-
电催化氧化技术(ECOP)广泛应用于难生化废水的处理,具有处理效率高、占地面积小、无药剂添加和易于管理等优点,ECOP可以将大分子污染物以及难生化有毒污染物分解成羧酸、丙酮等小分子物质,进而可以缓慢地将这些小分子有机物进一步氧化分解成CO2。在ECOP中起到主导作用的是阳极材料,钛基氧化物涂层电极(DSA)[1]是目前常用的阳极材料,其中Ti/SnO2-Sb电极被认为是DSA电极中氧化效率较高的电极。目前,钛基锡锑电极(Ti/SnO2-Sb)存在的主要问题是电极催化效率偏低和催化层容易脱落[2]。Ti/SnO2电极改性的研究很多都集中在掺杂稀土元素[3-6]以及使用新型的双向脉冲式电沉积工艺优化,以此提高电催化氧化效果[7-8],通过钛基底改性进而提高催化效率的研究[9-10]较少。为了提高Ti/SnO2-Sb电极的催化效率,本研究采用阳极氧化法[11],在钛板上制备垂直生长的空心二氧化钛纳米管(TiO2-NTs),并以此为基底,制备纳米管修饰的锡锑电极(TiO2-NTs/SnO2-Sb),进而通过与Pt、TiO2-NTs和Ti/SnO2-Sb电极对比研究,阐明其高效降解有机物的主要机制。
目前,对于锡锑电极的处理研究多停留在实验室处理模拟污染物,而对于实际工业废水的处理研究较少。有研究[12]指出,钻井废水中含有较多的高分子聚合物和丙烯腈等有害物质,具有色度深、高COD值和难生化处理的特点。国内对于钻井废水的处理技术主要有混凝法、臭氧氧化法、Fenton试剂氧化法和生物法等[13-16],但是这些方法单一使用存在处理效果差、易产生二次污染的缺点。本研究以制备出的高效TiO2-NTs/SnO2-Sb电极作为阳极材料处理实际钻井废水,考察TiO2-NTs/SnO2-Sb电极对于实际钻井废水的处理效果,这对该电极应用于难生化降解有机废水的ECOP处理有着重要的现实意义。
-
以电极基底为纯钛板(尺寸均为20 mm×20 mm),先用电磨机打磨钛板表面,之后用400目砂纸打磨,去除表面氧化物。将打磨后的钛板放入蒸馏水中,超声清洗15 min,最后在质量分数为5%的草酸溶液中,90 ℃下酸洗20 min,使钛板表面蚀刻成均匀的灰白色麻面。
以预处理后的钛板作为阳极,不锈钢板为阴极,通过阳极氧化实验,制备二氧化钛纳米管。在40 V电压条件下,氧化60 min。电解质溶液由300 mL乙二醇加入1.3 g的NH4F和11 mL超纯水组成。阳极氧化后的钛板,最初是无定形的。将阳极取出,在充满氮气的环境中干燥10 min后,放入马弗炉中,以5 ℃·min−1的升温速率加热至450 ℃,恒温煅烧1 h,即得到TiO2-NTs。
-
通过电沉积法制备SnO2-Sb电极,以上述制备的TiO2-NTs或Ti板作为阴极,以铂片为阳极,电沉积液由0.2 mol·L−1的SnCl4、0.008 mol·L−1的SbCl3和体积分数为2%的浓盐酸 (37%)组成,以正丁醇为溶剂,以5 mA·cm−2的电流密度电沉积40 min。取出沉积后的阴极,用蒸馏水冲洗表面残余的盐酸。将其放入100 ℃的干燥箱中,干燥40 min,随后放入马弗炉中,以10 ℃·min−1的升温速率加热至600 ℃,恒温煅烧2 h,即得到SnO2-Sb电极。
-
苯酚初始浓度为100 mg·L−1,实测COD为270 mg·L−1,反应体积为100 mL,取相同体积的苯酚溶液于200 mL电解槽中,以Pt、TiO2-NTs、Ti/SnO2-Sb和TiO2-NTs/SnO2-Sb电极作为待测阳极,钛板为阴极,0.05 mol·L−1硫酸钠为电解质,电流密度为20 mA·cm−2。使用4-氨基安替比林法[17]测定苯酚浓度和相同时刻的COD值,以此反映苯酚降解过程中的矿化效果。在电催化氧化反应中,苯酚浓度随电解时间的变化可用一级反应动力学的关系式(1)表示。
式中:
$C_{{\rm{b}}_0} $ 为初始的苯酚浓度,mg·L−1;$C_{{\rm{b}}_t} $ 为t时刻的苯酚浓度,mg·L−1;k为一级反应学动力常数,min−1;t为反应时间,min。基于COD测试结果,可以计算出不同电极降解苯酚过程中平均电流效率(η),从t0到t时刻之间的平均电流效率可用式(2)表示。式中:η为平均电流效率;C0和Ct分别是t0和t时刻的COD值,mg·L−1;I为电解时电流,A;t为时间,h;F为法拉第常数,取值96 485 C·mol−1;V为表示溶液体积,L;8为氧当量值。
-
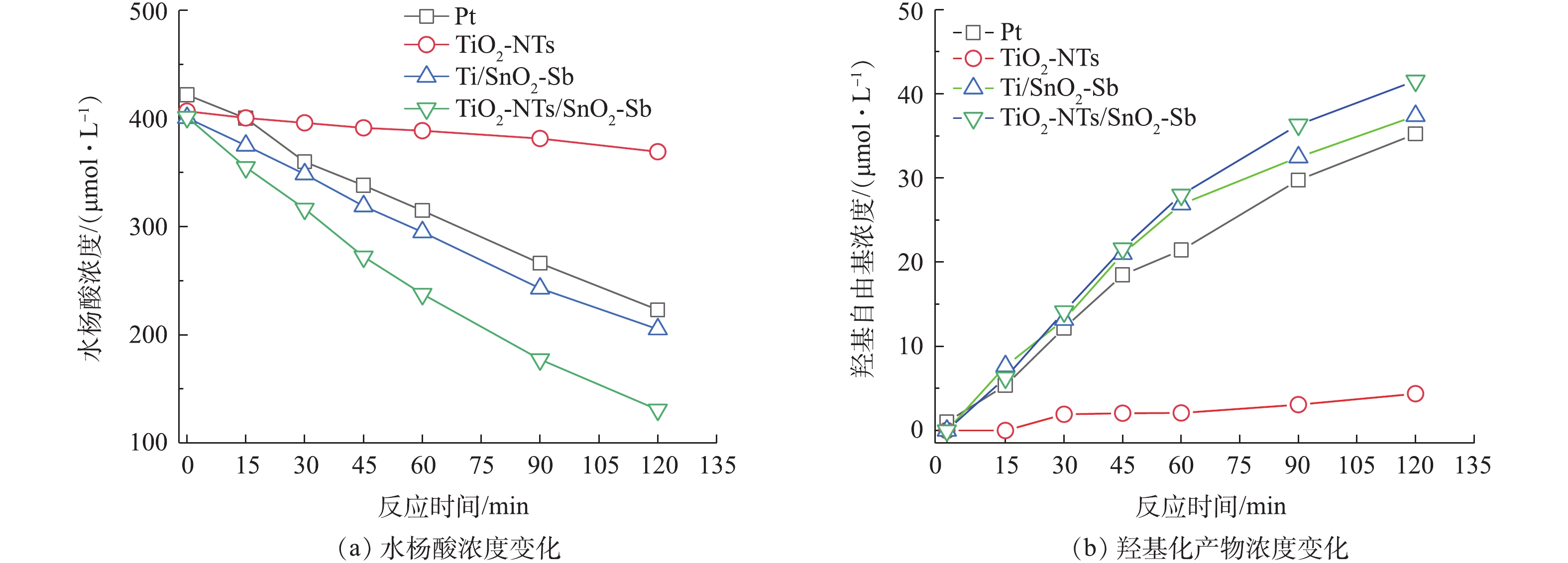
4种待测目标电极为阳极,钛板为阴极,0.05 mol·L−1硫酸钠为电解质,电流密度20 mA·cm−2。400 μmol·L−1水杨酸作为羟基自由基( · OH)捕获剂,阳极表面产生的 · OH与水杨酸反应,生成2,5-二羟基苯甲酸和2,3-二羟基苯甲酸2种羟基化产物,2种羟基化产物浓度的加和近似等于 · OH的浓度,通过高效液相色谱分析3种物质浓度随反应时间的变化。液相色谱分析条件:液相色谱柱采用Agilent Plus C18柱(4.6 mm×250 mm);流动相为超纯水和甲醇,体积比为65∶35,流速1.0 mL·min−1,柱温设置为30 ℃,进样量20 μL,紫外检测波长310 nm,数据采集频率20 Hz。
-
实验用水采自鄂尔多斯盆地某钻井废液池。处理条件:处理废液体积100 mL,以4种待测电极为阳极,钛板为阴极,电流密度40 mA·cm−2,使用稀释倍数法[18]测试水样色度,比较24 h的水样色度及COD去除效果。实际废水的电化学处理效果与很多因素有关,除了与废水中污染物的类型有关之外,也和废水中无机盐种类和含量以及pH有着密切关系[19],在电化学氧化过程中,电极表面可能会伴随着析氯析氧等多种副反应。钻井废液的各项水质指标如下:外观为黑色,TDS为16.48 g·L−1,COD为7 912.5 mg·L−1,色度为2 750倍。为探究4组电极对难生化有机废水的ECOP处理效果,实验将4组目标电极应用于钻井废液的实际处理。
-
采用SEM和XRD,表征分析TiO2纳米管及制备的SnO2-Sb电极的表面形貌和表面晶体组成。线性伏安扫描法LSV (CH Instruments Model 600E)实验分别测试4组电极的析氧电位,以Ag/AgCl为参比电极,待测目标电极为工作电极,钛板为对电极,溶液中含有0.25 mol·L−1的Na2SO4,扫描范围0.4~3.0 V,扫描速率为20 mV·s−1。为了研究不同电极处理钻井废液过程中污染物在电极表面的毒化作用可能对电极性能的影响,实验通过LSV结果数学线性拟合出Tafel图,并分析降解过程中电极析氧反应动力学(OER)参数的变化情况。在一定范围内,超电势(v)与电流密度(i)的关系见式(3)。
式中:v为超电势,V;a为Tafel曲线的截距,反映了电极体系中进行电子转移步骤的难易程度,V;b为Tafel曲线的斜率,反映了电催化氧化反应活性的高低,V;i0为交换电流密度,表征了平衡条件下电极进行电化学反应的速度和去极化能力,mA·cm−2。
-
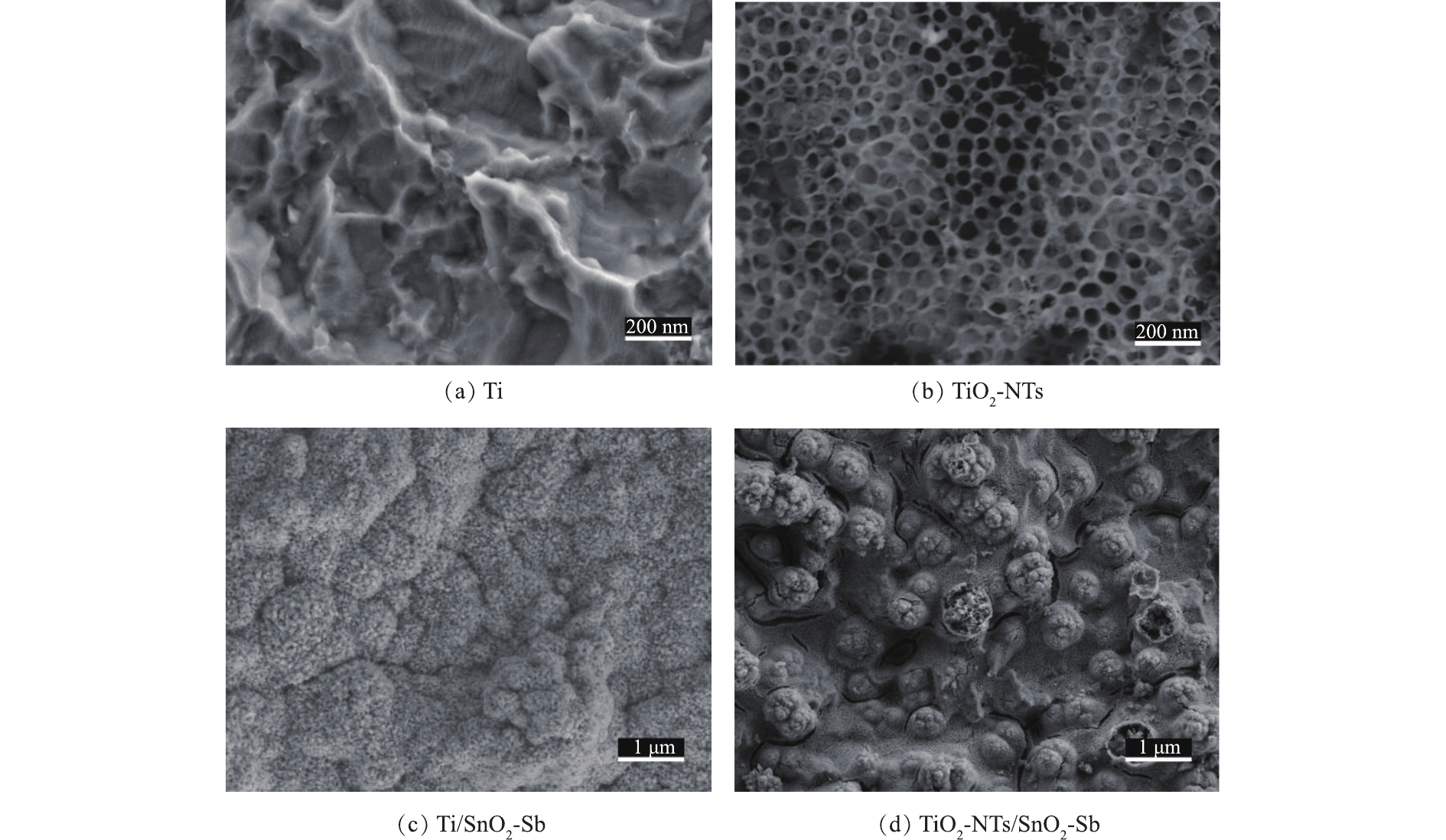
为了更直观地了解基底和SnO2-Sb电极的形貌,用SEM分别表征2种基底及对应SnO2-Sb电极的表面形态(见图1)。图1(a)为预处理后的钛基板,可以看出灰色麻面的钛板表面形貌比较粗糙。图1(b)为TiO2-NTs微观结构,可以看出钛基表面形成了高度有序垂直排列的具有顶部开口的纳米管状结构,纳米管顶部孔径为90~140 nm。图1(c)和图1(d)分别为Ti、TiO2-NTs基底经过电沉积后的SnO2-Sb电极形貌。由图1(c)可以看出,锡锑催化涂层均匀地覆盖在钛基表面,并没有发现表面涂层有裂缝,这样更有利于涂层保护基底不被氧化。图1(d)的表面有明显的圆球凸起状结构,这可能是纳米管顶部形成特有的凸起状结构。这样形成的凸起结构有可能为SnO2-Sb电极提供更多的活性位点[20]。
-
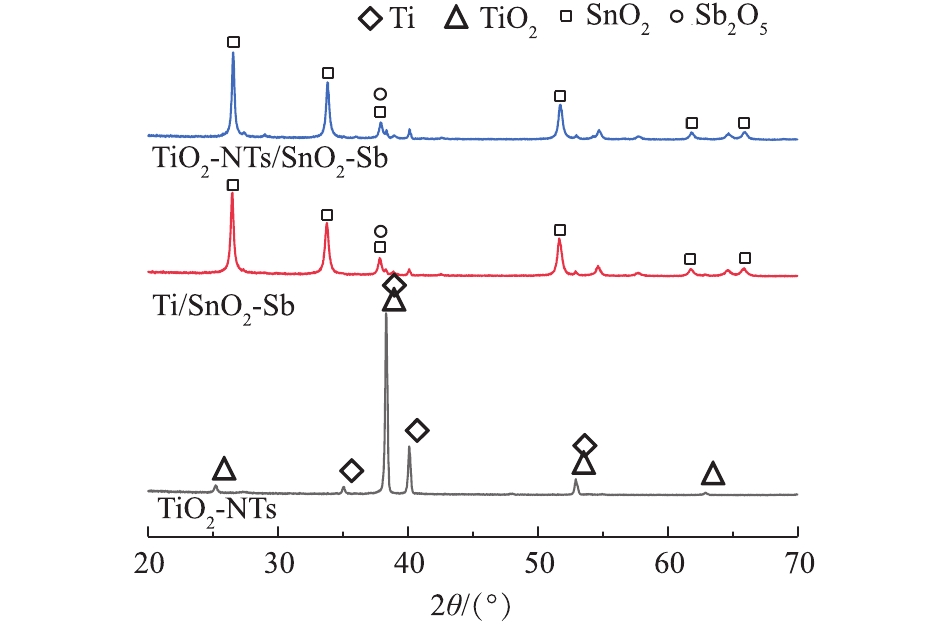
为了解基底和电极表面晶体的物相组成,对TiO2-NTs、Ti/SnO2-Sb和TiO2-NTs/SnO2-Sb进行了XRD分析。图2为其XRD衍射峰谱图。将TiO2-NTs的图谱与锐钛矿TiO2标准卡(PDF 21-1272)对比发现,其中25.3°、37.8°、53.9°和62.7°位置的衍射峰分别对应着锐钛矿TiO2(101)、(004)、(105)和(204)的晶格平面。其中25.3°为锐钛矿TiO2典型的特征峰,说明通过阳极氧化法在450 ℃高温热处理后得到的TiO2纳米管主要以锐钛矿的形式存在。锐钛矿TiO2作为一个拥有较宽带系的n型半导体材料,更容易被激发生成羟基自由基[21]。Ti/SnO2-Sb、TiO2-NTs/SnO2-Sb的XRD衍射峰谱图对比SnO2标准卡(PDF 46-1008)可以看出,26.5°、33.7°、37.8°、51.8°、61.8°和65.7°位置的衍射峰分别对应着SnO2(110),(101)、(200)、(211)、(310)和(301)的晶格平面。其中38.1°和40.1°的衍射峰对应着Sb2O5(111)和(202)的晶格平面,说明锡锑氧化物涂层在电极表面主要是SnO2和Sb2O5。Ti/SnO2-Sb和TiO2-NTs/SnO2-Sb的衍射峰的谱图几乎完全相同,说明2种电极表面锡锑氧化物催化涂层晶体组成相同。
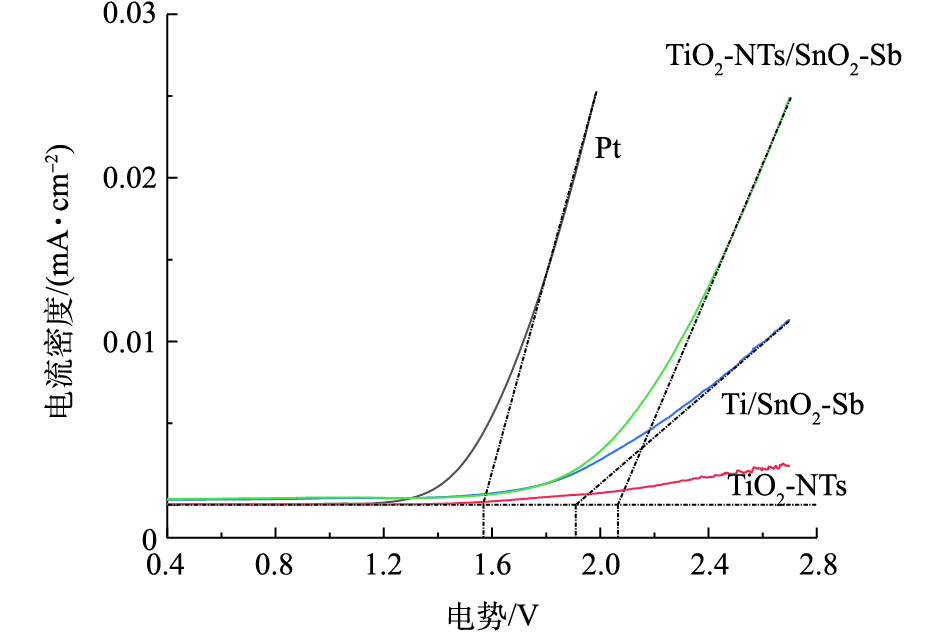
为了探究TiO2-NTs对于SnO2-Sb电极析氧电位的影响,分别测试了4组目标电极的析氧电位。图3为4组电极的线性伏安扫描曲线,阳极在电解过程中除了能产生 · OH,还会不可避免地发生析氧副反应,阳极析氧电位越高,相同条件下阳极的析氧副反应就会越弱。线性伏安扫描法(LSV)分析待测阳极的析氧电位,LSV曲线分成2部分:第1部分是储能阶段,随着电压的增加几乎没有电流通过;第2部分是析氧阶段,电流随着电压的升高而急剧升高。通常认为,析氧电流急剧上升段接近直线部分的反向延长线与横坐标轴的交点对应的电势即为析氧电位。由图3看出,Pt电极析氧电位接近1.6 V。Ti/SnO2电极的析氧电位在1.9 V左右,跟之前报道的结果[22]一致。TiO2-NTs修饰后电极的析氧电位明显增加,析氧电位在2.03 V左右。电极析氧电位的提高将减少析氧副反应的发生,更有利于电极产生更多的 · OH。
-
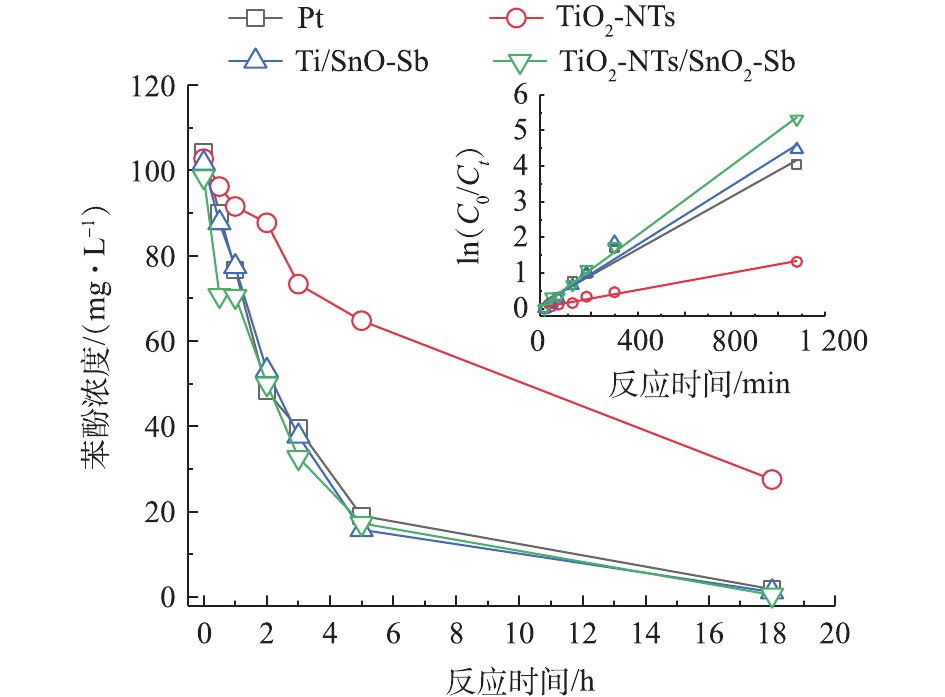
为了比较4组电极对模型有机物苯酚的降解及矿化效果,研究了不同电极对苯酚的降解速率。图4为待测目标电极降解苯酚随时间的变化曲线及一级动力学拟合曲线,降解苯酚一级动力学拟合结果表明,4组电极降解苯酚速率的顺序为TiO2/SnO2-Sb>Ti/SnO2-Sb>Pt>TiO2-NTs,对应的一级反应学动力常数k分别为0.004 9、0.004 1、0.003 7、0.001 2 min−1,且R2值均大于0.98。TiO2-NTs作为阳极,氧化苯酚速率最慢,这可能是由于TiO2自身作为半导体材料导电性较差,阻碍了电极的电子传递,不适合直接作为电极材料。SnO2-Sb电极氧化苯酚速率较快,降解速率略高于Pt电极。相比于Ti修饰SnO2-Sb电极,TiO2-NTs修饰的电极对苯酚的降解速率更高。
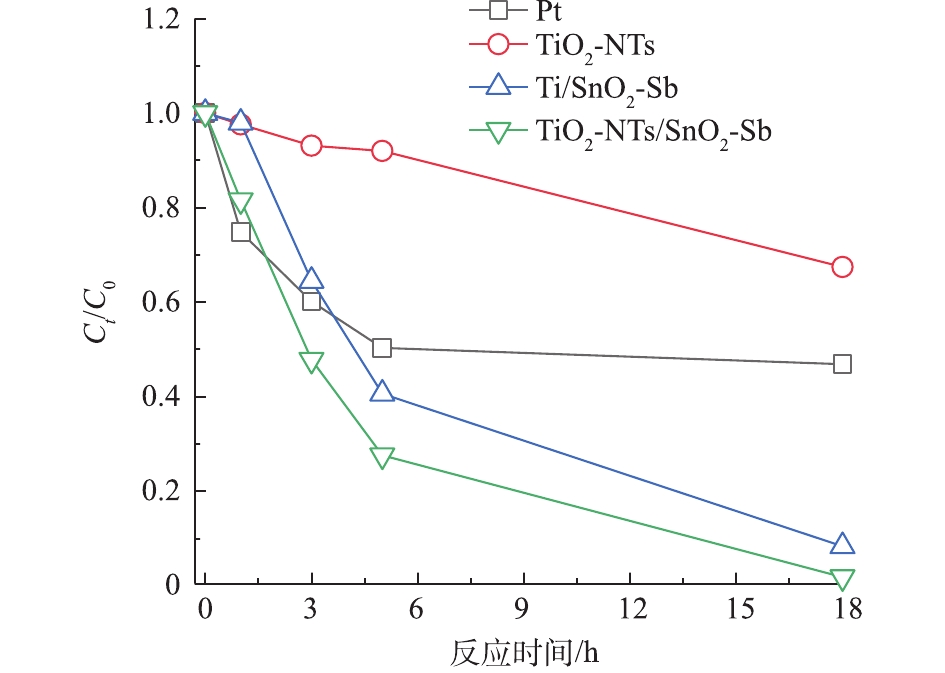
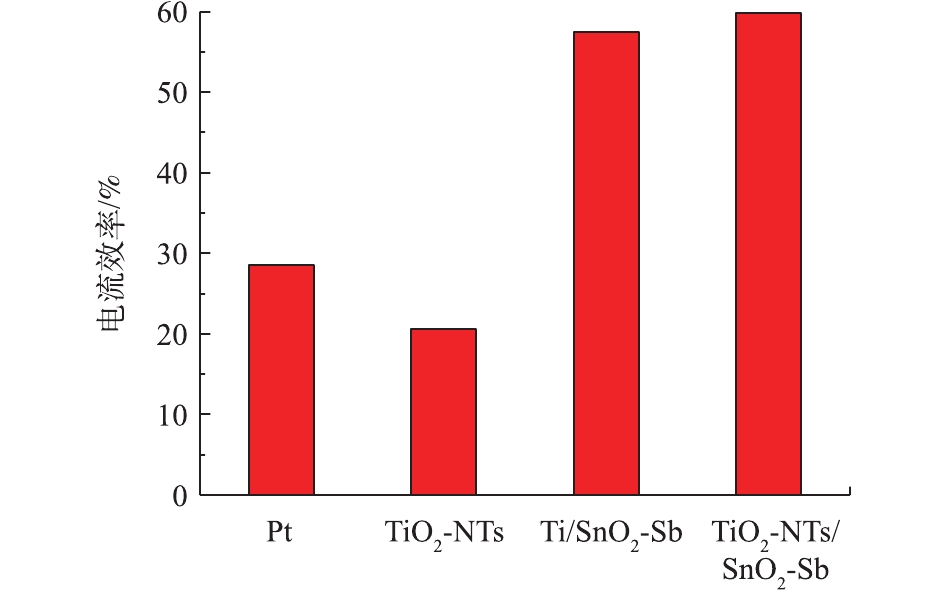
通过测定不同时间COD值来反映电极对苯酚的矿化效果。图5为待测目标电极在降解苯酚过程中COD的变化情况。可以看出,TiO2-NTs作为阳极,对苯酚的矿化效果最差,COD变化很小,这也与苯酚降解浓度变化结果一致。TiO2-NTs/SnO2-Sb要比Ti/SnO2-Sb和Pt电极矿化苯酚的效果好,说明TiO2-NTs的引入提高了电催化氧化能力,提高了对苯酚的矿化效果。由图6可以看出,不同电极降解苯酚18 h的电流效率大小顺序为TiO2/SnO2-Sb>Ti/SnO2-Sb>Pt>TiO2-NTs,这与测试电极析氧电位顺序结果一致。在降解苯酚过程中,电极的析氧电位越高,其析氧副反应越弱,对污染物的矿化能力越强,其电流效率也就越高。
-
通过测定4组目标电极羟基自由基的产生量来进一步说明TiO2-NTs改性钛基底后对电极性能的影响。图7为4组电极羟基自由基测试实验曲线图,由图7(b)看出,羟基自由基产生速率大小顺序为TiO2-NTs/SnO2-Sb> Ti/SnO2-Sb>Pt>TiO2-NTs。这与苯酚降解实验中COD矿化度效果一致,说明降解苯酚矿化度的差异主要是电极产生 · OH不同导致的, · OH产生量越多,对于苯酚的矿化效果越好。结果还发现,TiO2-NTs作为阳极也能产生少量的羟基自由基,说明TiO2-NTs可能对TiO2-NTs/SnO2-Sb电极产生 · OH具有一定的贡献。
-
表1为不同电极处理钻井废液24 h水样色度变化结果。可以看出,不同电极处理废水后色度差异较大,说明4组电极对于钻井废液的处理效果具有明显的差异。电化学氧化钻井废液处理6 h后,Pt、Ti/SnO2-Sb、TiO2-NTs/SnO2-Sb电极处理的钻井废液均变成砖红色,TiO2-NTs电极处理的水样仍为黑色。处理12 h后,TiO2-NTs/SnO2-Sb的水样经过0.45 μm玻璃纤维膜过滤后,水样呈无色透明。处理24 h后,Pt、Ti/SnO2-Sb、TiO2-NTs/SnO2-Sb作阳极处理水样均呈无色透明液体。
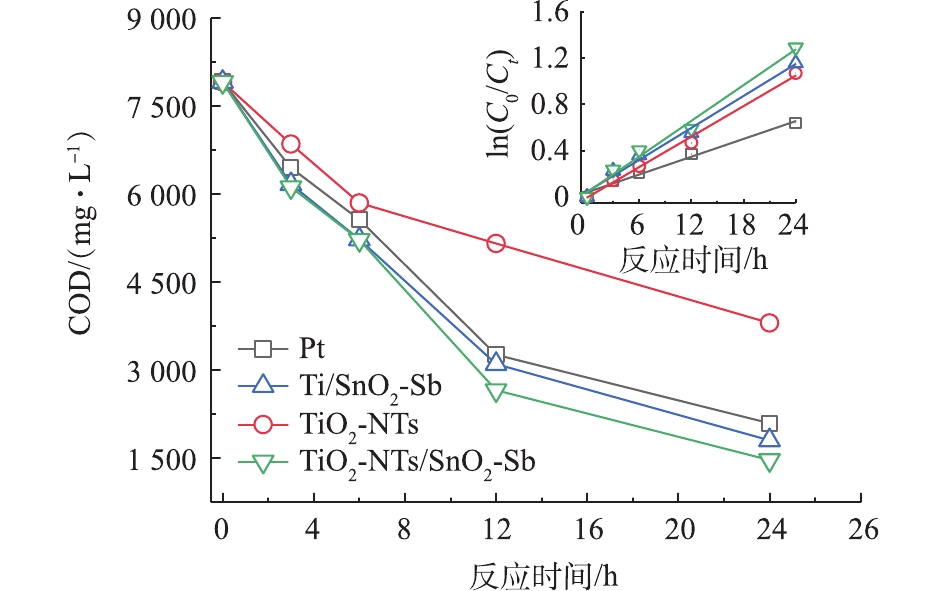
图8为4组目标电极处理钻井废水的COD降解曲线及一级动力学拟合曲线。拟合结果表明,4组电极对钻井废液COD降解符合一级反应动力学,反应速率的大小顺序为TiO2-NTs/SnO2-Sb>Ti/SnO2-Sb>Pt>TiO2-NTs,对应的一级反应学动力常数k分别为0.056 7、0.029 1、0.061 8、0.071 4 h−1,且R2值均大于0.97。这与之前的处理结果顺序相一致。有研究指出,TiO2-NTs作为阳极只会产生极少量的 · OH[21],因此,对于钻井废水的处理效果不佳。对于Pt电极作为阳极对其氧化作用机理并没有完全的定论,MARCO等[22]指出,Pt作为阳极材料,对于污染物的氧化是不完全的,只能将大分子有机物分解为小分子物质,对于醋酸和马来酸等小分子物质无法继续分解。但对于SnO2电极作为阳极的氧化是彻底的,可以实现对污染物的完全矿化,而TiO2-NTs修饰后提高了SnO2电极产生 · OH的能力,因此,处理效果最好,TiO2-NTs /SnO2-Sb对钻井废水的COD去除率最高,处理24 h后的COD去除率达到81.4%。
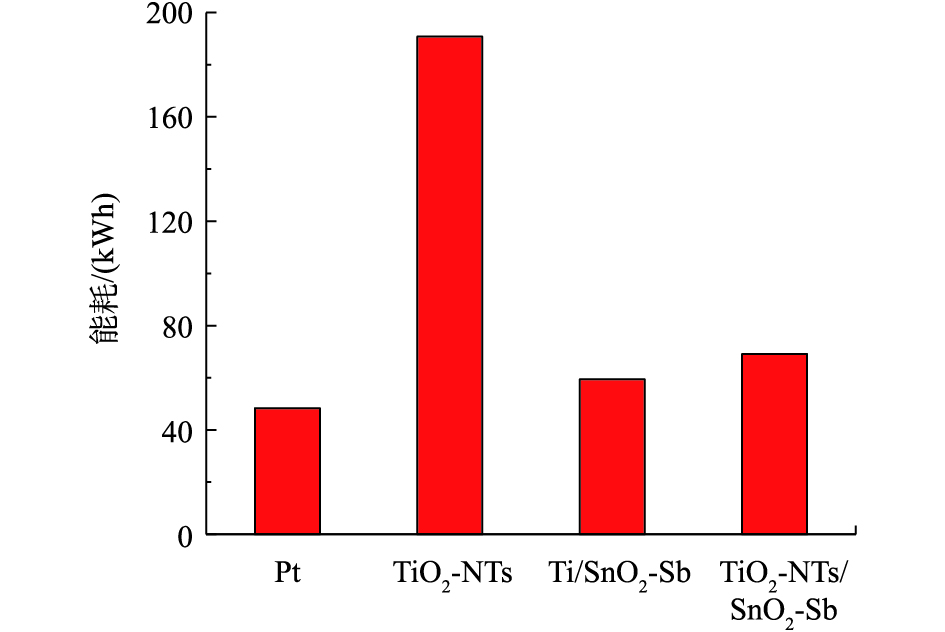
图9为4组目标电极处理钻井废液24 h的能耗计算。可以看出,TiO2-NTs的能耗最高,TiO2作为导电性较差的半导体材料,材料阻值较高,因此,在相同的电流条件下,需要外加更大的电压,能耗最高。其他3组电极的能耗接近,其中Pt电极能耗最小,这是由于Pt电极属于导电性较好的金属催化材料。TiO2-NTs/SnO2-Sb能耗略高于Ti/SnO2-Sb,这是由于TiO2-NTs修饰后,增加了电极的电子传递阻力,从而增加了能耗。
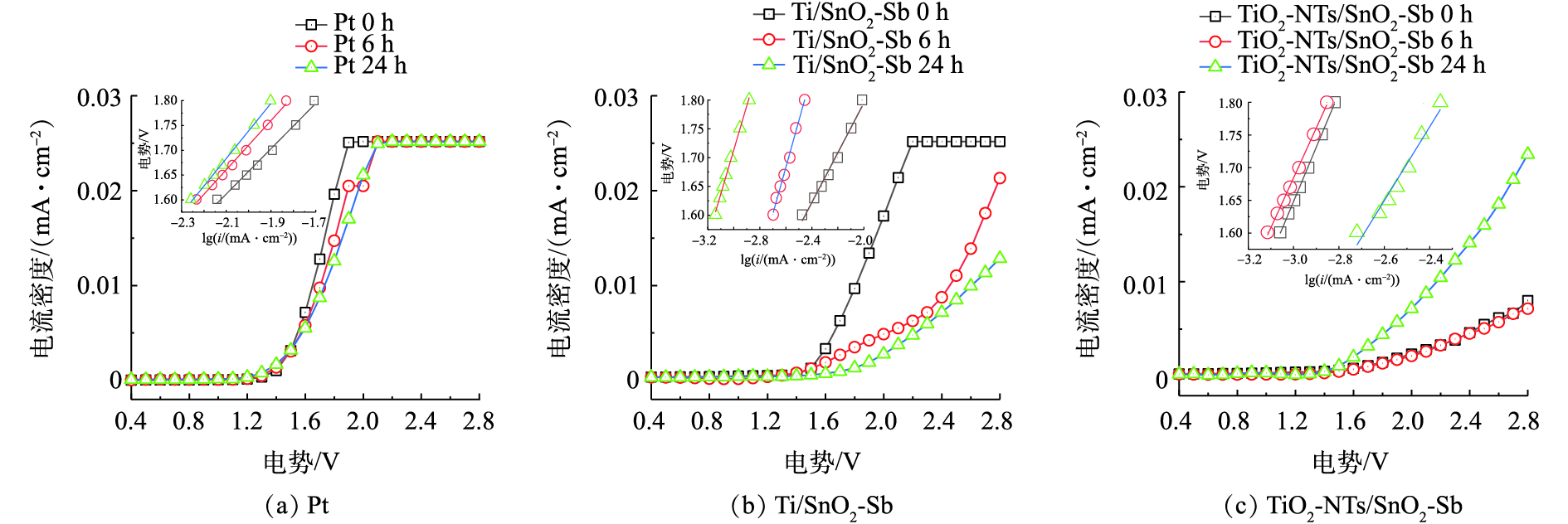
对于电催化氧化处理高COD污水,阳极表面会出现 “中毒效应”[22]:即在阳极表面会生成一层污染物薄膜,这层薄膜一般由有机聚合物组成,从而阻碍电子传递,降低催化氧化能力。为了探究电极材料在降解过程中毒化作用可能对电极造成的影响,考察电催化氧化钻井废液不同时间的LSV曲线,并拟合出对应的塔菲尔(Tafel)拟合图(图10),分析污染物对不同阳极材料的毒化作用情况。
由图10的Tafel拟合结果计算出拟合动力学参数(见表2),由塔菲尔拟合动力学参数看出,Pt电极和Ti/SnO2电极在电催化氧化处理钻井废液一段时间后,a值增大,说明电极的电子转移受到阻碍,且b值也增大,说明电极的催化活性也同时降低。这可能与电极表面的中毒效应有关。而TiO2-NTs/SnO2-Sb电极处理钻井废液后,催化活性反而会增加,这说明TiO2-NTs纳米管可以改善或避免阳极的中毒效应。
-
1)通过改变钛基底的微观结构制备了TiO2-NTs/SnO2-Sb电极,通过SEM和XRD表征分析可知,TiO2-NTs表面为垂直空心纳米管状形貌,孔径为90~140 nm,纳米管主要是锐钛矿TiO2。SnO2-Sb电极表面涂层致密均匀地覆盖在电极表面,涂层主要由SnO2和Sb2O5这2种晶体组成。对于纳米管修饰的TiO2-NTs/SnO2-Sb电极,推测锡锑氧化物可能进入纳米管内部,提高了电极表面垂直空间的利用效率,从而负载更多的锡锑氧化物,有利于提高电极催化效率。
2)从线性伏安扫描结果看出,析氧电位大小顺序为TiO2-NTs/SnO2-Sb>Ti/SnO2-Sb>Pt。说明TiO2-NTs修饰电极可以提高电极的析氧电位,从而减少电解中析氧副反应的发生,有利于产生更多的羟基自由基。
3)在降解苯酚实验中,4组目标电极对苯酚的降解速率和矿化效果为TiO2-NTs/SnO2-Sb>Ti/SnO2-Sb>Pt>TiO2-NTs,这与析氧电位的顺序结果一致。羟基自由基捕获实验结果表明,TiO2-NTs修饰电极提高了羟基自由基的产生量,这是电极提高苯酚矿化效果的主要原因。电流效率TiO2-NTs /SnO2-Sb>Ti/SnO2-Sb>Pt>TiO2-NTs。
4)在钻井废水处理实验中,除TiO2-NTs外,其他3种电极对钻井废水具有良好的脱色效果和高的COD去除率。TiO2-NTs/SnO2-Sb处理效果最好,处理24 h后,COD去除率达到81.4%,脱色效果良好,且能有效缓解阳极的“中毒效应”。
修饰型TiO2锡锑电极的制备及其在钻井废水处理中的应用
Preparation of Sb-doped SnO2 electrode with titanium dioxide nanotubes on Ti substrate and its application in drilling wastewater treatment
-
摘要: 为了解决传统锡锑电极电催化氧化效果偏低、涂层易脱落的问题,使用阳极氧化法,在钛基底表面制备垂直生长的二氧化钛纳米管(TiO2-NTs), 然后在此基底上采用电沉积法制备出锡锑电极(TiO2-NTs/SnO2-Sb)。结果表明,相比没有二氧化钛纳米管的锡锑电极(Ti/SnO2-Sb),TiO2-NTs/SnO2-Sb 电极的析氧电位从1.9 V 增加到2.03 V,电极具有较高的羟基自由基生成能力和更高的电流效率,促进了苯酚的矿化。TiO2-NTs/SnO2-Sb 电极对实际钻井废水具有较好的脱色效果,COD去除率高达81.4%,且能够有效地改善阳极的“中毒效应”。Abstract: This study aimed to address the low electro-catalytic oxidation effect and coating falling off easily of traditional Sb-doped SnO2 electrode. The vertically grown titanium dioxide nanotubes (TiO2-NTs) structure was first formed on the Ti plate by anodization, then the Sb-doped SnO2 electrode (TiO2-NTs/SnO2-Sb) was prepared on this modified Ti plate by electrodeposition. The results showed that compared to the Sb-doped SnO2 electrode without titanium dioxide nanotubes (Ti/SnO2-Sb) on the Ti plate, the oxygen evolution potential of TiO2-NTs/SnO2-Sb electrode increased from 1.9 V to 2.03 V, which contributed to the phenol mineralization enhancement due to its high capacity for hydroxyl radicals generation and higher current efficiency. The TiO2-NTs/SnO2-Sb electrode can completely remove the color of the drilling wastewater, and the COD removal rate was as high as 81.4%. In addition, the “Poisoning effect” of the anode was alleviated during the treatment of drilling wastewater.
-
Key words:
- electrochemical oxidation /
- TiO2 nanotube /
- SnO2 electrode /
- hydroxyl radical
-

-
表 1 不同阳极条件下钻井废水色度变化
Table 1. Color variation of the drilling wastewater with different anodes
反应时间/h 色度/倍 TiO2-NTs Pt Ti/SnO2-Sb TiO2-NTs/SnO2-Sb 0 2 750 2 750 2 750 2 750 6 2 600 2 000 1 500 1 500 12 1 000 600 400 400 24 800 50 0 0 表 2 不同电极电催化钻井液过程Tafel拟合动力学参数
Table 2. Tafel fitting kinetic parameters of anodes during the drilling wastewater treatment
电极材料 a/V b/V R2 Pt(0 h) 2.57 0.45 0.992 Pt(6 h) 2.70 0.49 0.995 Pt(24 h) 2.85 0.56 0.984 Ti/SnO2-Sb(0 h) 2.66 0.43 0.998 Ti/SnO2-Sb(6 h) 3.79 0.81 0.993 Ti/SnO2-Sb(24 h) 4.01 0.76 0.997 TiO2-NTs/SnO2-Sb(0 h) 4.13 0.83 0.999 TiO2-NTs/SnO2-Sb(6 h) 3.97 0.76 0.998 TiO2-NTs/SnO2-Sb(24 h) 3.11 0.56 0.960 -
[1] 孔德生, 吕文华, 冯媛媛, 等. DSA电极电催化性能研究及尚待深入探究的几个问题[J]. 化学进展, 2009, 21(6): 1107-1117. [2] 徐浩, 邵丹, 杨鸿辉, 等. Ti/Sb-SnO2电极电解后的表面状态变化[J]. 西安交通大学学报, 2014, 48(2): 93-98. [3] ZHU F L, MENG Y S, HUANG X Y. Electro-catalytic degradation properties of Ti/SnO2-Sb electrodes doped with different rare earths[J]. Rare Metals, 2016, 35(5): 412-418. doi: 10.1007/s12598-014-0397-x [4] LI S P, ZENG X Y, JIANG Y Y. The study on the effect of Er on the structure and properties of Ti/SnO2-Sb anode prepared by pechini’s method[J]. Advanced Materials Research, 2013, 699: 724-729. doi: 10.4028/www.scientific.net/AMR.699 [5] FENG Y, CUI Y H, LIU J, et al. Factors affecting the electro-catalytic characteristics of Eu doped SnO2/Sb electrode[J]. Journal of Hazardous Materials, 2010, 178(1): 29-34. [6] LI S, LI Y, WANG W, et al. Electro-catalytic degradation pathway and mechanism of acetamiprid using Er doped Ti/SnO2-Sb electrode[J]. RSC Advances, 2015, 5(84): 68700-68713. doi: 10.1039/C5RA09376G [7] MERATI Z, PARSA J B. Enhancement of the catalytic activity of Pt nanoparticles toward methanol electro-oxidation using doped-SnO2 supporting materials[J]. Applied Surface Science, 2017, 435: 535-542. [8] YING D, YE C, WEN Q, et al. Fabrication of dense spherical and rhombic Ti/Sb-SnO2 electrodes with enhanced electrochemical activity by colloidal electrodeposition[J]. Journal of Electroanalytical Chemistry, 2016, 768: 81-88. doi: 10.1016/j.jelechem.2016.02.044 [9] XIAO C, ZHAO G, LEI Y, et al. Novel vertically aligned TiO2 nanotubes embedded with Sb-doped SnO2 electrode with high oxygen evolution potential and long service time[J]. Materials Chemistry & Physics, 2009, 113(1): 314-321. [10] HAO A, HAO C, ZHANG W, et al. Fabrication and electrochemical treatment application of a microstructured TiO2-NTs/Sb-SnO2 /PbO2 anode in the degradation of C.I. reactive blue 194(RB 194)[J]. Chemical Engineering Journal, 2012, 209: 86-93. doi: 10.1016/j.cej.2012.07.089 [11] LIAO Y, YUAN B, ZHANG D, et al. Fabrication of heterostructured metal oxide/TiO2 nanotube arrays prepared via thermal decomposition and crystallization[J]. Inorganic Chemistry, 2018, 57(16): 10249-10256. doi: 10.1021/acs.inorgchem.8b01483 [12] 马文臣, 刘宜利, 徐世杰, 等. 催化氧化法在钻井废水处理中的应用[J]. 油气田环境保护, 2004, 14(1): 15-16. doi: 10.3969/j.issn.1005-3158.2004.01.005 [13] 刘音, 崔远众, 常青, 等. 油田钻井废液处理研究进展[J]. 石油化工应用, 2014, 33(10): 1-5. doi: 10.3969/j.issn.1673-5285.2014.10.001 [14] 张红岩, 吕荣湖, 郭绍辉. 混凝-臭氧氧化法处理三磺泥浆体系钻井废水[J]. 过程工程学报, 2007, 7(4): 718-722. doi: 10.3321/j.issn:1009-606x.2007.04.016 [15] 余晓霞, 蒲晓林. 化学破乳法在钻井废水处理中的应用综述[J]. 油气田环境保护, 2007, 17(2): 43-46. doi: 10.3969/j.issn.1005-3158.2007.02.013 [16] 邓磊, 蒋姝, 黄文章, 等. 臭氧-Fenton联合氧化处理钻井液废水研究[J]. 工业水处理, 2018, 38(2): 44-47. doi: 10.11894/1005-829x.2018.38(2).044 [17] 潘杰, 戴学文, 李晗, 等. 4-氨基安替比林分光光度法测定苯酚滴耳液中苯酚含量[J]. 天津医科大学学报, 2015, 21(1): 87-89. [18] 国家环境保护总局. 水和废水监测分析方法[M]. 4版. 北京: 中国环境科学出版社, 2002. [19] 蒋朦. 电化学体系中羟基自由基的检测及生成规律的影响研究[D]. 西安: 西安建筑科技大学, 2015. [20] CHAI S, ZHAO G, LI P, et al. Novel sieve-like SnO2/TiO2 nanotubes with integrated photoelectrocatalysis: Fabrication and application for efficient toxicity elimination of nitrophenol wastewater[J]. Journal of Physical Chemistry C, 2011, 115(37): 18261-18269. doi: 10.1021/jp205228h [21] CHEN A. Electrocatalytic enhancement of salicylic acid oxidation at electrochemically reduced TiO2 nanotubes[J]. ACS Catalysis, 2014, 4(8): 2616-2622. doi: 10.1021/cs500487a [22] MARCO P, GIACOMO C. Direct and mediated anodic oxidation of organic pollutants[J]. Chemical Reviews, 2009, 109(12): 6541-6569. doi: 10.1021/cr9001319 -



 下载:
下载: