-
新型烟碱类农药(neonicotinoid insecticides,NNIs)是一种新兴的神经性杀虫剂,由于其杀虫广谱、内吸性好且选择性高,因此,被广泛地应用于农作物、土壤及家庭等领域害虫的治理中[1]。正因其应用广泛,在蔬果[2]、饮用水[3]、牛奶[4]、地表水[5]中,NNIs均被检出。新型烟碱类化合物水溶性较高[2],生产及应用过程中产生的废水毒性大,具有生物累积性[6],并且存在生态风险[7]与健康风险[8],所以需要研究出一种有效去除水中此类物质的方法。
目前,含NNIs废水的处理方法主要有光降解[9]、反应活性氧化降解[10]等高级氧化法。然而由于NNIs的代谢产物毒性较大[11],高级氧化法耗时长、花费高,还需要利用复杂的设备。吸附法被认为是一种较有竞争力的除污方法[12]。已有学者研究了碳冷凝胶[13]、活性炭等[14]、纤维素[12]和金属有机骨架[15]等对新型烟碱类农药的吸附行为,但大部分研究集中于某一化合物的单一吸附,对于相关机制的探究较少,且吸附材料选择的范围有待扩大。因此,选择一种易合成、可回收利用的新型材料吸附处理NNIs废水具有实际意义。
金属有机骨架(metal-organic frameworks, MOFs)是一种多孔无机-有机杂化成分的材料。这种无机和有机相结合的结构,结构多样且材料内孔尺寸高度可调节,比传统多孔材料沸石具有更高的孔隙率和更复杂的孔道结构[16]。MOFs比表面积较大,拥有大量的不饱和金属位点,加上具有化学稳定性优越及孔道可调控等特征,这种材料非常适用于吸附去除水中的有机污染物[17]。有研究[18]利用MOF-5去除废水中苯酚,30 min内达到吸附平衡,去除率达到97%以上,且材料可多次重复使用;龚文朋等[19]使用MOF-508对水中染料进行去除,在2 min内达到100%的去除率;BHADRA等[20]研究指出bio-MOF-1可快速去除药物及个人护理产品,且去除效果优于其他吸附剂;QIN等[21]将MIL-101用于水中双酚A的去除,同样取得优异的效果,吸附可在60 min达到平衡,材料重复利用性好。因此,MOF具有吸附容量大、吸附速度快、重复利用率高等优点,广泛应用于水中污染物的去除。然而关于MOF吸附去除NNIs的研究还不多见。
使用不同的金属位点和配体构建MOFs,可以灵活改变其物理化学性质[22-23],因此,许多学者将其进行改性从而加强材料的吸附降解性能。ZHANG等[24]对铁基MOF进行氨基化处理促进了偶氮染料的降解;LI等[25]将TiO2包裹在用水杨醛改性氨基化MOF,增加了材料的光吸收性,显著提高了MB的降解;李超等[26]将MIL-101氨基化后,MIL-101对CO2的吸附率显著提高;龚文朋等[19]通过H6P2Mo15W3O62对 MOF-508进行改性,提高了其对水中亚甲基蓝的吸附。
MLI-101是MOFs的一类分支,其中氨基改性的MIL-101相较于MIL-101具有更丰富的官能团,材料氨基化可改变材料的吸附性能,还使其在光催化、碱催化等领域的应用具有潜在价值[27]。因此,使用氨基改性MIL-101去除水中NNIs具有现实意义。本研究以水热法制备NH2-MIL-101,对其微观结构进行表征分析,通过吸附实验对比NH2-MIL-101与MIL-101对不同结构NNIs的去除效果,并探讨溶液pH、共存阴离子对去除率的影响,从而分析影响去除效果的因素,探究材料氨基化的优势及相应去除机制,结合材料吸附动力学、热力学及重复利用率研究结果,分析评价NH2-MIL-101作为吸附剂去除水中NNIs的可行性,为解决含NNIs的废水处理问题提供参考。
全文HTML
-
6-氯烟酸(ChloA)、啶虫脒(Ace)、噻虫胺(Clo)、吡虫啉(Imi)、噻虫嗪(Thim)标准溶液(纯度大于95%,美国Standford Chemicals公司),将其配成1 000 mg·L−1混合溶液,备用。甲醇、乙腈(色谱纯,美国Tedia公司);甲酸(色谱纯,上海阿拉丁生化科技股份有限公司);无水乙醇、九水硝酸铬、2-氨基对苯二甲酸、氯化钠、氢氧化钠(分析纯,国药集团化学试剂有限公司);实验用水为德国赛多利斯集团Arium® Comfort纯水系统制备的超纯水。
-
本研究采用液相色谱质谱联用仪进行检测。在进行色谱检测时,样品采用Ultimate 3000高效液相色谱仪(美国戴安公司)测定。在使用Thermo Syncronls C18液相色谱柱(2.1 mm×100 mm,1.7 μm)和Ultra UHPLC系统进行检测时,柱温为30 ℃,流动相流速为0.2 mL·min−1,流动相为0.1%甲酸水(A)和乙腈(B)。采用梯度洗脱,洗脱程序为:在0~3 min时,流动相B的比列由10%增至81% B;在3~6 min时,流动相B的比例保持81% 不变;在6~6.5 min时,流动相B的比列由81%减至10% B;在6.5~11 min时,流动相B的比例保持10% 不变。
在进行质谱检测时,样品采用TSQ Quantum Ultra EMR三重四级杆质谱检测器检测,采用电喷雾离子源的正离子(ESI+)模式进行扫描,扫描方式为多反应监测(SRM)。喷雾电压为3 500 V,喷雾温度为300 ℃,鞘气压力为25 MPa,辅助气压力为10 MPa,离子传输管温度为350 ℃。质谱参数如表1所示。
-
在MIL-101(Cr)的制备过程中,称取4.0 g九水硝酸铬(Cr(NO3)3·9H2O)和1.5 g对苯二甲酸(H2BDC)于0.05 mol·L−1醋酸钠水溶液50 mL中混匀,于200 ℃下反应12 h[28]。冷却至室温后,离心得到的沉淀物经水和无水乙醇交替洗3次,再次加入60 mL的无水乙醇溶液,置于反应釜内,在90 ℃温度下,纯化4 h,冷却至室温后过滤,用无水乙醇洗涤,于150 ℃下真空干燥5 h,得到绿色粉末,即为MIL-101(Cr) [29]。
在NH2-MIL-101的制备过程中,采用水热法制备NH2-MIL-101。称取九水硝酸铬(Cr(NO3)3·9H2O)1.606 g(0.004 01 mol)、2-氨基对苯二甲酸(NH2BDC)0.32 g(0.000 18 mol)和氢氧化钠(NaOH)0.40 g(0.02 mol) 置于30 mL超纯水中,在572 r·min−1条件下,磁力搅拌30 min,得均相反应溶液。将混合溶液转移至高压反应釜中,于150 ℃下反应12 h,冷却至室温后,抽滤沉淀物,经无水乙醇洗涤2次后,再次放置与反应釜中纯化,在90 ℃下反应4 h之后继续抽滤,抽滤物经无水乙醇洗涤2次后,在60 ℃下真空干燥8 h,研磨备用,最终得到绿色粉末产品,即为NH2-MIL-101。
在进行官能团表征时,为分析材料表面官能团的分布,本研究测定了材料改性前后的傅里叶变换红外光谱(FT-IR)。在实验中,利用溴化钾对材料进行混合压片,然后采用傅里叶变换红外光谱(Nicolet-6700,美国Thermo公司)对样品进行表征,扫描波数为400~4 000 cm−1。为了进一步说明改性前后材料表面元素含量和N元素存在状态变化,采用X射线光电子能谱仪(Thermo Scientific K-Alpha+,美国Thermo公司)对样品C、N、O、Cr元素进行测定,在此基础上分析N元素的存在状态及分布比例。
在进行材料形貌特征分析时,为获取材料的形貌特征,本研究对材料进行了电镜分析。将烘干后的样品进行喷金处理,采用扫描电子显微镜(S-4800,日本日立)对其表面形态进行表征;取少量烘干样品,在200 kV下,用透射电子显微镜(Tecnai G2F20S-TWIN,美国FEI公司)对其形貌进行观察。此外,本研究采用X射线衍射仪(Bruker D8 Advance,美国Bruker公司)对材料晶型进行了测定,扫描角度为4°~90°,扫描速度为6(°)·min−1。
在进行微观结构分析时,利用比表面积测定仪(Micrromeritics ASAP 246,美国麦克仪器公司)对材料微观比表面积和微观孔隙分布进行分析,测定前,将样品干燥后,在105 °C下脱气12 h,随后在77 K条件下测定氮气吸附-脱附实验曲线,然后利用BET及BJH模型获取材料的比表面积和孔隙分布。
-
取100 mL 1 mg·L−1 NNIs工作溶液于锥形瓶中,将NH2-MIL-101投入到配置好的溶液即开始计时,调节磁力搅拌转速为500 r·min−1,搅拌30 min后取样,经0.22 μm水系滤膜过滤后上机测样。对5种化合物的单化合物体系的吸附实验操作同上。实验首先比较了MIL-101与NH2-MIL-101(投加量均为0.04 g)对NNIs的吸附差异,将相同质量的MIL-101与NH2-MIL-101(0.04 g)分别投入100 mL 1 mg·L−1 NNIs工作溶液,进行上述吸附实验,后选取去除较好的材料,依次进行吸附投加量(0.01、0.015、0.02、0.03、0.04、0.05、0.06 g)、溶液初始pH (3、5、7、9、11)、阴离子(Cl−、
${\rm{SO}}_4^{2 - }$ 、${\rm{HCO}}_3^ - $ )等单因子对吸附影响的实验,均采用控制变量法。采用优化好的条件进行动力学及吸附等温线实验。所有样品均进行3组平行实验。 -
NNIs的吸附率用式(1)计算,吸附量qe通过式(2)计算。
式中:η为NNIs的吸附率;C0为NNIs溶液的初始浓度,mg·L−1;Ct为不同反应时间下溶液中NNIs的浓度,mg·L−1;qe为NH2-MIL-101的饱和吸附量,mg·g−1;V为反应溶液的体积,L;m为材料的投加量,mg。
准一级动力学、准二级动力学模型表达式分别见式(3)和式(4)。
式中:qe和qt分别为平衡时和t时刻的吸附量,mg·g−1;k1为准一级动力学方程的吸附速率常数, min−1;k2为准二级动力学的吸附速率常数,g·(mg·min)−1。
吸附等温线Langmuir拟合模型如式(5)所示。
式中:Ce为吸附平衡时NNIs的浓度,mg·L−1;qe为吸附平衡时的吸附量,mg·g−1;qm为NH2-MIL-101的饱和吸附量,mg·g−1;K为Langmuir常数,L·mg−1。
Langmuir拟合模型可以用参数RL来拟合结果可信度,如RL值=0~1,说明该拟合结果可取。RL的计算方法见式(6)。
式中:RL为衡量可信度的参数;Cmax为目标污染物最高浓度,mg·L−1。
1.1. 试剂
1.2. 检测条件
1.3. 金属有机骨架材料的制备及表征方法
1.4. 吸附性能分析
1.5. 计算方法
-
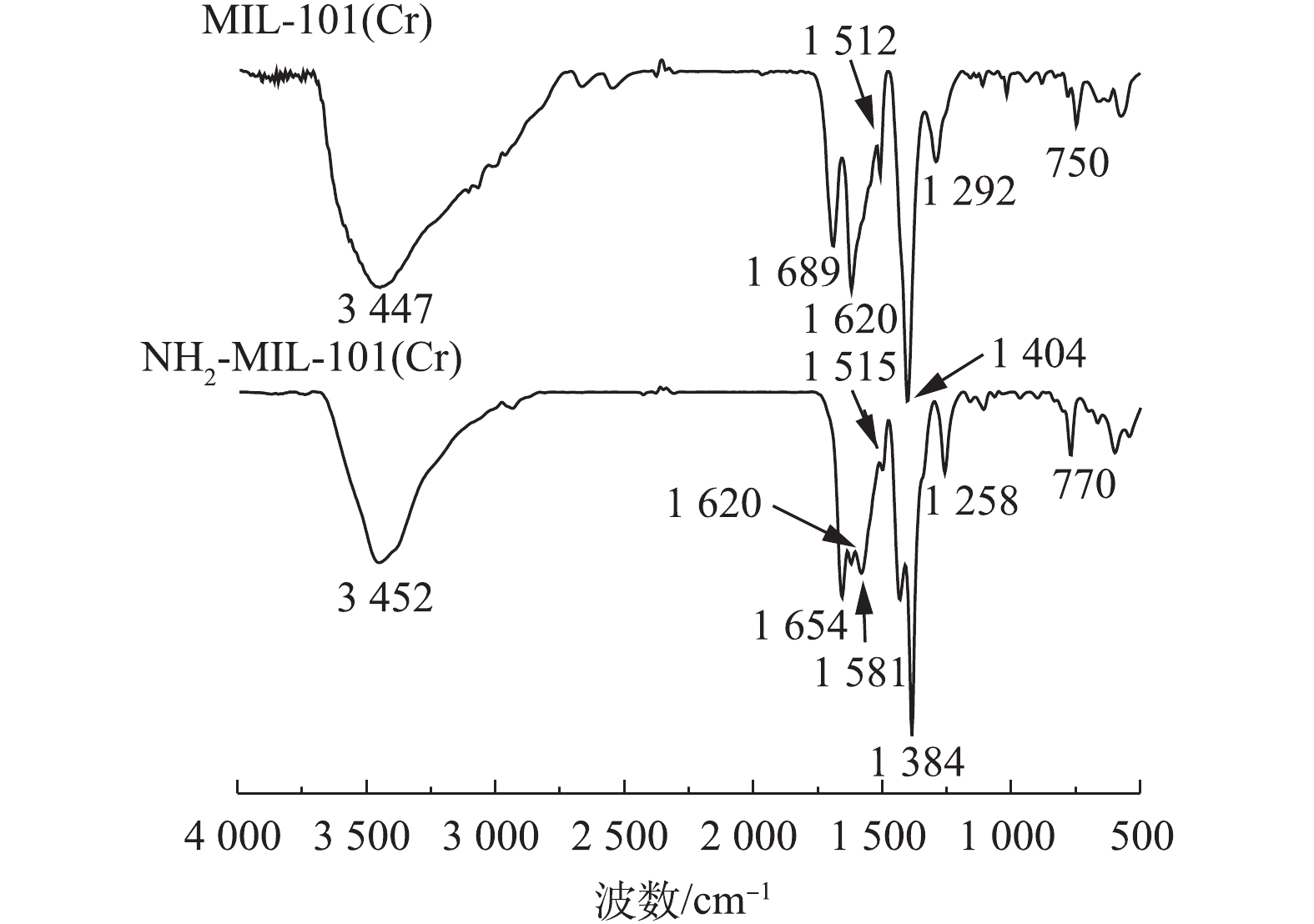
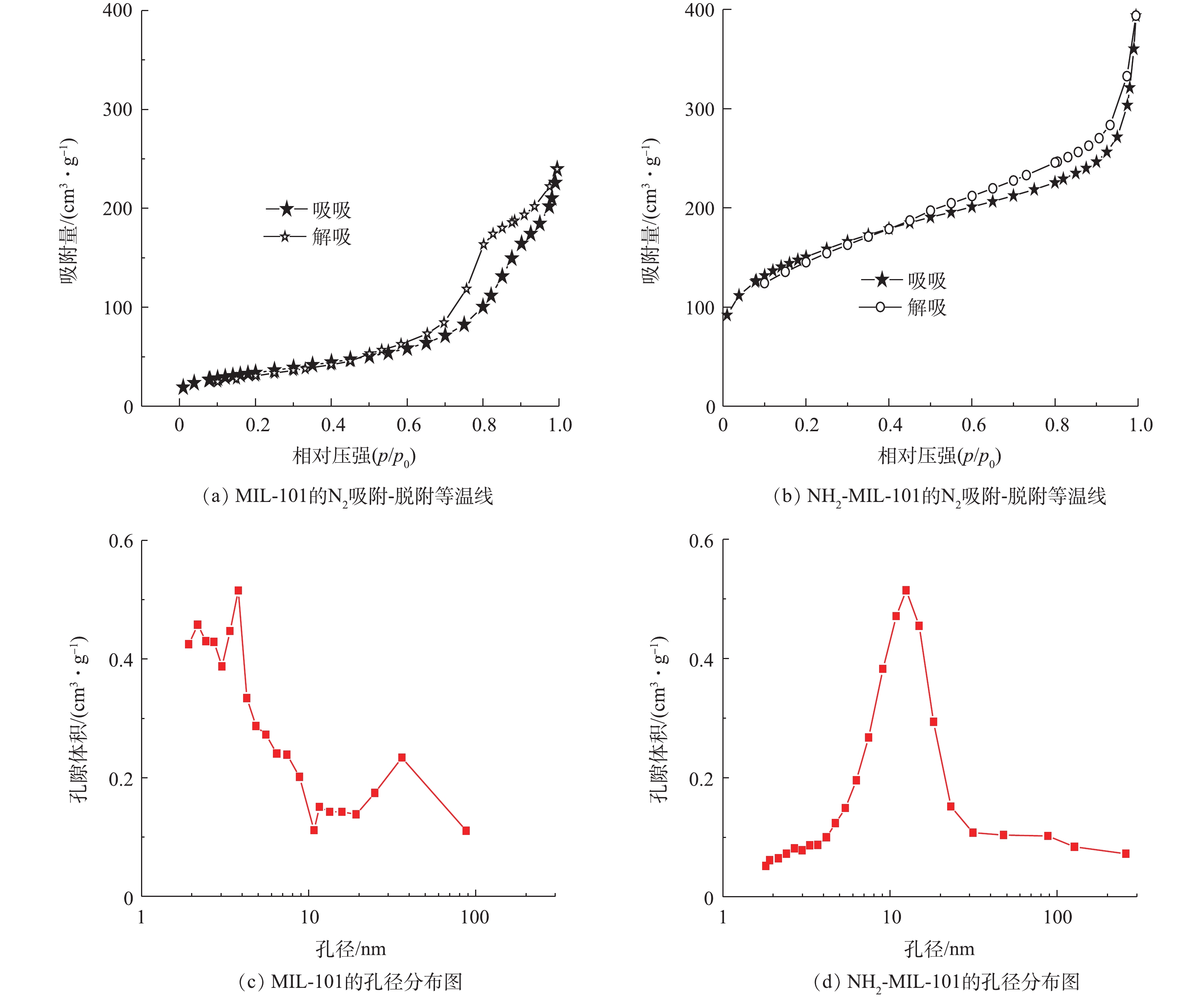
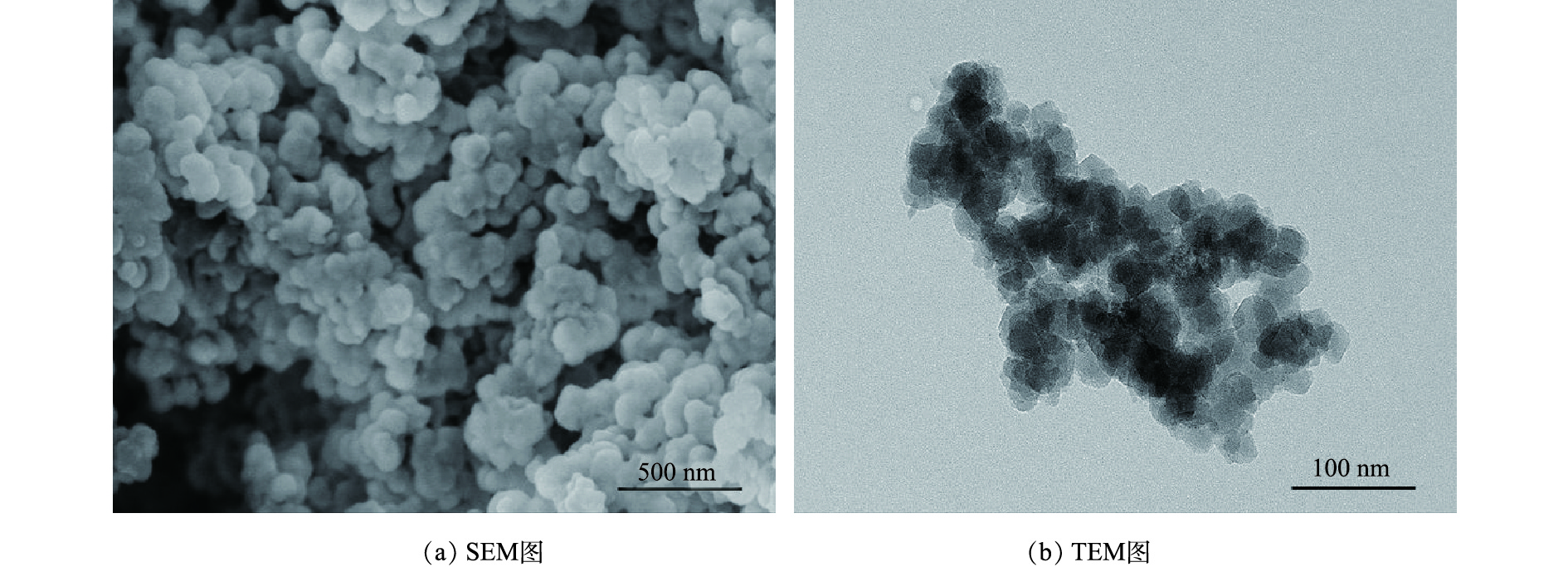
通过扫描电镜(SEM)和透射电镜(TEM)对NH2-MIL-101的表面形态分析结果见图1。可以看出材料为团簇状。图2为MIL-101和NH2-MIL-101的FT-IR谱图。通过傅里叶变换红外光谱(FT-IR)对NH2-MIL-101改性前后的官能团进行分析发现:750 cm−1和 770 cm−1处的吸收峰反映了苯环的C—H弯曲振动[30],表明材料存在一定的芳香性;1 292 cm−1与1 258 cm−1处为苯环上C—N的伸缩振动[31];1 404 cm−1与1 384 cm−1为C—O的伸缩振动,1 620 cm−1为苯环的骨架振动峰,由于氨基基团的引入,其与芳环发生p-π共轭作用,吸收峰发生分裂,形成1 581 cm−1及1 620 cm−1 2个吸收峰;而1 689 cm−1和1 654 cm−1分别为 HBDC–和 NH2-BDC–的C=O伸缩振动峰,吸收峰在改性后发生红移现象,可能是因为氨基的引入后氮原子的孤对电子与C=O双键发生p-π共轭,导致C=O伸缩振动吸收峰向长波长方向移动;3 447 cm−1和3 452 cm−1处的吸收峰可能为材料表面吸附了水分子导致的,导致N—H的伸缩振动难以观察。以上现象证明材料合成成功。MIL-101改性前后的N2吸附/脱附等温线及孔洞分布结果如图3所示。经 BET 和 BJH 方法拟合计算:MIL-101的BET比表面积为302.3 m2·g−1,平均孔径为5.1 nm,孔体积为0.15 cm3·g−1;NH2-MIL-101的BET比表面积为526.39 m2·g−1,平均孔径为5.59 nm,孔体积为0.46 cm3·g−1,改性后比表面积变大,孔洞结构更加丰富。
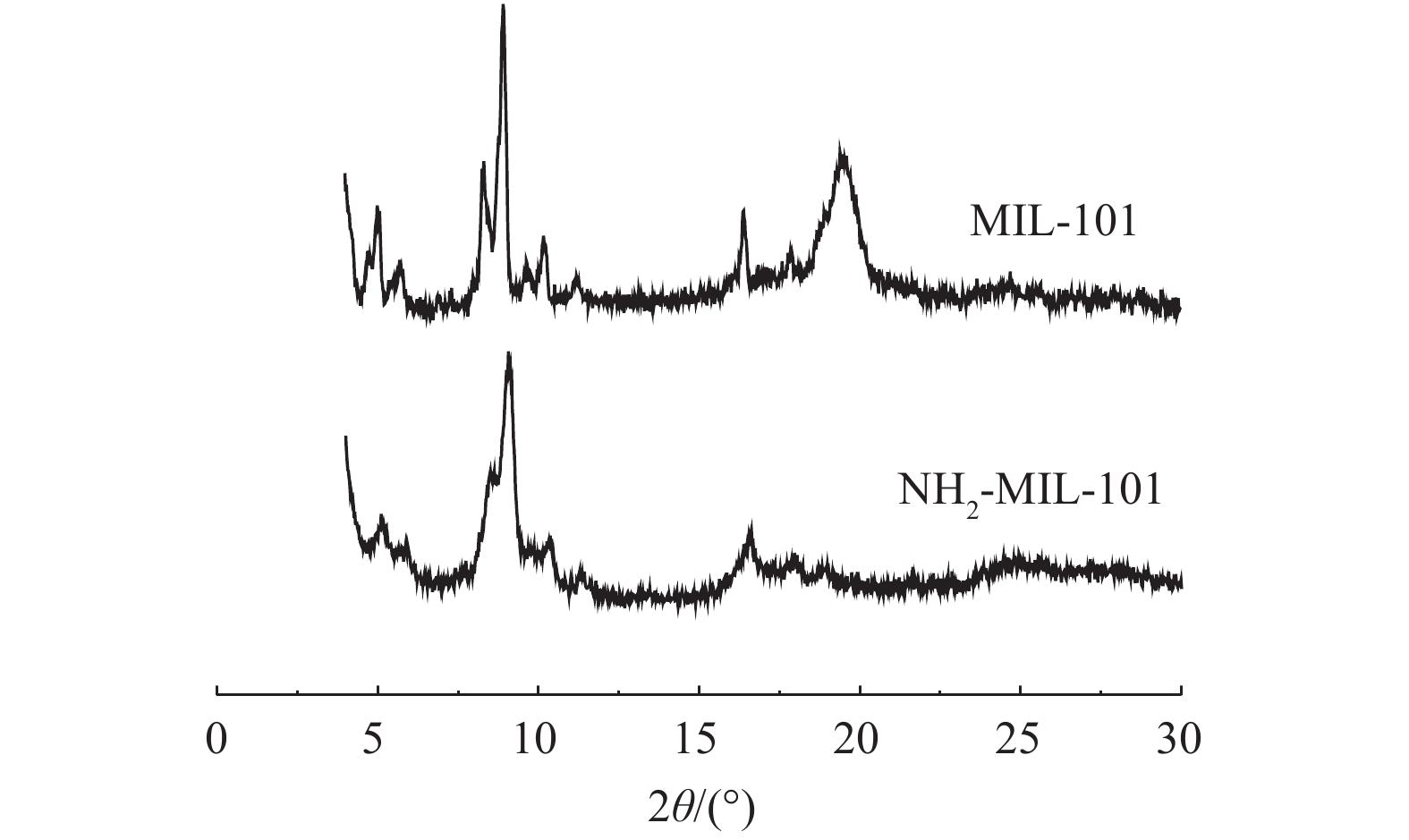
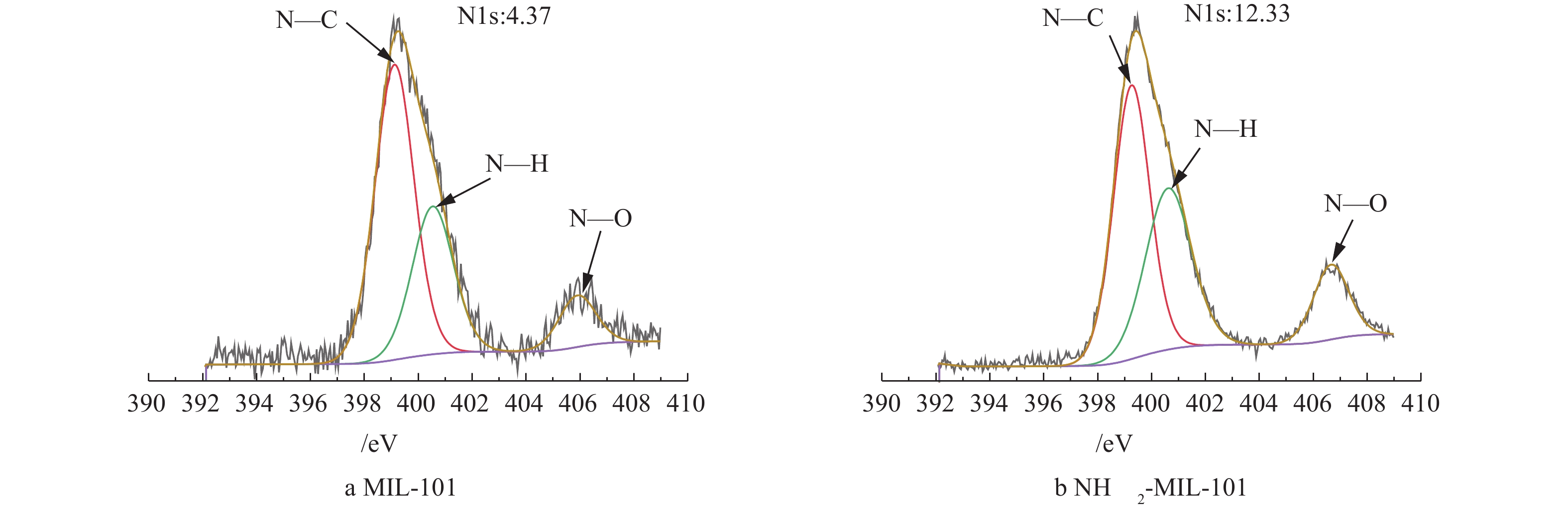
MIL-101改性前后的XRD谱图如图4所示。图4中显示的特征峰与已有报道[32-33]相似,说明材料成功合成。此外,NH2-MIL-101的主要衍射峰与MIL-101基本相同且没有额外的峰出现,这说明材料基本骨架得以保持并且在改性过程没有出现其他结晶相[31],但峰强度下降,说明部分孔道被占据[34]。XPS表征材料改性前后表面元素含量分布结果表明,氨基化后材料中Cr、O、N、C元素的含量从改性前的5.64%、28.5%、4.37%、61.49%变为4.15%、27.55%、12.33%、55.97%,氨基化后的N元素含量比原材料高出近3倍。此外,对比分析材料改性前后N元素的存在状态(图5),包括C—N(399.24,399.38 eV)、N—H(400.78,410.13 eV)和N—O(405.9,406.48 eV)[24,35],C—N、N—H、N—O存在状态的氮元素含量从改性前的67.59%、21.74%、10.67%变为77.2%、11.2%、11.6%。材料改性后N含量显著增加,且与改性前相比,与C结合的氮元素比例增加,与H结合的氮元素比例降低,这说明更多的氨基参与了骨架的形成。
-
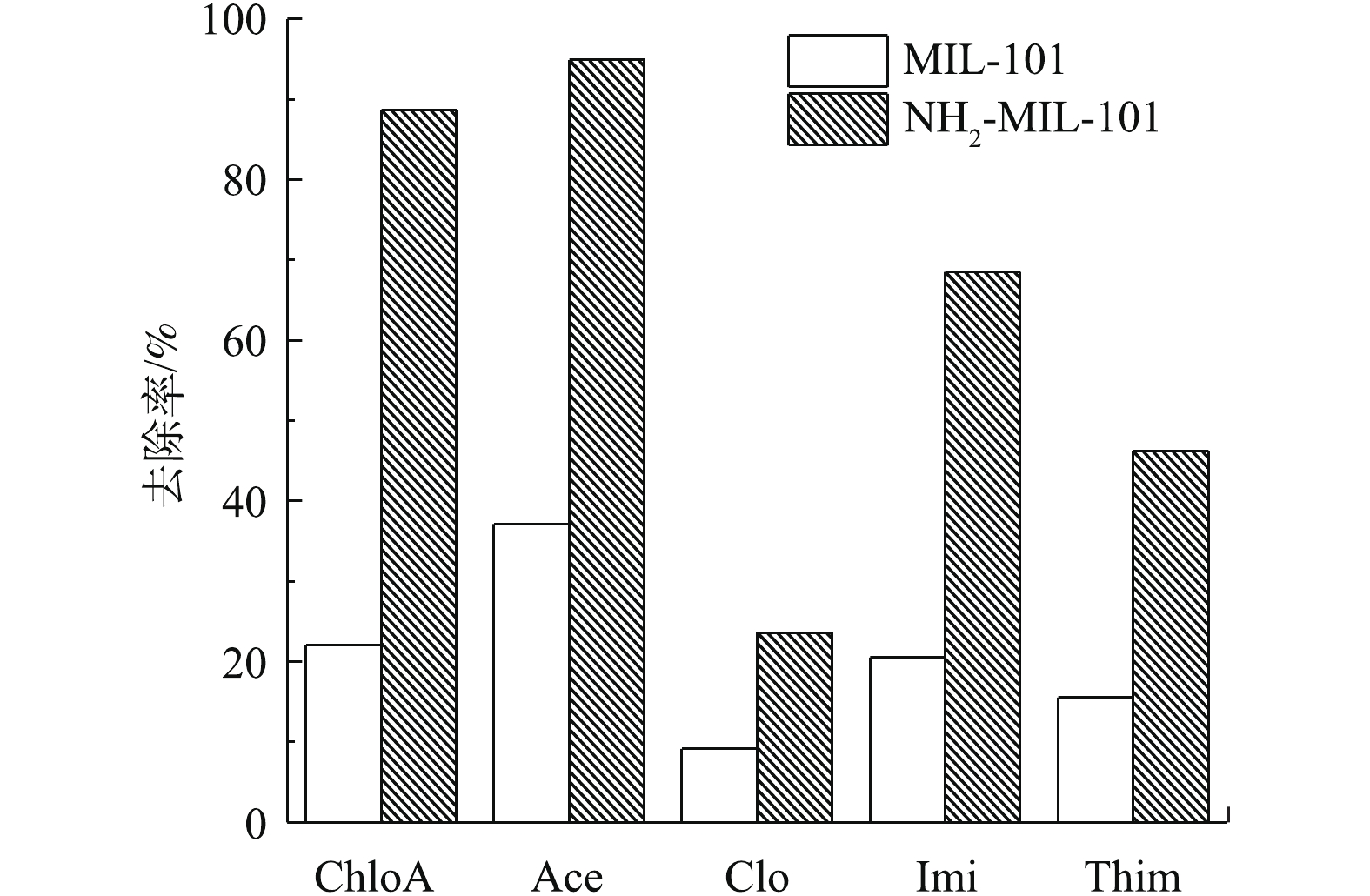
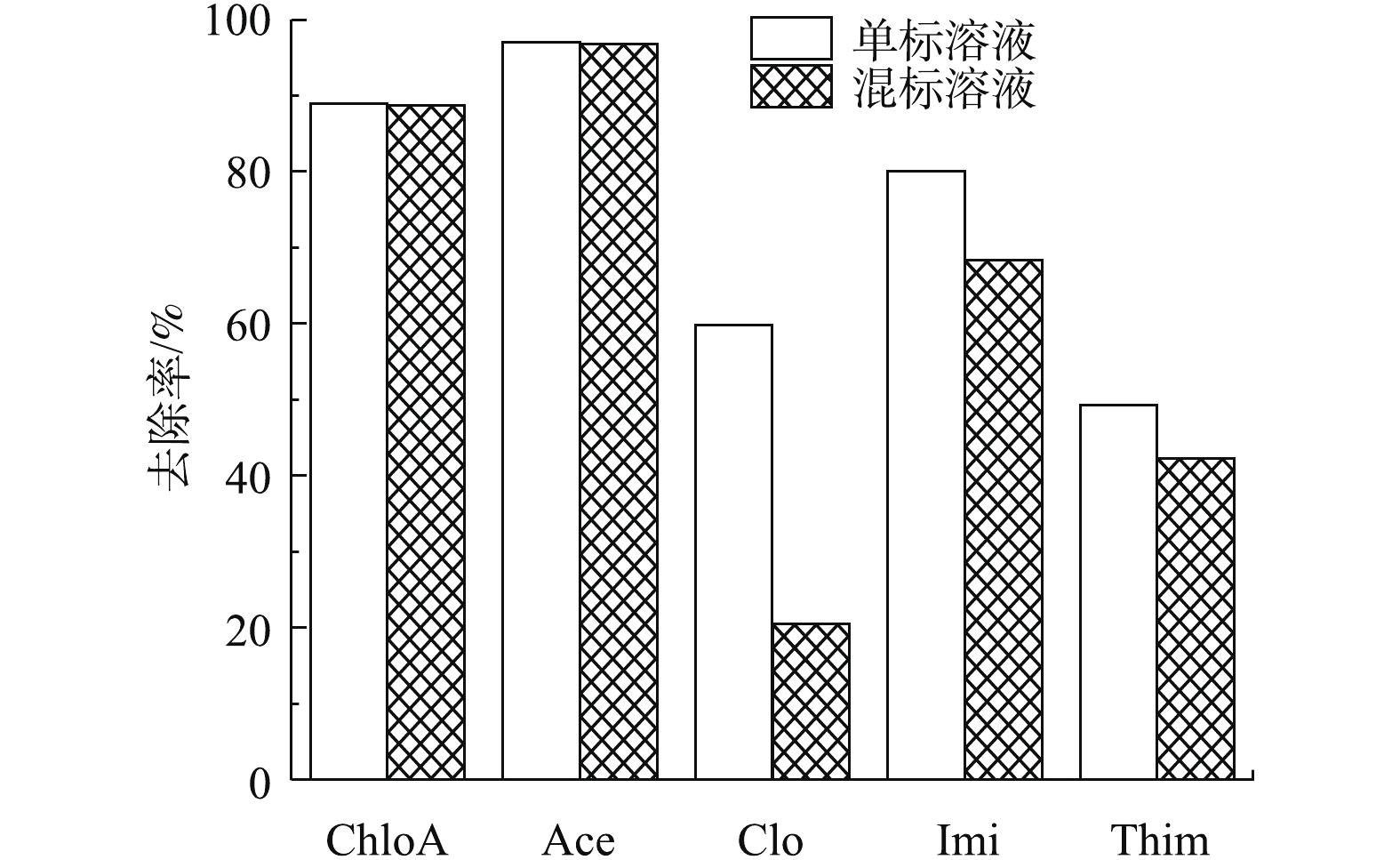
分别用MIL-101(0.04 g)与NH2-MIL-101(0.04 g)进行吸附实验,结果见图6。可以看出,NH2-MIL-101对NNIs的去除效果显著优于MIL-101。一方面,从上述BET表征结果发现,氨基的引入使得材料比表面积有所提高,增加了材料的吸附性能;另一方面,XPS表征结果表明,在氨基化后的MIL-101中,N元素含量有大幅提高,氨基为亲水基团,氨基化可增加材料的亲水性能,而5种NNIs同样具有一定亲水性,因此,氨基化后,材料表面吸附位点可利用性有所增加[36]。另外,氨基的给电子性使NH2-MIL-101苯环上的电子云密度增加,而NNIs共轭环上具有强吸电子效应的氯原子,氨基的引入增强了NH2-MIL-101与NNIs之间的π-π EDA相互作用,导致氨基化的MIL-101的去除效果更为显著,后续实验选取NH2-MIL-101作为吸附剂。同时,实验表明Clo、Imi、Thim在单化合物体系中的去除率大于在混合溶液中的去除率(图7),表明共存化合物对Clo、Imi、Thim在NH2-MIL-101上的吸附存在竞争性[37],然而,ChloA与Ace的去除率在2种体系中没有明显差别。这可能是由于这2种化合物分子体积较小,且ChloA与其他化合物分子结构差异较大,所以其余化合物的存在对其可利用的吸附位点影响较小,导致共存化合物对ChloA与Ace的竞争性很弱。以上结果表明,NH2-MIL-101在去除新烟碱类农药的复合污染方面具有优势。
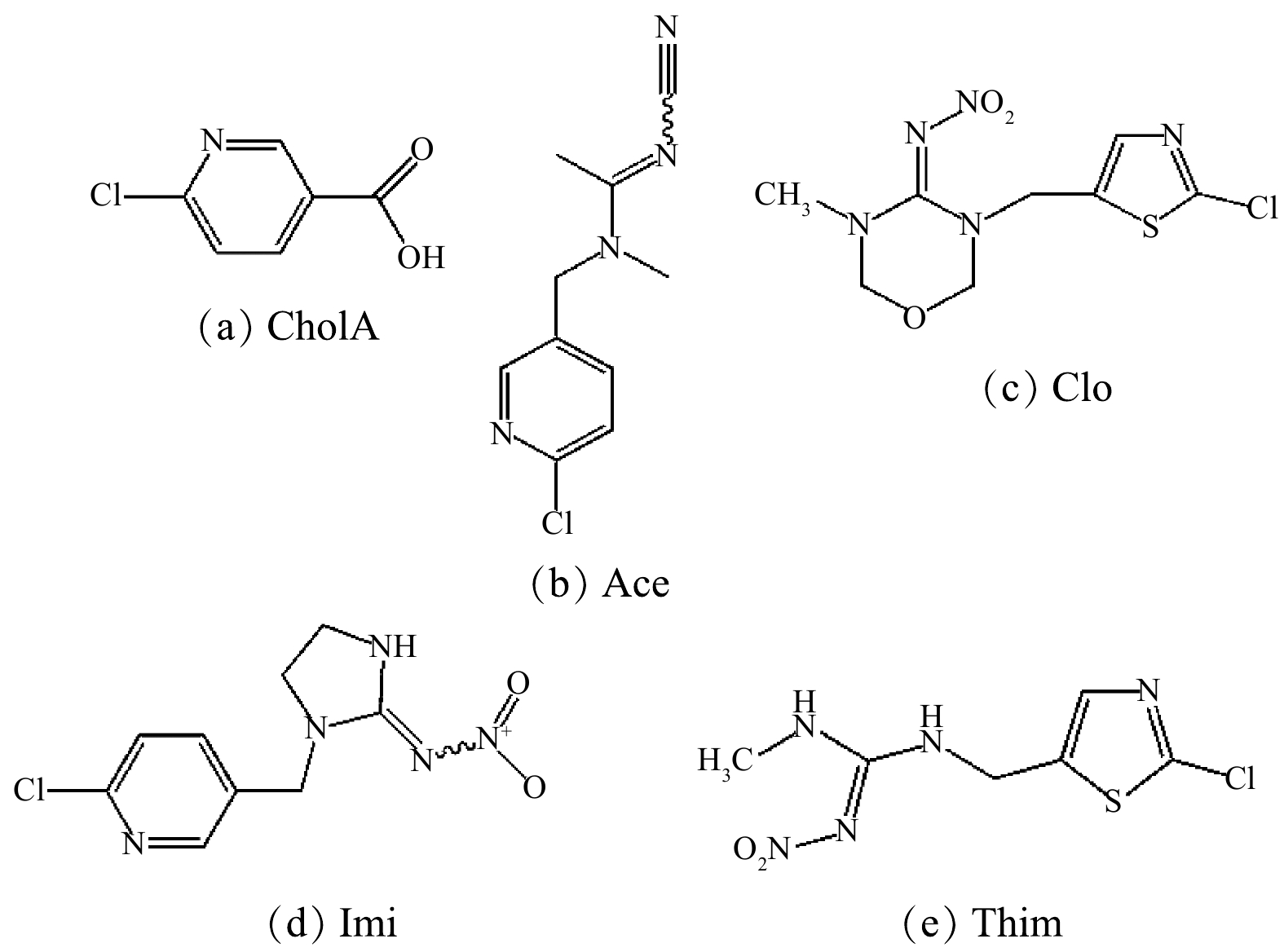
与ChloA及Ace相比,Imi、Thim和Clo的去除率较低。研究表明,芳香性结构的π-π作用在碳质材料吸附有机化合物的过程中起着重要作用[38]。由于ChloA、Ace及Imi分子结构中均存在类似苯环的六元共轭芳香性结构[3](表2和图8),该结构与NH2-MIL-101的芳香性基团可形成较强的π-π作用,增强了吸附作用。虽然Thim和Clo也存在S、N五元共轭环,但其共轭较弱(表2和图8),使其在NH2-MIL-101上的吸附性弱于ChloA、Ace及Imi的吸附性。此外,分子空间位阻在化合物有效利用吸附位点过程中也起着重要作用[39]。与ChloA和Ace相比,Imi分子体积较大,具有较大的空间位阻,减弱了其在材料上的吸附性(表2和图8)。
-
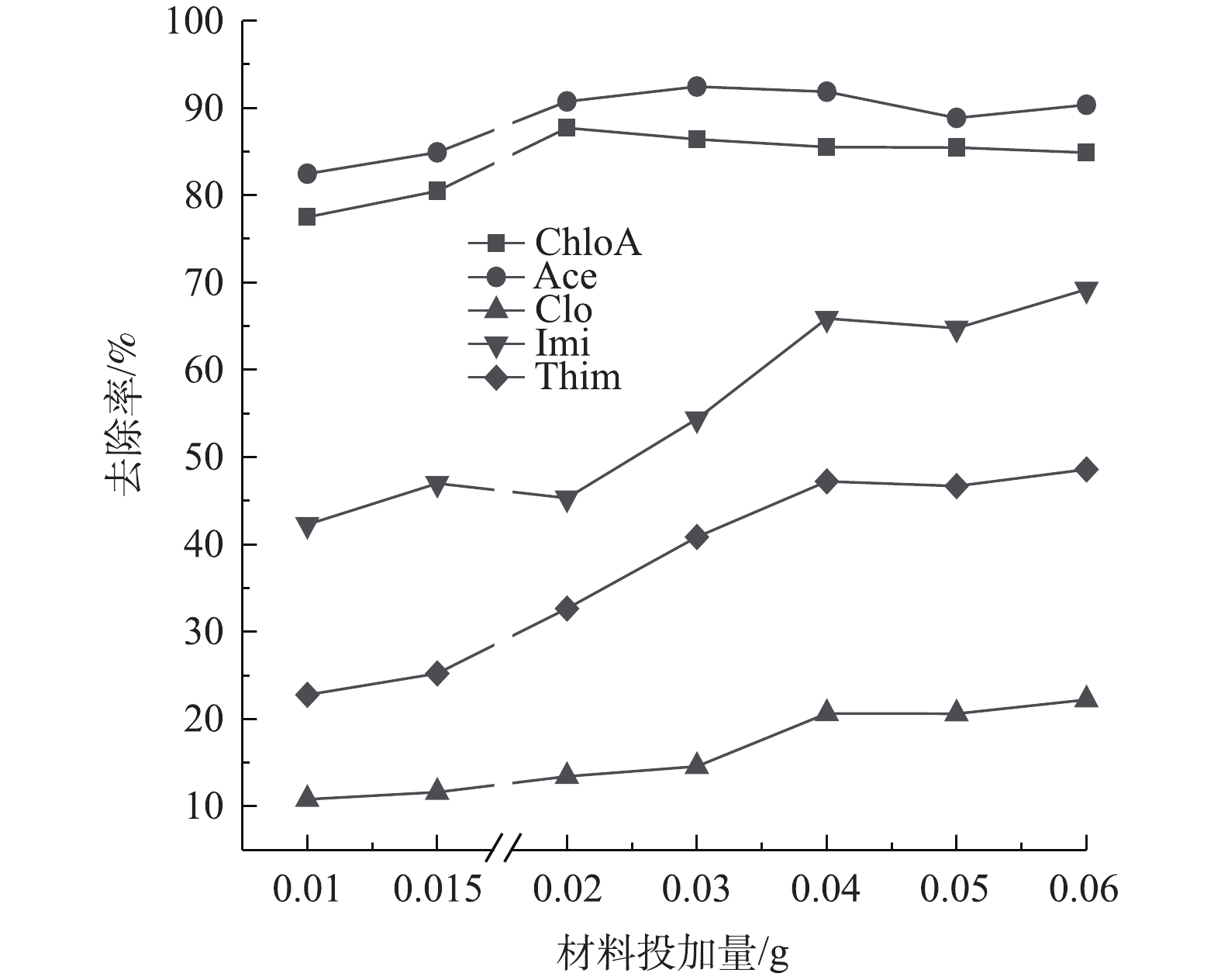
吸附剂的用量是影响吸附效果的重要因素。由图9可知,在溶液pH=6.5,温度T=25 ℃的条件下,当NH2-MIL-101的投加量从0.01 g增至0.04 g,ChloA、Ace、Imi、Thim、Clo的最终去除率分别从77.5%、82.4%、42.2%、22.7%、10.8%增加到86.4%、92.5%、65.9%、47.2%、20.6%。原因主要是:随着吸附剂投加量的增加,吸附剂材料上的活性吸附位点有所提高,从而提高了污染物的吸附去除[40];当NH2-MIL-101的投加量从0.04 g继续增加至0.06 g,ChloA、Ace、Thim、Clo的去除率无明显上升趋势,Imi的去除率上升4%。随着投加量的增加,去除率的上升逐渐趋于平缓[41]。当投加量大于0.04 g后,5种NNIs的去除率上升较小。综合考虑上述结果,选择NH2-MIL-101最佳投加量为0.04 g进行后续实验。
-
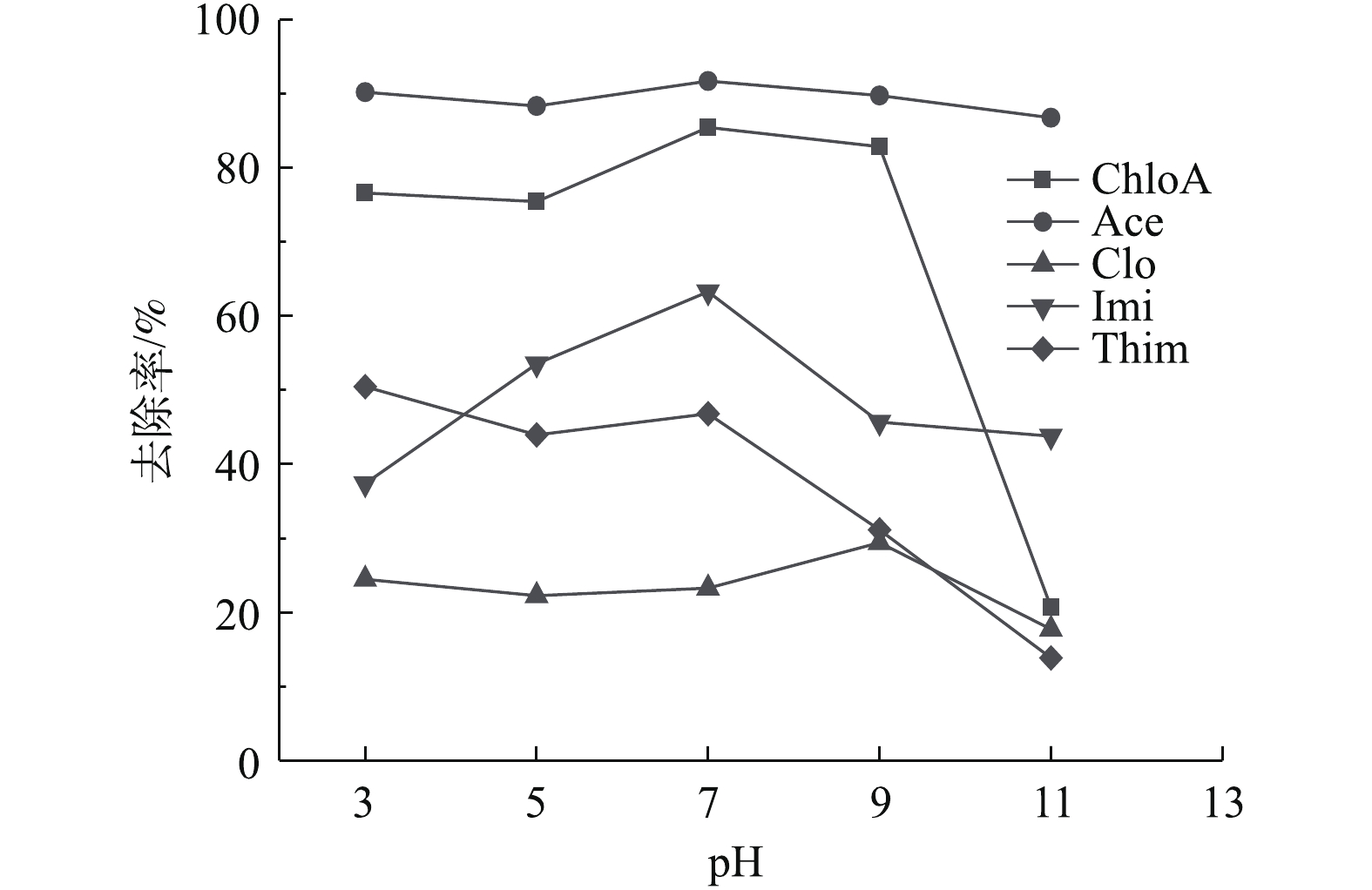
初始pH是影响材料去除可电离有机污染物效果的重要因素[42]。由图10可知,pH对Ace、Clo的去除率影响较小。随着溶液pH的升高,NH2-MIL-101对ChloA、Imi的去除效果先上升后下降,在pH = 7时均可达最佳,分别为85.5%、63.3%;Thim的去除与pH的变化呈负相关关系。吸附性随着环境pH的变化关系与材料表面电荷和物质的结构有关[41]。本研究使用Zeta电位和激光粒度分析仪(美国布鲁克)测定了NH2-MIL-101的表面零电荷点pHpzc = 8.4。当溶液pH为3~8.4时,NH2-MIL-101表面氨基结合氢离子使材料表面带正电荷,而使ChloA、Ace、Imi、Thim带负电荷(表2)。因此,当pH在此范围时,NH2-MIL-101与ChloA、Ace、Imi、Thim发生强烈的静电作用,吸附效果好。而当溶液pH>8.4时,材料表面负电荷逐渐增强,与同样带负电荷的ChloA、Ace、Imi、Thim之间的静电引力作用减弱,因此,其去除效果下降。由表2可以看出,Clo的pKa为11.09,与其他目标化合物相比,pKa较高,其吸附性随pH的变化与其他4种化合物相比存在不同。当pH为3~7时,材料与大部分Clo分子均带正电,材料与Clo分子之间静电斥力占主导;当溶液pH由7转变为9时,材料表面由正电转变为负电,而大部分Clo分子仍带正电,材料与Clo分子的静电作用逐渐由斥力转为引力,吸附性增强;当溶液pH由9变化到11时,带正电的Clo分子比例逐渐降低,可与带负电的材料之间发生吸附作用的分子数减少,导致吸附性减弱。因此,对于不同NNIs的去除,溶液最佳pH存在差异。综合考虑,当pH为3~7时,NH2-MIL-101对NNIs的去除效果好。后续选择水溶液pH=6.5进行实验。
-
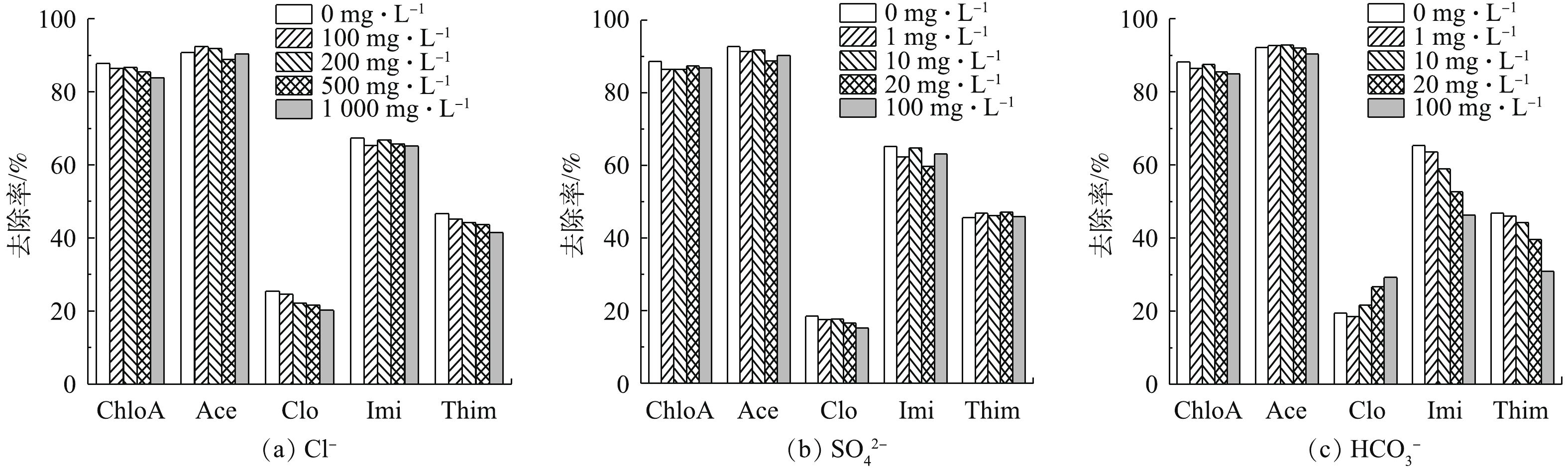
在实际中,废水中会存在一些阴离子(Cl−、
${\rm{SO}}_4^{2 - }$ 、${\rm{HCO}}_3^ - $ )影响材料对NNIs的去除效果。研究发现,随着水中Cl−浓度的升高,NH2-MIL-101对Ace、Imi的去除效果无明显变化。当Cl−浓度升高到500 mg·L−1时,材料对ChloA、Clo、Thim的去除率有所下降(图11),但总体下降不超过5%,影响不大;由图11可知,当${\rm{SO}}_4^{2 - }$ 浓度从0增至100 mg·L−1时,对NNIs的去除率影响较小;然而,由图11看出,随着${\rm{HCO}}_3^ - $ 浓度的升高,ChloA、Ace、Imi、Thim的去除率与之呈负相关,原因可能是随着${\rm{HCO}}_3^ - $ 浓度的升高,${\rm{HCO}}_3^ - $ 在溶液中水解生成H2CO3,使溶液pH升高至碱性,这在一定程度上抑制了NH2-MIL-101对这4种物质的吸附;然而随着溶液中${\rm{HCO}}_3^ - $ 浓度的增加,溶液pH由6.5增加至9.2,Clo的去除率反而略有上升,该结果与溶液pH对NNIs在NH2-MIL-101上吸附的影响结果一致。总的来说,NH2-MIL-101在高浓度Cl−、${\rm{SO}}_4^{2 - }$ 、${\rm{HCO}}_3^ - $ 废水中也具有较好的稳定性,适用于含高浓度Cl−、${\rm{SO}}_4^{2 - }$ 、${\rm{HCO}}_3^ - $ 的实际工农业废水。 -
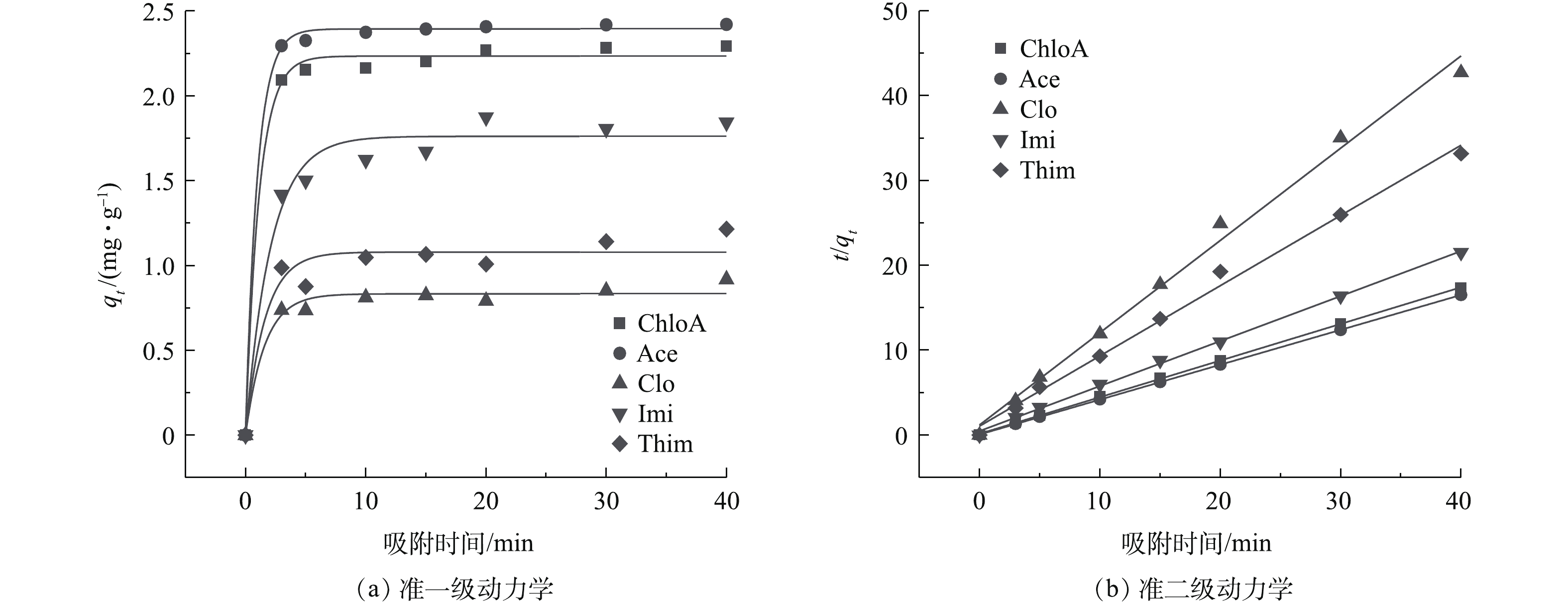
利用准一级、准二级动力学模型进一步分析NH2-MIL-101对NNIs的吸附行为。由图12可知,材料对NNIs的吸附在20 min就已达到平衡。LIU等[15]利用磁性石墨烯材料制备的金属有机骨架在吸附7种NNIs时,超过60 min达到平衡;MOMČILOVIĆ等[13]用活性炭吸附Imi,其吸附平衡时间大于20 h。而本研究吸附速率更快,准一级与准二级动力学模型对实验数据拟合的R2都较高(见表3),但准二级动力学模型(R2>0.99)更优于准一级动力学模型(R2>0.93)。且准二级动力学模型测定的qe值与计算值更接近,因此,准二级动力学模型能更好地描述NH2-MIL-101对NNIs的吸附过程。
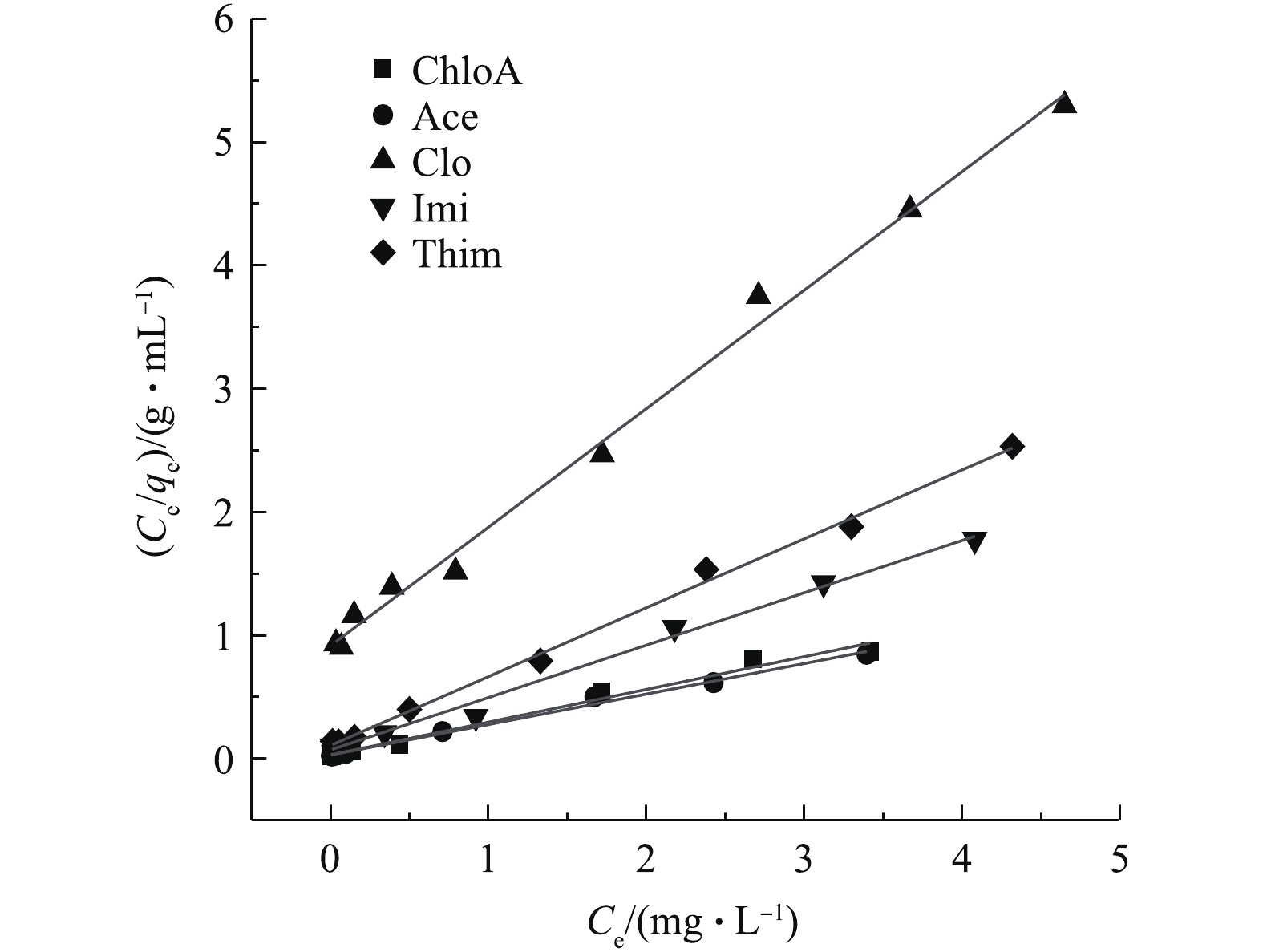
本研究对NH2-MIL-101对NNIs的吸附等温线进行了探讨,在室温下探索NH2-MIL-101的吸附机制以及NH2-MIL-101的表面特性,采用Langmuir [43]模型进行拟合。Langmuir 吸附等温式通常用于化学吸附和在常温、常压下的物理吸附,吸附剂表面性质均匀,为单层吸附[44]。
由图13可以看出,5种物质Langmuir等温拟合线性关系良好,可决系数R2>0.97;且RL均在区间内,说明NH2-MIL-101对于NNIs的吸附真实有效,符合Langmuir模型。由表4可知,NH2-MIL-101对ChloA、Ace、Clo、Imi、Thim的饱和吸附量分别为3.77、4.04、1.04、2.35、1.79 mg·g−1。通过等温线拟合结果计算得出,材料投加量理论值要增加到本实验最大投加量0.06 g的30倍以上,才可使5种NNIs的去除率达到100%。因此,采取低剂量增加NH2-MIL-101投加量的方式,难以实现进一步提升本实验浓度下的目标化合物的去除率。
-
去除剂的重复利用性在天然水体及实际废水污染修复方面至关重要,同时也是衡量其性能及经济性的重要指标[45]。取0.04 g NH2-MIL-101置于100 mL 1 mg·L−1的NNIs溶液中,搅拌吸附30 min后,用离心法将材料与溶液分离,然后用甲醇及超纯水交替洗脱2次,真空干燥。重复上述步骤6次,分别测量每次吸附后溶液中NNIs的浓度。在实验过程中,NH2-MIL-101保持了较高的去除性能(图14),重复利用5次后,5种NNIs去除率下降在5%以内。6次之后,NNIs的去除率明显降低,但下降程度仍在10%以内。总体来说,结果表明NH2-MIL-101材料具有良好的循环再生能力。
2.1. 材料的表面分析
2.2. NH2-MIL-101对不同NNIs的去除效果
2.3. NH2-MIL-101投加量对NNIs去除率的影响
2.4. 初始pH对NH2-MIL-101吸附性能的影响
2.5. 阴离子对去除率的影响
2.6. 动力学与吸附等温线
2.7. 材料再生实验
-
1)利用水热法技术制备的NH2-MIL-101,具有高比表面积和化学稳定性,且该材料对于水体中的NNIs具有优异的去除性能及循环再生能力。
2) NNIs的去除率与NH2-MIL-101投加量呈正相关关系,但本材料对于本研究的目标化合物在此浓度下的去除率难以达到100%;初始pH对NNIs的吸附效果具有较大影响,对于不同NNIs的去除,溶液最佳pH存在差异,综合而言,pH为3~7时去除效果较好。
3) NH2-MIL-101对NNIs的去除率受Cl−、
${\rm{SO}}_4^{2 - }$ 的影响较小,但${\rm{HCO}}_3^ - $ 的加入对ChloA、Ace、Imi、Thim在NH2-MIL-101 上的吸附具有明显的抑制作用,而${\rm{HCO}}_3^ - $ 的加入对Clo在该材料上的吸附有促进作用。4)温度为25 ℃时,NH2-MIL-101对ChloA、Ace、Clo、Imi、Thim的饱和吸附量分别为3.77、4.04、1.04、2.35 和1.79 mg·g−1,仅20 min就可达到吸附平衡,且吸附过程服从准二级动力学模型和Langmuir模型。







 下载:
下载: