-
石油炼化过程包含一系列分离、纯化操作,消耗大量水资源的同时也产生大量的废水[1-3]。据估计每处理1 t原油产生废水量为0.4~1.6 t,废水中含有大量的石油类、挥发酚、重金属等有毒、难降解物质,因此,炼化废水也被称为典型的工业危险废物[4-5]。未经处理直接排放不仅会污染环境,还会降低产品回收率,错失水资源回收利用的机会[6]。目前,炼化废水经隔油、气浮、生化单元处理后部分回用,外排水经由深度处理达到《石油炼制工业污染物排放标准》(GB 31570—2015)标准规定限值后外排[7-8]。非均相催化臭氧氧化技术由于其绿色、清洁、不产生二次污染等特点被广泛应用于废水的深度处理,但由于废水水质的复杂化程度加剧和外排水水质标准的不断提高,导致经由单一深度处理技术处理后达标较为困难,同时,对于深度处理单元的污染物去除率要求更高[9-11]。
东北某炼化公司深度处理单元主要包括多介质过滤器、臭氧催化氧化池、MBBR(moving-bed biofilm reactor移动床生物膜反应器)、电催化、活性碳滤罐,臭氧催化氧化池作为生化出水的第1个处理单元,对废水中微生物难降解的有机物具有较高的去除作用,同时可提高废水的可生化性[12-13]。该臭氧催化氧化单元采用传统非均相臭氧催化氧化技术,催化剂的优劣对臭氧催化氧化单元污染物去除率起到关键作用[9, 14]。工程化应用的非均相催化剂主要是金属离子负载型,基体普遍采用氧化铝基,但负载的金属离子种类繁多[15-18]。有研究[19-20]表明,负载不同金属离子的催化剂在催化臭氧氧化处理同一种废水时污染物去除率存在较大差异。负载锰氧化物的催化剂可显著提高苯类物质的矿化速率,负载铁氧化物的催化剂对酿酒废水有较高的COD去除率[15, 21]。WU等[22]研究表明,γ-Al2O3负载Mn、Ce催化臭氧氧化处理1-氨基-4-溴蒽醌-2-磺酸时,对污染物的去除率较高。LI等[23]研究表明,Al2O3负载Mn、Fe、Ce 3种金属处理奶牛养殖废水时,废水COD去除率可达48.9%。因此,如何做到将废水水质参数与催化剂负载组分相关联,得到与废水水质适配的负载金属离子成为亟需解决的关键问题。此外,催化剂制备过程中受焙烧温度、焙烧时间等因素的影响。
此次更换催化剂为某研究院根据炼化公司外排水水质参数针对性设计的催化剂制备方案和制备条件,催化剂生产完成后,随机抽样送至具有CMA (China metrology accreditation) 资质的第三方检测公司进行催化剂性能测定并出具测试报告。符合要求后送至炼化公司进行催化剂的更换,对更换后臭氧催化氧化单元污染物去除情况进行分析,以期为臭氧催化氧化单元的实际工程应用提供技术支撑。
-
废水来源为东北某炼化公司污水处理厂二级处理出水,经由调节池、多介质过滤器后进入臭氧催化氧化池,其深度处理单元来水主要水质指标为:COD值120~200 mg·L−1、TOC质量浓度40~60 mg·L−1、TN质量浓度25~35 mg·L−1、NH3-N质量浓度12~17 mg·L−1。
-
1)催化剂的制备。本次更换的臭氧催化剂为某研究院提供的铝基金属离子负载型催化剂(型号HKYWK-CCM-01),负载金属类型为过渡金属元素和稀土元素,堆密度0.65 g·cm−3,抗压强度184.35 N·颗−1,磨耗率0.83%,吸水率59.99%。该催化剂金属组分的负载方案和制备条件是基于该炼化公司二级出水水质特性研发确定。
2)臭氧催化氧化单元。工程应用构筑物为东北某炼化公司外排水臭氧催化氧化单元,其臭氧催化氧化池为钢筋混凝土结构(长×宽×高=9 m×3 m×6.5 m),每组池子分为大小相同的三间,两侧为进水池,中间为出水池。进水通过进水池顶部布水管均匀布水,从上到下穿过催化剂床层后从底部进入出水池,在出水池中从下到上穿过催化剂床层。出水池顶部设有出水廊道,两侧布设有溢流堰。催化剂装填量为:进水池催化剂床层高度3 m,装填27 m3,出水池催化剂床层高度1.3 m,装填11.7 m3。氧化池内部构造从下到上依次为反冲洗布水、布气管路,垫层,臭氧布气管路,催化剂床层。
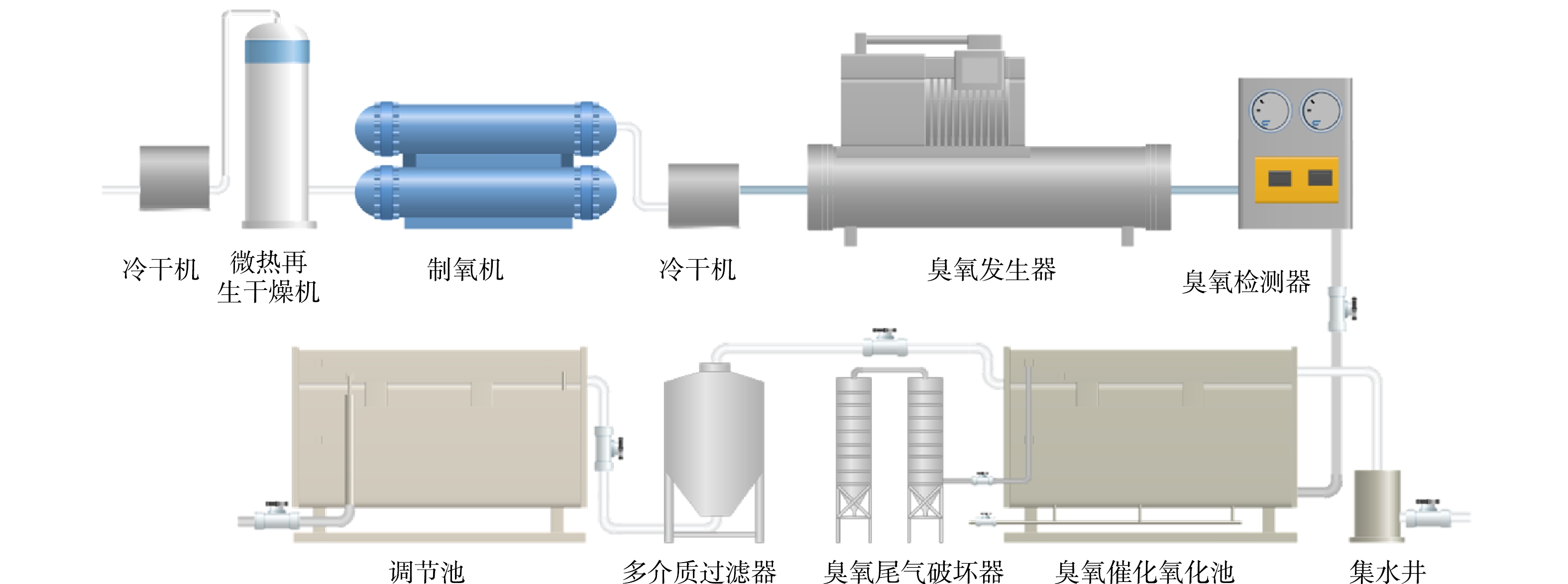
臭氧系统为空气经由冷干机、微热再生干燥机、制氧机、冷干机、臭氧发生器、臭氧浓度检测仪后通过管路输送到臭氧催化氧化池,臭氧曝气方式为穿孔管式。臭氧催化氧化单元整体流程示意图如图1所示。
-
COD值采用快速消解分光光度法进行测定,操作过程严格执行《水质 化学需氧量的测定 快速消解分光光度法》(HJ/T 399-2 007)标准。TOC浓度采用总有机碳分析仪(TOC-L,日本岛津企业管理公司)测定;UV254采用紫外可见分光光度计(UV-1 700,日本岛津企业管理公司)测定;三维荧光光谱分析采用三维荧光检测仪(HITACHI F-3 000,日本天美有限公司)测定,样品经0.45 μm滤膜过滤后测定,扫描速率12 000 nm·min−1,分析方法采用荧光区域积分法(FRI法)。FRI法将荧光光谱分为五个区域,分别代表类酪氨酸、类色氨酸、类富里酸、类溶解性代谢产物和类腐殖酸[24-25]。BOD5的测定采用稀释与接种法,测定过程严格执行《水质 五日生化需氧量(BOD5)的测定 稀释与接种法》(GB 7 488-87)标准。
催化剂的晶型和物相采用SmartLab SE型X-射线粉末衍射仪(日本Rigaku公司)测定,操作电流40 mA、操作电压40 kV。催化剂的比表面积和孔隙度采用ASAP2 460型比表面积仪(美国麦克公司)测定,孔隙度根据氮气吸附-脱附等温线和Barrett-Joyner-Halenda (BJH)方法得到。
-
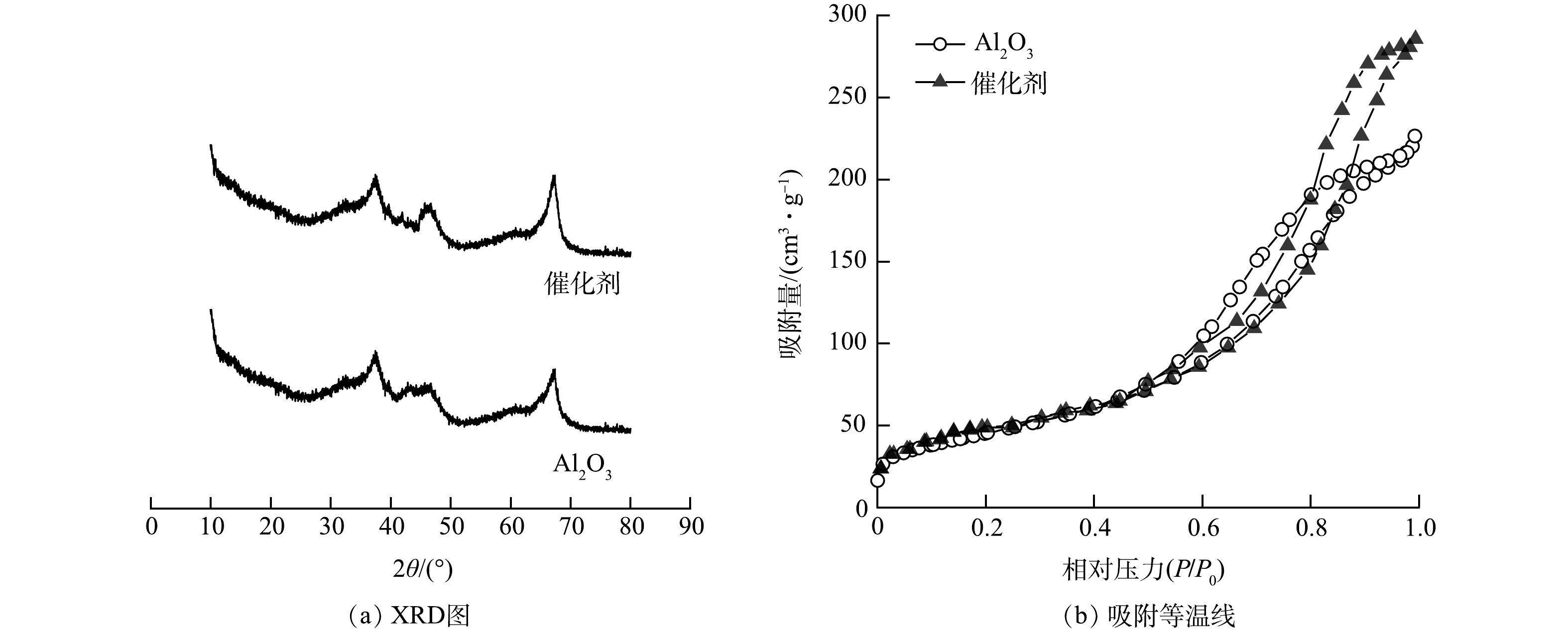
对氧化铝基体和催化剂的晶型结构和比表面积、孔径等进行表征分析,结果见图2。由图2(a)所示,氧化铝和催化剂在2θ为37.3°、45.6°、66.8°处有特征衍射峰,通过对比PDF卡号50-0741,这些特征峰为γ-Al2O3的特征衍射峰[19]。结晶度主要取决于制备过程中活性金属的负载量和焙烧温度、时间等因素,载体与催化剂所用焙烧温度和时间均一致,故晶型结构无明显差别[26]。此外催化剂并未出现其他活性金属的衍射峰,这是由于催化剂制备过程中采用混合法,活性金属组分含量较低且在载体中高度分散所致[27]。由图2(b)和表1可知,氧化铝和催化剂的吸附等温线均符合国际化学与应用化学(IUPAC)Ⅳ型曲线,催化剂的最大吸附量为285.50 cm3·g−1,孔容为0.48 cm3·g−1,表明催化剂具有较好的孔隙结构。催化剂的比表面积、孔径和孔容均优于氧化铝基体,这是因为催化剂在工厂加工过程中比氧化铝基体少了一步滚圆操作,氧化铝基体经过滚圆后,球体表面更为光滑,粉体结合更为紧密,导致氧化铝基体比表面积等有所降低。此外,负载适量的活性金属后,有助于提高氧化铝的催化性能。有研究表明氧化铝基体中引入活性金属组分可为催化剂表面提供活性位点[28-30]。在催化臭氧氧化过程中,这些活性位点可作为反应场所,吸附络合臭氧和有机污染物,增加气液固三相的传质效率[31]。
-
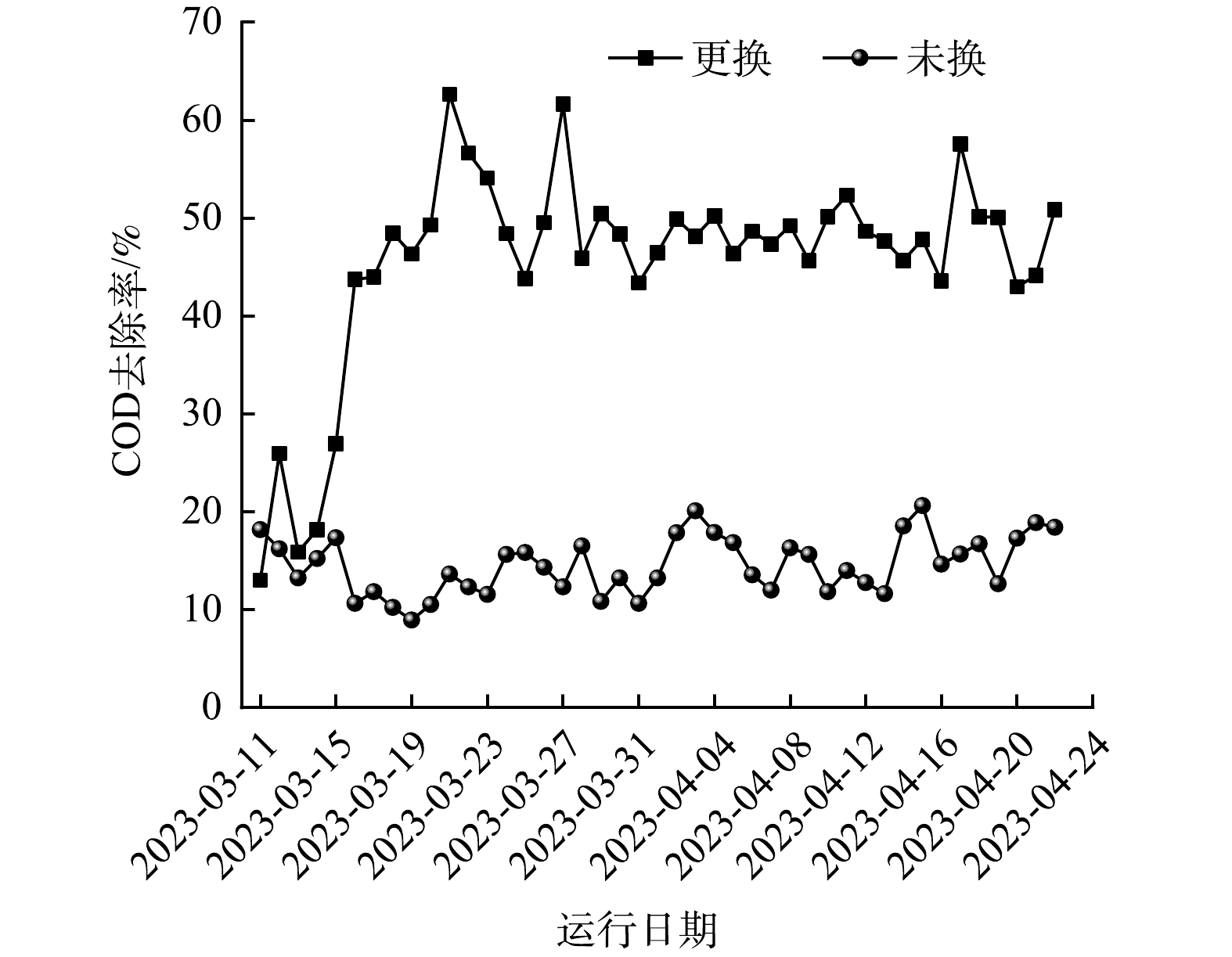
2023年3月10日臭氧催化氧化池完成催化剂更换(共4组,更换3组),11—15日期间进行运行调试,16日开始正常运行,对更换后43 d内COD数据进行分析,去除率结果如图3所示:催化剂更换过程中未对垫层和底部支撑结构进行彻底清洗,在更换施工过程中由于踩踏、翻动等使附着在垫层上的高浓度有机物部分脱落,在新催化剂更换完成后随出水逐渐排出。11日臭氧催化氧化池SS质量浓度达到91.0 mg·L−1,更换后5 d内SS质量浓度逐渐降低,16日SS质量浓度为6.0 mg·L−1,与未更换的臭氧催化氧化池(SS=9.0 mg·L−1)相近,16日更换催化剂后的臭氧催化氧化池出水COD去除率达到43.73%,远高于未更换氧化池的10.65%。
运行调试完成后,进入常态化运行,对常态化运行38 d内数据进行统计分析,更换后COD去除率最高达到62.63%(3月21日),最低为42.98%(4月20日),38 d内均值为48.95%,远高于未更换氧化池的COD去除率(最大值20.64%、最小值8.96%、均值14.58%)。上述结果表明该研究院提供的催化剂具有良好的催化活性和稳定性,更换后可大幅度提高臭氧催化氧化单元的污染物去除率,增加臭氧催化氧化单元利用率,减轻后续单元污染物处理负荷。
-
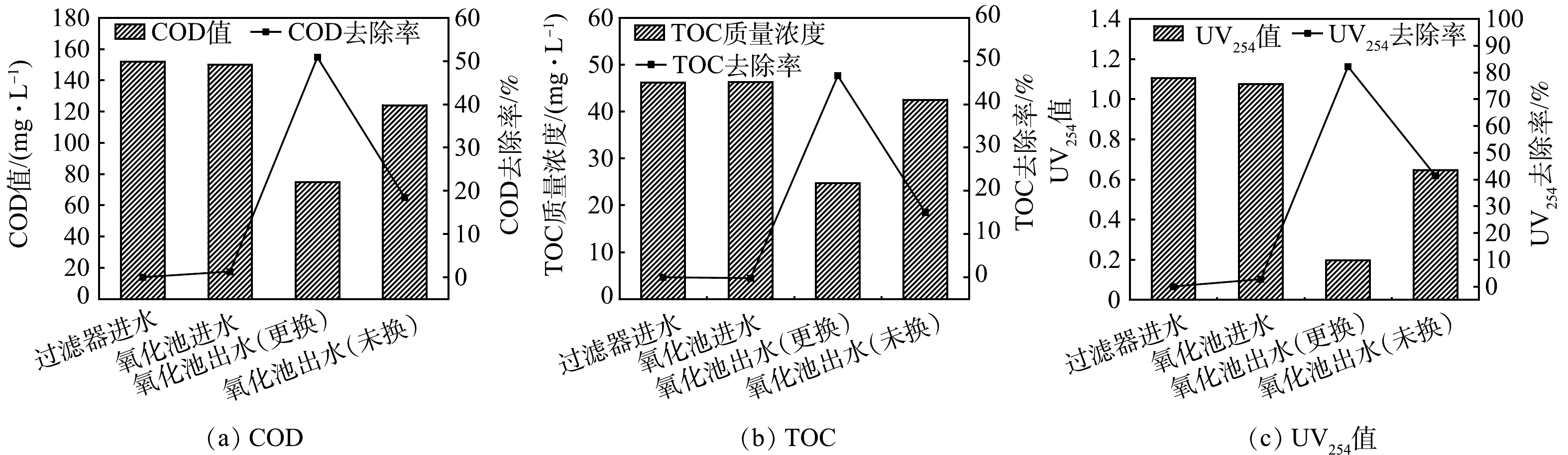
1) COD、TOC、UV254。混合调节池出水进入多介质过滤器,去除SS后出水进入臭氧催化氧化单元。2023年4月24日对多介质过滤器进水、臭氧催化氧化池进水、臭氧催化氧化池出水(更换、未换)的COD、TOC、UV254含量和去除率进行分析,结果如图4所示。由图4(a)可知,经过多介质过滤器处理后,COD值COD值从152.0 mg·L−1降至150.0 mg·L−1,去除率为1.32%。这说明多介质过滤器对COD无明显去除作用。更换催化剂后氧化池出水COD值为74.7 mg·L−1,去除率达到50.86%,远高于未更换的氧化池(去除率18.42%)。由图4(b)可知,经过多介质过滤器处理后,出水TOC质量浓度达到46.3 mg·L−1,稍高于进水(46.2 mg·L−1)。这可能是由于多介质过滤器在1个周期内截留污染物未及时反洗而有少量随水流出。经过臭氧催化氧化单元处理后,更换催化剂的氧化池出水TOC质量浓度为24.66 mg·L−1,去除率达到46.62%,而未更换催化剂的氧化池TOC去除率仅有15.03%。UV254为样品在254 nm处的吸光度值,可以反映废水中腐殖质类大分子物质以及含有双键的芳香族类化合物[32]。由图4(c)可知,多介质过滤器进水UV254值为1.105,出水为1.075,表明过滤器可截留少量腐殖质类大分子物质和芳香族类化合物。经过臭氧催化氧化单元处理后,出水UV254值为0.197(更换)、0.647(未换),更换催化剂后出水UV254去除率达到82.17%,远高于未换臭氧催化氧化池(41.45%)。
对多介质过滤器进水、臭氧催化氧化池进水、氧化池出水(更换、未换)的COD、TOC、UV254指标分析结果表明,多介质过滤器通过截留作用对有机污染物的去除贡献较小,反洗不及时情况下反而会存在部分截留物随水流出的情况。臭氧催化氧化单元更换催化剂后相比于未更换的臭氧催化氧化池,污染物去除率显著提高,表明所更换催化剂催化活性高,性能稳定。
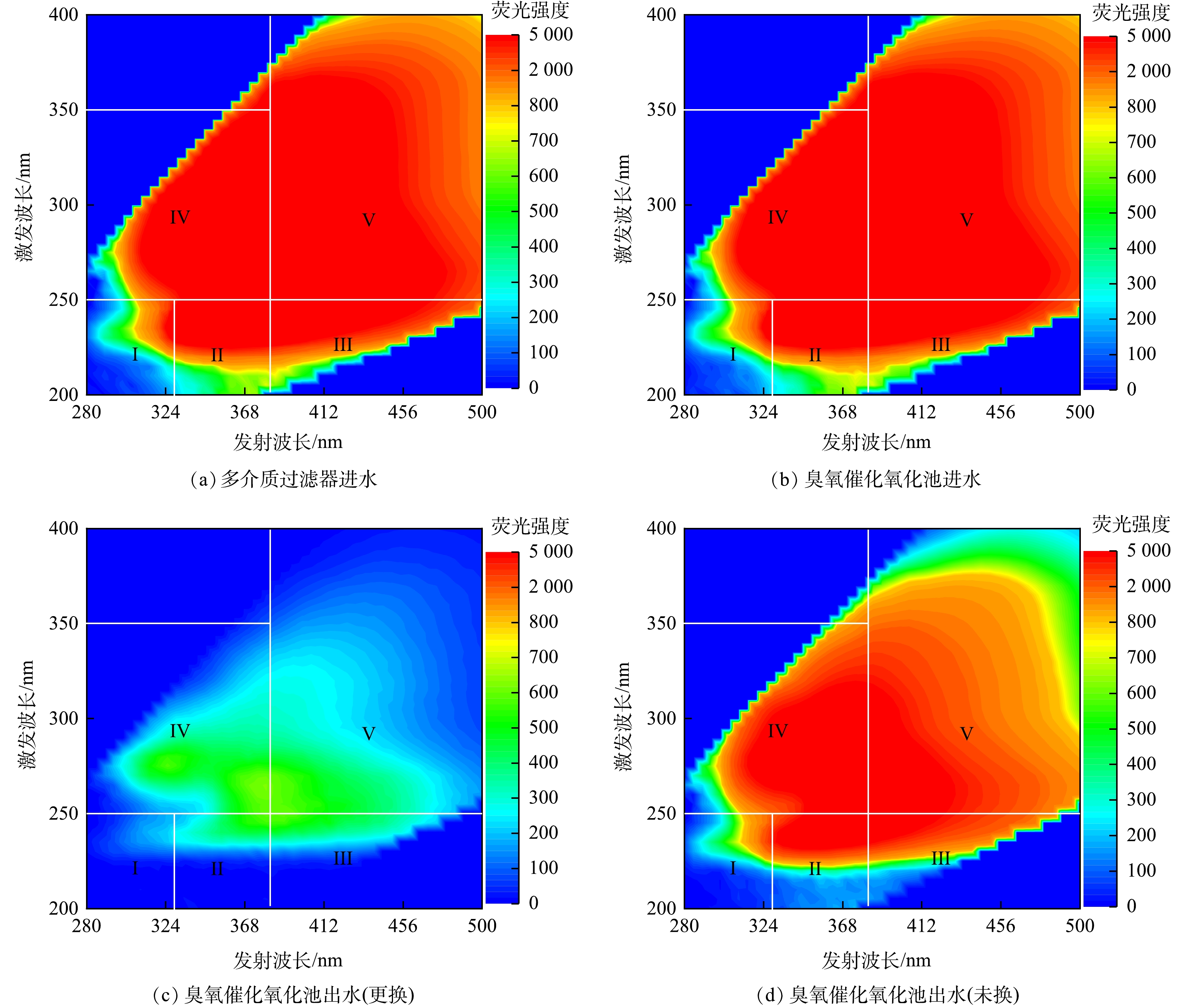
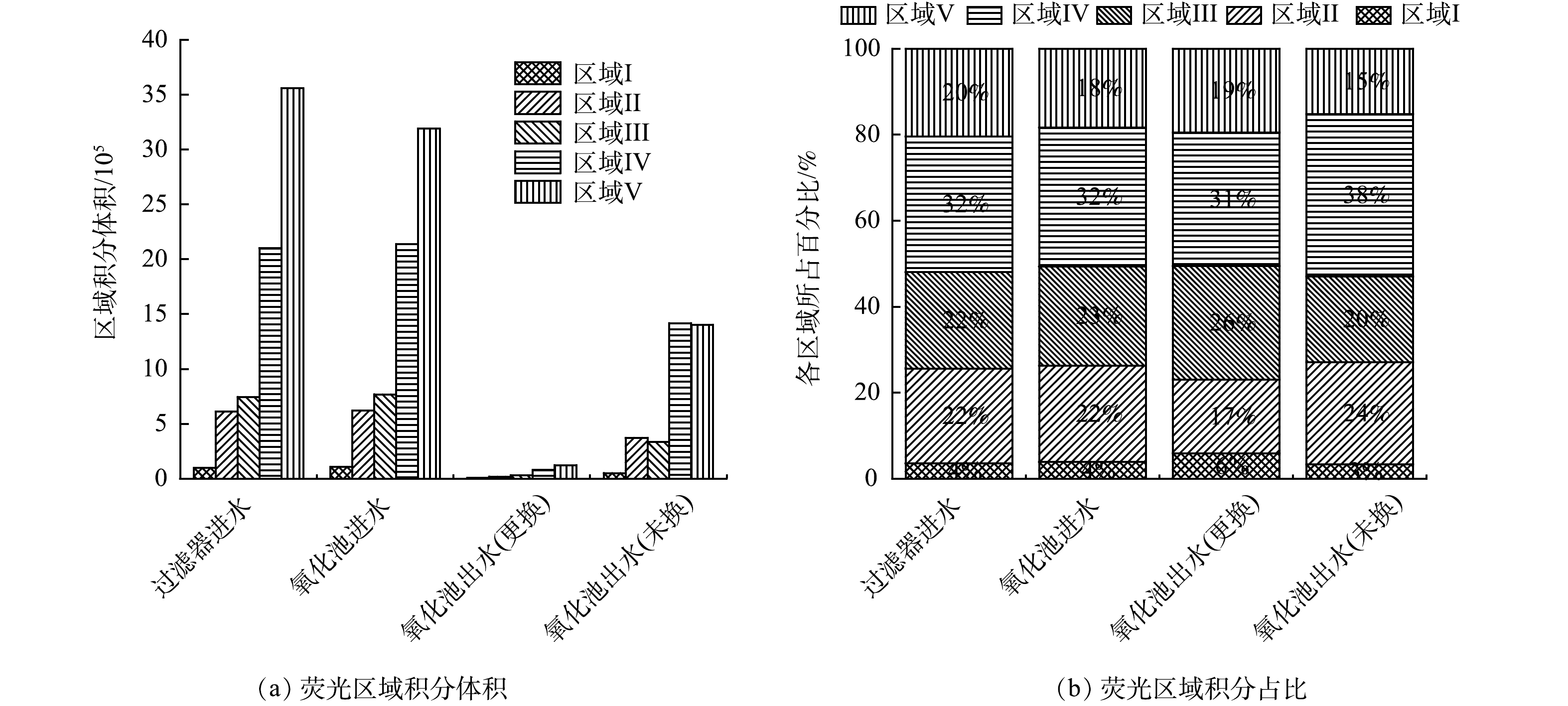
2)三维荧光光谱。对多介质过滤器进水、臭氧催化氧化池进水、臭氧催化氧化池出水(更换、未换)取样测定三维荧光光谱,并测定超纯水的荧光强度,便于修正拉曼和瑞利散射干扰,得到荧光光谱图如图5所示。可见,过滤器进水荧光强度≥2 000,经由臭氧催化氧化单元处理后,荧光强度≤1 000,表明石油炼化废水经生化单元处理后剩余的有机污染物中含有大量荧光类物质,经臭氧催化氧化单元处理后,荧光强度显著降低。
由图6可知,荧光类物质区域积分体积分别为:过滤器进水71.13×105、臭氧催化氧化池进水68.22×105、氧化池出水(更换) 2.57×105、氧化池出水(未换) 35.77×105。经由臭氧催化氧化处理后,荧光类物质去除率为96.23%(更换),相比于未更换的氧化池(去除率为47.56%),去除率提高48.67个百分点。表明更换催化剂对荧光类物质去除效果显著。荧光类物质中占比较高的为区域Ⅳ代表的类溶解性代谢产物和区域Ⅴ代表的类腐殖酸,这两部分均属于微生物难降解有机物,而臭氧对这两部分物质均具有较高的去除作用,同时臭氧催化氧化处理可调整5个区域之间的比例,区域Ⅰ、Ⅱ的占比增加。区域Ⅰ、Ⅱ代表的类酪氨酸、类色氨酸为微生物易降解的物质,因此,经臭氧催化氧化处理后,出水的可生化性得到了提高[33]。为了进一步证明经过臭氧催化氧化处理后废水可生化性的改善,对氧化池进水和氧化池出水(更换)取样测定BOD5,结果表明,氧化池进水BOD5质量浓度为31.5 mg·L−1,氧化池出水(更换)BOD5质量浓度为41.83 mg·L−1,氧化池进水和氧化池出水(更换)B/C值分别为0.21和0.56,证明经由臭氧催化氧化单元处理后,可生化性增加。有研究表明,臭氧催化氧化出水进入曝气生物滤池后,可显著降低有机物含量,这也表明经由臭氧催化氧化处理后,出水可生化性的改善[34-35]。
-
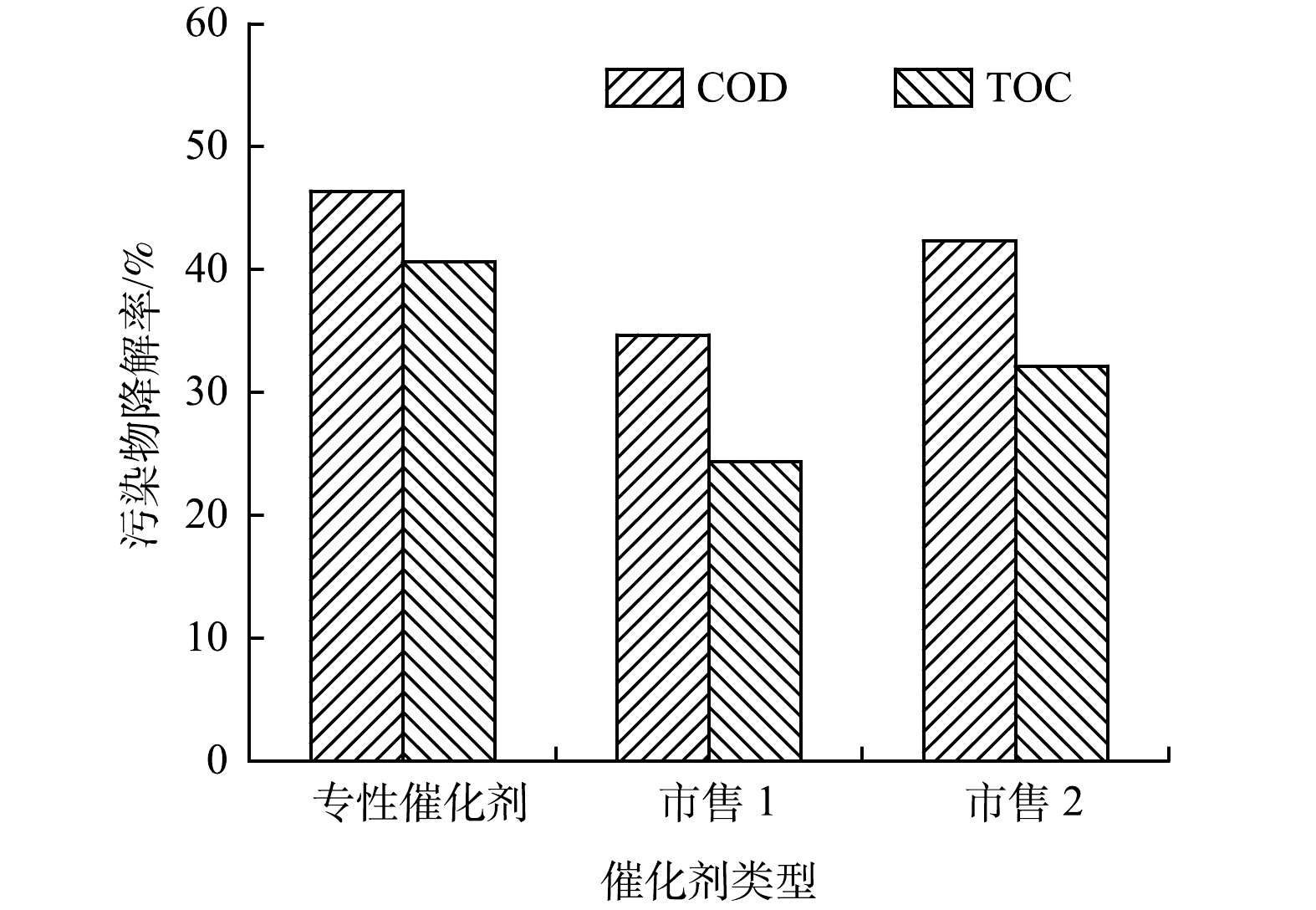
为更好比较此次更换专性催化剂(型号HKYWK-CCM-01)与市场上商品化臭氧催化剂催化效率的区别,在臭氧投加量为42 mg·(L·h)−1、停留时间60 min、催化剂填充量40%条件下,取1.0 L氧化池进水进行臭氧催化氧化实验,结果如图7所示。结果表明,炼化公司此次更换专性催化剂COD和TOC去除率分别为46.38%和40.62%,均高于市售催化剂(市售催化剂1对COD和TOC的去除率分别为34.68%和24.37%;市售催化剂2对COD和TOC的去除率分别为42.34%和32.13%)。说明此次某研究院根据炼化公司二级出水水质特性设计专性催化剂具有较高的催化效率。
-
臭氧剂量为去除每克COD投加的臭氧量,可根据式(1)进行计算。
式中:θ为臭氧剂量,g·g−1;N为臭氧投加量,g·h−1;C0为混合臭氧催化氧化池进水采样口COD值,mg·L−1;C1为混合臭氧催化氧化池A、C、D 3组出水采样口COD平均值,mg·L−1;Q为混合池进水流量,m3·h−1。
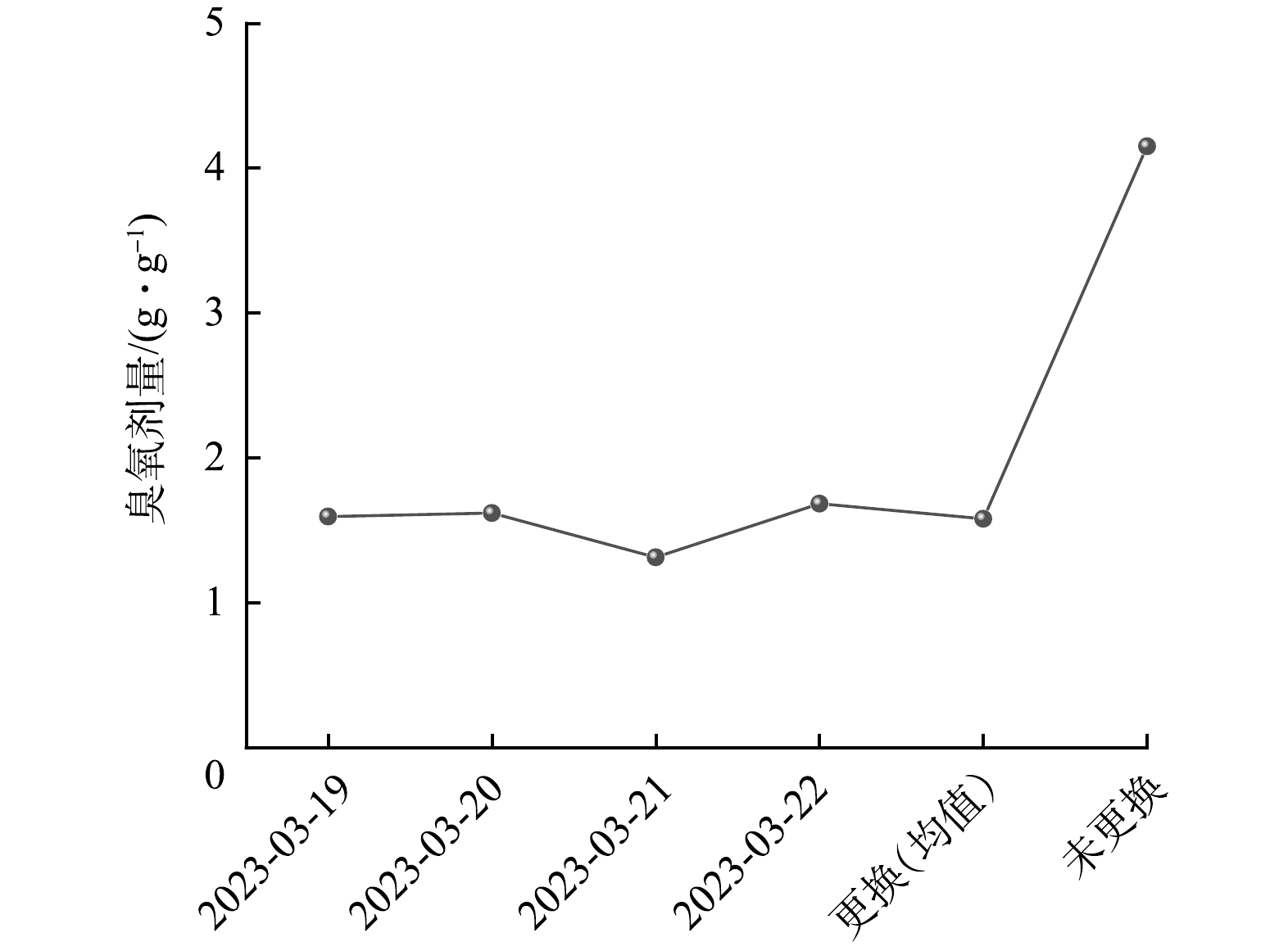
对19—22日臭氧催化氧化池的臭氧投加量和臭氧剂量进行分析,结果如图8所示。臭氧经由臭氧发生系统产生后,通过管道输送至臭氧催化氧化单元,经过气体流量计后进入氧化池。可见,常态化运行期间各臭氧催化氧化池的臭氧投加量均维持在9.70~10.45 kg·h−1,更换催化剂的臭氧催化氧化池臭氧剂量维持在1.31~1.68 g·g−1(以COD计),均值为1.58 g·g−1,远低于未更换催化剂臭氧催化氧化池的臭氧剂量(4.15 g·g−1)。这表明在更换催化剂后,污染物去除率增加的同时臭氧的消耗也显著降低,达到节能减排的目的。
-
在正常运行后,本研究对臭氧催化氧化池进、出水的金属组分浓度进行了为期1周的检测。结果表明,金属1和金属2的离子溶出质量浓度均值分别为72.60 μg·L−1和35.20 μg·L−1,且随着使用时间的延长,浓度逐渐降低。根据《污水综合排放标准》(GB 8978-1996)、《城镇污水处理厂污染物排放标准》(GB 18918-2002)标准要求,金属1和金属2的排放限值分别为2 000 μg·L−1和500 μg·L−1,金属离子溶出量远低于排放标准,对出水无影响。
-
1)该研究院提供铝基金属离子负载型催化剂具有良好的晶型和孔隙结构,负载金属组分可为催化剂提供大量的活性位点,从而大幅提高催化剂性能。常态化运行过程中,更换催化剂的臭氧催化氧化池COD去除率维持在42.98%~62.63%,远高于未更换的臭氧催化氧化池(8.96%~20.64%)。
2)多介质过滤器对污染物的去除贡献不大,主要通过截留作用去除部分污染物质。臭氧催化氧化池更换催化剂后,COD值由进水150.0 mg·L−1降至出水74.7 mg·L−1,去除率达到50.86%。TOC和UV254去除率分别达到46.62%和82.17%,远高于未更换催化剂的氧化池(COD、TOC、UV254去除率分别为18.42%、15.03%、41.45%)。荧光类物质经臭氧催化氧化池处理后,去除率达到96.23%(更换催化剂后),经由臭氧催化氧化处理后出水B/C值为0.56,可生化性提高。更换催化剂后臭氧剂量降至1.58 g·g−1(以COD计),远低于未更换催化剂的臭氧剂量(4.15 g·g−1),表明通过更换催化剂实现节能、降耗行之有效。催化剂的金属离子溶出浓度均低于排放标准限值,对出水无影响。
3)在运行过程中发现,臭氧曝气方式仍采用穿孔管式,导致臭氧利用率较低,可更换为曝气片、曝气头等方式提高臭氧利用率。此外,进水难以做到精细化调控,导致每组臭氧催化氧化池处理负荷存在差异,可增加进水流量计等实现布水均衡。
臭氧催化氧化池更换铝基催化剂的工程应用
Engineering application of ozone catalytic oxidation tank to replace alumina -based catalyst
-
摘要: 为解决某炼化公司外排水装置臭氧催化氧化单元污染物去除率低,能耗高的问题,将臭氧催化氧化池原有催化剂(氧化铝基+活性碳棒)更换为某研究院基于炼化公司外排水水质参数所制备的专性催化剂,对更换前后污染物去除率和臭氧剂量进行分析。结果表明:专性催化剂具有良好的晶型和发达的孔隙结构,常态化运行后,更换催化剂后的氧化池COD去除率达到42.98%~62.63%,远高于未更换的氧化池(8.96%~20.64%)。对多介质过滤器进水、臭氧催化氧化池进水、臭氧催化氧化池出水(更换、未换)分别取样测定COD、TOC、UV254和荧光光谱,发现多介质过滤器只可通过截留作用去除分子质量较大的类腐殖质等物质,去除率较低;更换催化剂后臭氧催化氧化池的COD去除率达50.86%、TOC去除率达46.62%、UV254去除率达82.17%、荧光类物质去除率可达96.23%。臭氧催化氧化池出水(更换)B/C值为0.56(臭氧催化氧化池进水B/C值为0.21),经由臭氧催化氧化处理后,出水可生化性提高。常态化运行期间臭氧剂量为:更换催化剂的氧化池1.31~1.68 g·g−1(以COD计),均值为1.58 g·g−1,未更换催化剂的氧化池为4.15 g·g−1,表明更换催化剂后可显著降低臭氧的消耗。以上结果表明,炼化公司外排水装置更换臭氧催化剂后污染物处理负荷大幅度提高,臭氧消耗量减少,节能减排成效显著。Abstract: In order to solve the problem of low pollutant removal rate and high energy consumption in the ozone catalytic oxidation unit of the external drainage device of a refining and chemical company. The original catalyst (alumina base+activated carbon rod) in the ozone catalytic oxidation tank was replaced with a specific catalyst prepared by a research institute based on the water quality parameters of the refining company's external drainage. And the pollutant removal rate and ozone dose were analyzed before and after the replacement. The results show that the specific catalyst has a good crystal form and a well-developed pore structure. After normal operation, the COD removal rate of the oxidation tank after the catalyst replacement could reach 42.98%~62.63%, which was much higher than that of the unreplaced oxidation tank (8.96%~20.64%). The water samples from the incoming water of the multi-media filter, the incoming water of the ozone catalytic oxidation tank, and the effluent water of the ozone catalytic oxidation tank (replaced and unreplaced) were taken and their COD, TOC, UV254 and fluorescence spectra were tested, respectively. It was found that the multi-media filter could only remove humic substances with higher molecular weight through interception, and the removal rate was low; After replacing the catalyst, the COD,TOC, UV254 and fluorescent substance removal rates of the ozone catalytic oxidation tank reached 50.86%, 46.62%, 82.17% and 96.23%, respectively. The B/C value of the effluent from the ozone catalytic oxidation tank (replaced) was 0.56 (the B/C value of the incoming water to the ozone catalytic oxidation tank was 0.21). After catalytic ozonation treatment, the biodegradability of the effluent increased. The ozone dose during normal operation was following: 1.31~1.68 g·g−1 (calculated by COD), for the oxidation tank with catalyst replacement with an average value of 1.58 g·g−1, 4.15 g·g−1 for the oxidation tank without catalyst replacement, which indicated that the replacement of catalyst could significantly reduce ozone consumption. Above results showed that after replacing the ozone catalyst in the external drainage device of the refining and chemical company, the pollutant treatment load has been greatly increased, the ozone consumption has been reduced, and the energy saving and emission reduction have achieved remarkable results.
-

-
表 1 Al2O3和催化剂的比表面积及孔径分布
Table 1. Specific surface area and pore size distribution of Al2O3 and catalysts
催化剂 比表面积/(m2·g−1) 平均孔径/nm 孔容/(cm3·g−1) 氧化铝 206.13 7.76 0.42 催化剂 228.50 7.99 0.48 -
[1] FETANAT A, TAYEBI M. A picture fuzzy set-based decision support system for treatment technologies prioritization of petroleum refinery effluents: A circular water economy transition towards oil & gas industry[J]. Separation and Purification Technology, 2022, 303(1): 1-16. [2] COELHO A, CASTRO A, DEZOTTI M, et al. Treatment of petroleum refinery sourwater by advanced oxidation processes[J]. Journal of Hazard Materials, 2006, 137(1): 178-184. doi: 10.1016/j.jhazmat.2006.01.051 [3] KARRAY F, ALOUI F, JEMLI M, et al. Pilot-scale petroleum refinery wastewaters treatment systems: Performance and microbial communities’ analysis[J]. Process Safety and Environmental Protection, 2020, 141(1): 73-82. [4] JAFARINEJAD S, JIANG S. Current technologies and future directions for treating petroleum refineries and petrochemical plants (PRPP) wastewaters[J]. Journal of Environmental Chemical Engineering, 2019, 7(5): 10332-10336. [5] ELNAAS M, ALHAIJA M, ALZUHAIR S. Evaluation of a three-step process for the treatment of petroleum refinery wastewater[J]. Journal of Environmental Chemical Engineering, 2014, 2(1): 56-62. doi: 10.1016/j.jece.2013.11.024 [6] ZHONG J, SUN X, WANG C. Treatment of oily wastewater produced from refinery processes using flocculation and ceramic membrane filtration[J]. Separation and Purification Technology, 2003, 32(1/2/3): 93-98. [7] 环境保护部. 石油炼制工业污染物排放标准: GB 31570-2015[S]. 北京: 中国环境科学出版社, 2015. [8] 宋佳宇, 张婷婷, 李立君, 等. 典型炼化废水微生物功能结构与主要致毒物质响应关系研究[J]. 环境科学研究, 2023, 36(5): 943-953. doi: 10.13198/j.issn.1001-6929.2023.02.04 [9] TURKAY O, INAN H, DIMOGLO A. Experimental and theoretical investigations of CuO-catalyzed ozonation of humic acid[J]. Separation and Purification Technology, 2014, 134(1): 110-116. [10] CHEN K, WANG Y. The effects of Fe–Mn oxide and TiO2/α-Al2O3 on the formation of disinfection by-products in catalytic ozonation[J]. Chemical Engineering Journal, 2014, 253(1): 84-92. [11] QI W, WANG J, QUAN X, et al. Catalytic ozonation by manganese, iron and cerium oxides on γ-Al2O3 pellets for the degradation of organic pollutants in continuous fixed-bed reactor[J]. Ozone: Science & Engineering, 2019, 42(2): 136-145. [12] UDREA I, BRADU C. Ozonation of substituted phenols in aqueous solutions over CuO-Al2O3 catalyst[J]. Ozone: Science & Engineering, 2003, 25(4): 335-343. [13] DAI Q, WANG J, CHEN J, et al. Ozonation catalyzed by cerium supported on activated carbon for the degradation of typical pharmaceutical wastewater[J]. Separation and Purification Technology, 2014, 127(1): 112-120. [14] MARTINS R, QUINTA-FERREIRA R. Remediation of phenolic wastewaters by advanced oxidation processes (AOPs) at ambient conditions: Comparative studies[J]. Chemical Engineering Science, 2011, 66(14): 3243-3250. doi: 10.1016/j.ces.2011.02.023 [15] EINAGA H, FUTAMURA S. Catalytic oxidation of benzene with ozone over alumina-supported manganese oxides[J]. Journal of Catalysis, 2004, 227(2): 304-312. doi: 10.1016/j.jcat.2004.07.029 [16] POCOSTALES P, ALVAREZ P, BELTRAN F. Catalytic ozonation promoted by alumina-based catalysts for the removal of some pharmaceutical compounds from water[J]. Chemical Engineering Journal, 2011, 168(3): 1289-1295. doi: 10.1016/j.cej.2011.02.042 [17] REZAEI E, SOLTAN J, CHEN N, et al. Effect of noble metals on activity of MnO x/γ-alumina catalyst in catalytic ozonation of toluene[J]. Chemical Engineering Journal, 2013, 214(1): 219-228. [18] KEYKAVOOS R, MANKIDY R, MA H, et al. Mineralization of bisphenol A by catalytic ozonation over alumina[J]. Separation and Purification Technology, 2013, 107(1): 310-317. [19] 胡映明, 王盼新, 付丽亚, 等. 不同制备方法对铝基催化剂臭氧催化氧化的效果研究[J]. 环境科学研究, 2022, 35(11): 2559-2567. doi: 10.13198/j.issn.1001-6929.2022.07.01 [20] LI M, FU L, DENG L, et al. A tailored and rapid approach for ozonation catalyst design[J]. Environmental Science and Ecotechnology, 2023, 15(1): 1-10. [21] SREETHAWONG T, CHAVADEJ S. Color removal of distillery wastewater by ozonation in the absence and presence of immobilized iron oxide catalyst[J]. Journal of Hazardous Materials, 2008, 155(3): 486-493. doi: 10.1016/j.jhazmat.2007.11.091 [22] WU Z, ZHANG G, ZHANG R, et al. Insights into mechanism of catalytic ozonation over practicable mesoporous Mn-CeOx/γ-Al2O3 catalysts[J]. Industrial & Engineering Chemistry Research, 2018, 57(6): 1943-1953. [23] LI J, SONG W, YU Z, et al. Preparation of the Mn-Fe-Ce/γ-Al2O3 ternary catalyst and its catalytic performance in ozone treatment of dairy farming wastewater[J]. Arabian Journal of Chemistry, 2020, 13(2): 3724-3734. doi: 10.1016/j.arabjc.2020.01.006 [24] CHEN W, WESTERHOFF P, LEENHEER J, et al. Fluorescence excitatione emission matrix regional integration to quantify spectra for dissolved organic matter[J]. Environmental Science & Technology, 2003, 37(1): 5701-5710. [25] 李敏, 付丽亚, 谭煜, 等. Mn-Ce/γ-Al2O3催化臭氧氧化深度处理石化废水中试研究[J]. 环境科学研究, 2021, 34(10): 2380-2388. [26] LI Y, XU J, QIAN M, et al. The role of surface hydroxyl concentration on calcinated alumina in catalytic ozonation[J]. Environmental Science and Pollution Research, 2019, 26(15): 15373-15380. doi: 10.1007/s11356-019-04909-5 [27] YANG L, HU C, NIE Y L, et al. Catalytic ozonation of selected pharmaceuticals over mesoporous alumina-supported manganese oxide[J]. Environmental Science & Technology, 2009, 42(1): 2525-2529. [28] ZHAO K, MA Y, LIN F, et al. Refractory organic compounds in coal chemical wastewater treatment by catalytic ozonation using Mn-Cu-Ce/Al2O3[J]. Environmental Science and Pollution Research, 2021, 28(30): 41504-41515. doi: 10.1007/s11356-021-13629-8 [29] NAWROCKI J, FIJOLEK L. Effect of aluminium oxide contaminants on the process of ozone decomposition in water[J]. Applied Catalysis B: Environmental, 2013, 142-143(1): 533-537. [30] NIE R, LEI H, PAN S, et al. Core–shell structured CuO–ZnO@H-ZSM-5 catalysts for CO hydrogenation to dimethyl ether[J]. Fuel, 2012, 96(1): 419-425. [31] IKHLAQ A, BROWN D, KASPRZYK-HORDERN B. Mechanisms of catalytic ozonation on alumina and zeolites in water: formation of hydroxyl radicals[J]. Applied Catalysis B: Environmental, 2012, 123-124(1): 94-106. [32] SHI Z, CHOW C, FABRIS R, et al. Evaluation of the impact of suspended particles on the UV absorbance at 254 nm (UV254) measurements using a submersible UV-Vis spectrophotometer[J]. Environmental Science and Pollution Research, 2020, 28(10): 12576-12586. [33] FU L, WU C, ZHOU Y, et al. Ozonation reactivity characteristics of dissolved organic matter in secondary petrochemical wastewater by single ozone, ozone H2O2, and ozonecatalyst[J]. Chemosphere, 2019, 233(1): 34-43. [34] 史振宇, 刘建伟, 王元月, 等. 混凝-臭氧催化氧化-曝气生物滤池处理工业园区污水[J]. 现代化工, 2019, 39(8): 203-209. doi: 10.16606/j.cnki.issn0253-4320.2019.08.042 [35] 郑垒, 郑旭文, 汪晓军, 等. 一体式臭氧催化氧化-曝气生物滤池深度处理印染废水[J]. 中国给水排水, 2019, 35(22): 105-107. doi: 10.19853/j.zgjsps.1000-4602.2019.22.022 -



 下载:
下载: