-
全氟辛酸(PFOA)和全氟辛基磺酸(PFOS)是两种全氟化合物,由于C—F键是自然界中极性最强的共价键之一[1],使得PFOA和PFOS具有优良的化学稳定性、耐热性和高表面活性,因而被广泛应用于化工电镀、消防设施、涂料、皮革、纺织、炊具制造和合成洗涤剂等诸多工业生产和生活消费领域[2]。然而,研究发现PFOA和PFOS具有环境持久性、长距离迁移性、生物累积性和毒性等特点,其广泛应用给环境造成了很大的负面影响。毒理学研究表明PFOA和PFOS对生物体具有脏器毒性、免疫和内分泌毒性、神经毒性、致癌性、生殖及发育毒性[3-4]。2009年5月,联合国环境规划署正式将PFOS及其盐类列为新型持久性有机污染物,列入斯德哥尔摩公约,160多个国家和地区同意减少并最终禁止使用该类物质[3]。
目前在大气[5]、水[6]、土壤[7]、生物[8]甚至极地冰川[9]都能检测到PFOA和PFOS,其普遍污染严重威胁生态环境和人类健康。PFOA和PFOS是人工合成的具有高稳定性的化合物,其中C—F键键能高达460 kJ·mol−1,氟原子最外层的3对未成键电子可以有效保护C—F键,因此很难通过自然分解和微生物处理的方式被降解。例如,有研究发现将PFOA和PFOS放入硝酸或者硫酸中煮沸,PFOA和PFOS中的C—F键没有断裂的迹象[10-11];还有研究表明,PFOA和PFOS热分解所需温度高达600 ℃[12]。此外,Schröder等对比研究了不同条件下微生物降解废水中PFOS和PFOA的效率,发现好氧条件下PFOA和PFOS都无法降解,厌氧条件下PFOS在两天内降解为PFOA,而PFOA完全消失需要25 d[13-14]。由于传统的污染物处理方法很难有效降解PFOA和PFOS,因此亟需开发有效的技术去除自然环境中的PFOA和PFOS,减少其对生态环境和人类健康的危害。
目前,PFOA和PFOS的降解方法主要有超声降解技术、光降解技术和电化学氧化法[15]。超声降解技术和电化学氧化法存在操作繁琐、试剂及装置费用高、反应条件较为苛刻、能耗大等缺点[16],极大地限制了这些方法的实际应用。光降解技术是利用光直接作用于污染物使其发生降解的一种技术[17],具有绿色环保、成本低、反应条件温和以及无二次污染等优点。因此,光催化降解技术对实现PFOA和PFOS的安全、有效降解具有重要意义。
本文总结了目前光降解PFOA和PFOS的研究现状,对比了不同催化剂、氧化剂和光敏剂作用下PFOA和PFOS的光降解效率,并总结了不同反应条件下PFOA和PFOS的光降解机理。同时分析了PFOA和PFOS光降解技术在实际应用中可能存在的问题及挑战,以期为PFOA和PFOS光降解技术的开发和安全有效应用提供科学参考。
-
光降解技术可分为直接光降解技术和间接光降解技术,直接光降解技术是指化合物分子吸收光子的能量跃迁至激发态后发生反应转化为产物的技术,间接光降解技术是指由另一种化合物吸收光子后,将能量转移给目标化合物而引起分解反应的技术[18]。PFOA和PFOS光降解技术一般是指在光的作用下,PFOA和PFOS逐步形成低分子中间产物,最终彻底矿化为CO2和F−的技术。根据反应条件不同,PFOA/PFOS光降解技术可分为PFOA/PFOS直接光降解技术和PFOA/PFOS间接光降解技术。
-
根据PFOA和PFOS的紫外-可见吸收光谱可以发现,PFOA和PFOS都不能吸收可见光,PFOA的紫外(UV)吸收光谱在185—300 nm范围内[19],而PFOS对波长在220—300 nm的UV有较弱的吸收,对波长小于220 nm的UV以及真空紫外(VUV)有较强的吸收[20]。由于PFOA和PFOS均不能吸收可见光发生光化学反应[21],所以目前关于PFOA和PFOS直接光降解的研究使用的光源均为UV或VUV。
-
2004年,Hori等首次研究了PFOA的UV光降解,发现1.35 mmol·L−1的PFOA水溶液在200 W氙汞灯发射的220—460 nm UV照射72 h后,PFOA的降解率和脱氟率分别达到了89.5%和33%[19]。该工作通过系统研究UV照射下PFOA的降解行为,证实了UV可以实现PFOA的安全有效降解,为PFOA UV光降解技术的可行性奠定了基础。但是,之后的UV光降解PFOA的研究中,可能由于光源的差异,UV直接降解PFOA的效果并不太理想。Song等发现,10 W低压汞灯发射的254 nm UV照射24 h后,50 μmol·L−1 PFOA的降解率仅为2.3%[22]。Giesy等也提出PFOA的直接光降解行为有待证实[23]。
为了改善PFOA的光降解效率,能量更高的VUV受到了研究者的关注。Chen等首次报道VUV光降解技术可以有效降解PFOA,在2 h内,15 W低压汞灯发射的185 nm紫外光对PFOA的降解率和脱氟率分别达到了61.7%和17.1%[24]。对比于UV光降解技术,VUV光降解技术不仅可加快PFOA的降解速率,还可实现C—F键的直接断裂形成F−,使PFOA降解得更彻底。
-
2007年,Yamamoto等研究了PFOS的UV降解过程,发现经32 W低压汞灯发射的254 nm UV照射240 h后,20 mg·L−1 PFOS的降解率和脱氟率分别为68%和71%[25]。虽然UV光降解PFOS已被证实具有可行性,但是PFOS对UV的吸收较弱,导致光利用率低,因此UV光降解PFOS效率低、降解所需时间长[26],也在一定程度上限制了这项技术的发展[25,27]。
从紫外吸收光谱可以发现,PFOS对VUV的吸收较强,而且VUV的能量也高于UV,因此VUV被用于提高PFOS的光降解效率。目前,研究者普遍认为,PFOS的直接光降解技术是一个还原过程,起主要作用的物质是水合电子(
$ {\rm{e}}_{{\rm{aq}}}^ - $ )。Lyu等系统地研究了VUV下PFOS的降解效果,发现光生还原物质,如$ {\rm{e}}_{{\rm{aq}}}^ - $ ,是加快PFOS降解效率的关键因素[26]。$ {\rm{e}}_{{\rm{aq}}}^ - $ 是一种强还原剂,其标准还原电位为−2.9 V,而PFOS的还原电势仅为−1.1 V[28],因此$ {\rm{e}}_{{\rm{aq}}}^ - $ 可与PFOS结合生成活性高的阴离子自由基,从而加快PFOS的降解。当pH 7.6和温度为90 ℃时,VUV照射可实现PFOS的快速光降解,其准一级降解速率常数为0.012 h−1,而在其他环境条件下,PFOS的准一级降解速率常数仅为0.0054 h−1[26]。虽然VUV降解PFOA/PFOS的效果比UV好,但是低压汞灯主要发射波长为254 nm的UV光,185 nm VUV仅占全部辐射能的12%—15%[20],因此提高光利用率或开发更高效的光源是提高PFOA和PFOS直接光降解效率的核心问题。
-
由于PFOA和PFOS直接光降解效果不理想,通过添加催化剂、氧化剂或光敏剂来提高PFOA和PFOS光降解效率的间接光降解技术受到了研究者的青睐。考虑到经济效应、环境友好性、可获得性等因素,目前光降解PFOA和PFOS所使用的催化剂、氧化剂和光敏剂主要有TiO2、Na2S2O8、FeCl3、KI、K3[Fe(CN)6]、
${\rm{SO}}_3^{2 - }$ 、碱性异丙醇、吲哚乙酸和腐殖酸(HA)等。根据PFOA和PFOS降解机理的不同,间接光降解可分为氧化性光降解和还原性光降解。其中,通过加入TiO2、FeCl3和Na2S2O8产生·OH、$ {\rm{SO}}_4^{-}\cdot $ 等氧化性活性物种的方法为氧化性光降解,而通过加入碱性异丙醇、KI、K3[Fe(CN)6]、吲哚乙酸和${\rm{SO}}_3^{2-} $ 生成$ {\rm{e}}_{{\rm{aq}}}^ - $ 等还原性活性物种的方法为还原性光降解。表1和表2分别列举了PFOA和PFOS氧化性和还原性光降解技术的反应条件和降解效率。后文将根据降解机理的不同,详细阐述PFOA和PFOS的氧化和还原降解过程和机理。
-
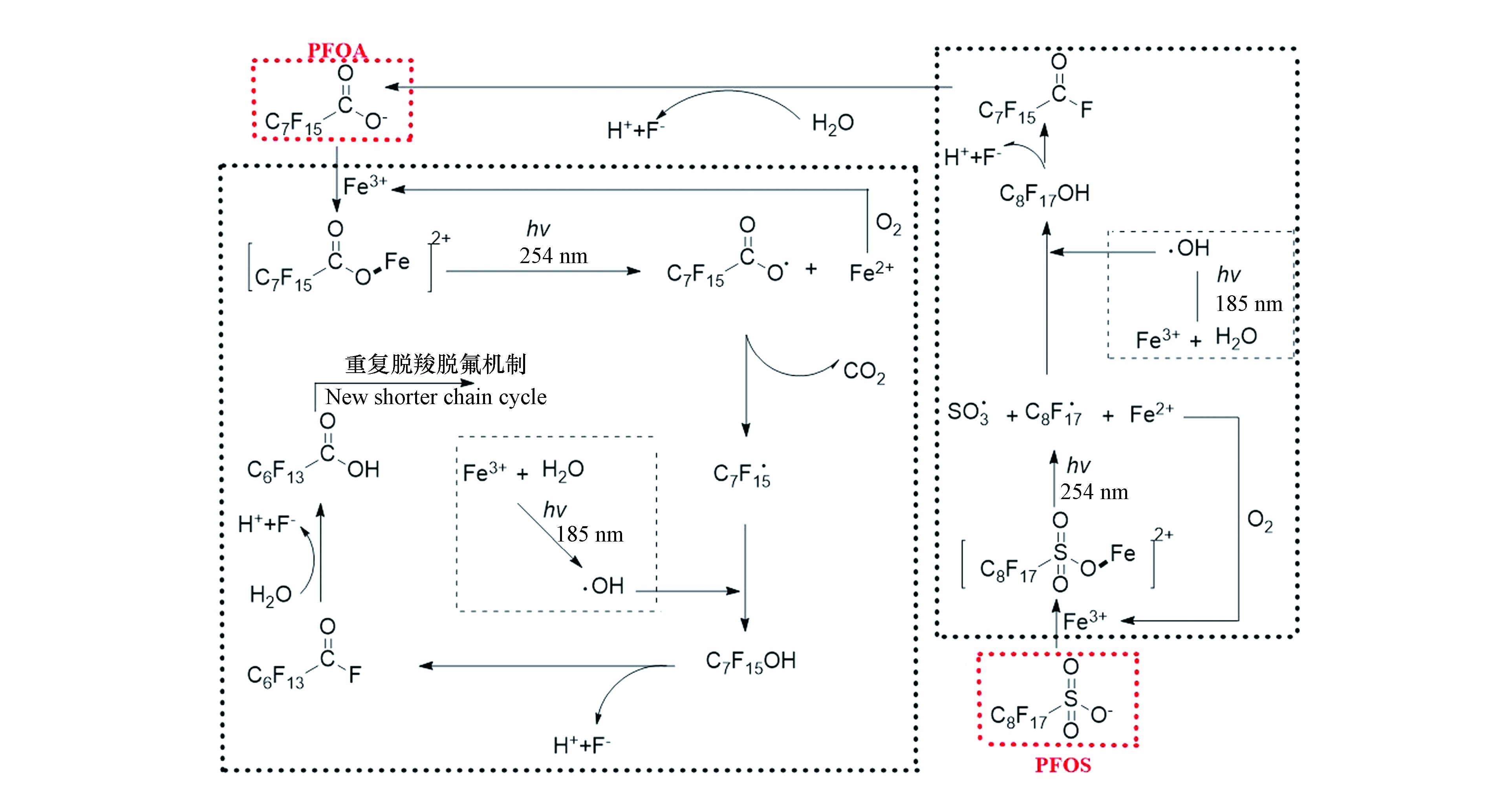
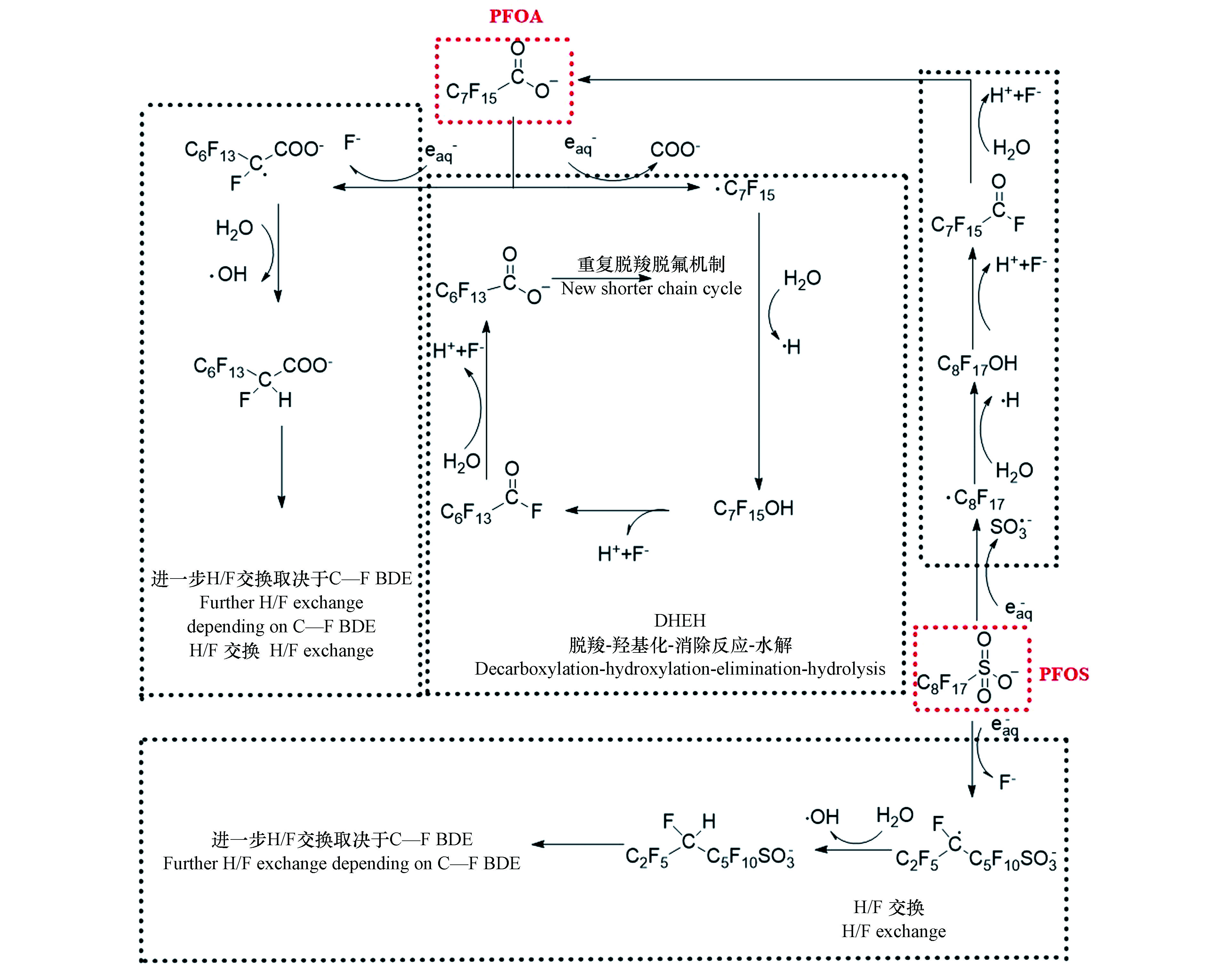
在早期的研究中,研究者推测PFOA和PFOS通过直接吸收UV/VUV后断裂C—C键,从而导致其降解[19,25]。近期研究表明,PFOA和PFOS通过直接吸收UV/VUV发生降解的可能性不大[41],其直接光降解机理有待进一步研究。目前,研究者普遍认为,PFOA、PFOS的直接光降解是一个还原过程,其中光生还原性物质,如
$ {\rm{e}}_{{\rm{aq}}}^ - $ ,起着主要的作用(式1和2)[26]。$ {\rm{e}}_{{\rm{aq}}}^ - $ 还原降解PFOA的降解机理如图1所示,主要包括两种途径:①脱羧-羟基化-消除反应-水解(decarboxylation-hydroxylation-elimination-hydrolysis,DHEH):PFOA受末端—COO—基团的影响,—CF2—链中与羧基相连的C原子具有较高的电子云密度,因此易被$ {\rm{e}}_{{\rm{aq}}}^ - $ 进攻,从而导致PFOA发生脱羧反应形成·C7F15[42];·C7F15具有很高的活性,易与·OH或H2O反应,羟基化形成C7F15OH;羟基会与连接在同一C原子上的F原子竞争电子,导致C7F15OH易发生消除反应脱去F原子和H原子生成C6F13COF;C6F13COF进一步水解生成C6F13COOH;在$ {\rm{e}}_{{\rm{aq}}}^ - $ 作用下,C6F13COOH会重复DHEH等一系列反应,形成碳链更短的全氟羧酸(PFCAs),直至彻底降解为F−和CO2[43]。②H/F交换:被$ {\rm{e}}_{{\rm{aq}}}^ - $ 进攻后,密度泛函理论计算结果显示,PFOA中靠近羧酸基团的碳原子具有较高的电子云密度,与此C原子连接的C—F键自发延伸[41],从而导致PFOA易发生H/F交换行为生成C7F14HCOO−;根据键离解能(BDE),C7F14HCOO−进一步与$ {\rm{e}}_{{\rm{aq}}}^ - $ 反应,重复H/F交换过程[41]。由于末端基团不同,
$ {\rm{e}}_{{\rm{aq}}}^ - $ 还原降解PFOS降解机理与PFOA略有不同,可分为2种途径(图1):①碳链断裂:$ {\rm{e}}_{{\rm{aq}}}^ - $ 进攻—CF2—链中与磺酸基团相连的具有较高电子云密度的C原子,C—S键自发延伸,从而导致PFOS脱去磺酸基团生成·C8F17[44];$ {\rm{F}}_{17}^ - $ 经过羟基化、消除反应和水解等一系列反应生成PFOA,PFOA重复DHEH反应最终降解为F−和CO2。②H/F交换:密度泛函理论计算显示PFOS中间的两个C具有较高的电子云密度,易被$ {\rm{e}}_{{\rm{aq}}}^ - $ 进攻,此C原子上C—F键自发延伸,从而导致PFOS发生H/F交换行为生成C8F16$ {\rm{HSO}}_{^3}^ - $ [41,44-45];根据键离解能(BDE),C8F16$ {\rm{HSO}}_{^3}^ - $ >进一步与$ {\rm{e}}_{{\rm{aq}}}^ - $ 反应,重复H/F交换过程[41]。虽然UV可以降解PFOA和PFOS,但是VUV对PFOA和PFOS的降解效果更好。这是因为VUV具有更高的辐射能,从而提高活性物种的产率,例如185 nm的VUV直接分解水分子产生H和·OH[46]。此外,185 nm的VUV既可以直接激发C—C键断裂,又可以激发C—F键断裂;而254 nm的UV很难激发C—F键断裂[24]。
-
为了进一步提高PFOA/PFOS光降解效率,HA、FeCl3、Na2S2O8、TiO2等光敏剂、氧化剂或催化剂被用于提高光利用率或·OH、
$ {\rm{SO}}_4^{-}\cdot $ 等氧化性活性物种的产率。下面列举了不同条件下PFOA/PFOS的氧化降解机理:(1)UV/Fe3+体系反应机理
如图2所示,Fe3+可以通过与PFOA/PFOS络合(式3)提高它们的光吸收和利用效率(式4),同时从羧基或磺酸基团相邻的C原子上得到电子氧化PFOA/PFOS,从而导致PFOA/PFOS失去一个电子形成C7F15COO·/C8F17SO3·,进而提高其降解效率[20,27,29-30,47]。另外,Fe3+氧化PFOA/PFOS后形成的Fe2+(式5),易被空气中的O2氧化生成Fe3+,Fe3+又可继续氧化目标化合物。但由于Fe(Ⅲ)不稳定,在中性或碱性条件下易沉淀,因此UV/Fe3+体系降解PFOA/PFOS的效率受pH值影响很大。当pH值为2—4时,PFOA的脱氟率约30%—40%;当pH值大于4时,氢氧化铁沉淀出现,Fe3+与目标化合物的络合效率会降低;当pH值大于5时,PFOA的脱氟率低于12%。
(2)UV/TiO2体系反应机理
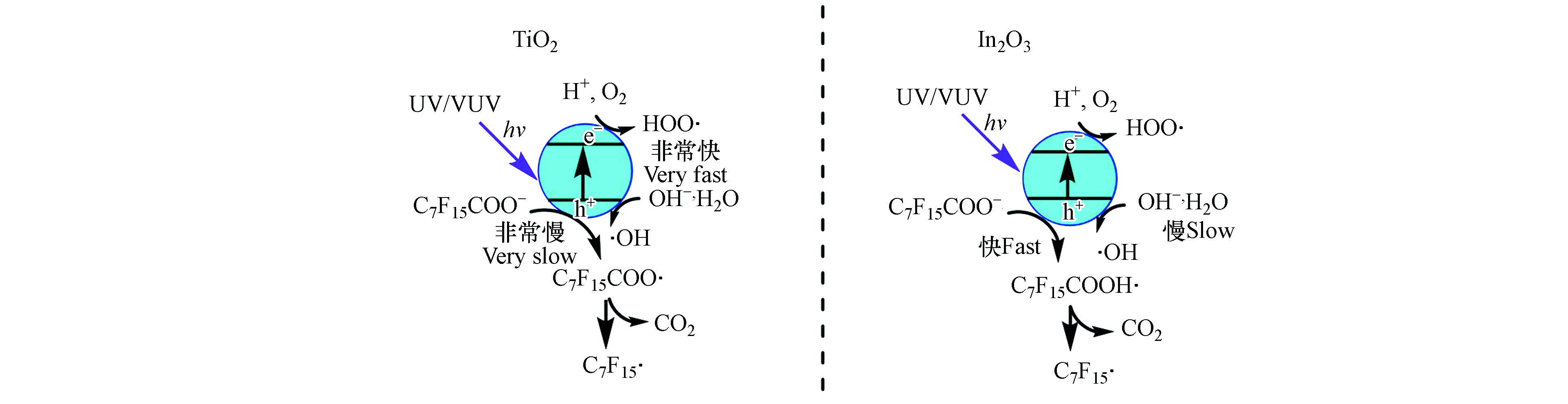
UV/TiO2体系光催化降解PFOA机理,如图3所示。吸收UV或VUV后[48],TiO2表面会形成光生空穴(h+)和电子(e−)(式6),h+与其表面的H2O或HO−反应形成游离的·OH(式7和8)。空穴可氧化吸附于TiO2表面的PFOA,PFOA被氧化成C7F15COO·(式9)[42]。而由于磺酸基团吸电子能力比羧酸基团强,PFOS比PFOA更稳定,不能被TiO2光催化降解[49-50]。
如图3所示,h+是氧化降解PFOA的关键活性物种,虽然·OH等自由基也参与PFOA的降解过程,但是很难直接与PFOA反应。TiO2对PFOA的吸附效果不佳、光利用率不高且光生载流子易复合导致量子效率低,因此TiO2复合催化剂被用于提高PFOA的光催化降解效率[50]。C-TiO2纳米管复合材料可通过增强对紫外-可见光吸收而提高对PFOA的降解效率[31];过渡金属修饰的TiO2催化剂,如Fe-TiO2和Cu-TiO2等,可以通过捕获光生电子抑制电子和h+复合促进PFOA的降解[33];沸石修饰的TiO2复合材料可以通过增加表面积来提高反应活性,进而达到增强PFOA降解效果的目的[34]。虽然TiO2比In2O3的量子产率高,但因为PFOA的羧基端与In2O3表面呈二齿配位连接,导致In2O3比TiO2更易直接氧化PFOA,因此In2O3对PFOA的光催化效率远高于TiO2[36,51],其主要降解途径如图3所示。有研究对比了纳米多孔微球、纳米片和纳米块等3种形态的In2O3对PFOA的光催化效果,发现纳米多孔微球因具有更高的氧空位浓度而对PFOA的光催化效率最高[37,52]。此外,Ca2O3也因其表面能与PFOA紧密键合而可以高效降解PFOA[53]。
(3)UV/S2
$ {\rm{O}}_8^{2-} $ 体系反应机理S2
$ {\rm{O}}_8^{2-} $ 吸收UV后生成$ {\rm{SO}}_4^{-}\cdot $ (式10),$ {\rm{SO}}_4^{-}\cdot $ 具有强氧化性,可从靠近羧酸或磺酸端的C原子上夺取一个电子氧化PFOA/PFOS(式11),从而导致PFOA/PFOS失去电子分别形成C7F15COO·、C8F17SO3·[35,54],进而提高光降解效率。从上述反应过程中可以看出,TiO2、FeCl3和Na2S2O8通过吸收UV产生h+、·OH、
$ {\rm{SO}}_4^{-}\cdot $ 等不同的活性物种降解PFOA/PFOS,但C7F15COO·/C8F17SO3·是这些降解途径中的共同降解中间体。C7F15COO·的降解机理:受羧酸基团的影响,C7F15COO·骨架中靠近羧基端的C—C键最不稳定,因此其末端C—C键最先断裂,继而生成C7F15·与CO2;生成的C7F15·不稳定,易水解或者与•OH反应生成C7F15OH;C7F15OH进一步发生消除反应生成C6F13COF,再通过水解生成C6F13COOH;C6F13COOH可以重复C—C键断裂、C—F键断裂过程,直至完全降解为CO2和F−。C8F17SO3·的降解机理:—CF2—链中与磺酸基团相连的具有较高电子云密度的C原子,C—S键自发延伸,从而导致C8F17SO3·脱去磺酸基团生成C8F17·;C8F17·通过水解、消除反应生成C6F13COOH;C7F15COOH的降解机理如前面所述。PFOA和PFOS具有较强的抗氧化性,即使使用强氧化试剂降解PFOA和PFOS,其降解效率也较低,降解所需时间较长,且脱氟率不高[43]。循环伏安曲线显示,诱导全氟烷基化合物的氧化电势为+2.0 V(标准氢电极),而还原电势仅为−1.0 V,说明从热动力学角度看,全氟烷基化合物发生更容易还原反应[28]。·OH难以直接氧化PFOA/PFOS,h+和
$ {\rm{SO}}_4^{-}\cdot $ 的氧化效果较·OH好,但是这些氧化性活性物种不能彻底降解PFOA/PFOS[55],因此还原性光降解技术引起了研究者的关注。 -
$ {\rm{e}}_{{\rm{aq}}}^ - $ 的产率是影响还原性光降解PFOA/PFOS效率的关键因素,因此许多研究者通过添加还原剂或光敏剂来提高$ {\rm{e}}_{{\rm{aq}}}^ - $ 的产率,从而提高PFOA/PFOS的降解效率。但这些方法只是提高了$ {\rm{e}}_{{\rm{aq}}}^ - $ 的产率,促进了·C7F15COO2-/·C8F17$ {\rm{SO}}_3^{2-}$ 的生成,后续·C7F15COO2−/·C8F17$ {\rm{SO}}_3^{2-} $ 的降解途径与直接光降解中所描述的降解途径一致。下面列举了不同还原剂和光敏剂作用下,$ {\rm{e}}_{{\rm{aq}}}^ - $ 的形成机理:(4)UV/
$ {\rm{SO}}_3^{2-} $ 反应机理在UV/
$ {\rm{SO}}_3^{2-} $ 体系中,$ {\rm{SO}}_3^{2-} $ 吸收UV后产生$ {\rm{e}}_{{\rm{aq}}}^ - $ 和$ {\rm{SO}}_3^{-} $ ·(式12),部分$ {\rm{e}}_{{\rm{aq}}}^ - $ 会被H+、OH−和副产物S2$ {\rm{O}}_6^{2-} $ (式13)消耗[22]。(5)UV/I−反应机理
在UV/I−体系中,I−水溶液在光照条件下通过电子转移跃迁生成激发态的I·H2O−*(14)。I·H2O−*包含(I·,e−)复合体和H2O(式15)。(I·,e−)复合体可衰减产生I·和
$ {\rm{e}}_{{\rm{aq}}}^ - $ (式16)[28,39],同时UV/I−体系也会产生消耗$ {\rm{e}}_{{\rm{aq}}}^ - $ 的$ {\rm{I}}_3^ - $ 副产物。(6)UV/
$ {\rm{Fe}}\left( {{\rm{CN}}} \right)_6^{4 - } $ 反应机理在UV/
$ {\rm{Fe}}\left( {{\rm{CN}}} \right)_6^{4 - } $ 体系中,$ {\rm{Fe}}\left( {{\rm{CN}}} \right)_6^{4 - } $ 吸收UV产生$ {\rm{e}}_{{\rm{aq}}}^ - $ 和$ {\rm{Fe}}\left( {{\rm{CN}}} \right)_6^{3 - } $ (式17),但是此方法产生的${\rm{e}}_{{\rm{aq}}}^ - $ 很容易被氧化且半衰期短,需要引入其他物质(如三乙酸)来提高电子利用效率[56]。(7)UV/吲哚反应机理
在UV/Indole体系,吲哚吸收UV转变为激发态,激发态通过电荷分离产生
$ {\rm{e}}_{{\rm{aq}}}^ - $ 和Indole+(式18)[57-58]。研究表明,蒙脱土可以吸附PFOA和吲哚,从而增加紫外光下吲哚产生的
$ {\rm{e}}_{{\rm{aq}}}^ - $ 与PFOA的接触几率,进而提高$ {\rm{e}}_{{\rm{aq}}}^ - $ 的利用率,加快PFOA的光降解过程,并且此反应只受溶解氧和pH的影响[38,59]。另外,HA也可通过与蒙脱土类似的机理提高$ {\rm{e}}_{{\rm{aq}}}^ - $ 的利用率,加快PFOS的降解速度,降解0.03 mmol·L−1 PFOS的最佳HA浓度为1.0 mg·L−1,环境中的HA浓度也在mg·L−1级别[39],说明此研究具有很重要的环境意义。值得注意的是,在UV/KI体系中,PFOS对$ {\rm{e}}_{{\rm{aq}}}^ - $ 的利用率较高,其光降解效率甚至比PFOA更高[60]。 -
PFOA和PFOS是广泛存在于自然界的典型持久性有机污染物,其光降解技术和机理研究早已成为环境领域持续关注的重点。本文重点介绍了PFOA/PFOS光降解技术和机理的研究现状,以期为开发更安全、高效的光降解技术提供有用的信息。
同时,光降解技术也存在一些需要解决的问题。现阶段大部分PFOA/PFOS光降解技术的研究还处于实验层面,光降解技术在污水处理工艺中的应用有一定的难度。通过以下几点,对今后的研究工作提出展望:
(1)PFOA/PFOS直接光降解技术面临光源能量不足的问题,因此提高光利用率或开发更高效的光源是解决这一问题的重要策略。使用功率更大和发光波长短于220 nm的光源,将有助于解决这一问题。
(2)PFOA氧化性光降解技术大多采用非均相催化剂吸附并氧化目标化合物,面临催化剂氧化效果不佳的问题。提高催化剂吸附效果和光利用率、抑制光生载流子复合将有助于提高光量子效率。例如,过渡金属修饰的TiO2催化剂可以通过捕获光生电子抑制光生载流子复合促进PFOA的降解[33];沸石修饰的TiO2复合材料可以通过增加表面积来提高反应活性[34];纳米多孔微球形的In2O3因比块状和片状的In2O3具有更高的氧空位浓度而对PFOA的光催化效率更高[37,52]。一方面,对氧化性光催化剂进行改性,如金属掺杂、非金属掺杂、稀土金属掺杂、贵金属沉积等措施,可能会抑制光生载流子复合达到提高光降解效率的目的。另一方面,纳米形态的催化剂具有独特的性质,在PFOA/PFOS废水处理中的应用已成为当前的研究热点。
(3)PFOA/PFOS还原性光降解技术比较成熟,并且已经应用到PFOA污水处理的工艺中。其面临的问题是过程中的副产物有可能对环境造成威胁。例如,PFOA还原性光降解技术中,以KI为催化剂时,产物中可能出现CFHI2、C2F4HI、C5F6HI、C6F13I和C6F10HI等化合物;以亚硫酸盐为催化剂时,产物中可能出现C7F15
$ {\rm{SO}}_3^{-} $ 、C6F13$ {\rm{SO}}_3^{-} $ 、C5F11$ {\rm{SO}}_3^{-} $ 、C4F9$ {\rm{SO}}_3^{-} $ 、和C3F7$ {\rm{SO}}_3^{-} $ 等化合物[41]。如何彻底去除PFOA/PFOS还原性光降解过程中的副产物,也是当前研究者们关注的热点。(4)PFOA和PFOS主要吸收波长小于220 nm的UV以及VUV,而地球表面不存在这些高能紫外光。目前已有少量研究表明,可见光可用于光催化降解PFOA。若能实现可见光在PFOA和PFOS降解中的应用,对开发新型PFOA/PFOS光降解技术和实现PFOA/PFOS的安全高效降解具有重要意义。
(5)PFOA/PFOS光降解后得到的部分短链PFCAs和短链全氟磺酸化合物(PFSAs)仍具有毒性和环境持久性,因此PFOA/PFOS降解产物的鉴别和进一步处理应引起研究者的关注,而实现其彻底矿化是PFOA/PFOS降解的终极挑战。
(6)在开发高效的PFOA/PFOS降解技术的同时,寻找替代品减少其排放也是研究者关注的重点。有研究表明,F/H交换行为不利于PFOA后续脱氟[44],而醚键的引入有助于促进PFOA深度脱氟[45],种类繁多的含醚键替代品也应运而生。但也有研究表明,部分全氟化合物在引入醚键后,其生物累积性反而增加了。深入研究PFOA/PFOS的降解机理,阐明其结构与稳定性和生物毒性之间的内在联系,将有助于开发新型PFOA/PFOS替代品。
全氟辛酸和全氟辛基磺酸的光降解技术及机理研究进展
Photodegradation technology and mechanism of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS):A critical review
-
摘要: 目前,普遍存在于各种环境介质中的全氟辛酸(PFOA)和全氟辛基磺酸(PFOS)造成的环境污染问题已引起全球的广泛关注。PFOA和PFOS具有稳定性、持久性和生物累积性等特点。常规的方法如:超声降解法、电化学氧化法和微生物降解法等,很难将其彻底降解,因此开发有效的PFOA和PFOS降解技术成为了环境领域的研究重点。近年来,光降解技术在有效去除环境介质中的PFOA和PFOS方面显示出巨大潜力和优良的应用前景,并引起科学研究者的广泛关注。因此,本论文系统综述了近年来国内外关于PFOA和PFOS光降解方面的研究报道,重点对比了不同催化剂、氧化剂和光敏剂对PFOA和PFOS光降解效率的影响及降解机理。同时系统分析了目前PFOA和PFOS光降解技术可能存在的问题及其应用前景,以期为开发安全有效的PFOA和PFOS光降解技术提供全面的科学信息,并为光降解PFOA和PFOS的发展方向提供参考意见。Abstract: At present, the ubiquitous occurrence of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS) has resulted in global environmental pollution and caused worldwide concerns. Owing to their stability, persistence and bioaccumulation, PFOA and PFOS are difficult to be degraded by using conventional methods, such as ultrasonic degradation, electrochemical oxidation or microbial degradation. Therefore, development of effective technologies for decomposing PFOA and PFOS into harmless species is now the research priority. Currently, the great potential and excellent application prospects of photodegradation technology on the PFOA and PFOS degradation have received considerable attentions from the researchers. Here we systematically summarized the domestic and overseas studies on the photodegradation of PFOA and PFOS done in recent years. The photodegradation efficiencies and mechanisms of PFOA and PFOS with different catalysts, oxidants, or photosensitizer were reviewed in details. The possible problems and application potentials of the PFOA and PFOS photodegradation technology were systematically discussed. The aim of this review is to provide comprehensive information for the development of effective and safe photodegradation technology for PFOA and PFOS degradation, and provide scientific guidance for the direction of these technologies.
-
Key words:
- perfluorinated compounds /
- PFOA /
- PFOS /
- photodegradation /
- degradation mechanism
-

-
表 1 PFOA/PFOS氧化性光降解技术
Table 1. PFOA/ PFOS oxidative photo degradation technology
化合物
Compounds催化剂
Catalysts初始浓度
Initial concentration功率/波长
Power/ Wavelength反应时间
Reaction time降解率/脱氟率
Degradation ratio/Defluorination ratio参考文献
ReferencesPFOA Fe2(SO4)3 48.3 μmol·L−1 实际太阳光 672 h 97.8%/12.7% [29] PFOA FeCl3·6H2O 36 μmol·L−1 12 W/185 nm 4 h 51.21%/46.7% [20] PFOA FeSO4/ ${ {\rm{S} }_2}{\rm{O} }_8^{2 - }$ 20.0 mmol·L−1 9 W/254 nm 5 h 93.9%/63.3% [30] PFOA TiO2纳米管 72.46 μmol·L−1 300 W/365 nm 8 h 60%/— [31] PFOA TiO2 1208 μmol·L−1 23 W/254 nm 3 h 100%/16.5% [32] PFOA Fe/TiO2 120.8 μmol·L−1 400 W/254 nm 12 h 91%/19% [33] PFOA (Pt, Pd, Ag)-TiO2 144.9 μmol·L−1 18 W/365 nm 7 h 100%/34.8% [34] PFOA Na2S2O8 1350 μmol·L−1 200 W/254 nm 4 h 100%/59.1% [35] PFOS FeCl3 20 μmol·L−1 23 W/254 nm 72 h 100%/58.2% [27] PFOS 碱性异丙醇 40 μmol·L−1 32 W/254 nm 240 h 92%/— [25] PFOA In2O3 100 μmol·L−1 23 W/254 nm 4 h 78.9%/19.7% [36] PFOA In2O3 1208 μmol·L−1 15 W/254 nm 2 h 100%/— [37] —:无数据,no data. 表 2 PFOA/PFOS还原性光降解技术
Table 2. PFOA/ PFOS reductive photo degradation technology
化合物
Compounds催化剂
Catalysts初始浓度
Initial concentration功率/波长
Power/ Wavelength降解时间
Reaction time降解率/脱氟率
Degradation ratio/
Defluorination ratio参考文献
ReferencesPFOA Na2SO3 20 μmol·L−1 10 W/254 nm 24 h 100%/88.5% [22] PFOA KI 24 μmol·L−1 8 W/254 nm 2.5 h 15%/— [28] PFOA 蒙脱土 24.1 μmol·L−1 36 W/254 nm 10 h 100%/98% [38] PFOS KI 20 μmol·L−1 8 W/254 nm 2.5 h 30%/— [28] PFOS KI&HA 300 μmol·L−1 14 W/254 nm 1.5 h 86%/55.6% [39] PFOS Na2SO3 37.2 μmol·L−1 10 W/185 nm 4 h 97.3%/68.5% [40] —:无数据,no data. -
[1] LAU C, ANITOLE K, HODES C, et al. Perfluoroalkyl acids: A review of monitoring and toxicological findings [J]. Toxicological Sciences:An Official Journal of the Society of Toxicology, 2007, 99(2): 366-394. doi: 10.1093/toxsci/kfm128 [2] 罗梅清, 卓琼芳, 许振成, 等. 全氟化合物处理技术的研究进展 [J]. 环境科学与技术, 2015, 38(8): 60-67. LUO M Q, ZHUO Q F, XU Z C, et al. Research trends on degradation of perfluorinated compounds-a review [J]. Environmental Science & Technology, 2015, 38(8): 60-67(in Chinese).
[3] 张美, 楼巧婷, 邵倩文, 等. 全氟化合物污染现状及风险评估的研究进展 [J]. 生态毒理学报, 2019, 14(3): 30-53. ZHANG M, LOU Q T, SHAO Q W, et al. Research progress of perfluorinated compounds pollution status and risk assessment [J]. Asian Journal of Ecotoxicology, 2019, 14(3): 30-53(in Chinese).
[4] ZHANG Y Y, MOORES A, LIU J X, et al. New insights into the degradation mechanism of perfluorooctanoic acid by persulfate from density functional theory and experimental data [J]. Environmental Science & Technology, 2019, 53(15): 8672-8681. [5] GE H, YAMAZAKI E, YAMASHITA N, et al. Particle size specific distribution of perfluoro alkyl substances in atmospheric particulate matter in Asian cities [J]. Environ Science:Process & Impacts, 2017, 19(4): 549-560. [6] JOVICIC V, KHAN M J, ZBOGAR-RASIC A, et al. Degradation of low concentrated perfluorinated compounds (PFCs) from water samples using non-thermal atmospheric plasma (NTAP) [J]. Energies, 2018, 11(5): 1290-1304. doi: 10.3390/en11051290 [7] CHEN S, JIAO X C, GAI N, et al. Perfluorinated compounds in soil, surface water, and groundwater from rural areas in eastern China [J]. Environmental Pollution, 2016, 211: 124-131. doi: 10.1016/j.envpol.2015.12.024 [8] LAM J C W, LYU J L, KWOK K Y, et al. Perfluoroalkyl substances (PFASs) in marine mammals from the South China Sea and their temporal changes 2002–2014: concern for alternatives of PFOS? [J]. Environmental Science & Technology, 2016, 50(13): 6728-6736. [9] MACINNIS J J, FRENCH K, MUIR D C G, et al. Emerging investigator series: A 14-year depositional ice record of perfluoroalkyl substances in the High Arctic [J]. Environmental Science:Processes & Impacts, 2017, 19(1): 22-30. [10] RENNER R. Growing concern over perfluorinated chemicals [J]. Environmental Science & Technology, 2001, 35(7): 154-160. [11] GIESY J P, KANNAN K. Global distribution of perfluorooctane sulfonate in wildlife [J]. Environmental Science & Technology, 2001, 35(7): 1339-1342. [12] LASSEN C J A A, POTRYKUS A, CHRISTENSEN F, et al. Survey of PFOS, PFOA and other perfluoroalkyl and polyfluoroalkyl substances[R]. Environmental Project No. 1475, 2013. [13] SCHRÖDER H F. Determination of fluorinated surfactants and their metabolites in sewage sludge samples by liquid chromatography with mass spectrometry and tandem mass spectrometry after pressurised liquid extraction and separation on fluorine-modified reversed-phase sorbents [J]. Journal of Chromatography A, 2003, 1020(1): 131-151. doi: 10.1016/S0021-9673(03)00936-1 [14] LIOU J S C, SZOSTEK B, DERITO C M, et al. Investigating the biodegradability of perfluorooctanoic acid [J]. Chemosphere, 2010, 80(2): 176-183. doi: 10.1016/j.chemosphere.2010.03.009 [15] 卢丽娟, 唐敏康, 陈瑛, 等. 典型全氟化合物的去除技术研究进展 [J]. 四川环境, 2016, 35(5): 135-141. doi: 10.3969/j.issn.1001-3644.2016.05.027 LU L J, TANG M K, CHEN Y, et al. Research progress on the removal technologies of typical perfluorinated compounds [J]. Sichuan Environment, 2016, 35(5): 135-141(in Chinese). doi: 10.3969/j.issn.1001-3644.2016.05.027
[16] 王飞, 李晓明, 李建勇, 等. 水中全氟化合物的污染处理研究进展 [J]. 水处理技术, 2016, 42(11): 5-11. WANG F, LI X M, LI J Y, et al. Research progress on degradation of perfluorinated compounds in water [J]. Technology of Water Treatment, 2016, 42(11): 5-11(in Chinese).
[17] 许晨敏. 水中典型全氟化合物(PFCs)的吸附及光催化降解研究[D]. 南京: 南京理工大学, 2018. XU C M. Removal of typical perfluorinated compounds(PFCs) by adsorption and photocatalysis[D]. Nanjing: Nanjing University of Science & Technology, 2018 (in Chinese).
[18] 黄宏, 李圆杏, 杨红伟. 水环境中抗生素的光降解研究进展 [J]. 环境化学, 2013, 32(7): 1335-1341. doi: 10.7524/j.issn.0254-6108.2013.07.029 HUANG H, LI Y X, YANG H W. Research progress on photodegradation of antibiotics in aqueous solution [J]. Environmental Chemistry, 2013, 32(7): 1335-1341(in Chinese). doi: 10.7524/j.issn.0254-6108.2013.07.029
[19] HORI H, HAYAKAWA E, EINAGA H, et al. Decomposition of environmentally persistent perfluorooctanoic acid in water by photochemical approaches [J]. Environmental Science & Technology, 2004, 38(22): 6118-6124. [20] CHENG J H, LIANG X Y, YANG S W, et al. Photochemical defluorination of aqueous perfluorooctanoic acid (PFOA) by VUV/Fe3+ system [J]. Chemical Engineering Journal, 2014, 239: 242-249. doi: 10.1016/j.cej.2013.11.023 [21] CHATTERJEE D, DASGUPTA S. Visible light induced photocatalytic degradation of organic pollutants [J]. Journal of Photochemistry and Photobiology C:Photochemistry Reviews, 2005, 6(2): 186-205. [22] SONG Z, TANG H, WANG N, et al. Reductive defluorination of perfluorooctanoic acid by hydrated electrons in a sulfite-mediated UV photochemical system [J]. Journal of Hazardous Materials, 2013, 262: 332-338. doi: 10.1016/j.jhazmat.2013.08.059 [23] GIESY J P, MABURY S A, MARTIN J W, et al. Perfluorinated compounds in the great lakes[M]. Springer Berlin Heidelberg, 2006: 391-438. [24] CHEN J, ZHANG P Y, LIU J. Photodegradation of perfluorooctanoic acid by 185 nm vacuum ultraviolet light [J]. Journal of Environmental Sciences, 2007, 19(4): 387-390. doi: 10.1016/S1001-0742(07)60064-3 [25] YAMAMOTO T, NOMA Y, SAKAI S I, et al. Photodegradation of perfluorooctane sulfonate by UV irradiation in water and alkaline 2-propanol [J]. Environmental Science & Technology, 2007, 41(16): 5660-5665. [26] LYU X J, LI W W, LAM P K, et al. Insights into perfluorooctane sulfonate photodegradation in a catalyst-free aqueous solution [J]. Scientific Reports, 2015, 5(1): 9353. doi: 10.1038/srep09353 [27] JIN L, ZHANG P Y, SHAO T, et al. Ferric ion mediated photodecomposition of aqueous perfluorooctane sulfonate (PFOS) under UV irradiation and its mechanism [J]. Journal of Hazardous Materials, 2014, 271: 9-15. doi: 10.1016/j.jhazmat.2014.01.061 [28] PARK H, VECITIS C D, CHENG J, et al. Reductive degradation of perfluoroalkyl compounds with aquated electrons generated from iodide photolysis at 254 nm [J]. Photochemical & Photobiological Sciences:Official Journal of the European Photochemistry Association and the European Society for Photobiology, 2011, 10(12): 1945-1953. [29] LIU D D, XIU Z M, LIU F, et al. Perfluorooctanoic acid degradation in the presence of Fe(Ⅲ) under natural sunlight [J]. Journal of Hazardous Materials, 2013, 262: 456-463. doi: 10.1016/j.jhazmat.2013.09.001 [30] SONG Z, TANG H Q, WANG N, et al. Activation of persulfate by UV and Fe 2+ for the defluorination of perfluorooctanoic acid [J]. Advances in Environmental Research, 2014, 3(3): 185-197. doi: 10.12989/aer.2014.3.3.185 [31] SONG C, CHEN P, WANG C Y, et al. Photodegradation of perfluorooctanoic acid by synthesized TiO2–MWCNT composites under 365nm UV irradiation [J]. Chemosphere, 2012, 86(8): 853-859. doi: 10.1016/j.chemosphere.2011.11.034 [32] WANG Y, ZHANG P Y. Photocatalytic decomposition of perfluorooctanoic acid (PFOA) by TiO2 in the presence of oxalic acid [J]. Journal of Hazardous Materials, 2011, 192(3): 1869-1875. doi: 10.1016/j.jhazmat.2011.07.026 [33] CHEN M J, LO S L, LEE Y C, et al. Photocatalytic decomposition of perfluorooctanoic acid by transition-metal modified titanium dioxide [J]. Journal of Hazardous Materials, 2015, 288: 168-175. doi: 10.1016/j.jhazmat.2015.02.004 [34] ESKANDARIAN M R, FAZLI M, RASOULIFARD M H, et al. Decomposition of organic chemicals by zeolite-TiO2 nanocomposite supported onto low density polyethylene film under UV-LED powered by solar radiation [J]. Applied Catalysis B:Environmental, 2016, 183: 407-416. doi: 10.1016/j.apcatb.2015.11.004 [35] HORI H, YAMAMOTO A, HAYAKAWA E, et al. Efficient decomposition of environmentally persistent perfluorocarboxylic acids by use of persulfate as a photochemical oxidant [J]. Environmental Science & Technology, 2005, 39(7): 2383-2388. [36] LI X Y, ZHANG P Y, JIN L, et al. Efficient photocatalytic decomposition of perfluorooctanoic acid by indium oxide and its mechanism [J]. Environmental Science & Technology, 2012, 46(10): 5528-5534. [37] LI Z M, ZHANG P Y, SHAO T, et al. Different nanostructured In2O3 for photocatalytic decomposition of perfluorooctanoic acid (PFOA) [J]. Journal of Hazardous Materials, 2013, 260: 40-46. doi: 10.1016/j.jhazmat.2013.04.042 [38] TIAN H T, GU C. Effects of different factors on photodefluorination of perfluorinated compounds by hydrated electrons in organo-montmorillonite system [J]. Chemosphere, 2018, 191: 280-287. doi: 10.1016/j.chemosphere.2017.10.074 [39] SUN Z Y, ZHANG C J, CHEN P, et al. Impact of humic acid on the photoreductive degradation of perfluorooctane sulfonate (PFOS) by UV/Iodide process [J]. Water Research, 2017, 127: 50-58. doi: 10.1016/j.watres.2017.10.010 [40] GU Y R, LIU T Z, WANG H J, et al. Hydrated electron based decomposition of perfluorooctane sulfonate (PFOS) in the VUV/sulfite system [J]. Science of the Total Environment, 2017, 607-608: 541-548. doi: 10.1016/j.scitotenv.2017.06.197 [41] CUI J K, GAO P P, YANG D. Destruction of per- and polyfluoroalkyl substances (PFAS) with advanced reduction processes (ARPs): A critical review [J]. Environmental Science & Technology, 2020, 54(7): 3752-3766. [42] QU Y, ZHANG C, LI F, et al. Photo-reductive defluorination of perfluorooctanoic acid in water [J]. Water Research, 2010, 44(9): 2939-2947. doi: 10.1016/j.watres.2010.02.019 [43] 杨波, 李影影, 余刚, 等. 物化作用氧化降解PFOA/PFOS [J]. 化学进展, 2014, 26(7): 1265-1274. YANG B, LI Y Y, YU G, et al. Oxidative degradation of PFOA /PFOS with physicochemical techniques [J]. Progress in Chemistry, 2014, 26(7): 1265-1274(in Chinese).
[44] BENTEL M J, YU Y C, XU L H, et al. Defluorination of per- and polyfluoroalkyl substances (PFASs) with hydrated electrons: Structural dependence and implications to PFAS remediation and management [J]. Environmental Science & Technology, 2019, 53(7): 3718-3728. [45] BAO Y X, HUANG J, CAGNETTA G, et al. Removal of F-53B as PFOS alternative in chrome plating wastewater by UV/Sulfite reduction [J]. Water Research, 2019, 163: 114907. doi: 10.1016/j.watres.2019.114907 [46] ZOSCHKE K, BÖERNICK H, WORCH E. Vacuum-UV radiation at 185 nm in water treatment-a review [J]. Water Research, 2014, 52: 131-145. doi: 10.1016/j.watres.2013.12.034 [47] HORI H, NAGAOKA Y, YAMAMOTO A, et al. Efficient decomposition of environmentally persistent perfluorooctanesulfonate and related fluorochemicals using zerovalent iron in subcritical water [J]. Environmental Science & Technology, 2006, 40(3): 1049-1054. [48] KORMANN C, BAHNEMANN D W, HOFFMANN M R. Photolysis of chloroform and other organic molecules in aqueous titanium dioxide suspensions [J]. Environmental Science & Technology, 1991, 25(3): 494-500. [49] HIDAKA H, JOU H, NOHARA K, et al. Photocatalytic degradation of the hydrophobic pesticide permethrin in fluoro surfactant / TiO2 aqueous dispersions [J]. Chemosphere, 1992, 25(11): 1589-1597. doi: 10.1016/0045-6535(92)90307-D [50] YUAN Q Z, RAVIKRISHNA R, VALSARAJ K T. Reusable adsorbents for dilute solution separation. 5. Photodegradation of organic compounds on surfactant-modified titania [J]. Separation and Purification Technology, 2001, 24(1): 309-318. [51] DA SILVA F L, LAITINEN T, PIRILÄ M, et al. Photocatalytic degradation of perfluorooctanoic acid (PFOA) from wastewaters by TiO2, In2O3 and Ga2O3 Catalysts [J]. Topics in Catalysis, 2017, 60(17-18): 1345-1358. doi: 10.1007/s11244-017-0819-8 [52] ZHANG W L, EFSTATHIADIS H, LI L Y, et al. Environmental factors affecting degradation of perfluorooctanoic acid (PFOA) by In2O3 nanoparticles [J]. Journal of Environmental Sciences, 2020, 93(7): 48-56. [53] TAN X J, CHEN G H, XING D Y, et al. Transition metal-modified Ga2O3 hierarchical nanosheets as efficient photocatalysts for the degradation of perfluorooctanoic acid [J]. Environmental Science:Nano, 2020, 7(8): 2229-2239. doi: 10.1039/D0EN00259C [54] YANG S W, CHENG J H, SUN J, et al. Defluorination of aqueous perfluorooctanesulfonate by activated persulfate oxidation [J]. Plos One, 2013, 8(10): 74877-74886. doi: 10.1371/journal.pone.0074877 [55] TROJANOWICZ M, BOJANOWSKA C A, BARTOSIEWICZ I, et al. Advanced oxidation/reduction processes treatment for aqueous perfluorooctanoate (PFOA) and perfluorooctanesulfonate (PFOS) – a review of recent advances [J]. Chemical Engineering Journal, 2018, 336: 170-199. doi: 10.1016/j.cej.2017.10.153 [56] HUANG L, DONG W B, HOU H Q. Investigation of the reactivity of hydrated electron toward perfluorinated carboxylates by laser flash photolysis [J]. Chemical Physics Letters, 2007, 436(1): 124-128. [57] MIALOCQ J C, AMOUYAL E, BERNAS A, et al. Picosecond laser photolysis of aqueous indole and tryptophan [J]. The Journal of Physical Chemistry, 1982, 86(16): 3173-3177. doi: 10.1021/j100213a022 [58] TIAN H T, GUO Y, PAN B, et al. Enhanced photoreduction of nitro-aromatic compounds by hydrated electrons derived from indole on natural montmorillonite [J]. Environmental Science & Technology, 2015, 49(13): 7784-7792. [59] TIAN H, GAO J, LI H, et al. Complete defluorination of perfluorinated compounds by hydrated electrons generated from 3-indole-acetic-acid in organomodified montmorillonite [J]. Scientific Reports, 2016, 6(1): 32949-32958. doi: 10.1038/srep32949 [60] PARK H, VECITIS C D, CHENG J, et al. Reductive defluorination of aqueous perfluorinated alkyl surfactants: Effects of ionic headgroup and chain length [J]. The Journal of Physical Chemistry, 2009, 113: 690-696. doi: 10.1021/jp807116q -



 下载:
下载: