-
水体氟污染是全世界广泛关注的环境问题[1]。据统计,全球有近2亿人长期饮用氟化物超标的地下水[2]。氟摄入过量会引起氟斑牙、骨质疏松、脆性骨骼等疾病,对人体健康造成严重危害[3]。世界卫生组织(WHO)要求饮用水中氟化物的质量浓度不高于1.5 mg·L−1,而我国则制定了更为严格的饮用水氟化物标准(≤1.0 mg·L−1)[4]。工业生产是水体氟污染的重要来源之一,其中金属冶炼、化肥、电镀、半导体等行业在生产过程中会产生大量的酸性含氟废水,该类废水的稳定达标处理是相关行业可持续发展的重要保障[5]。
当前常用的污水除氟技术包括:沉淀法[6]、膜分离法[7]、离子交换法[8]、电絮凝法[9]以及吸附法[10]等。其中吸附法简单高效、运行稳定,常用于含氟废水的深度处理[1]。近年来,纳米金属氧化物(nanosized metal oxides, NMOs)因比表面积大、活性位点多、吸附速率快、吸附容量大等特点[11-12],成为吸附除氟领域的研究热点,其中最具代表性的有Al[13]、Mg[14]、La[15]、Ce[16]和Zr[17]等金属氧化物吸附材料。NMOs能够通过表面羟基的配体交换作用与氟离子形成稳定的M–F内核配位结构,实现污水中氟的选择性吸附[18-19]。然而,NMOs在酸性溶液中缺乏稳定性,溶解的金属离子将导致二次污染,这极大限制了NMOs在复杂工业废水处理中的适用性[20-21]。
近期的研究表明,磷酸铈(cerium phosphate, CeP)对重金属离子表现出良好的吸附性能,且在酸性或有机配体共存的溶液中具有优异的化学稳定性[22-23]。氧化铈能通过羟基配体交换、配位络合等作用实现氟的高效吸附[24],而CeP与氧化铈具有类似的Ce–O结构[25],由此推测CeP也能拥有良好的除氟性能。目前,环境领域有关CeP的研究多集中于阳离子污染物的吸附去除,对阴离子吸附行为的研究较少[26-27]。因此,本研究拟采用液相沉淀法制备CeP纳米吸附剂,考察其对酸性废水中氟的吸附特性,探究其理化性质及除氟机制,以期为酸性含氟废水的深度处理提供技术支撑。
-
实验所用硝酸铈铵(Ce(NH4)2(NO3)6)、磷酸、氟化钠、无水乙醇、冰醋酸、氯化钠、柠檬酸钠、硫酸钠、氢氧化钠、硝酸钠均为分析纯,硝酸铈铵购自Sigma-Aldrich公司,其余试剂均购自国药集团有限公司,实验用水为去离子水。离子交换法是污水除氟最常用的技术之一,而水合氧化铈(hydrated cerium oxides, HCO)是目前研究较多的Ce基吸附材料,因此本研究选择阴离子交换树脂D201(浙江争光实业股份有限公司)和HCO(自制)开展对比实验。
-
CeP的制备:将5 g硝酸铈铵溶解于50 mL 10%乙醇的去离子水中,随后将其与200 mL 12 moL·L−1的磷酸溶液混合,反应生成淡黄色的CeP凝胶;室温条件下继续搅拌反应12 h,离心去除上清液得到CeP沉淀,用去离子水冲洗沉淀直到出水pH达中性,然后将其置于105 ℃的鼓风干燥箱内干燥12 h,研磨后制得CeP纳米吸附剂。
HCO的制备[24]:将5 g硝酸铈铵溶解于50 mL含10%乙醇的去离子水中,向溶液中滴加1 mol·L−1的氢氧化钠溶液至pH达12,继续搅拌反应12 h,离心去除上清液得到Ce(OH)4沉淀,用去离子水冲洗沉淀直到出水pH达中性,105 ℃烘干12 h后研磨得到HCO纳米吸附剂。
-
实验中吸附剂用量均为0.50 g·L−1,反应在含有100 mL溶液的锥形瓶中进行,无特殊说明溶液初始氟质量浓度均为10 mg·L−1,pH=3.0,吸附反应温度为298 K,使用浓度为1 mol·L−1的HCl或NaOH调节溶液pH。分别调节不同锥形瓶中溶液的pH至1~12,于298 K条件下恒温振荡24 h,考察pH对CeP除氟性能的影响。分别加入不同浓度的共存离子(SO42−、Cl−、NO3− 和HCO3−),考察CeP对氟的选择性吸附性能。吸附动力学实验中,将0.5 g CeP放入含1 000 mL氟溶液的三口烧瓶中,每隔一段时间取5 mL溶液测定氟离子质量浓度;取吸附平衡后的CeP样品进行FT-IR和XPS分析,研究其吸附机理。等温吸附实验分别在温度为298、303和308 K的条件下开展,并控制溶液氟的初始质量浓度为10~100 mg·L−1。采用1 mol·L−1的NaOH溶液对吸附饱和的CeP进行脱附,考察材料的再生和重复利用性能。
-
氟浓度使用氟离子选择电极(PXS-270,INESA,上海仪电)测量;pH稳定性实验中Ce浓度采用ICP-MS(ICP-Optima 7300 DV,PerkinElmer, USA)测定。采用Nova-3000氮气吸附仪(Quantachrome,USA)测定CeP的比表面积;CeP的颗粒形貌通过扫描电镜SEM(S-4800 II,17 Hitachi,Japan)和透射电子显微镜TEM(Tecnai 12,Philips,Netherlands)测定;使用多晶X射线衍射仪XRD(D8 Advance,Bruker-AXS,Germany)测定CeP的晶体形态,采用傅里叶变换红外光谱FT-IR(Cary 5000, Varia, USA)分析CeP吸附前后表面化学基团的变化,利用X射线光电子能谱(ESCALAB250Xi,ThermoFisher,USA)分析CeP吸附前后的能谱变化。
-
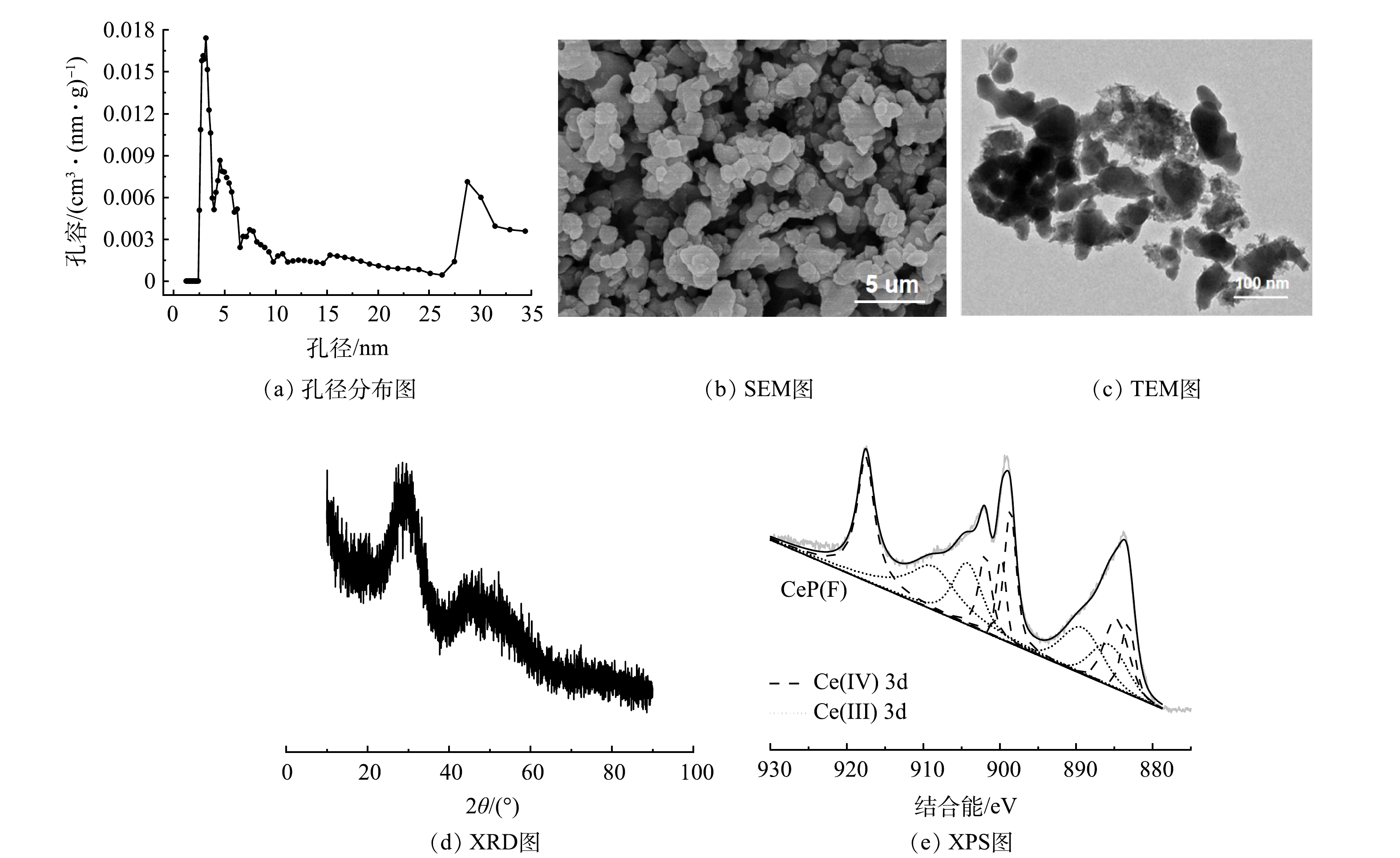
制得的CeP纳米吸附剂比表面积为95.48 m2·g−1,且孔径在微孔和中孔区域呈现双峰分布(图1(a))。由SEM(图1(b))和TEM(图1(c))表征结果可知,CeP以不规则纳米片的形式存在,粒径约为50~100 nm。CeP的XRD衍射图(图1(d))中没有出现明显的衍射峰,说明CeP主要为无定形形态[28]。样品Ce3d的XPS高分辨能谱(图1(e))可分解为10个特征峰,分别为Ce(IV)和Ce(III),表明制得的CeP中铈元素以Ce(IV)和Ce(III)的混合价态存在,这可能与制备过程中CeP表面产生了部分氧空位有关[29]。
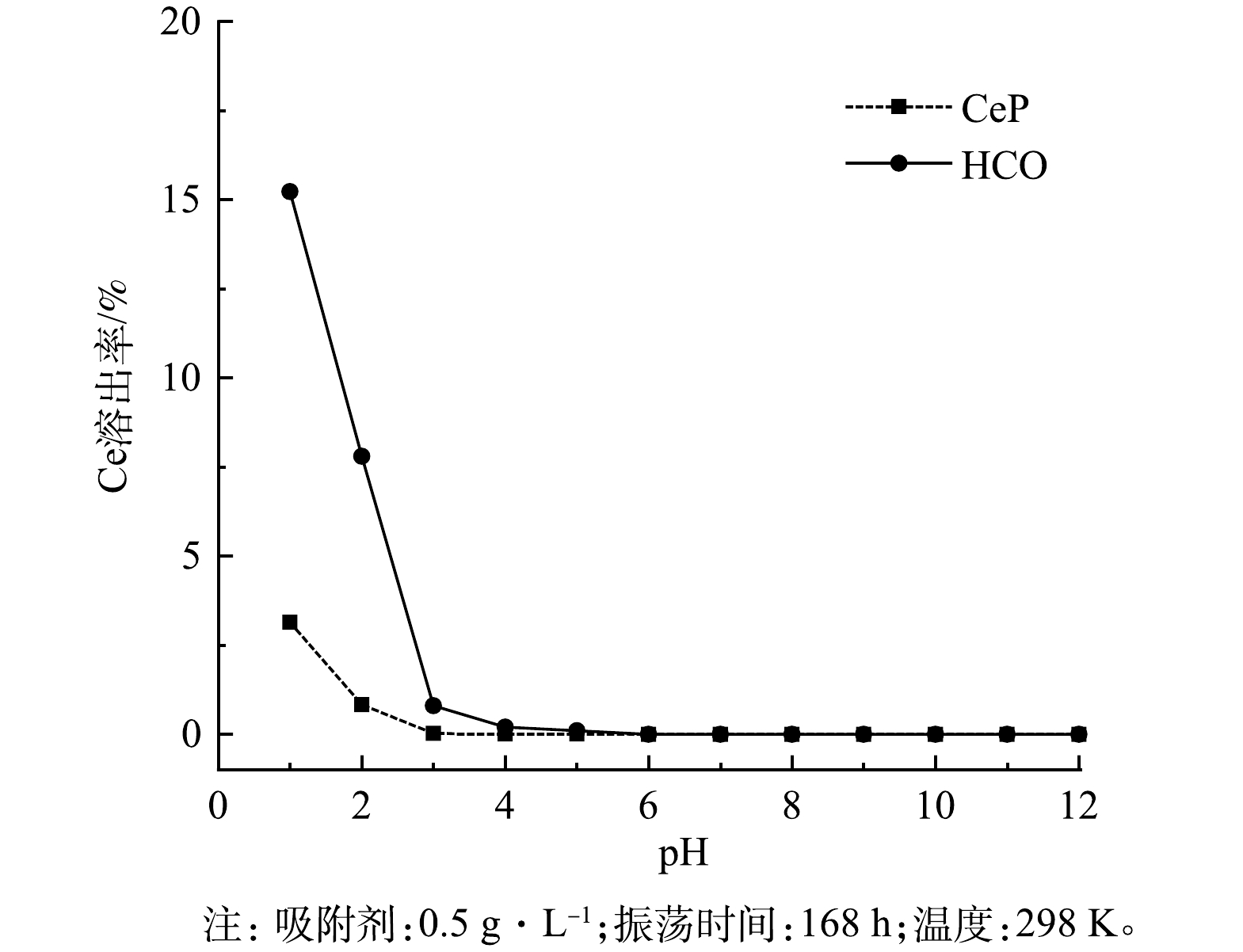
为进一步考察CeP在酸碱废水中长期使用的稳定性,本研究测定了CeP在溶液pH=1~12条件下Ce的溶出率,并与HCO进行对比分析。由图2可知,当溶液pH≥5.0时,CeP与HCO均未检测到有Ce溶出;当pH=3.0时,CeP仍未检测到Ce的溶出,而HCO中Ce溶出率上升至0.8%;进一步降低pH到1.0时,CeP的Ce溶出率仅为3.14%,而HCO的Ce溶出率则高达15.23%。上述实验结果表明,CeP在酸性溶液中的稳定性明显优于HCO,可在酸性废水处理中长期稳定使用。
-
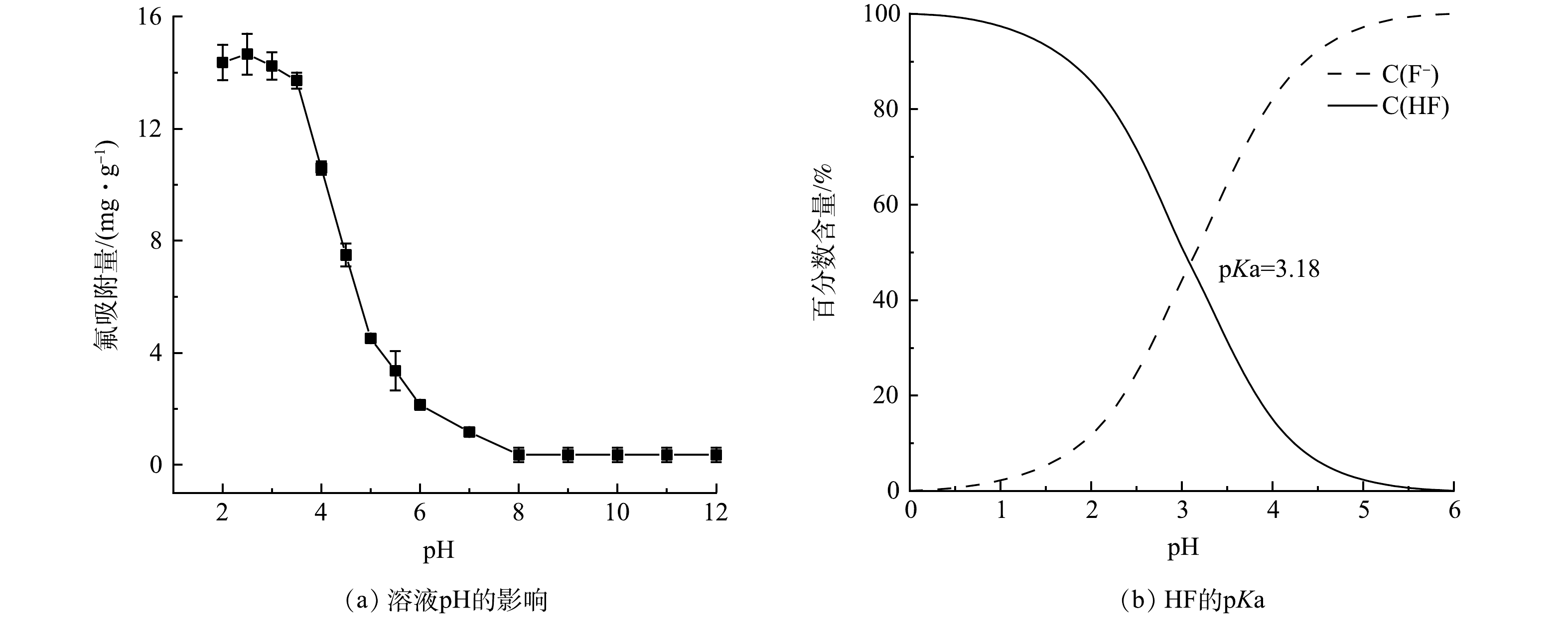
溶液pH不仅会改变CeP的荷电状态,还会影响氟化物在溶液中的赋存形态,从而对CeP的除氟性能产生显著影响。由图3(a)可知,CeP在酸性条件下(pH≤4)具有较好的除氟性能,当pH=2.5时,其除氟吸附量达到最大(14.67 mg·g−1)。随着溶液pH的升高,CeP的除氟性能开始急剧下降,当pH>8.0时其吸附量接近于零。上述现象与不同溶液pH条件下CeP的荷电状态以及氟化物的赋存形态有关(图3(b))[30]。具体而言,酸性条件下CeP因质子化而带正电,这有利于CeP对负电性氟离子的吸附(式(1)~(2));随着溶液pH升高至4以上,CeP对氟的吸附性能开始快速下降,这主要是由于CeP去质子化后表面带负电,对F−产生静电排斥效应[31]。当溶液pH进一步升高至碱性范围,CeP对氟的吸附量降至接近于零。这主要由于pH升高后溶液中OH−浓度增加,与F−竞争CeP的表面吸附位点(式(3))。值得注意的是,当pH低于3.18时溶液中氟主要以HF的形式存在,而此时CeP对氟仍然具有较高的吸附容量,说明CeP和HF之间存在较强的内配位络合作用(式(4))[32]。综合考虑材料的稳定性和除氟性能,后续实验均在pH=3.0的条件下开展。
-
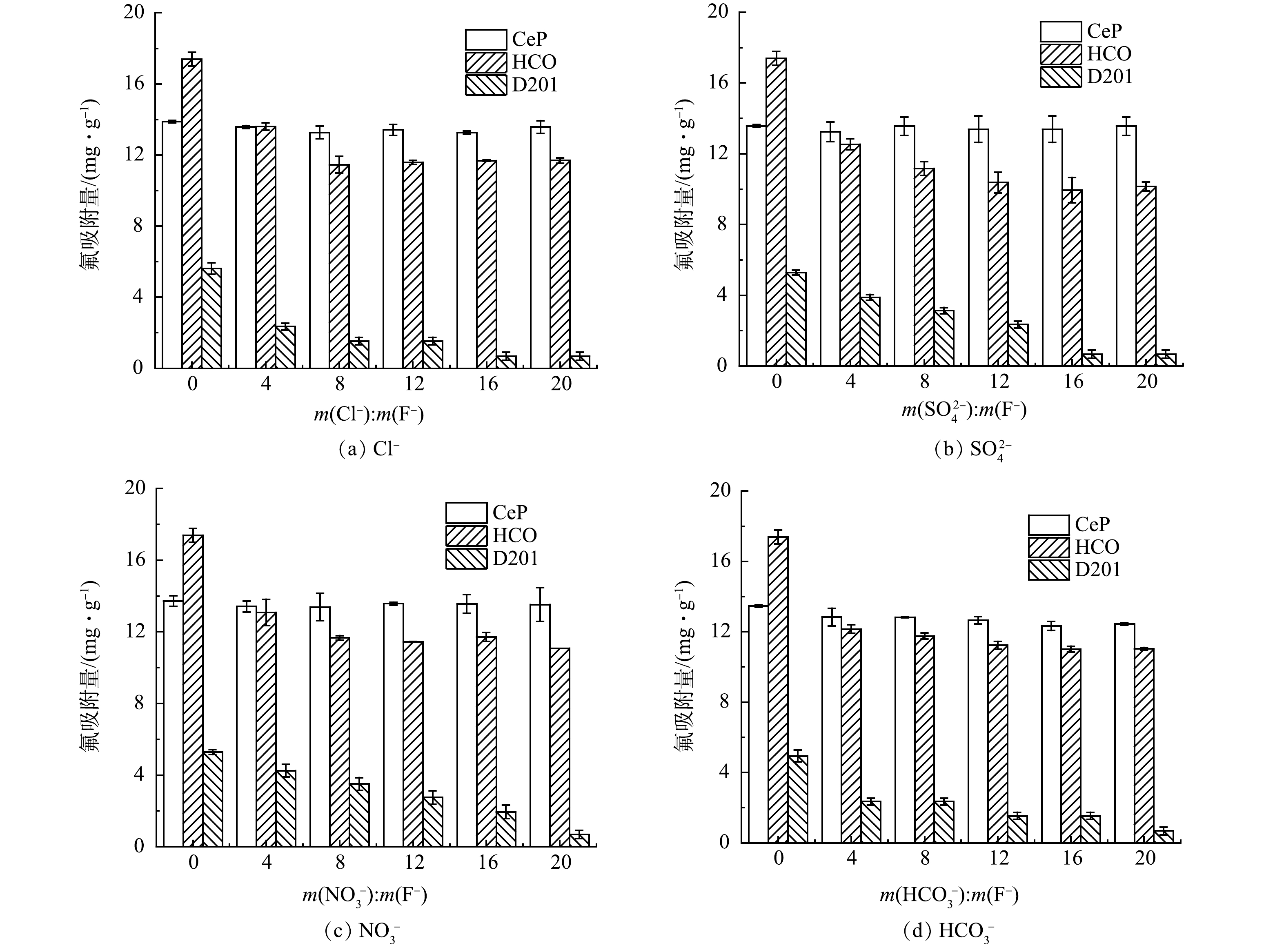
真实含氟废水水质较为复杂,除氟化物外一般还含有一定量的共存离子。吸附过程中共存离子会与氟竞争吸附位点,从而影响吸附剂的除氟性能[33]。本研究选择废水中普遍存在的SO42−、Cl−、NO3− 和HCO3−作为代表性离子,考察不同浓度共存离子对CeP除氟性能的影响,并选择HCO和D201进行对比研究。如图4所示,CeP的除氟性能几乎不受共存离子的影响。表明CeP对氟的吸附具有良好的选择性,这是由于氟离子可以提供共用电子对与CeP形成较强的内配位络合物,从而实现对氟的特异性吸附[34]。当HCO3−与F−摩尔比大于16后CeP除氟性能出现小幅下降,这与HCO3−具有一定的碱性缓冲作用有关。HCO的除氟性能在共存离子与氟的摩尔比升高至4时出现一定程度的下降,继续增加共存离子浓度HCO的除氟性能保持基本稳定,说明HCO对氟的吸附也具有一定的选择性。与CeP和HCO形成鲜明对比的是,D201的除氟性能随着共存离子浓度的升高显著下降。这是由于D201仅能通过静电吸引或离子交换作用除氟,而高浓度共存离子会对氟的吸附产生强烈的竞争。
-
吸附动力学实验结果如图5所示。可见,CeP在前10 min对氟的吸附速率较快,吸附量呈线性上升,随后吸附速率逐渐下降并在90 min达到吸附平衡。前10 min较快的吸附速度是由于CeP具有较高的比表面积,在溶液中能够暴露氟的吸附的活性位点;当CeP表面的活性位点被氟占据后,溶液中游离态的氟必须扩散到CeP内部才能够被吸附,导致10 min后吸附速率逐渐减缓直至达到吸附平衡。分别采用伪一阶动力学(式(5))和伪二阶动力学(式(6))模型对吸附数据进行拟合[28],结果如表1所示。可以看出,伪二阶动力学模型能更好的拟合实验数据,且拟合所得平衡吸附量更接近实验结果。
式中:qt和qe分别为t时刻和平衡时的氟吸附量,mg·g−1;k1为伪一阶动力学反应速率常数,min−1;k2为伪二阶动力学反应速率常数,g·(mg·min) -1。
-
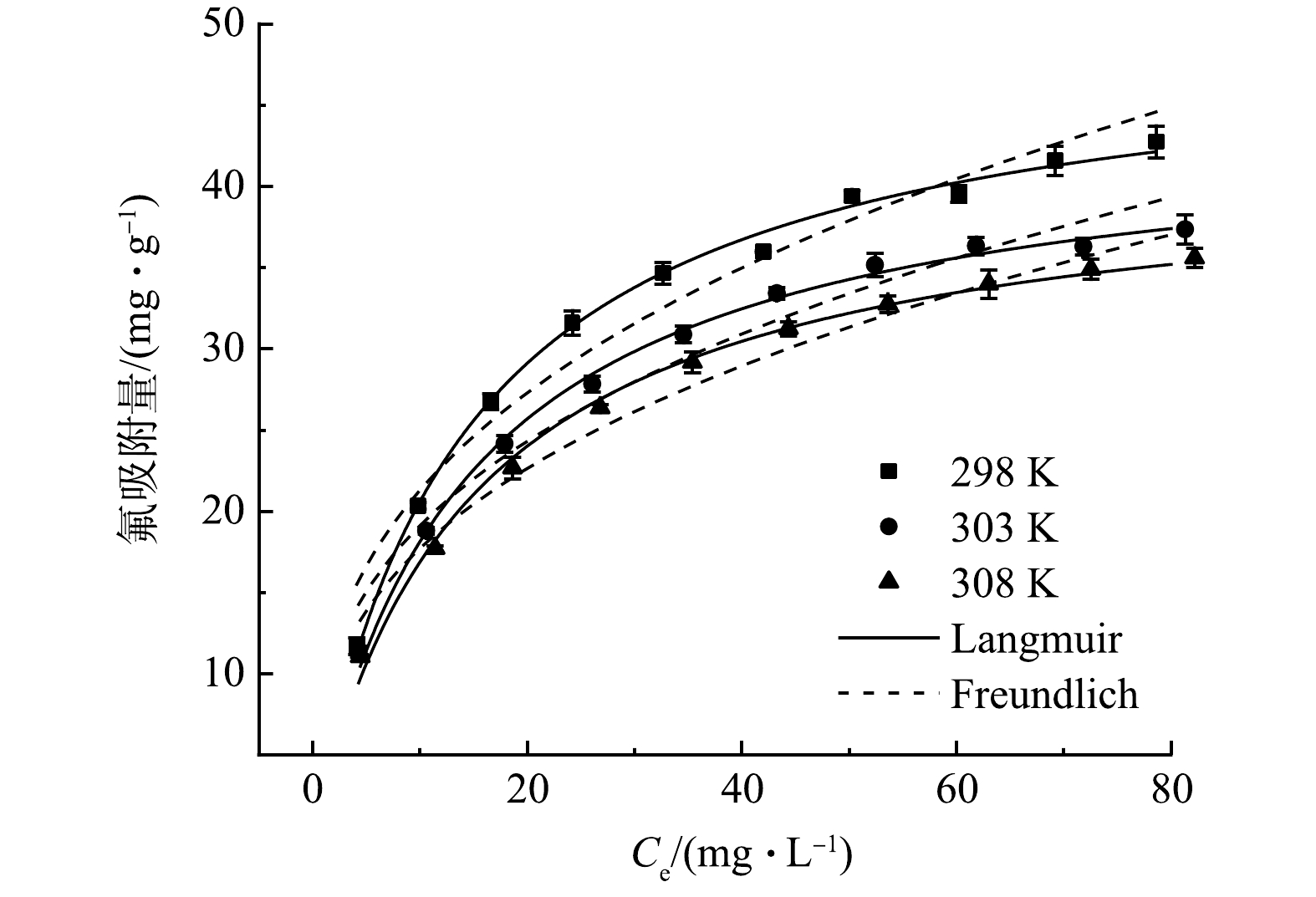
温度也是影响吸附性能的重要因素之一。本研究分别考察了298、303和308 K下CeP的除氟性能,结果如图6所示。分别采用Langmuir(式(7))和Freundlich(式(8))等温吸附模型对实验数据进行拟合[35],拟合结果如表2所示。结果表明,CeP对氟的等温吸附过程更符合Langmuir模型,所得R2值均高于0.99,且在298 K条件下拟合所得最大吸附量为49.72 mg·g−1。表3对比了不同吸附材料对氟的吸附性能,可以看出CeP的除氟性能明显优于其他材料。
式中:Ce为吸附平衡时氟化物的质量浓度,mg·L−1;qe为平衡吸附量,mg·g−1;qmax为最大吸附量,mg·g−1;KL为Langmuir模型的平衡常数,L·mg−1;KF为Freundlich模型的平衡常数,mg·g−1;
$ \dfrac{1}{n} $ 为异质性因子。为进一步阐明CeP对氟的吸附热力学机制,根据式(9)和式(10)计算CeP吸附氟过程中的自由能变化(ΔG)、焓变(ΔH)和熵变(ΔS)[38],热力学平衡常数可根据式(11)计算。
式中:R为气体常数,为8.314 J·(mol·K)−1;T为温度,K;K为热力学平衡常数。
式中:ρ为水的密度,为1 000 g·L−1。
假设在所研究的范围内温度恒定,且忽略∆H随温度变化的影响[43]。CeP吸附氟的热力学计算结果如表4所示。吸附过程中ΔG<0表明吸附过程为自发性的;ΔH<0表示吸附过程是放热反应,低温有利于CeP对氟的吸附。此外,焓变的负值较大,说明焓变是自发反应的主要原因,其对吸附过程的影响远远大于熵变的影响[44]。
-
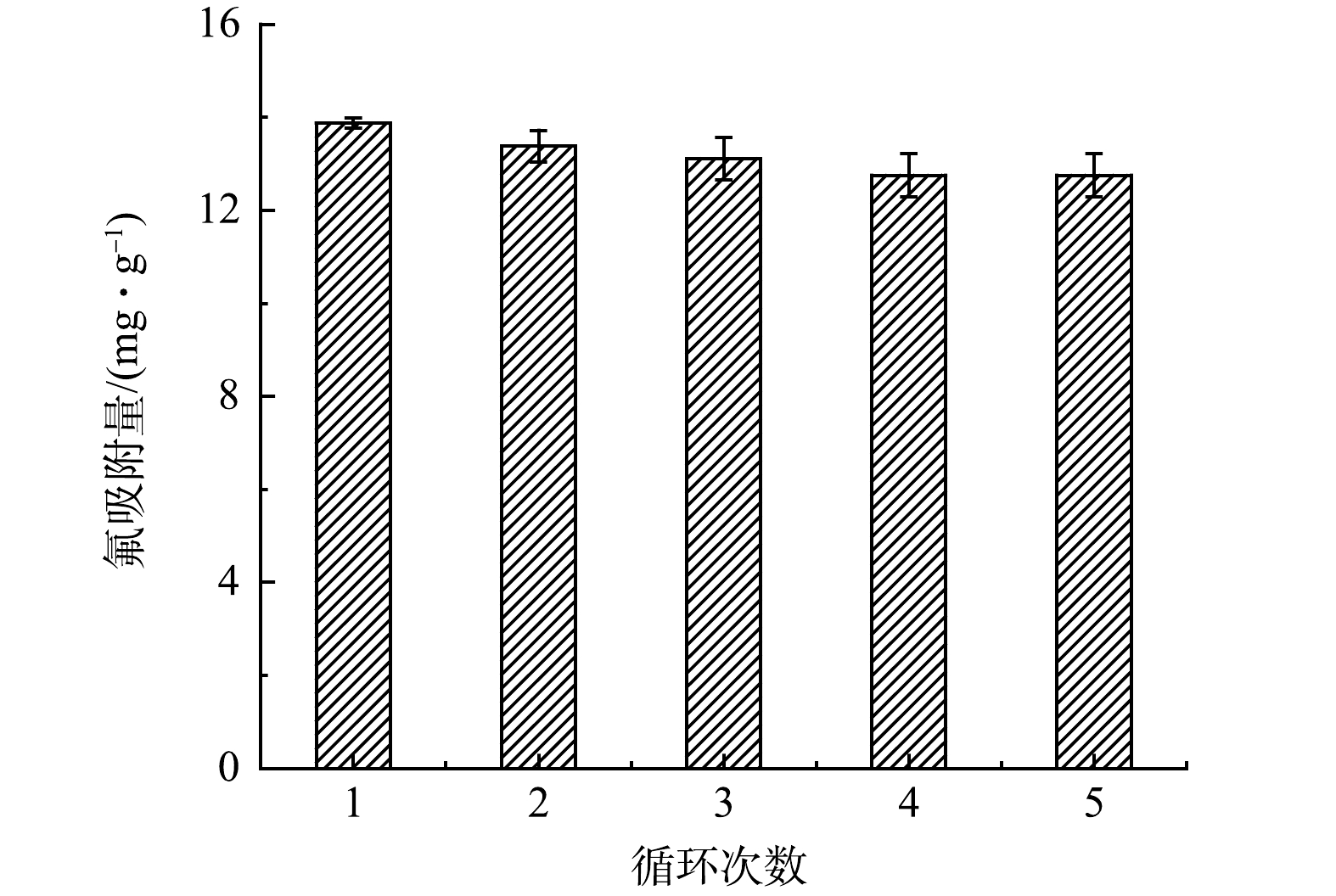
吸附-再生循环实验有助于考察CeP在含氟废水处理中的实际应用潜力。由pH影响实验可知,CeP在碱性条件下对氟几乎完全丧失吸附能力,因此,本研究选择1 mol·L−1的NaOH为脱附剂对吸附饱和的CeP进行脱附再生,并在脱附后依次采用5%的NaCl溶液和去离子水对CeP进行漂洗,以消除残留NaOH对下一批次吸附实验的影响。如图7所示,经过连续5次吸附-再生循环后,CeP仍可保留初始吸附量的90%。表明CeP具有良好的再生性能和稳定性,可循环用于含氟废水的处理。
-
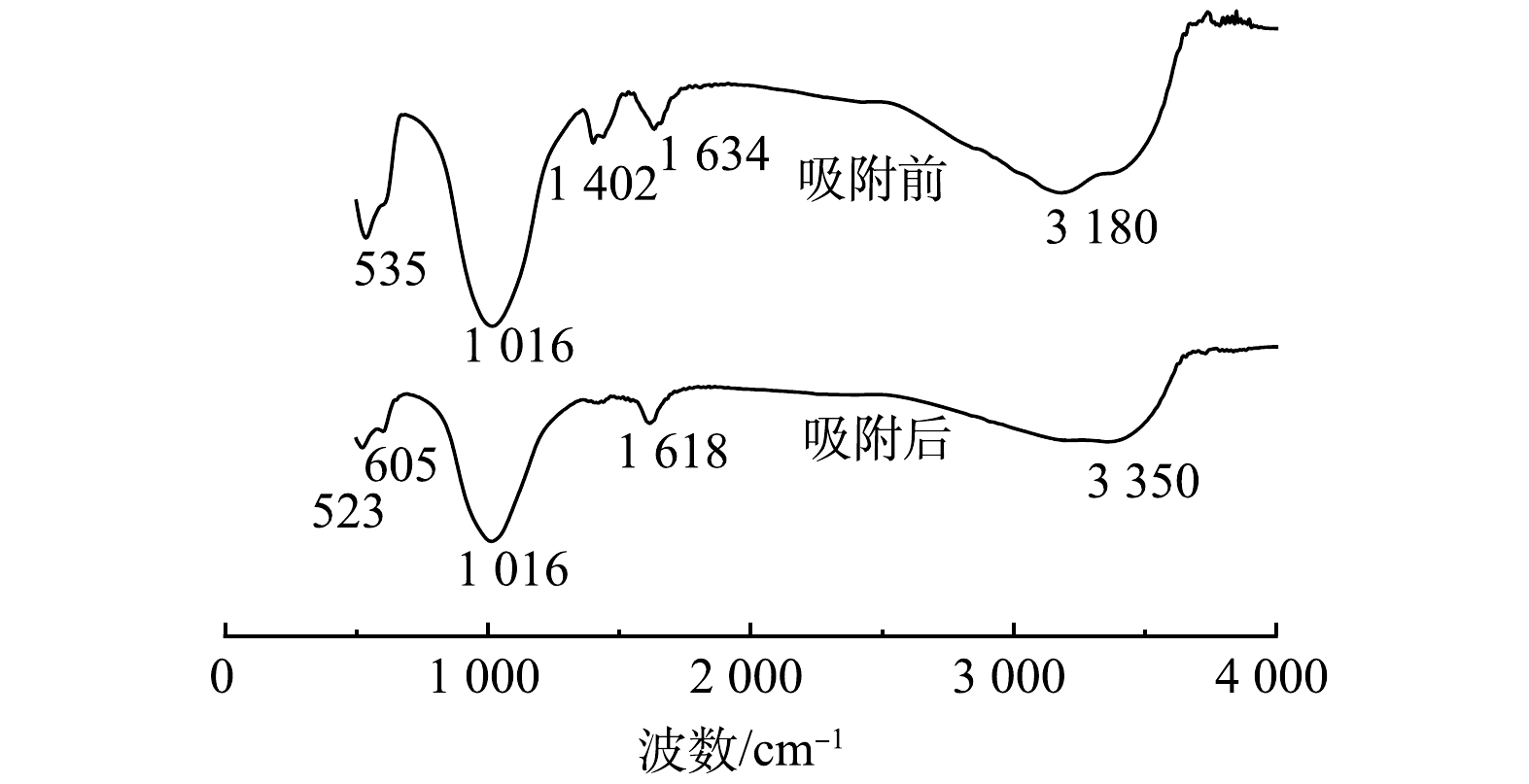
1) FTIR光谱分析。CeP吸附氟前后的红外光谱如图8所示,3 150~3 400 cm−1处的吸收带以及1 630 cm−1处的吸收峰归属于CeP结合水中O—H键的伸缩与弯曲振动峰[43-45];1 016 cm−1处的强吸收峰归因于PO43-中P—O键的对称伸缩振动[21];在1 402 cm−1处的特征吸收峰对应Ce—OH的弯曲振动峰,吸附后Ce—OH吸收峰强度显著降低,这是由于CeP表面羟基被吸附的氟取代所致[25]。吸附前CeP在535 cm−1处的吸收峰主要源自Ce—O的伸缩振动[21],吸附后吸收峰强度明显降低并发生一定程度的蓝移(523 cm−1),同时在605 cm−1处出现一个新的吸收峰,说明可能形成了Ce—F配合物[46]。综上所述,CeP对氟的吸附机制包括表面羟基与氟离子的配体交换作用,这种吸附作用符合路易斯酸碱配位理论(金属配位),相比于单纯的离子交换或者静电吸附,配体交换存在较强的选择性[47]。通过XPS能谱的表征分析,可以进一步阐明其吸附机制。
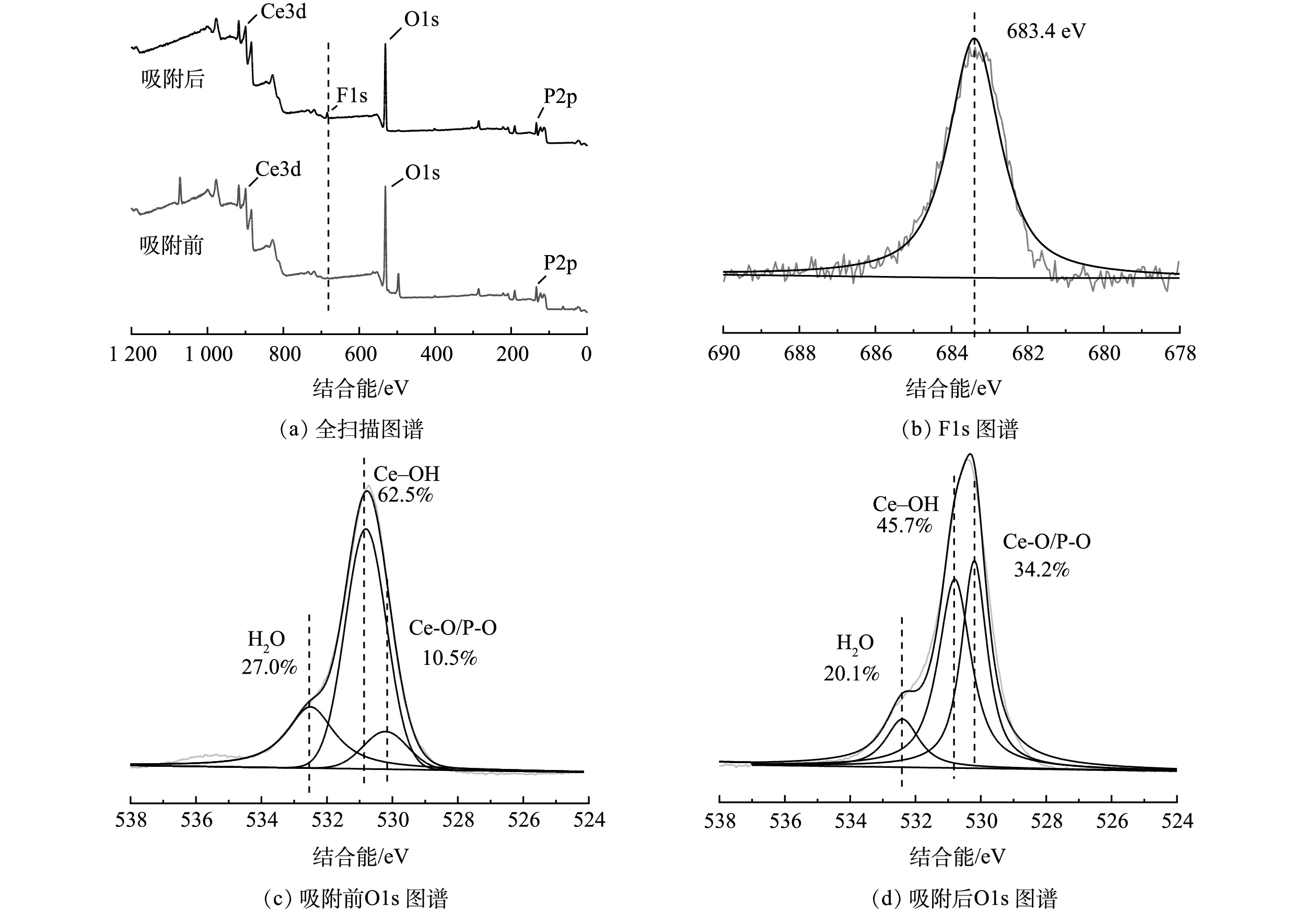
2) XPS能谱分析。CeP吸附氟前后的XPS能谱如图9所示,吸附后在683.4 eV处出现了1个新的F1s峰(图9(a)~(b)),表明氟已被CeP成功吸附;该峰与氟化钠的标准F1s峰(684.9 eV)相比向低结合能方向偏移了1.5 eV,表明CeP和氟之间存在较强的吸附亲和力[48]。吸附前,O1s能谱可分解为532.5 eV(结合水)、530.8 eV(Ce—OH)和530.2 eV(Ce—O/P—O)3个特征峰[49],吸附后Ce—OH的峰面积占比由62.5%下降到45.7%(图9 (c)~(d)),表明CeP的表面羟基(—OH)通过配体交换被氟离子取代,这与FT-IR分析结果一致。
-
1)通过液相沉淀法制备纳米磷酸铈吸附剂(CeP),其比表面积为95.48 m2·g−1,主要以50~100 nm纳米片的形式存在,在酸碱溶液中具有良好的化学稳定性。
2)溶液pH对CeP的除氟性能有较大的影响,当pH=2.5时,CeP的吸附量达到最大;CeP对氟的吸附属于自发放热反应,当温度为298 K时CeP的最大拟合吸附量可达49.72 mg·g−1。
3) CeP的除氟机制主要包括静电吸引、配体交换和内配位络合等作用,其中内配位络合具有较强的特异性,有助于CeP在高浓度竞争离子存在下对氟的选择性吸附。
4)采用NaOH溶液可以实现吸附饱和CeP的高效再生,再生后其除氟性能没有明显下降,在酸性含氟废水的深度处理领域具有良好的应用潜力。
纳米磷酸铈的制备及其对酸性废水中氟的吸附性能
Preparation of nano-cerium phosphate and its adsorption properties for fluoride in acidic wastewater
-
摘要: 采用液相沉淀法制备了磷酸铈纳米吸附剂(CeP),并研究了CeP对酸性废水中氟化物的去除特性。结果表明,与纳米水合氧化铈(HCO)相比,CeP在酸性条件下具有更强的稳定性。溶液pH对CeP的除氟性能有较大影响,酸性条件更有利于CeP对氟的吸附,在pH=2~3时其除氟吸附量达到最大值。基于CeP与氟之间的静电吸引、配体交换和内配位络合等作用,CeP对氟表现出优异的吸附选择性。CeP对氟的吸附符合伪二阶吸附动力学模型,Langmuir模型能较好地描述等温吸附过程,298 K条件下由Langmuir模型拟合所得最大吸附量为49.72 mg·g−1,热力学计算结果表明CeP对氟的吸附属于自发放热过程。吸附饱和的CeP可采用NaOH溶液进行高效再生,再生后CeP的除氟性能没有明显下降,可长期重复使用,在酸性含氟废水处理领域具有良好的应有潜力。Abstract: In this study, the cerium phosphate (CeP) nano adsorbent was synthesized by liquid-phase precipitation method for efficient fluoride removal from acidic wastewater. The results showed that CeP exhibited better chemical stability under acidic conditions compared with nano hydrated cerium oxides (HCO). Fluoride uptake onto CeP was a pH-dependent process and acidic conditions were more conducive to its adsorption towards fluoride, which could reach the maximum capacity at pH 2~3. Based on the effects of electrostatic attraction, ligand exchange and inner-sphere complexation, CeP showed a conspicuous adsorption affinity towards fluoride. The adsorption of fluoride onto CeP followed pseudo-second-order kinetic model, and the Langmuir model could better describe the adsorption isotherm process than other thermodynamic models. The maximum adsorption capacity of CeP towards fluoride calculated by the Langmuir model was 49.72 mg·g−1, and the thermodynamic calculation results demonstrated the adsorption of fluoride was a spontaneous exothermic process. The exhausted CeP could be readily regenerated using NaOH solution, the fluoride removal performance of the regenerated CeP decreased slightly, and could be adapted for a long-term reutilization. The above results verified that CeP is an efficient adsorbent for fluoride removal from acid wastewater.
-
Key words:
- nano-cerium phosphate /
- acid wastewater /
- fluoride /
- preferential adsorption
-

-
表 1 CeP对氟的吸附动力学参数
Table 1. Adsorption kinetic parameters of CeP for fluoride
伪一阶动力学 伪二阶动力学 实验结果
qm/(mg·g−1)k1/min−1 qe/( mg·g−1) R2 k2/(g·(mg·min)−1) qe/( mg·g−1) R2 0.734 13.416 0.902 0.113 13.600 0.988 13.80 表 2 CeP对氟的等温吸附参数
Table 2. Adsorption isotherm parameters of CeP for fluoride
温度/K Langmuir 模型 Freundlich 模型 qm/(mg·g−1) KL/(L·mg−1) R2 KF/(mg·g−1) 1/n R2 298 49.720 0.070 5 0.996 6 9.308 0 0.358 6 0.962 4 303 44.105 0.069 6 0.997 4 8.451 7 0.351 2 0.959 7 308 41.673 0.067 9 0.991 8 7.942 5 0.350 9 0.976 8 表 3 不同吸附材料对氟吸附性能的对比
Table 3. Comparison of fluoride adsorption properties by different adsorbents
表 4 CeP对氟的吸附热力学参数
Table 4. Thermodynamic parameters of fluoride adsorption by CeP
初始氟质量浓度 /(mg·L−1) ∆H/(kJ·mol−1) ∆S /(J·(K·mol)−1) ∆G/(kJ·mol−1) 298 K 303 K 308 K 20 −21.868 −9.947 −18.908 −18.846 −18.809 40 −21.723 −13.401 −17.776 −17.566 −17.645 90 −17.041 −4.150 −15.853 −15.682 −15.815 -
[1] BHATNAGAR A, KUMAR E, SILLANPää M. Fluoride removal from water by adsorption: A review[J]. Chemical Engineering Journal, 2011171(3): 811-840. doi: 10.1016/j.cej.2011.05.028 [2] YANG W, TIAN S, TANG Q, et al. Fungus hyphae-supported alumina: An efficient and reclaimable adsorbent for fluoride removal from water[J]. Journal of Colloid and Interface Science, 2017496: 496-504. doi: 10.1016/j.jcis.2017.02.015 [3] BISWAS G, KUMARI M, ADHIKARI K, et al. A critical review on occurrence of fluoride and its removal through adsorption with an emphasis on natural minerals[J]. Current Pollution Reports, 20173(2): 104-119. doi: 10.1007/s40726-017-0054-8 [4] ROZOV I. New WHO (World Health Organization) guidelines for drinking water quality[D]. World Health Organization1995. [5] KU Y, CHIOU H. The Adsorption of fluoride ion from aqueous solution by activated alumina[J]. WaterAirSoil Pollution, 2004133(4): 349-361. [6] JIANG K, ZHOU K, YANG Y, et al. A pilot-scale study of cryolite precipitation from high fluoride-containing wastewater in a reaction-separation integrated reactor[J]. Journal of Environmental Sciences, 201325(7): 1331-1337. doi: 10.1016/S1001-0742(12)60204-6 [7] QIANG L, WANG B, LI W, et al. Performance evaluation of magnetic anion exchange resin in removing of fluoride[J]. Journal of Chemical Technology and Biotechnology, 201591(6): 1747-1754. [8] BOUBAKRI A, BOUCHRIT R, HAFIANE A, et al. Fluoride removal from aqueous solution by direct contact membrane distillation: Theoretical and experimental studies[J]. Environmental Science and Pollution Research International, 201421(17): 10493-10503. doi: 10.1007/s11356-014-2858-z [9] ARAHMAN N, MULYATI S, LUBIS M, et al. The removal of fluoride from water based on applied current and membrane types in electrodialyis[J]. Journal of Fluorine Chemistry, 2016191(5): 97-102. [10] PAN B, XU J, WU B, et al. Enhanced removal of fluoride by polystyrene anion exchanger supported hydrous zirconium oxide nanoparticles[J]. Environmental Science and Technology, 201347(16): 9347-9354. doi: 10.1021/es401710q [11] WANG J, WU L, LI J, et al. Simultaneous and efficient removal of fluoride and phosphate by Fe-La composite: Adsorption kinetics and mechanism[J]. Journal of Alloys and Compounds, 2018753(5): 422-432. [12] DISHA K, SOMNATH M. A review of metal oxide nanomaterials for fluoride decontamination from water environment[J]. Materials Today: Proceedings, 201918(7): 1146-1155. [13] 赵晓钰, 宋兆阳, 乔庆东, 等. 吸附除氟氧化铝改性研究进展[J]. 当代化工, 202251(9): 2166-2170. [14] BARTLET J, CHAPMAN R. The removal of fluoride ion from aqueous solution by magnesium oxide[J]. Canadian Journal of Chemistry, 201133(10): 1629-1630. [15] RAO C, KARTHIKEYAN J. Removal of fluoride from water by adsorption onto lanthanum oxide[J]. WATER AIR AND SOIL POLLUTION, 2012223(3): 1101-1114. doi: 10.1007/s11270-011-0928-0 [16] XU Y, NING A, ZHAO J. Preparation and defluorination performance of activated cerium (IV) oxide/SiMCM-41 adsorbent in water[J]. Journal of Colloid and Interface Science, 2001235(1): 66-69. doi: 10.1006/jcis.2000.7344 [17] DOU X, MOHAN, PITTMAN C, et al. Remediating fluoride from water using hydrous zirconium oxide[J]. Chemical Engineering Journal, 2012198(8): 236-245. [18] ELHALIL A, QOURZAL S, MAHJOUBI F, et al. Defluoridation of groundwater by calcined Mg/Al layered double hydroxide[J]. Emerging Contaminants, 20162(1): 42-48. doi: 10.1016/j.emcon.2016.03.002 [19] 田追, 张震, 卢嫚, 等. 新型除氟吸附材料的研究进展[J]. 化工进展, 202241(6): 3051-3062. [20] GUO H, LI W, CHANG Z, et al. Mechanism study of fluoride adsorption by hydrous metal oxides[J]. Spectroscopy and Spectral Analysis, 201131(8): 2210-2214. [21] ZHANG Q, LI Y, PHANLAVONG P, et al. Highly efficient and rapid fluoride scavenger using an acid/base tolerant zirconium phosphate nanoflake: Behavior and mechanism[J]. Journal of Cleaner Production, 2017161: 317-326. doi: 10.1016/j.jclepro.2017.05.120 [22] VARSHNEY K G, RAFIQUEE M Z, SOMYA A. Effect of surfactants on the adsorption behaviour of cerium (IV) phosphatecation exchanger for alkaline earths and heavy metal ions[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2007301(1/2/3): 69-72. [23] VARSHNEY K G, RAFIQUEE M Z, SOMYA A. Triton X-100 based cerium (IV) phosphate as a new Hg (II) selectivesurfactant based fibrous ion exchanger: Synthesischaracterization and adsorption behaviour[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2008317(1-3): 400-405. [24] KANG D, YU X, GE M. Morphology-dependent properties and adsorption performance of CeO2 for fluoride removal[J]. Chemical Engineering Journal, 2017330: 36-43. doi: 10.1016/j.cej.2017.07.140 [25] SHAKSHOOKI S K, EL-AKARI F A, EL-FITURI S M, et al. Fibrous cerium (IV) hydrogen phosphate membrane self-supported benzimidazole polymerization agent[J]. Advanced Materials Research, 2014856(5): 3-8. [26] VINISHA V P, CHATHOTH J. Polyaniline modified tin cerium phosphate composite cation exchanger: an eco-friendly adsorbent for lead and bismuth metal ions[J]. Astronomy and astrophysics, 2015409(1): 275-286. [27] SAHU S, BISHOYI N, PATEL R K. Cerium phosphate polypyrrole flower like nanocomposite: a recyclable adsorbent for removal of Cr (VI) by adsorption combined with in-situ chemical reduction[J]. Journal of Industrial and Engineering Chemistry, 202199(1): 117-130. [28] YANG W, SHI X, WANG J, et al. Fabrication of a novel bifunctional nanocomposite with improved selectivity for simultaneous nitrate and phosphate removal from water[J]. ACS ACS Applied Materials & Interfaces, 201911(38): 35277-35285. [29] WU X, ZHANG Y, DOU X, et al. Fluoride adsorption on an Fe–Al–Ce trimetal hydrous oxide: Characterization of adsorption sites and adsorbed fluorine complex species[J]. Chemical Engineering Journal, 2013223(3): 364-370. [30] 许乃才, 史丹丹. 介孔氧化铝纳米材料的制备及除氟性能研究[J]. 应用化工, 202352(2): 464-470. doi: 10.3969/j.issn.1671-3206.2023.02.027 [31] ESKANDARPOUR A, ONYANGO M S, OCHIENG A, et al. Removal of fluoride ions from aqueous solution at low pH using schwertmannite[J]. Journal of Hazardous Materials, 2008152(2): 571-579. doi: 10.1016/j.jhazmat.2007.07.020 [32] 史新星. 聚合物基水合氧化铈复合纳米材料对废水中磷和氟的深度去除[D]. 扬州: 扬州大学2021. [33] YANG W, SHI X, DONG H, et al. Fabrication of a reusable polymer-based cerium hydroxide nanocomposite with high stability for preferable phosphate removal[J]. Chemical Engineering Journal, 2021405(7): 1249-1266. [34] ZHANG Y, QIAN Y, LI W, et al. Fluoride uptake by three lanthanum based nanomaterials: Behavior and mechanism dependent upon lanthanum species[J]. Science of the Total Environment, 2019683: 609-616. doi: 10.1016/j.scitotenv.2019.05.185 [35] ZHANG Q, DU Q, HUA M, et al. Sorption enhancement of lead ions from water by surface charged polystyrene-supported nano-zirconium oxide composites[J]. Environmental Science and Technology, 201347(12): 6536-6544. doi: 10.1021/es400919t [36] SUNDARAM C S, VISWANATHAN N, MEENAKSHI S. Defluoridation chemistry of synthetic hydroxyapatite at nano scale: equilibrium and kinetic studies[J]. Journal of Hazardous Materials, 2008155(1/2): 206-215. [37] SWAIN S K, PATNAIK T, SINGH V K, et al. Kineticsequilibrium and thermodynamic aspects of removal of fluoride from drinking water using meso-structured zirconium phosphate[J]. Chemical Engineering Journal, 2011171(3): 1218-1226. doi: 10.1016/j.cej.2011.05.030 [38] DASH S S, SAHU M K, SAHU E, et al. Fluoride removal from aqueous solutions using cerium loaded mesoporous zirconium phosphate[J]. New Journal of Chemistry, 201539(9): 7300-7308. doi: 10.1039/C5NJ01030F [39] TENG S, WANG S, GONG W, et al. Removal of fluoride by hydrous manganese oxide-coated alumina: performance and mechanism[J]. Journal of Hazardous Materials, 2009168(2/3): 1004-1011. [40] ZHANG Y, LIN X, ZHOU Q, et al. Fluoride adsorption from aqueous solution by magnetic core-shell Fe3O4@alginate-La particles fabricated via electro-coextrusion[J]. Applied Surface Science, 2016389: 34-45. doi: 10.1016/j.apsusc.2016.07.087 [41] GOSWAMI A, PURKAIT M K. The defluoridation of water by acidic alumina[J]. Chemical Engineering Research and Design, 201290(12): 2316-2324. doi: 10.1016/j.cherd.2012.05.002 [42] KUMAR E, BHATNAGAR A, JI M, et al. Defluoridation from aqueous solutions by granular ferric hydroxide (GFH) [J]. Water Research, 200943(2): 490-498. doi: 10.1016/j.watres.2008.10.031 [43] XU L, CHEN G, PENG C, et al. Adsorptive removal of fluoride from drinking water using porous starch loaded with common metal ions[J]. Carbohydrate Polymers, 2017160(15): 82-89. [44] MONDAL N K, BHAUMIK R, DATTA J K. Removal of fluoride by aluminum impregnated coconut fiber from synthetic fluoride solution and natural water[J]. Alexandria Engineering Journal, 201554(4): 1273-1284. doi: 10.1016/j.aej.2015.08.006 [45] MARKO C, HUGUES K P, MADHUMITA B, et al. Hydrous CeO2-Fe3O4 decorated polyaniline fibers nanocomposite for effective defluoridation of drinking water[J]. Journal of Colloid and Interface Science, 2018532(7): 500-516. [46] ZHAO Y, LI X, LIU L, et al. Fluoride removal by Fe (III) -loaded ligand exchange cotton cellulose adsorbent from drinking water[J]. Carbohydrate Polymers, 200878(72): 144-151. [47] NEHRA S, RAGHAV S, KUMAR D. Biomaterial functionalized cerium nanocomposite for removal of fluoride using central composite design optimization study[J]. Environmental Pollution, 2020258: 113773-113780. doi: 10.1016/j.envpol.2019.113773 [48] DENG S, LIU H, ZHOU W, et al. Mn-Ce oxide as a high-capacity adsorbent for fluoride removal from water[J]. Journal of Hazardous Materials, 2011186(2): 1360-1366. [49] WANG X, PAN S, ZHANG M, et al. Modified hydrous zirconium oxide/PAN nanofibers for efficient defluoridation from groundwater[J]. Science of the Total Environment, 2019685(8): 401-410. -



 下载:
下载: