-
抗生素等新污染物引起的环境问题已引起广泛关注. 四环素(TC)是目前应用最多,最广泛的广谱抗生素之一[1],主要用于临床医疗和畜禽养殖,而废水中残留的TC进入环境水体后对水生生物和人体存在潜在威胁[2]. 但已有污水处理设施对TC的去除效果有限,基于活化过一硫酸盐(PMS)的高级氧化技术(AOPs)可高效降解四环素类抗生素,在含TC污水的治理中具有广阔应用前景[3].
合适的催化剂可实现PMS的高效活化,进而促进TC的降解. 生物炭具有理化性质易调控,原料来源广泛,环境友好且成本低廉的优点,是一种良好的PMS催化剂[4],但生物炭的催化效能和循环利用性能有待进一步提升. 零价铁具有强还原性和环境友好的优点[5],也存在不稳定和易团聚的缺点[6-7],通过耦合生物炭和零价铁,可实现PMS的高效活化,如陈家玮教授等团队研究发现零价铁修饰可显著改善生物炭的催化效能[8-10]. 值得注意的是,本课题组近期发现球磨可改变生物炭的表面含氧官能团和缺陷结构,进而调控其活化PMS的效能[11],因此推测使用球磨生物炭负载零价铁可获得高效的PMS催化剂. 但目前球磨生物炭负载零价铁活化PMS降解TC的性能和内在驱动机制尚需进一步的探究.
本研究利用水热法制备了球磨生物炭负载零价铁复合材料,并将其作为PMS催化剂用于降解TC. 结合SEM、TEM、EDS mapping、BET、FTIR、XRD、XPS 和EPR等一系列表征,分析催化剂的理化性质. 研究复合材料活化PMS降解TC的效能,探寻最佳反应条件,并对其循环利用性能及抗干扰能力进行测试,有望为生物质的资源化利用,生物炭负载零价铁材料/PMS类芬顿体系去除水中抗生素提供理论支持.
-
商用椰壳生物炭购买于江苏宜青活性炭有限公司. 盐酸四环素(TC)、过一硫酸氢钾复合盐(K5H3S4O18,PMS)、 腐殖酸(HA)、L-组氨酸(L-histidine)、聚乙二醇-4000、对苯醌(1,4-Benzoquinone)、超氧化物歧化酶(SOD)购买于上海麦克林生化科技有限公司. 乙醇(EtOH)、叔丁醇(TBA)购于天津富宇精细化工有限公司. Na2SO4、NaCl、NaNO3、NaBH4、NaOH、HCl购于天津大茂化学试剂有限公司. FeSO4·7H2O购于阿拉丁试剂(上海)有限公司.
-
(1)球磨生物炭的制备:首先将生物炭(BC)通过行星式球磨机球磨处理. 将总质量为100 g的氧化锆球放入容积为100 mL的不锈钢球磨罐中,再放入生物炭开始球磨. 然后制备不同球质比的球磨生物炭,将2.5、1.25、0.5 g生物炭和氧化锆球加入球磨罐中,转速设置为500 r·min−1,球磨时间为2 h,所得球磨生物炭分别命名为BC40-2、BC80-2和BC200-2. 最后制备不同球磨时间的球磨生物炭. 将2.5 g生物炭和氧化锆球加入球磨罐中,在转速500 r·min−1时,球磨时间分别为0.5、2、4 h条件下制备的球磨生物炭命名为BC40-0.5、BC40-2和BC40-4.
(2)球磨生物炭负载零价铁复合材料的制备:将4.96 g FeSO4·7H2O溶解到100 mL乙醇水溶液中(乙醇:水=3:7,体积比),加入0.25 g BC40-0.5,同时加入0.5 g聚乙二醇-4000,通入氮气,在四口烧瓶中搅拌40 min,用0.1 mol·L−1的NaOH溶液调节溶液pH值至6,随后,将新配制的1 mol·L−1 NaBH4 溶液50 mL以1—2滴·s−1的速度逐滴滴加到上述溶液中. NaBH4溶液滴加完成后,继续搅拌30 min,用磁铁将得到的材料与溶液分离,用去离子水与无水乙醇交替清洗3次,冷冻干燥后存放在充满氮气的棕色玻璃瓶中储存备用. 得到的复合材料命名为ZVI/0.25BC40-0.5. 当加入的球磨生物炭的质量为0.5、0.75、1.00、1.25 g时,得到的复合材料分别命名为ZVI/0.5BC40-0.5、ZVI/0.75BC40-0.5、ZVI/1BC40-0.5和ZVI/1.25BC40-0.5.
(3)零价铁(ZVI)的制备:在上述制备步骤中不添加生物炭和聚乙二醇-4000即可得到ZVI.
-
在室温条件下,将100 mL的20 mg∙L−1的TC溶液置于锥形瓶中,加入2 mg催化剂(如球磨生物炭,ZVI和球磨生物炭负载零价铁复合材料),调节溶液pH值到5.0±0.2. 在溶液中加入1 mL的100 mmol·L−1的PMS溶液,开始降解实验. 分别在0、5、10、20、30 min取2 mL溶液,并用0.45 μm的PES膜过滤. 用紫外分光光度计测量样品的吸光度,波长设定为357 nm.
为了考察实验条件对反应体系的影响,改变了催化剂用量,PMS用量,TC浓度和溶液pH,以及添加阴离子(SO42−、Cl−和NO3−)或HA进行抗干扰实验. 通过自由基猝灭实验对反应体系中活性氧物种的存在和作用进行分析探究.
将反应后的溶液通过离心处理后,倒去上清液,剩余的材料用磁铁回收,蒸馏水和无水乙醇交替洗涤3次,烘干后进行循环实验.
-
使用扫描电镜(SEM)和透射电镜(TEM)对催化剂的表面形貌和结构进行表征. 催化剂的晶体结构采用X射线衍射(XRD)分析表征. 催化剂的比表面积、总孔体积和孔径通过比表面积和孔径分析仪(BET)测定. 催化剂的官能团类型采用傅里叶变换红外光谱(FTIR)分析表征. 催化剂的表面元素及化学形态利用X光电子能谱(XPS)分析表征. 反应体系中的活性氧物种采用电子顺磁共振波谱仪(EPR)分析表征,捕获剂为DMPO和TEMP.
-
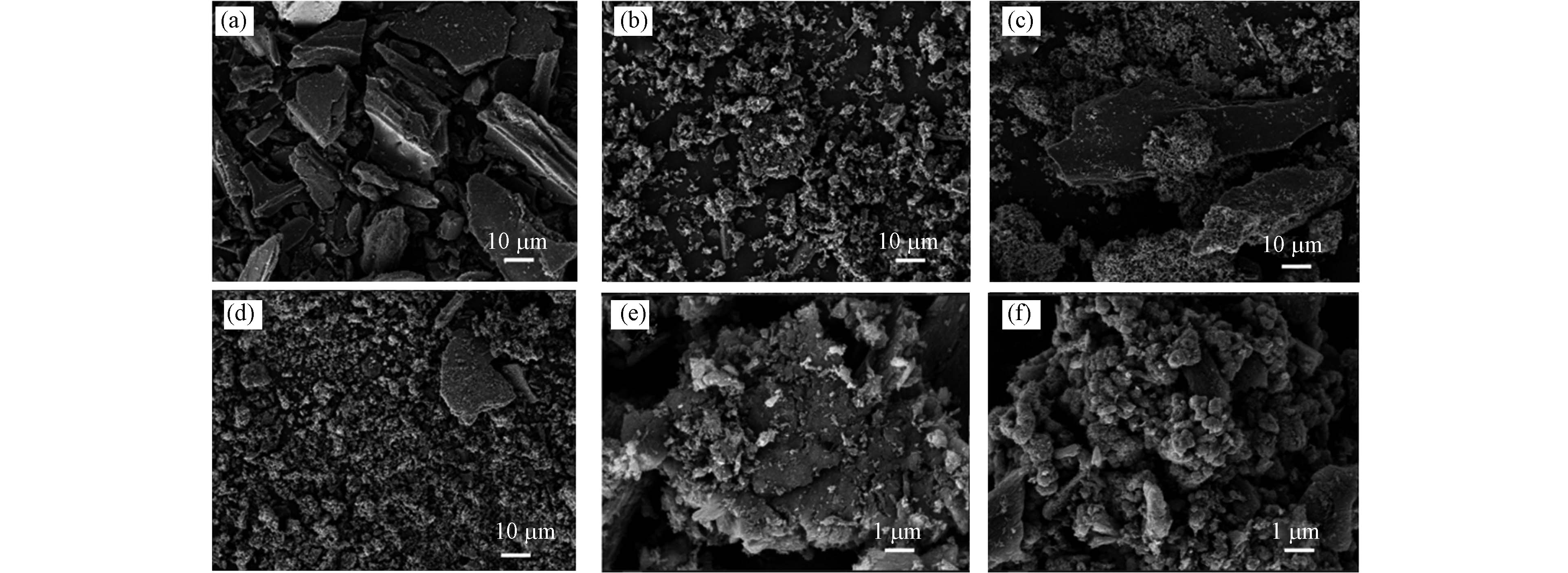
通过SEM观察催化剂微观尺度的变化和特性. 由图1(a)和图1(b)可知,生物炭经过球磨处理后被破碎成了粒径更小的块状结构. 生物炭负载零价铁后,通过图1(c,e)和图1(d,f)可以观察到在其的表面有不规则的球状颗粒,球状颗粒是结合在生物炭表面的铁氧化物. 图1(c,e)和图1(d,f)分别对应未球磨生物炭负载零价铁复合材料(ZVI/BC)和催化剂ZVI/0.25BC40-0.5的SEM图.
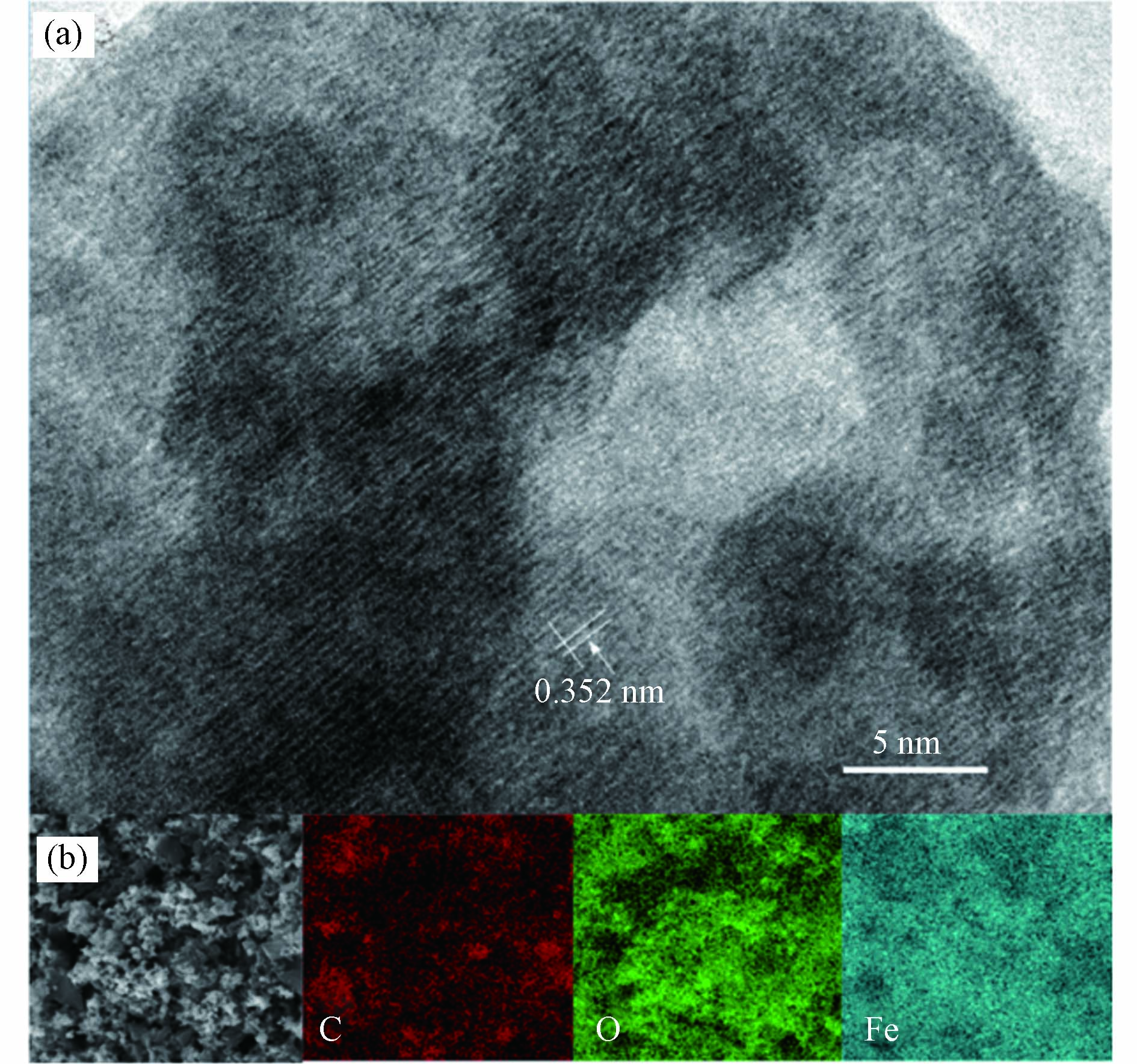
通过TEM进一步观察ZVI/0.25BC40-0.5的表面形貌. 从图2(a)观察到了无定形形态的生物炭,生物炭边缘带有明显晶格条纹的黑色颗粒为铁氧化物,其晶格间距为0.352 nm. 通过EDS Mapping表征催化剂ZVI/0.25BC40-0.5表面的元素分布情况. 如图2(b)所示,催化剂ZVI/0.25BC40-0.5的表面均匀分布着C、O、Fe元素. 以上结果证明了复合材料ZVI/0.25BC40-0.5的成功制备.
生物炭具有巨大的比表面积和极高的总孔体积,表1列出了几种催化剂的比表面积和孔隙参数. BC和BC40-0.5的比表面积分别为1263 m2·g−1和1136 m2·g−1,总孔体积分别为1.105 cm3·g−1和1.059 cm3·g−1. 而负载之后的ZVI/0.25BC40-0.5的比表面积和总孔体积只有78 m2·g−1和0.148 cm3·g−1,这可能是因为在生物炭负载零价铁的过程中,金属Fe和铁氧化物Fe3O4的含铁基团结合在生物炭的表面上和孔隙中,导致比表面积减小.
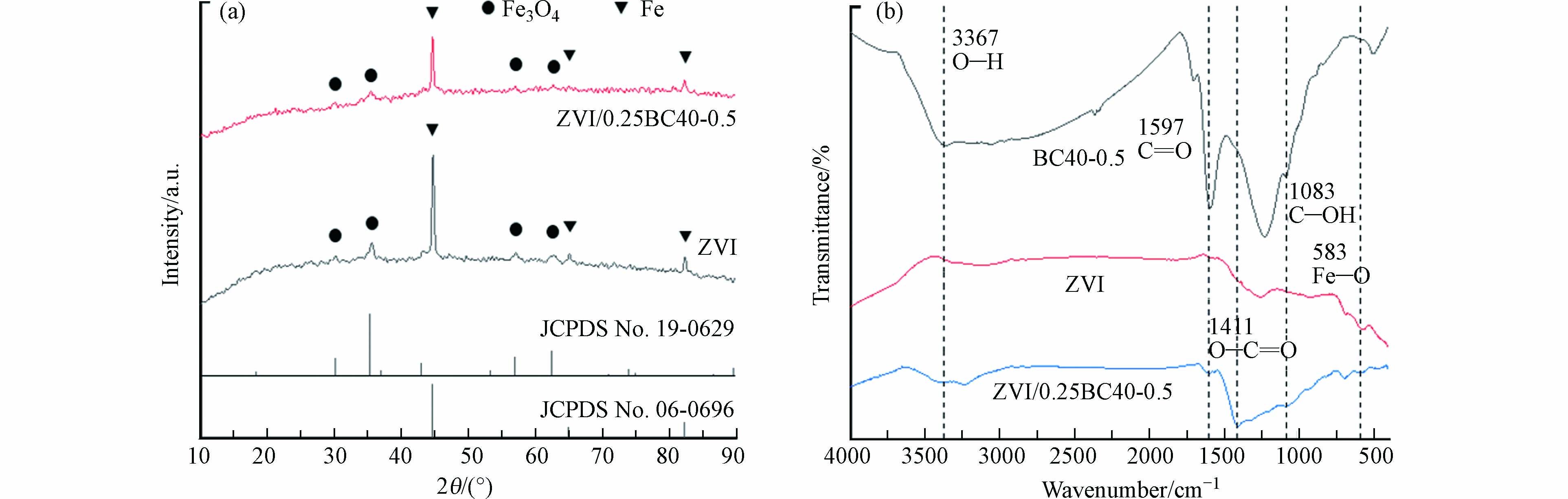
由于ZVI/0.25BC40-0.5中含有铁氧化物,对催化剂进行XRD分析. 如图3(a)所示,2θ为44.7°、65.0°和82.3°位置的峰对应金属Fe(Fe0)的(110)、(200)和(211)晶面 (JCPDS No.06-0696)[12]. 2θ为30.1°、35.4°、43.1°、56.9°和62.5°的峰对应Fe3O4的(220)、(311)、(400)、(511)和(440)晶面 (JCPDS No.19-0629)[13-14]. 复合材料ZVI/0.25BC40-0.5的特征峰和ZVI对应,说明ZVI/0.25BC40-0.5是将零价铁负载在生物炭上得到的,包含了Fe0和Fe3O4.
通过FTIR分析催化剂表面的官能团. 生物炭表面具有丰富的官能团,图3(b)中在3367 cm−1处的特征峰为O—H振动[15],1597 cm−1处的特征峰为C=O拉伸振动[15],1411 cm−1处的特征峰为O—C=O拉伸振动[15],1083 cm−1处的特征峰为C—OH拉伸振动[16],583 cm−1处的特征峰为Fe—O振动[17]. 即ZVI/0.25BC40-0.5具有ZVI的官能团和BC40-0.5的官能团,说明复合材料的成功制备.
通过XPS技术分析ZVI/0.25BC40-0.5中所含元素的化学键和电子结构. 图4(a)为ZVI/0.25BC40-0.5的XPS总谱图,表明ZVI/0.25BC40-0.5表面的主要元素为C、O和Fe. 图4(b)所示,C 1s谱图出现在284.8、286.1、289.5 eV的特征峰分别对应C—C/C=C、C—O和C=O/O—C=O[18-19]. 图4(c)所示,530.2、531.5、532.0 eV处的特征峰分别为Fe—O、C—O和C=O,表明Fe和生物炭成功结合在一起[19-20]. C=O/O—C=O基团可以激活PMS产生1O2,有利于有机物的降解. 图4(d)所示,结合能分别为710.8 eV和724.0 eV的两个特征峰分别为Fe2+的Fe 2p3/2和Fe 2p1/2,结合能为713.0 eV和726.1 eV的两个特征峰属于Fe3+的Fe 2p3/2和Fe 2p1/2,结合能为719.0 eV和732.4 eV的两个特征峰为Fe3+的卫星峰,表明ZVI/0.25BC40-0.5表面的铁以Fe3O4的形式存在[21]. 从图3(a)可以看出,ZVI/0.25BC40-0.5上也存在Fe0,但XPS谱图上Fe0峰值不明显,这可能是Fe0被氧化铁层包裹所致[22].
-
球磨改性会影响生物炭的理化性质,其中球质比和球磨时间对生物炭的催化能力影响较大[11]. 如图5(a)所示,当球磨时间2 h时,球磨生物炭的催化效果随球质比的增大逐渐降低,且球质比为40:1时催化效果最佳,TC去除率为57.0%. 然后,固定球质比为40:1,研究球磨时间对催化效果的影响,球磨生物炭的催化效果呈现随球磨时间延长去除率逐渐降低的趋势,球磨时间为0.5 h时效果最佳,TC去除率为59.4%(图5(b)). 这可能是因为随着球磨时间的延长,生物炭在球磨过程中被过度球磨,导致被破碎的生物炭得到压实和团聚,从而抑制了生物炭的效果[23]. 选择效果最好的球磨生物炭BC40-0.5,作为载体负载ZVI.
不同ZVI负载比例会对复合材料的催化能力产生影响. 图5(c)中,复合材料的效果呈现随ZVI负载比例增大去除率逐渐升高的趋势,ZVI/0.25BC40-0.5/PMS体系的催化效果最好,对TC的去除率达到87.1%.
图5(d)研究了不同体系对TC的去除效果. 在仅有TC存在的情况下,TC自降解30 min后去除率只有2.4%. 当体系中存在PMS时,TC的去除率为35.1%. 在PMS存在的情况下,BC40-0.5和ZVI作为催化剂时,TC去除率分别为59.4%和79.5%. 在PMS存在的情况下,ZVI/0.25BC40-0.5作为催化剂时,TC去除率为87.1%. ZVI/0.25BC40-0.5/PMS体系降解TC的去除率高于BC40-0.5/PMS和ZVI/PMS体系的去除率,ZVI/0.25BC40-0.5/PMS体系具有更高的催化效果. 此外,为了进一步证明ZVI/0.25BC40-0.5/PMS体系具有更高的催化效果,引入了拟一级动力学模型,对不同催化剂/PMS体系进行拟合,计算各体系的表观速率常数(kobs)(图5(e)和(f)). 经过计算,ZVI/0.25BC40-0.5/PMS体系的kobs=0.0883 min−1,高于其他催化剂/PMS体系.
此外,该反应体系的矿化率较高,可以有效降解TC. 例如,反应30 min后,ZVI/0.25BC40-0.5/PMS体系中的总有机碳(TOC)减少了48.3%. 将时间延长至120 min时,TOC的去除率达到了56.2%,说明TOC的去除主要是在30 min内.
-
实验条件(TC、PMS、催化剂浓度)会影响反应体系的催化效果. 首先研究了不同催化剂用量和PMS用量对反应体系的影响. 如图6(a),TC去除率随着催化剂用量增加而升高. 当催化剂ZVI/0.25BC40-0.5用量为20 mg·L−1时,TC去除率为87.1%,这是由于催化剂用量的增加促进了催化剂表面的催化位点与PMS接触,更快产生活性氧物种,使TC去除率升高. 如图6(b),随PMS用量增加,TC去除率呈现先增后减的趋势,这可能是因为溶液中过量的PMS会与产生的活性氧物种反应,抑制部分活性氧物种和TC反应. 如式(1)—(5)所示[24-26]:
图6(c)中,当初始TC浓度为20、30、40 mg·L−1时,四环素的去除率分别为87.1%、90.8%和91.9%,可以看出不同的TC浓度对该反应体系的TC去除率影响较小,说明该反应体系具有较好的降解能力. 此外,溶液pH也是影响反应体系TC降解效果的因素之一. 图6(d)中,当溶液pH为3—10时,TC去除率维持在83.7%—87.1%之间,说明该反应体系稳定,pH适应范围广.
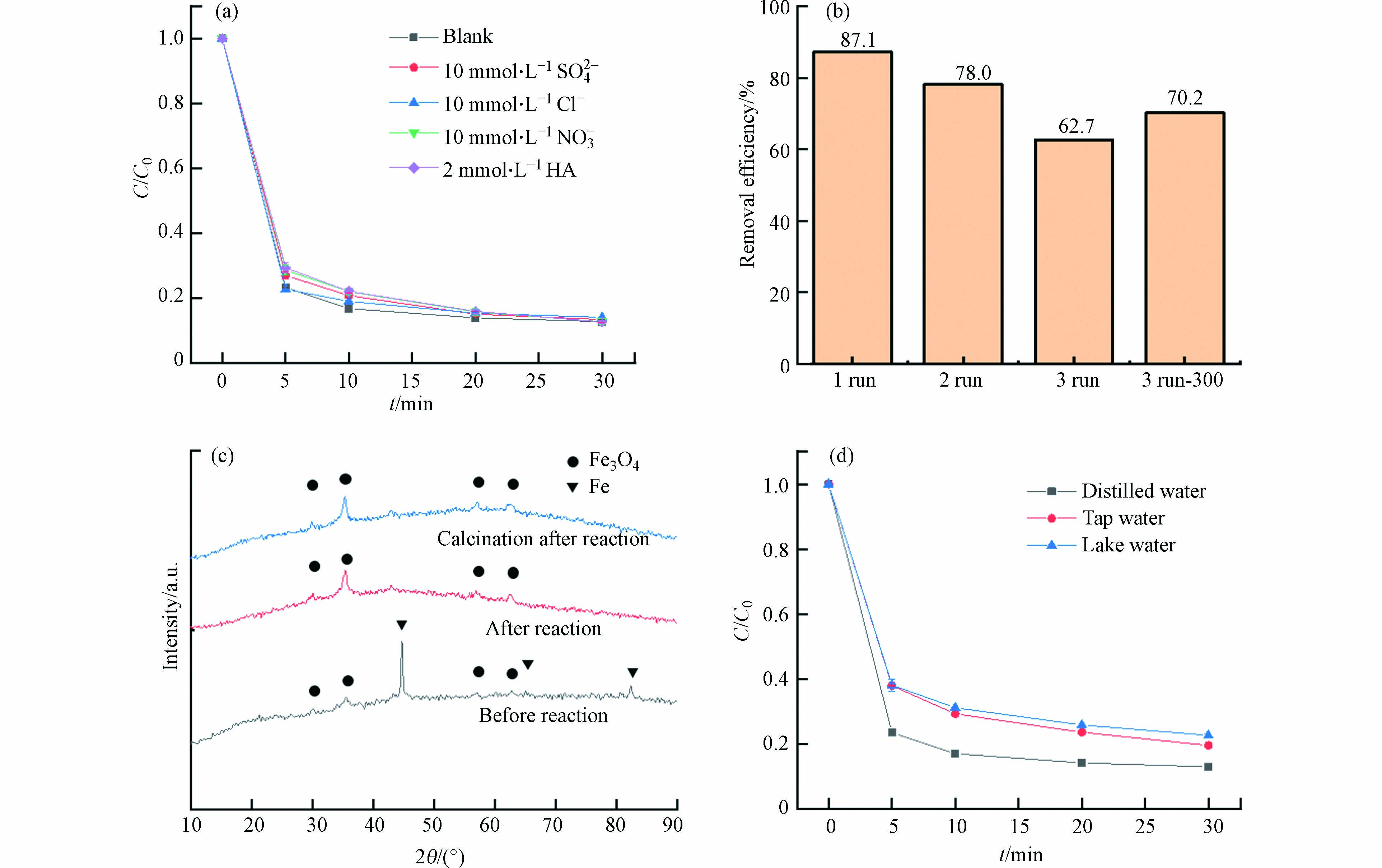
为了研究反应体系的抗干扰性,在反应体系中加入共存阴离子和腐殖酸进行实验. 从图7(a)可以看到,当反应体系中存在10 mmol·L−1 SO42−、Cl−和NO3−时,TC去除率分别为86.5%、85.8%和87.1%,这与反应体系中不存在共存阴离子时的TC去除率几乎一致,说明该反应体系对共存阴离子SO42−、Cl−和NO3−都具有抗干扰性,可以在复杂环境下稳定运行. 此外,选择了环境中较为常见的腐殖酸(HA)作为共存有机物进行实验. 当反应体系中存在2 mg·L−1 HA时,TC的去除率为87.2%,没有对反应体系产生明显作用,说明该反应体系对HA也具有抗干扰性.
为了研究催化剂的可循环使用性,将反应后的催化剂进行回收,再用于TC的降解实验. 图7(b)为催化剂循环实验的效果. 第二次循环的去除率为78.0%,仍具有较高的TC去除效果. 但随着催化剂的回收次数增加,效果也逐渐降低到62.7%,这可能是由于反应中催化剂表面的催化位点转化或被降解产物覆盖. 为了进一步验证催化剂活性下降的原因,将第二次循环后的催化剂在300 ℃下煅烧,去除催化剂表面的降解产物,催化能力得到部分恢复,TC去除率为70.2%. 此外,通过图7(c)的催化剂XRD图可以发现催化剂表面的Fe0转化为Fe3O4,这也是催化剂催化效果下降的原因之一. Huang等制备的磷掺杂生物炭纳米零价铁复合材料在反应后也出现了Fe0转化为Fe3O4的情况[27]. 为了研究催化剂ZVI/0.25BC40-0.5的实际应用潜力,还进行了实际水体实验和金属浸出实验. 在实际水体实验中,ZVI/0.25BC40-0.5/PMS体系的适应性良好,在自来水和湖水环境下分别有80.5%和77.5%的TC去除率. 通过ICP-MS分析溶液中催化剂的金属浸出量,Fe的浸出量为0.014 mg·L−1.
-
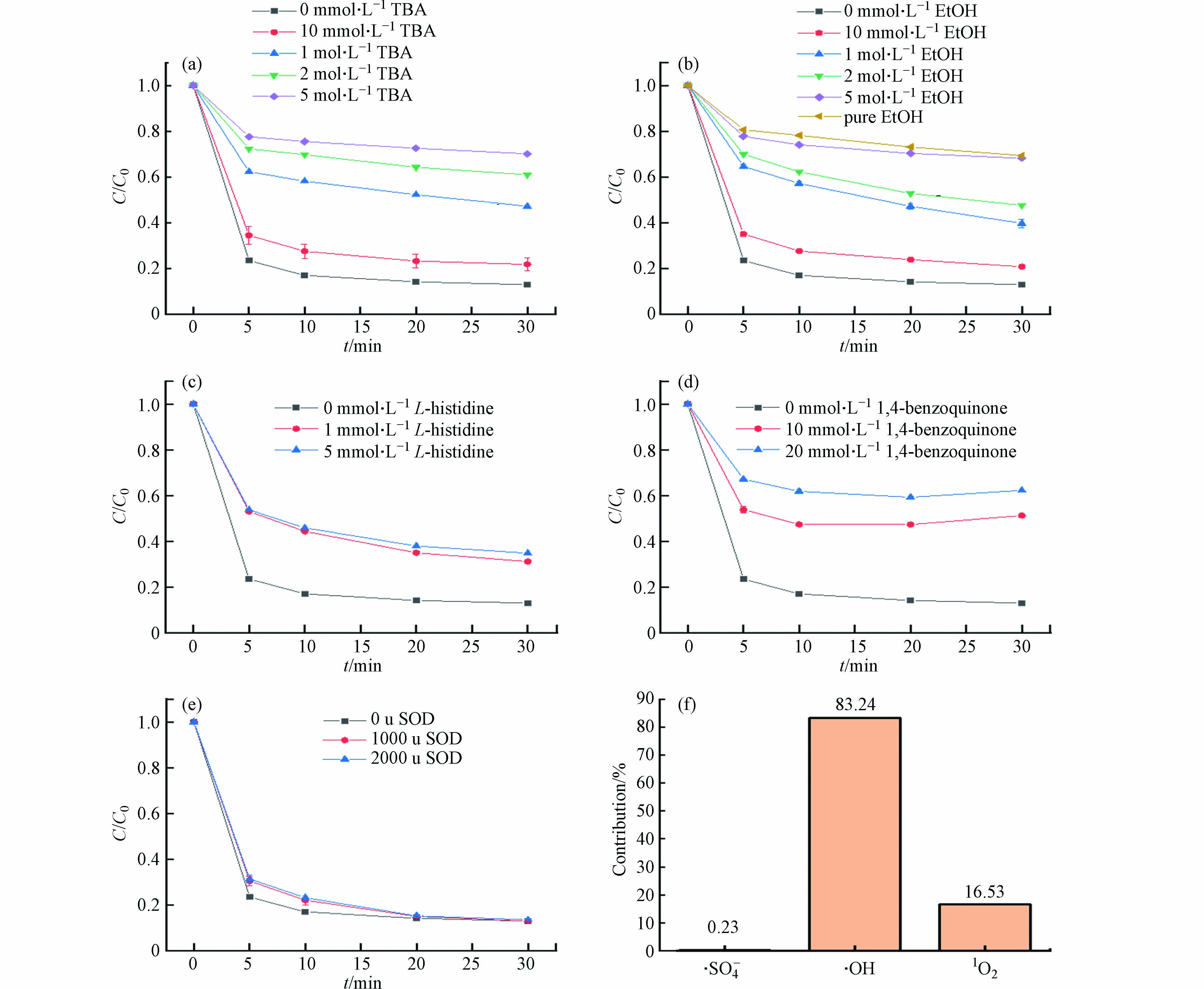
进行猝灭实验来判断反应体系中活性氧物种的类别和作用. 基于PMS的高级氧化技术可能有硫酸根自由基(SO4−·),羟基自由基(·OH),单线态氧(1O2)以及超氧自由基(·O2−)等活性氧物种参与反应. 叔丁醇(TBA)和乙醇(EtOH)可作为·OH和SO4−·的猝灭剂[28-29]进行自由基猝灭实验. TBA对·OH有猝灭作用, EtOH对·OH和SO4−·都有猝灭作用. 图8(a)所示,当TBA的浓度为10 mmol·L−1和1 mol·L−1时,TC的降解得到不同程度的抑制,TC去除率分别为78.3%和52.8%,说明·OH参与了降解反应. 图8(b)所示,当EtOH的浓度为10 mmol·L−1和1 mol·L−1时,TC去除率分别为79.3%和60.4%,说明SO4−·和·OH可能都参与了降解反应. 进一步提高TBA和EtOH的浓度,使猝灭剂能够完全猝灭溶液中的自由基. 当TBA和EtOH的浓度都达到5 mol·L−1时,与使用EtOH作为溶剂时的抑制效果相当,而使用EtOH作为溶剂可以完全猝灭溶液中的SO4−·和·OH,因此可以认为TBA的浓度为5 mol·L−1时溶液中的·OH已被完全猝灭. L-组氨酸(L-histidine)通常作为1O2的猝灭剂使用[30]. 图8(c)所示,当L-histidine的浓度为1 mmol·L−1和5 mmol·L−1时,对TC降解也有抑制作用,TC去除率分别为68.9%和65.3%,说明1O2参与了降解反应. 此外,使用对苯醌(1,4-benzoquinone)作为猝灭剂[31]来研究·O2−的作用. 图8(d)所示,当对苯醌的浓度为10 mmol·L−1和20 mmol·L−1时,对TC降解具有较强的抑制作用,去除率分别为48.8%和37.8%. 但是,对苯醌不仅对·O2−有猝灭作用,对SO4−·和·OH也有一定的猝灭作用,而SOD只对·O2−有猝灭作用[32],因此,进一步使用SOD作为·O2−的猝灭剂再次进行猝灭实验. 由图8(e)所示,使用不同剂量的SOD都没有对TC降解产生抑制作用,因此,·O2−对TC降解的作用可以忽略.
为了计算各活性氧物种在TC降解中的贡献度,选择使用5 mol·L−1 TBA和使用纯EtOH作为自由基猝灭剂的ZVI/0.25BC40-0.5/PMS体系的kobs计算SO4−·和·OH的贡献度(图8(f)). SO4−·、·OH和1O2的贡献度由公式(6)—(8)计算[33]. k0为不含猝灭剂的ZVI/0.25BC40-0.5/PMS体系的表观速率常数. k1和k2分别为5 mol·L−1 TBA和纯EtOH的猝灭实验的表观速率常数. λ[·OH]、λ[SO4−·]和λ[1O2]分别表示·OH、SO4−·和1O2的贡献度. 计算得k0、 k1和 k2分别为0.0883 min−1、0.0148 min−1和0.0146 min−1. 从图8(f)可以看出,SO4−·、·OH和1O2在ZVI/0.25BC40-0.5/PMS体系中的贡献度不同. 其中,ZVI/0.25BC40-0.5/PMS体系中·OH的贡献率最高(83.24%),其次是1O2 (16.53%)和SO4−· (0.23%). 因此,基于上述结果,说明自由基途径(SO4−·和·OH)和非自由基途径(1O2)对降解过程都有重要作用,·OH是起主要降解作用的活性氧物种.
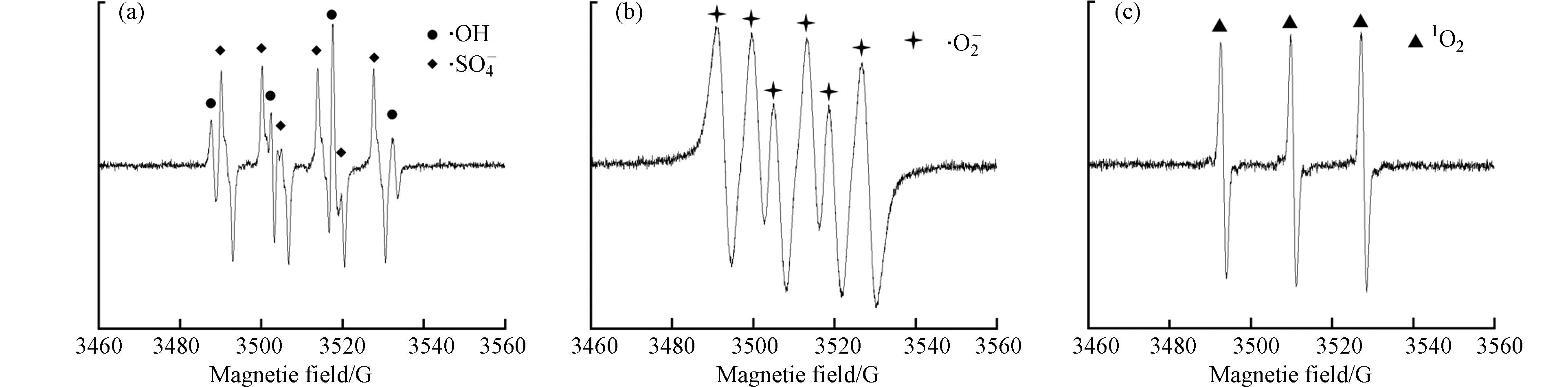
通过EPR光谱分析进一步验证反应体系中产生的活性氧物种. 使用DMPO作为SO4−·,·OH和·O2−的捕获剂,TEMP作为1O2的捕获剂. 从图9(a)可以看出,检测到峰值强度比为1:2:2:1的DMPO-·OH,DMPO-·SO4−的特征峰型,证明反应体系中存在SO4−·和·OH. 从图9(b)观察到·O2−的峰值强度比为1:1:1:1:1:1的特征峰型,说明反应体系中存在·O2−. 此外,从图9(c)可以看出,1O2的峰值强度比为1:1:1的特征峰型也被检测到,证明反应体系中存在1O2.
因此,基于上述结果,说明自由基途径(SO4−·和·OH)和非自由基途径(1O2)对降解过程都有重要作用. 结合猝灭实验结果,·OH是起主要降解作用的活性氧物种.
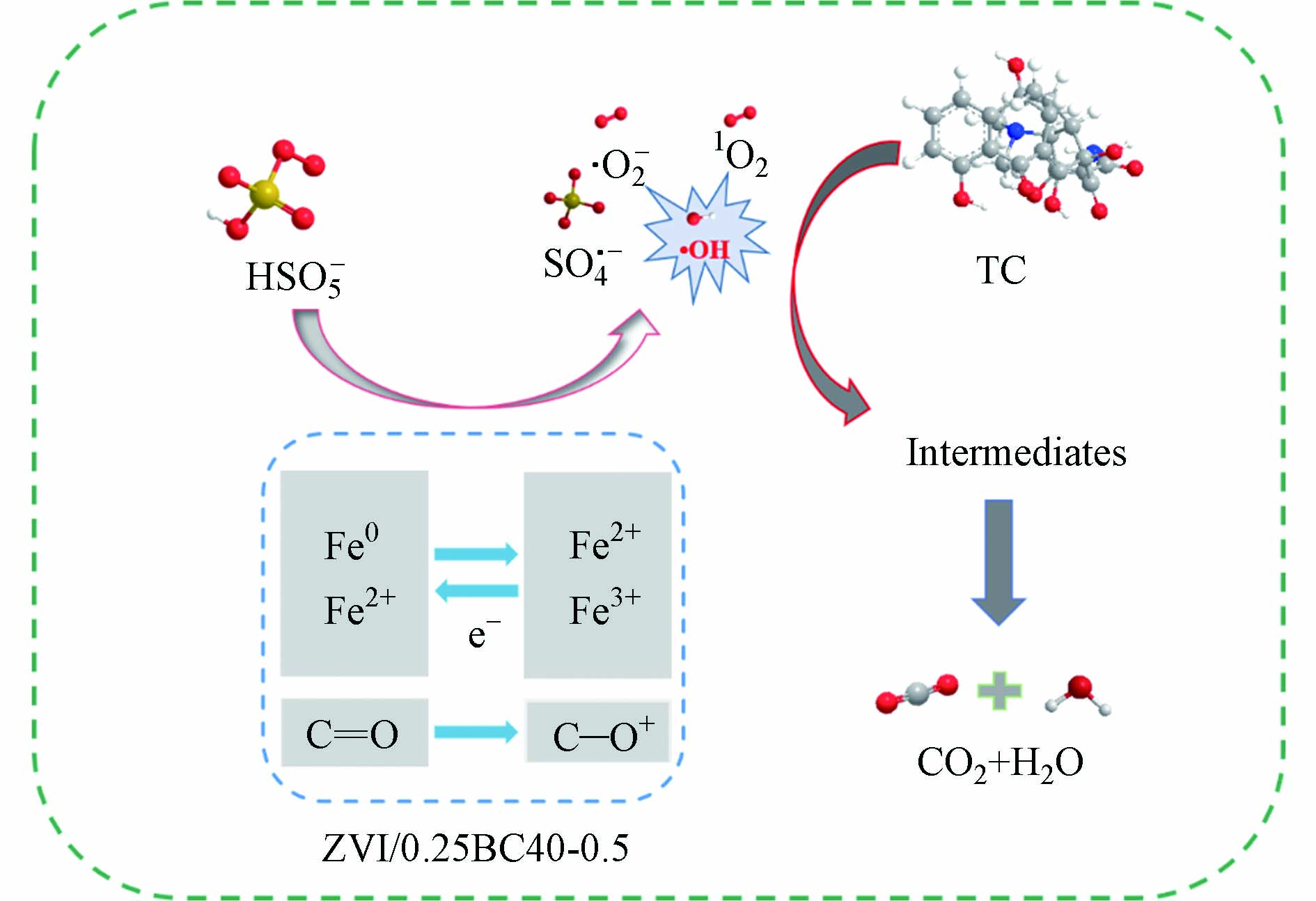
ZVI/0.25BC40-0.5/PMS体系的催化机制如图10所示. 生物炭的C=O可作为催化位点催化PMS产生活性氧物种,如式(9)所示[11]. 同时,生物炭作为电子供体,经球磨处理之后,缺陷程度增加[11],其缺陷结构可作为催化位点催化PMS,同时还原Fe3+,如式(10)—(12)所示[34]. 复合材料上的Fe物种,如Fe0和Fe2+也可作为催化位点催化PMS,如式(13)—(18)[35]. 式(19)—(23)为反应体系中可能的1O2产生途径[34,36].
-
(1)ZVI/0.25BC40-0.5可高效催化PMS降解TC,在30 min内,ZVI/0.25BC40-0.5/PMS体系对TC的去除率可达87.1%(TC浓度为20 mg∙L−1,催化剂用量为20 mg∙L−1,PMS用量为1 mmol·L−1,溶液pH=5).
(2)ZVI/0.25BC40-0.5/PMS反应体系抗干扰能力强,受pH、共存阴离子和腐殖酸影响小.
(3)催化剂ZVI/0.25BC40-0.5表面的C=O和Fe物种是主要的催化位点.
(4)ZVI/0.25BC40-0.5催化PMS降解TC的机理包括自由基途径和非自由基途径,其中·OH是主要的活性物种.
球磨生物炭负载零价铁催化过一硫酸盐降解四环素
Degradation of tetracycline by peroxymonosulfate catalyzed by ball-milled biochar loaded with zero-valent iron
-
摘要: 生物炭负载零价铁(ZVI/BC)在高级氧化处理有机污水领域具有广阔的应用前景,但通过球磨改性载体生物炭,进而提升ZVI/BC的催化效能尚缺乏系统研究. 首先通过球磨处理得到系列球磨生物炭,再利用水热法制备球磨生物炭负载零价铁(ZVI/0.25BC40-0.5),采用SEM、TEM、EDS Mapping、XRD、BET和FTIR对复合材料的理化性质进行表征. 研究结果表明,ZVI/0.25BC40-0.5/PMS体系可实现四环素(TC)的高效降解,当催化剂、PMS和TC的浓度分别为20 mg∙L−1、1 mmol∙L−1和20 mg∙L−1,pH = 5.0,反应时间为30 min时,TC的降解效率可达87.1%;催化剂和PMS浓度的增加均能够促进TC在ZVI/0.25BC40-0.5/PMS体系中的降解;水体中共存阴离子和腐殖酸对反应体系影响较小;自由基猝灭实验和EPR分析证实ZVI/0.25BC40-0.5/PMS体系中存在自由基途径和非自由基途径,其中•OH起主要作用;综合XPS结果说明C=O和Fe物种是ZVI/0.25BC40-0.5表面主要的催化位点.Abstract: Biochar supported zero-valent iron (ZVI/BC) is promising in treating organic wastewater through advanced oxidation treatment. However, the catalytic performance of ZVI/BC by modifying biochar through ball milling lacks systematic study so far. A series of ball-milled biochar was prepared by ball milling, and then the zero-valent iron/ball-milled biochar hybrid (ZVI/0.25BC40-0.5) was prepared using a hydrothermal method. The physicochemical properties of the composite were characterized by SEM, TEM, EDS Mapping, XRD, BET and FTIR. Tetracycline (TC) could be efficiently degraded in ZVI/0.25BC40-0.5/PMS system. Specially, the removal efficiency of TC reached 87.1% at pH 5.0 after 30 min when the concentrations of TC, PMS and catalyst were 20 mg∙L−1, 1 mmol∙L−1 and 20 mg∙L−1, respectively. In ZVI/0.25BC40-0.5/PMS system, the removal efficiencies of TC increased with the increase of catalyst and PMS concentration, while the anion and humic acid had little effect on the catalytic efficiency. Furthermore, free radical quenching experiment and EPR analysis confirmed the existence of free radical and non-free radical pathways, and •OH played a major role in ZVI/0.25BC40-0.5/PMS system. The results of XPS showed that C=O and Fe species were the main catalytic sites on ZVI/0.25BC40-0.5 surface.
-
Key words:
- biochar /
- zero-valent iron /
- peroxymonosulfate /
- catalytic degradation /
- advanced oxidation process.
-

-
图 5 (a)不同球质比,(b)不同球磨时间对球磨生物炭/PMS去除四环素的影响,(c)不同ZVI负载比例对复合材料/PMS去除四环素的影响,(d)不同反应体系的去除效果;(e)不同复合材料/PMS体系的kobs和(f)不同催化剂/PMS体系的kobs
Figure 5. Effects of (a) different ball-material ratio, (b) different milling-time on tetracycline removal of ball-milled biochar/PMS, (b) effects of different ZVI loading ratio on tetracycline removal of composite materials/PMS and (d) removal effects of different reaction systems. (e) kobs values of different composites materials/PMS and (f) kobs values of different catalysts/PMS
图 7 (a)共存阴离子和腐殖酸的影响;(b)催化剂ZVI/0.25BC40-0.5的循环实验;(c)催化剂ZVI/0.25BC40-0.5反应前后的XRD图;(d)实际水体实验
Figure 7. (a) Influence of co-existing anions and humic acids on the reaction system; (b) Cycle experiment of ZVI/0.25BC40-0.5;(c) XRD images of ZVI/0.25BC40-0.5 before and after reaction; (d) Actual water body experiment
表 1 催化剂的比表面积和孔隙参数
Table 1. The specific surface area and pore parameters of catalysts
催化剂
Catalyst比表面积/ (m2·g−1)
BET总孔体积/(cm3·g−1)
Vtotal平均孔径/nm
Pore sizeBC 1263 1.105 3.502 BC40-0.5 1136 1.059 3.063 ZVI/0.25BC40-0.5 78 0.148 3.810 -
[1] DAGHRIR R, DROGUI P. Tetracycline antibiotics in the environment: A review [J]. Environmental Chemistry Letters, 2013, 11(3): 209-227. doi: 10.1007/s10311-013-0404-8 [2] KOVALAKOVA P, CIZMAS L, MCDONALD T J, et al. Occurrence and toxicity of antibiotics in the aquatic environment: A review [J]. Chemosphere, 2020, 251: 126351. doi: 10.1016/j.chemosphere.2020.126351 [3] LEE J, von GUNTEN U, KIM J H. Persulfate-based advanced oxidation: Critical assessment of opportunities and roadblocks [J]. Environmental Science & Technology, 2020, 54(6): 3064-3081. [4] 唐亚鑫, 黄雯, 张建强, 等. Fe0/Fe3C羊粪生物炭复合材料的制备及其活化过一硫酸盐降解磺胺嘧啶 [J]. 环境化学, 2022, 41(12): 3991-4005. doi: 10.7524/j.issn.0254-6108.2021081602 TANG Y X, HUANG W, ZHANG J Q, et al. Preparation of Fe0/Fe3C sheep manure biochar composites for activating peroxymonosulfate to degrade sulfadiazine [J]. Environmental Chemistry, 2022, 41(12): 3991-4005(in Chinese). doi: 10.7524/j.issn.0254-6108.2021081602
[5] 张永祥, 杜伟, 李雅君, 等. 纳米零价铁在水处理中的应用研究综述 [J]. 中国环境科学, 2022, 42(11): 5163-5178. doi: 10.3969/j.issn.1000-6923.2022.11.023 ZHANG Y X, DU W, LI Y J, et al. A review of nano zero valent iron in water treatment [J]. China Environmental Science, 2022, 42(11): 5163-5178(in Chinese). doi: 10.3969/j.issn.1000-6923.2022.11.023
[6] REDDY A V B, YUSOP Z, JAAFAR J, et al. Recent progress on Fe-based nanoparticles: Synthesis, properties, characterization and environmental applications [J]. Journal of Environmental Chemical Engineering, 2016, 4(3): 3537-3553. doi: 10.1016/j.jece.2016.07.035 [7] SUN Y Q, LEI C, KHAN E, et al. Aging effects on chemical transformation and metal(loid) removal by entrapped nanoscale zero-valent iron for hydraulic fracturing wastewater treatment [J]. Science of the Total Environment, 2018, 615(15): 498-507. [8] LI Z, SUN Y Q, YANG Y, et al. Biochar-supported nanoscale zero-valent iron as an efficient catalyst for organic degradation in groundwater [J]. Journal of Hazardous Materials, 2020, 383(5): 121240. [9] DIAO Z H, ZHANG W X, LIANG J Y, et al. Removal of herbicide atrazine by a novel biochar based iron composite coupling with peroxymonosulfate process from soil: Synergistic effect and mechanism [J]. Chemical Engineering Journal, 2021, 409(1): 127684. [10] JIANG S F, LING L L, CHEN W J, et al. High efficient removal of bisphenol A in a peroxymonosulfate/iron functionalized biochar system: Mechanistic elucidation and quantification of the contributors [J]. Chemical Engineering Journal, 2019, 359(1): 572-583. [11] ZHANG W, YAN L G, WANG Q D, et al. Ball milling boosted the activation of peroxymonosulfate by biochar for tetracycline removal [J]. Journal of Environmental Chemical Engineering, 2021, 9(6): 106870. doi: 10.1016/j.jece.2021.106870 [12] LI M Q, LUO R, WANG C H, et al. Iron-tannic modified cotton derived Fe0/graphitized carbon with enhanced catalytic activity for bisphenol A degradation [J]. Chemical Engineering Journal, 2019, 372(15): 774-784. [13] XU X M, CHEN W M, ZONG S Y, et al. Atrazine degradation using Fe3O4-sepiolite catalyzed persulfate: Reactivity, mechanism and stability [J]. Journal of Hazardous Materials, 2019, 377(5): 62-69. [14] TANG S F, ZHAO M Z, YUAN D L, et al. Fe3O4 nanoparticles three-dimensional electro-peroxydisulfate for improving tetracycline degradation [J]. Chemosphere, 2021, 268: 129315. doi: 10.1016/j.chemosphere.2020.129315 [15] YI Y, WANG X Y, MA J, et al. Fe(Ⅲ) modified Egeria najas driven-biochar for highly improved reduction and adsorption performance of Cr(Ⅵ) [J]. Powder Technology, 2021, 388: 485-495. doi: 10.1016/j.powtec.2021.04.066 [16] PENG R H, XU X J, AN J J, et al. Research on Treatment of leaded wastewater by process combined Fe/C micro-electrolysis with microbial adsorbent-flocculant [J]. Applied Mechanics and Materials, 2014, 700: 470-474. doi: 10.4028/www.scientific.net/AMM.700.470 [17] QIAN J, YANG X W, JIANG L, et al. Facile preparation of Fe3O4 nanospheres/reduced graphene oxide nanocomposites with high peroxidase-like activity for sensitive and selective colorimetric detection of acetylcholine [J]. Sensors and Actuators B:Chemical, 2014, 201(1): 160-166. [18] ZANG T C, WANG H, LIU Y H, et al. Fe-doped biochar derived from waste sludge for degradation of rhodamine B via enhancing activation of peroxymonosulfate [J]. Chemosphere, 2020, 261: 127616. doi: 10.1016/j.chemosphere.2020.127616 [19] HAN Q, WANG Z H, XIA J F, et al. Facile and tunable fabrication of Fe3O4/graphene oxide nanocomposites and their application in the magnetic solid-phase extraction of polycyclic aromatic hydrocarbons from environmental water samples [J]. Talanta, 2012, 101(15): 388-395. [20] CAO J Y, LAI L D, LAI B, et al. Degradation of tetracycline by peroxymonosulfate activated with zero-valent iron: Performance, intermediates, toxicity and mechanism [J]. Chemical Engineering Journal, 2019, 364(15): 45-56. [21] LONG Y K, HUANG Y X, WU H Y, et al. Peroxymonosulfate activation for pollutants degradation by Fe-N-codoped carbonaceous catalyst: Structure-dependent performance and mechanism insight [J]. Chemical Engineering Journal, 2019, 369(1): 542-552. [22] WU Y W, CHEN X T, HAN Y, et al. Highly efficient utilization of nano-Fe(0) embedded in mesoporous carbon for activation of peroxydisulfate [J]. Environmental Science & Technology, 2019, 53(15): 9081-9090. [23] WANG Y, DENG W Q, LIU X W, et al. Electrochemical hydrogen storage properties of ball-milled multi-wall carbon nanotubes [J]. International Journal of Hydrogen Energy, 2009, 34(3): 1437-1443. doi: 10.1016/j.ijhydene.2008.11.085 [24] TAKDASTAN A, KAKAVANDI B, AZIZI M, et al. Efficient activation of peroxymonosulfate by using ferroferric oxide supported on carbon/UV/US system: A new approach into catalytic degradation of bisphenol A [J]. Chemical Engineering Journal, 2018, 331(1): 729-743. [25] DU J K, BAO J G, LIU Y, et al. Efficient activation of peroxymonosulfate by magnetic Mn-MGO for degradation of bisphenol A [J]. Journal of Hazardous Materials, 2016, 320(15): 150-159. [26] WANG X, WANG L G, LI J B, et al. Degradation of Acid Orange 7 by persulfate activated with zero valent iron in the presence of ultrasonic irradiation [J]. Separation and Purification Technology, 2014, 122(10): 41-46. [27] HUANG P, ZHANG P, WANG C P, et al. P-doped biochar regulates nZVI nanocracks formation for superefficient persulfate activation [J]. Journal of Hazardous Materials, 2023, 450(15): 130999. [28] ZHU S J, XU Y P, ZHU Z G, et al. Activation of peroxymonosulfate by magnetic Co-Fe/SiO2 layered catalyst derived from iron sludge for ciprofloxacin degradation [J]. Chemical Engineering Journal, 2020, 384(15): 123298. [29] TAO S Y, YANG J K, HOU H J, et al. Enhanced sludge dewatering via homogeneous and heterogeneous Fenton reactions initiated by Fe-rich biochar derived from sludge [J]. Chemical Engineering Journal, 2019, 372(15): 966-977. [30] MISKOSKI S, GARCÍA N A. Influence of the peptide bond on the singlet molecular oxygen-mediated (O2[1Δg]) photooxidation of histidine and methionine dipeptides. A kinetic study [J]. Photochemistry and Photobiology, 1993, 57(3): 447-452. doi: 10.1111/j.1751-1097.1993.tb02317.x [31] LEE H, KIM H I, WEON S, et al. Activation of persulfates by graphitized nanodiamonds for removal of organic compounds [J]. Environmental Science & Technology, 2016, 50(18): 10134-10142. [32] QIN W X, FANG G D, WANG Y J, et al. Mechanistic understanding of polychlorinated biphenyls degradation by peroxymonosulfate activated with CuFe2O4 nanoparticles: Key role of superoxide radicals [J]. Chemical Engineering Journal, 2018, 348(15): 526-534. [33] LI X G, WANG L, GUO Y X, et al. Goethite-MoS2 hybrid with dual active sites boosted peroxymonosulfate activation for removal of tetracycline: The vital roles of hydroxyl radicals and singlet oxygen [J]. Chemical Engineering Journal, 2022, 450(15): 138104. [34] CHEN L K, HUANG Y F, ZHOU M L, et al. Nitrogen-doped porous carbon encapsulating iron nanoparticles for enhanced sulfathiazole removal via peroxymonosulfate activation [J]. Chemosphere, 2020, 250: 126300. doi: 10.1016/j.chemosphere.2020.126300 [35] XU L, FU B R, SUN Y, et al. Degradation of organic pollutants by Fe/N co-doped biochar via peroxymonosulfate activation: Synthesis, performance, mechanism and its potential for practical application [J]. Chemical Engineering Journal, 2020, 400(15): 125870. [36] GUO Y X, YAN L G, LI X G, et al. Goethite/biochar-activated peroxymonosulfate enhances tetracycline degradation: Inherent roles of radical and non-radical processes [J]. Science of the Total Environment, 2021, 783(20): 147102. -



 下载:
下载: