-
随着我国矿业得到更多重视和发展,采矿业发展必然带来大量的尾矿产生[1]。2019年我国铁尾矿产生量 5.36×108 t,综合利用量 1.16×108 t,铁尾矿综合利用率不足30%造成其堆积[2],而尾矿堆积引起的环境问题如累积潜在有毒元素等已成为全球性问题[3-4]。铁尾矿的铅、锌、镉、铜、镍、铬和锰等重金属,受到风化和沥滤等自然环境作用时,会产生具有毒性的酸性重金属废水污染地表水和地下水,而产生不可忽视地经济损失[5]。随着国家人民健康发展需求的日益增长,铁尾矿安全处置已引起广大关注。
目前,学者对铁尾矿资源化利用研究已有报道,如烧结固化技术[6-7]、制备改性材料[8]和磁化回收铁资源等。在烧结固化技术中,WANG等[5]在铁尾矿中添加高岭土和飞灰制备烧结砖,满足重金属浸出和抗压标准;在制备改性材料中,LI等[8]以铁尾矿和粉煤灰制备高比表面积(1.185 m2·g−1)和高孔隙率(62%)的多孔人工陶粒滤料。但以上两种途径对铁尾矿资源化回收方式没利用铁尾矿中赋存价值高的矿物,或是存在高能耗低价值等缺点[5, 8]。因而高效利用铁尾矿中赋存价值较高的铁元素显得格外重要。
中国因高品质铁矿石产量少,而成为高度依赖高品质铁矿石进口大国[1]。我国政协十三届全国委员会第四次会议也将铁矿列为战略性矿产,并大力加强铁矿石理论研究及其创新。可见,通过回收国内铁尾矿的铁以补充国内高品质铁矿石需求符合当代提倡的内循环模式。目前,学者通过磁化焙烧,对铁矿中的铁进行还原回收。按照还原剂不同,LI等[9]采用50% H2磁化焙烧铁尾矿获品位65.30%,回收率39.79%的铁精矿;YUAN等[10]采用20% CO磁化焙烧铁尾矿获品位68.31%,回收率96.34%的铁精矿;HUANG等[11]采用15%木屑磁化焙烧铁尾矿获品位62.84%,回收率94.58%的铁精矿。按照焙烧方式不同,其中YUAN等[10]采用悬浮磁化焙烧铁尾矿;HUANG等[11]采用固定床磁化焙烧铁尾矿。新颖的悬浮磁化焙烧法具有传热传质效率高等优点[12],但目前使用的还原剂多为单一还原剂或为理想性比例混气为主[9-10]。若采用还原性废气如高炉尾气和生物质造气等,按其主要成分为CO、H2、CO2和N2进行模拟还原混气研究[13-14],可寻找到一种低成本、节能、环保的工艺解决铁尾矿堆存资源浪费问题。
本研究以CO、H2、CO2和N2混气作为还原混气,研究不同温度、时间、混气H2和CO占比对铁尾矿磁化焙烧后铁品位和回收率的影响。利用X射线衍射(XRD)和扫描电子显微镜(SEM)研究焙烧前后铁尾矿基本特性和晶相结构,利用振动样品磁强计(VSM)测试样品磁性变化,利用光电子能谱仪(XPS)测试元素价态变化,利用N2吸脱附等温仪(BET)测试样品孔隙变化。本研究结果可为铁尾矿的资源化利用提供参考。
-
供试样品为广东省韶关市大宝山早期铁尾矿。铁尾矿元素含量分析如表1所示。可见,铁尾矿中铁为主要金属元素,品位为43.71%。二氧化硅及其氧化铝为主要杂质,并含有重金属。铁主要以赤铁矿、褐铁矿形式存在,占97.92%。本实验使用一氧化碳(CO, 99.95%);二氧化碳(CO2, 99.9%);氮气(N2, 99.9%)和由氢气发生装置(SPH – 300A,北京中惠普)制备的H2。
-
实验系统如图1所示。铁尾矿风干干燥,研磨至40%过200目,混合均匀储存密封袋中。待立式悬浮焙烧炉(SK–G03123K–D,天津中环)达到规定温度时,将10 g铁尾矿加入悬浮管,并组装悬浮焙烧炉。通N2(0.5 L·min−1)使物料保持悬浮,排出管中空气。通过4路混气系统(GSL–4Z,合肥科晶)将实验所配比的还原性混气(CO、H2、CO2、N2)通入悬浮管后反应一定时间。焙烧完成后,通入N2来及时排走过多还原混气,并对焙烧矿进行冷却至常温。随后将焙烧矿研磨过200目,依次使用磁场强度为120、80和60 mT的无极调节磁选管(XCGS–50,永盛选矿设备)湿法磁选获得铁精矿和磁选渣。最后在60 ℃烘箱处理12 h获得铁精矿和磁选渣固体,以待后续实验使用。
-
铁矿石品位测定依据《铁矿石全铁含量的测定三氯化钛还原重铬酸钾滴定法(常规方法)》(GB/T 6730.65-2009)标准[15],回收率计算公式如式(1)所示。
式中:R是精矿铁回收率; c0是磁化焙烧前原铁尾矿样品铁品位;M0是磁化焙烧前铁尾矿样品质量,g;c是铁精矿样品铁品位;M是铁精矿总质量,g。
采用XRD分析铁尾矿悬浮磁化焙烧和磁选工艺前后物相结构变化;采用XPS分析悬浮磁化焙烧和磁选前后元素含量和价态变化;采用VSM对铁尾矿悬浮磁化焙烧和磁选前后磁性变化进行分析;采用比表面积分析仪测定悬浮磁化焙烧前后铁尾矿比表面积和孔径分布;采用SEM – EDS观察悬浮磁化焙烧前后铁尾矿的表面结构、形态特征和元素含量。
-
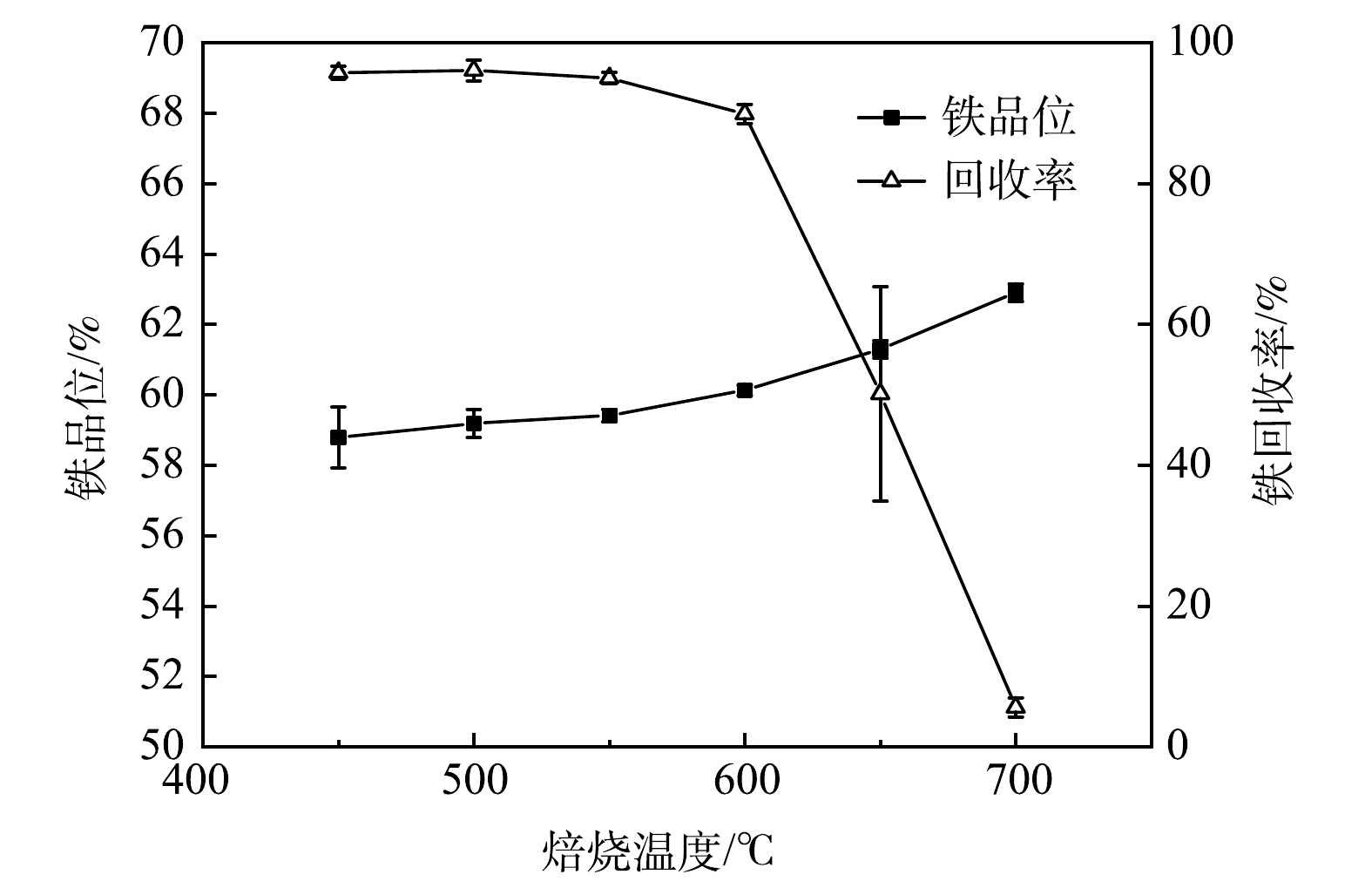
1)焙烧温度。在焙烧10 min、混气体积比H2∶CO∶CO2∶N2为10∶20∶15∶55条件下探究温度对铁精矿铁品位和回收率影响如图2所示。从图2可知,温度对铁精矿铁品位和回收率影响较大,随着温度上升,铁品位上升,而回收率稳定后急速下降。450 ℃时,铁尾矿磁化焙烧还原反应已能进行,获得铁品位和回收率分别为58.78%和95.73%的铁精矿,但此时较低的铁品位将导致利用率低下而增加炼铁厂成本[16];从450 ℃到600 ℃的过程中,铁品位提高至60.13%,而回收率仅下降5.83%,可知样品中更多的Fe以磁性Fe3O4的形式回收到铁精矿中;从600 ℃提升至700 ℃过程中,铁精矿回收率急速降至5.61%,即当前磁场无法回收铁精矿,而对于铁品位则提升至62.90%。600 ℃后,过高温度会让磁化焙烧副反应快速进行,导致铁尾矿原有的Fe2O3生成磁性Fe3O4后迅速生成弱磁性FeO等导致无法磁选收集[12,17];而铁品位的增加可能因为高温导致过还原的焙烧矿经过弱磁场磁选后,有大部分Fe以弱磁性铁相流失,表现为回收率低下,但弱磁场保留铁精矿虽质量少,但物质较纯,以强磁Fe3O4形式存在,所以铁品位有所上升。此时物质较纯的原因可能是:1)降低了精矿质量回收,从而降低了硅酸盐夹带的概率[18];2)降低了精矿质量回收,从而削弱了磁团聚现象[19]。综合考虑铁精矿产品质量和成本要求,选择600 ℃为最佳温度,此时铁品位和回收率分别为60.13%和89.90%。
2)反应时间。在焙烧温度600 ℃、混气体积比H2∶CO∶CO2∶N2为10∶20∶15∶55条件下探究磁化焙烧时间对铁精矿铁品位和回收率的影响。从图3可知,随着时间从5 min提升到30 min,铁精矿品位维持在60.00%~61.30%,而回收率总体随时间呈现先上升后下降趋势。当时间从5 min增加到10 min时,回收率从79.36%提升到92.57%,提高了13.21%,而铁精矿的品位几乎不变。而从10 min继续增加焙烧时间时,回收率总体呈现下降趋势至69.35%。可知,焙烧时间对铁精矿回收率影响较大,在5 min时,磁化焙烧进行未完全,仍然残留着Fe2O3未被还原,而10 min时,铁尾矿的Fe2O3几乎以强磁Fe3O4形式存在,能尽可能回收;而继续增加焙烧时间后,磁化焙烧的副反应导致生成的Fe3O4过还原,降低了磁性,导致回收率下降[12]。综合考虑铁精矿产品质量和成本要求,选择10 min为最佳时间,此时铁品位和回收率分别为61.21%和92.57%。
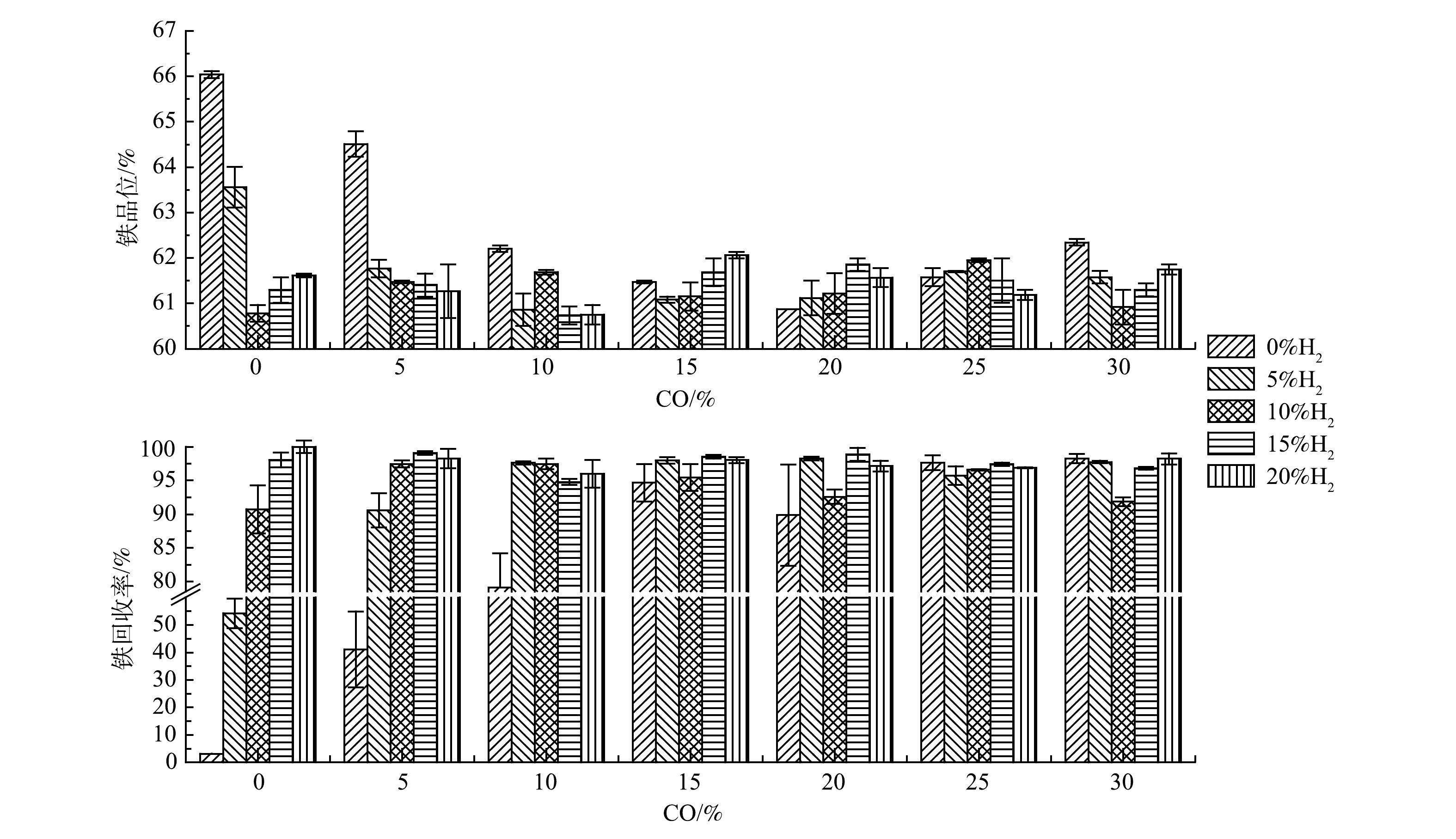
3)还原混气配比。在焙烧温度600 ℃、焙烧时间10 min条件下探究混气中2种还原性气体H2和CO占比对铁精矿铁品位和回收率的影响。从图4中可以看出,随着H2或CO占比的上升,铁精矿铁品位先下降后于61.00%~62.00%间波动;而回收率则先上升后稳定于95.00%以上。对比600 ℃下,H2∶CO为5∶0、10∶0、15∶0和20∶0时的回收率均比对应的0∶5、0∶10、0∶15和0∶20高,可见此温度下,单独还原气H2的还原性比单独还原气CO的强;同时,对比H2∶CO为10∶0、0∶10与5∶5的回收率可知,在还原性气体总占比一定时,H2与CO混合气的共同作用与H2单独作用对回收率影响不明显,而远远高于CO单独作用[20]。当总还原气体未过剩时,随着还原气体占比增大,铁品位从66.04%下降至60.00%~62.00%,而回收率从3.16%上升至95.00%。这是因为还原气体未过剩,随着其占比增加,更多Fe2O3被还原成Fe3O4,从而回收率上升,并随着铁精矿质量增加,物质间包夹作用和磁团聚增强[18, 19],铁精矿铁品位下降。当总还原性气体过剩且占比逐渐加大时,铁品位和回收率会趋于稳定区间,可见在最佳温度和时间下,过还原反应受还原性气体浓度小幅度过量影响较小。这是因为在适宜温度600 ℃下,过还原反应不发生或反应较慢[10]。从铁精矿产品最佳而言,最佳理论还原性混气比例应为H2∶CO∶CO2∶N2为20∶15∶15∶50,此时铁精矿的铁品位和回收率分别为62.06%和98.03%。
-
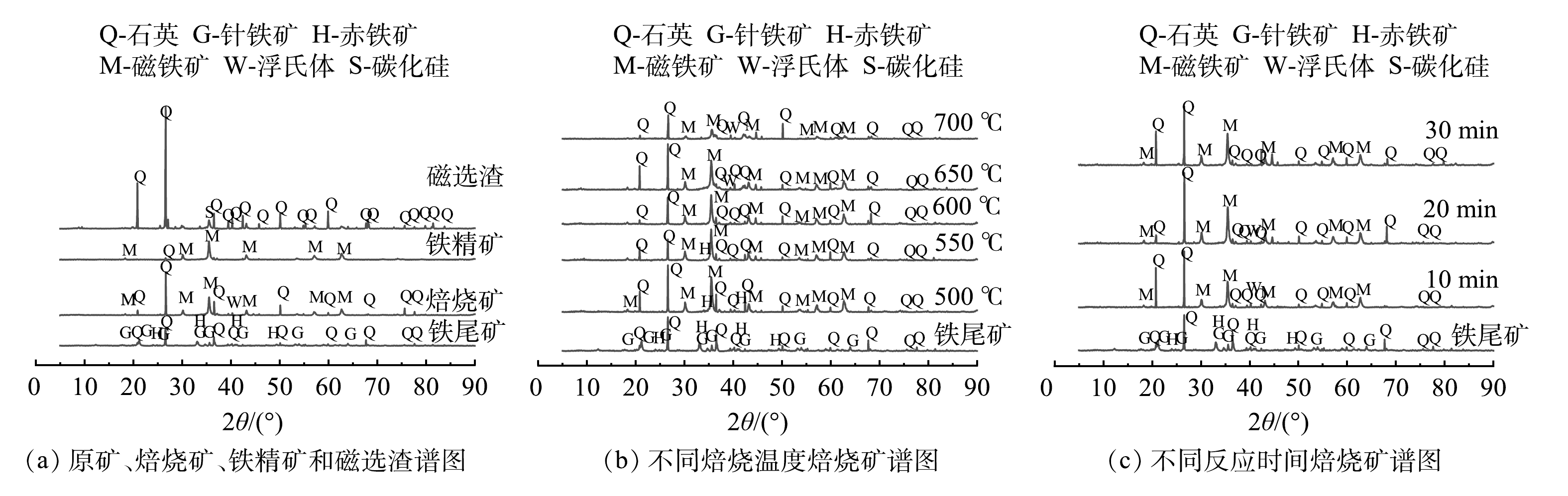
1)物相分析。悬浮磁化焙烧磁选工艺出现的各种矿物形式、不同温度下焙烧矿和不同时间下焙烧矿的x射线衍射谱图见图5。可知,铁尾矿通过悬浮磁化焙烧磁选后,铁相从赤铁矿和针铁矿的形式转换成磁铁矿的形式富集于铁精矿中。原矿中含有石英相,是导致铁尾矿铁品位较低的主要原因[21]。悬浮磁化焙烧后,从焙烧矿衍射图谱与原矿对比可知,赤铁矿针铁矿的铁相消失,取代其的是磁铁矿出现,完成铁相转变[22]。并通过磁选过后,从铁精矿和磁选渣谱图对比可以看出,几乎所有石英相均于磁选渣中,而铁精矿以主要相磁铁矿的形式存在,完成铁的富集[12]。综上, 悬浮磁化焙烧磁选工艺确实能对铁尾矿进行铁富集回收,有效去除石英,提高精矿质量。
从图5(b)可知,温度变化对铁尾矿磁化焙烧铁相存在形式影响较大。随着温度从500 ℃上升到600 ℃,赤铁矿针铁矿衍射峰强度逐渐降低至消失,取而代之的是磁铁矿衍射峰增强,可知磁化反应顺利进行;提高温度至600 ℃时,焙烧矿不再出现赤铁矿衍射峰,铁相几乎全以磁铁矿的形式存在;随温度的继续上升,焙烧矿中磁铁矿衍射峰强度减弱,而出现了浮氏体这一弱磁性铁相。这就表明,600 ℃后提高温度会让过还原反应加快进行,导致磁铁矿含量下降,出现弱磁性浮氏体,导致铁流失在磁选渣中[23]。这一现象也能说明最佳温度为600 ℃,如继续提高温度导致铁回收率急速下降。
从图5(c)可知,时间变化对铁尾矿磁化焙烧铁相的存在形式影响较小,这也与随着时间变化,铁品位变化不大的现象一致。在焙烧时间为10 min时,焙烧矿中赤铁矿针铁矿衍射峰消失,铁相主要以磁铁矿的形式存在,但出现了微弱的浮氏体衍射峰,表明焙烧已经进行完全。结合继续提高焙烧时间时焙烧矿的磁铁矿衍射峰无明显变化,表明10 min焙烧时间已经足够。此时若继续提高焙烧时间不仅会提高工艺成本,而且无利于提高铁精矿质量[24]。
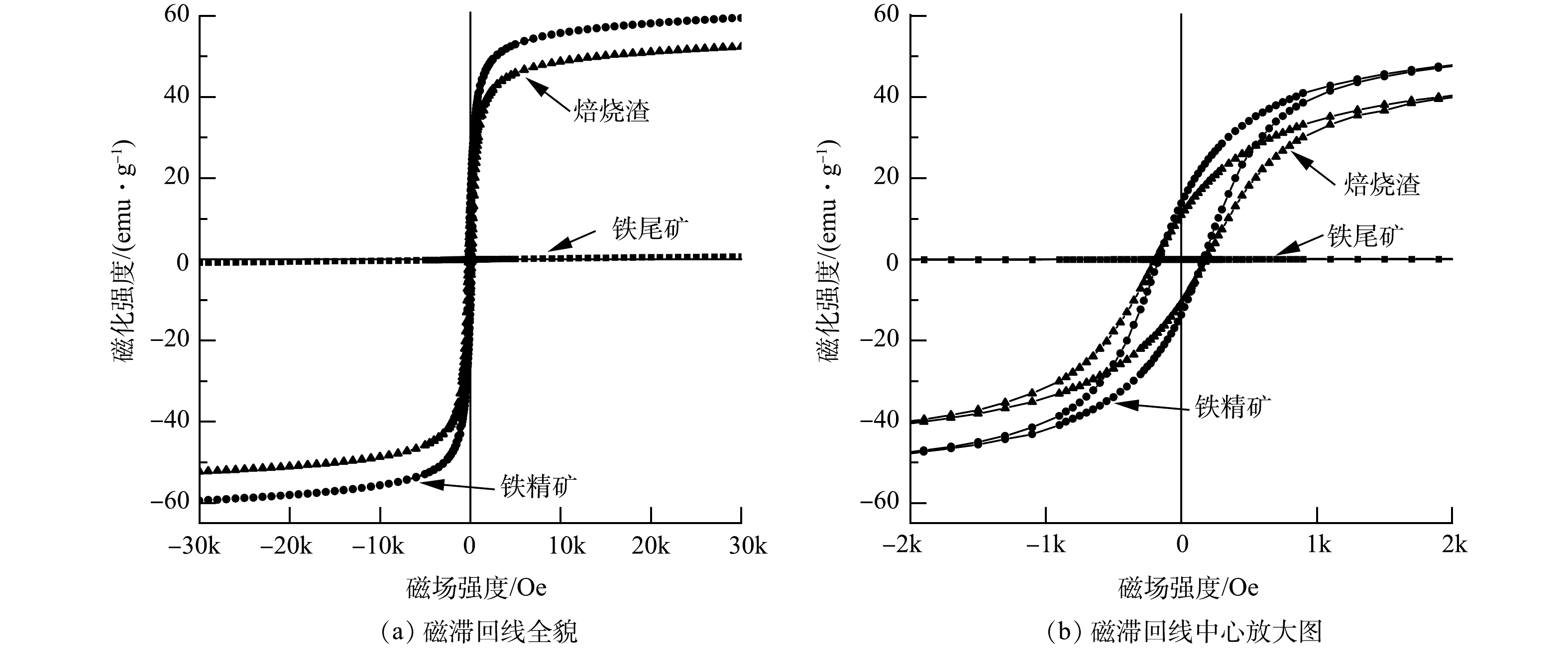
2) 磁性分析VSM。样品磁滞回线如图6所示,磁性参数如表2所示。由图6和表2可知,经过悬浮磁化焙烧的焙烧矿相比原矿,磁性有较大提升。饱和磁化强度和剩磁强度分别由0.77和0.05 Am2·kg−1提升至52.31和10.82 Am2·kg−1,提升了51.54和10.77 Am2·kg−1。这就说明,悬浮磁化焙烧能较高提升矿物磁性能[10, 12]。通过磁选工艺,能将磁性差异较大的磁铁矿和石英等进行分离,从而得到饱和磁化强度更接近纯磁铁矿的铁精矿,其饱和磁化强度和剩磁强度达到59.43和13.72Am2·kg−1。这也能侧面印证出悬浮磁化焙烧磁选工艺能提高铁精矿铁品位,同时保证较高的铁回收率,从而满足低成本炼钢炼铁要求。
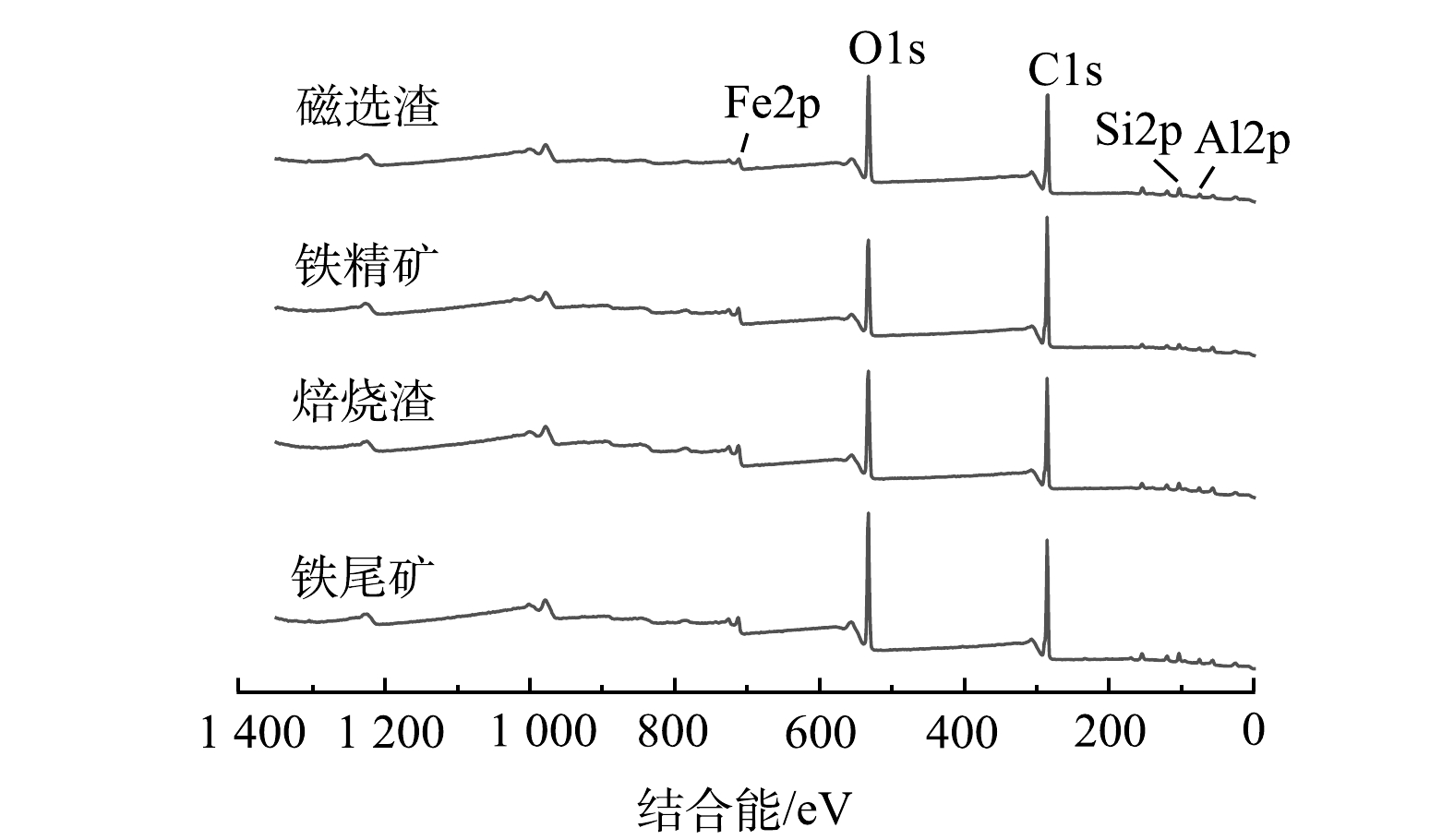
3) XPS分析。样品XPS分析总谱如图7所示,谱图均采用C1s的284.80 eV进行荷电校正。由总谱可知,元素Fe、O、Si、Al和C都有对应峰响应,且样品不同,Fe2p、O1s、Si2p和Al2p都有不同程度变化。Fe2p峰响应强度在焙烧矿中比原铁尾矿高,且结合O1s峰响应强度随着悬浮磁化焙烧磁选过程逐渐降低,可揭示过程中还原气CO和H2夺走铁尾矿部分O而生成Fe3O4[21]。Si2p和Al2p的峰响应强度在原铁尾矿和焙烧矿中变化不大,而铁精矿中几乎不存在Si2p和Al2p的峰响应,同时磁选渣中有较强的Si2p和Al2p的峰响应。可知,磁选这一过程能很好分离出Si和Al物质,以达到提高铁精矿品质[12]。这一结果与XRD分析结果保持一致。
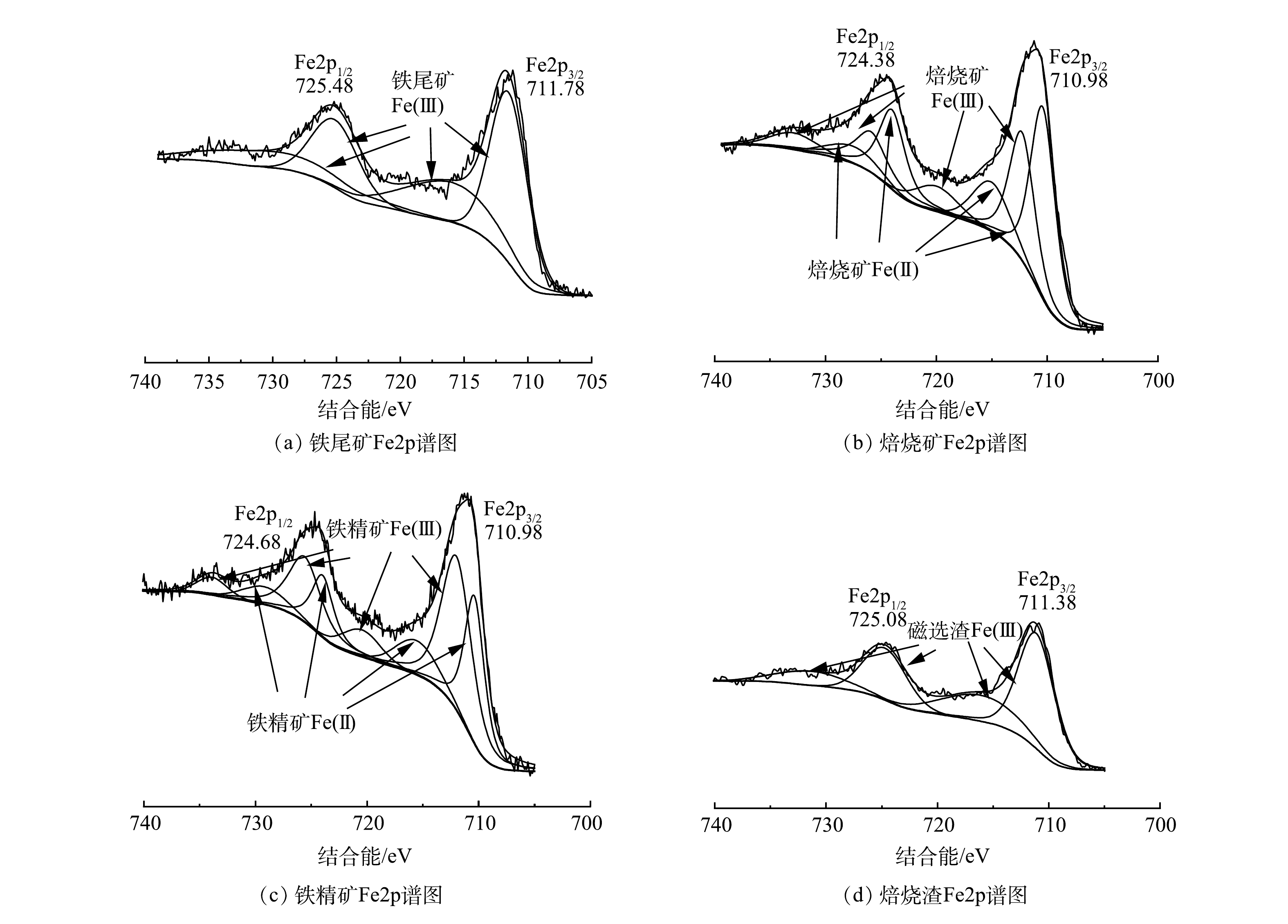
样品XPS分析的Fe2p窄谱如图8所示,谱图均采用C1s的284.80 eV进行荷电校正。从图8(a)可知,原铁尾矿含有Fe2p3/2和Fe2p1/2结合能,分别在711.78和725.48 eV,两者间相差13.7(~13.6)eV。这些结合能位置信息暗示着原铁尾矿的Fe以Fe2O3和FeOOH的Fe+3存在[25],这也与XRD分析结果高度一致。对于图8(b)和8图(c)所示焙烧矿和铁精矿而言,Fe2p3/2和Fe2p1/2分别为710.98、724.38 eV和710.98、724.68 eV,其结合能对比原铁尾矿发生明显向右位移,即结合能下降,谱图出现了Fe2+。这是因为,在悬浮磁化焙烧过程中,一部分的Fe3+被还原成Fe2+,形成Fe3O4,所以导致Fe2p3/2和Fe2p1/2结合能右移[26-27]。8(d)图磁选渣中Fe2p响应相比较低,且不存在Fe2+。综上,XPS分析结果进一步证实了悬浮磁化焙烧Fe相从Fe2O3、FeOOH到Fe3O4转变的机理,并可明确通过悬浮磁化焙烧磁选工艺,获得高品质铁精矿是可行的。
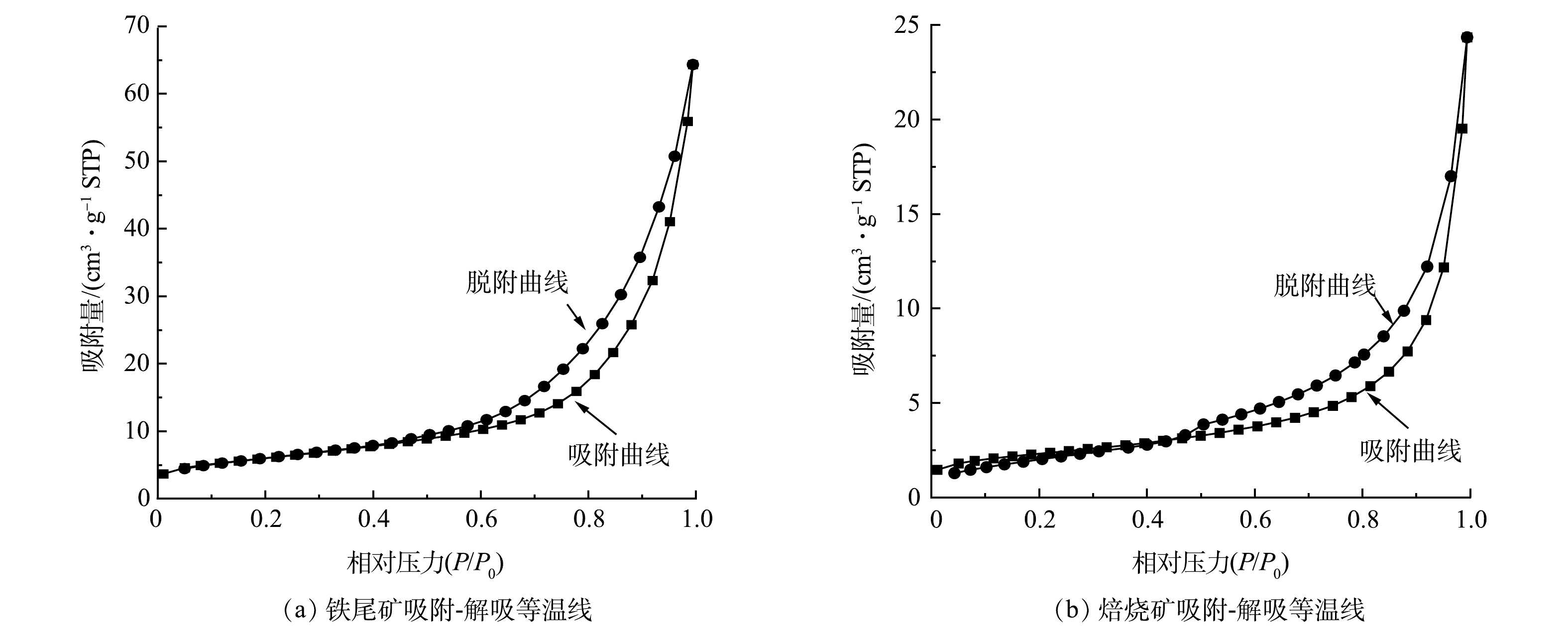
4)微观结构分析。原铁尾矿和焙烧矿的N2吸附-脱附等温曲线如图9所示。从图9可知,铁尾矿和焙烧矿的等温曲线类型是Ⅱ与Ⅳ型结合。在相对压力(P/P0)较低的情况下,原铁尾矿和焙烧矿的吸附容量均表现较低,微孔数量较少,这是Ⅱ型的表现;在相对压力(P/P0)较高的情况下,原铁尾矿和焙烧矿等温线出现H3型滞后环,表明产物存在狭缝状孔,这是Ⅳ型的表现[12]。由表3可知,铁尾矿经过悬浮磁化焙烧后BET表面积、朗缪尔表面积、孔隙体积和孔径分别提升了13.167 6 m2·g−1、274.842 2 m2·g−1、0.044 121 cm3·g−1和2.599 8 nm。较大孔隙能大大降低后续焙烧矿的研磨成本[23]。原铁尾矿和焙烧矿的BET表面积、朗缪尔表面积、孔隙体积和孔径如表4所示。综上,铁尾矿悬浮磁化焙烧过程,能较大程度提高铁尾矿的比表面积和孔隙等微观性能,可为后续焙烧矿研磨磁选提供便利。
原铁尾矿和焙烧矿的SEM-EDS分析如图10所示。由图10(a)和(b)可知,原铁尾矿以有棱角的块状结构存在,质地紧密,表面吸附着片状块状且尺寸较小的物质;由图10(c)和(d)可知,焙烧矿以块状棒状团聚的形式存在,质地疏松。综上所述,经过悬浮磁化焙烧后,铁尾矿块状结构发生气固反应破裂,以尺寸更小的块状和棒状团聚形式存在,存在更多的孔隙[23]。这也和BET分析结果一致。从图10(e)原铁尾矿和图10(f)焙烧矿EDS对比可知,经过悬浮磁化焙烧后,Fe的相对含量上升,O相对含量下降。这个揭示了焙烧过程中Fe2O3经过CO和H2还原生成Fe3O4的反应机理,这也与XRD、XPS分析结果相一致。
-
1) 焙烧温度600 ℃、焙烧时间10 min、H2∶CO∶CO2∶N2体积比为20∶15∶15∶50时,铁精矿产品铁品位和回收率分别为62.06%和98.03%达到最优。
2) 悬浮磁化焙烧能有效将铁尾矿铁相转化成磁铁矿相,使饱和磁化强度由0.77 Am2·kg−1提升到59.43 Am2·kg−1,且磁选能有效将磁铁矿和石英分离。过高温度和过长时间会产生弱磁性浮氏体而阻碍铁的回收。
3) 经悬浮磁化焙烧后,焙烧矿颗粒出现裂缝,比表面积和孔隙率均有较大提升。
混气悬浮磁化焙烧铁尾矿及其磁分选效果
Recycling Fe3O4 from iron tailings via suspension magnetized roasting by mixed gas and magnetic separation
-
摘要: 为实现铁尾矿资源化回收利用,以H2、CO、CO2和N2模拟还原混气对铁尾矿进行悬浮磁化焙烧,通过磁选获得铁精矿。探究温度、时间、H2和CO占比对铁精矿铁品位和回收率的影响,采用X射线衍射、振动样品强磁计、X射线光电子能谱、BET表面分析和扫描电子显微镜X光微区分析方法,探究悬浮磁化焙烧磁选过程中晶相结构变化和反应机理。结果表明,铁尾矿在温度、时间和H2∶CO∶CO2∶N2(体积比)分别为600 ℃、10 min和20∶15∶15∶50时,铁精矿铁品位和回收率最优分别为62.06%和98.03%。铁精矿饱和磁化强度由0.77 Am2·kg−1提升到59.43 Am2·kg-1。悬浮磁化焙烧能有效将赤铁矿针铁矿还原为磁铁矿,且BET表面积提升了13.1676 m2·g−1,并能通过磁选有效分离Fe3O4和SiO2等脉石。本研究可为从铁尾矿中回收铁资源提供参考。Abstract: For recycling Fe3O4 from iron tailing, iron tailing was reduced by mixed gas of H2, CO, CO2 and N2, and then magnetic separated. In this study, the effects of temperature, time, proportion of H2 and CO on iron grade and recovery ratio were investigated. XRD, VSM, XPS, BET and SEM-EDS methods were used to reveal the diversification of crystal structure and reaction mechanisms in the process of suspension magnetized roasting and magnetic separation. The optimal iron grade of 62.06% and recovery ratio of 98.03% was obtained via suspension magnetized roasted at 600 ℃, 10 min, and H2: CO: CO2: N2 of 20: 15: 15: 50. The saturation magnetization of iron concentrate had been increased from 0.77 to 59.43 Am2·kg−1. The suspension magnetized roasting can effectively reduce the hematite goethite into magnetite, and the BET surface area of tailing was increased by 13.1676 m2·g−1. Finally, magnetic separation was used for effectively separating Fe3O4 and SiO2 form reduced iron tailing. This study provided an effective method to recovery iron form the disposed iron tailing for sustainable development of the mine environment.
-

-
表 1 铁尾矿元素质量分数
Table 1. Element content of iron tailings %
Fe2O3 SiO2 Al2O3 SO3 K2O ZnO CuO 其他 46.78 27.90 19.59 4.11 0.54 0.20 0.17 0.71 表 2 样品及纯物质磁性参数
Table 2. Magnetic parameters of samples and pure substances Am2·kg-1
样品 饱和磁化强度 剩磁 原矿 0.77 0.05 焙烧矿 52.31 10.82 铁精矿 59.43 13.72 纯赤铁矿 0.40 - 纯磁铁矿 92.00 - 表 4 原矿焙烧矿EDS分析Fe、O元素占比
Table 4. EDS analysis of raw ore roasted ore Fe and O elements account wt%
样品 Fe O 铁尾矿 61.98 33.71 焙烧矿 66.01 24.02 表 3 BET分析的相关参数
Table 3. Relevant parameters of bet analysis
供试样品 BET表面积/
(m2·g−1)朗缪尔表面积/
(m2·g−1)孔隙体积/
(cm3·g−1)孔径/
nm铁尾矿 8.122 6 81.491 1 0.018681 9.199 4 焙烧矿 21.290 2 356.333 3 0.0628 02 11.799 2 -
[1] SUN Y S, ZHU X R, HAN Y X, et al. Iron recovery from refractory limonite ore using suspension magnetization roasting: A pilot-scale study[J]. Journal of Cleaner Production, 2020, 261: 121221. doi: 10.1016/j.jclepro.2020.121221 [2] 2020中国环境统计年鉴[EB/OL]. [2020-1-1]. http://www.mee.gov.cn. [3] BUCH A C, NIEMEYER J C, MARQUES E D, et al. Ecological risk assessment of trace metals in soils affected by mine tailings[J]. Journal of Hazardous Materials, 2021, 403: 123852. doi: 10.1016/j.jhazmat.2020.123852 [4] ŽIBRET G, GOSAR M, MILER M, et al. Impacts of mining and smelting activities on environment and landscape degradation—Slovenian case studies[J]. Land Degradation & Development, 2018, 29(12): 4457-4470. [5] WANG G W, NING X A, LU X W, et al. Effect of sintering temperature on mineral composition and heavy metals mobility in tailings bricks[J]. Waste Management, 2019, 93: 112-121. doi: 10.1016/j.wasman.2019.04.001 [6] 周伟伦, 廖正家, 陈涛, 等. 利用铁尾矿制备烧结砖的可行性及烧结固化机理[J]. 环境工程学报, 2021, 15(5): 1670-1678. [7] 严捍东, 陈秀峰. 粉煤灰和铁尾矿对烧结海泥多孔砖泛霜程度的影响[J]. 环境工程学报, 2012, 6(8): 2846-2852. [8] LI P W, LUO S H, ZHANG L, et al. Study on preparation and performance of iron tailings-based porous ceramsite filter materials for water treatment[J]. Separation and Purification Technology, 2021, 276: 119380. doi: 10.1016/j.seppur.2021.119380 [9] LI W B, HAN Y X, LIU X, et al. Effect of fluidized magnetizing roasting on iron recovery and transformation of weakly magnetic iron mineral phase in iron tailings[J]. Physicochemical Problems of Mineral Processing, 2019, 55(4): 906-916. [10] YUAN S, ZHOU W T, HAN Y X, et al. Individual enrichment of manganese and iron from complex refractory ferromanganese ore by suspension magnetization roasting and magnetic separation[J]. Powder Technology, 2020, 373: 689-701. doi: 10.1016/j.powtec.2020.07.005 [11] 黄玥, 陈海斌, 蒙李燕, 等. 木屑对铁尾矿磁化焙烧磁选工艺的影响[J]. 环境工程学报, 2020, 14(11): 1-8. [12] LI Y J, ZHANG Q, YUAN S, et al. High-efficiency extraction of iron from early iron tailings via the suspension roasting-magnetic separation[J]. Powder Technology, 2021, 379: 466-477. doi: 10.1016/j.powtec.2020.10.005 [13] SKRINSKY J, VERES J, KOLONICNY J. Explosion characteristics of blast furnace gas[J]. Inzynieria Mineralna, 2018, 19(1): 131-136. [14] COUHERT C, COMMANDRE J-M, SALVADOR S. Is it possible to predict gas yields of any biomass after rapid pyrolysis at high temperature from its composition in cellulose, hemicellulose and lignin?[J]. Fuel, 2009, 88(3): 408-417. doi: 10.1016/j.fuel.2008.09.019 [15] 中华人民共和国质量监督检验检疫总局, 中国标准化管理委员会. 铁矿石《总铁含量的测定》三氯化钛还原重铬酸钾滴定法(常规方法): GB/T 6730.65-2009 [S]. 北京: 中国环境科学出版社, 2009 [16] ZHOU D D, CHENG S S, WANG Y S, et al. The production of large blast furnaces during 2016 and future development of ironmaking in China[J]. Ironmaking & Steelmaking, 2017, 44(10): 714-720. [17] YUAN S, ZHOU W T, HAN Y X, et al. Selective enrichment of iron from fine-grained complex limonite using suspension magnetization roasting followed by magnetic separation[J]. Separation Science and Technology, 2019, 55(18): 3427-3437. [18] DWORZANOWSKI M. Maximizing the recovery of fine iron ore using magnetic separation[J]. Journal - South African Institute of Mining and Metallurgy, 2012, 112(3): 197-202. [19] GUO X F, ZHANG M R, REN W J, et al. Influence of particle size on the magnetism of magnetite and the development of an energy-efficient three-product magnetic separator[J]. Separation Science and Technology, 2020, 56(8): 1397-1406. [20] TANG Z D, ZHANG Q, SUN Y S, et al. Pilot-scale extraction of iron from flotation tailings via suspension magnetization roasting in a mixture of CO and H2 followed by magnetic separation[J]. Resources, Conservation and Recycling, 2021, 172: 105680. doi: 10.1016/j.resconrec.2021.105680 [21] YUAN S, ZHOU W T, HAN Y X, et al. Separation of manganese and iron for low-grade ferromanganese ore via fluidization magnetization roasting and magnetic separation technology[J]. Minerals Engineering, 2020, 152: 106359. doi: 10.1016/j.mineng.2020.106359 [22] YUAN S, LIU X, GAO P, et al. A semi-industrial experiment of suspension magnetization roasting technology for separation of iron minerals from red mud[J]. Journal of Hazardous Materials, 2020, 394: 122579. doi: 10.1016/j.jhazmat.2020.122579 [23] YUAN S, ZHANG Q, YIN H, et al. Efficient iron recovery from iron tailings using advanced suspension reduction technology: A study of reaction kinetics, phase transformation, and structure evolution[J]. Journal of Hazardous Materials, 2020, 404(Pt B): 124067. [24] YUAN S, ZHOU W T, HAN Y X, et al. An innovative technology for full component recovery of iron and manganese from low grade iron-bearing manganese ore[J]. Powder Technology, 2020, 373: 73-81. doi: 10.1016/j.powtec.2020.06.032 [25] GAO W G, YAN J C, QIAN L B, et al. Surface catalyzing action of hematite (α-Fe2O3) on reduction of Cr(VI) to Cr(III) by citrate[J]. Environmental Technology & Innovation, 2018, 9: 82-90. [26] KENDELEWICZ T, LIU P, DOYLE C S, et al. Spectroscopic study of the reaction of aqueous Cr(VI) with Fe3O4 (111) surfaces[J]. Surface Science, 2000, 469(2-3): 144-163. doi: 10.1016/S0039-6028(00)00808-6 [27] OMRAN M, FABRITIUS T, ELMAHDY A M, et al. XPS and FTIR spectroscopic study on microwave treated high phosphorus iron ore[J]. Applied Surface Science, 2015, 345(1): 127-140. -




 下载:
下载: