-
近年来,由于水环境中营养盐过量及气候变暖,导致湖泊和水库甚至河流有害藻华频繁暴发,对水生生态系统、渔业和人类健康造成了严重威胁[1],因而藻类污染治理已成为当今国内外面临的一大难题[2-3]。尽管控制污染源可以从根本上解决藻类污染,但时间长,见效慢。而在当前,我国江河湖库大多趋于富营养状态,难以避免藻类灾害的突发,而有些藻类灾害突发会造成重大损失[4]。因此,开展各种综合除藻方法和应急除藻技术研究显得尤为重要。
目前,研究者们已开发了多种应急除藻方法。其中,天然无毒的黏土矿物材料絮凝去除水体中藻类是治理藻华的有效方法[5-8]。这种方法因其成本低、吸附容量高、阳离子交换容量高和比表面积大,尤其是当藻华的规模大到一定程度时,黏土除藻优势更加明显。然而,将黏土用于藻类去除上仍存在一些问题[9-10],如沉降至水底的大量黏土及有害藻类的聚集体可能会影响水生生物的呼吸和摄食。虽然改性黏土可以减小黏土的使用量并提高除藻效率,但黏土改性过程中需要复杂的预处理过程和高成本,同时改性黏土中的掺杂物对底栖生物的影响难以评价[9-11]。因此,开发一种经济、高效、安全的水华控制技术尤为迫切。
近年来,一些矿物因其有良好的吸附能力而被应用于水污染治理中。GHOSAL等[12]采用响应面法和人工神经网络法研究了铁/橄榄石对砷的吸附去除过程;SHABAN等[13]研究了天然蛇纹石对常见的水体污染物染料和重金属离子的吸附性能;BAHABADI等[14]通过修饰海泡石、沸石、坡缕石去除水环境中的重金属Cd、Cu、Zn;BANDURA等[15]归纳了天然矿物在水体中吸附石油的研究进展;XIAO等[16]将磁铁矿用于污水处理厂吸附水中的磷。另外,有学者发现天然矿物可以有效去除水中藻。WANG等[17]考察了不同理化条件下天然磁闪锌矿去除有害藻类海洋卡盾藻;SUKENIK等[18]采用阳离子表面活性剂改性的膨润土用于湖泊水体中蓝藻和蓝藻毒素的去除等。不同种类的矿物由于其表面结构和理化性质差异,其对藻类的去除效果及去除速率差异较大,而且一些天然矿物表面带正电荷,利用其吸附去除表面带负电荷的藻细胞具有独特优势。因此,开展天然矿物除藻研究具有重要的现实意义。
本研究以铜绿微囊藻为去除目标,测定了10种天然矿物对铜绿微囊藻叶绿素a的去除情况,探讨了矿物成分与去除铜绿微囊藻效果的相关性并发现天然橄榄石具有优良的除藻性能,系统地研究了反应条件如矿物用量、藻密度、光强、水温及介质pH对其除藻效率的影响,同时从反应前后细胞形态、Zeta电位及营养盐的变化等方面探讨和揭示天然橄榄石对铜绿微囊藻的去除机理,为去除铜绿微囊藻的矿物选择以及改性提供参考。
全文HTML
-
天然矿物样品由中南冶金地质研究所、宜昌地质矿产研究所提供,粒径为180~200目。矿物包括铝土矿、磷矿(CaO 31.56%,MgO 0.99%,Fe2O3 4.46%,SiO2 25.04%)、磷铁矿、重晶石(BaSO4 98.00%,Al2O3 0.02%,Fe2O3 0.02%,SiO2 0.30%)、方解石、白云石(CaO 33.67%,Al2O3 0.12%,MgO 17.82%,Fe2O3 0.55%,SiO2 1.61%)、石灰石(CaO 53.38%,Al2O3 0.68%,MgO 0.62%,Fe2O3 0.43%,SiO2 1.57%)、方铅矿(Pb 32.44%)、黄铁矿(S 37.20%,F 0.030%,Se 0.0059%)、橄榄石(CaO 1.53%,Al2O3 3.44%,MgO 35.04%,Fe2O3 4.13%,SiO2 39.06%)。铜绿微囊藻(Microsystisaeruginosa)(编号:FACHB-905)购于中国科学院水生生物研究所国家淡水藻种库。
-
X射线衍射仪(XRD,Ultima IV,Rigaku,日本);冷场扫描电子显微镜(SEM,JSM-7500F,JEOL,Japan);紫外-可见分光光度计(UV-Vis DRS,Lambda25,美国);pH计(Delta 320,Mettler-Toledo,上海有限公司);Zeta电位分析仪(Malvern Zetasizer,2000HSA,UK);高压灭菌锅(SYQ-DSY-280B,上海申安医疗器械厂);光照培养箱(PGX-250B,宁波海曙赛佛实验仪器厂)。
-
取200 mL灭菌后的培养基,向培养基中加入处于对数生长期的藻细胞,使得初始藻密度为1.7×106 cells·mL−1,初始pH为7.5,温度为25 ℃,天然矿物投加量一定,放入光照培养箱。反应一定时间,静置30 min,于液面下3 cm处取样,每个实验组设置3个重复。通过测定叶绿素a的变化来计算矿物对藻的去除率,计算方法见式(1)。
式中:R为藻去除率;C为实验组的藻类叶绿素a含量;C0为对照组藻类叶绿素a含量。
针对筛选出的最佳矿物天然橄榄石,考察不同反应条件对其除藻性能的影响。具体考察参数及设置条件如下:矿物浓度为0.5、1.0、1.5和2.0 g∙L‒1;反应的初始藻密度为0.9×106、1.7×106、5.1×106、8.5×106和1.7×107 cells∙mL‒1;光强为0 (黑暗)、500、1 000、1 500和2 000 lx;温度为15、20、25、30和35 ℃;pH为5、6、7、8、9、10和11。所有实验均在培养箱中进行,时间为60 min,其他条件及方法同上。
在天然橄榄石对藻细胞去除机理研究中,取200 mL灭菌后的培养基,向培养基中加入处于对数生长期的藻细胞,控制初始藻密度为1.7×106 cells∙mL‒1、初始pH为7.5,温度为25 ℃,矿物投加量一定,放入光照培养箱反应60 min。测定反应过程中铜绿微囊藻Zeta电位、培养基中营养盐以及藻细胞形貌。
-
天然橄榄石的表征:天然橄榄石的晶形采用X射线衍射仪(XRD,Ultima IV,Rigaku,日本)测定,扫描2θ为20°~60°;采用冷场扫描电子显微镜(SEM,JSM-7500F,JEOL,Japan)。
藻细胞表面结构观察:分别取100 mL空白藻液和加入矿物处理的藻液,在4 000 r·min−1下离心10 min,弃去上清液,向收集的藻细胞中加入1 mL 2%的戊二醛固定藻细胞2 h,然后进行电镜制样(由三峡大学医学院基础医学实验教学省级示范中心完成)并在扫描电子显微镜(SEM, JSM-7500F, JEOL,日本)下观察拍照。
Zeta电位的变化:取生长对数期的铜绿微囊藻和反应过程的铜绿微囊藻,离心弃去上清液,藻泥用超纯水配制成一定浓度,再将该浓度下的藻液pH分别调节至2、4、6、8、10左右,采用Zeta电位分析仪(Malvern Zetasizer, 2000HSA, UK)测定不同pH条件下的铜绿微囊藻Zeta电位,仪器自动测定3次,取平均值。
除藻前后培养基中营养盐的测定:在实验中,分别于0、20、40和60 min时,在正常生长的藻液和天然橄榄石处理的藻液中,各取10 mL溶液,经0.45 µm膜过滤,滤液进行总磷及总氮测定。其中,总磷的测定采用GB 11893-1989中的钼酸铵分光光度法,总氮的测定采用GB 11894-1989中的碱性过硫酸钾消解紫外分光光度法。
1.1. 实验材料
1.2. 实验仪器
1.3. 实验方法
1.4. 分析方法
-
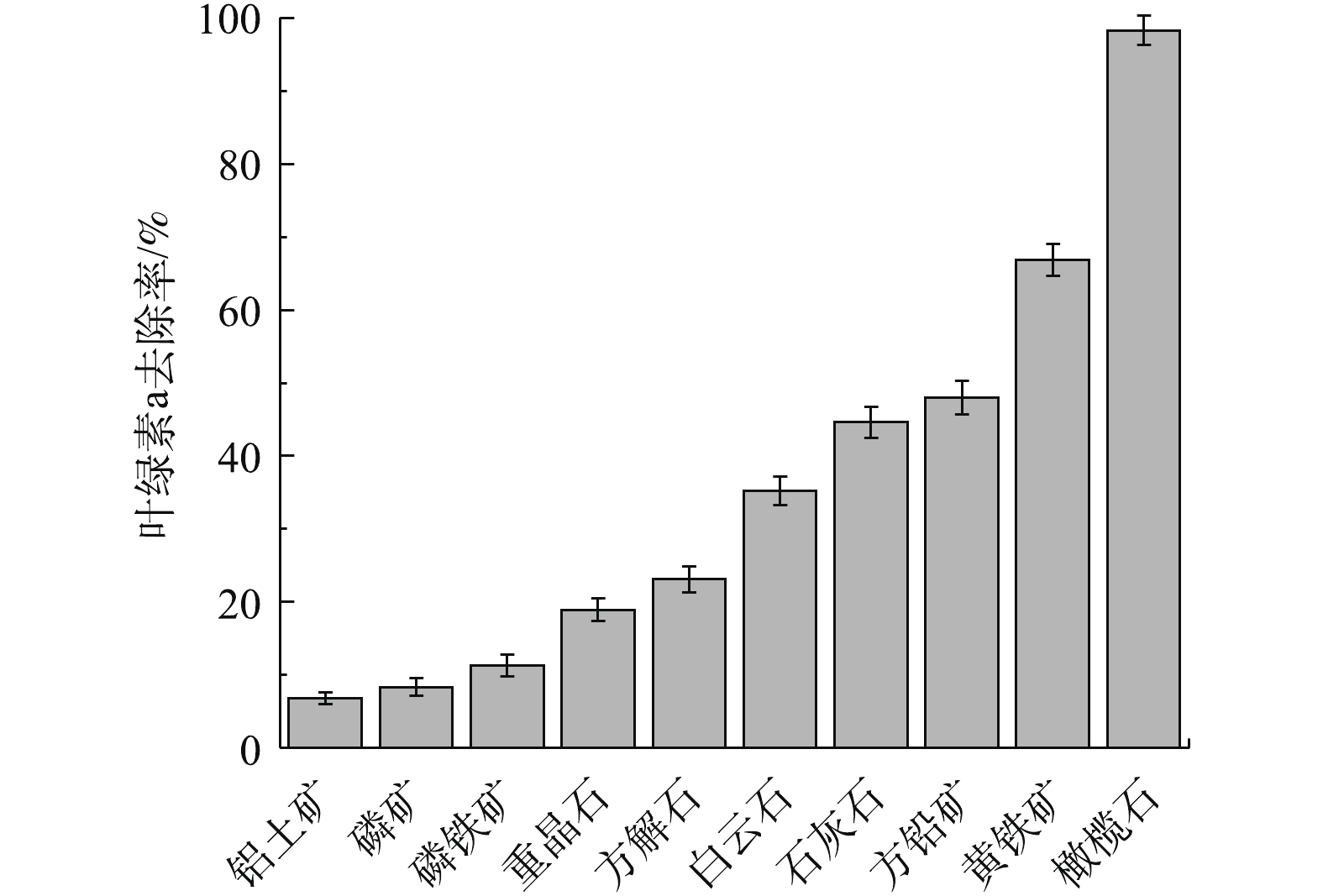
图1反映了10种天然矿物对铜绿微囊藻叶绿素a的去除率。可以看出,不同矿物对铜绿微囊藻的作用显著不同。在10种天然矿物中,对铜绿微囊藻叶绿素a去除率从高到低依次为橄榄石>黄铁矿>方铅矿>石灰石>白云石>方解石>重晶石>磷铁矿>磷矿>铝土矿。其中,橄榄石对叶绿素a去除率最高,达98.32%;去除效果最差的为铝土矿,叶绿素a去除率仅为6.81%。这可能是由于不同矿物中的金属氧化物类型、金属价态、晶型结构、表面性质存在着较大差异,导致其对叶绿素a去除效果差异显著[19]。使用SPSS12软件,计算相应矿物中各组分含量与叶绿素a去除率间的相关性,结果见表1。可以看出,铜绿微囊藻叶绿素a去除率与矿物的SiO2、Al2O3、Fe2O3含量之间具有显著相关性(P<0.05),而与MgO含量无显著相关性 (P>0.05)。由于天然橄榄石对铜绿微囊藻的去除效果最好,因此,在后续实验中,以天然橄榄石为吸附剂,探讨其去除铜绿微囊藻的作用机理。
-
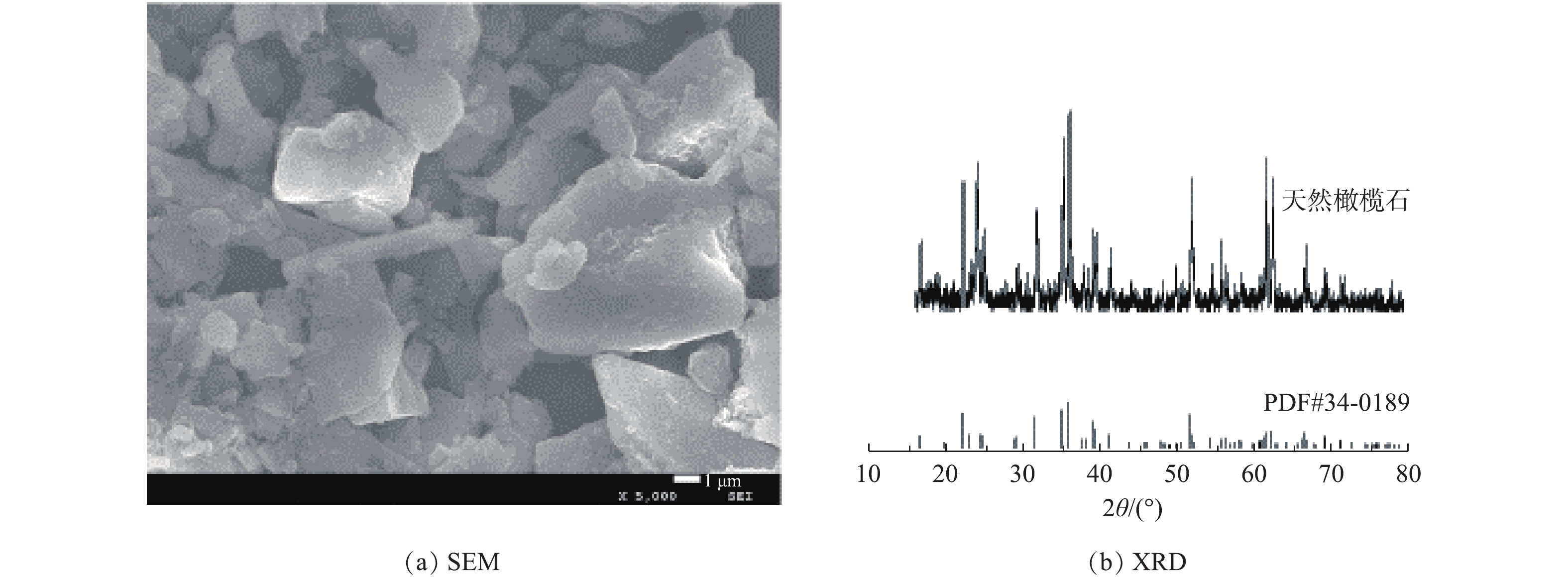
图2为天然橄榄石的SEM及XRD图。由图2(a)可看出,天然橄榄石呈现大小不一的块状结构,且分布不均匀。图2(b)所示的XRD图与标准卡PDF#34-0189的天然橄榄石一致,在2θ为22.89°、32.35°、35.75°、36.55°、52.28°等出现的衍射峰与天然橄榄石的标准卡相对应。
不同橄榄石浓度对铜绿微囊藻的叶绿素a去除率如图3所示。叶绿素a的去除率依赖于橄榄石的用量,当用量小于1.5 g∙L‒1时,随着橄榄石用量的增加,叶绿素a的去除率增加。在用量为1.5 g∙L‒1时,叶绿素a的去除率达到90.2%;继续增加橄榄石的用量时,叶绿素a的去除率不再增大,趋于稳定。这是由于矿物颗粒与藻细胞之间的吸附作用取决于接触和黏附[20],在藻密度一定时,增加橄榄石的用量,可以增大矿物颗粒与藻细胞之间的碰撞概率,从而实现吸附的效果;而继续增加橄榄石导致藻液浊度增大,而参与反应的有效橄榄石量不变[21]。因此,在橄榄石用量为1.5 g∙L‒1后,叶绿素a去除率趋于平衡,故在后续实验中橄榄石用量选为1.5 g∙L‒1。
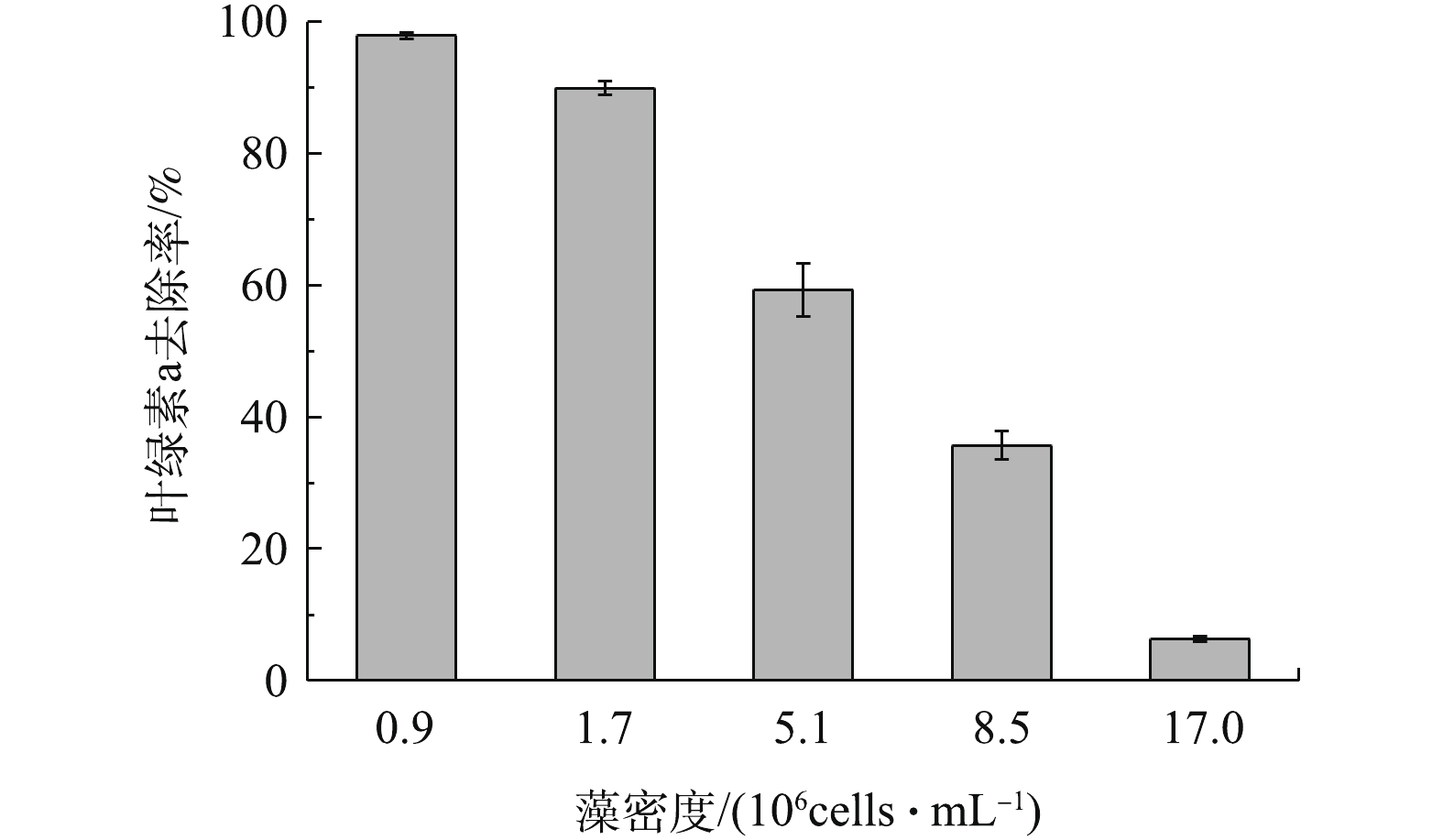
在天然水体中,不同地区不同季节会出现不同的藻密度[22]。因此,实验考察了天然橄榄石对初始藻细胞密度不同的藻去除率,结果如图4所示。在反应60 min内,随着藻密度的增大,藻液中叶绿素a的去除率逐渐降低。具体来讲,藻密度低于1.7×106 cells∙mL‒1时,橄榄石能高效除藻,叶绿素a去除率达到89.9%及以上。当藻密度上升到5.1×106、8.5×106和1.7×107 cells∙mL‒1时,藻去除率迅速下降,叶绿素a去除率分别为59.3%、35.7%和6.3%。这是因为,随着藻细胞个数的增加,橄榄石表面的吸附位点逐渐被占据,吸附开始减弱,因而随着藻密度增大,橄榄石的除藻性能逐渐降低[23]。
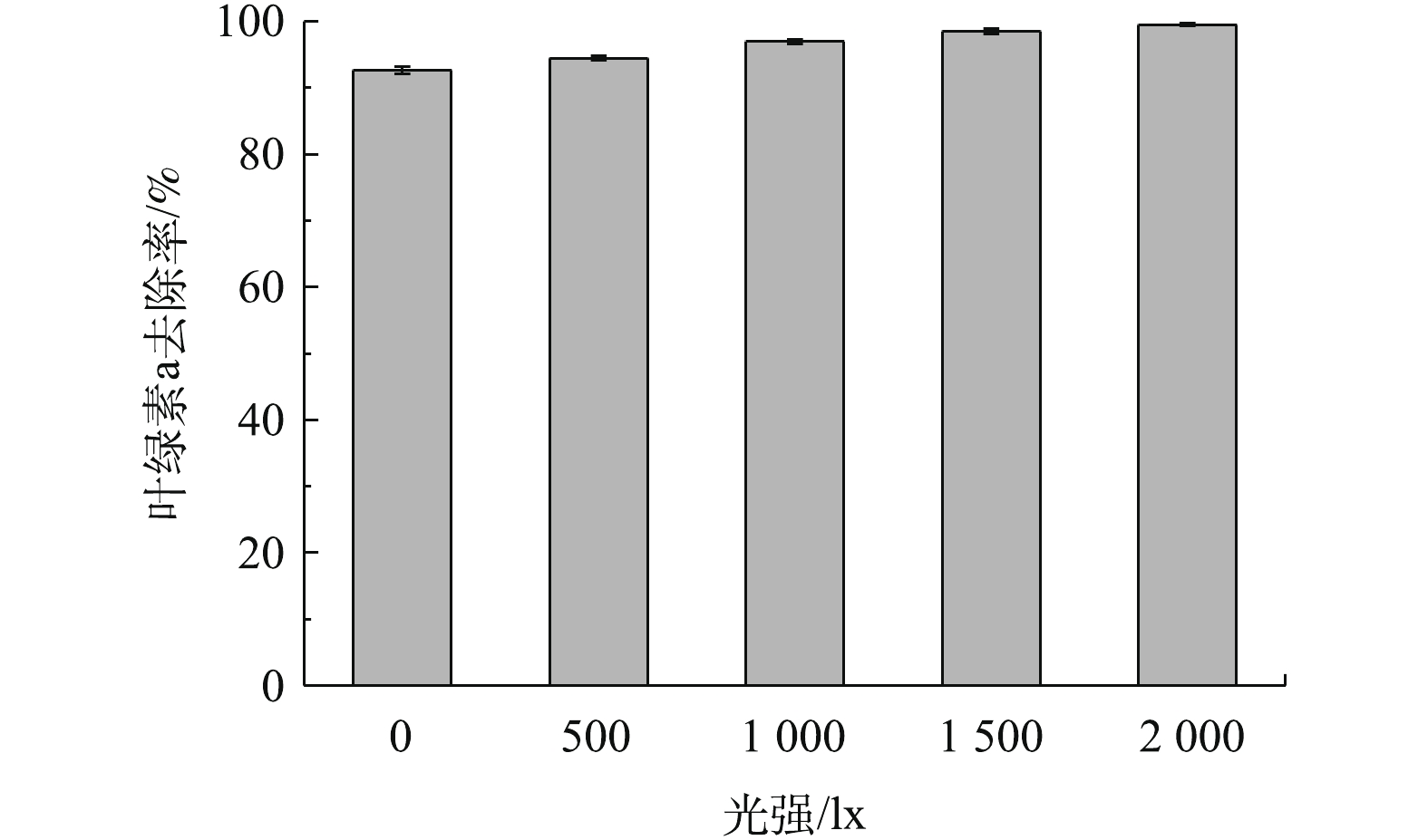
光强是影响铜绿微囊藻生长的环境因子之一,光强的大小不仅直接影响藻类的光合作用,还与藻体内色素含量、脂类含量的合成密切相关[24]。因此,本实验考察了橄榄石在除藻过程中是否会受到光强的影响,结果见图5。由图5明显看出,随着光强的增加,去除率依次增大,最大值为99.5%,说明光对橄榄石除藻有一定的促进作用。但在光强为0(即黑暗)情况下,橄榄石对叶绿素a的去除率就达92.6%,说明光强对橄榄石除藻的影响较小,橄榄石对铜绿微囊藻的去除主要为吸附作用,而非光催化反应。
温度是决定水体中藻类演替的主要因素,藻类的生长速率随温度的变化而变化[25]。温度会影响藻类生理反应、代谢率和营养的利用系数[26]。图6显示了不同温度的培养基培养下,橄榄石吸附铜绿微囊藻的结果。由图6可以看出,在温度为15 ℃时,叶绿素a的去除率最高;20~35 ℃下,叶绿素a的去除率随着温度的升高而升高,在35 ℃时趋于平衡。这是因为随着水温的升高,加速了橄榄石与铜绿微囊藻之间分子的碰撞速率[27],导致藻细胞在很短时间内出现了自溶现象,细胞内物质释放到水体。因此,藻细胞的叶绿素a去除率升高。而当水温为15 ℃时,低温刺激铜绿微囊藻的代谢活动不利于其生长繁殖,因此,水体中叶绿素a去除率高。
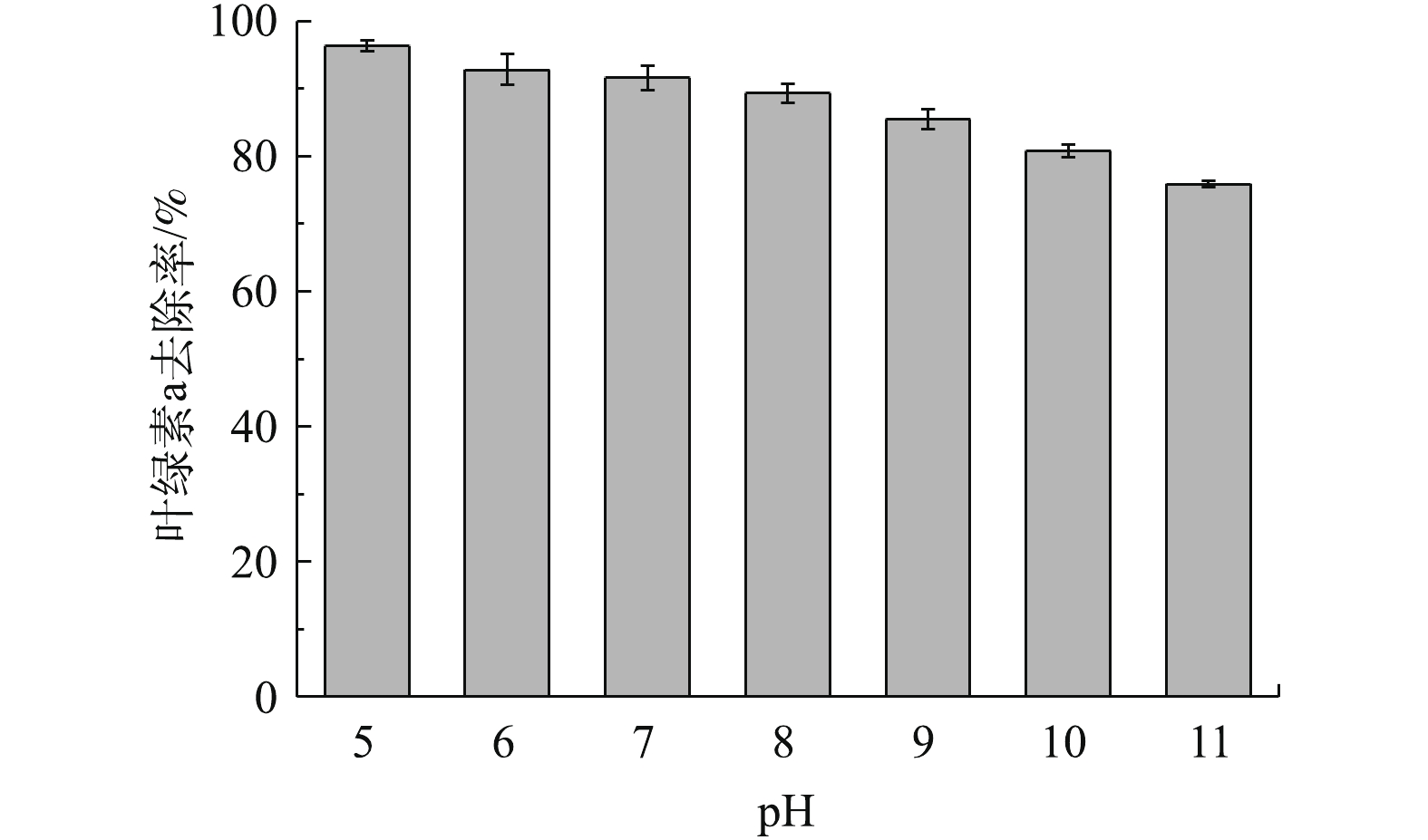
矿物表面基团在pH不同时会分解、质子化或羟基化,从而使其表面呈现不同的基团特性和带电性质[28-30],因此,藻液初始pH对吸附的效果会有一定的影响。不同pH下橄榄石对叶绿素a去除率的影响如图7所示。在酸性和弱碱性条件下,叶绿素a去除率均在90%以上;随着碱性的增强,去除率逐渐下降,在pH=11时去除率为75.9%。
经Zeta电位测定,橄榄石的零电位点pH为10.5。因此,当藻液pH<10.5时,随着酸性的增强,橄榄石表面所带的正电荷越多,与带负电荷的铜绿微囊藻结合的概率越大[31];当pH>10.5时,橄榄石表面带负电荷,与同样带负电荷的藻细胞相互排斥,从而导致藻去除率降低;当pH=11时,藻的去除率为75.9%。另一方面,铜绿微囊藻在酸性条件下不适宜生长,因此,水体pH为弱酸性时藻去除率较高[17]。在铜绿微囊藻水华暴发时,水体pH通常偏碱性,藻密度越大,水体pH越高。因此,采用橄榄石进行控藻时,须控制水体pH以达到较好的除藻效果。
为了比较所考察因子对橄榄石去除藻性能影响的大小,计算了不同因素下橄榄石去除叶绿素a的方差。在所考察范围内,橄榄石用量、藻密度、光强、水温及pH对叶绿素a去除率的方差分别为183×104、1 445×104、8×104、13×104和52×104,说明在橄榄石除藻过程中,橄榄石用量及藻密度影响最大,其次为pH及水温,光强影响最小。
-
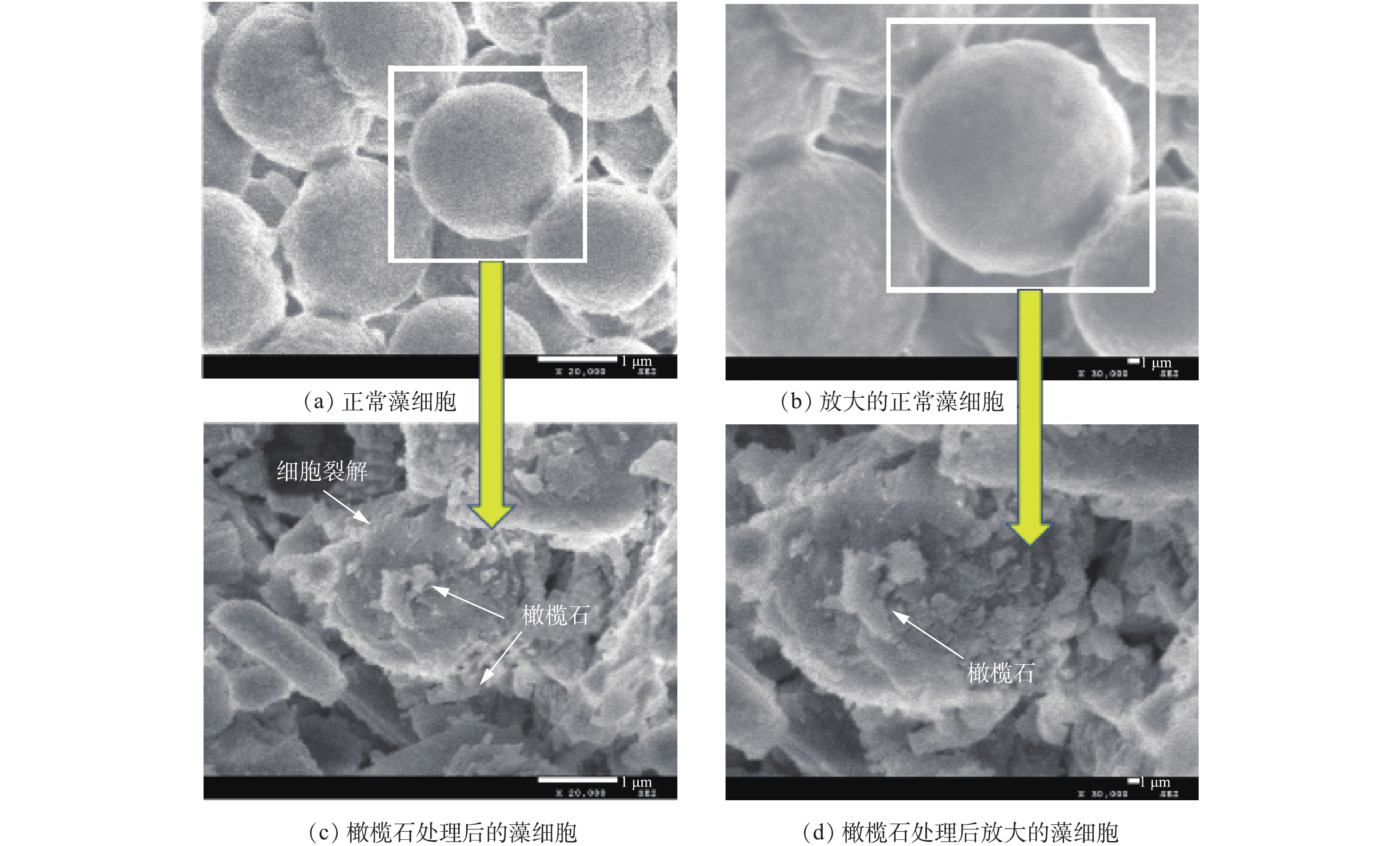
采用扫描电子显微镜(SEM)评估藻细胞被橄榄石处理后的损伤情况,结果如图8所示。图8(a)和图8(b)为正常生长繁殖状态下的铜绿微囊藻SEM图,可以看出,铜绿微囊藻单个细胞近球形,直径为1.8~2.2 µm,细胞圆润饱满,外表光滑。图8(c)和图8(d)为天然橄榄石处理后的铜绿微囊藻细胞微观结构,可以发现,橄榄石处理后的藻细胞受到很大程度破坏,细胞壁和细胞膜不仅破损皱缩,而且被橄榄石的片状结构穿插、嵌入细胞内部,不再具有完整的细胞结构,最终导致藻细胞死亡。处理后的藻细胞周边布满了橄榄石,说明橄榄石可以与藻细胞絮凝在一起,部分藻细胞发生破裂分解,进而沉降[32-35]。此结果与ZHAO等[36]报道的还原氧化石墨烯薄片作用于铜绿微囊藻后的SEM图类似。
图9(a)给出了不同pH下处于对数期的铜绿微囊藻和天然橄榄石的Zeta电位图。可以看出,天然橄榄石的Zeta电位在pH为2~10时均为正电性,而铜绿微囊藻的Zeta电位均呈负电性。这主要是因为,处于对数期的铜绿微囊藻生长繁殖能力旺盛,会分泌大量的黏液物质,它们含有羟基、羧基、氨基等官能团,这些基团会发生水合和电离作用使得藻体表面负电性增加,且pH越高,负电性越强[37]。
天然橄榄石处理后的藻细胞Zeta电位变化如图9(b)所示。随着反应的进行,空白藻液的Zeta电位维持在‒40~‒45 mV,而加入橄榄石处理的藻液,其Zeta电位随着反应的进行从‒43 mV逐渐上升,反应到75 min时,藻液的Zeta电位值为‒27.2 mV。在反应过程中,橄榄石表面的电正性与带负电的铜绿微囊藻由于电荷相互吸引而中和,造成负电性逐渐减少,Zeta电位向正值移动。随着藻细胞表面的电荷减少,细胞失去稳定性,形成聚集体而沉降,推测这种聚集效应有助于藻类去除[38]。
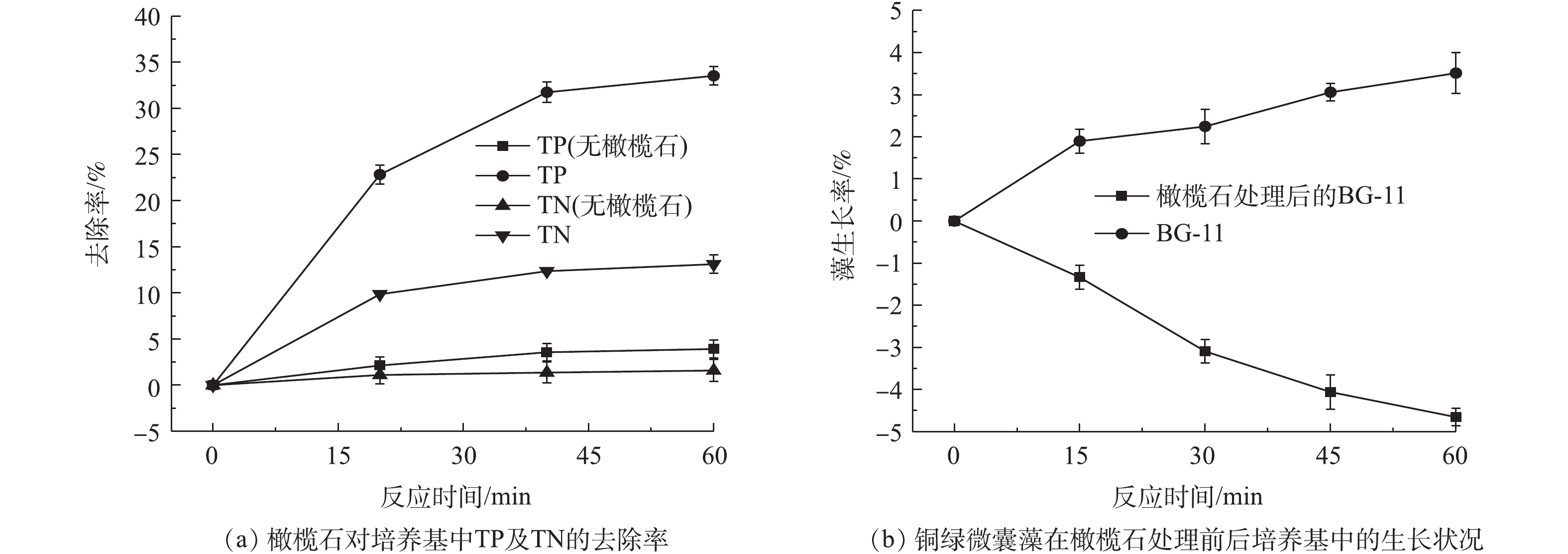
藻类通过磷和氮进行能量传输以满足其生长需求,因此,磷和氮的控制是缓解水体富营养的关键[39-40]。已有很多学者对矿物材料吸附磷的性能进行了研究,如硅藻土/伊利石[41]、磁铁矿[42]、铁氧化物[43]、水铁矿[44]等对营养盐具有很好的吸附效果。为了解橄榄石除藻过程中其对氮、磷的去除情况,在实验过程中同时监测了BG-11培养基中氮磷浓度的变化情况,培养基中初始总磷(TP)和总氮(TN)的含量分别为5.49 mg·L‒1和251.93 mg·L‒1,实验结果如图10所示。在60 min内,BG-11培养基中的总磷和总氮去除率分别为33.52%和13.11%,藻正常的生长繁殖消耗培养基中的总磷和总氮去除率仅为3.92%和1.59%(图10(a))。利用橄榄石吸附后的BG-11培养基二次接种藻细胞,发现藻细胞的生长受到一定抑制(图10(b)),这可能由于橄榄石除藻过程中同时吸附氮、磷,使得培养基营养不足而导致。上述结果说明,橄榄石去除藻细胞的同时对总磷及总氮也有一定的去除率。
2.1. 不同天然矿物去除铜绿微囊藻的效果比较
2.2. 天然橄榄石对铜绿微囊藻的去除
2.3. 橄榄石对铜绿微囊藻的去除机制
-
1)通过对比10种天然矿物对铜绿微囊藻叶绿素a的去除率发现,不同的天然矿物对铜绿微囊藻均有一定的去除效果,其中橄榄石去除效果最佳。
2)考察了反应条件(橄榄石用量、藻液初始藻密度、光强、水温和pH)对橄榄石除藻过程的影响,结果发现,橄榄石用量及藻密度影响最大,其次为pH及水温,光强影响最小。在橄榄石浓度为1.5 g·L‒1、藻密度<1.7×106 cells·mL‒1、温度控制在15 ℃、反应介质pH为弱酸性或中性时,叶绿素a去除率最大,去除率高于96%。
3)对反应过程中Zeta电位和营养盐的监测以及反应后铜绿微囊藻的表面结构观察发现,天然橄榄石主要通过静电作用吸附去除铜绿微囊藻,同时橄榄石在反应过程中吸附培养基中的营养盐,造成藻细胞营养缺少,从而抑制藻细胞的生长。



 下载:
下载: