-
铁尾矿是工业选矿后产生的固体废物,通常在尾矿库中进行露天堆放处置,除了长期占用大量土地外,还容易产生含有大量重金属的废水,对环境造成污染[1]。以粤北大宝山尾矿库为例,参照《GBT 3838-2002地表水环境质量标准》[2],尾矿区废水中大量重金属元素超标,其中Cu平均含量是Ⅴ类水体标准的6倍,平均Mn含量是标准的40倍[3]。
铁尾矿的还原磁化焙烧则是回收铁元素的有效技术之一。其中气基还原的主要还原剂为CO,具有高反应效率、还原更充分均匀的特点,但工业CO和H2的价格昂贵,不适合大量生产[4]。自然界中的生物质简单易得,在300~400 ℃下热解即可产生大量CO,在高温条件下更可生成一定量的H2,在还原焙烧铁尾矿从而生产磁铁矿上有巨大的应用潜力[5]。TAN等[6]利用N2为载气在650 ℃下对榴莲壳进行无氧热解,在GC-TCD检测中发现热解气中含有大约50%的CO2与35%的CO;AL[7]利用快速热解技术,在680 ℃的条件下对甘蔗渣进行热解,最终得到的还原气中含有45.3%的H2和20.5%的CO;MALIUTINA等[8]在600~900 ℃的条件下分别对小球微藻和棕榈壳进行了闪速热解,在800 ℃的条件下,微藻产生的热解气中含有44.33%的H2,而棕榈壳在900 ℃下产生的热解气中H2的含量为26.62%。
以往研究大多关注生物质热解后各组分的含量,针对如何提高热解气中还原性气体占比的研究较少(本研究中还原性气体含量指CO与H2总量),也少有将动力学机理纳入热解气的生成探讨中。本研究针对广东省韶关地区大量种植的杉树与青竹进行单因素试验,热重分析与红外光谱分析,考察不同反应条件下杉树木屑与竹屑的热解产气情况,并与热解动力学协同研究,探讨这两种生物质的产气机理与一般规律。
-
实验所用生物质样品为杉树木屑和竹屑,从广东省韶关地区采得,经破碎至粒径小于50目(小于0.297 mm),于烘箱内105 ℃下烘干后储存至干燥箱备用。工业分析与成分分析分别依据《GBT 28731-2012 固体生物质燃料工业分析方法》[9]以及《GBT 35818-2018 林业生物质原料分析方法 多糖及木质素含量的测定》[10]进行测定,结果见表1。
-
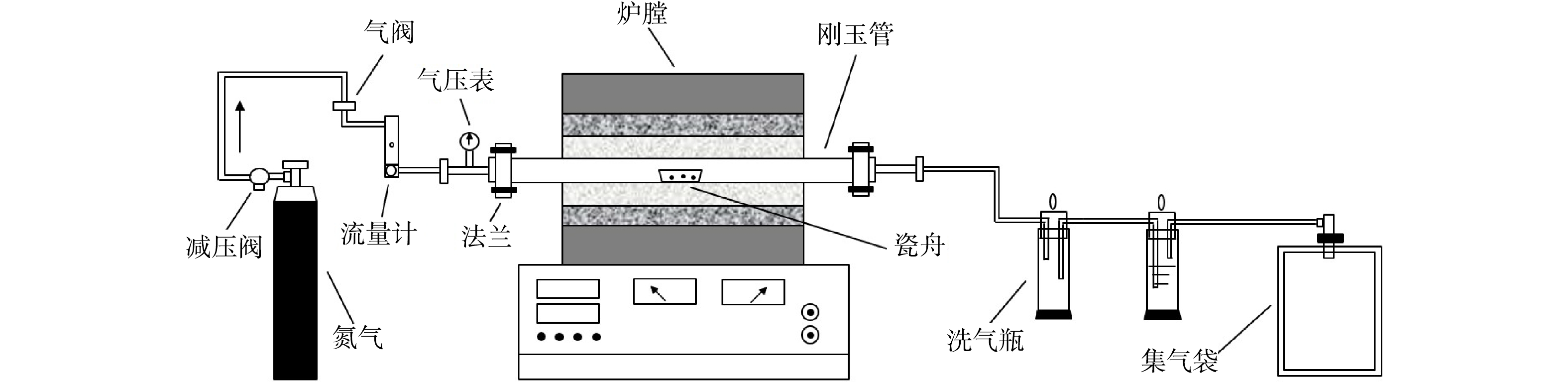
生物质热解实验系统如图1所示,主要由载气输送部分、真空气氛管式电炉(SK-G08143,最大功率为5 kW,最高温度为1 400 ℃,刚玉管内径50 mm)以及气体收集部分3部分组成。
-
实验前从干燥箱内称取一定量的烘干样品,以10%为梯度,用移液枪加入相应用量的去离子水,制备含水率为10%~50%的生物质样品,将样品混匀后加盖,置于阴暗无风处备用。含水率计算方法如式(1)所示。
式中:
$\omega $ 为生物质样品的含水率;m1为添加的去离子水质量,g;m2为烘干的生物质样品质量,g。利用管式炉对不同含水率梯度与催化剂(Na2CO3粉末,AR)含量梯度的杉树木屑与竹屑进行热解实验,以高纯氮气为载气,氮气流量为100 mL·min−1,实验温度为500~900 ℃,恒温热解15 min;每次实验称取1.0 g的生物质(湿基)于瓷舟中,在管式炉加热至指定温度后迅速将瓷舟放入进行热解,同时利用集气袋收集尾气,每次试验确保集气袋密封完好后置于阴暗无风处备用。
-
采用元素分析仪(Vario MACRO cube型,德国Elementar公司)对生物质样品进行元素分析;采用气相色谱仪(7890B型,美国Agilent公司)对热解气体进行气相色谱分析,检测器为FID与TCD,载气为高纯氮气;采用热重分析仪(TGA 4000型,美国PerkinElmer公司)对烘干样品进行热重分析,载气为高纯氮气,升温速率分别为10、20、40 ℃·min−1,实验温度为30~900 ℃;采用热重分析仪(TG 209 F1型,德国NETZSCH公司)与红外光谱仪(Nicolet IS 50型,美国Thermo Fisher Scientific公司)对烘干样品进行联用分析,热重分析仪以高纯氮气为载气,升温速率为40 ℃·min−1,实验温度为30~900 ℃;红外光谱仪波长扫描范围为500~4 000 cm−1,分辨率为4 cm−1,扫描次数设置为32。
生物质热解涉及多种反应,其组分之间存在复杂的互相作用[11],动力学分析能方便的描述热解过程中的反应机理与耗能情况,等转化率法为常用计算方法之一[12]。
生物质样品在恒定的升温速率下进行热解,根据阿伦尼乌斯公式,某一时刻t其分解速率如式(2)所示。
式中:k为反应速率常数;f(α)为反应模型函数;β为升温速率,K·min−1;R为理想气体常数,8.314 J·K−1·mol−1;T为绝对温度,K;A为指前因子,s−1;Ea为反应活化能,kJ·mol−1;α为转化率,定义如式(3)所示。
式中:w0为样品的初始质量,g;wt为样品在t时刻的质量,g;w∞为样品反应后的最终质量,g。
式(2)即为固体可燃材料热解的基本方程,本研究采取无模型法与主图法来求解式(2)中的动力学参数和确定反应机理。
1)无模型法。无模型法是基于等转化率和多重升温速率的方法,其优点在于它无需选择反应机理函数而避免引入误差。为避免活化能计算中的偶然性,本研究选用KAS法、FWO法和Friedman法3种方法[13]。
KAS法公式如式(4)所示。
式中:Tmax为DTG曲线的峰值温度,K;G(α)为f(α)的积分形式,其方程如式(5)所示。
FWO法公式如式(6)所示。
Friedman法表达式可由公式(2)移项并取对数求得,其公式如式(7)所示。
对式(4)、式(6)~式(7)进行线性拟合,可根据斜率求得活化能E,选择合适的机理函数后便可求得指前因子A。
2)主图法。主图法是一种能够估计反应机理函数f(α)的模型拟合法。使用p(x)的合理逼近可以得到更准确的反应机理模型[14]。p(x)表达式如式(8)所示。
以转化率α=0.5时为参考点,结合式(5)与式(8),可得主图法表达式,其公式如式(9)所示。
根据
$ \dfrac{G\left(\alpha \right)}{G\left(0.5\right)} $ 可作出理论主图,根据$ \dfrac{p\left(x\right)}{p\left(0.5\right)} $ 可作出实验主图,若两个图像相近则表示选择了合适的机理函数。 -
生物质热解是一个复杂的反应体系,除了本体的热分解外[15],还涉及了碳气化、水煤气变换、甲烷重整反应等[16],其化学方程式如式(10)~ 式(13)所示。
通过单因素实验探究热解温度、生物质含水率与催化剂添加量对生物质热解制备还原性气体的影响。
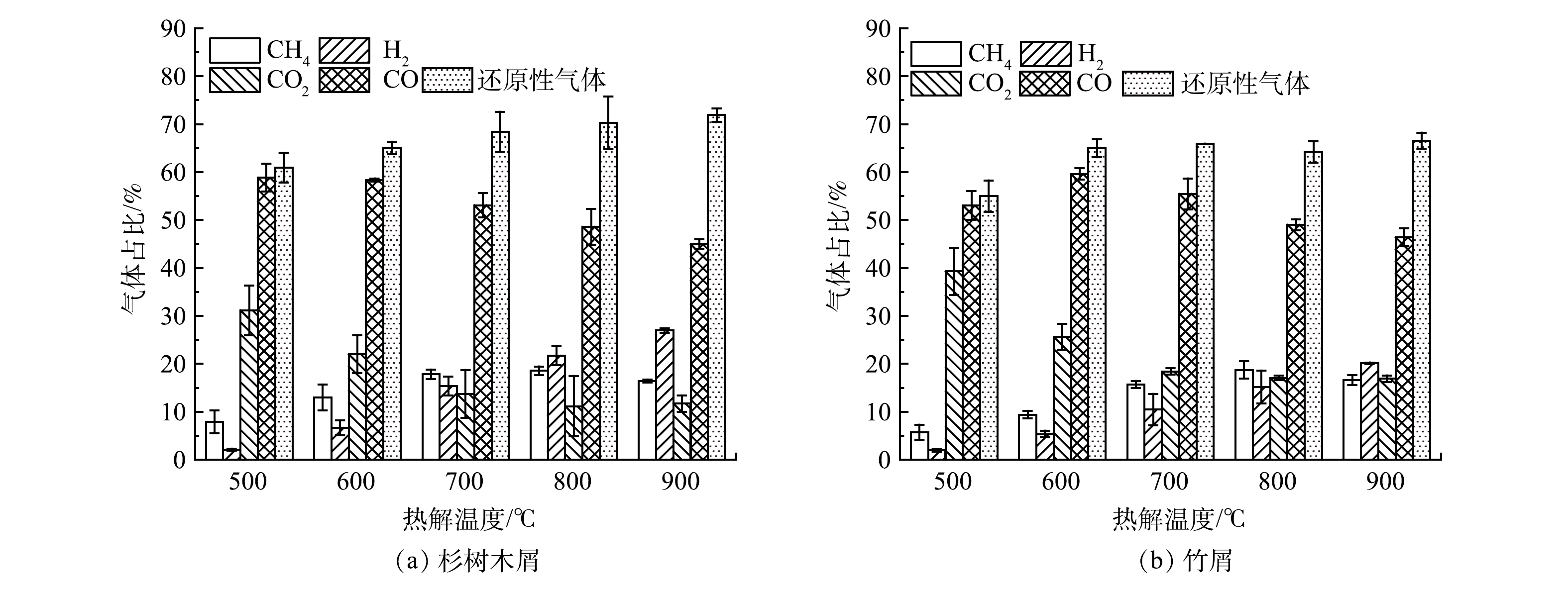
1)温度。对不添加催化剂的烘干生物质样品进行热解,不同热解温度对还原气占比的影响见图2。由图2可看出,两种生物质热解总气量中CO和CO2占比随温度升高而下降,而H2占比则从5%以下提升到20%以上,故还原性气体占比随温度呈上升趋势。CO2的生成主要在低温阶段,温度的升高对CO2的产率无显著影响[17];CO则由于水煤气反应而有所消耗。值得注意的是,杉树木屑的H2占比提升更为显著,在900 ℃下达到26.96%,其可能原因是杉树木屑中的纤维素与木质素要多于竹屑,二者共热解过程中所形成的低聚物通过断裂氢键,使左旋葡萄糖末端中间体上的H迁移至木质素自由基,从而生成酚类与丁香醇类,进一步分解成小分子物质[18]。
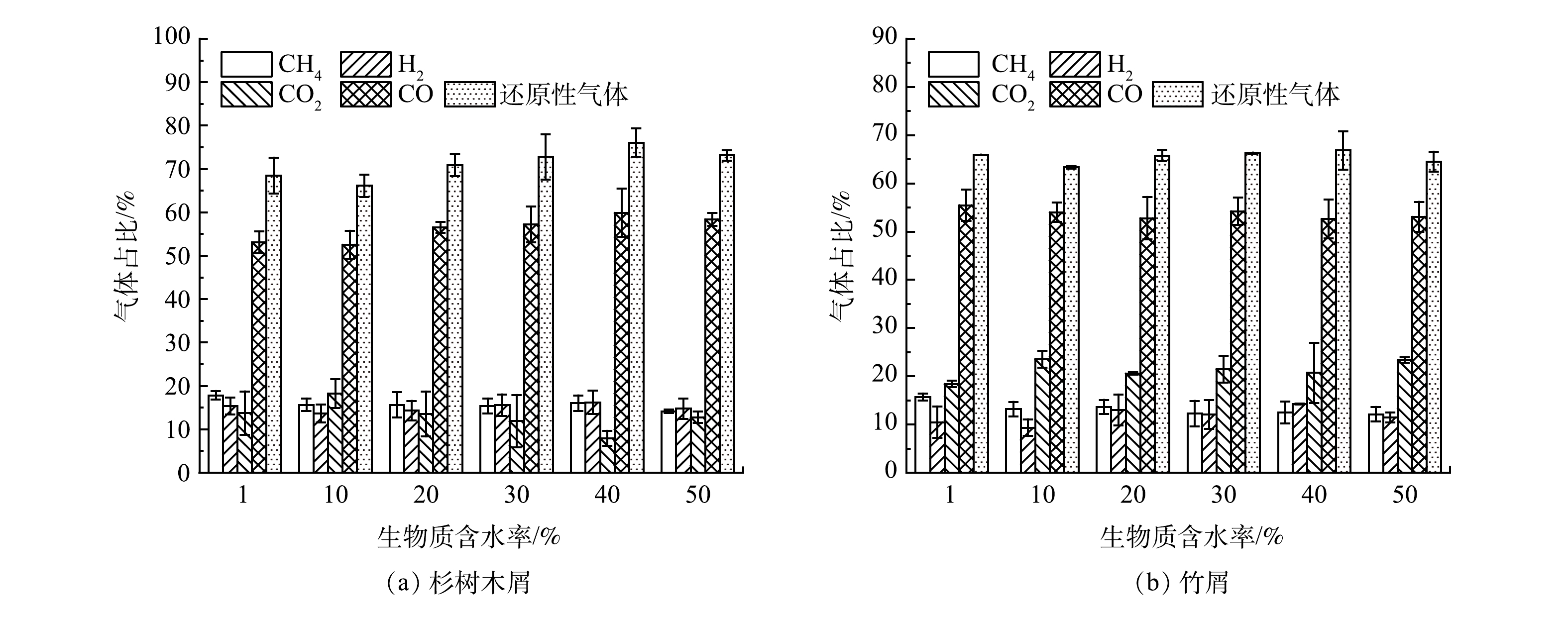
2)含水率。对不添加催化剂的生物质样品进行热解,不同含水率对还原气占比的影响见图3。由图3(a)可看出,随着含水率的提高,杉树木屑热解气中CO与H2的占比逐渐升高,CO提升幅度较为明显,还原性气体的占比最高达到76.05%,而在含水率达到50%时,CO和H2的占比反而有所降低。水分的增多提高了热解气氛中的水蒸气含量,促进了碳气化反应与甲烷重整反应[19],但过多的水蒸气会阻碍传热,从而影响还原性气体的生成[20]。ZHANG等[21]在研究生物质蒸汽汽化的过程中,同样指出了在高浓度水蒸气的条件下,H2的占比会有所下降。
在图3(b)中,随着含水率的提高,竹屑热解气中H2的占比呈先升高后下降的趋势,与杉树木屑较为一致,但CO的占比变化不明显,推测是由于竹屑的热解成碳率较低,其碳气化反应不如杉树木屑剧烈,导致其还原性气体峰值占比仅为66.84%,比杉树木屑要低。
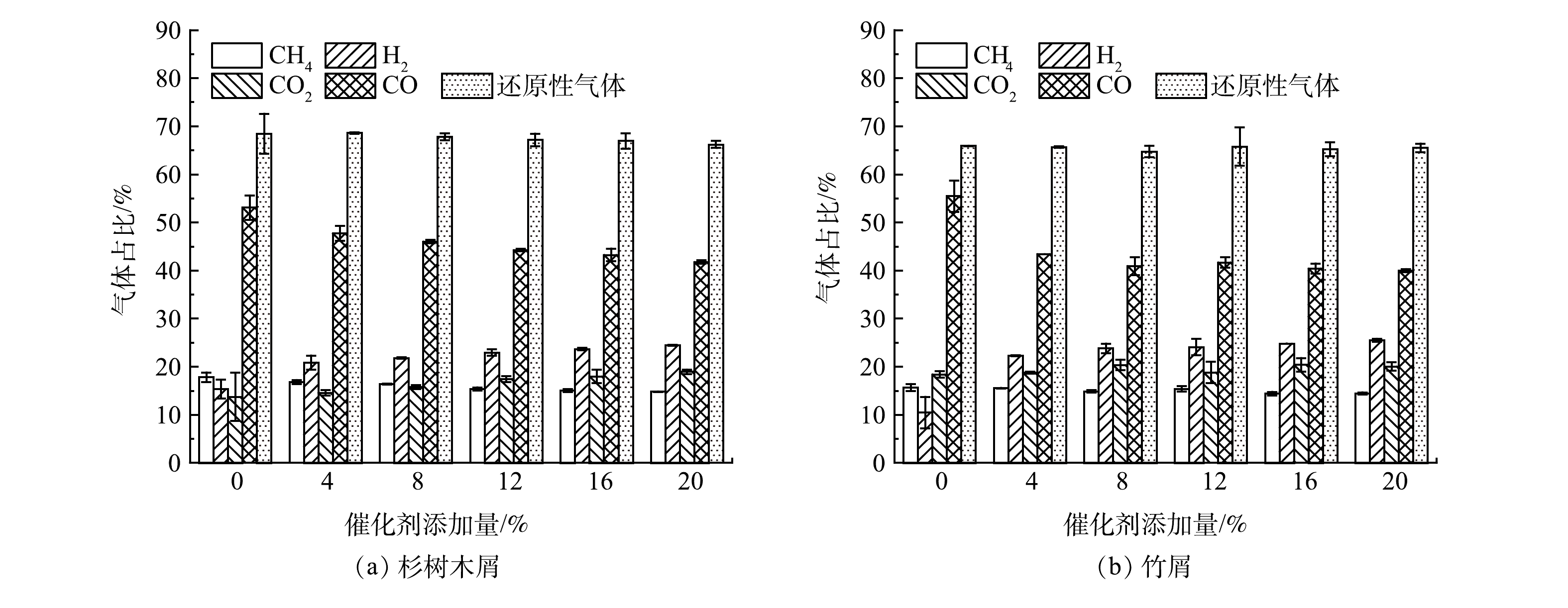
3)催化剂。为防止杂元素干扰,以Na2CO3粉末为催化剂,在700 ℃下对烘干的生物质样品进行热解,不同催化剂添加量对还原气占比的影响见图4。由图4中可看出,随着催化剂加量的增大,虽然H2占比得到提升,但CO的占比反而有所下降,因此还原性气体的占比几乎没有变化。随着反应体系内Na2CO3的引入,CO2的气体占比呈小幅上升趋势,且由于Na+的催化作用,反应体系的活化能降低,碳气化反应、水煤气变换反应与甲烷重整反应更为剧烈;Na2CO3促进了木质素中芳香环的开裂,Na+与-OH或-COOH反应,生成-ONa或-COONa,释放H+,进而促进了H2的生成[18]。
根据单因素实验,生物质热解制备还原气的最优工艺参数为,热解温度900 ℃,生物质含水率为40%,Na2CO3催化剂添加量为4%。杉树木屑制备还原气效果要优于竹屑,还原气占比能达76%以上,这是由于杉树木屑中有较多的纤维素与木质素。
-
为进一步了解生物质热解过程与气体产生情况,对生物质进行热重分析与热重-红外光谱联用分析。
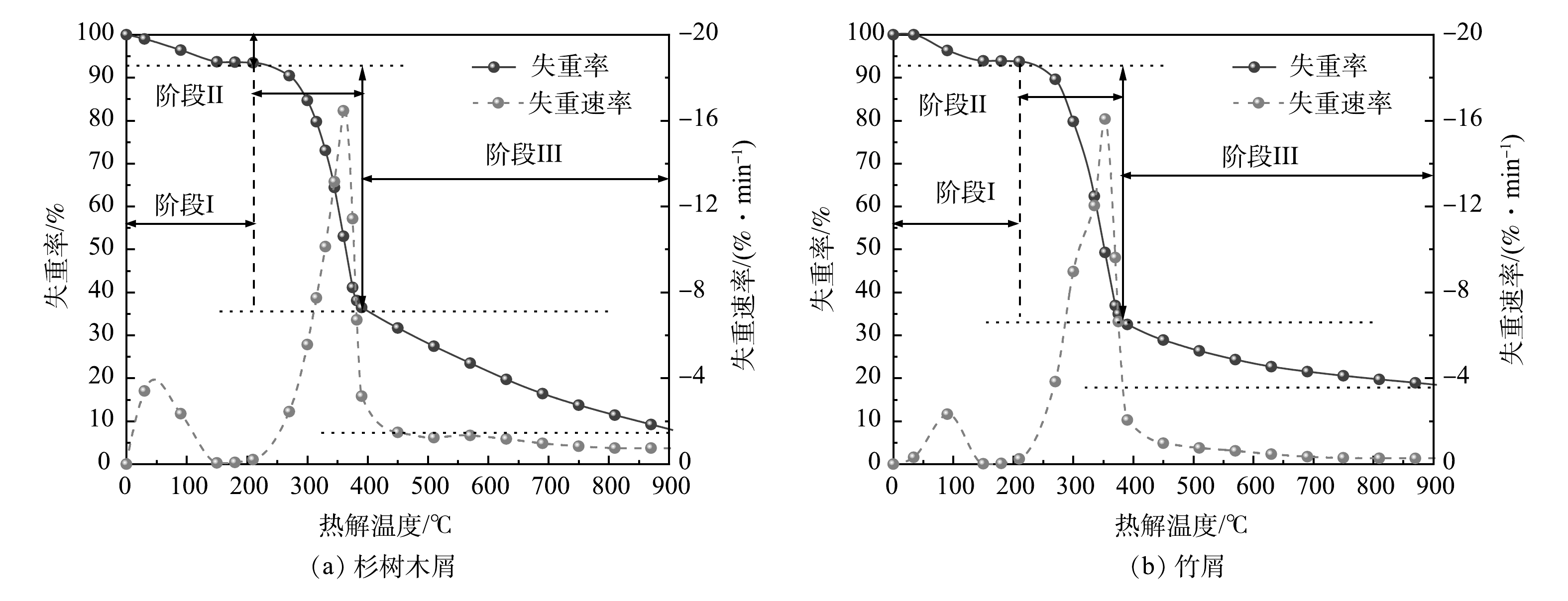
1)生物质热解特性分析。生物质失重过程一般遵循三阶段热解机理[22]。热解第一阶段主要为生物质内部水分的挥发以及部分内含物与半纤维素的分解反应;在第二阶段热解过程中,生物质中的半纤维素几乎全部分解,纤维素大量分解,木质素部分分解[23],生成大量的CO、CO2与CH4[24];第三阶段则为残余纤维素与木质素的热解。
在升温速率为20 ℃·min−1的条件下对经过烘干处理的生物质样品进行热重分析,其热重图像见图5。由图5可看出,2种生物质210 ℃前均处于热解脱水阶段,质量损失约为6%;在210~400 ℃温度段迎来第二阶段热解,最大失重率温度点同样出现在此温度段,质量损失也较为接近,均为60%左右,值得注意的是,在300 ℃左右竹屑的DTG曲线中出现了肩峰,这是由于竹屑中的半纤维素多于杉木,半纤维素大量分解所致[25];第三阶段热解中两种生物质表现出了较大差异,杉树木屑的质量损失为28.38%,竹屑的质量损失仅为13.97%,这是由于杉树木屑中大量的木质素抑制了纤维素生成的左旋葡聚糖的热聚合,促进了纤维素生成低分子量产物,降低了生物质炭的形成;大量的纤维素则降低了木质素的成碳率,促进木质素进一步分解[26]。
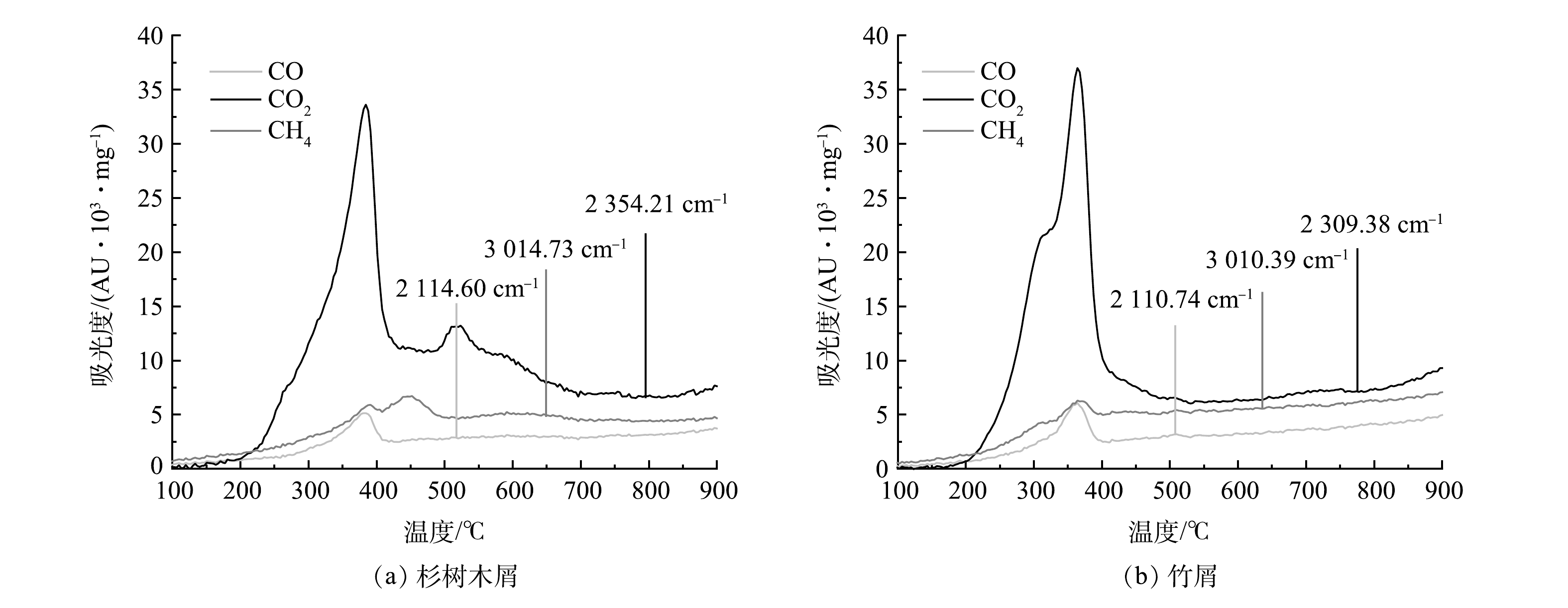
2)生物质产气特性分析。对杉树木屑与竹屑的烘干样品进行TG-IR分析,CO、CO2与CH4的气体生成规律见图6。由图6可知,在200~450 ℃热解温度区间中,杉树木屑与竹屑均在波长为2 000~2 250 cm−1范围内有明显的CO吸收峰,在波长为2 250~2 400 cm−1范围内有明显的CO2吸收峰,在波长为2 700~3 100 cm−1范围内有明显的CH4吸收峰[27],图中所显示的气体生成规律与热重分析结果较为一致。
-
为探究生物质热解产气过程的耗能情况与反应机理,需要对热解过程进行动力学分析。
1)无模型法。KAS法、FWO法和Friedman法3种方法相应的活化能随着转化率改变的计算结果见表2。活化能代表反应能进行的最低能量要求。由表2可看出,杉树木屑与竹屑KAS法活化能计算结果分别为118.32和117.17 kJ·mol−1,二者热解耗能较为相近;在FWO法与Friedman法中,2种生物质均在转化率α=0.1~0.7时有较好的线性拟合度,取该区间进行计算并取平均值,前者活化能平均值计算结果分别为130.42和117.84 kJ·mol−1,杉树木屑热解耗能略高于竹屑;后者活化能平均值计算结果分别为131.83和118.75 kJ·mol−1,杉树木屑热解耗能同样高于竹屑。2种生物质的活化能均随转化率的提高而增大,这是由于在热解前期,短链的半纤维素因其较差的热稳定性而迅速分解,所需能量较少;而在热解中期,较长链的纤维素大量分解,所需活化能增加;热解后期,复杂三维结构的木质素缓慢热解,活化能进一步增加[28]。
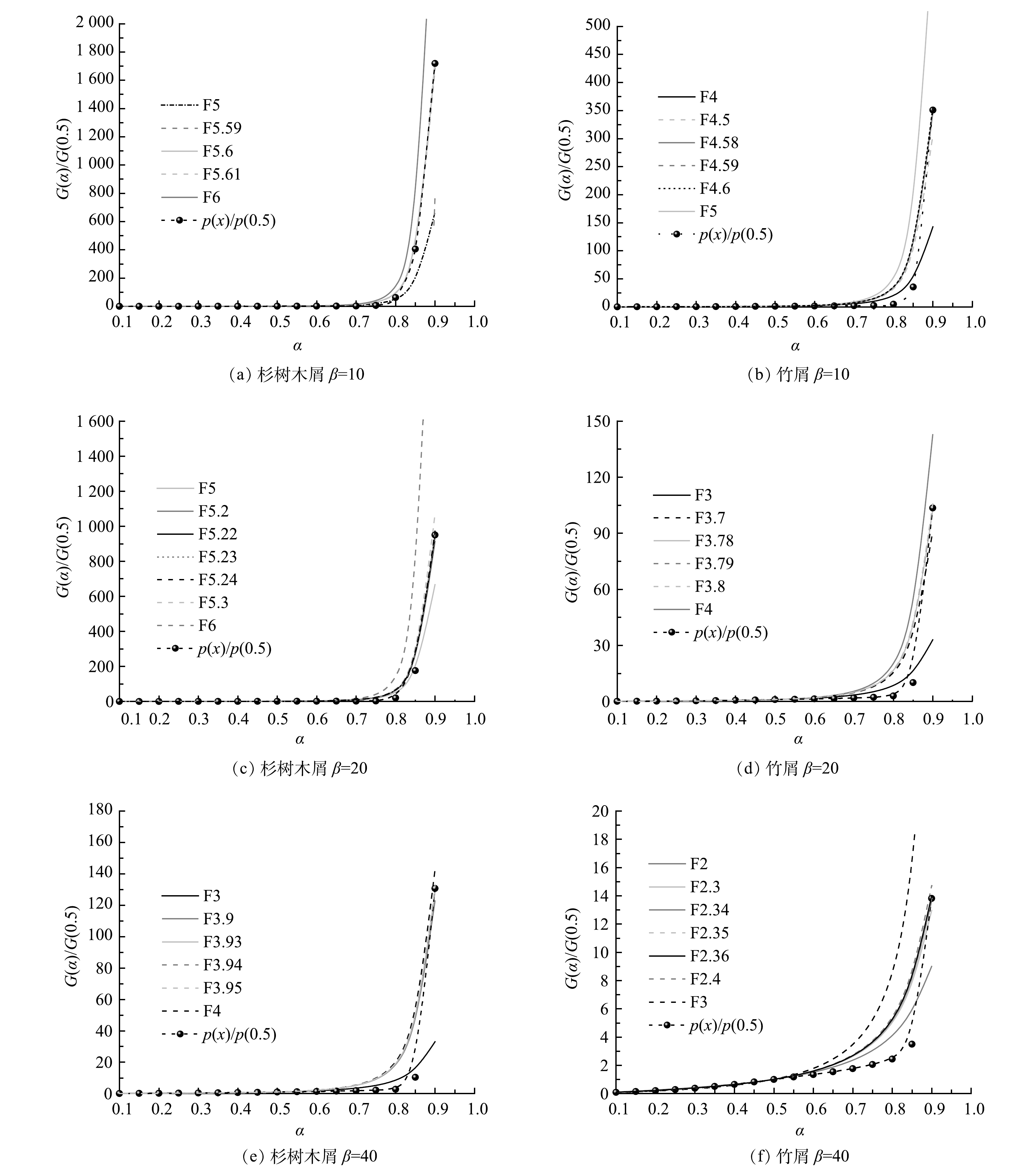
2)主图法。以往生物质的热解反应过程普遍被视为一级反应[29],但后来又有学者指出,生物质的热解中存在较为复杂多步分解,而反应级数函数能较好地描述这一过程[30]。对升温速率分别为10、20、40 K·min−1的生物质热解过程进行主图法拟合,所用活化能值为KAS法、FWO法和Friedman法3种方法所求活化能的平均值。主图法拟合曲线见图7,生物质热解活化能随着机理函数的选取而改变的计算结果见表3。由图7可看出,级数反应函数的确能够较好地描述热解过程,且反应级数较高,YEO等[31]也指出,半纤维素的反应级数为1.5~4.0,而木质素的反应级数则在4以上;由表3中可看出,随着升温速率的提高,热解反应的反应级数逐渐下降,可能是由于外界温度的剧烈变化促进了生物质组分间的互相反应,阻碍了生物质热解过程中衍生物或中间体的生成,导致其无法长时间停留,从而使热解过程向一级反应趋近,与上文提出的说法较为吻合,ZHOU等[32]对小麦秸秆动力学研究中同样出现了这种现象。
经动力学分析,确定级数反应为生物质的热解反应机理函数,其中杉树木屑的活化能计算结果为118.32~135.43 kJ·mol−1,相对误差为14.46%;竹屑的活化能计算结果为112.39~118.75 kJ·mol−1,相对误差为5.66%,二者相对误差均小于20%,计算结果较为可信[33],且杉树木屑的热解活化能要高于竹屑。
-
1)随着热解温度的提高,生物质热解气中H2气体占比与总还原性气体占比有所提高,且H2的提升幅度较大。
2)生物质在含水率为40%以下的条件下热解时,随着含水率的提高,热解气中H2气体占比与CO气体占比均有所提高,且CO的提升幅度较大。
3)随着催化剂添加量的增大,生物质热解气中H2气体占比有所提升,CO气体占比则下降,总还原性气体占比基本保持不变。
4)杉树木屑与竹屑热解行为均属于三阶段热解,气体主要产生于热解第二阶段,其热解过程符合反应级数机理,且随着升温速率的提高趋近于一级反应,而杉树木屑的活化能要略高于竹屑。
木屑生物质热解制备高还原性气体机理
Mechanism of pyrolysis of sawdust biomass to produce highly reducing gas
-
摘要: 针对气基磁化焙烧还原铁尾矿成本昂贵的问题,基于生物质廉价易得且具有较好的还原性气体制备潜力的特点进行产气机理探究。通过热解实验研究了温度、含水率、Na2CO3催化剂添加量对杉树木屑与竹屑热解制备还原性气体的影响,利用TG-IR探究二者的产气特征,并结合动力学分析确定杉树木屑与竹屑的热解机理函数与活化能。结果表明,生物质在较低含水率(40%以下)的条件下提高热解温度,有利于还原气的制备;当热解温度为700 ℃、Na2CO3添加量为4%时,烘干的杉树木屑热解气中还原性气体占比约为76%,竹屑则约为66%;二者的还原性气体均集中产生于热解第二阶段,其热解过程符合反应级数模型;经计算,杉树木屑的活化能为118.32~135.43 kJ·mol−1,竹屑的活化能为112.39~118.75 kJ·mol-1。本研究结果可为生物质热解制备还原性气体提供技术指导。Abstract: Due to the high cost for reducing iron tailings by gas-based magnetization roasting, this paper explored the mechanism of gas production, which was based on the cheap, easily obtaining, and good potential for reducing gas production characteristics of biomass. Through single-factor experiments, the effects of temperature, water content, and Na2CO3 catalyst addition on the fir sawdust and bamboo sawdust were explored. Besides, the gas production characteristics between these two sawdust were investigated by TG-IR. By kinetic analysis, the pyrolysis mechanism function and activation energy of fir sawdust and bamboo sawdust were determined. The results indicated that the moisture content below 40% was conducive to reduce gas production. When the pyrolysis temperature was 700 ℃ and the addition amount of Na2CO3 was 4%, the proportion of reducing gas in the pyrolysis gas of dried fir sawdust was about 76%, and that of bamboo sawdust was about 66%. Both of the reducing gases are concentrated in the second stage of pyrolysis, which can be better described by the reaction series model. By calculated, the activation energy of Chinese fir sawdust was 118.32~135.43 kJ·mol-1; while the activation energy of bamboo sawdust was 112.39~118.75 kJ·mol-1. The results can provide technical guidance for the preparation of reductive gases by biomass pyrolysis.
-
Key words:
- fir sawdust /
- bamboo sawdust /
- pyrolysis /
- reducing gas /
- kinetics
-

-
表 1 样品的元素分析、工业分析与成分分析
Table 1. Ultimate, proximate and material analysis of samples
%(质量分数) 样品 元素分析 工业分析 成分分析 C H O N S Mad Aad Vad FCad 多糖 木质素 杉木 48.60 6.20 44.84 0.26 0.03 8.12 0.40 75.23 16.25 53.97 34.70 竹屑 47.46 6.62 45.66 0.24 0.03 6.64 1.56 75.97 15.83 65.26 25.70 表 2 无模型法计算得到的热解动力学参数
Table 2. pyrolysis kinetic parameters by model-free method
无模型法 杉树木屑 竹屑 α R2 E/ kJ·mol−1 α R2 E/ kJ·mol−1 KAS法 − 0.996 79 118.32 − 0.999 59 117.17 FWO法 0.1 0.998 01 118.48 0.1 0.987 24 91.88 0.2 0.998 66 123.12 0.2 0.986 96 101.10 0.3 0.998 28 127.01 0.3 0.988 65 114.80 0.4 0.998 75 130.49 0.4 0.987 14 123.21 0.5 0.998 06 131.92 0.5 0.986 06 126.01 0.6 0.998 37 134.87 0.6 0.989 52 130.81 0.7 0.998 15 147.02 0.7 0.988 41 137.05 平均 − 130.42 平均 − 117.84 Friedman法 0.1 0.997 81 119.69 0.1 0.985 78 91.84 0.2 0.998 53 124.37 0.2 0.985 58 101.36 0.3 0.998 11 128.31 0.3 0.987 57 115.64 0.4 0.998 62 131.86 0.4 0.985 98 124.35 0.5 0.997 87 133.28 0.5 0.984 82 127.19 0.6 0.998 21 136.30 0.6 0.988 59 132.17 0.7 0.997 98 148.99 0.7 0.987 43 138.67 平均 − 131.83 平均 − 118.75 表 3 不同反应级数的热解动力学参数
Table 3. Pyrolysis kinetic karameters of different reactions orders
升温速率/(K·min−1) 杉树木屑 竹屑 G(α) R2 E/ kJ·mol−1 A/s−1 G(α) R2 E/ kJ·mol−1 A/s−1 10 F5.6 0.957 57 137.87 3.66×1011 F4.59 0.945 29 122.05 2.60×1010 20 F5.23 0.951 07 139.25 2.25×1011 F3.79 0.933 25 116.42 3.38×109 40 F3.94 0.925 52 129.18 1.00×1010 F2.35 0.916 18 98.71 2.48×107 -
[1] 周伟伦, 廖正家, 陈涛等. 利用铁尾矿制备烧结砖的可行性及烧结固化机理[J]. 环境工程学报, 2021, 15(5): 1670-8. [2] 国家环境保护总局, 中华人民共和国国家质量监督检验检疫总局. 地表水环境质量标准: GB 3838-2002[S]. [3] 陈莹, 陈炳辉, 邹琦等. 粤北大宝山AMD水-表层沉积物的重金属分布特征及其影响因素[J]. 环境科学学报, 2018, 38(01): 133-41. [4] 孙旭. 包头含稀土选铁尾矿磁化焙烧及物相转变实验研究[D]. 东北大学, 2014. [5] DAI L, WANG Y, LIU Y, et al. A review on selective production of value-added chemicals via catalytic pyrolysis of lignocellulosic biomass[J]. Science of The Total Environment, 2020, 749: 142386. doi: 10.1016/j.scitotenv.2020.142386 [6] TAN Y L, ABDULLAH A Z, HAMEED B H. Fast pyrolysis of durian (Durio zibethinus L) shell in a drop-type fixed bed reactor: Pyrolysis behavior and product analyses[J]. Bioresource Technology, 2017, 243: 85-92. doi: 10.1016/j.biortech.2017.06.015 [7] AL ARNI S. Comparison of slow and fast pyrolysis for converting biomass into fuel[J]. Renewable Energy, 2018, 124: 197-201. doi: 10.1016/j.renene.2017.04.060 [8] MALIUTINA K, TAHMASEBI A, YU J, et al. Comparative study on flash pyrolysis characteristics of microalgal and lignocellulosic biomass in entrained-flow reactor[J]. Energy Conversion and Management, 2017, 151: 426-38. doi: 10.1016/j.enconman.2017.09.013 [9] 中华人民共和国国家质量监督检验检疫总局, 中国国家标准化管理委员会. 固体生物质燃料工业分析方法: GB 28731-2012[S]. [10] 中华人民共和国国家质量监督检验检疫总局, 中国国家标准化管理委员会. 林业生物质原料分析方法 多糖及木质素含量的测定: GB 35818-2018 [11] HILBERS T J, WANG Z, PECHA B, et al. Cellulose-Lignin interactions during slow and fast pyrolysis[J]. Journal of Analytical and Applied Pyrolysis, 2015, 114: 197-207. doi: 10.1016/j.jaap.2015.05.020 [12] SONG Y, HU J, LIU J, et al. Catalytic effects of CaO, Al2O3, Fe2O3, and red mud on Pteris vittata combustion: Emission, kinetic and ash conversion patterns[J]. Journal of Cleaner Production, 2020, 252: 119646. doi: 10.1016/j.jclepro.2019.119646 [13] LIU H, LIU J, HUANG H, et al. Optimizing bioenergy and by-product outputs from durian shell pyrolysis[J]. Renewable Energy, 2021, 164: 407-18. doi: 10.1016/j.renene.2020.09.044 [14] HU J, SONG Y, LIU J, et al. Combustions of torrefaction-pretreated bamboo forest residues: Physicochemical properties, evolved gases, and kinetic mechanisms[J]. Bioresource Technology, 2020, 304: 122960. doi: 10.1016/j.biortech.2020.122960 [15] ZHANG Z, PANG S. Experimental investigation of biomass devolatilization in steam gasification in a dual fluidised bed gasifier[J]. Fuel, 2017, 188: 628-35. doi: 10.1016/j.fuel.2016.10.074 [16] PARTHASARATHY P, NARAYANAN K S. Hydrogen production from steam gasification of biomass: Influence of process parameters on hydrogen yield-A review[J]. Renewable Energy, 2014, 66: 570-9. doi: 10.1016/j.renene.2013.12.025 [17] YU D, JIN G, PANG Y, et al. Gas Characteristics of Pine Sawdust Catalyzed Pyrolysis by Additives[J]. Journal of Thermal Science, 2021, 30(1): 333-42. doi: 10.1007/s11630-020-1244-z [18] WU S, SHEN D, HU J, et al. Cellulose-lignin interactions during fast pyrolysis with different temperatures and mixing methods[J]. Biomass and Bioenergy, 2016, 90: 209-17. doi: 10.1016/j.biombioe.2016.04.012 [19] CAO L, YU I K M, XIONG X, et al. Biorenewable hydrogen production through biomass gasification: A review and future prospects[J]. Environmental Research, 2020, 186: 109547. doi: 10.1016/j.envres.2020.109547 [20] 郭朝强, 尚双, 兰奎等. 不同含水率污泥和小麦秸秆混合热解制备富氢合成气[J]. 环境工程, 2020, 38(05): 160-4+214. [21] ZHANG B, ZHANG L, YANG Z, et al. Hydrogen-rich gas production from wet biomass steam gasification with CaO/MgO[J]. International Journal of Hydrogen Energy, 2015, 40(29): 8816-23. doi: 10.1016/j.ijhydene.2015.05.075 [22] ZHOU H, LONG Y, MENG A, et al. The pyrolysis simulation of five biomass species by hemi-cellulose, cellulose and lignin based on thermogravimetric curves[J]. Thermochimica Acta, 2013, 566: 36-43. doi: 10.1016/j.tca.2013.04.040 [23] YU J, PATERSON N, BLAMEY J, et al. Cellulose, xylan and lignin interactions during pyrolysis of lignocellulosic biomass[J]. Fuel, 2017, 191: 140-9. doi: 10.1016/j.fuel.2016.11.057 [24] ZHAO C, JIANG E, CHEN A. Volatile production from pyrolysis of cellulose, hemicellulose and lignin[J]. Journal of the Energy Institute, 2017, 90(6): 902-13. doi: 10.1016/j.joei.2016.08.004 [25] YUAN X, HE T, CAO H, et al. Cattle manure pyrolysis process: Kinetic and thermodynamic analysis with isoconversional methods[J]. Renewable Energy, 2017, 107: 489-96. doi: 10.1016/j.renene.2017.02.026 [26] HOSOYA T, KAWAMOTO H, SAKA S. Cellulose–hemicellulose and cellulose–lignin interactions in wood pyrolysis at gasification temperature[J]. Journal of Analytical and Applied Pyrolysis, 2007, 80(1): 118-25. doi: 10.1016/j.jaap.2007.01.006 [27] WU X, BA Y, WANG X, et al. Evolved gas analysis and slow pyrolysis mechanism of bamboo by thermogravimetric analysis, Fourier transform infrared spectroscopy and gas chromatography-mass spectrometry[J]. Bioresource Technology, 2018, 266: 407-12. doi: 10.1016/j.biortech.2018.07.005 [28] CHEN C, QU B, WANG W, et al. Rice husk and rice straw torrefaction: Properties and pyrolysis kinetics of raw and torrefied biomass[J]. Environmental Technology & Innovation, 2021, 24: 101872. [29] 林顺洪, 李伟, 柏继松, 等. TG-FTIR研究生物质成型燃料热解与燃烧特性[J]. 环境工程学报, 2017, 11(11): 6092-7. [30] HE Y, CHANG C, LI P, et al. Thermal decomposition and kinetics of coal and fermented cornstalk using thermogravimetric analysis[J]. Bioresource Technology, 2018, 259: 294-303. doi: 10.1016/j.biortech.2018.03.043 [31] YEO J Y, CHIN B L F, TAN J K, et al. Comparative studies on the pyrolysis of cellulose, hemicellulose, and lignin based on combined kinetics[J]. Journal of the Energy Institute, 2019, 92(1): 27-37. doi: 10.1016/j.joei.2017.12.003 [32] ZHOU L, ZHANG G, SCHURZ M, et al. Kinetic study on CO2 gasification of brown coal and biomass chars: reaction order[J]. Fuel, 2016, 173: 311-9. doi: 10.1016/j.fuel.2016.01.042 [33] 姚丛雪. 水稻和玉米的热解动力学及机理研究[D]. 中国科学技术大学, 2020. -



 下载:
下载: