-
近年来,随着工业化与城市化进程的不断加快,化工、医药、农药、纺织和印染等行业的废水排放量也日益增多。上述废水中有机物的种类复杂,可生化性差,进入城镇污水处理厂后,若仅采用传统的生化处理工艺进行处理,已不能满足越来越严格的市政污水排放标准[1]。探索高效、经济的方法,对城镇污水处理厂出水进行深度处理,以进一步去除其中的难降解污染物,实现出水COD的达标排放,已成为目前研究的热点。
活性炭是一种具有多孔结构的水处理材料,因具有较强的吸附能力,广泛应用于排放标准严格地区的污水处理厂深度处理提标改造中[2]。当活性炭因吸附出水中的难降解COD达到饱和时,若将饱和活性炭直接丢弃,会对环境造成二次污染,并提高活性炭使用的成本[3]。因此,饱和活性炭的再生是活性炭高效应用的关键。
活性炭主要的再生方法有电加热再生、过热蒸汽再生等[4]。其中,过热蒸汽再生是指使用锅炉运行产生130 ℃的蒸汽,再通过线性加热器将该蒸汽加热至500 ℃,并将蒸汽通过活性炭层的布汽器来均匀冲洗活性炭,使其呈流化状态,将活性炭炭床温度加热至400~500 ℃进行再生。过热蒸汽再生的原理是利用过热蒸汽对活性炭吸附的有机物进行热解和气提,将有机物从活性炭中解吸出去[5]。电加热再生需在密闭条件下将活性炭炭床温度先加热至700~800 ℃,然后再通入水蒸气与活性炭发生水煤气反应进行再生。2种方法相比较,过热蒸汽再生具有能耗低、可进行活性炭的原位再生、避免炭转移质量损失等优点[6]。采用过热蒸汽进行原位再生的设备包括水蒸气发生器、水蒸气过热管和吸附-再生一体罐,其中水蒸气可通过电加热锅炉或外接热电厂产生的蒸汽获得。过热蒸汽再生活性炭在国外已有较多的应用[7],近2年也在我国开始应用[8]。
目前,关于活性炭热再生的研究均以吸附饱和的活性炭为研究对象,但在实验过程中发现,未吸附饱和的活性炭若干次再生后,其再生效果要优于吸附饱和的活性炭。因此,本研究以某城镇污水处理厂吸附二级出水的不同吸附饱和度活性炭为研究对象,以过热蒸气加热为再生方式,考察了再生温度、再生时间、多次再生等因素对不同吸附饱和度活性炭再生效率及得率的影响,并探究过热蒸汽再生过程中活性炭吸附动力学及表面结构特征,以期为过热蒸汽再生活性炭的实际工程应用提供参考。
全文HTML
-
实验中,将活性炭放置于吸附柱内吸附某城镇污水处理厂二级出水,出水COD为30~45 mg·L−1、BOD5为6~8 mg·L−1、TP为0.1~0.3 mg·L−1、TN为8~10 mg·L−1、SS为6~9 mg·L−1。实验用活性炭为某公司生产的商品煤质活性炭,碘吸附值为1 020.0 mg·g−1,亚甲基蓝吸附值为123.5 mg·g−1,粒径为0.38~1.7 mm,水分为1.25%。再生前,将活性炭筛分至1 mm粒度以上,并放入烘箱以105 ℃烘干3 h作为实验原料。活性炭不同吸附饱和度的计算方法如式(1)所示,不同吸附饱和度下活性炭的碘吸附值和亚甲基蓝吸附值如表1所示。
式中:p为活性炭吸附饱和度;n为活性炭吸附柱运行时间,d;Qi为单位活性炭在运行期间内的COD吸附量,mg;Qmax为单位活性炭的饱和COD吸附量,mg。
-
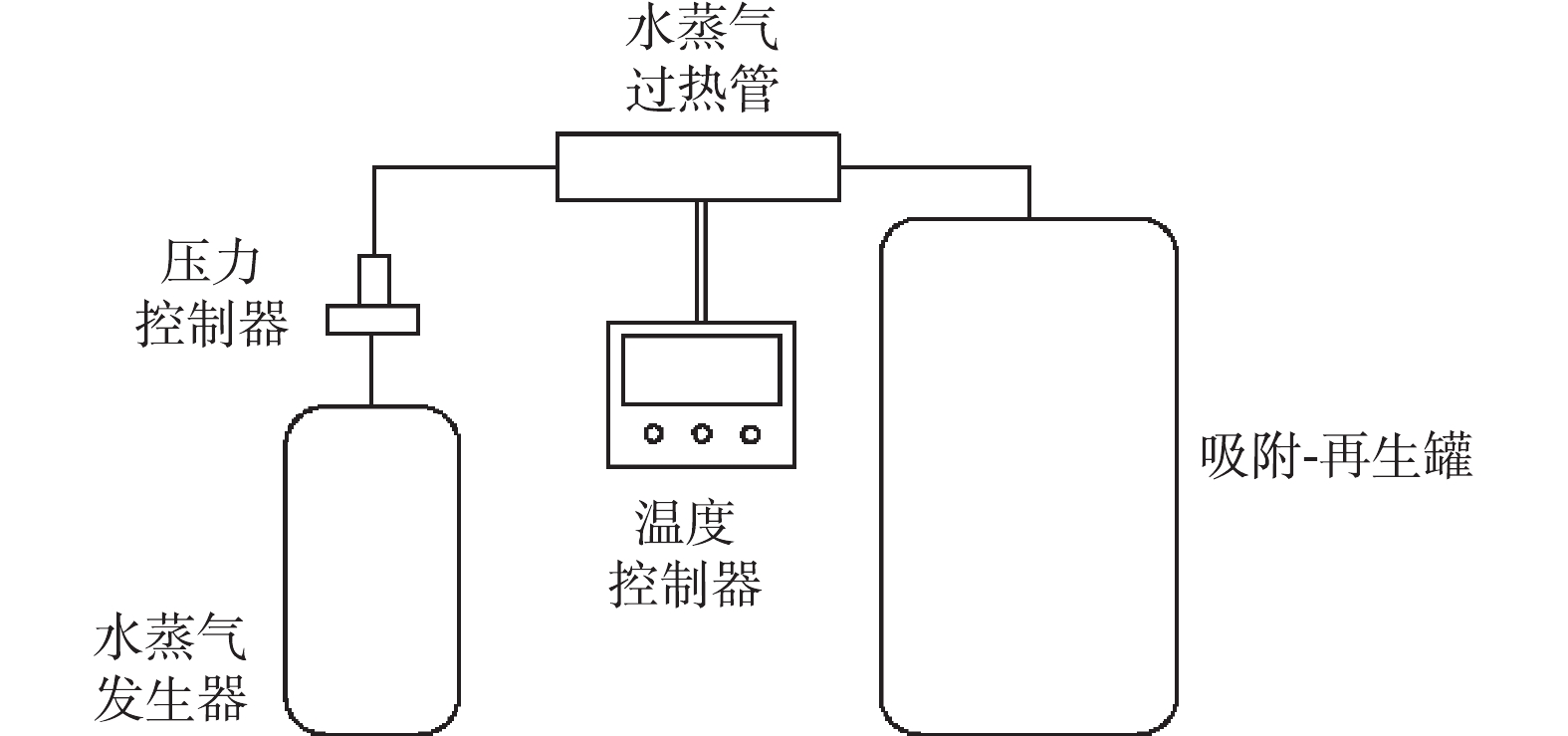
实验装置如图1所示。装置主体部分由水蒸气发生器、水蒸气过热管、吸附-再生罐组成,吸附-再生罐中装入不同吸附饱和度的活性炭。再生开始时,先开启水蒸气发生器,待产生的水蒸气温度升至130 ℃时,开启水蒸气过热管,加热水蒸气变为过热蒸汽,使得活性炭炭床升温,待炭床温度达到设定温度即开始保温并计时。
准确称取活性炭150 mg放入定量瓶内,瓶内加入1 L某城镇污水处理厂二级出水,置于恒温振荡器中(振荡条件:温度为25 ℃、转速为200 r·min−1)。在设定时间后,分别取出样品,经0.45 μm滤膜过滤后测定溶液的COD。
-
活性炭的碘吸附值可体现活性炭微孔的发达程度,亚甲基蓝吸附值可体现活性炭中孔的发达程度[9]。由表1可知,相比于碘吸附值,不同吸附饱和度活性炭亚甲基蓝值变化并不显著。由此可推测,活性炭中的微孔结构对该污水处理厂二级出水吸附起主要作用。选择活性炭的碘吸附值作为活性炭再生效率的评价标准,具体由式(2)计算。活性炭得率由式(3)计算。
式中:η为再生效率;q为再生后活性炭的碘吸附量,mg·g−1;q0为新活性炭的碘吸附量,mg·g−1。
式中:Y为再生活性炭得率;M为再生后活性炭的质量,g;M0为再生前活性炭的质量,g。
分别采用伪一级动力学方程与伪二级动力学方程对吸附数据进行拟合,计算方法见式(4)和式(5)。
式中:k1为伪一级吸附速率常数,min−1;k2为伪二级吸附的速率常数,g·(mg·min)−1;qe、qt分别为平衡时和t时刻的吸附量,mg·g−1。
采用日本BEL公司的物理吸附仪(Belsorp-Max型)测定活性炭的比表面积,活性炭的孔体积分布由非定域化密度函数理论(NLDFT)进行表征,相对压力为0.99的氮吸附量决定了总孔体积的大小。采用日本日立扫描电镜(TM3030型)对活性炭的形貌进行表征。
1.1. 实验材料
1.2. 实验装置与方法
1.3. 活性炭特性表征
-
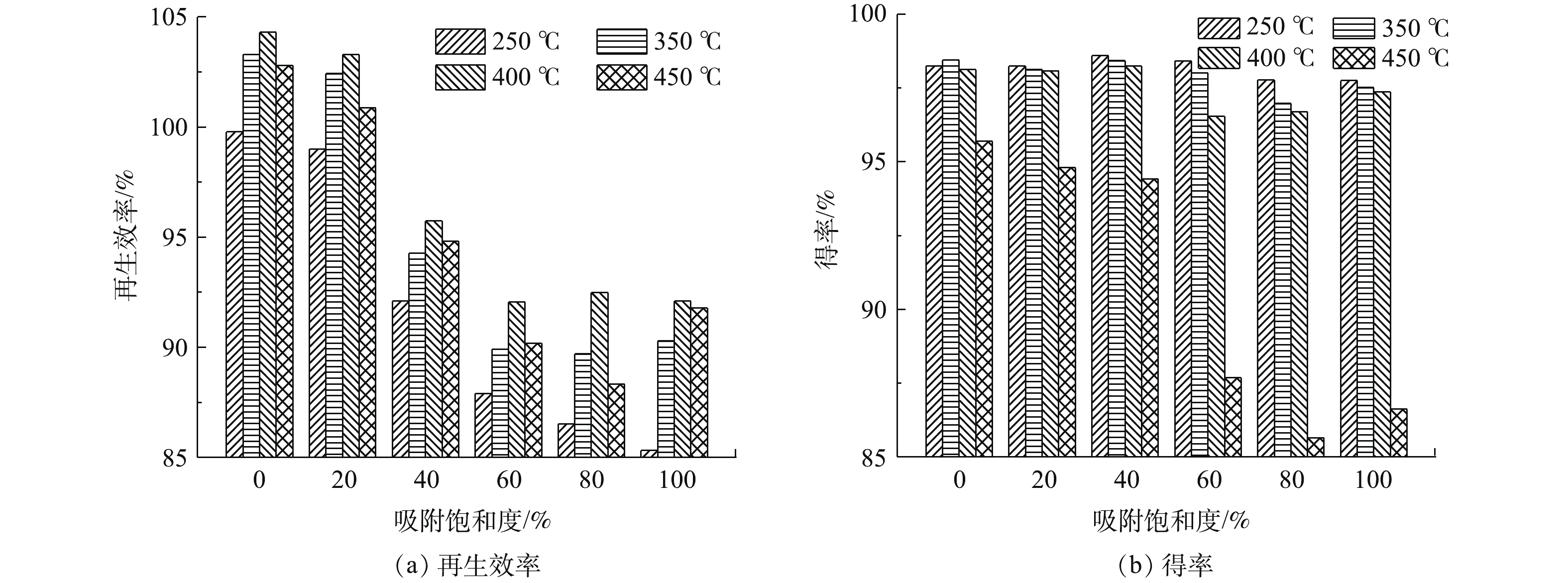
称取吸附饱和度为0、20%、40%、60%、80%和100%的活性炭各100 g分别放置于吸附-再生罐中,控制再生时间为6 h,活性炭的再生温度分别设置为250、350、400和450 ℃,考察不同再生温度对不同吸附饱和程度活性炭再生效率和得率的影响,并选取吸附饱和度为60%的活性炭测定不同再生温度下的比表面积和孔体积分布,相关结果如图2和表2所示。可以看出,随着再生温度的升高,活性炭的再生效率呈现出2个明显的变化阶段。在250~400 ℃,活性炭的再生效率显著上升;而在400~450 ℃,活性炭的再生效率则有所下降。这是由于活性炭的再生是一个炭化与解吸的过程,随着温度的升高,炭化过程使活性炭所吸附的大量有机物分解,并使挥发分大量逸出,为活性炭所吸附的有机物解吸创造了条件[10]。结合表2可知,当再生温度由250 ℃升至400 ℃时,活性炭的比表面积和总孔体积逐渐增加,而平均孔径则逐渐减小,表明在此区间内,随着温度的升高,活性炭的比表面积会相应提高。然而,当温度超过400 ℃时,活性炭的比表面积则出现了下降,总孔体积减小,平均孔径增大。这可能是因为进一步提高温度会使得活性炭过度热解,过热蒸汽与活性炭表面较活跃的碳原子发生了剧烈反应,引起了活性炭的炭损失,降低了孔的数量,从而导致了比表面积的下降[11]。由此可见,400 ℃是过热蒸汽炭化-解吸活性炭的温度平衡点,在此温度下可以获得最佳再生效率。同时,对比吸附饱和度为0的新炭与20%和40%的低吸附饱和度活性炭的再生情况可发现,吸附饱和度为20%的活性炭再生效率相比新炭略有降低,而40%吸附饱和度的活性炭有明显下降。这说明吸附饱和度为20%时活性炭孔道吸附的有机物相对较少,在该吸附饱和度下过热蒸汽对活性炭内部基本没有发生炭化与解吸反应。
不同吸附饱和度的活性炭得率随着再生温度的升高均有所下降。再生温度为250~400 ℃时,活性炭的得率随再生温度的升高略微下降;而当再生温度升高至450 ℃时,活性炭的得率则明显下降。这是因为温度较低时,活性炭解吸过程不充分,使炭化过程中一些挥发性气体及有机物残留在活性炭内;随着温度的升高,活性炭吸附的有机物逐步炭化充分并被过热蒸汽解吸,并在400 ℃达到临界值[12]。继续升高温度时,过热蒸汽则会进一步热解活性炭,导致得率急剧下降。综合考虑再生活性炭的再生效率和得率,可选取400 ℃作为不同吸附饱和度活性炭的最佳再生温度。
-
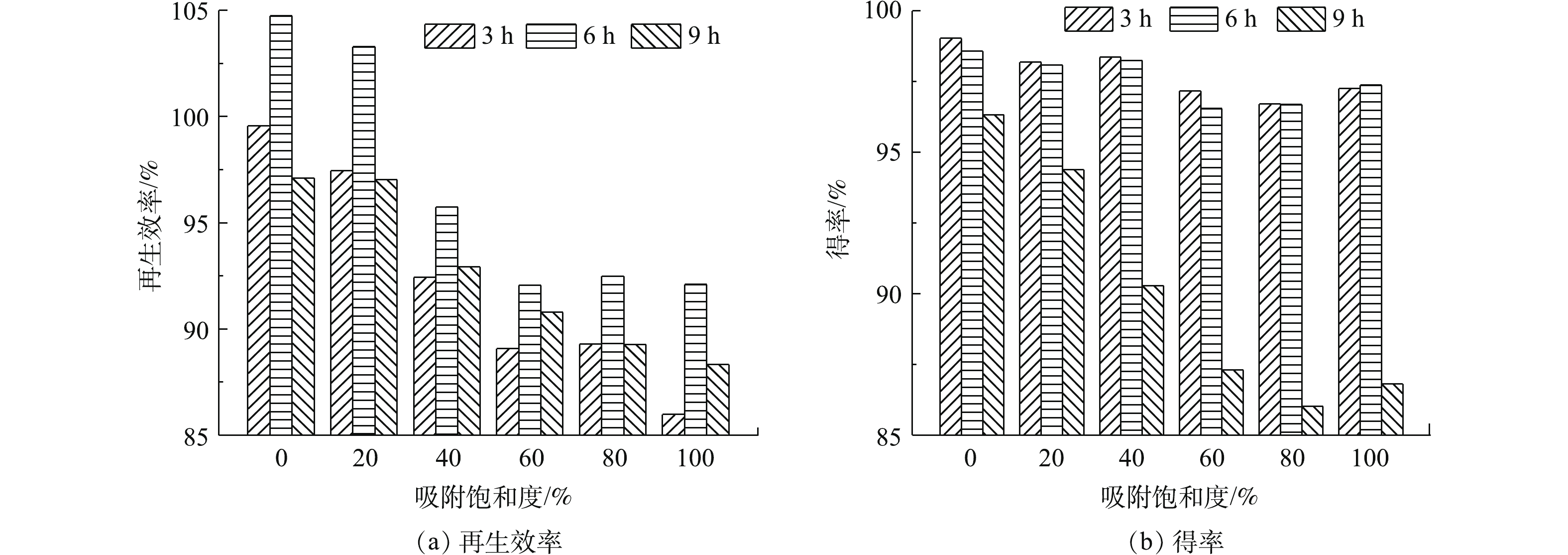
称取吸附饱和度为0、20%、40%、60%、80%和100%的活性炭各100 g分别放置于吸附-再生罐中,控制再生温度为400 ℃,分别调节活性炭的再生时间为3、6和9 h,考察不同再生时间对不同吸附饱和程度活性炭再生效率和得率的影响,并选取吸附饱和度为60%的活性炭测定不同再生时间下的比表面积和孔体积分布,相关结果如图3和表3所示。可以看出,再生时间对不同吸附饱和度活性炭再生效率有较大影响。再生时间为6 h时,再生效率最高,随后出现下降。这是由于再生时间为3 h时,再生时间较短,活性炭的炭化与解吸反应尚未完全进行。由表3可知,再生时间为3 h时,活性炭的比表面积相对较低,平均孔径也低于再生时间为6 h和9 h时的活性炭。当再生时间为6 h时,解吸反应进行完全,再生效率达到最高,比表面积和总孔体积也最大。但随着再生时间的进一步延长,过热蒸汽开始进一步热解活性炭,造成活性炭平均孔径增大,比表面积减小,反而降低了再生效率。
当再生时间为3 h 和6 h时,不同吸附饱和度活性炭的得率基本相近;而延长再生时间至9 h时,活性炭的得率则出现了下降,均低于95%以下。这是因为再生时间为3~6 h时,活性炭的炭化与解吸过程逐渐充分,吸附的有机物变为气体排出。随着再生时间延长至9 h,吸附的有机物解吸已经完全,过热蒸汽进而开始热解活性炭的炭骨架,使得活性炭的得率出现了明显下降的趋势[13]。因此,可选取再生时间为6 h作为不同吸附饱和度活性炭的最佳再生时间。
-
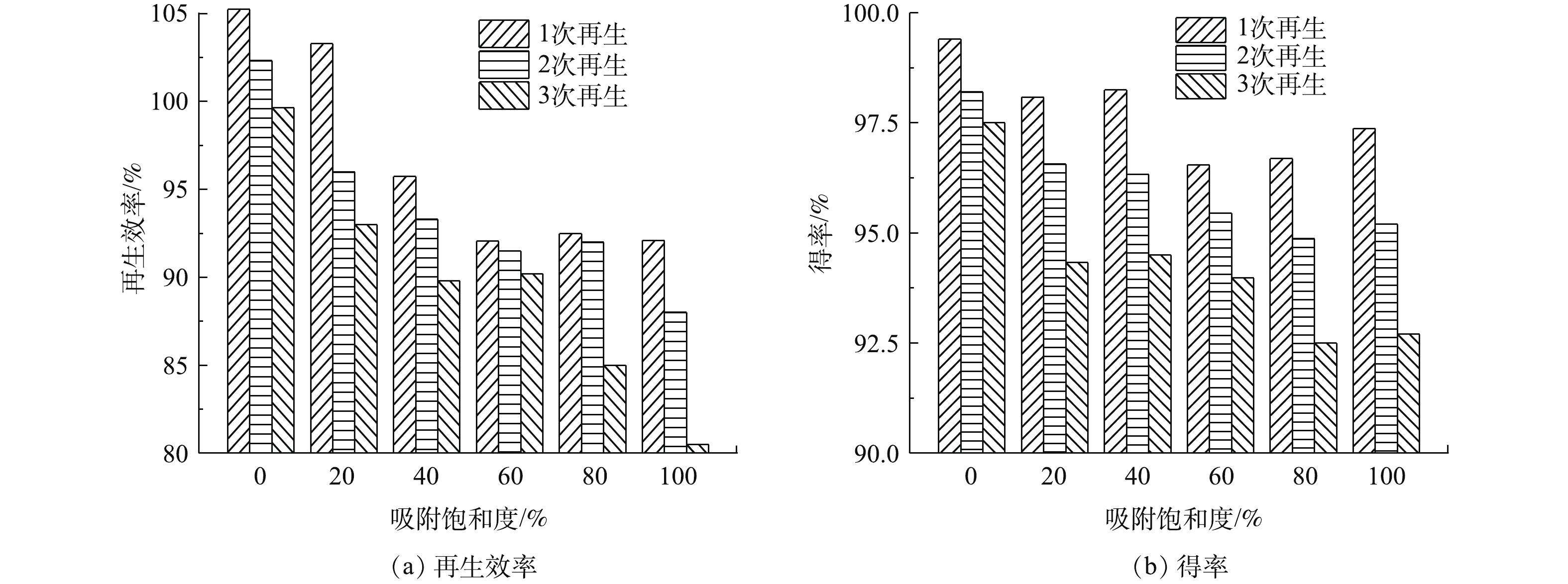
将再生后不同吸附饱和度活性炭分别再吸附至上一次的吸附饱和度,控制再生温度为400 ℃,再生时间为6 h,考察多次吸附-再生对不同吸附饱和程度活性炭再生效率和得率的影响,结果如图4所示。可以看出,不同吸附饱和程度的活性炭再生效率和得率随再生次数的增加而降低。经过3次再生后,吸附饱和度为60%活性炭表现出最好的再生效果,再生效率仅降低1.86%,得率降低2.56%;而吸附饱和度为100%的活性炭再生效果最差,再生效率降低了11.61%,得率降低了4.67%。这一现象与活性炭吸附有机物后的结构有关。当吸附饱和度为0的新炭多次再生时,新炭的再生效率虽然随着再生次数的下降,但相比于自身原有的碘吸附值则几乎没有发生变化。当活性炭的吸附饱和度为20%~40%时,由于活性炭上吸附的有机物较少,活性炭自身结构紧密部分易被过热蒸汽热解。而经过多次再生后,结构紧密部分被逐渐解吸完全,并有部分变为气体排出[14]。吸附饱和度为80%~100%的活性炭因吸附的有机物较多,在炭化过程中这些有机物转化为结构疏松部分,解吸过程中易使活性炭表面上相近的孔连接在一起,从而降低了孔的数量[15]。同时,由于大量吸附的有机物炭化,多次再生后得率出现大幅下降。而发生在吸附饱和度为60%的活性炭上再生反应正好处在炭化与解吸过程的最佳平衡状态,使其得率的损失在不同吸附饱和度中处于最低的水平。因此,选取吸附饱和度为60%的活性炭,在再生温度400 ℃,再生时间6 h下,多次再生可获得最佳的再生效果和较低的得率损失。
-
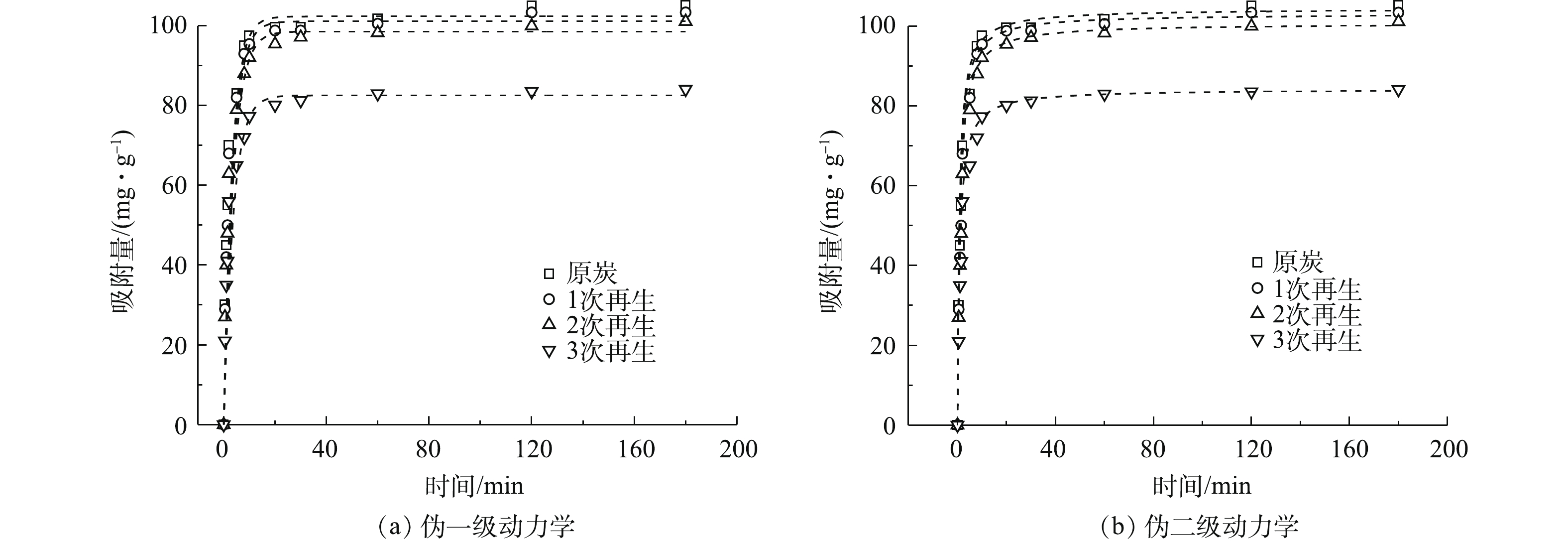
选取原炭和吸附饱和度为60%的多次再生活性炭对污水处理厂二级出水COD进行吸附动力学研究,伪一级动力拟合结果如图5(a)所示,伪二级动力拟合结果如图5(b)所示,动力学模型参数见表4。在25 ℃时,原炭与多次再生炭对二级出水中COD的吸附量随着时间的延长而不断增大,吸附前30 min速率较快,60 min后吸附量趋于平衡。吸附平衡时原炭对COD的吸附量(105.13 mg·g−1)高于3次再生后活性炭的吸附量(84.10 mg·g−1)。
分析图5(a)和图5(b)的数据可发现,伪一级、伪二级动力学模型所拟合的动力学方程的R2均高于0. 99,模型的拟合度较高。但伪一级动力学方程计算得到的吸附量qe小于伪二级动力学方程计算得到的吸附量qe,伪二级动力学方程模型更接近实验值qe,这表明活性炭吸附二级出水COD符合伪二级动力学模型。这是由于伪一级动力学模型有其局限性,一般只适合吸附初始阶段的动力学描述,并不能准确地描述吸附的全过程。而活性炭对二级出水COD的伪二级动力学吸附模型可描述为受2种主要因素影响的吸附过程。该模型可描述外部液膜扩散、颗粒内部扩散和表面吸附等吸附全过程[16]。
-
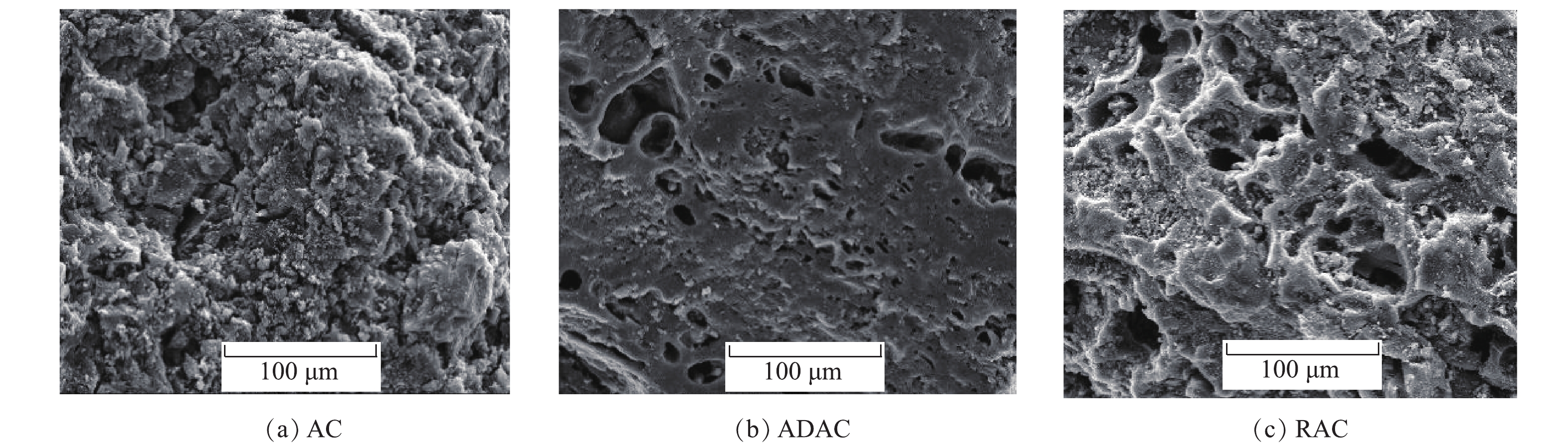
将原炭(activated carbon,AC)经过2次吸附-再生后再吸附的吸附炭(adsorbent activated carbon,ADAC)和经过3次吸附-再生的再生活性炭(regenerate activated carbon,RAC)进行了理化特征测试,吸附条件均为吸附至60%饱和度,再生条件均为再生温度400 ℃,再生时间6 h。根据国际纯粹与应用化学联合会(IUPAC)的分类标准,吸附剂的孔径分为3类:孔径小于2 nm的微孔、2~50 nm的中孔和大于50 nm的大孔[17]。由图6(a)可知,AC、ADAC和RAC的吸附等温曲线都属于I型(Langmuir型)等温线,在相对压力低于0.1时,吸附量基本达到饱和,表明这3种活性炭都含有大量的微孔[18]。RAC的微孔含量相比吸附炭明显提高,相对压力大于0.1后,AC、ADAC和RAC的吸附量随着相对压力的增大而缓慢增加;在相对压力接近1时,氮气的吸附量未出现急剧增加,这表明这时的RAC基本不存在中孔和大孔,以微孔为主[19]。图6(b)证明了AC、ADAC和RAC以2 nm左右微孔为主,ADAC微孔的体积较AC减少了57%,而RAC微孔体积恢复到原炭的77%。
由表5可知,RAC相比ADAC的比表面积增加了413.9 m2·g−1,总孔体积增加了0.232 9 cm3·g−1,说明采用过热蒸汽再生具有较好的再生效果,再生后活性炭的比表面和孔体积均有明显增加。分析AC、ADAC和RAC的氧化基团量和零电荷点值pHpzc可发现,吸附和再生过程使得AC表面的羧基和酚羟基减少,进而导致酸性官能团的减少。pHpzc与Boehm滴定数据一致,相比AC的pHpzc(5.01),RAC的pHpzc略有增加(5.10),这说明活性炭表面的含氧基团的数量在过热高温蒸汽下有所减少。
-
由图7(a)可知,AC表面存在发达的孔结构,孔道较为清晰,炭骨架结构较为紧密。进而观察图7(b),ADAC内大部分孔道被有机物堵塞,孔道结构并不明显,活性炭表面光滑。对比图7(c)还发现,经过再生后,活性炭孔道内吸附的有机物明显较少,堵塞的孔被打开,孔结构也显得更加清晰。但由于多次再生,ADAC经过多次炭化与解吸后,孔径较AC也随之增大,炭骨架结构变得疏松。
2.1. 再生温度对活性炭再生效率和得率的影响
2.2. 再生时间对活性炭再生效率和得率的影响
2.3. 多次再生对活性炭再生效率和得率的影响
2.4. 多次再生对活性炭吸附动力学影响
2.5. 多次再生后活性炭孔结构分析
2.6. 再生活性炭的表面形貌结构分析
-
1)通过实验确定的活性炭最佳再生条件为:再生温度400 ℃,再生时间6 h。在此条件下,对不同吸附饱和度的活性炭有最佳的再生效率和较高的得率。经过3次再生,吸附饱和度为60%活性炭的再生效率与得率损失最少,分别为1.86%和3.18%。
2)吸附动力学实验表明,原炭与多次再生吸附饱和度为60%活性炭在60 min对二级出水COD达到吸附平衡,采用伪二级动力学模型对实际数据的拟合度更高,因此,活性炭对二级出水COD的吸附符合伪二级级动力学模型。
3)活性炭的表征说明,多次再生后的活性炭孔径以2 nm左右微孔为主,再生活性炭相比吸附炭的比表面积增加了413.9 m2·g−1,再生炭表面酸性官能团减少,pHpzc值有所增加。由SEM可以发现,经过多次再生后,相比于再生前,活性炭发生了较好的炭化与解吸,孔结构清晰。



 下载:
下载: