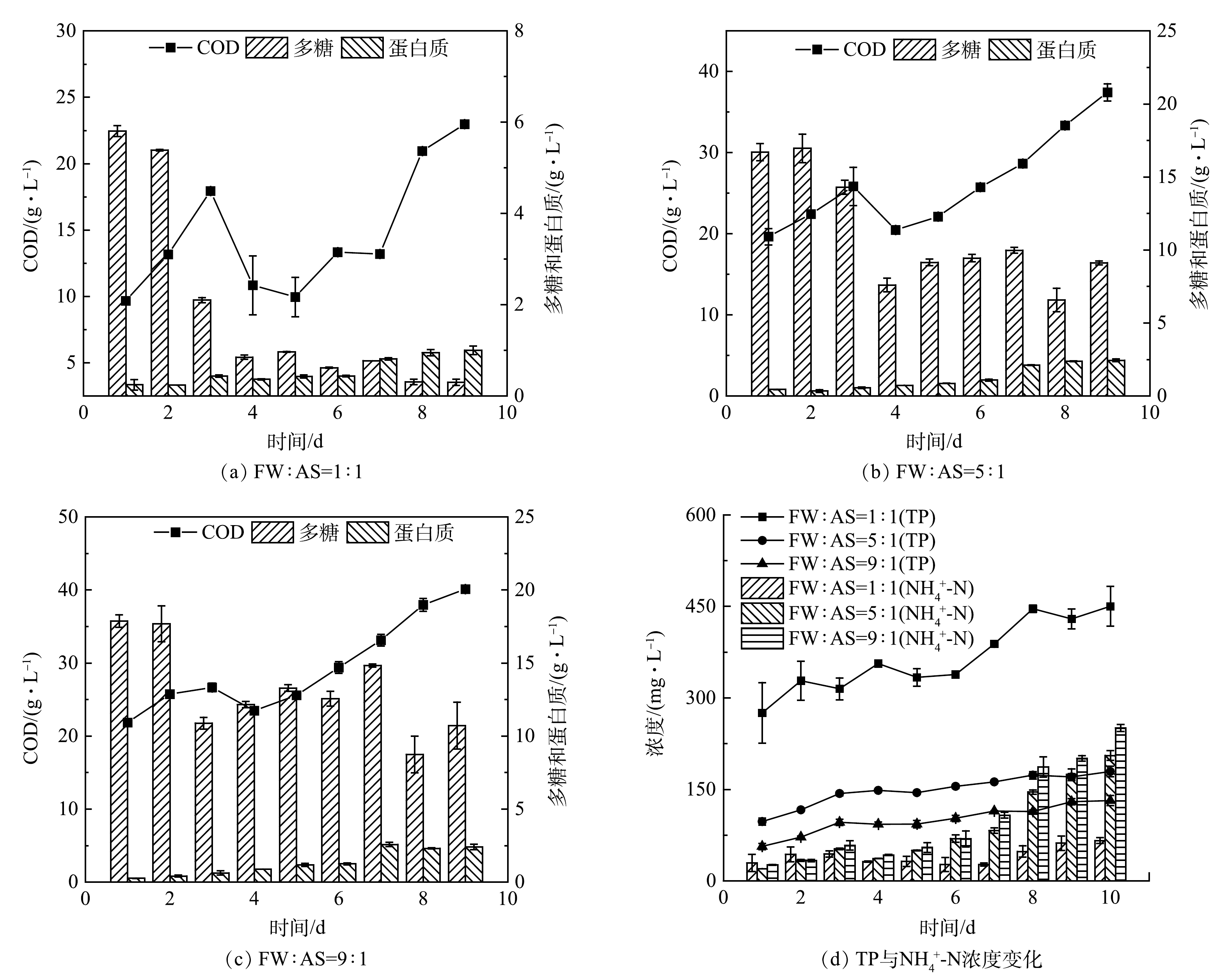
TN和TP随发酵时间的质量浓度变化如图1所示。FW:AS=5:1(体积比)的FWFL 中COD值为FW:AS=1:1时的1.4~2.2倍,而FW:AS=9:1时FWFL 中COD值为FW:AS=5:1时的1.5~2.6倍,体积比超过5:1时COD值的增加并不显著。此外,多糖与蛋白质为FWFL的主要成分,而乙酸虽然随着发酵时间延长质量浓度增加(表2),但总体VFAs的质量浓度并不高。FWFL中NH4+-N和TP主要来自于AS,随着FW比例增大,NH4+-N质量浓度随发酵时间增加质量浓度增加明显。考虑FWFL加入人工合成废水后对其的影响,选择FW:AS=5:1发酵3 d的发酵液作为外加碳源。
-
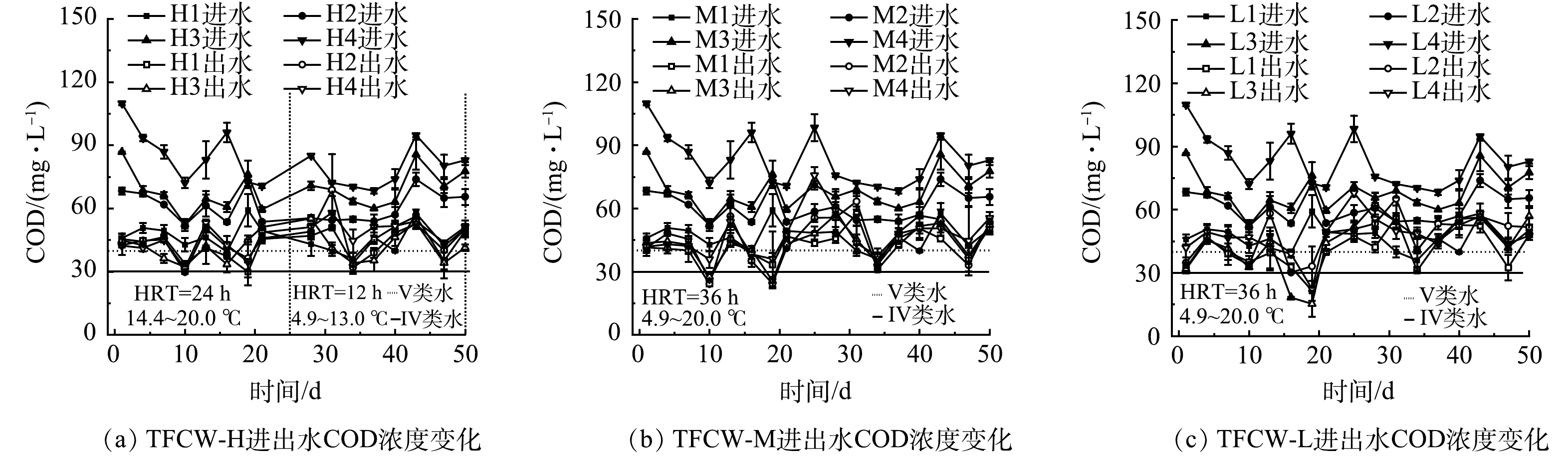
图2为不同处理TFCW进出水COD的变化情况。可见,添加FWFL后,各组人工湿地进水COD升高,H2、H3、H4湿地的COD去除负荷从3.4 g∙(m2∙d)−1分别增至32.0、69.8、64.6 g∙(m2∙d)−1,M2、M3、M4的COD去除负荷从1.3 g∙(m2∙d)−1分别增至6.9、9.8、14.1 g∙ (m2∙d)−1,L2、L3、L4的COD去除负荷从1.2 g∙(m2∙d)−1分别增至2.7、4.9、7.0 g∙(m2∙d)−1。出水COD与对照组均无显著性差异(P>0.05),表明投加FWFL增加进水有机负荷后,不会显著影响出水COD。缺氧或厌氧环境有利于反硝化脱氮,此过程需要有机质作为电子供体,这也是添加FWFL后各组人工湿地均表现出稳定的有机物降解能力的原因。
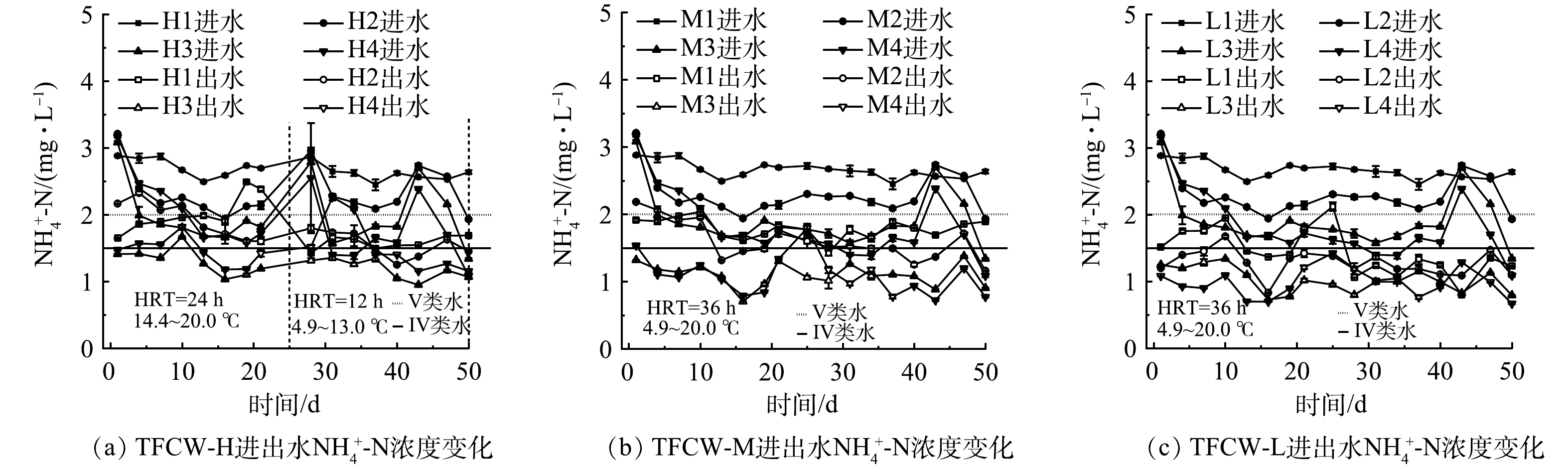
图3为不同处理TFCW的NH4+-N进出水质量浓度变化情况。由于人工湿地处于缺氧或厌氧状态,对NH4+-N的去除率最高仅为46.2%。此外,TFCW-L的NH4+-N去除率(43.5%~46.2%)高于TFCW-H(22.8%~32.9%)和TFCW-M(29.0%~40.1%)的去除率。
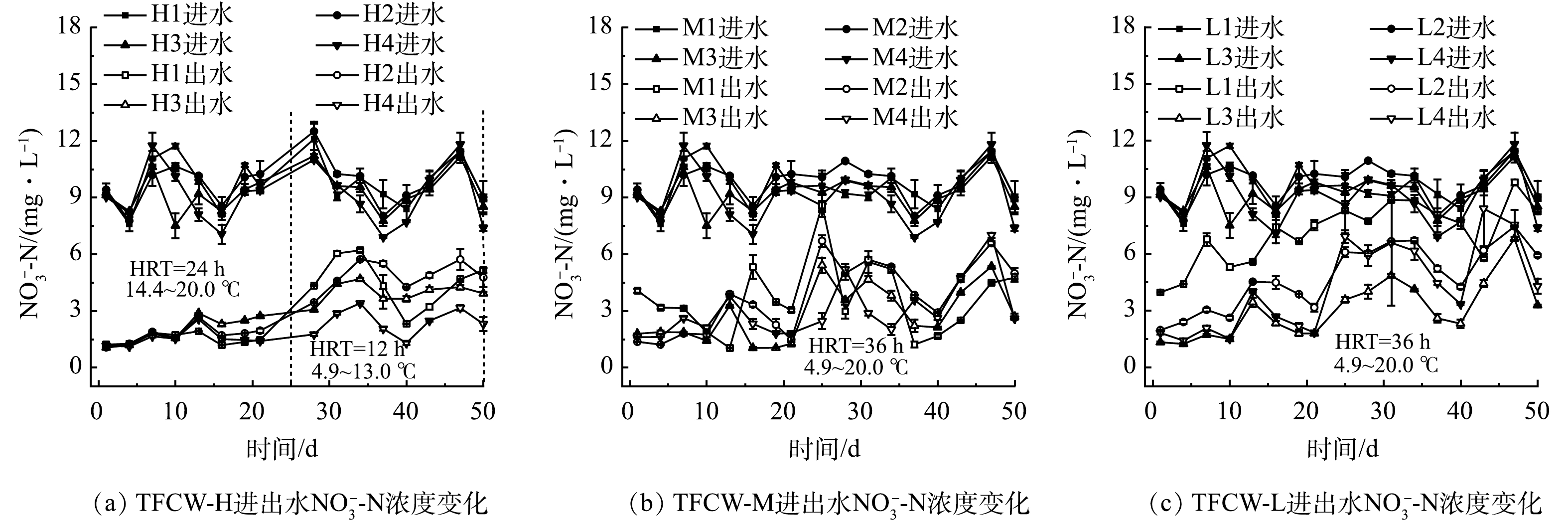
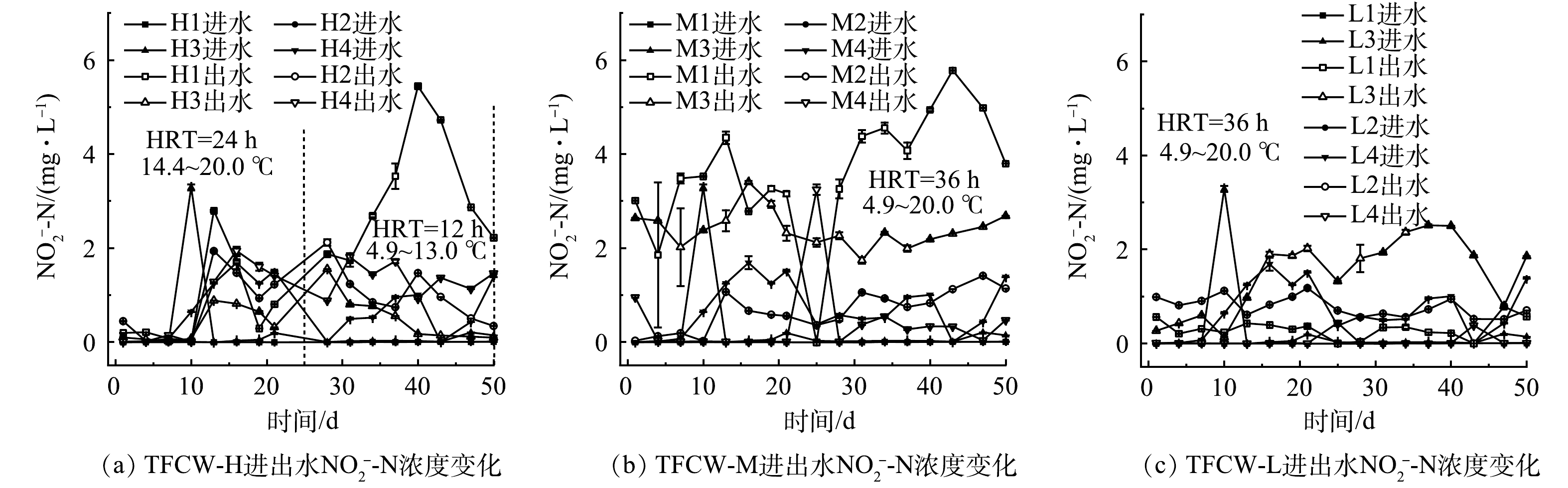
图4和图5分别为NO3−-N和NO2−-N进出水质量浓度的变化情况。淹水时间为12 h时,H1、H2、H3、H4湿地的平均出水NO3−-N分别为4. 6、5.1、4.1、2.5 mg∙L−1。M1、M2、M3、M4的平均出水NO3−-N分别为3.7、3.7、2.8、3.0 mg∙L−1,L1、L2、L3、L4的分别为7.1、4.7、3.0、4.2 mg∙L−1。这表明添加FWFL后,促进了NO3−-N的去除,且对不同高度比较发现,湿地越高越有利于NO3−-N的去除。当进水碳源不足时,硝酸盐还原酶(nitrate reductase, NAR)与亚硝酸盐还原酶(nitrite reductase, NIR)在竞争电子的过程中,前者处于优势,这也是H1、M1湿地出现NO2−-N积累(0.2~5.4 mg∙L−1、1.9~5.8 mg∙L−1)的原因。
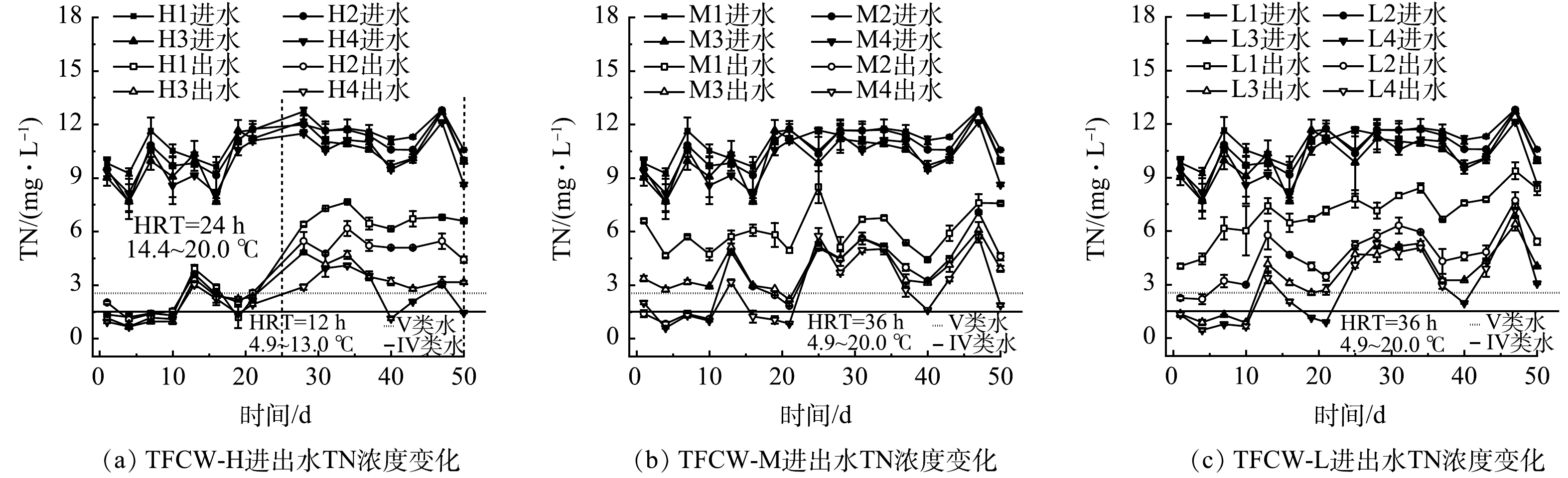
图6为不同处理人工湿地进出水TN质量浓度变化情况,与对照组相比,H2、H3、H4湿地的TN平均去除率从61.7%提高到67.1%、74.4%和78.9%,M2、M3、M4湿地的TN平均去除率从45.1%提高到61.5%、67.2%和74.0%,L2、L3、L4湿地的平均去除率从36.4%提高到56.9%、66.9%和72.4%,这表明添加FWFL能有效提高脱氮效率,其中C/N=8时脱氮效果最佳。污水厂尾水碳源不足限制了反硝化作用,而FWFL的添加弥补了污水厂尾水碳源不足的缺点,从而提高了脱氮效率。实验后期人工湿地内水温逐渐降低(<10 ℃)。有研究[23]表明,当温度低于15 ℃时会显著抑制反硝化作用。但在本实验后期,添加FWFL的湿地仍然具有良好的脱氮效果,且人工湿地高度增加,脱氮效率也有所增加(图6),表明提高人工湿地高度或添加FWFL可以有效改善低温对TN去除效果的影响。
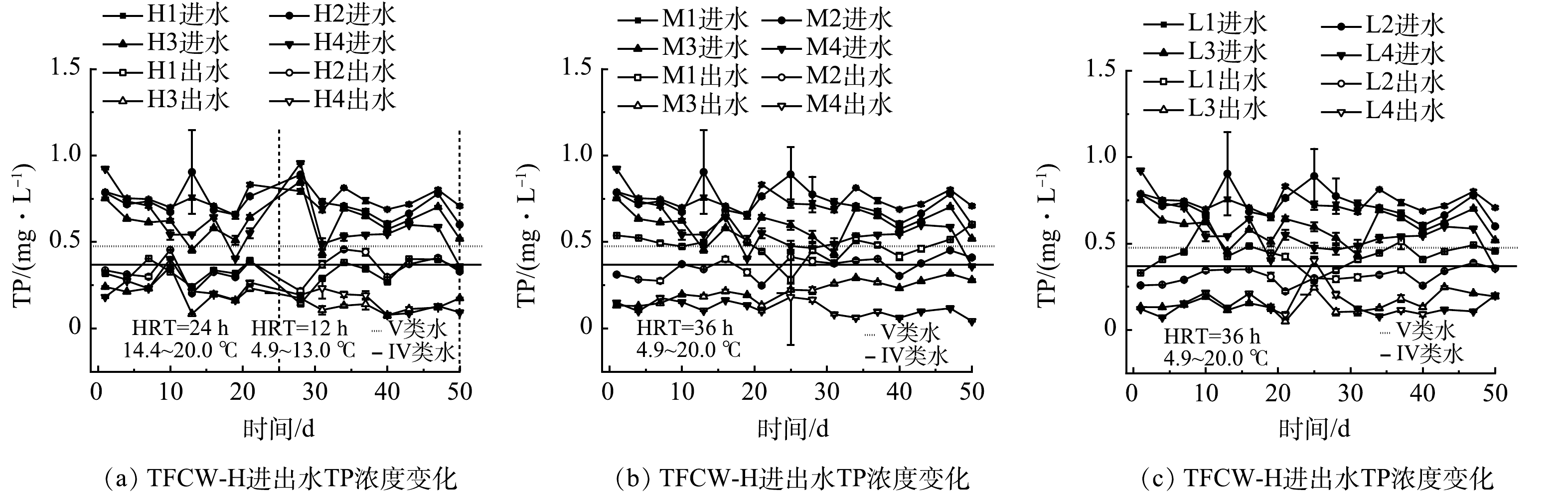
不同处理TFCW进出水TP质量浓度随时间的变化情况如图7所示。TFCW-H、M、L湿地的出水质量浓度分别0.1~0.3、0.1~0.5、0.1~0.4 mg∙L−1。随着C/N的升高,H3、H4湿地对TP的去除率从55.4%提高到72.9%、66.5%,M2、M3、M4湿地对TP的去除率从34.7%提高到50.9%、60.3%、80.1%,L2、L3、L4湿地对TP的去除率从40.9%提高到57.2%、75.8%、72.8%,H3、H4、M3、M4、L3、L4湿地出水TP质量浓度均能优于《地表水环境质量标准》(GB 3838-2002)V类水限值。FWFL的添加为湿地中微生物提供了丰富的底物,促进了湿地中生物膜的生长,也有助于除磷菌的繁殖。此外,TFCW较好的复氧效果促进了噬磷菌的代谢活性,从而提升了湿地的除磷效果。
-
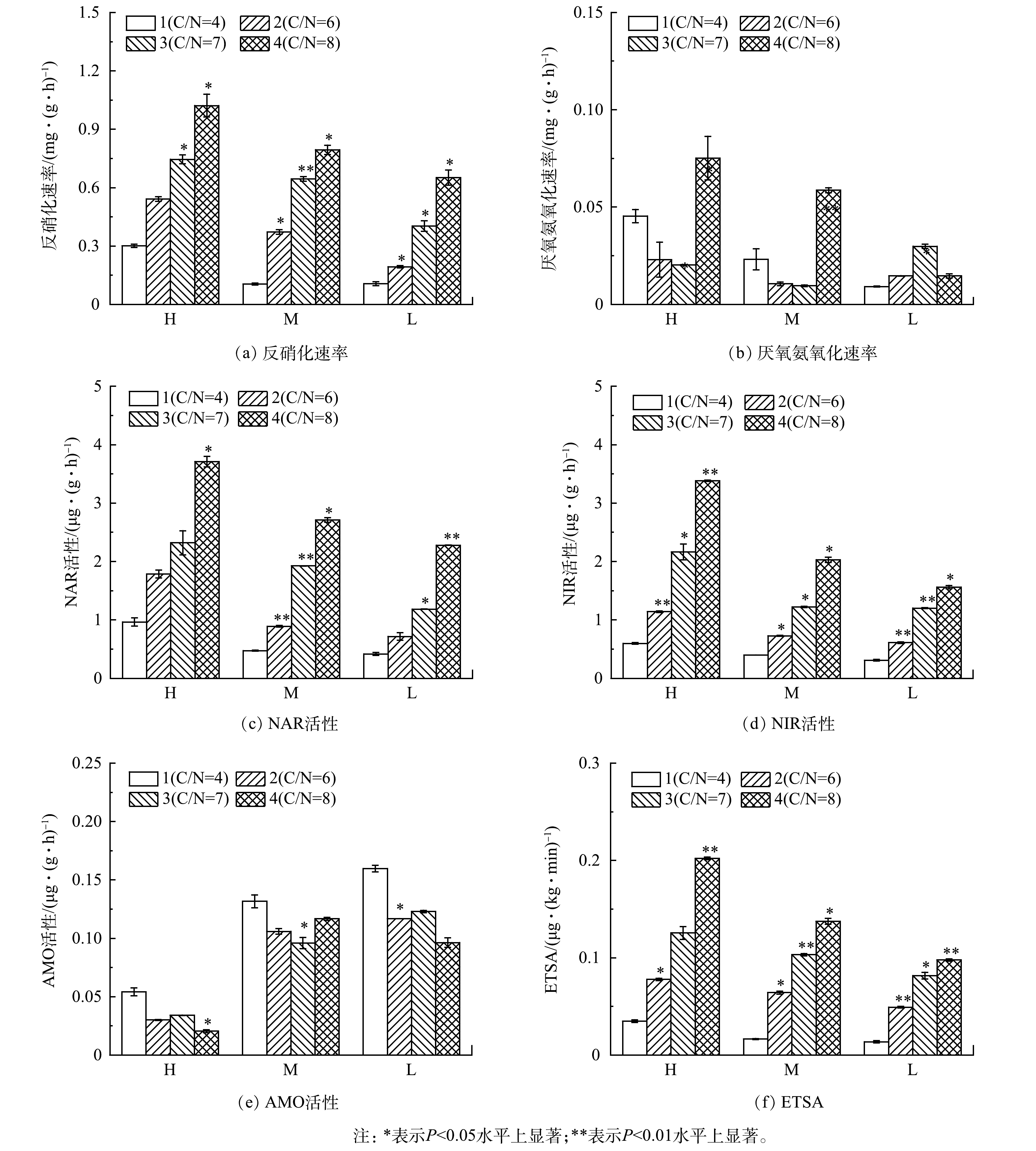
人工湿地反硝化速率(以NO3−-N计,生物量以MLVSS(Mixed Liquid Volatile Suspended Solids)计)测定结果如图8(a)所示。可见,在同一高度水平上比较发现,H1湿地反硝化速率最低,H3、H4与H1湿地的反硝化速率具有显著性差异(P<0.05),H2、H3、H4湿地的反硝化速率分别是H1的1.7、2.5与3.5倍;M1反硝化速率最低,M2、M3、M4与M1的反硝化速率具有显著性差异(P<0.05),分别是M1的3.5、6.0与7.5倍;L1反硝化速率最低,L2、L3、L4与L1的反硝化速率具有显著性差异(P<0.05),分别是M1的1.7、3.7与5.9倍。这表明加入的FWFL为反硝化过程提供了充足的电子供体,强化了反硝化过程中的电子传递,进而使得湿地反硝化速率大幅度提升。相同C/N时,反硝化速率随人工湿地高度增加而增加。反硝化过程需要缺氧或厌氧环境,大气复氧和植物根系泌氧是人工湿地中DO的主要来源,而人工湿地高度越高,越不利于大气复氧,DO含量越低,因此,人工湿地高度越高越有利于反硝化反应的进行。
如图8(b)所示,对相同高度的湿地比较发现,H4、M4、L3湿地厌氧氨氧化速率(以NO2−-N计,生物量以MLVSS计)最高,且H4、M4湿地的厌氧氨氧化速率高于L3,这是由于厌氧氨氧化反应需要严格的厌氧环境,H4、M4湿地的平均DO质量浓度分别为0.1 mg∙L−1、0.2 mg∙L−1,更有利于厌氧氨氧化的发生。
人工湿地填料生物膜硝化速率(以NH4+-N计,生物量以MLVSS计) 测定结果很低(<0.05 mg∙(g∙h)−1),表明氮素转化过程中,硝化作用不是主导过程。反硝化速率、厌氧氨氧化速率数据结果显示,反硝化速率平均值为厌氧氨氧化速率平均值的23.3倍,说明本实验中反硝化是人工湿地脱氮的主导过程。
反硝化过程需要反硝化酶的参与,其中NAR与NIR(以NO3−-N和NO2−-N计,生物量以MLVSS计)至关重要,NAR负责将NO3−-N还原成NO2−-N,NIR负责将NO2−-N还原成一氧化二氮(N2O)。由图8(c)~(d)可见,H4、M2、M3、M4、L3、L4湿地的NAR与NIR活性均与对照组有显著性差异(P<0.05),添加FWFL后,湿地填料生物膜NAR与NIR活性显著提高,其中H4、M4、L4湿地添加FWFL体积最大,NAR与NIR活性也最高。反硝化速率与反硝化酶活性密切相关,该结果与反硝化速率结果相一致。
硝化反应是氨被微生物氧化为NO3−-N的过程,其中氨单加氧酶(ammonia monooxygenase, AMO)是该过程的关键酶之一。由图8(e)可知,AMO活性(以NO2−-N计,生物量以MLVSS计)随人工湿地的高度增加而降低。这是由于硝化反应需要好氧环境,而随着人工湿地高度增加,DO含量逐渐降低,不利于硝化反应的发生。总体来看,人工湿地的AMO活性均不高,这是由于湿地的DO含量较低(<0.5 mg∙L−1),不利于硝化反应的进行。
反硝化反应是NO3−-N经过一系列还原反应最终被还原成一氧化氮(nitric oxide, NO)或N2的过程,该过程需要电子的参与,通过人工湿地中的电子传递系统转移,最终被反硝化酶消耗。ETSA(以O2计,生物量以蛋白质计)可以反映硝化和反硝化过程中电子的有效传递效率和活性。人工湿地ETSA测定结果如图8(f)所示。可见,湿地的ETSA随着FWFL添加量的增加而增加,其中H4、M4、L4湿地的ETSA最高,与对照组均有显著性差异(P<0.05),表明添加FWFL有利于人工湿地脱氮过程中的有效电子传递。
-
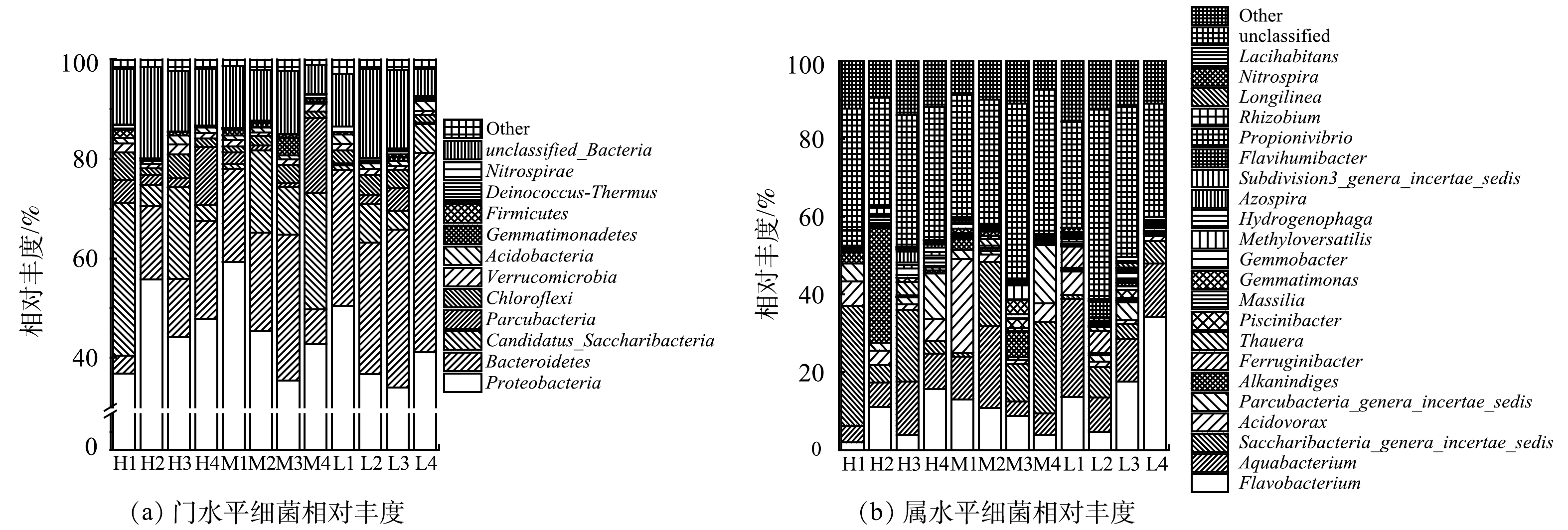
人工湿地填料生物膜微生物群落结构测定结果如图9(a)所示。变形菌门(Proteobacteria,相对丰度为34.1%~59.3%)、拟杆菌门(Bacteroidetes,相对丰度为3.4%~40.2%)、念珠菌门(Candidatus Saccharribacteria,相对丰度为0.9%~30.8%)以及细微杆菌门(Parcubacteria,相对丰度为0.3%~15.0%)为门水平优势菌,比例之和均超过70.0%。此外,在门水平上还发现了较高比例的反硝化菌门,即酸杆菌门(Acidobacteria,相对丰度为0.4%~2.1%)和厚壁菌门(Firmicutes,相对丰度为0.3%~1.6%)。
在湿地系统中变形菌门(Proteobacteria)通常是最大的门类[24]。有研究[25]表明,变形菌门(Proteobacteria)不仅包括反硝化细菌,还包括亚硝化和硝化细菌。拟杆菌门(Bacteroidetes)是具有降解有机物功能的菌群,一些反硝化细菌和大多数固氮微生物也属于这类微生物[26-27]。念珠菌门(Candidatus Saccharribacteria)是反硝化污泥中的主要细菌,主要降解生物难降解的有机物[28-29]。加入FWFL后,湿地中拟杆菌门(Bacteroidetes)与念珠菌门(Candidatus Saccharribacteria)相对丰度分别增加了1.0%~16.1%、2.9%~22.5%,有利于有机物的降解。绿弯菌门(Chloroflexi)、疣微菌门(Verrucomicrobia)均与多糖降解有关,其相对丰度的增加了0.2%~2.5%,可为COD的去除提供证据[30-31]。细微杆菌门(Parcubacteria)、酸杆菌门(Acidobacteria)、芽单胞菌门(Gemmatimonadetes)均具有反硝化菌属[32-34],其相对丰度在FWFL加入后分别提高了1.2%~12.7%、0.1%~0.7%、0.04%~2.4%。
在属水平上进一步研究了不同C/N湿地中微生物的群落结构。如图9(b)所示,其他菌属与未知菌属占比较高(>35.0%)。已知菌属以黄杆菌属(Flavobacterium,相对丰度为2.0%~34.3%)、水杆菌属(Aquabacterium,3.7%~20.9%)、产丁酸盐细菌属(Saccharibacteria genera incertae sedis,相对丰度为0.9%~30.8%)、食酸菌属(Acidovorax,相对丰度为1.0%~24.2%)、Parcubacteria genera incertae sedis(相对丰度为0.3%~15.0%)为主要菌属。黄杆菌属(Flavobacterium)是具有反硝化作用的菌属,拥有很强的硝酸盐还原能力,能够在脱氮中其中起重要作用[35],该菌属大多数为好氧菌,故在TFCW-H、M湿地中的相对丰度少于TFCW-L湿地。水杆菌属(Aquabacterium)与产丁酸盐细菌属(Saccharibacteria genera incertae sedis)都可将NO3−-N还原为NO2−-N[36]。研究表明食酸菌属(Acidovorax)对整个反硝化过程均有贡献[37]。陶厄氏菌属(Thauera)也是反硝化脱氮的主要细菌[38]。此外,双鱼杆菌属(Piscinibacter)、芽单胞菌属(Gemmatimonas)、芽殖杆菌属(Gemmobacter)、噬氢菌属(Hydrogenophaga)等均具有反硝化功能[39-43]。除反硝化细菌外,有研究[44]表明,Parcubacteria genera incertae sedis可在一定程度上进行厌氧氨氧化。在H4(11.7%)、M4(15.0%)湿地中的相对丰度最高,可能是由于TFCW-H、M湿地中DO质量浓度(≤0.3 mg∙L−1)较低,更有利于其生长。该结果也与图8(b)中厌氧氨氧化速率的变化结果相似。
-
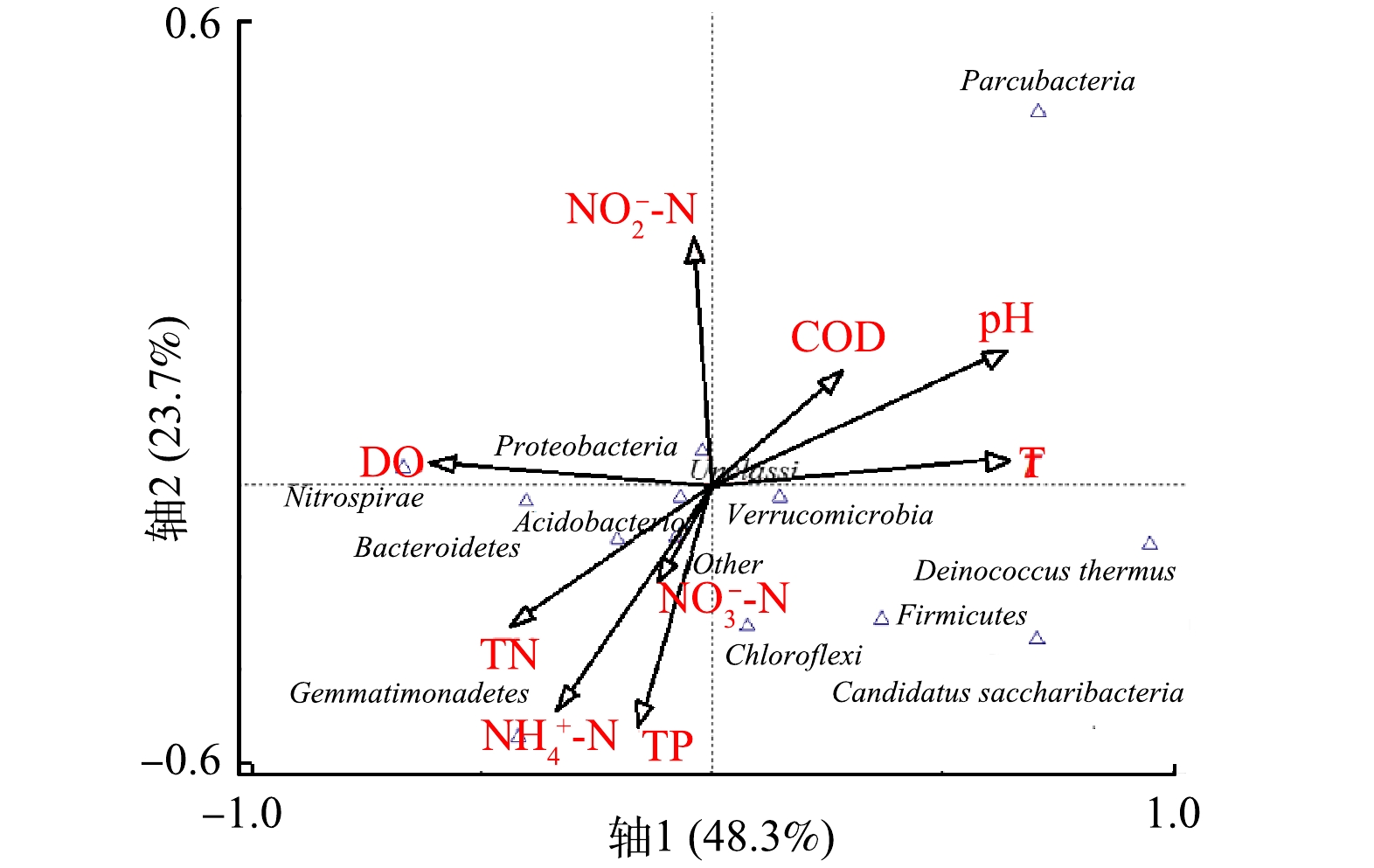
图10反映了人工湿地门水平微生物群落与9种环境因子间的关系。主成分(轴1和轴2)可以解释72.0%的微生物菌群,表明结果具有一定参考意义。轴1与水温、pH、COD为正相关,与DO、NH4+-N、NO3−-N、NO2−-N、TN、TP为负相关。轴2与水温、pH、DO、COD、NO2−-N为正相关,与NH4+-N、NO3−-N、TN、TP为负相关。同时,人工湿地门水平微生物群落与环境因子的CCA分析结果较好的反映了人工湿地门水平微生物群落与环境因子的相关性。
结果表明,变形菌门(Proteobacteria)、拟杆菌门(Bacteroidetes)、酸杆菌门(Acidobacteria)、芽单胞杆菌门(Gemmatimonadetes)、硝化螺旋菌门(Nitrospirae)与DO、NH4+-N、TN、TP呈显著正相关,与NO2−-N呈负相关,与水温、pH呈强烈负相关。念珠菌门(Candidatus Saccharribacteria)、厚壁菌门(Firmicutes)、疣微菌门(Verrucomicrobia)与水温、pH、COD呈显著正相关,与DO、NO2−-N呈显著负相关。绿弯菌门(Chloroflexi)与NH4+-N、TN、TP呈显著正相关,与NO2−-N呈显著负相关。细微菌门(Parcubacteria)与水温、pH、COD、NO2−-N呈显著正相关,与DO、NH4+-N、TN、TP呈显著负相关。总的来看,DO、TN、水温、pH对人工湿地门水平微生物群落结构均具有显著影响。
-
1)添加FWFL至C/N为8时可显著提高人工湿地脱氮效率,TN平均去除率可达72.4%~78.9%。水温低于15 ℃时,FWFL的添加可以改善低温对脱氮的不利影响。C/N相同时,适当提高人工湿地高度也有助于提高脱氮效率。
2)添加FWFL未对湿地出水NH4+-N与COD产生显著影响;添加FWFL后湿地NO3−-N的去除率提高了3.3%~42.3%,TP去除率提高了11.2%~45.8%。
3)添加FWFL后,湿地填料表面附着生物膜的反硝化速率、反硝化酶活性以及ETSA活性均显著升高(P<0.05),进而提高了人工湿地的脱氮效率。
4)湿地中的优势反硝化菌属为黄杆菌属(Flavobacterium)、水杆菌属(Aquabacterium)、产丁酸盐细菌属(Saccharibacteria genera incertae sedis)、铁杆菌属(Ferruginibacter)、陶厄氏菌属(Thauera)。添加FWFL后人工湿地中反硝化菌的相对丰度也有所增加。DO、TN、水温、pH是影响湿地门水平微生物群落结构的主要因素。
Enhanced denitrification of sewage treatment plant effluent by tidal flow constructed wetland with food waste fermentation liquid addition
- Received Date: 25/07/2022
- Available Online: 26/02/2023
-
Key words:
- tidal flow constructed wetland /
- food waste fermentation liquid /
- effluent of sewage treatment plant /
- denitrification /
- denitrifying bacteria
Abstract: The food waste fermentation liquid (FWFL) was used as the carbon source of the tidal flow constructed wetland (TFCW), and the effect of FWFL addition on nitrogen removal of sewage treatment plant effluent was investigated. Furthermore, the denitrification mechanism was explored through the determination of wetland nitrogen conversion rate, enzyme activity and microbial community structure. The results showed that, after adding FWFL in the TFCW, the removal rates of TN, NO3−-N and TP in sewage treatment plant effluent increased by 15.7%~36.2%, 3.3%~42.3% and 11.2%~45.8%, respectively, and the addition of FWFL did not have a significant impact on effluent NH4+-N and COD, while it could improve the denitrification effect of the TFCW at low temperatures; the rates of denitrification, denitrification enzyme activity and electron transport system activity of the TFCW increased with FWFL addition; the richness and diversity of wetland microorganisms were significantly improved, the microbial community structure also tended to be stable, and the denitrifying microbiota increased significantly. Proteobacteria, Bacteroidetes, Candidatus Saccharribacteria were the dominant phylums, Aquabacterium and Saccharibacteria genera incertae sedis were the dominant genus.





 DownLoad:
DownLoad:
