-
虽然在我国颁布的《污水综合排放标准》中,已明确二级标准下磷酸盐的排放需小于1 mg·L−1. 但是现阶段由于农业磷肥的施用,以及日常生产生活中含磷废水的无序排放,导致实际进入自然水体中的磷酸盐远超过执行标准. 磷元素是水生生物生长的重要营养因子,当过多的磷酸盐进入湖泊、海湾等缓流水体,并超过了水体的自净能力时,将导致水体富营养化,从而引发水华或者赤潮,造成水体溶解氧量下降,水质恶化. 这不仅会造成鱼类及其他生物大量死亡,形成黑臭水体,而且由爆发性生长的浮游植物产生的藻毒素还会危害人类的健康[1]. 因此,在富营养化水体的防治中,减少外源磷的输入、降低上覆水体中的磷浓度非常重要. 此外,还需控制富营养化水体底泥中内源磷的释放.
上覆水体中磷酸盐去除方法包括:膜过滤法、化学沉淀法、生物修复法、电化学法和吸附法等,其中吸附法由于修复周期短、操作简单、成本低廉等优势引起广泛关注[2]. 底泥中的内源磷可采用原位钝化技术,利用钝化剂对内源磷进行吸附封存处理[3 − 4]. 值得注意的是,吸附法和原位钝化技术的核心均是吸附材料的研发.
类水滑石材料(HTLs, hydrotalcite-like materials)具有成本低,合成简单, 结构可调,“记忆效应”等优点,被广泛应用于催化、医药、阻滞剂等领域[5 − 8]. 该类材料被报道可有效吸附水体中的亚砷酸盐、砷酸盐、铬酸盐、磷酸盐、亚硒酸盐、硒酸盐、硼酸盐、硝酸盐等[2, 9- 10],且对于底泥中的磷酸盐也有较好的结合作用[11 − 12].
为了更好了解和应用HTLs,本文系统综述了HTLs的合成方法、HTLs的结构特性,以及常用的改性方法,并对比了同类改性材料间以及不同改性材料之间的吸附效果的差异,讨论了HTLs吸附水体中磷酸盐的影响因素和吸附机理. 同时,归纳了目前HTLs对内源磷的控制效果,分析了其作用机理,指出了HTLs用于吸附/钝化水体中磷酸盐的不足,进而对 HTLs在吸附/钝化水体中的磷酸盐的实际应用方面进行了展望.
-
常用的HTLs吸附剂的合成方法包括共沉淀法、尿素分解法、水热法、电磁波辅助法、电化学法、溶胶-凝胶法. 其合成原理、特点及影响因素见表1.
-
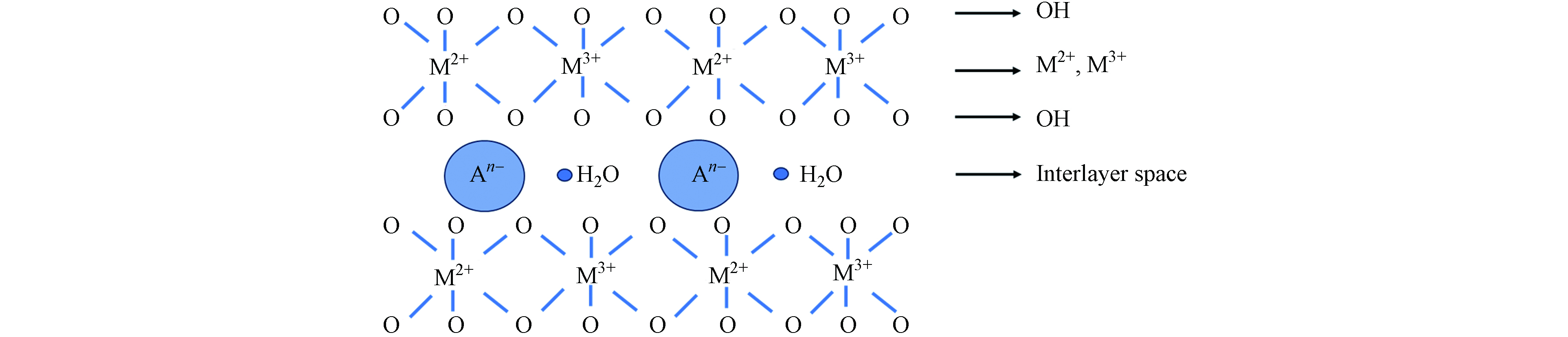
HTLs属于阴离子黏土,其通用分子式为[M2+1−x M3+ x (OH)2]x+ An− x/n · mH2O,其中M2+和M3+表示位于层中的金属二价离子和金属三价离子,二价金属和三价金属与羟基基团八面体配位形成带正电荷的层板,An−表示位于层间的阴离子,层间由阴离子和层间水组成,层板的正电荷被阴离子平衡[5]. 层间金属离子可以被替换成为不同的金属组分,并且层板中二价金属离子和三价金属离子的配比可以被调整,研究表明,HTLs通用分子式中x的数值一般是0.17—0.33,m一般在0—6[28]. 整体来看,层板和层间阴离子的结合使得HTLs呈现一种三明治结构(图1)[29]. HTLs层间阴离子,包括无机阴离子,金属氧酸盐,有机阴离子以及带负电的生物大分子等,可以通过一步合成、阴离子置换或者“记忆效应”来插入合适的种类[6]. 由于HTLs的化学组成和结构具有高度可调的特点, 所以可以合成出多种HTLs材料.
HTLs的特点可以总结为:1)二维结构可以具有比较高的比表面积(100—600 m2·g-1)和相当大的层间空间(0.73—2.28 nm)[30 − 31],这种结构有利于吸附水体污染物;2)层板金属阳离子的成分和比例可以调控,层间阴离子的种类也可以改变,从而可以根据吸附的实际需要来调整HTLs的理化性质;3)具有“记忆效应”,即HTLs通过焙烧形成混合金属氧化物之后,可以通过再次浸润到含有阴离子的水溶液中,完成结构的重建,新的阴离子可以通过“记忆效应”插入层间,因此HTLs焙烧材料理论上可以吸附更多的阴离子污染物;4)强吸附能力,HTLs材料具有弱碱性,从而更容易吸附一些无机或者有机含氧阴离子,包括但不限于砷酸盐、铬酸盐、磷酸盐,和阴离子表面活性剂等,并且这种吸附同时存在于层板的内外两侧[32].
-
首先,可通过精细调控HTLs层板的金属种类,金属比例,以及层间阴离子种类,提高类水滑石对水体中磷酸盐的吸附能力. Ashekuzzaman等[33]合成了钙基和镁基两种不同体系的HTLs吸附剂,并研究了它们的吸附性能. 实验结果表明,钙基HTLs显示出比镁基HTLs更高的对磷酸盐的吸附能力,但是钙基HTLs的可重复利用率比镁基HTLs低. 同时,Ashekuzzaman等[33]指出如果需要大量吸附剂的情况下,应优先考虑镁基HTLs,因为镁基HTLs相较于钙基HTLs,在水体中更加稳定. 随后,Jaberi等[34]研究了Ca-Fe HTLs材料在含磷水中的形态变化. 在磷酸盐浓度很高([
${\rm{PO}}_4^{2-} $ ]>100 mg·L−1)的情况下,溶解性钙离子浸出HTLs,与水体中的磷酸盐共沉淀形成羟基磷灰石,从而移除水体中的磷酸盐,去除理论值约为130 mg·g−1;在低磷酸盐浓度溶液中,磷酸盐的去除主要靠水铁矿类似物Fe-Ca复合物的吸附. 作者指出,钙离子的浸出是Ca-Fe HTLs吸附水体中磷酸盐的主要缺陷. Seftel等[35]研究了锌铝水滑石层板锌铝比例、层间阴离子种类以及焙烧对磷酸盐吸附的影响,结果表明铝离子比例越高,吸附能力越高,且${\rm{NO}}_3^{-} $ 型锌铝水滑石对磷酸盐离子有很好的吸附性能. Othman等[36]研究了Ni、Cu、Zn的二价离子和Al、Cr和Fe的三价离子不同组合所合成的HTLs吸附剂的吸附性能. 他们观察到Cu-Zn-Cr三元HTLs吸附剂具有最佳吸附性能,作者认为这是因为Cu、Zn 和 Cr三者之间的协同作用增强了磷酸盐的吸附. 拟合Freundlich 等温线Kf值显示Cu-Zn-Cr HTLs具有最大磷酸盐亲和力,反应动力学可用伪二级反应速率表达式拟合. 但是作者不建议使用铜离子构造HTLs吸附剂,因为层板中铜离子的结构稳定性较差,在水中易浸出.表2总结了近年来不同HTLs材料作为磷酸盐吸附剂,Langmuir等温线模拟下磷酸盐的最佳吸附容量. 如表所示,不同金属组成,比例以及阴离子种类均会影响吸附效果. 对比研究发现,Zn-Al HTLs的吸附效果最佳. 一般来说,三价金属比例高的HTLs吸附效果较好,这是因为三价金属越高,层板所带正电荷越多,越有利于磷酸盐阴离子的吸附. 但也要考虑到HTLs材料结构的稳定性,在合理的范围内下调控三价金属的比例.
其次,可通过焙烧法,以HTLs为前驱体,制备出混合金属氧化物,利用HTLs的“记忆效应”提高吸附剂的吸附能力. Cheng等[42]合成了Zn-Al HTLs及其焙烧材料并将其用于吸附市政黑臭水体中磷酸盐的研究. 他们发现,经过300 ℃焙烧之后,Zn-Al HTLs材料的吸附能力从26.9 mg·g−1提升到42.2 mg·g−1,提高了近1.5倍. 作者认为可能原因包括:1)“记忆效应”会自发吸附水中阴离子来恢复HTLs层状结构;2) 焙烧之后的混合金属氧化物具有更大的比表面积,能更大吸附磷酸盐离子;3)焙烧去除了层间阴离子,减少了阴离子之间的竞争吸附[42]. 与HTLs类似,HTLs焙烧材料吸附剂的金属成分和比例也会影响吸附效果. Das等[43]发现,Mg-Al吸附能力大于Zn–Al, Ni–Al, Co–Al, Zn–Fe, Co–Fe, Mg–Fe和 Ni–Fe;并且二价金属与三价金属比例也对吸附能力有影响,研究表明,Mg∶Al =2∶1的吸附效果高于3∶1和4∶1,这是因为三价金属比例高会使得层板所带正电荷更多,从而对阴离子磷酸盐有更好的吸附效果. 此外,He等[44]等研究了
${\rm{CO}}_3^{2-} $ 型锌铝水滑石在200、400、600、800 ℃下焙烧后的吸附效果. 如表3所示,通过对比不同焙烧材料吸附剂的吸附效果,可知煅烧后的锌铝水滑石对磷酸盐离子有很高的选择性,且具有良好的稳定性和可重复利用性能.值得注意的是,HTLs焙烧温度需要控制在合理的范围内,Seftel等[35]发现,325 ℃焙烧Zn-Al HTLs后,最大吸附容量能达到172 mg·g−1,但是用更高的温度焙烧(500 ℃)则会导致吸附能力的下降. 前人研究过HTLs材料在焙烧过程中的结构变化:用温度200 ℃焙烧HTLs时,仅仅损失材料表面的吸附水和孔隙水[48];当温度升高到250—450 ℃时,HTLs材料损失层间水并发生脱羟基化,层板结构遭到破坏[49];当温度继续升高,HTLs层状结构会完全被破坏并形成复合金属氧化物,在这个阶段,材料具有“记忆效应”,可以通过补液后的记忆重建来恢复层状结构. 然而,Miyata等[50]研究发现,当焙烧温度过高(800 ℃)时,HTLs会转化成为稳定的尖晶石固体,从而丧失“记忆效应”,即使重新放置在溶液中,也无法重建层状结构. 因而,焙烧温度的范围需要控制在既要温度升高到能够去除层间大部分原有阴离子的温度,又要低于晶相转变的临界温度,在此温度范围内,可增加吸附剂的比表面积,且能够允许重建HTLs层状结构,从而提高HTLs焙烧材料的吸附能力.
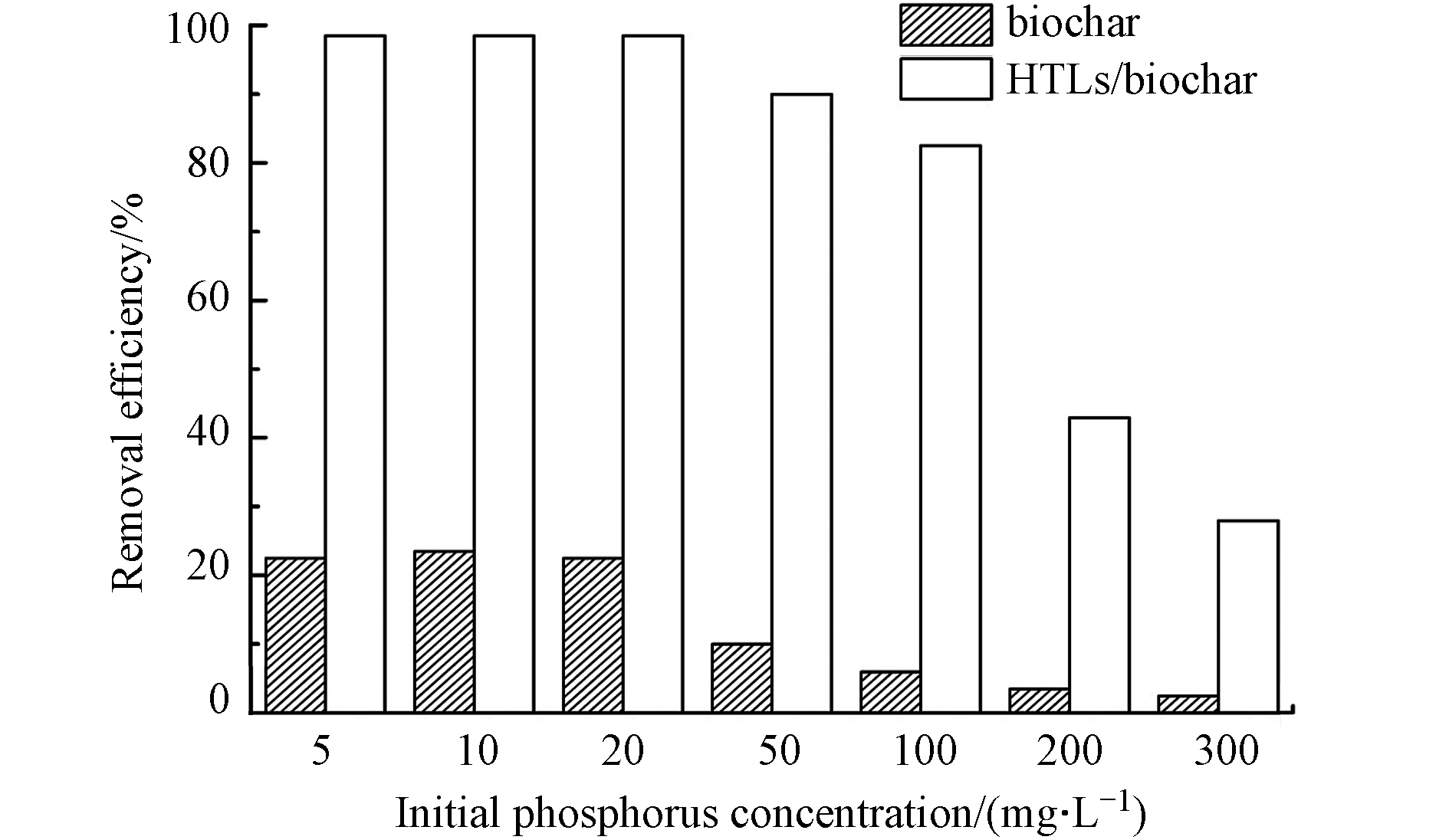
除了组分调控和焙烧等方法,还可通过复合其他类型的材料(如生物炭和磁性材料)完成HTLs的改性,从而增强吸附效果. Bolbol 等[51]合成了Mg-Fe HTLs/生物炭复合吸附剂,将此复合材料用于磷酸盐的吸附去除实验中. 如图2所示,结合了HTLs之后,生物炭的吸附能力提高了12倍. Yang等[52]以不同金属成分HTLs做为前驱体合成了一系列玉米秸秆生物炭混合金属氧化物,并测试了它们的磷酸盐吸附能力. 实验结果表明,以Zn/Al HTLs做为前驱体合成出的吸附剂(BC-Zn/Al-HTLs)其最大吸附量能达到152.1 mg·g−1 (pH 5), 明显高于其他组分同类型吸附剂(BC-Mg/Al-HTLs,BC-Ni/Fe-HTLs)[52]. Li等[53]合成了Mg-Al HTLs混合生物炭吸附剂,吸附水体中磷酸盐的效果可以达到81.83 mg·g−1(pH 3). Mg/Al 比例影响了复合吸附剂的吸附效果,与Das结果不同,Li 的实验结果表明,Mg/Al = 4∶1 的HTLs复合生物炭吸附剂的吸附效果优于Mg/Al 比例为3∶1 和2∶1[53]. 随着 Mg/Al 摩尔比的增加,生物炭复合材料的 HTLs 的层间空间增加,导致更大的磷酸盐吸附能力. HTLs还可以与磁性材料进行复合,方便回收利用[53]. Cui等[26]用电化学法合成了磁性HTLs/biochar复合吸附材料,吸附等温线符合langmuir-freundlich 曲线,对磷酸盐的最大理论吸附值可以达到252.88 mg·g−1. Alagha等[54]制备了磁性镁铁/活性炭复合吸附剂,对磷酸盐的最大吸附容量可以达到110 mg·g−1,且具有磁性易回收,实验结果表明可“吸附-再生”5 次.
HTLs与各种常见吸附剂的吸附能力比较见表4. 表中的吸附剂按介质分类,包含了沸石、多孔硅、生物炭、氧化物、黏土类物质等. 由于不同的实验条件(例如 pH 值和温度)和正在测试的对象不同(例如地表水、饮用水、实际废水和模拟废水),导致HTLs 与其他吸附剂的直接比较是困难的,所以表中只比较了文献中各吸附剂在最佳条件下的最大磷酸盐吸附容量. 通过对比各种吸附剂的对磷酸盐的最大理论吸附量,可以看出, HTLs焙烧材料在吸附水体中的磷酸盐的应用中具有巨大的应用潜力.
-
环境因素对HTLs的吸附效果具有较大的影响,包括溶液的pH、溶液中的竞争阴离子、吸附温度等.
首先,HTLs吸附磷酸盐受到 pH 值的显著影响. 一方面水体的pH值会影响磷酸盐的存在形式,根据磷酸的三级解离方程式(如下式所示,其中Ka1=2.1, Ka2=7.2, Ka3=12.7),过低的pH值(pH<2.1)使得溶液中磷酸的含量较高,HTLs溶解在磷酸中而导致其对磷酸盐的吸附量降低;在酸性条件(2.1<pH<5)下,磷酸盐在水溶液中主要以
${\rm{H}}_{2} {\rm{PO}}_{4}^{-} $ 的形式存在,同时HTLs因表面质子化带正电荷,通过静电引力和离子交换作用,表现出对磷酸盐较强的吸附力;随着水体pH值的增加,水体pH值逐渐增至HTLs的等电点附近时(pHzpc ≈ 8)[26],水体中的${\rm{H}}_{2} {\rm{PO}}_{4}^{-} $ 逐渐转化为${\rm{H}}_{2} {\rm{PO}}_{4}^{2-} $ ,而这种转变使得磷酸盐的吸附自由能升高,且此时溶液中的H+含量减少,HTLs表面质子化程度降低,以上两点均不利于HTLs对磷酸盐的吸附;当溶液的pH> pHzpc时,HTLs表面吸附的负电荷增加,抑制了静电引力对磷酸盐的吸附,导致吸附性能的显著降低,同时,溶液中存在的OH−离子还会与${\rm{H}}_{2} {\rm{PO}}_{4}^{2-} $ 、${\rm{PO}}_4^{3-} $ 竞争吸附位点,降低HTLs对磷的吸附.因此,已报道的HTLs吸附剂的最佳pH值通常呈酸性. Das等[43]发现,用焙烧镁铝HTLs作为吸附剂,pH值为5时,达到磷酸盐的最大去除率. Bolbol等[51]报道的HTLs/biochar复合吸附剂在pH 为 2—4时达到最大磷酸盐去除量,随着 pH 从 4 增加到 10,吸附容量从16.32 mg·g−1下降到 6.52 mg·g−1. 这是因为在酸性条件下,吸附剂表面有更多的 H+ 离子,表面质子化而带正电,从而有利于磷酸盐的吸附;而在碱性条件下,吸附剂表面带负电荷,导致磷酸盐离子的静电排斥增加,从而降低了磷酸盐的吸附. 此外,在较高的 pH 范围内,磷酸盐离子与羟基阴离子竞争吸附剂的表面活性位点,也会导致磷酸盐吸附量的减少[53].
其次,温度变化会改变HTLs吸附剂达到吸附平衡时的最大吸附量. 温度对 HTLs吸附的影响较为复杂,可能与离子水解有关,具体影响机制还有待进一步研究. Das等[43]发现,焙烧HTLs吸附磷酸盐离子是一个放热过程,其吸附量随着溶液温度的升高而降低. 而Halajnia等[41]的研究却发现,HTLs对磷和硫的吸附为吸热过程,对氮的吸附属于放热过程;他们还发现温度的升高可以使HTLs吸附磷酸盐的吸附速率增大,从而有利于提高吸附性能,但同时也指出,当温度达到一定程度时,吸附量可能随温度的升高而降低.
此外,溶液中的竞争阴离子也有可能影响HTLs及其衍生材料吸附剂对磷酸盐的吸附. 在自然界的污水中,除了磷酸盐离子,也同时存在其他阴离子,如Cl−、
${\rm{SO}}_4^{2-} $ 、${\rm{NO}}_3^{2-} $ 、${\rm{NO}}_2^{-} $ 、F−等. Li等[53]研究发现水体中Cl−的存在,对镁铝HTL/生物炭复合材料吸附剂对磷酸盐的吸附几乎无影响,而${\rm{SO}}_4^{2-} $ 和F-的存在则极大降低了材料的吸附性能. 他们认为,与磷酸盐离子半径相近的氧阴离子,如${\rm{SO}}_4^{2-} $ ,会和磷酸盐竞争吸附位点,从而降低吸附量;其他离子(Cl−、${\rm{NO}}_3^{2-} $ 、${\rm{NO}}_2^{-} $ )的离子半径小于磷酸盐,所以影响很小. 并且HTLs在这些离子半径小的共存阴离子溶液中,会对磷酸盐选择性吸收. F−则是因为它的电负性很大,所以更易被吸附,从而降低了HTLs吸附剂对磷酸盐的吸附量[53]. -
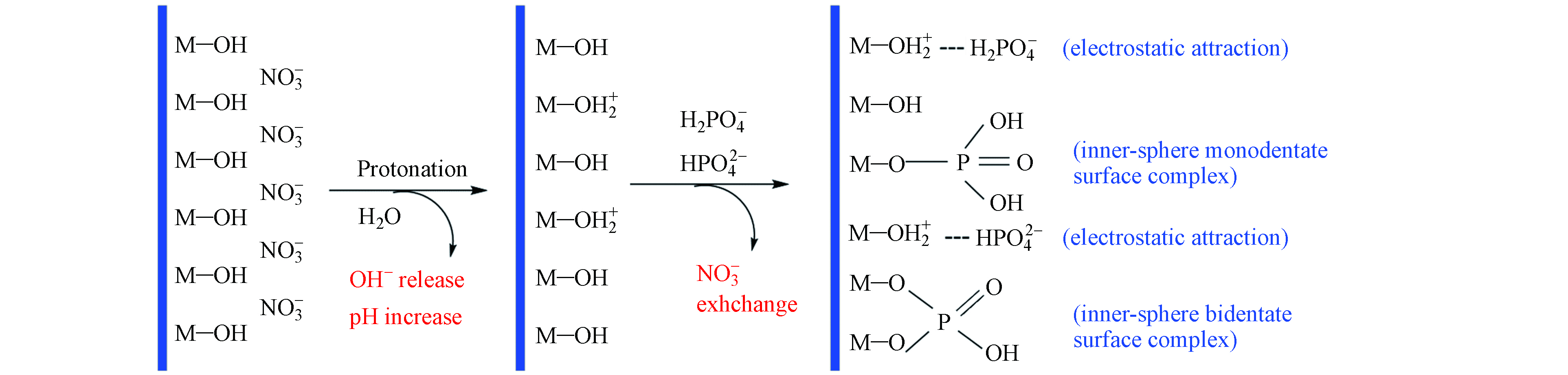
HTLs吸附水体中磷酸盐的作用机理包括静电吸引、配体交换和离子交换等[53]. 静电吸引主要是利用正负电荷之间的相互吸引力, 将磷酸盐吸附于HTLs上. 静电吸引通常受HTLs表面电荷和磷酸盐形态的影响, 在“2.2”的分析中可知溶液的pH显著影响HTLs水体中磷酸盐的吸附效果. 如图3所示,除了静电吸引外,HTLs表面丰富的羟基基团可与磷酸盐阴离子中未质子化的氧原子发生配体交换,形成单齿和双齿内球表面复合物;并且,HTLs的层间阴离子能与溶液中的磷酸盐阴离子进行离子交换,磷酸盐阴离子替代原有的HTLs层间阴离子进入HTLs层状结构中.
配体交换与离子交换的过程可通过FTIR、XPS等技术手段进行表征. Li等[53]发现,在吸附磷酸盐后,HTLs/生物炭复合材料的红外光谱发生变化:Al—O 在 557、630 、 683 cm−1 处的伸缩振动强度有所降低,这证实了 Al—O 或 Mg—O 键参与了磷酸盐的吸附. 伴随着P—O 键的强不对称振动峰的出现,Al—OH (Mg—OH)的伸缩振动峰位移或者降低,这表明磷酸盐通过形成潜在的单齿和双齿内球表面复合物被金属氧化物表面(Mg—OH 和 Al—OH)强烈吸附. 层间硝酸根N—O的伸缩振动峰消失,这一结果符合HTLs的层间阴离子交换特性.
Cui等[26]研究了磁性HTLs/生物炭复合材料吸附磷酸盐前后的XPS能谱的变化,在吸附了磷酸盐后,磁性HTLs/生物炭复合材料的P2p谱图可明显分为两个峰,分别分配给M—P(磷酸盐与金属结合键)和M—O—P(磷酸盐与金属氧化物结合键),且O1s中分配给O2-的积分面积较吸附前有所降低,分配给OH-积分面积有所升高,以上数据证明了磁性HTLs/生物炭吸附剂与磷酸盐之间的配体交换作用. 材料吸附磷酸盐前后O1s能谱图中可分配给CO32-的峰面积明显降低(从37.89%降低为31.5%)表明磁性HTLs/生物炭复合材料对磷酸盐的吸附机制还包括阴离子交换作用[26]. 进一步研究磁性HTLs/生物炭吸附剂中Mg1s,Al2p和Fe2p的光电子能谱后发现,在吸附后Mg1s和Al2p的能谱峰位置均有所偏移,而Fe2p的能谱峰位置没有明显变化,说明吸附过程主要发生与HTLs层板中的镁铝氢氧化物与磷酸盐之间[26]. 焙烧HTLs材料吸附剂除了上述几个吸附途径外,还包括由“记忆效应”导致的混合金属氧化物的再水合和氧阴离子同时插入中间层以重建HTLs[35, 51 − 53].
-
如图4所示,在截断外源磷的输入之后,除了需要降低水体中的磷酸盐的浓度外,还应该考虑阻断内源磷的释放,即防止沉积物向水体中释放磷酸盐[63]. 沉积物中的磷酸盐溶解到间隙水中,通过间隙水与上覆水之间的分子扩散作用释放到上覆水体中;可溶性磷直接穿过沉积物和上覆水之间的界面向上覆水体中扩散[64]. 沉积物原位化学控制技术是目前公认的比较有效的内源磷释放控制技术,主要通过磷钝化剂吸附封存沉积物中的磷酸盐,防止其释放到上覆水体中.
-
在实验室水平的研究中,吴俊麟等[65]制备的镁铁HTLs吸附剂不仅可以降低上覆水和间隙水中溶解性活性磷(正磷酸盐)的浓度,还可以显著增强湖泊底泥对水体中的磷酸盐的吸附能力. 通过对镁铁HTLs吸附的磷酸盐的进一步分析,发现被镁铁HTLs吸附的磷酸盐一半以上是稳定的金属氧化物结合态磷(52.3%),另外结合的磷属于不稳定结合态磷,具有重新释放的风险,包括NH4Cl提取态磷和氧化还原态敏感磷,分别占比13.7 %和34.0 %[65]. 作者建议,用镁铁HTLs吸附剂降低富营养化湖泊中的磷酸盐的浓度时,需要将吸附饱和的镁铁HTLs吸附剂及时清除,可考虑将镁铁HTLs吸附剂与磁性材料进行结合,提高回收效率[65]. 在随后的研究中,吴俊麟等[66]通过在镁铁HTLs上负载四氧化三铁粒子,进一步开发了磁性镁铁HTLs吸附剂,实验结果表明,所制备的磁性镁铁HTLs吸附剂即使在溶解氧很低的情况下,也可有效削减底泥向上覆水体中释放的可溶性活性磷,削减率达到94 %以上. 与镁铁HTLs吸附剂相类似,被磁性镁铁HTLs所吸附的可移动态磷的占比较大. 因此,考虑到浅水水体容易受到自然因素的扰动(风、水流、生物等)而造成沉积物再悬浮,以及为了便于吸附剂的回收,可将磁性镁铁HTLs与编织材料混合制备成为磁性织物包裹镁铁HTLs,并采用顶端覆盖的方式投加到沉积物上[66]. 实验结果表明,磁性织物包裹镁铁HTLs覆盖底泥,可有效控制内源磷释放到上覆水体中,削减率达到80 %以上[66]. 作者认为,从拦截效率、应用方便性和沉积物修复的可持续性的角度来看,织物包裹磁性镁铁HTLs覆盖沉积物是一种很有前景的控制沉积物中磷释放的方法[66].
与含铁钝化剂相比,含铝钝化剂中,铝离子的性质受到氧化还原条件的影响较小,含铝钝化剂吸附的磷的状态较为稳定[67]. 因此,可考虑使用铝盐代替铁盐合成镁铝HTLs,作为钝化剂用于控制内源磷的释放. Lin等[63]将镁铝HTLs与四氧化三铁(Fe3O4)以及氢氧化镧(La(OH)3)进行复合,得到磁性Fe3O4@MgAl-HTLs@ La(OH)3材料(MMAL). 所制备的MMAL可有效吸附上覆水体和间隙水中的溶解态磷,Langmuir模型下MMAL对磷酸盐的最大理论吸附量为66.5 mg·g−1,且水体中的磷被MMAL吸附后,可稳定保持10 d以上. 实验表明,MMAL覆盖层还可以捕获沉积物中移动态的磷,显著减少沉积物向上覆水体释放磷. 吸附饱和的MMAL可通过外加磁场进行快速回收,并且可以对MMAL进行解吸和再生,具有良好的回收利用性能[63]. He等[11]通过孔隙水采样和薄膜扩散梯度技术分析对比农业排水沟中添加了镁铝HTLs的点位和对照组点位的沉积物中的可溶活性磷和铁离子的浓度,发现添加镁铝HTLs吸附剂可以有效地降低农业排水沟中上覆水中可溶性活性磷的浓度,同时有效地阻碍其沉积物中的内源磷从间隙水中扩散到到上覆水体中. 与以往实验室水平下的研究结果不同, He等[11]分析了时间跨度为44d的沉积物中吸附磷的状态的变化,研究表明镁铝HTLs作为盖层材料覆盖在底泥上可以逐渐将沉积物中的可移动磷转化成为稳态磷[11]. Wu等[12]以沸石作为载体,合成了钙铁HTLs/沸石复合钝化剂(CFL-Z),在为期35 d的修复实验中,所制备的CFL-Z表现出优异的钝化性能. 作为盖层材料覆盖在底泥表面的CFL-Z,可有效减少上覆水和间隙水中可溶解活性磷的浓度,且数据表明,添加了CFL-Z的实验组,沉积物中的可移动磷将逐渐转化成为稳态磷[12].
-
磷钝化机制包括化学沉淀、吸附作用和共沉淀[4]. HTLs钝化内源磷的机制主要是吸附作用[12, 63, 66]. 在pH值大于3时,溶液中存在的无机磷的形式主要是带负电荷的各级解离的磷酸根离子,由于HTLs层板带正电荷,所以磷酸根离子可通过静电引力被吸附;同时,磷酸盐还可以与HTLs层板上的羟基基团发生配体交换. 通过镧改性后的HTLs增加了镧离子活性位点,镧离子是一种路易斯酸,可以结合作为路易斯碱的磷酸盐阴离子,形成镧与磷酸盐的内层配合体(LaPO4),增加对水中的磷酸盐的吸附[63]. 除了静电引力、配体交换外,磷酸盐还可以通过与层板上的金属离子形成氧桥(Fe—O—P、Mg—O—P),以及与HTLs的层间阴离子发生阴离子交换的方式被吸附. 吴俊麟等[66]通过镁铁HTLs吸附磷酸盐前后的XPS能谱发现,吸附了磷酸盐之后的HTLs的层间氯离子的含量伴随着磷的含量的升高而降低,且层板金属元素Mg、Fe的结合能均在吸附磷酸盐之后有明显的位移,位移后的结合能对应于氧桥配合物的形成.
-
虽然HTLs及其衍生材料有较好的吸附效果,且文献报道这类吸附剂的再生效率较高,然而,HTLs的某些缺陷可能会限制它们在去除水中磷酸盐离子方面的广泛应用. 1)HTLs在水溶液中不稳定[38]. HTLs是一种固体碱性材料,当放置于弱酸性水溶液中,固体会有部分溶解. Jobbágy等 [68]发现即使在轻度酸化下,也存在Mg-Al HTLs中Mg2+ 异构溶解的问题. 在HTLs材料用于除磷的研究中,Ashekuzzaman等 [33]也发现钙基HTLs浸出严重的现象. 2)HTLs吸附剂导致水体pH升高. 尤其是HTLs焙烧材料,它的碱性较强,当将HTLs焙烧材料添加到水中时,水体pH可达到10. pH增高一方面会导致吸附效率下降,另一方面会改变水体环境,有可能造成难以估测的生态影响. 3)HTLs可能的生态毒性. 在以往的研究中发现,Cu-Ni-Al HTLs对D.magna有明显的生态毒性[69]. 如果要将HTLs作为吸附/钝化材料用于水体富营养化的防治,考虑到它要进入水体中,HTLs的毒性机理需要进一步研究.
-
HTLs是一种多功能阴离子粘土层状材料,它可以采用不同的金属阳离子和金属比例,以及不同的阴离子,制备出种类繁多的HTLs材料. HTLs成分的高度可调性,以及“记忆效应”,使其非常适合做为水中含氧阴离子污染物的吸附剂. 在吸附磷的研究中,HTLs层板带正电荷,容易和磷酸盐离子存在静电吸附作用而固定磷. 而焙烧HTLs材料的“记忆效应”,可以让磷酸盐离子被吸附在层间. HTLs材料还可以与其他材料一起形成复合物,发生协同效应,增强吸附效果. HTLs不仅对水体中的磷酸盐有较高的吸附性能,对内源磷的释放也可以起到一定的控制作用. 此外,HTLs材料可以用简便的方法合成,成本低廉,容易实现量产被推广应用.
为了促进 HTLs在水体富营养化防治中的应用, 未来HTLs 可以从以下方面进行探索:1)增强 HTLs 材料的稳定性,从而解决因为HTLs材料溶解导致的吸附能力降低,以及金属离子浸出导致的不利环境影响. 在这方面,可以开发HTLs复合材料,增强结构稳定性. 2)HTLs材料的广泛应用评估主要在实验室条件中进行,少有关于HTLs材料应用于湖泊水体实际控磷的实验报道. 应当开展相关方面实验,以评估HTLs材料在实际水环境中的应用效果. 随着对 HTLs 材料研究进一步深入以及生态修复技术的不断发展, HTLs 必将为净化环境水体事业发挥重要力量.
类水滑石材料吸附/钝化磷酸盐的研究进展
Progress of research on phosphate adsorption/passivation by hydrotalcite-like materials
-
摘要: 控磷是治理富营养化水体的一个关键步骤. 类水滑石材料(HTLs,Hydrotalcite-like materials)由于其特殊的理化结构和性质,可有效地吸附水体中的磷酸盐,且可通过结合沉积物中磷酸盐的方式,抑制沉积物中的磷向水体释放,是一种具有巨大应用潜力的控磷材料. 近年来,围绕HTLs控磷的主题所发表的研究论文日益增多. 为了更好地理解HTLs,并将其应用到水体富营养化的防治中,本文介绍了HTLs的组成、特性、制备方法;梳理了提高HTLs对磷酸盐阴离子吸附性能的改性方法,对比了不同HTLs对水体中磷酸盐阴离子的吸附性能和对内源磷的控制效果;总结了HTLs对水体中和沉积物中磷酸盐阴离子的吸附机理、影响HTLs吸附效果的因素;最后分析了HTLs在应用过程中可能存在的问题. 论文对今后 HTLs在水体控磷领域中的研究与应用方向进行了展望.Abstract: Phosphorus control is a key step in the management of eutrophic water. Hydrotalcite-like materials (HTLs) can effectively adsorb phosphate from water due to their special physicochemical structure and properties, and can inhibit the release of phosphorus from sediments into water by binding phosphate in sediments, which is a phosphorus control material with great potential for application. In recent years, an increasing number of research papers have been published on the topic of phosphorus control by HTLs. In order to better understand HTLs and apply them to the prevention and treatment of eutrophic water, this paper firstly introduces the composition, characteristics, and preparation methods of HTLs; secondly, it sorts out the altered performance methods to improve the adsorption performance of HTLs on phosphate anions, compares the adsorption performance of different HTLs on phosphate anions in water, and compares the control effect of different HTLs on endogenous phosphorus; then, it summarizes the adsorption mechanism of HTLs on phosphate anions in water, sediments, and the factors affecting the adsorption effect of HTLs due to the experimental results; and then it analyzes the possible problems of HTLs in the application process. Finally, this paper provides an outlook on the future research and application directions of HTLs in aqueous phosphorus control field.
-
Key words:
- hydrotalcite-like materials /
- phosphate /
- adsorbent /
- endogenous control /
- eutrophication.
-

-
表 1 HTLs的合成方法
Table 1. The synthetic methods of HTLs
方法
Method合成过程
Synthetic process优点
Advantage缺点
Disadvantage共沉淀法[13] 同时滴定金属盐溶液与碱液,发生共沉淀反应,老化一定时间后得到HTLs材料 操作步骤简单,容易量产 滴定的过程中,在强力搅拌的条件下,碱液并不是均匀分散在溶液中,从而导致合成的HTLs结晶度低;由于成核时间不均一,导致HTLs的晶粒大小不均匀 尿素分解法[14 − 17] 用尿素替代碱液,在尿素缓慢水解的过程中,保持溶液的pH在9-10,发生共沉淀反应,得到HTLs 操作步骤简单,容易量产,且结晶度高,晶粒均一 尿素水解速度缓慢,在整个结晶过程中过饱和度差,晶核生长缓慢,导致晶粒尺寸变大,合成的HTLs比表面积变小 水热法[18 − 19] 将一定比例的金属盐与尿素混合均匀的水溶液转移至高压釜中. 在水热条件下,尿素分解成氨,产生OH-和CO32-,然后金属离子与OH-逐渐沉淀,形成水镁石状层, 同时碳酸盐与水一起插层到层间,生成HTLs 提供高压环境有利于晶核生长,减少了合成时间,提高结晶度 水热釜高压存在安全问题 电磁波辅助法[20 − 23] 将金属盐与碱沉淀剂混合后,放入微波加热器中采用一定频率的电磁波作为热源
进行电磁加热减少了合成时间,增加比表面积,减小了晶粒尺寸 用微波加热器代替传统油浴/水浴锅进行加热,易造成合成反应速率过快产生安全问题 电化学法[24 − 26] 给电极上一定的电压, 从而水分解产生羟基基团. 可选用硫酸盐,金属硝酸盐,卤盐等不同溶液,电解产生的羟基基团与液中的金属离子结合, 然后在导电基底上形成 HTLs 纳米阵列或薄膜 简单快速,可制备出HTLs纳米阵列和薄膜 需要特定的电化学设备,一般用于订制特别需求的HTLs材料 溶胶-凝胶法[27] 采用金属乙酸盐在乙醇介质中共沸形成溶胶,进而在凝胶化过程中往体系添加计算量的去离子水、乙酸钠与氢氧化钠溶液等,控制金属离子配比、含水量,进而实现对产物组成、物相和结构的调控 产出的HTLs材料纯度高,比表面积高 合成步骤相对复杂 表 2 HTLs吸附剂去除磷酸盐离子的比较评价
Table 2. Comparative evaluation of phosphate ion removal by HTLs adsorbents
吸附剂
Adsorbent比表面积/ (m2·g−1)
Specific surface area饱和吸附量/(mg·g−1)
Saturation adsorption
capacity Qm吸附条件
Adsorption conditions动力学模型
Kinetic model参考文献
Ref.Ca-Al HTL NF 66.7 pH 7; 0.3 g·L−1; 0—20 mg·L−1; 2 h P-S [33] Zn1.25Al-NO3 HTL 40.0 90.9 pH NF; 1 g·L−1; 5—200 mg·L−1;
24 h; RTP-F [35] Mg-Al HTL 104.0 31.3 NF P-S [37] Zn-Al HTL 135.0 68.4 NF P-S [37] Mg-Al HTL 26 228 pH 5; 0.3 g·L−1;
0.06—3.2 mmol·L−1; RTNF [38] 均苯四甲酸插层
Zn-Al HTLNF 64.3 pH 5; 1 g·L−1; 5—100 mg·L−1;
4 h; 50 ℃P-S [39] FeMg2Mn HTL NF 34.3 pH 6.5; 1 g·L−1; 0.5—100 mg·L−1;
4 h; 25 ℃P-S [40] Mg-Al HTL 64.4 26.6 pH 7; 5 g·L−1; 0.2—5 mmol·L−1;
2 h; 25 ℃P-S [41] HTL, 类水滑石吸附剂. HTL, Hydrotalcite-like material. RT, 室温. RT, room temperature. NF, 无相关信息. NF, no information; P-S: 准二级动力学模型. P-S:pseudo second-order model. P-F: 准一级动力学模型. P-F: pseudo first-order model. 表 3 HTLs焙烧材料吸附剂去除磷酸盐离子的比较评价
Table 3. Comparative evaluation of phosphate ion removal by adsorbents of HTLs calcined materials
HTLs焙烧材料
Calcinated HTLs比表面积/(m2·g−1)
Specific surface area饱和吸附量/(mg·g−1)
Saturation adsorption
capacity Qm吸附条件
Adsorption conditions动力学模型
Kinetic model焙烧温度/℃
Calcinated Temperature参考
文献
Ref.Zn1.25Al-NO3 90 172.2 pH NF; 1 g·L−1;
5—200 mg·L−1 24 hP-S 325 [35] Zn-Al 85.5 ~247 pH NF; 1 g·L−1
0.25—2.5 mmol·L−1P-S 200 [45] Zn-Al NF 92.58 pH NF; 0—20 mg·L−1; 72 h; 50 ℃ P-S 300 [46] Mg-Fe NF 9.8 pH ~7; 2 g·L−1;
5—40 mg·L−1 2 h; RTP-S 450 [47] 表 4 不同吸附剂对磷酸盐的吸附能力比较
Table 4. Comparison of phosphate adsorption capacity of various adsorbents
吸附剂
Adsorbent比表面积/ (m2·g−1)
Specific surface area饱和吸附量/(mg·g−1)
Saturation adsorption capacity Qm吸附条件
Adsorption conditions参考文献
Ref.LA@TCAP 71.9 10.6 pH 7; 1 g·L−1; 10—100 mg·L−1; 24 h; 25 ℃ [55] TCAP 29.2 4.1 pH 7; 1 g·L−1; 10—100 mg·L−1; 24 h; 25 ℃ [55] Al@TCAP 72.3 8.8 pH 7; 1 g·L−1; 10—100 mg·L−1; 24 h; 25 ℃ [55] 沸石 500 25.9 NF [56] 商用氢氧化镧 31.1 55.6 pH NF; 2.5 g·L−1; 5—500 mg·L−1; 25 ℃ [57] 改性氢氧化镧 153.3 107.5 pH NF; 2.5 g·L−1; 5—500 mg·L−1; 25 ℃ [57] 羟基铝膨润土 200 12.7 pH 3; 4 g·L−1; 25—60 mg·L−1; RT [58] 镧改性介孔二氧化硅 67.4 42.8 pH 5; 0.5 g·L−1; 0—57 mg·L−1; 25 °C [59] 木质生物炭 147 12.0 pH 7; 12.5 g·L−1; 25—150 mg·L−1; 25 °C [60] 商用树脂材料 NF 48 pH 7.2—7.6; 0.1—10 g·L−1; 10 mg·L−1; 24 °C [61] 蛋壳焙烧材料 19.32 32.6 pH 2—10; 2 g·L−1; 65 mg·L-1 ;25 °C [62] 锌铝HTL焙烧材料 90 172.2 pH NF; 1 g·L−1; 5—200 mg·L−1; 24 h [35] 锌铝HTL焙烧材料 85.5 ~247 pH NF; 1 g·L−1; 0.25—2.5 mmol·L−1 [45] TCAP:热处理富钙凹凸棒石. TCAP: thermally treated calcium-rich attapulgite.
-
[1] BACELO H, PINTOR A M A, SANTOS S C R et al. Performance and prospects of different adsorbents for phosphorus uptake and recovery from water[J]. Chemical Engineering Journal, 2020, 381: 122566. doi: 10.1016/j.cej.2019.122566 [2] 任树鹏, 齐宇彤, 石瑶, 等. 层状双氢氧化物负载生物炭对磷酸盐的吸附性能研究进展[J]. 环境化学, 2023, 42(2): 575-584. doi: 10.7524/j.issn.0254-6108.2021101904 REN S P, QI Y T, SHI Y, et al. The adsorption performance of layered double hydroxides functionalized biochar on phosphate: Research advances[J]. Environmental Chemistry, 2023, 42(2): 575-584(in Chinese). doi: 10.7524/j.issn.0254-6108.2021101904
[3] 张巧颖, 杜瑛珣, 罗春燕, 等. 镧改性膨润土钝化湖泊中的磷及其生态风险的研究进展[J]. 湖泊科学, 2019, 31(6): 1499-1509 doi: 10.18307/2019.0620 ZHANG Q Y, DU Y X, LUO C Y, et al. Advances in researches on phosphorus immobilization by lanthanum modified bentonite in lakes and its ecological risk[J]. Journal of Lake Sciences, 2019, 31(6): 1499-1509(in Chinese). doi: 10.18307/2019.0620
[4] 马鑫雨, 杨盼, 张曼, 等. 湖泊沉积物磷钝化材料的研究进展[J]. 湖泊科学, 2022, 34(1): 1-17. doi: 10.18307/2022.0101 MA X Y, YANG P, ZHANG M, et al. Advances in researches on phosphorous inactivation materials in lake sediment[J]. Journal of Lake Sciences, 2022, 34(1): 1-17(in Chinese). doi: 10.18307/2022.0101
[5] TAMPIERI A, RUSSO C, MAROTTA R, et al. Microwave-assisted condensation of bio-based hydroxymethylfurfural and acetone over recyclable hydrotalcite-related materials[J]. Applied Catalysis B: Environmental, 2021, 282: 119599. doi: 10.1016/j.apcatb.2020.119599 [6] KANEDA K, MIZUGAKI T. Design of high-performance heterogeneous catalysts using hydrotalcite for selective organic transformations[J]. Green Chemistry, 2019, 21(6): 1361-1389. doi: 10.1039/C8GC03391A [7] SONG Y, BEAUMONT S K, ZHANG X, et al. Catalytic applications of layered double hydroxides in biomass valorisation[J]. Current Opinion in Green and Sustainable Chemistry, 2020, 22(11): 29-38. [8] SARDAR B, SRIMANI D. Concept and progress on the de(hydrogenation) and hydrogenation reactions using transition metal integrated layered double hydroxides (LDHs). Tetrahedron, 2023, 138: 133414. [9] 蒋柱武, 吴梦帆, 李登胜, 等. 层状双金属氢氧化物吸附剂的功能化改性策略[J]. 精细化工, 2023, 40(6): 1239-1252. doi: 10.13550/j.jxhg.20220846 JIANG Z W, WU M F, LI D S, et al. Functional modification strategies of layered double-metal hydroxide adsorbents[J]. Fine Chemicals, 2023, 40(6): 1239-1252(in Chinese). doi: 10.13550/j.jxhg.20220846
[10] 杨靖, 范议议, 王赛娣, 等. 二维层状双金属氢氧化物在去除磷酸盐中的应用[J]. 化工进展, 2022, 41(7): 3689-3706. YANG J, FAN Y Y, WANG S D, et al. Layered double hydroxide(LDH) for phosphate removal[J]. Chemical Industry and Engineering Progress, 2022, 41(7): 3689-3706(in Chinese).
[11] HE H J, LIU L, LI Q, et al. Feasibility of using Mg/Al-based layered double hydroxides as an inactivating agent to interrupt phosphorus release from contaminated agricultural drainage ditch sediments[J]. Ecotoxicology and Environmental Safety, 2021, 223: 112599. doi: 10.1016/j.ecoenv.2021.112599 [12] WU Y H, SONG L, SHI M F, et al. Ca/Fe-layered double hydroxide-zeolite composites for the control of phosphorus pollution in sediments: Performance, mechanisms, and microbial community response[J]. Chemical Engineering Journal, 2022, 450: 138277. doi: 10.1016/j.cej.2022.138277 [13] NGUYEN-QUANGQ M, AZZOLINA-JURY F, SAMOJEDEN B, et al. On the influence of the preparation routes of NiMgAl-mixed oxides derived from hydrotalcite on their CO2 methanation catalytic activities[J]. International Journal of Hydrogen Energy, 2022, 47(89): 37783-37791. doi: 10.1016/j.ijhydene.2022.08.278 [14] DAI R R, ESER B E, GUO Z, Beyond flower-like structure – The synergy within Pd/Ni-Al hydrotalcite for base-free oxidation of benzyl alcohols[J]. Applied Catalysis A: General, 2021, 610: 117972. [15] GUO C Y, SHEN S G, LI M N, et al. Rapid in situ synthesis of MgAl-LDH on η-Al2O3 for efficient hydrolysis of urea in wastewater[J]. Journal of Catalysis, 2021, 395: 54-62. doi: 10.1016/j.jcat.2020.12.024 [16] NASEEM S, GEVERS B, BOLDT R, et al. Comparison of transition metal (Fe, Co, Ni, Cu, and Zn) containing tri-metal layered double hydroxides (LDHs) prepared by urea hydrolysis[J]. RSC Advances, 2019, 9(6): 3030-3040. doi: 10.1039/C8RA10165E [17] QUISPE-DOMINGUEZ R, NASEEM S, LEUTERITZ A, et al. Synthesis and characterization of MgAl-DBS LDH/PLA composite by sonication-assisted masterbatch (SAM) melt mixing method[J]. RSC Advances, 2019, 9(2): 658-667. doi: 10.1039/C8RA08780F [18] YANG R, ZHOU Y M, XING Y G, et al. Synergistic coupling of CoFe-LDH arrays with NiFe-LDH nanosheet for highly efficient overall water splitting in alkaline media[J]. Applied Catalysis B: Environmental, 2019, 253(4): 131-139. [19] LIU S X, ZHANG H W, HU E L, et al. Boosting oxygen evolution activity of NiFe-LDH using oxygen vacancies and morphological engineering[J]. Journal of Materials Chemistry A, 2021, 9(41): 23697-23702. doi: 10.1039/D1TA06263H [20] SAKR A A E, ZAKI T, ELGABRYl O, et al. Enhanced CO2 capture from methane-stream using MII -Al LDH prepared by microwave-assisted urea hydrolysis[J]. Advanced Powder Technology, 2021, 32(11): 4096-4109. doi: 10.1016/j.apt.2021.09.016 [21] KUANG H, ZHANG H, LIU X, et al. Microwave-assisted synthesis of NiCo-LDH/graphene nanoscrolls composite for supercapacitor[J]. Carbon, 2022, 190: 57-67. doi: 10.1016/j.carbon.2021.12.097 [22] GANDAMALLA A, MANCHALA S, VERMA A, et al. Microwave-assisted synthesis of ZnAl-LDH/g-C3N4 composite for degradation of antibiotic ciprofloxacin under visible-light illumination[J]. Chemosphere, 2021, 283: 131182. doi: 10.1016/j.chemosphere.2021.131182 [23] ARSHAD F, MUNIR A, TAHIR A, et al. Microwave-assisted growth of spherical core-shell NiFe LDH@CuxO nanostructures for electrocatalytic water oxidation reaction[J]. International Journal of Hydrogen Energy, 2023, 48(12): 4719-4727. doi: 10.1016/j.ijhydene.2022.10.252 [24] YIN Z Z, HE R Z, ZHANG Y C, et al. Electrochemical deposited amorphous FeNi hydroxide electrode for oxygen evolution reaction[J]. Journal of Energy Chemistry, 2022, 69: 585-592. doi: 10.1016/j.jechem.2022.01.020 [25] JIANG K, LIU W J, LAI W, et al. NiFe layered double hydroxide/FeOOH heterostructure nanosheets as an efficient and durable bifunctional electrocatalyst for overall seawater splitting[J]. Inorganic Chemistry, 2021, 60(22): 17371-17378. doi: 10.1021/acs.inorgchem.1c02903 [26] CUI Q L, JIAO G J, ZHENG J Y, et al. Synthesis of a novel magnetic Caragana korshinskii biochar/Mg-Al layered double hydroxide composite and its strong adsorption of phosphate in aqueous solutions[J]. RSC Advances, 2019, 9(32): 18641-18651. doi: 10.1039/C9RA02052G [27] SOKOL D, VIEIRA D E L, ZARKOV A, et al. Sonication accelerated formation of Mg-Al-phosphate layered double hydroxide via sol-gel prepared mixed metal oxides[J]. Scientific Reports, 2019, 9: 10419. doi: 10.1038/s41598-019-46910-5 [28] BODHANKAR P M, SARAWADE P B, SINGH G, et al. Recent advances in highly active nanostructured NiFe LDH catalyst for electrochemical water splitting[J]. Journal of Materials Chemistry A, 2021, 9(6): 3180-3208. doi: 10.1039/D0TA10712C [29] CANTRELL D G, GILLIE L J, LEE A F, et al. Structure-reactivity correlations in MgAl hydrotalcite catalysts for biodiesel synthesis[J]. Applied Catalysis A: General, 2005, 287(2): 183-190. doi: 10.1016/j.apcata.2005.03.027 [30] YANG Z Z, WEI J J, ZENG G M, et al. A review on strategies to LDH-based materials to improve adsorption capacity and photoreduction efficiency for CO2[J]. Coordination Chemistry Reviews, 2019, 386: 154-182. doi: 10.1016/j.ccr.2019.01.018 [31] LI J X, LI B, WANG J K, et al. Recent advances in layered double hydroxides and their derivatives for biomedical applications[J]. Acta Chimica Sinica, 2021, 79(3): 238-256. doi: 10.6023/A20090441 [32] SAHOO D P, DAS K K, MANSINGH S, et al. Recent progress in first row transition metal Layered double hydroxide (LDH) based electrocatalysts towards water splitting: A review with insights on synthesis[J]. Coordination Chemistry Reviews, 2022, 469: 214666. doi: 10.1016/j.ccr.2022.214666 [33] ASHEKUZZAMAN S M, JIANG J Q. Study on the sorption-desorption-regeneration performance of Ca-, Mg- and CaMg-based layered double hydroxides for removing phosphate from water[J]. Chemical Engineering Journal, 2014, 246: 97-105. doi: 10.1016/j.cej.2014.02.061 [34] AL JABERI M, MALLET M, GREENWELL H C et al. Using Ca-Fe layered double hydroxide transformation to optimise phosphate removal from waste waters[J]. Applied Clay Science, 2019, 182: 105281. doi: 10.1016/j.clay.2019.105281 [35] SEFTEL E M, CIOCARLAN R G, MICHIELSEN B, et al. Insights into phosphate adsorption behavior on structurally modified ZnAl layered double hydroxides[J]. Applied Clay Science, 2018, 165: 234-246. doi: 10.1016/j.clay.2018.08.018 [36] ALMOJIL S F, OTHMAN M A. Screening different divalent and trivalent metals containing binary and ternary layered double hydroxides for optimum phosphate uptake[J]. Scientific Reports, 2019, 9(1): 15511. doi: 10.1038/s41598-019-52031-w [37] YANG K, YAN L G, YANG Y M, et al. Adsorptive removal of phosphate by Mg-Al and Zn-Al layered double hydroxides: Kinetics, isotherms and mechanisms[J]. Separation and Purification Technology, 2014, 124: 36-42. doi: 10.1016/j.seppur.2013.12.042 [38] LUENGO C V, VOLPE M A, AVENA M J. High sorption of phosphate on Mg-Al layered double hydroxides: Kinetics and equilibrium[J]. Journal of Environmental Chemical Engineering, 2017, 5(5): 4656-4662. doi: 10.1016/j.jece.2017.08.051 [39] YU Q Q, ZHENG Y Q, WANG Y P, et al. Highly selective adsorption of phosphate by pyromellitic acid intercalated ZnAl-LDHs: Assembling hydrogen bond acceptor sites[J]. Chemical Engineering Journal, 2015, 260: 809-817. doi: 10.1016/j.cej.2014.09.059 [40] ZHOU H G, TAN Y L, YANG Y M, et al. Application of FeMgMn layered double hydroxides for phosphate anions adsorptive removal from water[J]. Applied Clay Science, 2021, 200: 105903. doi: 10.1016/j.clay.2020.105903 [41] HALAJNIA A, OUSTAN S, NAJAFI N, et al. Adsorption-desorption characteristics of nitrate, phosphate and sulfate on Mg-Al layered double hydroxide[J]. Applied Clay Science, 2013, 80/81: 305-312. doi: 10.1016/j.clay.2013.05.002 [42] CHENG X, HUANG X R, WANG X Z, et al. Influence of calcination on the adsorptive removal of phosphate by Zn-Al layered double hydroxides from excess sludge liquor[J]. Journal of Hazardous Materials, 2010, 177(1/2/3): 516-523. [43] DAS J, PATRA B S, BALIARSINGH N, et al. Adsorption of phosphate by layered double hydroxides in aqueous solutions[J]. Applied Clay Science, 2006, 32(3/4): 252-260. [44] HE H M, KANG H L, MA S L, et al. High adsorption selectivity of ZnAl layered double hydroxides and the calcined materials toward phosphate[J]. Journal of Colloid and Interface Science, 2010, 343(1): 225-231. doi: 10.1016/j.jcis.2009.11.004 [45] IFTEKHAR S, KUCUK M E, SRIVASTAVA V, et al. Application of zinc-aluminium layered double hydroxides for adsorptive removal of phosphate and sulfate: Equilibrium, kinetic and thermodynamic[J]. Chemosphere, 2018, 209: 470-479. doi: 10.1016/j.chemosphere.2018.06.115 [46] CHENG X, HUANG X R, WANG X Z, et al. Phosphate adsorption from sewage sludge filtrate using zinc–aluminum layered double hydroxides[J]. Journal of Hazardous Materials, 2009, 169(1/2/3): 958-964. [47] ASHEKUZZAMAN S M, JIANG J Q. Strategic phosphate removal/recovery by a re-usable Mg-Fe-Cl layered double hydroxide[J]. Process Safety and Environmental Protection, 2017, 107: 454-462. doi: 10.1016/j.psep.2017.03.009 [48] LIU T, CHEN Y X, YU Q L, et al. Effect of MgO, Mg-Al-NO3 LDH and calcined LDH-CO3 on chloride resistance of alkali activated fly ash and slag blends[J]. Construction and Building Materials, 2020, 250: 118865. doi: 10.1016/j.conbuildmat.2020.118865 [49] KAMEDA T, KURUTACH T, TAKAHASHI Y, et al. Thermal decomposition behavior of MnO2/Mg-Al layered double hydroxide after removal and recovery of acid gas[J]. Results in Chemistry, 2022, 4: 100310. doi: 10.1016/j.rechem.2022.100310 [50] MIYATA S. Physico-chemical properties of synthetic hydrotalcites in relation to composition[J]. Clays and Clay Minerals, 1980, 28(1): 50-56. doi: 10.1346/CCMN.1980.0280107 [51] BOLBOL H, FEKRI M, HEJAZI-MEHRIZI M. Layered double hydroxide-loaded biochar as a sorbent for the removal of aquatic phosphorus: Behavior and mechanism insights[J]. Arabian Journal of Geosciences, 2019, 12(16): 1-11. [52] YANG F, ZHANG S S, SUN Y Q, et al. Assembling biochar with various layered double hydroxides for enhancement of phosphorus recovery[J]. Journal of Hazardous Materials, 2019, 365: 665-673. doi: 10.1016/j.jhazmat.2018.11.047 [53] LI R H, WANG J J, ZHOU B Y, et al. Enhancing phosphate adsorption by Mg/Al layered double hydroxide functionalized biochar with different Mg/Al ratios[J]. Science of the Total Environment, 2016, 559: 121-129. doi: 10.1016/j.scitotenv.2016.03.151 [54] ALAGHA O, MANZAR M S, ZUBAIR M, et al. Magnetic Mg-Fe/LDH intercalated activated carbon composites for nitrate and phosphate removal from wastewater: Insight into behavior and mechanisms[J]. Nanomaterials, 2020, 10(7): 1361. doi: 10.3390/nano10071361 [55] YIN H B, YANG P, KONG M, et al. Use of lanthanum/aluminum co-modified granulated attapulgite clay as a novel phosphorus (P) sorbent to immobilize P and stabilize surface sediment in shallow eutrophic lakes[J]. Chemical Engineering Journal, 2020, 385: 123395. doi: 10.1016/j.cej.2019.123395 [56] ONYANGO M S, KUCHAR D, KUBOTA M, et al. Adsorptive removal of phosphate ions from aqueous solution using synthetic zeolite[J]. Industrial & Engineering Chemistry Research, 2007, 46(3): 894-900. [57] XIE J, WANG Z, LU S Y, et al. Removal and recovery of phosphate from water by lanthanum hydroxide materials[J]. Chemical Engineering Journal, 2014, 254: 163-170. doi: 10.1016/j.cej.2014.05.113 [58] YAN L G, XU Y Y, YU H Q, et al. Adsorption of phosphate from aqueous solution by hydroxy-aluminum, hydroxy-iron and hydroxy-iron-aluminum pillared bentonites[J]. Journal of Hazardous Materials, 2010, 179(1/2/3): 244-250. [59] HUANG W Y, YU X, TANG J P, et al. Enhanced adsorption of phosphate by flower-like mesoporous silica spheres loaded with lanthanum[J]. Microporous and Mesoporous Materials, 2015, 217: 225-232. doi: 10.1016/j.micromeso.2015.06.031 [60] KIZITO S, LUO H Z, WU S B, et al. Phosphate recovery from liquid fraction of anaerobic digestate using four slow pyrolyzed biochars: Dynamics of adsorption, desorption and regeneration[J]. Journal of Environmental Management, 2017, 201: 260-267. doi: 10.1016/j.jenvman.2017.06.057 [61] NUR T, JOHIR M A H, LOGANATHAN P, et al. Phosphate removal from water using an iron oxide impregnated strong base anion exchange resin[J]. Journal of Industrial and Engineering Chemistry, 2014, 20(4): 1301-1307. doi: 10.1016/j.jiec.2013.07.009 [62] KÖSE T E, KıVANÇ B. Adsorption of phosphate from aqueous solutions using calcined waste eggshell[J]. Chemical Engineering Journal, 2011, 178: 34-39. doi: 10.1016/j.cej.2011.09.129 [63] LIN Z G, CHEN J. Magnetic Fe3O4@MgAl-LDH@La(OH)3 composites with a hierarchical core-shell structure for phosphate removal from wastewater and inhibition of labile sedimentary phosphorus release[J]. Chemosphere, 2021, 264(Pt 2): 128551. [64] SONG K, ADAMS C J, BURGIN A J. Relative importance of external and internal phosphorus loadings on affecting lake water quality in agricultural landscapes[J]. Ecological Engineering, 2017, 108: 482-488. doi: 10.1016/j.ecoleng.2017.06.008 [65] 吴俊麟, 林建伟, 詹艳慧, 等. 镁铁层状双金属氢氧化物对磷酸盐的吸附作用及对内源磷释放的控制效果及机制[J]. 环境科学, 2020, 41(1): 273-283. doi: 10.13227/j.hjkx.201907174 WU J L, LIN J W, ZHAN Y H, et al. Adsorption of phosphate on Mg/Fe layered double hydroxides(Mg/Fe-LDH) and use of Mg/Fe-LDH as an amendment for controlling phosphorus release from sediments[J]. Environmental Science, 2020, 41(1): 273-283(in Chinese). doi: 10.13227/j.hjkx.201907174
[66] WU J L, LIN J W, ZHAN Y H. Interception of phosphorus release from sediments using Mg/Fe-based layered double hydroxide (MF-LDH) and MF-LDH coated magnetite as geo-engineering tools[J]. The Science of the Total Environment, 2020, 739: 139749. doi: 10.1016/j.scitotenv.2020.139749 [67] WANG J F, CHEN J G, CHEN Q, et al. Assessment on the effects of aluminum-modified clay in inactivating internal phosphorus in deep eutrophic reservoirs[J]. Chemosphere, 2019, 215: 657-667. doi: 10.1016/j.chemosphere.2018.10.095 [68] JOBBÁGY M, REGAZZONI A E. Dissolution of nano-size Mg-Al-Cl hydrotalcite in aqueous media[J]. Applied Clay Science, 2011, 51(3): 366-369. doi: 10.1016/j.clay.2010.11.027 [69] DAI R R, LIU B G, ZHANG Y, et al. Copper-based ternary hydrotalcite as a catalyst for hydroxylation of phenolic compounds[J]. Journal of Environmental Chemical Engineering, 2021, 9(6): 106390. doi: 10.1016/j.jece.2021.106390 -



 下载:
下载: