-
新烟碱类杀虫剂化学成分是一种类似于尼古丁结构的化合物,其通过作用于昆虫神经系统中烟碱型乙酰胆碱酯酶受体,阻断昆虫中枢神经系统信号的传导,从而导致害虫麻痹并死亡[1]。自1991年吡虫啉上市以来,新烟碱类杀虫剂迅速发展成为全球第一大类杀虫剂。随着新烟碱类杀虫剂的不断使用,其对环境的危害也进一步凸显出来。一些研究[2-5]表明,新烟碱类杀虫剂不仅对非目标生物(包括蜜蜂、鸟类、水生昆虫等)造成致死作用,而且对人类和哺乳动物的健康也会造成潜在的损害。关于环境中新烟类杀虫剂的迁移转化及去除研究已陆续开展,主要包括化学还原、微生物降解、光催化等,但是存在产物毒性强、降解速率慢、降解产物复杂等方面不足[6-8]。因此,如何高效安全地去除环境中残留的新烟碱类杀虫剂是目前亟待解决的问题之一。
吸附作为去除环境中污染物的有效方法之一,特别是采用环境友好型低成本的吸附剂,由于其使用简单、适用性强、操作灵活、效率高而被广泛应用[9]。生物炭是在缺氧条件下,将生物质经高温裂解产生的富碳产物,具有比表面积大、孔隙发达和含氧官能团丰富等优点,对环境中大部分污染物有良好的吸附和去除效果[10-14]。目前,研究较多的是人工制备的生物炭,对自然环境中生成的生物炭的研究相对较少。自然界中的森林火灾等在一定条件下也能够产生生物炭,且能长时间在自然生态系统中存在,其对环境中污染物迁移转化有着持久的影响[15]。基于此,本研究选用亚热带栽培广、生长快的杉木为原料,通过实验室马弗炉和自然环境下分别制备了2种生物炭,考察了2种生物炭对新烟碱类杀虫剂(啶虫脒(ACE),噻虫胺(CLO))的吸附性能及影响因素,比较了2种生物炭的吸附性能,为新烟碱类杀虫剂在环境中的迁移转化及污染修复提供参考。
-
ACE(C10H11CIN4, >97.0%)和CLO(C6H8CIN5O2S, >97.0%)均购自上海阿拉丁生化科技股份有限公司,2种农药的基本理化性质参数见表1。NaNO3为分析纯,购自无锡市晶科化工有限公司;Ca(NO3)·4H2O为分析纯,购自上海阿拉丁生化科技股份有限公司;HNO3为分析纯,购自无锡市展望化工试剂有限公司。实验用水均为超纯水。
-
高效液相色谱仪(e2695,美国Waters),超纯水装置(Millipore Integral 3,美国Milli-Q),组合式恒温摇床(ZQZY-BF8,上海知楚),马弗炉(SX2-8-10N/NP,上海一恒),型材切割机(355切割机,浙江欧莱德),pH计(FE28,瑞士Mettler Toledo)。
-
本实验的生物炭原料为杉木,生物炭分别采用2种方法制备。方法1:将木材经型材切割机切割成方形小薄块,放入150 mL石英坩埚内,盖上盖子,外层用锡箔纸包裹,置于马弗炉中,于700 ℃条件下高温热解6 h,冷却24 h后取出,在研钵中研磨过100目筛,装于自封袋中备用。将此生物炭标记为BC1。方法2:将木材经型材切割机切割成木块,置于自然环境中燃烧,冷却后刮去表层白色飞灰,研磨过100目筛,装于自封袋中备用。将此生物炭标记为BC2。
采用扫描电子显微镜(SU8100,日本Hitachi)观察BC1和BC2表面形貌;采用傅里叶红外光谱仪(Cary660,美国Agilent)分析生物炭表面官能团;采用全自动比表面和孔隙度测定仪 (Mini Ⅱ,日本Belsorp)测定BC1和BC2的比表面积(SBET)、孔体积和孔径分布。
-
所有实验在250 mL的具塞锥形瓶中进行,反应总体积为100 mL,2种新烟碱类杀虫剂母液浓度为100 mg·L−1,使用0.01 mol·L−1 HNO3和NaOH溶液调节溶液pH,使用1.0 mol·L−1 NaNO3溶液调节离子强度。吸附反应在组合式恒温摇床中进行,摇床转速为200 r·min−1。采用5 mL的针筒注射器取样,经0.45 μm的混合纤维素滤膜过滤后,使用高效液相色谱检测溶液中剩余污染物浓度。
在进行吸附动力学实验时,溶液初始pH为5.0,温度为25 ℃,2种新烟类杀虫剂的浓度初值均为10 mg·L−1,生物炭添加量为0.3 g·L−1,取样时间分别为1.0、2.0、5.0、10、15、20、30、45、60、90、120 min。测定各时间点时溶液中剩余污染物浓度,用准一级动力学模型和准二级动力学模型对实验数据进行拟合,每组实验设置3个平行组和1个空白对照组。
在进行吸附等温实验时,2种新烟类杀虫剂的浓度分别为3.0、5.0、8.0、10、15、18、20 mg·L−1,生物炭添加浓度为0.3 g·L−1,温度分别为15、25和35 ℃。吸附24 h后取样,测定溶液中剩余污染物浓度,用Langmuir和Freundlich模型拟合实验数据。
在考察初始pH和离子强度对吸附的影响时,由于pH≤9,24 h内,ACE基本不会发生水解[19],因此,本实验使用0.01 mol·L−1 HNO3和0.01 mol·L−1 NaOH分别将ACE、CLO溶液初始pH调节为5.0、6.0、7.0、8.0、9.0和3.0、5.0、7.0、9.0、11.0,以考察初始pH对吸附效果的影响。用1.0 mol·L−1的NaNO3和Ca(NO3)2调节溶液中金属离子浓度分别为0、0.01、0.1和0.5 mol·L−1,以考察离子强度对吸附效果的影响,其余条件与吸附动力学实验一致。
-
ACE、CLO采用高效液相色谱检测,吸收波长分别为245 nm和265 nm,色谱柱为XBridge@C18(250 mm×4.6 mm,5 μm),柱温为30 ℃,流动相甲醇与水的体积比分别为70∶30和45∶55。生物炭对ACE和CLO的去除率(R)和吸附量(qe)分别根据式(1)和式(2)进行计算。
式中:R为生物炭对ACE和CLO的去除率;qe为生物炭平衡吸附量,mg·g−1;C0和Ce分别为2种新烟碱类杀虫剂的初始浓度和平衡浓度,mg·L−1;V为反应溶液体积,L;m为生物炭添加量,g。
在吸附动力学实验中,采用准一级动力学方程(式(3))、准二级动力学方程(式(4))和内部扩散方程(式(5))对吸附过程进行拟合[20]。
式中:qt和qe分别为ACE和CLO在t时刻和吸附平衡时新烟碱类杀虫剂被吸附的量,mg·g−1;k1为准一级吸附动力学速率常数,min−1;k2为准二级吸附动力学速率常数,g·(mg·min)−1;k3为颗粒内扩散速率常数,mg·(g·min1/2)−1。
在等温吸附实验中,采用Langmuir方程(式(6))和Freundlich方程(式(7))吸附模型对吸附过程进行拟合[21]。
式中:qe和qm分别为生物炭吸附平衡和最大吸附量,mg·g−1;Ce为吸附平衡时的浓度,mg·L−1;KF和KL分别是Langmuir和Freundlich方程的相关系数;1/n为Freundlich的经验系数。
在吸附热力学实验中,利用式(8)~式(10)计算吸附过程中的自由能ΔG0、焓变ΔH0和熵变ΔS0[22]。
式中:R为理想气体摩尔常数,取值8.31 J·(mol·K)−1;T为热力学温度,K;Kd为吸附平衡常数。
-
图1是BC1和BC2的微观表面形貌图。可以看出,厌氧热解和自然环境下燃烧所形成的炭材料均保持着杉木的自然结构,在形态上无显著差异,杉木细胞空腔呈现出蜂窝状,破碎的导管在BC1和BC2表面形成了大量类似于隧道一样的沟壑,且沟壑中沉积着许多片状颗粒。
采用N2吸附-脱附法分析BC1和BC2的比表面积和孔隙结构,结果如图2(a)和图2(b)所示。根据国际纯粹与应用化学联合会(IUPAC)提出的6种物理吸附等温线[23],BC1和BC2均属于Ⅰ型吸附等温线,表明2种生物炭均具有微孔结构,N2在生物炭表面发生微孔填充现象。BC1吸附-脱附曲线形成了闭合回滞环,属于H4型回滞环。该型回滞环多出现在微孔和中孔混合的吸附剂上和含有狭窄的裂隙孔的固体中。BC2吸附-脱附曲线未形成闭环,这可能是由于BC2中热解未完全的有机物的作用。对比两者的BJH孔径分布曲线图,BC1的孔径主要分布在0~10 nm,没有出现明显的峰,说明孔径分布较均匀,主要以微孔结构为主。BC2的孔径主要分布在0~8 nm,且在2.37 nm时出现尖锐的峰,说明BC2主要含有2 nm左右的孔隙结构,微孔结构更小。根据表2中的数据可知,BC1的BET比表面积明显大于BC2,分别为440.27 m2·g−1和329.15 m2·g−1,主要原因可能是由于马弗炉的热解温度较高且稳定,自然开放环境下烧制的温度差异较大,导致部分生物炭热解不完全。BC1和BC2的平均孔径均在2 nm左右,但是,BC1总孔容大于BC2,这有利于BC1对污染物的吸附。
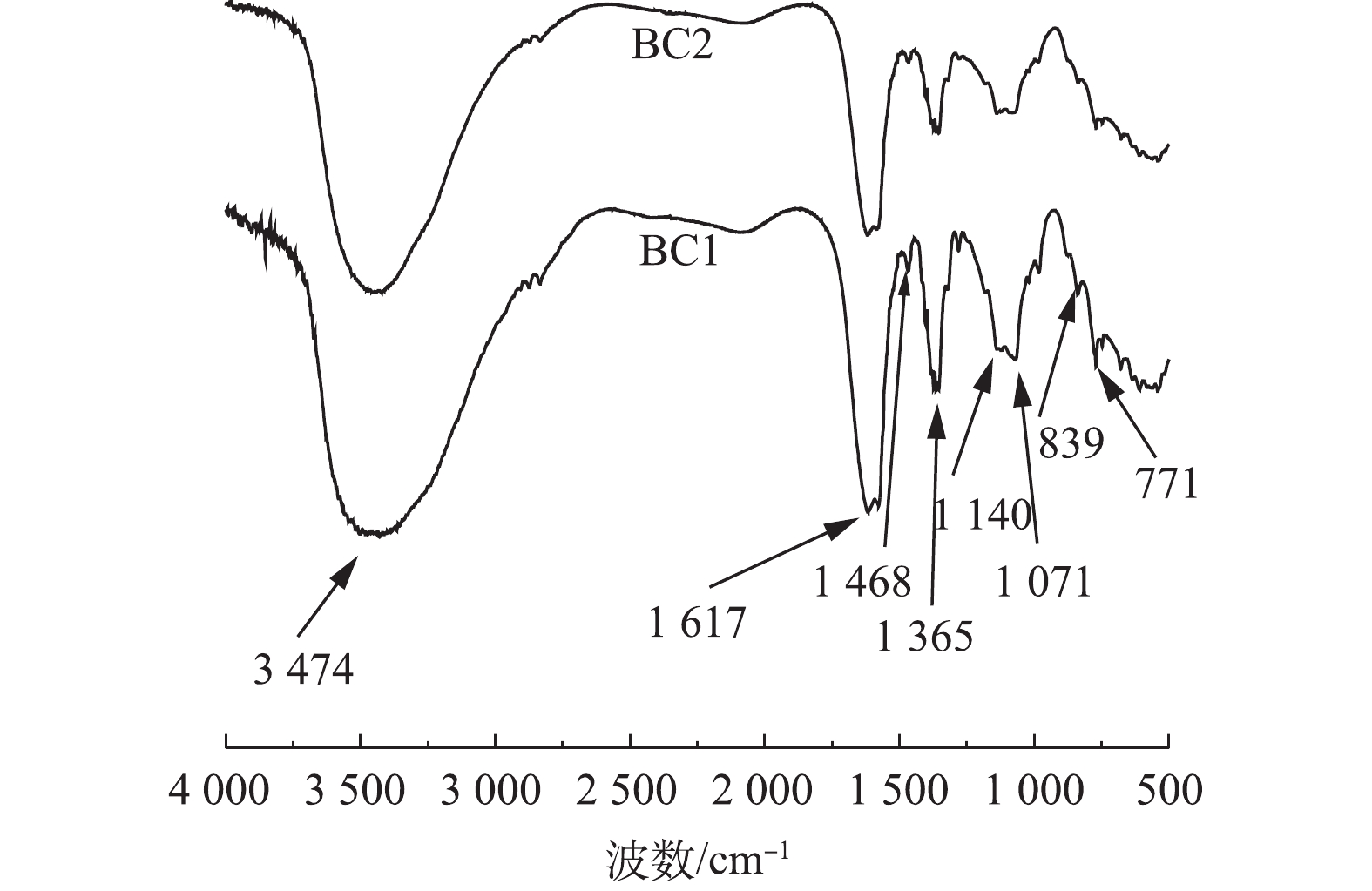
图3是BC1和BC2的傅里叶红外光谱图。可以看出,BC1和BC2具有许多相似的特征峰,这表明BC1和BC2表面含有丰富的官能团。3 474 cm−1处的宽峰是酚羟基(—OH)的伸缩振动峰,1 617 cm−1和1 468 cm−1处的吸收峰是芳香环中的C=O、C=C的伸缩振动峰,1 365、1 140和1 071 cm−1处的吸收峰可以归因于羧基(—OH)的弯曲模式和C—O拉伸振动,839 cm−1和771 cm−1附近的吸收峰主要是由于C—H伸缩振动[12,24-26]引起的。由此得出,BC1和BC2上主要的含氧官能团均为—OH、C=O、—COOH,此外,通过对比BC1和BC2峰强度的大小,可以看出,BC1在1 365、1 140和1 071 cm−1处的吸收峰强度高于BC2,这说明BC1表面的羧基数量可能大于BC2的羧基数量。
-
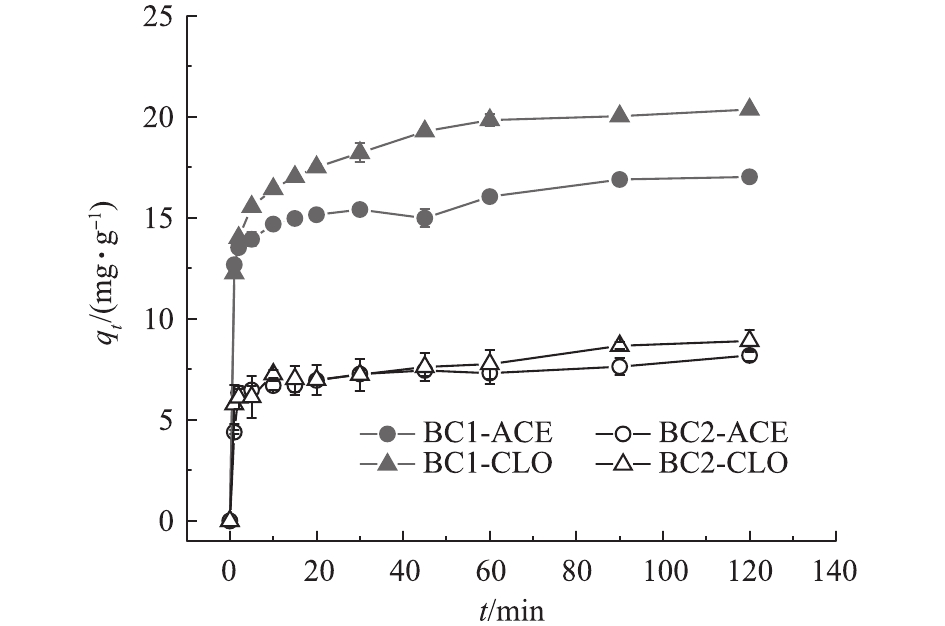
BC1和BC2对2种新烟碱类杀虫剂的吸附动力学曲线见图4。2种生物炭对溶液中的新烟碱类杀虫剂呈现出相近的吸附动力学过程。生物炭对杀虫剂的吸附量在短时间内(10 min)随着时间的增加迅速增大,之后吸附量增加的速度逐渐变缓,在45 min后,吸附量趋于稳定,此现象与生物炭表面的活性位点的有限性有关。在吸附初始阶段,生物炭表面的活性位点充分,目标污染物能够迅速占据活性位点而呈现出吸附量的急速上升;随着吸附的继续进行,可供吸附的活性位点逐渐减少,进而吸附速率开始下降,最终导致吸附趋于平衡[27]。
为进一步评价2种生物炭的吸附特性及其速率控制步骤和机理,采用准一级动力学方程和准二级动力学方程对2种生物炭吸附新烟碱类杀虫剂的动力学过程进行拟合,结果见表3。由表3可知,准二级动力学的R2均大于准一级动力学,且准二级动力学计算出的理论值qe更加接近于实验测得的数据,这说明准二级动力学方程能够更好地拟合BC1和BC2对2种杀虫剂的吸附过程。BC1的吸附速率普遍小于BC2,但是吸附容量大于BC2。这可能是由于BC1具有更高的孔容,污染物在孔隙中的吸附达到平衡需要更长的时间,总吸附容量也较大,BC2的孔容较小,污染物能够较快地达到吸附平衡,导致吸附容量相对有限。
颗粒内扩散模型反映了吸附过程中的实际控速步骤和吸附机理[28]。由于实验条件及吸附材料和目标污染物的理化性质不同,吸附过程通常可分为吸附2阶段过程和吸附3阶段过程[29-30]。吸附2阶段过程机理为污染物通过液膜扩散到吸附剂表面和污染物在吸附剂表面发生吸附的2个过程。吸附3阶段过程机理为污染物跨水膜扩散到吸附剂表面、污染物在吸附剂孔隙内扩散和吸附趋平衡的3个过程。本研究中生物炭对新烟碱类杀虫剂的吸附过程存在3个阶段,结果如图5所示,线性拟合数据见表4。整体上,BC1吸附过程中的3个阶段较BC2更加明显,BC2的阶段2和阶段3相对模糊,可能是由于BC1具有相对较大的平均孔径和总孔容,致使在阶段2中,新烟碱类杀虫剂于BC1孔隙内扩散积累的量大于BC2所造成。每种生物炭吸附拟合的斜率大小顺序均为k1>k2>k3,这说明污染物向吸附剂的扩散是速率逐渐减小直至达到吸附平衡的过程。BC1和BC2在阶段1中的模型拟合程度均较低,这可能由于该阶段污染物跨水膜扩散到吸附剂表面的作用复杂,影响因素较多所致。2种生物炭在阶段2中的拟合程度最优,R2接近1,这可能是因为孔隙中的环境较稳定,污染物在吸附剂孔隙内能够平稳的扩散所导致。模型对BC1和BC2的阶段3拟合差异最大,BC2的阶段3数据拟合程度明显低于BC1,表明新烟碱类杀虫剂在热解程度较低的生物炭上达到吸附平衡的过程更为复杂。
-
为进一步了解BC1和BC2对ACE、CLO的吸附容量和吸附机理,采用Langmuir和Freundlich 2种吸附模型对数据进行拟合,结果见图6和表5。可以看出,随着ACE和CLO初始浓度的增加,BC1和BC2的吸附量均呈现出先上升后趋于稳定的趋势。此外,随着温度的上升,BC1和BC2的吸附量也同样有所增加,对于BC1,Langmuir模型拟合的相关系数均高于Freundlich,且在3个温度条件下呈现相同的大小关系,说明BC1对2种杀虫剂的吸附属于单分子层吸附,温度的变化对杀虫剂在BC1上的吸附形式没有影响。对于BC2,Freundlich模型拟合的相关系数与Langmuir拟合相关系数并没有一致的大小关系,且随着温度的变化,2种模型相关性系数的大小关系也产生了变化。这说明BC2对目标污染物的吸附存在单分子层和多分子层吸附,温度变化对吸附形式具有一定的影响。BC1对每种杀虫剂的最大理论吸附量均高于BC2,呈现出更好的吸附能力,2种生物炭对杀虫剂的最大吸附量均为qm(CLO)>qm(ACE),此结果与动力学实验一致。KL和KF可指示吸附能力的强弱,KL和KF越大,吸附剂的吸附能力越强。BC1的吸附参数大于BC2,这说明马弗炉高温热解比自然环境烧制的生物炭吸附能力更强。Freundlich拟合模型常数1/n均为0~0.2,这说明BC1和BC2均能较好地吸附2种新烟碱类杀虫剂[31]。
通常使用热力学参数来描述生物炭对新烟碱类杀虫剂的吸附过程中热量的变化情况。在15、25和35 ℃下,2种生物炭吸附ACE和CLO的相关热力学参数见表6。在所选温度下,吉布斯自由能ΔG0均为负值,表明反应是自发进行的。ΔG0在所选温度下基本保持恒定,可见2种生物炭对新烟碱类杀虫剂的吸附受温度影响较小。ΔH0>0,且BC1在吸附过程中的ΔH0明显大于BC2,说明吸附过程需吸收热量,BC1对目标污染物具有更强的吸附能力。ΔS0>0表明在固液界面处生物炭的结合位点上被吸附的2种新烟碱类类杀虫剂的自由度降低,这表明目标污染物分子与生物炭表面活性位点具有较强的结合能力[32]。
-
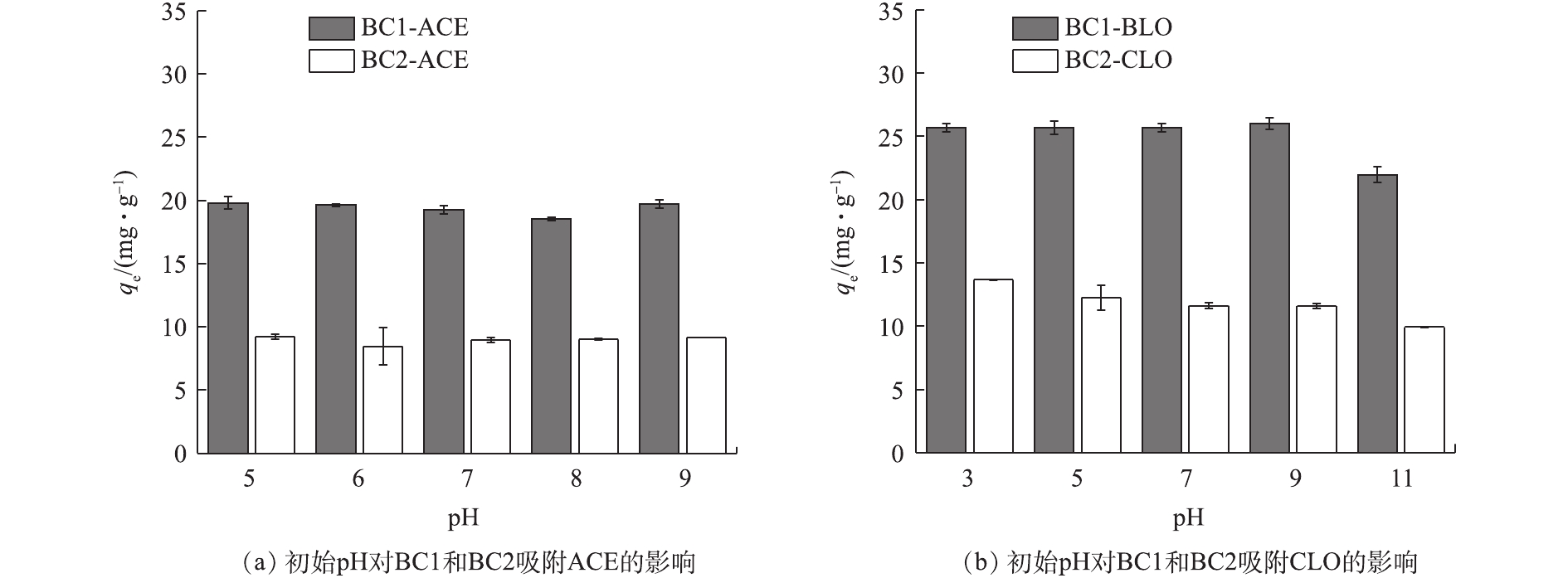
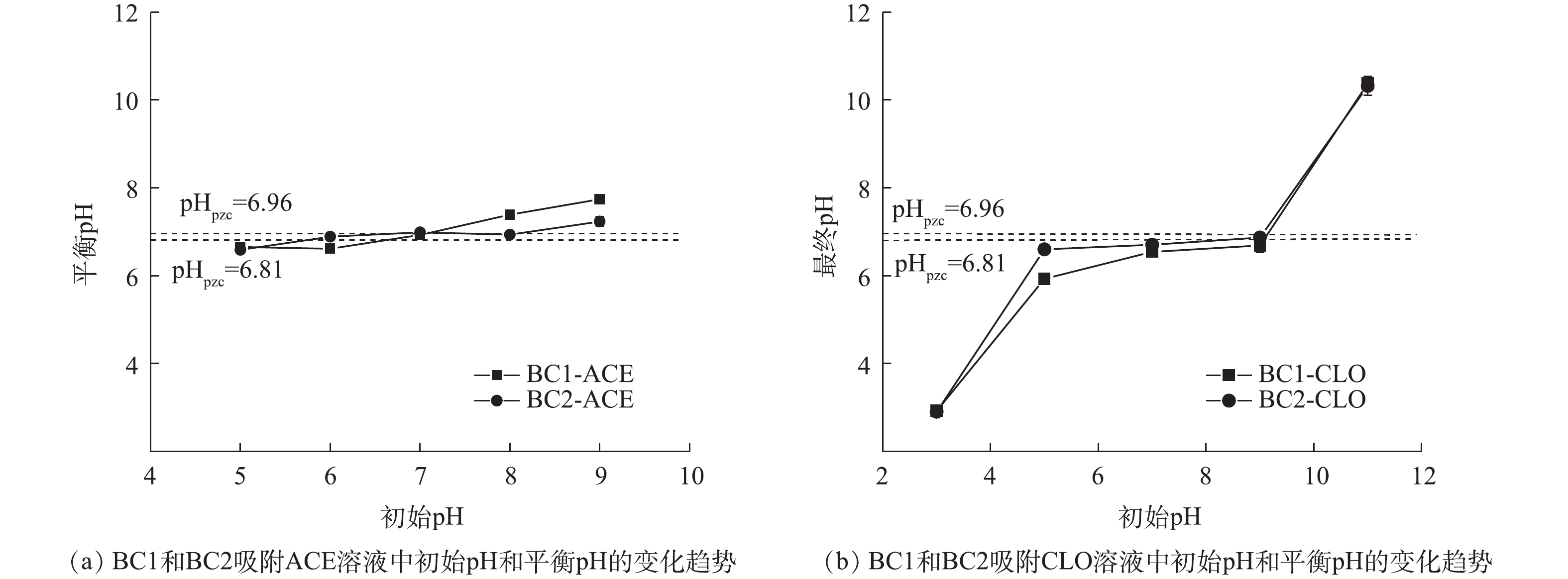
pH是影响吸附效果的重要因素之一,其可影响目标污染物的解离程度和生物炭的表面电荷[33]。由表1可知,2种新烟碱类杀虫剂的解离常数存在很大差异,ACE无pKa,CLO的pKa为11.1,即在pH为5~9和pH为3~11的范围内,ACE、CLO分别以分子、阳离子的形式存在于水溶液中。初始pH对BC1和BC2吸附ACE、CLO的影响如图7(a)和图7(b)所示。由吸附实验结果可见,随着初始pH的上升,2种生物炭的吸附量均未发生明显变化,为10%~20%,这说明2种生物炭均能在较宽的pH范围内对新烟碱类杀虫剂有良好的吸附效果。溶液初始pH和平衡pH的变化如图8(a)和图8(b)所示,当初始pH为11时,吸附能力的小幅度下降可能是由于吸附平衡后溶液体系为碱性,钝化了生物炭表面的活性位点和减弱了BC1和BC2与CLO分子中O、N原子间的氢键作用力,从而导致生物炭吸附能力的下降[20, 34]。
-
溶液离子强度对BC1和BC2去除2种新烟碱类杀虫剂的影响结果如图9所示。由图9可知,随着Na+和Ca2+浓度的增加,BC1和BC2的吸附容量均有所下降。当Na+离子浓度从0增加到0.5 mol·L−1时,BC1对ACE、CLO的吸附量分别下降了11.29%和5.74%,BC2对ACE、CLO的吸附量分别下降了10.80%和11.44%。Ca2+离子浓度从0增加到0.5 mol·L−1时,BC1对ACE、CLO的吸附量分别下降了20.07%和16.24%,BC2对ACE、CLO的吸附量分别下降了27.66%和18.69%。由图9可知,Na+和Ca2+的添加对2种生物炭吸附新烟碱类杀虫剂均有一定的抑制作用,且Ca2+的抑制作用大于Na+。根据离子强度的定义,相同浓度的Ca2+呈现的离子强度是Na+的4倍,且Ca2+可能会更有效地占据生物炭表面的活性位点[35]。
-
1) 自然条件下烧制的生物炭(BC2)比表面积小于马弗炉烧制的生物炭(BC1),分别为329.15 m2·g−1和440.27 m2·g−1。BC1的总孔容大于BC2,两者官能团种类基本一致,BC1的官能团含量高于BC2,这表明实验室条件下的生物炭较自然条件下的生物炭具有更优的结构和性质,有利于其对污染物的吸附和去除。
2) BC1对2种新烟碱类杀虫剂的吸附能力明显高于BC2。在25 ℃,pH=5时,BC1和BC2对ACE和CLO最大吸附量分别为24.46、31.56、11.13、12.24 mg·g−1。吸附过程更好地符合准二级动力学模型,2种新烟碱类杀虫剂在BC1上发生的是单分子层吸附,在BC2上存在单分子层和多分子层吸附。孔隙结构发达的BC1比BC2呈现出更加明显的跨水膜扩散、孔隙内扩散和吸附平衡3阶段过程。
3) 热力学实验结果表明,2种生物炭对新烟碱类杀虫剂的吸附是自发、吸热的过程。初始pH的升高和离子强度的增加均抑制了BC1和BC2对新烟碱类杀虫剂的吸附作用。
杉木生物炭对水中啶虫脒和噻虫胺的吸附特性
Adsorption properties of acetamiprid and clothianidin in water by Cunninghamia lanceolata biochar
-
摘要: 自然界中生物炭有多种产生途径,影响污染物的迁移转化。为比较实验室和在自然条件下生成的生物炭的吸附性能,以杉木为原料,分别于马弗炉(700 ℃)和自然开放环境中制备了2种生物炭(分别标记为BC1和BC2)。运用氮吸附(BET)、扫描电子显微镜(SEM)和红外光谱(FT-IR)表征分析了生物炭的结构与性质,研究了其对2种新烟碱类杀虫剂(啶虫脒(ACE)和噻虫胺(CLO))的吸附行为,分别考察了初始pH、温度和共存离子对吸附行为的影响。结果表明,700 ℃下裂解制备的BC1吸附能力明显优于自然条件下制备的BC2。BC1对ACE和CLO最大吸附量分别为24.46 mg·g−1和31.56 mg·g−1,BC2对ACE和CLO最大吸附量分别为11.13 mg·g−1和12.24 mg·g−1。BC1和BC2对2种新烟碱类杀虫剂的吸附过程较好地符合准二级吸附动力学模型。颗粒内扩散模型分析结果表明,BC1的吸附较BC2存在更明显的3阶段过程。Langmuir和Freundlich模型拟合结果表明,BC1对2种杀虫剂的吸附属于单分子层吸附,BC2的吸附过程同时存在单分子层和多分子层吸附。热力学研究表明,BC1和BC2对新烟碱类杀虫剂的吸附为自发的吸热过程。随着初始pH的升高和离子强度的增加抑制了生物炭的吸附能力,相同浓度Na+的抑制作用小于Ca2+。以上结果可为水中新烟碱类杀虫剂的去除提供参考。Abstract: Biochar can be formed by many processes in nature and affect the migration and transformation of pollutants in environment. In order to compare the adsorption performance of biochar formed under laboratory and natural conditions, two types of Cunninghamia lanceolata biochar were prepared in a muffle furnace at 700 ℃ and open environment, respectively, which were marked as BC1 and BC2. The structures and properties of these two types of biochar were characterized by nitrogen adsorption (BET), scanning electron microscope (SEM), and Fourier transform infrared spectroscopy (FTIR). The adsorption behaviors of chosen neonicotinoid insecticides (acetamiprid (ACE), clothianidin (CLO)) on biochar were studied, and the effects of initial pH, temperature and coexisting ions on the adsorption behaviors were also investigated. The results showed that the adsorption capacity of BC1 was much greater than BC2, the maximum adsorption capacities of BC1 toward ACE and CLO were 24.46 mg·g−1 and 31.56 mg·g−1, respectively, while the maximum adsorption capacities of BC2 toward ACE and CLO were 11.13 mg·g−1 and 12.24 mg·g−1, respectively. The adsorption kinetics of ACE and CLO on BC1 and BC2 fitted well with the pseudo-second order adsorption model. The intraparticle diffusion model analysis indicated that a more significant three-stage process occurred for BC1 adsorption than BC2 adsorption. The fitting results of Langmuir and Freundlich model showed that the adsorption of two insecticides on BC1 belonged to a single molecular layer adsorption, and on BC2 belonged to both single and multi-molecular layer adsorptions. The thermodynamics experiments indicated that the adsorption processes of neonicotinoid insecticides on BC1 and BC2 were Spontaneous endothermic ones. With the increase of initial pH and ionic strength, the adsorption ability of biochar was inhibited. The inhibitory effect of Na+ was lower than that of Ca2+ at the same concentration. The results can provide a reference for the removal of neonicotinoid insecticides in water.
-
Key words:
- biochar /
- acetamiprid /
- clothianidin /
- adsorption
-

-
表 1 2种农药基本的理化性质
Table 1. Physical and chemical properties of two neonicotinoid pesticides
表 2 BC1和BC2的BET比表面积和孔隙结构参数
Table 2. BET specific surface areas and pore structure parameters of BC1 and BC2
吸附剂 比表面积/(m2·g−1) 平均孔径/nm 总孔容/(cm3·g−1) BC1 440.27 2.06 0.227 BC2 329.15 1.94 0.160 表 3 BC1和BC2吸附ACE、CLO动力学参数
Table 3. Kinetic parameters of ACE and CLO adsorption on BC1 and BC2
吸附剂 杀虫剂 准一级动力学方程 准二级动力学方程 qe/(mg·g−1) k1/h−1 R2 qe/(mg·g −1) k2/(g·(mg·min)−1) R2 BC1 ACE 14.07 61.08 0.988 14.57 5.92 0.997 CLO 16.42 65.85 0.953 17.43 6.29 0.984 BC2 ACE 6.86 59.97 0.992 7.26 14.06 0.996 CLO 6.82 103.22 0.971 7.09 31.30 0.985 表 4 BC1和BC2吸附ACE、CLO的颗粒内扩散方程拟合参数
Table 4. Fitting parameters of intraparticle diffusion model of ACE and CLO adsorption on BC1 and BC2
吸附剂 杀虫剂 阶段1 阶段2 阶段3 k1 C1 R2 k2 C2 R2 k3 C3 R2 BC1 ACE 10.140 10.644 0.913 3.433 12.521 0.998 0.382 16.205 0.995 CLO 16.858 9.920 0.976 6.423 13.570 0.999 0.227 18.986 0.993 BC2 ACE 6.667 3.605 0.956 0.941 6.618 0.994 0.397 7.745 0.899 CLO 3.242 5.337 0.984 2.483 5.456 0.997 0.149 9.375 0.879 表 5 BC1和BC2吸附ACE、CLO的吸附等温线拟合参数
Table 5. Fitting parameters of adsorption isotherms of ACE and CLO on BC1 and BC2
吸附剂 杀虫剂 温度/℃ Langmuir模型 Freundlich模型 qm/(mg·g−1) KL/(L·mg−1) R2 1/n KF R2 BC1 ACE 15 19.99 3.61 0.951 0.14 14.31 0.891 25 24.47 3.87 0.979 0.15 17.34 0.866 35 24.95 7.31 0.897 0.14 18.45 0.869 BC2 ACE 15 11.82 2.28 0.953 0.12 8.58 0.890 25 11.13 1.85 0.895 0.14 7.62 0.962 35 13.35 1.62 0.968 0.16 8.62 0.942 BC1 CLO 15 23.96 2.70 0.939 0.14 18.49 0.869 25 31.56 1.20 0.902 0.16 16.51 0.835 35 30.00 4.88 0.949 0.18 20.10 0.900 BC2 CLO 15 13.05 1.31 0.954 0.18 7.95 0.866 25 12.25 3.60 0.930 0.11 9.29 0.881 35 14.11 3.28 0.835 0.12 10.53 0.910 表 6 BC1和BC2吸附ACE、CLO的热力学参数
Table 6. Thermodynamic parameters for ACE and CLO adsorption onto BC1 and BC2
吸附剂 杀虫剂 温度/℃ lnKd ΔG0/(kJ·mol−1) ΔH0/(kJ·mol−1) ΔS0/(kJ·(mol·K)−1) BC1 ACE 15 15.50 −21.02 38.14 0.262 1 25 16.33 −21.75 35 16.53 −22.48 BC2 ACE 15 14.44 −19.44 12.73 0.164 0 25 14.57 −20.12 35 14.79 −20.79 BC1 CLO 15 17.01 −11.52 16.63 0.180 0 25 16.73 −11.92 35 17.34 −12.32 BC2 CLO 15 14.86 −18.38 13.23 0.168 9 25 14.82 −19.01 35 15.22 −19.65 -
[1] JESCHKE P, NAUEN R, SCHINDLER M, et al. Overview of the status and global strategy for neonicotinoids[J]. Journal of Agricultural and Food Chemistry, 2011, 59(7): 2897-2908. doi: 10.1021/jf101303g [2] MITCHELL E A D, MULHAUSER B, MULOT M, et al. A worldwide survey of neonicotinoids in honey[J]. Science, 2017, 358(6359): 109-111. doi: 10.1126/science.aan3684 [3] HALLMANN C A, FOPPEN R P B, VAN TURNHOUT C A M, et al. Declines in insectivorous birds are associated with high neonicotinoid concentrations[J]. Nature, 2014, 511(7509): 341-343. doi: 10.1038/nature13531 [4] CAVALLARO M C, MAIN A R, LIBER K, et al. Neonicotinoids and other agricultural stressors collectively modify aquatic insect communities[J]. Chemosphere, 2019, 226: 945-955. doi: 10.1016/j.chemosphere.2019.03.176 [5] HAN W C, TIAN Y, SHEN X M. Human exposure to neonicotinoid insecticides and the evaluation of their potential toxicity: An overview[J]. Chemosphere, 2018, 192: 59-65. doi: 10.1016/j.chemosphere.2017.10.149 [6] 刘晓, 田颖, 周晓慧, 等. 零价铝在碱性条件下还原吡虫啉[J]. 化工学报, 2017, 68(4): 384-389. [7] MULLIGAN R A, TOMCO P L, HOWARD M W, et al. Aerobic versus anaerobic microbial degradation of clothianidin under simulated california rice field conditions[J]. Journal of Agricultural & Food Chemistry, 2016, 64(38): 7059-7067. [8] MIR N A, KHAN A, MUNEER M, et al. Photocatalytic degradation of a widely used insecticide thiamethoxam in aqueous suspension of TiO2: Adsorption, kinetics, product analysis and toxicity assessment[J]. Science of the Total Environment, 2013, 458-460: 388-398. doi: 10.1016/j.scitotenv.2013.04.041 [9] LI H, DONG X, DA SILVA E B, et al. Mechanisms of metal sorption by biochars: Biochar characteristics and modifications[J]. Chemosphere, 2017, 178: 466-478. doi: 10.1016/j.chemosphere.2017.03.072 [10] 程扬, 沈启斌, 刘子丹, 等. 两种生物炭的制备及其对水溶液中四环素去除的影响因素[J]. 环境科学, 2019, 40(3): 1328-1336. [11] NIE T, HAO P, ZHAO Z, et al. Effect of oxidation-induced aging on the adsorption and co-adsorption of tetracycline and Cu2+ onto biochar[J]. Science of the Total Environment, 2019, 673: 522-532. doi: 10.1016/j.scitotenv.2019.04.089 [12] YIN Y, GUO X, PENG D. Iron and manganese oxides modified maize straw to remove tylosin from aqueous solutions[J]. Chemosphere, 2018, 205: 156-165. doi: 10.1016/j.chemosphere.2018.04.108 [13] 李蕊宁, 王兆炜, 郭家磊, 等. 酸碱改性生物炭对水中磺胺噻唑的吸附性能研究[J]. 环境科学学报, 2017, 37(11): 4119-4128. [14] 李政剑, 石宝友, 苏宇, 等. 粉末活性炭粒径对水中菲吸附动力学的影响效应研究[J]. 环境科学学报, 2013, 33(1): 70-75. [15] PRESTON C M, SCHMIDT M W I. Black (pyrogenic) carbon: A synthesis of current knowledge and uncertainties with special consideration of boreal regions[J]. Biogeosciences, 2006, 3: 397-420. doi: 10.5194/bg-3-397-2006 [16] 邵翼飞, 张鹏, 刘爱菊. 生物炭对噻虫胺在土壤中吸附和降解的影响[J]. 农业环境科学学报, 2019, 38(11): 2520-2527. doi: 10.11654/jaes.2019-0460 [17] 庾琴, 周华, 王静, 等. 啶虫脒在环境中的降解代谢及其安全性的研究进展[J]. 农药, 2007, 46(4): 223-226. doi: 10.3969/j.issn.1006-0413.2007.04.002 [18] 管欢, 黄慧俐, 行艳景, 等. 噻虫胺在甘蔗和土壤中的残留分析及消解动态[J]. 现代农药, 2015, 14(2): 48-51. [19] 谢国红, 刘国光, 孙德智, 等. 啶虫脒水解动力学研究[J]. 安徽农业科学, 2007, 35(30): 9629-9630. doi: 10.3969/j.issn.0517-6611.2007.30.078 [20] 杨奇亮, 吴平霄. 改性多孔生物炭的制备及其对水中四环素的吸附性能研究[J]. 环境科学学报, 2019, 39(12): 3973-3984. [21] 张学良, 徐建, 占新华, 等. 微波辅助合成γ-Fe2O3/花生壳磁性生物炭对水体中环丙沙星吸附的研究[J]. 环境科学学报, 2019, 39(11): 3811-3820. [22] 郑晓青, 韦安磊, 张一璇, 等. 铁锰氧化物/生物炭复合材料对水中硝酸根的吸附特性[J]. 环境科学, 2018, 39(3): 262-274. [23] MUTTAKIN M, MITRA S, THU K, et al. Theoretical framework to evaluate minimum desorption temperature for IUPAC classified adsorption isotherms[J]. International Journal of Heat and Mass Transfer, 2018, 122: 795-805. doi: 10.1016/j.ijheatmasstransfer.2018.01.107 [24] OUYANG D, CHEN Y, YAN J, et al. Activation mechanism of peroxymonosulfate by biochar for catalytic degradation of 1,4-dioxane: Important role of biochar defect structures[J]. Chemical Engineering Journal, 2019, 370: 614-624. doi: 10.1016/j.cej.2019.03.235 [25] TAN X L, FANG M, CHEN C L, et al. Counterion effects of nickel and sodium dodecylbenzene sulfonate adsorption to multiwalled carbon nanotubes in aqueous solution[J]. Carbon, 2008, 46(13): 1741-1750. doi: 10.1016/j.carbon.2008.07.023 [26] LIU S, XU W H, LIU Y G, et al. Facile synthesis of Cu(II) impregnated biochar with enhanced adsorption activity for the removal of doxycycline hydrochloride from water[J]. Science of the Total Environment, 2017, 592: 546-553. doi: 10.1016/j.scitotenv.2017.03.087 [27] 孙航, 蒋煜峰, 石磊平, 等. 不同热解及来源生物炭对西北黄土吸附敌草隆的影响[J]. 环境科学, 2016, 37(12): 4857-4866. [28] ZHOU Y Y, LIU X C, XIANG Y J, et al. Modification of biochar derived from sawdust and its application in removal of tetracycline and copper from aqueous solution: Adsorption mechanism and modelling[J]. Bioresource Technology, 2017, 245: 266-273. doi: 10.1016/j.biortech.2017.08.178 [29] 孙绪兵, 吴雪梅, 朱建发, 等. 羧基甲壳素对Pb(Ⅱ)的吸附性能及机理研究[J]. 中国环境科学, 2018, 38(8): 3018-3028. doi: 10.3969/j.issn.1000-6923.2018.08.030 [30] 张连科, 王洋, 王维大, 等. 生物炭负载纳米羟基磷灰石复合材料的制备及对铅离子的吸附特性[J]. 化工进展, 2018, 37(9): 3492-3501. [31] DAI L C, ZHU W K, HE L, et al. Calcium-rich biochar from crab shell: An unexpected super adsorbent for dye removal[J]. Bioresource Technology, 2018, 267: 510-516. doi: 10.1016/j.biortech.2018.07.090 [32] PENG X, HU F, LAM F L, et al. Adsorption behavior and mechanisms of ciprofloxacin from aqueous solution by ordered mesoporous carbon and bamboo-based carbon[J]. Journal of Colloid Interface Science, 2015, 460: 349-360. doi: 10.1016/j.jcis.2015.08.050 [33] LIU P, LIU W J, JIANG H, et al. Modification of biochar derived from fast pyrolysis of biomass and its application in removal of tetracycline from aqueous solution[J]. Bioresource Technology, 2012, 121: 235-240. doi: 10.1016/j.biortech.2012.06.085 [34] LI H Q, HU J T, MENG Y, et al. An investigation into the rapid removal of tetracycline using multilayered graphene-phase biochar derived from waste chicken feather[J]. Science of the Total Environment, 2017, 603-604: 39-48. doi: 10.1016/j.scitotenv.2017.06.006 [35] PEIRIS C, GUNATILAKE S R, MLSNA T E, et al. Biochar based removal of antibiotic sulfonamides and tetracyclines in aquatic environments: A critical review[J]. Bioresource Technology, 2017, 246: 150-159. doi: 10.1016/j.biortech.2017.07.150 -




 下载:
下载: