-
氯苯类有机化合物是染料、农药、医药等行业中广泛应用的原料和有机溶剂,也是产品合成的中间体,已经成为我国化工场地常见的污染物之一[1-2]。氯苯类化合物作为典型的重质非水相液体 (Dense Non-Aqueous Phase Liquids,DNAPLs) ,进入土壤及地下水后,易在重力作用下向深层地下水迁移并累积[3],因其毒性大,化学性质稳定,难降解,早在1990年,氯苯、1,2-二氯苯、1,4-二氯苯、和六氯苯已经被我国列入水中优先控制污染物[4]。
目前针对土壤和地下水中氯苯类污染物的常用修复方法有化学氧化技术、热脱附技术和生物修复技术[5]。活化过硫酸盐技术是目前修复氯苯类污染地下水最常用的技术,其中包括热活化过硫酸盐、亚铁离子活化过硫酸盐[6-7]。原位氧化等措施会改变原生土壤性质、微生物群落结构等,甚至有可能产生有毒副产物[8]。因此,为了降低修复成本及环境影响,对暂不利用开发地块的修复,监控自然衰减 (monitored natural attenuation,MNA) 技术通常与其他修复技术联合使用。MNA是一种成本低、对地块环境扰动小的被动修复技术,主要通过原地块的微生物降解作用、稀释作用、吸附作用、蒸发作用和化学反应作用对污染物进行去除[9-10]。
美国超级基金场地修复报告统计结果显示,1990年地下水修复案例中MNA的应用低于10%,1995年增长至30%左右,2012至2014年该技术的应用比例进一步增加至33%,2015至2017年MNA占比为20%,2018至2020年MNA占比为31%[11]。南京市六合区某工业污染场地2012年至2016年监测了场地内1,2,4-三氯苯的脱氯转化,初步探索了由Dehalococcoides和Dehalobacter引导的降解机制[12]。江苏某化工场地2016年至2021年监测了场地内氯代烃的自然衰减,揭示了污染物的时空特征和不同区域微生物群落结构变化及其制约因素[13-14]。国内该技术起步较晚,监测时间较短,应用案例较少,且多用于氯代烃、石油烃、苯系物污染场地污染物质量浓度变化监测[15-19]。针对氯苯类污染地块自然衰减机制及其影响因素,目前研究还存在空白。
本研究以上海市某化工污染地块修复中试工程结束后2年的自然衰减监测为基础,从时间角度出发,探索1,2-二氯苯、1,4-二氯苯的降解速率。通过典型地球化学指标的变化,讨论其与地块微生物降解能力的关系,对比不同时间地下水细菌多样性,探讨其中可能存在的自然衰减规律和微生物群落结构变化的环境影响因素。
-
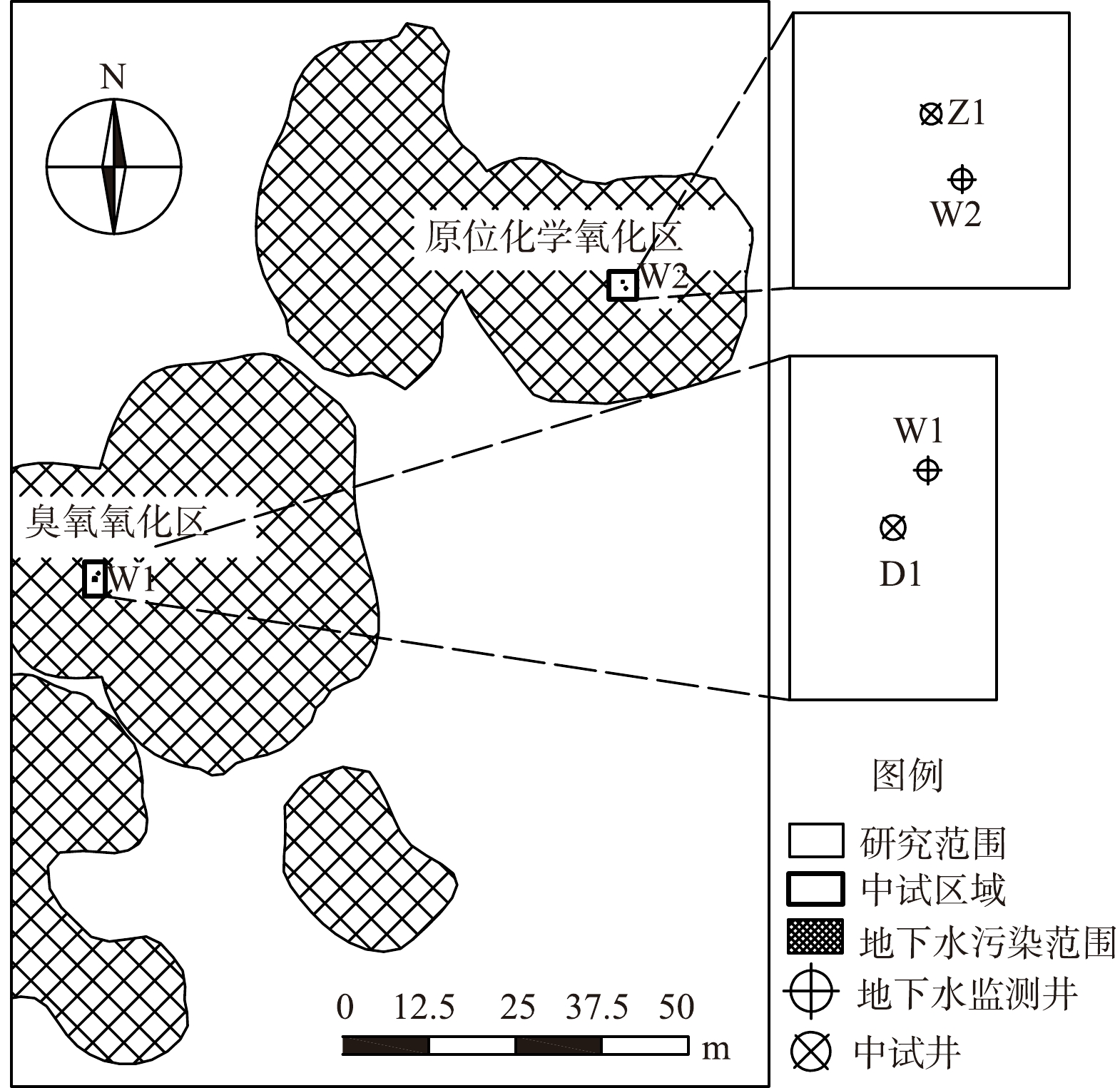
本研究地块位于上海市奉贤区,占地面积约为16 500 m2,该地块化工企业生产历史长达20年,2009年原厂搬迁、拆除,后地块闲置。经前期土壤和地下水污染状况调查和风险评估,地下水污染严重,主要污染物为1,2-二氯苯、1,4-二氯苯,最大检出质量浓度分别为41 300和9 050 μg·L−1 (W2地下水监测点) ,对比《地下水质量标准》 (GB/T 14848-2017) 中Ⅳ类标准[20],1,2-二氯苯、1,4-二氯苯最大超标倍数分别为20.65倍和15.08倍。采用kriging插值法绘制的地下水污染合并范围见图1。
地块地层从上至下分别为杂填土 (厚度约1.5 m) 、吹填土 (厚度约1.2 m) 、粘质粉土 (厚度约10.0 m) 、淤泥质粘土 (厚度约3.8 m) 、粘土 (未穿透) ,地下水稳定水位埋深为0.6~1.3 m,流向自北向南,水力坡度约为0.6%。研究地区地下水最高水位一般发生在每年11月至次年3月,地表易形成多个沼泽水洼,最低水位一般发生在每年6月至8月,地表沼泽水洼都已干涸。
在地下水中氯苯类污染物质量浓度较低的区域进行电解臭氧氧化中试,监测较低浓度的1,2-二氯苯、1,4-二氯苯 (W1监测点原始质量浓度分别为1 350和497 μg·L−1) 的变化情况。通过电极井D1制造的臭氧氧化污染物,臭氧电极共运行10 d,中试结束时W1地下水监测点的1,2-二氯苯、1,4-二氯苯质量浓度分别为354和148 μg·L−1。
选择1,2-二氯苯、1,4-二氯苯污染质量浓度最高的区域于2021年12月底开展原位化学氧化中试 (W2监测点1,2-二氯苯、1,4-二氯苯的原始质量浓度分别为9.1、41.3 mg·L−1) ,在药剂注入点Z1注入了700 L 3%的七水硫酸亚铁溶液和1 400 L 10%的过硫酸钠溶液。中试运行结束时W2地下水监测点的1,2-二氯苯、1,4-二氯苯质量浓度分别为12 425和3 015 μg·L−1。
-
在中试开始前和结束后的当天 (第0天) 、第290天、第416天、第529天、第652天、第727天分别在W1和W2地下水监测井 (地下6 m) 采样,地下水样品采集时避开降雨天气,建井及采样方法满足《地下水环境监测技术规范》 (HJ/T 164-2020) 中的相关要求[21]。
在中试开始前和结束后的第416天、第529天、第652天、第727天,在现场对W1和W2监测井分别采样,使用抽滤瓶将约1 L地下水微生物富集到0.22 μm的滤膜上,每个样品2个平行,采集后立刻将滤膜用干冰保存送至商业实验室进行测序分析。
在地块周边选取了1个未受污染的对照点CK,采集地下水样品分析其中的污染物含量及微生物种类。
-
地下水中的1,2-二氯苯、1,4-二氯苯的分析测试参照《水质 挥发性有机物的测定 吹扫捕集气相色谱-质谱法》 (HJ 639-2012) 测定[22]。参照国家相关测试方法对典型地球化学指标进行测定,包括pH值、氧化还原电位 (ORP) 、温度、总有机碳 (TOC) 、溶解氧 (DO) 、碱度、CO2、NO3−、Fe2+、SO42−、S2−、Cl−等。环境微生物多样性测序方法见参考文献[23-24]。
-
使用fastp (version 0.19.6) 进行质控,FLASH (version 1.2.11) 软件进行pair-end双端序列拼接,剔除嵌合体并根据97%的相似度对拼接后的序列划分操作分类单元OTU (Operational taxonomic unit)。为了尽量减少测序深度对后续Alpha多样性和Beta多样性数据分析的影响,将所有样本序列数抽平至20306,抽平后,每个样本的平均序列覆盖率仍可达98.3%。利用R语言 (version 3.3.1) 软件进行群落组成分析、主坐标分析 (PCoA) 和冗余分析 (RDA) ,群落组成组间差异性检验分析方法采用One-way ANOVA。使用BugBase表型预测进行16S rRNA功能预测分析。细菌多样性数据分析在majorbio平台(http://www.majorbio.com/) 进行操作。
-
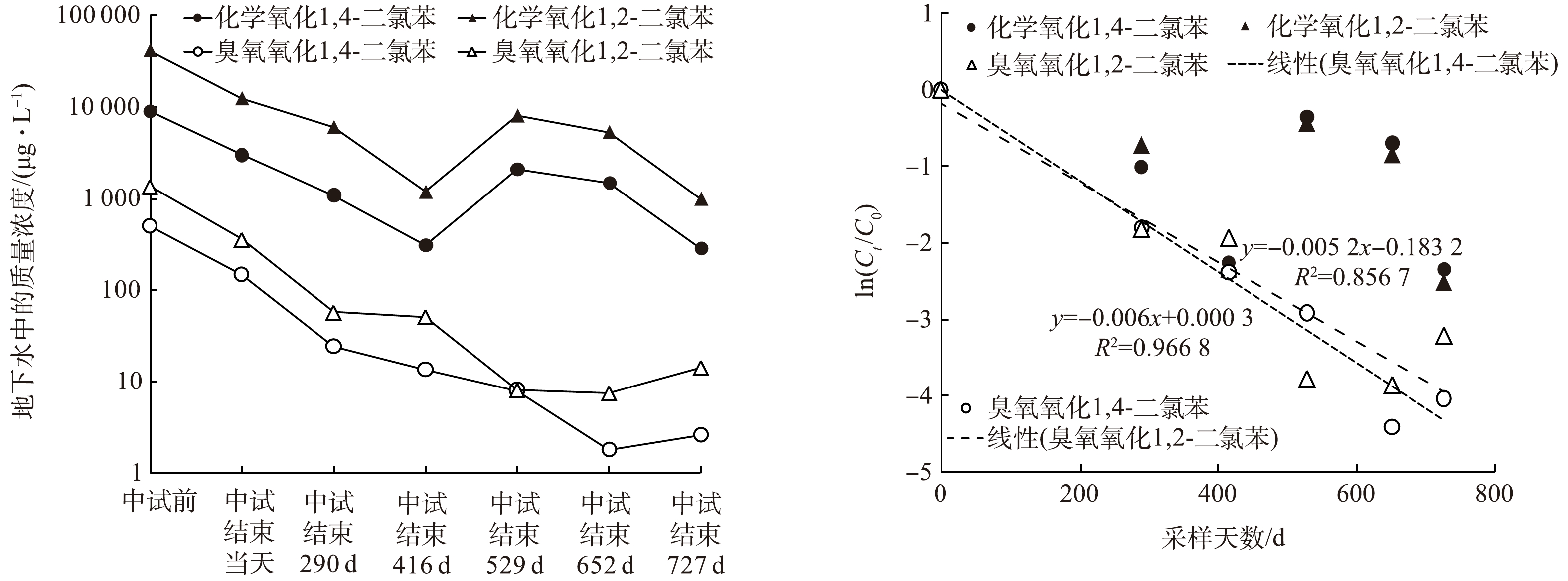
中试开始之前、中试结束的当天 (第0天) 、第290天、第416天、第529天、第652天、第727天,臭氧氧化和原位化学氧化区地下水监测井中1,2-二氯苯、1,4-二氯苯的质量浓度变化见图2(a)。臭氧氧化阶段1,2-二氯苯、1,4-二氯苯的去除率分别为73.8%和70.2%,过硫酸盐氧化阶段1,2-二氯苯、1,4-二氯苯的去除率分别为65.9%和70%。臭氧氧化过程主要由产生的羟基自由基氧化1,2-二氯苯、1,4-二氯苯[25-26],而过硫酸盐降解污染物由活化产生的硫酸根自由基作为氧化剂,其氧化能力与羟基自由基等同但稳定性大于羟基自由基,氧化能力羟基自由基(E0 = 2.7~2.8 V) ≈ 硫酸根自由基(E0 = 2.6 V)>过硫酸根离子(E0 = 2.1 V) ≈ 臭氧(E0 = 2.07 V) [27]。
在自然衰减阶段,整体上污染物质量浓度都呈现下降趋势,到中试后727 d,化学氧化区域1,2-二氯苯、1,4-二氯苯质量浓度降至1 000、286 μg·L−1,臭氧氧化区1,2-二氯苯和1,4-二氯苯质量浓度降至14.1、2.6 μg·L−1,臭氧氧化区中试后727 d的自然降解率分别为96.02%和98.24%,高于化学氧化区的91.95%和90.51%。原位化学氧化区W2监测井地下水中1,2-二氯苯、1,4-二氯苯的初始质量浓度较高,第290天、第416天监测数据持续下降,416 d后质量浓度出现回升,可能与监测井周边高浓度污染物的回流有关 (表1) 。臭氧氧化地块污染物自然衰减优于化学氧化地块,一方面,臭氧氧化区的污染物浓度低于化学氧化区,对于降解微生物的抑制作用较弱[28],更有利于污染物的去除;另一方面,化学氧化区的污染物浓度出现回升现象,可能与地块水文或地质情况相关[29],在416 d时,化学氧化区污染物的自然降解率可达90%。
在中试结束后的727 d内,臭氧氧化区中1,2-二氯苯、1,4-二氯苯的质量浓度呈现持续下降趋势,符合一阶衰减模型[30] (图2(b)) 。
式中:k为有机物的降解速率常数,d−1;C0为有机物的初始质量浓度,μg·L−1;Ct为有机物衰减后的质量浓度,μg·L−1;t为降解时间,d。
臭氧氧化区中1,2-二氯苯的一阶降解速率常数为0.005 2 d−1 (R2=0.857) ,1,4-二氯苯的一阶降解速率常数为0.006 0 d−1 (R2=0.967) 。李岩云[31]计算出在实验室条件下原状土介质中氯苯的降解速率常数为0.081 d−1,且证明土壤介质的存在会加快氯苯降解速率。实验室柱迁移试验计算得到的1,2,4-三氯苯的一阶降解速率常数为0.001 925 d−1[32]。江苏某农药污染场地原位化学氧化后5年自然衰减监测得到,浅层地下水中四氯化碳和氯仿的降解速率常数分别为0.002和0.001 3 d−1[14],与本研究中得到一阶降解速率常数数量级相当。地层条件与地球物理化学性质对污染物衰减速率的影响很大,然而目前地下水氯苯类污染物的自然衰减数据仍然缺乏[31,33]。
-
本研究地块中试前后的地下水中典型地球化学指标监测数据见表1,微生物降解能力得分见表2,评价方法参考美国环保署制定的标准[34]。根据标准,6~14分为微生物降解证据有限,15~20为微生物降解证据充足。w1-ck和w2-ck代表了中试开始前污染地块样品,中试前的地下水微生物降解能力得分11~14,其生物降解能力低于中试后529 d的地下水,臭氧氧化区和化学氧化区得分都是17。
一方面,这与其注入的药剂改变了土壤和地下水的地球化学特性有关,中试后529 d地下水中硫离子的质量浓度由0.21~8.8 mg·L−1上升到53.9~59.6 mg·L−1。原位化学氧化中试后地下水中SO42−和S2−质量浓度都显著升高,注入的硫酸亚铁和过硫酸钠在降解污染物的过程中被还原为硫化物。臭氧氧化中试也会使地下水中原有的SO42−作为电子受体被还原,SO42−质量浓度由442下降至204 mg·L−1,同时S2−质量浓度由8.8上升至59.6 mg L−1。与石油烃污染场地自然衰减监测发现SO42−、Fe (III) 和NO3−都发生还原现象不同[35],本地块中试后727 d,SO42−浓度升高,S2−浓度下降,Fe2+浓度升高。地下水中的DO质量浓度在529 d内一直<0.20 mg·L−1,后升高,ORP由中试前的−44.4~3.3 mV先降低至−350.4~−268.3 mV后升高,这也与氯代烷烃类污染场地的自然衰减一直维持厌氧还原状态不同[14,36]。
另一方面,中试注入的药剂可以强化刺激微生物生长,增强对污染物的降解[37]。硫酸亚铁、过硫酸钠和臭氧使低氯代苯氧化开环,中间产物自由基为土著微生物降解提供了电子供体,促进微生物共代谢降解和还原脱氯代谢[31,38]。污染物自然衰减前的强化氧化虽然会使土著微生物暂时性的减少,但微生物的数量和降解能力能逐渐恢复[37]。相反,强化氧化对后续微生物的降解有着促进作用,其不但可以降低污染物对微生物的毒性,还可以提高污染物的生物可利用性,产生生物可利用和可降解的氧化产物,给污染物的好氧生物代谢提供氧气[39]。
-
门分类水平下的微生物群落结构构成如图3 (a)所示,细菌OTU分配为67个门,193个纲,1637个属。在自然衰减的652 d内,相对丰度较高的变形菌门Proteobacteria、厚壁菌门Firmicutes在臭氧氧化区随时间呈现增加趋势,在化学氧化区变化无规律,可能与药剂的注入和污染物浓度的回升有关。
常见的有机物脱氯降解菌的相对丰度变化如图3 (b)所示,本研究选取的11种有机物脱氯降解菌参考以往文献[13,31]。中试后臭氧氧化区的常见有机物脱氯降解菌Comamonas、Desulfovibrio、Dehalobacter、Geobacter、Desulfitobacterium、Desulfomonile的相对丰度从0~3.27‰达到最高时的28.98‰,且相对丰度的最高值大部分出现在中试后416 d时;中试后化学氧化区的Dehalogenimonas、Sulfurospirillum、Desulfitobacterium、Desulfomonile、Pseudomonas的相对丰度从0.02~18.07‰达到最高时189.08‰,且最高相对丰度大部分出现在中试后416 d和529 d时。化学氧化区的Pseudomonas的相对丰度在中试后416 d增加了80倍,Desulfitobacterium的相对丰度在中试后529 d增加了391倍,本研究地块可能的降解优势菌属在化学氧化中试后的自然过程中从Desulfomonile转变为Pseudomonas。与化学氧化区相比,臭氧氧化区降解菌群的增长趋势更为均匀,可能的降解优势菌属从416 d的Geobacter、Desulfomonile和Pseudomonas转变为Desulfitobacterium再到Desulfomonile。此外,Pseudomonas sp. P51、Burkholderia sp. JS150、Ralstonia sp. JS705、Pseudomonas putida、Escherichia hermanii等在自然界中被发现可以降解低氯代苯[40-42]。
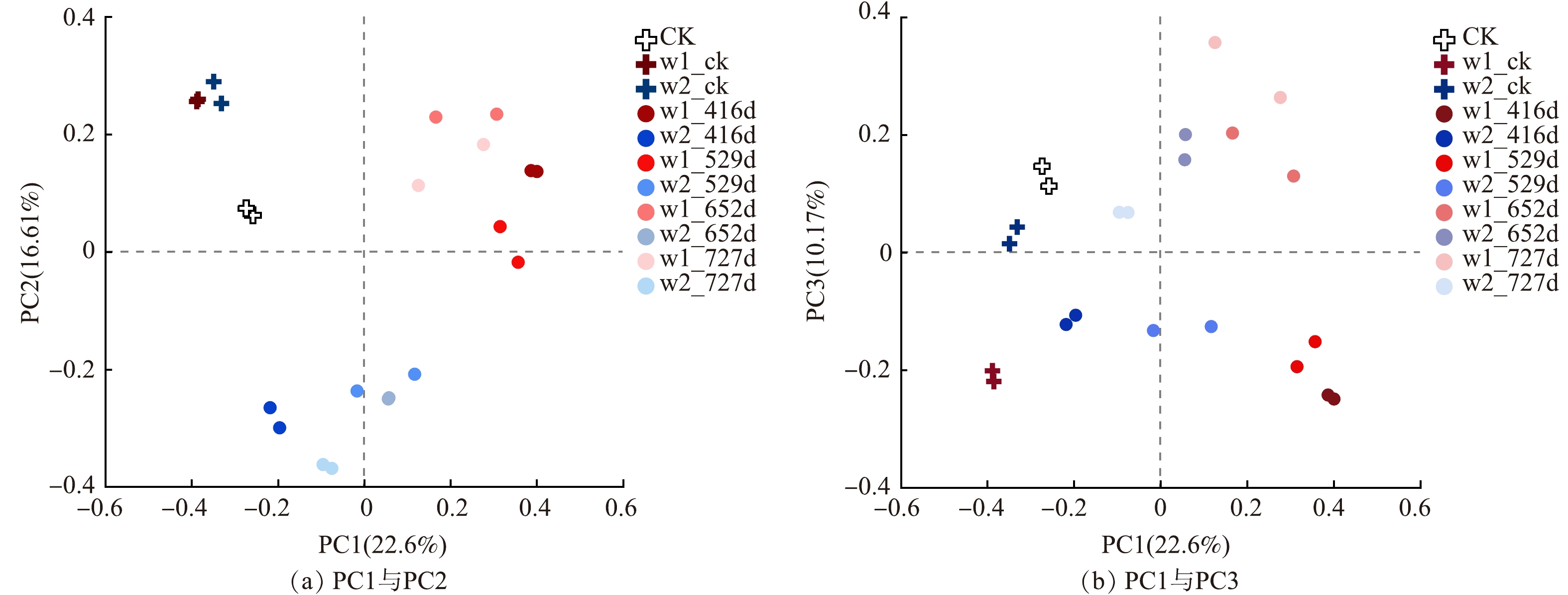
OTU水平下的微生物群落主坐标分析见图4,PC1、PC2 (图4 (a)) 、PC3 (图4 (b)) 贡献率分别为22.6%、16.61%和10.17%。通过Bray-Curtis距离算法,得到臭氧氧化区的w1-416d、w1-529d、w1-652d、w1-727d与无污染区域CK的距离分别为 0.913±0.013、0.883±0.009、0.892±0.006、0.872±0.004,化学氧化区的w2-416d、w2-529d、w2-652d、w2-727d与CK的距离分别为0.830±0.029、0.820±0.018、0.813±0.012、0.817±0.008。臭氧氧化区相较于无污染对照组的距离随着时间推移呈现显著缩小趋势,而化学氧化区变化趋势较小。对比中试实施前w1-ck、w2-ck与CK的距离 (分别为 0.827±0.012和0.801±0.020) ,说明中试的实施加剧了地下水中微生物群落结构的变化,在中试后群落结构逐渐恢复。
地下水中细菌的多样性统计结果见表3。对比中试修复前和中试后的数据,2个区域中试后416 d的Sobs指数 (观测到的OTU数) 、群落多样性指数Shannon指数、群落丰富度指数Chao1指数、ACE指数都比中试前高,最接近未污染区域,416 d后数据有所回落。其中臭氧氧化区的Sobs指数、Chao1指数、ACE指数、Shannon指数在529 d时对比中试前都显著降低;化学氧化区的样品Sobs指数、Shannon指数、ACE指数和Chao1指数在中试后727 d的数据都显著高于中试前。研究结果说明中试的实施有利于该污染区域微生物群落多样性的恢复,但在416 d后的自然衰减过程中受降解机制的影响,多样性下降,优势菌丰度增加。
-
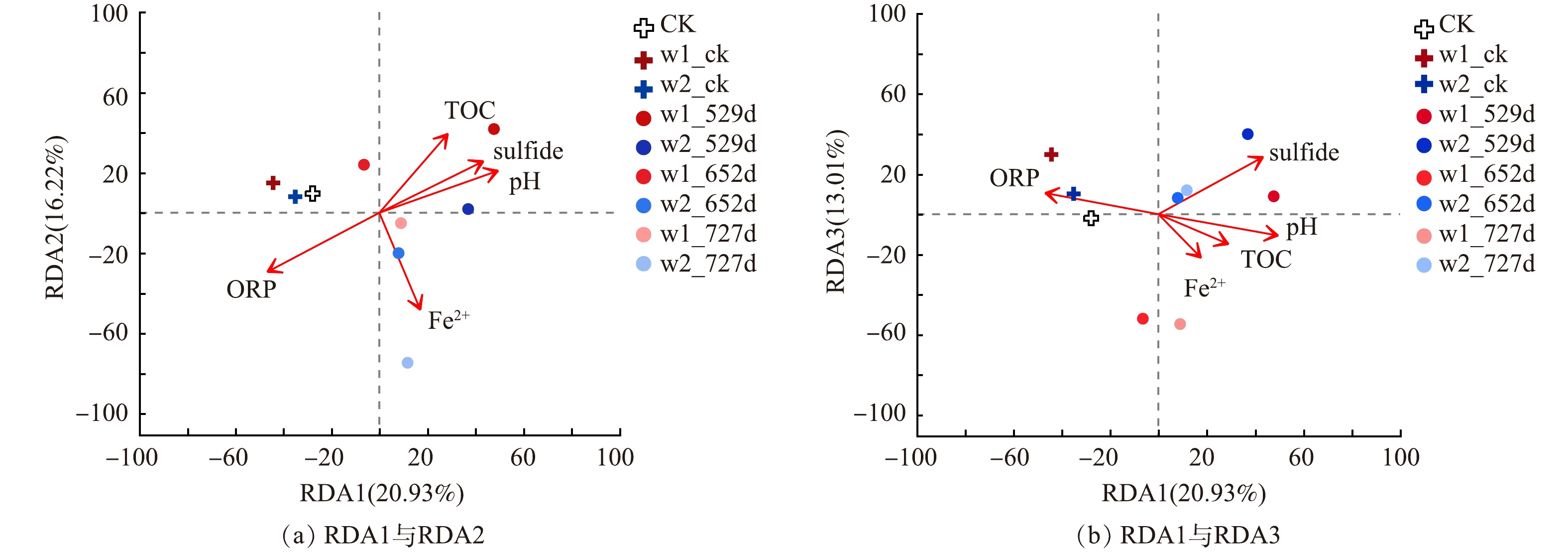
基于本研究的氯苯类污染地块特性,探究地块环境因子对地下水中微生物群落的影响关系,其中S2− (R2 = 0.714,p = 0.022) 、ORP (R2 = 0.859,p = 0.002) 和pH值 (R2 = 0.818,p = 0.005) 与微生物群落结构有显著性相关关系 (图5) 。之前的研究表明污染物浓度和Mn显著性影响氯代烃类污染场地地下水中微生物群落结构[13],溶解性有机碳、SO42−、和污染物浓度显著性影响溴代烃类污染场地地下水中微生物群落结构[43]。
此外,对地下水中微生物表型结果进行了预测,见表4。中试后416 d内2个区域都以有氧型细菌为主,后逐渐向厌氧型转变。通常氯苯类污染物的厌氧降解的速率低于好氧降解,这也意味着在中试后727 d内,氯苯类污染物的自然衰减速率先增加再降低,这与一阶自然衰减模型趋势一致。氯代烃自然衰减污染场地中微生物表型预测细菌群落逐渐向厌氧型转化,其ORP也是逐渐降低[14]。虽然本地块地下水中ORP变化与微生物表型预测结果的变化相反,这可能是受到了氧化中试注入的影响,但是本地块的ORP也一直维持较低的数值。
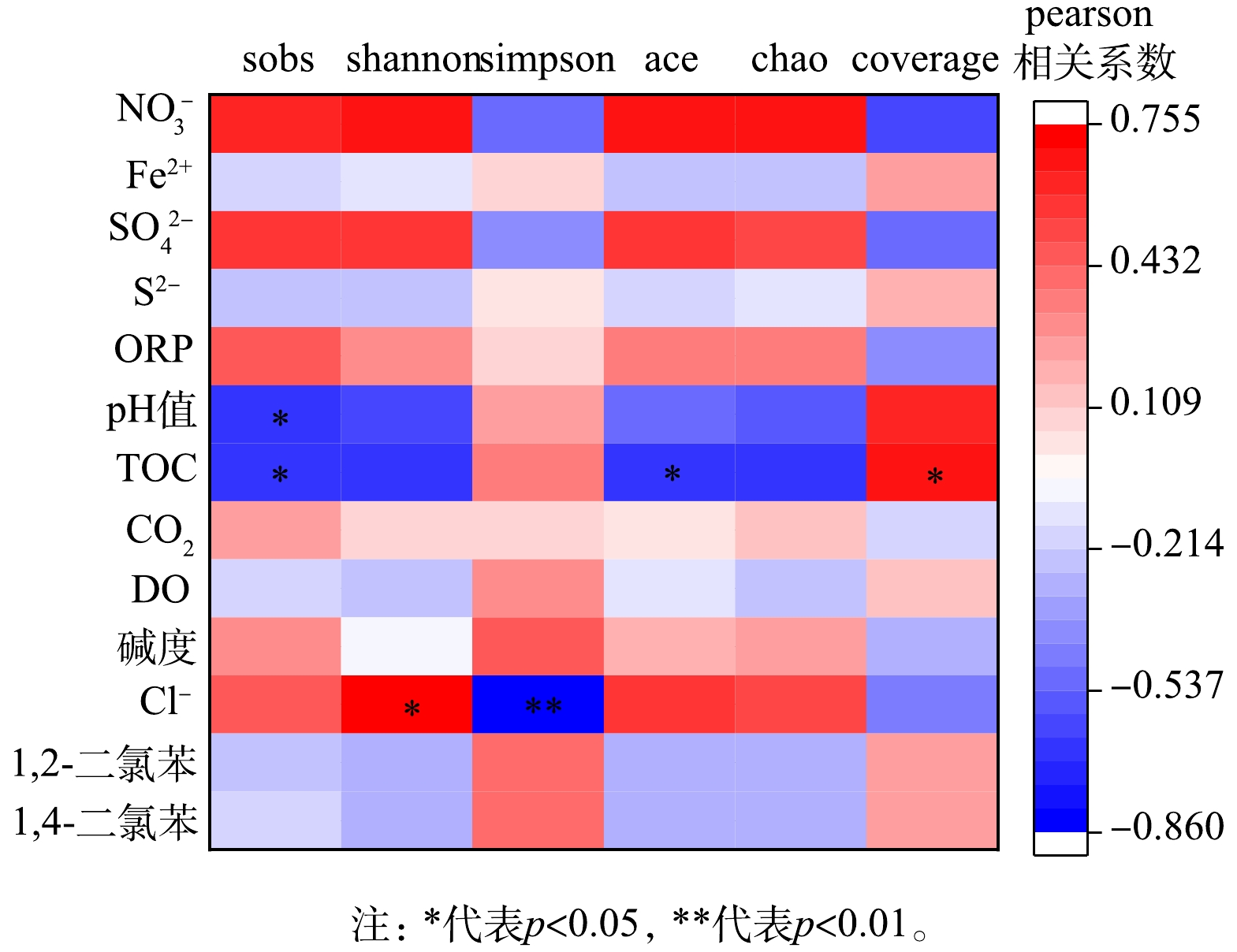
从地下水微生物群落与环境因子、污染物质量浓度相关性分析可以看出,Sobs指数、Shannon指数、Simpson指数和ACE指数分别与pH值、TOC、Cl−呈现显著线性相关关系 (图6) 。本地块地下水中微生物群落Alpha多样性指数与污染物质量浓度无显著线性相关关系,之前的研究表明石油烃和氯代烃污染场地污染物浓度越高,微生物多样性降低,优势菌群丰度增加[13,28]。本研究416 d后臭氧氧化区域污染物浓度降低,但同时多样性也降低,说明优势菌群丰度增加,这可能与416 d后厌氧菌丰度大幅增加有关, 416 d后由厌氧菌主导氯苯类污染物的厌氧降解,有机物脱氯降解菌Desulfitobacterium在416 d后相对丰度突增也与此规律一致。
之前的研究认为氯苯类污染物的生物降解可能更接近石油烃类的好氧降解,而非氯代烃类的厌氧降解[44],但氯苯类污染物的微生物降解过程可能更复杂。结合本地块环境因子和微生物表型预测的变化,本地块2个区域中试后416 d内都以化学氧化和微生物好氧降解为主,后向微生物厌氧降解转变。这也可能是由于氧化中试使氯苯类污染物的前期自然降解受到了人为影响。氯苯类污染物分子水平的自然降解机制有待进一步研究。本研究结论可为氯苯类污染地下水修复工程的绿色高效实施提供参考依据,控制发挥地块自身降解能力以经济效益最大化,同时也可指导自然衰减监测的精准实施,为进一步探究氯苯类污染物的自然衰减机制奠定基础。
-
1) 氯苯类污染地块臭氧氧化区地下水中1,2-二氯苯和1,4-二氯苯的一阶降解速率常数分别为0.005 2和0.006 0 d−1。化学氧化区地下水中污染物自然降解416 d后质量浓度出现回升,不符合一阶衰减模型。2) 在实施臭氧氧化和化学氧化中试后的529 d内,微生物降解能力有所提高,注入药剂改变了土壤和地下水的地球化学特性,同时可强化刺激微生物对污染物的降解。3) 中试的实施加剧了地下水中微生物群落结构的变化,在中试后群落结构逐渐恢复,环境因子中的S2−、ORP和pH与本研究中氯苯类污染地块地下水微生物群落结构有显著线性相关关系。氧化中试后416 d内,本地块地下水都以化学氧化和微生物好氧降解为主,后向微生物厌氧降解转变。
强化氧化后污染源浅层地下水中氯苯类污染物的自然衰减与微生物群落演化
Natural attenuation and microbial community evolution of chlorobenzene pollutants in shallow groundwater after enhanced oxidation
-
摘要: 监控自然衰减技术通常与其他地下水修复技术联用,以降低修复成本、减少环境影响,目前该技术在氯苯类污染地块的实践应用案例存在空白,其效果及作用机制尚不明确。以原位臭氧氧化和化学氧化中试后的氯苯类污染地块为研究对象,从实际工程案例的角度,监测强化氧化措施后2年内的氯苯类化合物的降解速率、细菌组成,评价不同修复措施后污染物自然衰减能力、探讨作用机制。结果显示,经过727 d的自然衰减,1,2-二氯苯和1,4-二氯苯在臭氧氧化区和化学氧化区中的降解率都超过了90%。臭氧氧化区地下水中1,2-二氯苯和1,4-二氯苯的一阶降解速率常数分别为0.005 2 d−1 (R2=0.857) 和0.006 0 d−1 (R2=0.967) ;化学氧化区地下水中污染物质量浓度在416 d后出现回升,不符合一阶衰减模型。细菌16S rRNA的高通量分析表明,臭氧和过硫酸钠氧化改变了地下水中细菌群落结构,在自然衰减过程中细菌群落结构逐渐恢复,环境因子S2−、ORP和pH值与细菌群落结构显著相关。本地块2个区域中试后416 d内都以化学氧化和微生物好氧降解为主,后向微生物厌氧降解转变。本研究可为氯苯类污染地下水修复工程的绿色高效实施提供参考。Abstract: The monitored natural attenuation technique is usually used in conjunction with other groundwater remediation technologies to reduce remediation costs and environmental impacts. However, there is still a gap in its practical application cases in chlorobenzenes contaminated sites, and the natural attenuation mechanism of chlorobenzenes is not clear. Therefore, this study took chlorobenzenes contaminated sites after pilot tests of in situ ozone oxidation or chemical oxidation as the research object. From the perspective of practical engineering cases, the bacterial composition of groundwater and degradation rate of chlorobenzene compounds in the two years after the intensified oxidation measures were monitored, the natural attenuation capacity of pollutants after different remediation measures were evaluated, and the mechanism of action was discussed. The results showed that after 727 days of natural decay, the degradation rates of 1, 2-dichlorobenzene and 1, 4-dichlorobenzene in the ozone oxidation zone and chemical oxidation zone both exceeded 90%. The first-order degradation rate constants of 1, 2-dichlorobenzene and 1, 4-dichlorobenzene in ozonation zone were 0.005 2 d−1 (R2=0.857) and 0.006 0 d−1 (R2=0.967), respectively. The concentration of pollutants in the groundwater in the chemical oxidation zone rose after 416 days, which was inconsistent with the one-order attenuation model. The results of bacterial 16S rRNA showed that ozone and sodium persulfate oxidation changed the bacterial community structure in groundwater, and the bacterial community structure gradually recovered during natural decay. The S2−, ORP and pH values of environmental factors were significantly correlated with bacterial community structure of groundwater in chlorobenzenes contaminated sites in this study. In 416 days after the pilot test, chemical oxidation and microbial aerobic degradation were dominant in two zones, and then changed to microbial anaerobic degradation. This study provides a reference for the green and efficient implementation of chlorobenzene contaminated groundwater remediation projects.
-

-
表 1 地下水中典型地球化学指标表
Table 1. Typical geochemical indexes in groundwater
样品
编号NO3−/
(mg·L−1)Fe2+/
(mg·L−1)SO42-/
(mg·L−1)S2-/
(mg·L−1)ORP/
mVpH值 TOC/
(mg·L−1)CO2/
(mg·L−1)DO/
(mg·L−1)碱度/
(mg·L−1)Cl−/
(mg·L−1)温度/
℃CK 10.6 <0.010 1 400 0.09 −131.4 6.8 41 113.8 <0.20 241 586.2 25.5 w1_ck 6.12 <0.010 442 8.8 −44.4 6.8 21.8 109.8 <0.20 386 49 24.6 w1_529d <0.05 0.283 204 59.6 −350.4 8.4 492 118.2 <0.20 275 37.6 26.5 w1_652d 4.06 0.194 243 0.01 −251.6 8.2 212 <4.0 1.45 134.3 145.4 21.5 w1_727d 0.56 2.16 697 0.502 −157.1 7.5 124 47.4 1.89 93 221.5 13.7 w2_ck 5.58 <0.010 74.3 0.21 3.3 7 138 145.3 <0.20 454 43 25.7 w2_529d 9.6 0.095 406 53.9 −268.3 8.2 42.4 21.9 <0.20 210 379.7 25.6 w2_652d 0.78 0.728 634 <0.01 −97.1 7.6 25.6 <4.0 2.08 177.8 205.3 21.4 W2_727d 1.59 2.52 1 540 1.04 −79.9 7.2 15.9 84.6 3.5 486 149.1 11 表 2 地下水生物降解能力得分表
Table 2. Biodegradability scores in groundwater
样品
编号分项指标得分 生物降解
能力得分*NO3− Fe2+ SO42- S2− ORP pH值 TOC CO2 DO 碱度 Cl− 温度 w1_ck 0 0 0 3 1 0 2 0 3 0 0 1 14 w1_529d 2 0 0 3 2 0 2 0 3 0 0 1 17 w1_652d 0 0 0 0 2 0 2 0 0 0 2 1 11 w1_727d 2 3 0 0 2 0 2 0 0 0 2 0 15 w2_ck 0 0 0 0 1 0 2 0 3 0 0 1 11 w2_529d 0 0 0 3 2 0 2 0 3 0 2 1 17 w2_652d 2 0 0 0 1 0 2 0 0 0 2 1 8 W2_727d 0 3 0 3 1 0 0 0 0 0 2 0 13 注:*参考EPA评分方法[33],因本地块关注污染物为氯苯类,挥发性脂肪酸和苯系物浓度指标都满足得分要求,统一总分加4,在此不单列。 表 3 地下水中微生物高通量数据统计结果 (每组样品2个平行)
Table 3. Statistical results of high-throughput data in groundwater. Data are given as mean±SD (n = 2)
生物多样
性指数CK 臭氧氧化区 化学氧化区 w1_ck w1_416d w1_529d w1_652d w1_727d w2_ck w2_416d w2_529d w2_652d w2_727d Sobs 2 910±
1292 909±
862 928±
2151 160±
241 281±
51 336±
661 470±
152 315±
4402 025±
2241 884±
1062 132±
231Shannon 6.576±
0.0825.949±
0.0896.755±
0.1904.800±
0.1074.888±
0.1555.470±
0.0044.988±
0.3225.362±
0.6755.656±
0.4245.492±
0.3785.313±
0.198Simpson 0.005±
0.0000.022±
0.0030.004±
0.0010.027±
0.0040.029±
0.0110.014±
0.0010.031±
0.0160.040±
0.0270.016±
0.0070.022±
0.0130.030±
0.002Ace 5 025.511±
955.3104 559.222±
190.4214 221.697±
176.2982 064.522±
551.1972 753.818±
78.8072 071.863±
158.7792 390.025±
374.3725 036.760±
350.8073 804.575±
845.1003 683.329±
76.4853 641.956±
221.255Chao1 4 335.848±
318.0774 430.149±
146.0284 189.969±
113.3611 822.019±
226.1292 100.149±
104.7711 913.870±
37.0792 042.517±
45.9853 820.097±
468.1993 280.057±
141.2282 980.934±
114.2673 027.674±
199.119Coverage 0.939±
0.0040.937±
0.0030.944±
0.0020.977±
0.0030.972±
0.0010.976±
0.0010.972±
0.0020.946±
0.0070.956±
0.0020.959±
0.0010.955±
0.003表 4 地下水中微生物表型预测相对丰度结果 (每组样品2个平行)
Table 4. Predicted relative abundance results of microbial phenotype in groundwater. Data are given as mean±SD (n = 2)
微生物
表型预测CK 臭氧氧化区 化学氧化区 w1_ck w1_416d w1_529d w1_652d w1_727d w2_ck w2_416d w2_529d w2_652d w2_727d 有氧菌 0.295±
0.0280.337±
0.0230.265±
0.0320.049±
0.0120.088±
0.0270.191±
0.0980.249±
0.1140.582±
0.0960.206±
0.1220.218±
0.0260.399±
0.031厌氧菌 0.294±
0.050.148±
0.0020.174±
0.0030.822±
0.0020.691±
0.0680.534±
0.1150.280±
0.1530.197±
0.0650.568±
0.1420.391±
0.0100.331±
0.044兼性厌氧菌 0.072±
0.0140.365±
0.0260.220±
0.0040.028±
0.0030.051±
0.0150.073±
0.0590.055±
0.0170.074±
0.0030.089±
0.0130.063±
0.0020.101±
0.022革兰氏阴性菌 0.874±
0.0270.933±
0.0070.849±
0.0160.928±
0.0100.836±
0.0340.708±
0.0640.913±
0.0160.958±
0.0190.754±
0.0830.793±
0.0060.958±
0.008革兰氏阳性菌 0.126±
0.0270.067±
0.0070.151±
0.0160.072±
0.0100.164±
0.0340.292±
0.0640.087±
0.0160.042±
0.0190.246±
0.0830.207±
0.0060.042±
0.008 -
[1] 李佳斌. 北京某染料厂污染地块土壤和地下水6种氯苯类化合物的分布特征及迁移转化分析[J]. 环境工程学报, 2022, 16(7): 2296-2307. [2] 孟宪荣, 许伟, 张建荣. 化工污染场地氯苯分布特征[J]. 土壤, 2019, 51(6): 1144-1150. [3] 张婉莹. 基于EVS的上海某化工污染场地中1, 4-二氯苯空间分布模拟研究[J]. 环境卫生工程, 2021, 29(3): 31-38. [4] 周文敏, 傅德黔, 孙宗光. 水中优先控制污染物黑名单[J]. 中国环境监测, 1990(4): 1-3. doi: 10.19316/j.issn.1002-6002.1990.04.001 [5] 刘乐, 张国良, 王芳, 等. 氯苯类化合物污染现状及其修复技术研究进展[J]. 湖北农业科学, 2022, 61(5): 91-97. doi: 10.14088/j.cnki.issn0439-8114.2022.05.018 [6] 刘小宁. 利用热活化过硫酸盐修复氯苯污染地下水的研究[D]. 硕士, 华东理工大学, 2013. [7] 王继鹏, 虞敏达, 蔡小波, 等. Fe~(2+)活化过硫酸钠原位修复氯苯污染地下水[J]. 环境工程学报, 2016, 10(3): 1276-1280. [8] 侯德义. 我国工业场地地下水污染防治十大科技难题[J]. 环境科学研究, 2022, 35(9): 2015-2025. [9] US EPA, Use of Monitored Natural Attenuation at Superfund, RCRA Corrective Action, and Underground Storage Tank Sites[S]. U. S. Environmental Protection Agency, Washington, DC, EPA 20460. [10] 李元杰, 王森杰, 张敏, 等. 土壤和地下水污染的监控自然衰减修复技术研究进展[J]. 中国环境科学, 2018, 38(3): 1185-1193. [11] US EPA. Superfund Remedy Report 17th Edition[R]. EPA-542-R-23-001 2023, Office of Land and Emergency Management. [12] QIAO W, LUO F, LOMHEIM L, MACK E E, et al. Natural Attenuation and Anaerobic Benzene Detoxification Processes at a Chlorobenzene-Contaminated Industrial Site Inferred from Field Investigations and Microcosm Studies[J]. Environmental Science & Technology, 2018, 52(1): 22-31. [13] FAN T, YANG M, LI Q, et al. A new insight into the influencing factors of natural attenuation of chlorinated hydrocarbons contaminated groundwater: A long-term field study of a retired pesticide site[J]. Journal of Hazardous Materials, 2022, 439: 129595. doi: 10.1016/j.jhazmat.2022.129595 [14] 范婷婷, 夏菲洋, 孔令雅, 等. 场地地下水中氯代甲烷烃自然衰减机制[J]. 环境工程学报, 2021, 15(12): 3934-3945. doi: 10.12030/j.cjee.202108083 [15] CHIU H Y, VERPOORT F, LIU J K, et al. Using intrinsic bioremediation for petroleum–hydrocarbon contaminated groundwater cleanup and migration containment: Effectiveness and mechanism evaluation[J]. Journal of the Taiwan Institute of Chemical Engineer, 2017, 72: 53-61. doi: 10.1016/j.jtice.2017.01.002 [16] LV H, SU X, WANG Y, et al. Effectiveness and mechanism of natural attenuation at a petroleum-hydrocarbon contaminated site[J]. Chemosphere, 2018, 206: 293-301. doi: 10.1016/j.chemosphere.2018.04.171 [17] 马欣程, 徐红霞, 孙媛媛, 等. 氯代烃污染场地生物自然衰减修复研究进展[J]. 中国环境科学, 2022, 42(11): 5285-5298. doi: 10.3969/j.issn.1000-6923.2022.11.035 [18] 周睿, 赵勇胜, 任何军, 等. BTEX在地下环境中的自然衰减[J]. 环境科学, 2009, 30(9): 2804-612808. doi: 10.13227/j.hjkx.2009.09.002 [19] 周艳, 姜登登, 孔令雅, 等. 典型农药污染场地地下水中苯系物监控自然衰减研究[J]. 环境科学学报, 2022, 42(7): 380-388. doi: 10.13671/j.hjkxxb.2021.0547 [20] 国家质量监督检验检疫总局, 国家标准化管理委员会. 地下水质量标准: GB/T 14848-2017 [S]. 北京: 中国环境科学出版社, 2017. [21] 生态环境部. 地下水环境监测技术规范: HJ/T 164-2020 [S]. 北京: 中国环境出版集团, 2020. [22] 生态环境部. 水质 挥发性有机物的测定 吹扫捕集/气相色谱-质谱法: HJ 639-2012 [S]. 北京: 中国环境科学出版社, 2012. [23] EDGAR, ROBERT C. UPARSE: highly accurate OTU sequences from microbial amplicon reads.[J]. Nature Methods, 2013, 10(10): 996-998. doi: 10.1038/nmeth.2604 [24] LIU C, ZHAO D, MA W, et al. Denitrifying sulfide removal process on high-salinity wastewaters in the presence of Halomonas sp[J]. Applied microbiology and biotechnology, 2016, 100(3): 1421-1426. doi: 10.1007/s00253-015-7039-6 [25] 黄先锋. 酸性条件下臭氧强化氧化缺电子芳香类和富电子胺类化合物的特性与机理[D]. 博士, 南京大学, 2016. [26] 刘芬, 魏东洋, 许振成, 等. 催化臭氧降解氯苯类化合物的研究进展[J]. 广东化工, 2009, 36(9): 62-63,99. doi: 10.3969/j.issn.1007-1865.2009.09.027 [27] 王冠群. 地下水中1, 2, 4-三氯苯污染的化学修复研究[D]. 硕士, 南京大学, 2015. [28] NING Z, ZHANG M, HE Z, et al. Spatial Pattern of Bacterial Community Diversity Formed in Different Groundwater Field Corresponding to Electron Donors and Acceptors Distributions at a Petroleum-Contaminated Site[J]. Water, 2018, 10(7): 842. doi: 10.3390/w10070842 [29] 李坡. 活化过硫酸盐原位修复苯胺污染地下水研究[D]. 硕士, 常州大学, 2022. [30] US EPA. Calculation and Use of First-Order Rate Constants for Monitored Natural Attenuation Studies[S]. United States Environmental Protection Agency, 2002. [31] 李岩云. 低渗透粘土介质中微生物介导的氯苯降解研究[D]. 武汉:中国地质大学, 2021. [32] DONG W, ZHANG Y, LIN X, et al. Prediction of 1, 2, 4-trichlorobenzene natural attenuation in groundwater at a landfill in Kaifeng, China[J]. Environmental Earth Sciences, 2014, 72(3): 941-948. doi: 10.1007/s12665-014-3386-3 [33] DONG W H, ZHANG P, LIN X Y, et al. Natural attenuation of 1, 2, 4-trichlorobenzene in shallow aquifer at the Luhuagang's landfill site, Kaifeng, China[J]. Science of the Total Environment, 2015, 505: 216-222. doi: 10.1016/j.scitotenv.2014.10.002 [34] WIEDEMEIER T H, SWANSON M A, MOUTOUX D E, et al. Technical Protocol for Evaluating Natural Attenuation of Chlorinated Solvents in Ground Water[S]. United States Environmental Protection Agency, 1998. [35] ZHANG M, GUO C, SHI C, et al. A Quantitative Redox Zonation Model for Developing Natural Attenuation-Based Remediation Strategy in Hydrocarbon-Contaminated Aquifers[J]. Journal of Cleaner Production, 2021, 290(8): 125743. [36] 郑昭贤, 苏小四, 王鼐, 等. 浅层地下水中氯代烷烃生物降解的地下水化学响应规律研究[J]. 地球学报, 2014, 35(2): 230-238. doi: 10.3975/cagsb.2014.02.16 [37] 罗俊鹏, 赵一澍, 廖晓勇, 等. 化学预氧化-生物强化-生物刺激对土壤中菲降解的联合效应[J]. 环境工程学报, 2019, 13(10): 2521-2529. doi: 10.12030/j.cjee.201902033 [38] AULENTA F, MAJONE M, TANDOI V, Enhanced anaerobic bioremediation of chlorinated solvents: environmental factors influencing microbial activity and their relevance under field conditions[J]. Journal of Chemical Technology And Biotechnology, 2006, 81: 1463-1474. [39] SUTTON N B, GROTENHUIS J T C, LANGENHOFF A A M, et al. Efforts to improve coupled in situ chemical oxidation with bioremediation: a review of optimization strategies[J]. Journal of Soils And Sediments, 2011, 11: 129-140. doi: 10.1007/s11368-010-0272-9 [40] 甘平, 樊耀波, 王敏健. 氯苯类化合物的生物降解[J]. 环境科学, 2001(3): 93-96. doi: 10.3321/j.issn:0250-3301.2001.03.020 [41] MULLER T A, WERLEN C, SPAIN J, et al. Evolution of a chlorobenzene degradative pathway among bacteria in a contaminated groundwater mediated by a genomic island in Ralstonia[J]. Environmental Microbiology, 2003, 5(3): 1462-2920. [42] HAIGLER B E, PETTIGREW C A, SPAIN J C. Biodegradation of Mixtures of Substituted Benzenes by Pseudomonas sp. strain JS150[J]. Applied and Environmental Microbiology, 1992, 58(7): 2237-2244. doi: 10.1128/aem.58.7.2237-2244.1992 [43] BALABEN N, YANKELZON I, ADAR E, et al. The spatial distribution of the microbial community in a contaminated aquitard below an industrial zone[J]. Water, 2019, 11(10): 2128. doi: 10.3390/w11102128 [44] ZOHRE K and JIM C S. Biodegradation of Chlorobenzene, 1, 2-Dichlorobenzene, and 1, 4-Dichlorobenzene in the Vadose Zone[J]. Environmental Science & Technology, 2013, 47: 3846-6854. -



 下载:
下载: