-
环境污染治理包括防治在生产建设或其他活动中产生的废气、废水、废渣、医疗废物、粉尘、恶臭气体、放射性物质等污染物,以及降低噪声、振动、光辐射、电磁辐射等对人体与环境的危害。其中,工业污染治理是环境污染治理的重点内容。燃油燃气锅炉、焦化、玻璃、陶瓷、水泥、垃圾焚烧、危废、酸洗、燃气发电等行业会排放大量气态硫化物、氮化物和汞,造成生态环境持久破坏,并危害人体健康,引起急、慢性中毒和致癌、致畸等远期危害[1]。现有的气体污染物净化方法包括:催化氧化还原、催化燃烧、利用吸收和吸附等[2]。然而,由于环境污染的复杂性,这些技术都不同程度地存在净化不彻底、投资成本高、操作复杂、回收率低、能耗高等缺点[2],也可能产生二次污染,所以需要探索和开发新技术、新方法,解决现有技术的问题。除反应机理和条件外,引入外加场也是提高反应效率和改善性能的方法,如引入磁场、电场等外加场会影响反应过程,并提高去除效率。
磁场是一种特殊物质,也是一种能量场,已被化学家和物理学家广泛应用于化学和物理控制过程。永磁铁或电磁铁在空间上产生磁场强度较为均匀的磁场。电子的运动伴生着磁场,磁性来自电子的运动,物质中带电粒子的运动形成物质的元磁矩。当这些元磁矩取向有序时,便形成了物质的磁性。通常物质的磁性分为顺磁性、抗磁性以及铁磁性[3]。顺磁性物质具有固定磁矩,在外加磁场中呈现微弱磁性,并产生与外加磁场同方向的附加磁场,能被磁体轻微吸引。抗磁性物质在外加磁场中呈现微弱磁性,并产生与外加磁场反方向的附加磁场,能被磁体轻微排斥。抗磁性在所有物质中都存在,由于抗磁性极其微弱,故常常被掩盖。铁磁性物质在外加磁场中呈现很强的磁性,并产生与外加磁场同方向的附加磁场,能被磁体强烈吸引,具有磁矩的分子表现为顺磁性,外磁场会影响磁性分子的取向,亦即影响反应体系的熵[4-5]。磁场能有效控制某些反应的速率,影响反应历程[6]。由经典电磁理论,磁场会对运动的化学粒子产生洛仑兹力。仅从能量看,磁场提供的能量较热运动的能量,不足以影响化学反应。但量子力学认为,化学反应还取决于化学粒子的电子自旋,磁场会影响化学反应粒子未成对电子的自旋状态,改变反应体系的熵,从而影响化学反应的进程和结果。磁场对化学反应影响是量子力学效应和磁流体力学效应之一或共同作用的结果[7]。有关磁场效应(磁场效应主要来自量子效应、磁热力学效应、磁矩、洛伦兹力、法拉第力、涡流和能量输入)的研究也是一个重要领域[8-9]。磁场的这些特性逐渐被研究开发,因提升了污染物处理效果和去除效率,而被运用于工业废气和废水处理、分选、高分子聚合以及催化剂制备等领域,成为一种新型污染控制技术。
近年来,利用磁场对磁性物质力的作用、对水中污染物的高能破坏作用和对微生物生长和酶活性的正向刺激作用,实现工业废水中污染物的去除已有一些研究进展[10]。在分选中利用其本身作用力,与磁场相互作用,受到转磁力矩和平动磁力而产生粒子聚集现象(粒子间磁偶极子力、拖曳力、布朗力、引力和范德华力),可实现杂质颗粒快速便捷分离和废物循环利用[11-13]。磁场在以自由基反应占主导地位的高分子聚合领域获得了一定成果,获得了产率高、分子量高且分布较窄的高聚物,且聚合物结晶度、大分子链的规整性、热性等都因磁场得到有效控制[14-16]。然而,磁场对工业废气污染的治理还未得到总结,目前磁场在废气治理研究方面,更多集中在废气脱硫脱硝和脱汞。
磁场应用于水处理的研究早已兴起,而利用磁场处理工业废气的研究发展缓慢。最开始,陈凡植等[17]使用高梯度磁分离器处理氧气顶吹转炉和电弧炼钢炉产生的烟尘,取得较好的粉尘去除效果。俞明等[18]研究发现磁场运用于汽车尾气中能够对HC及CO排放量和燃油经济性有一定改善。朱传征等[19]发现,常压下磁场能够提高合成氨反应的反应速率和转化率。随着研究人员对磁场去除污染物影响规律和作用机理的研究深入,磁场应用于工业废气处理有了一定的发展。本文介绍磁场对二氧化硫、氮氧化物和汞这3种气体污染物去除的影响效果和作用机理,为实际利用磁场治理工业废气提供理论和技术参考,为工业废气净化应用研究提供新思路与方向。
全文HTML
-
含硫工业废气源是导致雾霾和酸雨问题的一个重要因素。除硫方法主要分为干法、湿法、半干法等。近年来,出现了在反应器外部加磁场对颗粒磁性材料进行的气固流化,以磁流化床(magnetically fluidized bed,MFB)为反应器的半干式烟气脱硫(fiuidizedbedgas des,FGD)系统[20]。该技术克服了干法和湿法的局限,具有脱硫反应速度快、脱硫效率高,无污水废酸排出、脱硫后产物易于处理,适应范围广,投资、运行成本低等优点,成为MFB在FGD工艺(石灰石或石灰为吸收剂的强制氧化湿式脱硫方式)中的新应用。在20世纪80年代,WYLOCK等[21]就概念性地提出了逆流MFB,并用于连续吸收分离纯化乙烯、低温植物气、天然气和烟气等气体的方法,并在实验上进行了验证,成功推动MFB在烟气脱硫过程中的应用,以下将从MFB反应器处理SO2的吸收过程,磁助脱硫机理和磁助脱硫影响因素几个方面详细介绍。
-
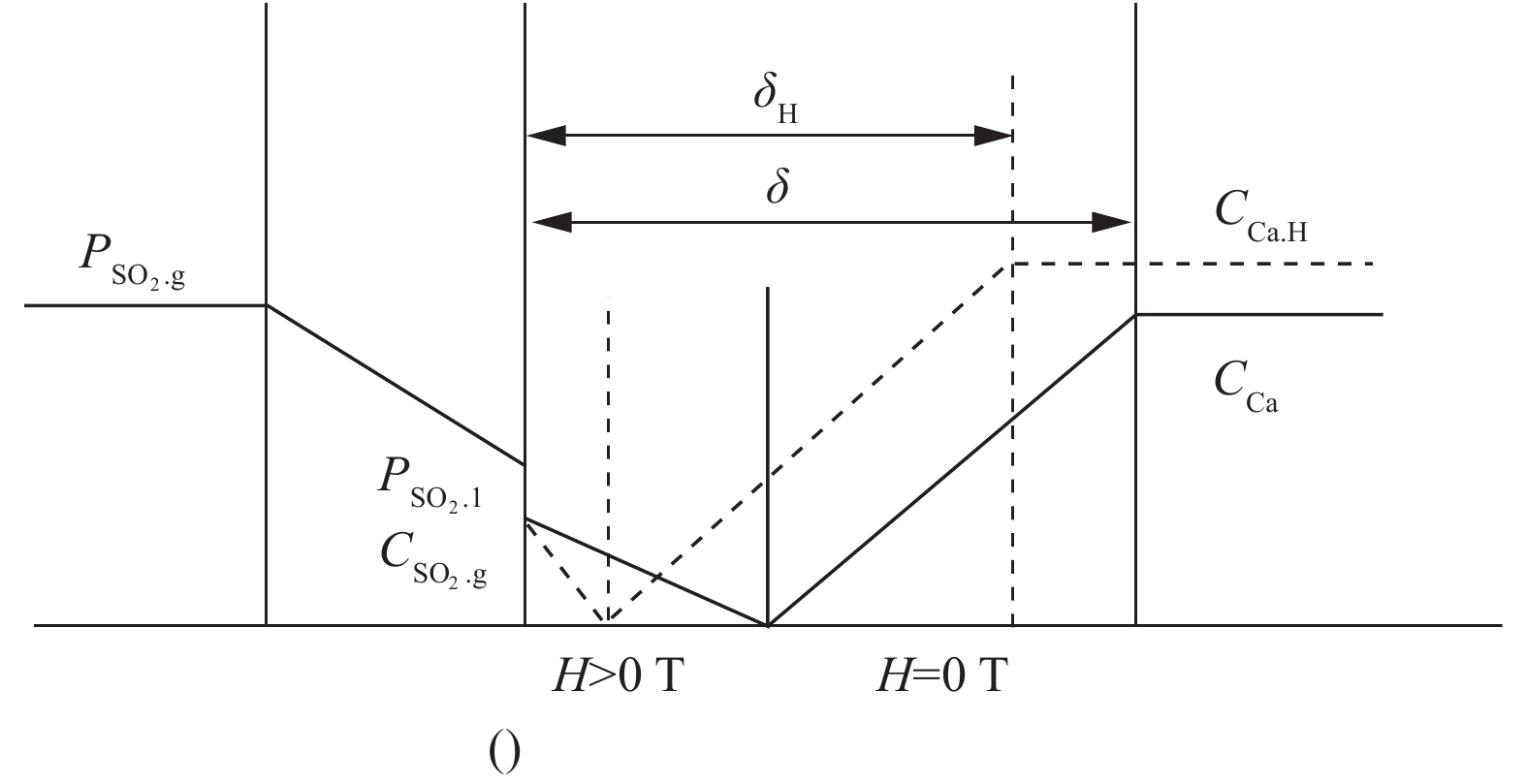
反应塔是由不锈钢柱组成的磁性流化床,石灰浆作为吸收剂被连续地喷入反应器中,反应装置[22]如图1所示。铁磁粒子表面SO2的吸收过程[22]如图2所示。气相中的SO2向铁磁粒子表面覆盖的液相扩散,溶解和电离SOx2−液膜。SO2溶解度高,而H2SO3电离度低。同时,Ca(OH)2分子向液相扩散。它在液体膜中溶解并电离成Ca2+,Ca(OH)2的溶解度较低,而电离度较高。
${\rm{SO}}_x^{2-} $ 和Ca2+在液膜中扩散,并立即反应。这些积极和消极的电离相遇,密度下降为零。因此,H2SO3电离度和Ca(OH)2溶解度成为控制液膜中离子迁移的主要因素,从而控制脱硫效率[23]。H2SO3增强电离,转让${\rm{SO}}_x^{2-} $ 于液相中,使液膜变薄。另一方面,有外部磁场时,液相Ca2+密度和液膜中Ca2+密度梯度增大,从而促进了液膜中Ca2+的转移,中和反应界面向气液界面移动,从而改善硫与吸附剂之间的传质,提高了脱硫效率[22]。一般来说,外加磁场强度的增加可以提高脱硫效率。实验中,外加磁感应强度由9 T增加到40 T,SO2去除效率提高近30%。一方面由于磁场会影响气固流化床铁磁颗粒的运动,随着磁场的增大,MFB的流动状态由“气泡床”变为“磁稳定床”;铁磁颗粒分布较好[22],附着在其表面的液滴越多;与烟气发生反应,脱硫产物在其表面聚集越多,反应后比表面积越大,从而使烟气和石灰充分有效接触。另一方面,磁场改善了S(IV)的氧化,液相中催化氧化S(IV)生成S(VI),导致SO2溶解度增加;从而增强SO2与Ca(OH)2的反应,促进了铁磁颗粒表面液膜中硫及其吸附剂之间的传质,SO2去除效率提高。具体反应见式(1)~式(4)所示。
-
1)铁磁粒子。在MFB脱硫过程中,铁磁颗粒本身也参与脱硫,而铁磁颗粒不仅可作为石灰浆液在类似石英颗粒上的沉淀平台,还参与脱硫反应。微量铁磁粒子溶解在水相中产生铁离子,Fe3+通过催化剂自氧化引发的自由基机制催化S4+氧化为S6+。ZHANG等[24]利用磁流化床脱除SO2,铁磁颗粒代替石英颗粒对SO2的去除率比石英颗粒高14%,且随其平均粒径的增大,去除率降低。
2)磁感应强度。外加磁场对流化床物理状态有不同程度影响。随着磁场的增强,磁流化床经历了3个阶段:在鼓泡流化状态下,床层压降不稳定,脱硫效率低;在磁稳流化状态下,床层压降小,脱硫效率快速提高;在磁聚状态下,床层压降较大,脱硫效率提高。ZHANG等[24]发现随着磁感应强度的增加,反应后铁磁粒子比表面积增大,磁感应强度为0、10、20 T时,反应前后比表面积之比分别为1.96、2.2、5.5,铁磁颗粒表面的形貌变得疏松,降低液相传质阻力,促进了流化床内的气固接触。张琦等[25]研究磁流化床脱硫过程中外加磁场对脱硫副产物影响时发现,在没有磁场的情况下,铁磁粒子表面会形成一个副产物的固体壳层,一旦固体壳层开始形成,随着液相传质阻力的大幅增加,SO2的吸收率急剧下降。在磁场的作用下,析出副产物团聚在一起,不能形成固体壳体,从而降低液相传质阻力,提高脱硫效率。另外,外加磁强度的增加不仅可以提高脱硫效率,对脱硫产物成分也有影响。实验中,当外加磁感应强度为0时,CaSO3衍射强度最大,说明其在反应产物中占主导地位。而当磁感应强度为40 T时,CaSO3在脱硫产物中只占很小比例,而CaSO4的衍射强度在所有成分中最高,已成为产品的主要成分。即外加磁场可以促进S(IV)的氧化,使更多的H2SO3和CaSO3分子转化为H2SO4和CaSO4。由于CaSO4·2H2O溶解度高于CaSO3·0.5H2O,石灰颗粒可溶性面积增大,溶解的石灰颗粒增多[24]。
3)磁场种类。磁场对除硫效果显著,但不同种类磁场去除机理也有所不同。在这里主要介绍直流磁场和交流磁场的不同。ZHANG等[24]发现提高直流磁场强度能提高Fe(II)和Fe(III)在S(IV)氧化反应中的催化活性,从而提高脱硫反应速率。当直流磁场应用于实际工业脱硫中,在90 T磁感应强度下,对于314 mg·m−3 SO2气体和1 145 mg·m−3CO气体,获得了100%的去除率[26]。而交流磁场对SO2的去除则有不同作用。交流磁场会引起电子回旋和漂移运动,使气隙空间中的剩余时间更长,增强电子能量和电物理化学作用,有效去除污染物气体。
1.1. 磁助脱硫机理
1.2. 磁助脱硫的影响因素
-
氮氧化物(NOx)是大气主要污染物之一。目前,比较成熟的烟气脱硝技术主要为选择性催化还原、选择性非催化还原和SNCR/SCR组合技术。其中,选择性催化还原脱硝效率可达90%,属于较成熟的烟气脱硝技术,但仍然存在着缺点。按脱硝剂和脱硝反应产物的状态可以分为干法、湿法两类。利用磁场处理NOx,可从新角度研究对污染物的作用机理,将成为未来的研究方向。
利用磁场处理NOx可影响化学反应的过程和结果[27],如反应与速率、取向、性质和结构,以及反应动力学等。在SCR中,反应速率、反应温度与SCR反应活化能有关,故将磁场用于SCR反应可增加反应活化能,提高SCR对中低温NO的脱除效率。BUSCA等[28-29]和RAMIS等[30]证明了温度为600 K时,外加磁场后SCR脱硝性能较好;APOSTOLESCI等[31]发现以ZrO2为载体负载Fe2O3的SCR系统进行烟气脱硝,275 ℃时去除率可达90%,320~365 ℃可达到完全去除;LARRUBIA等[32]研究了负载铁氧化物的TiO2用于磁助SCR系统的烟气脱硝,负载率为6%时效果较好;YAMAZAKI等[33]发现纯Fe2O3在催化脱硝中具有中等活性,且Fe2O3表现出较好的热稳定性,580 ℃下负载6.6%Fe2O3的Pt/Ba/(Al2O3-CeO2-Fe2O3)催化剂对NO去除率可达85%。以上研究表明,金属铁及其氧化物对NO的去除具有较好活性,故MFB能用于中低温状态下的NOx处理。以下主要介绍利用MFB处理NOx的4种主要机理。
-
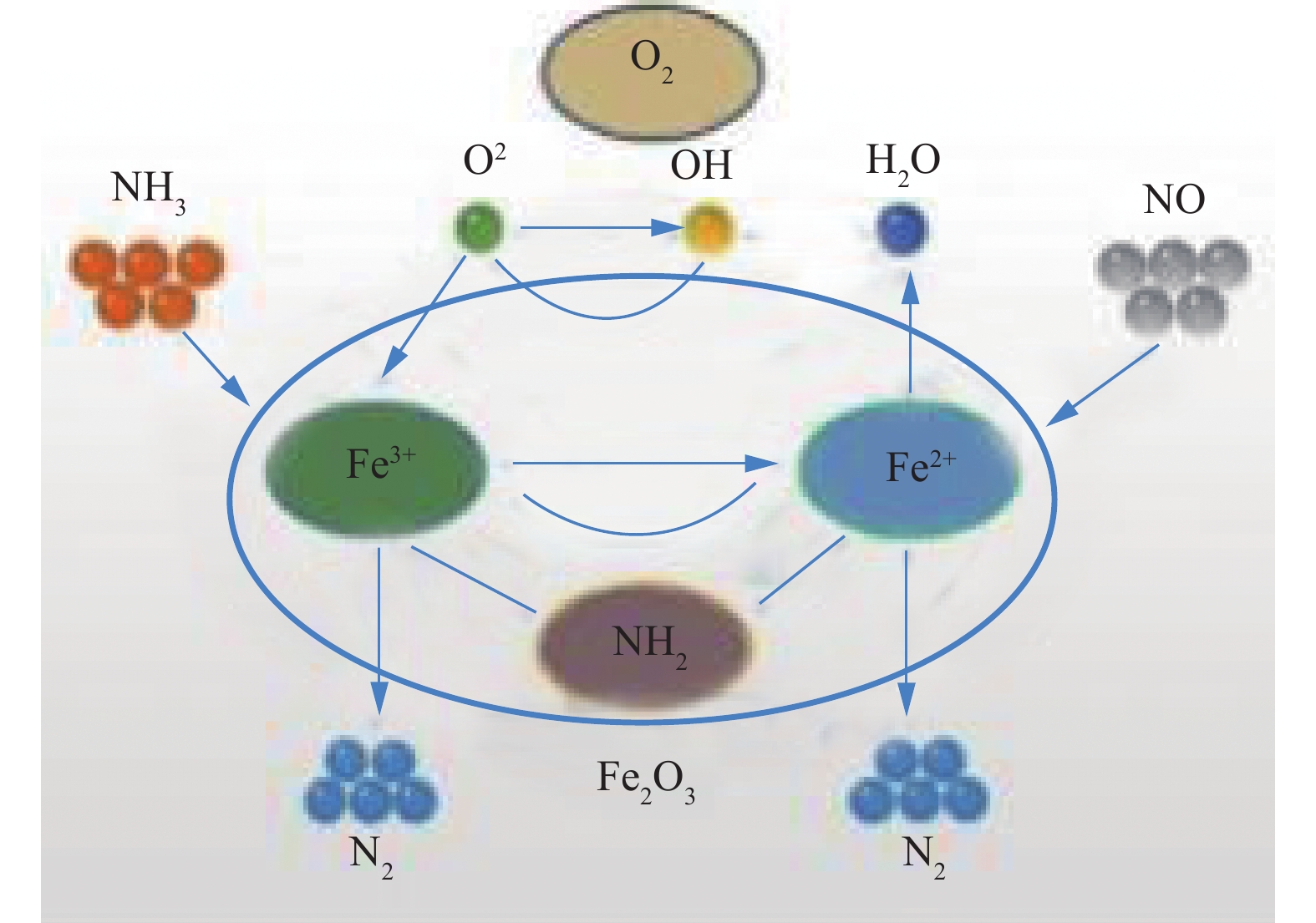
YAO等[34]利用MFB磁性Fe2O3催化剂选择性催化还原NO,外加磁场降低了SCR的表观活化能,提高了Fe2O3催化剂的低温SCR活性,即利用磁性能将NO去除率较高的温度范围从493~523 K扩展至453~523 K。用磁感应强度为0.01~0.015 T的外加磁场,温度453~493 K时,NO去除率提高到90%以上,比不加磁场时提高了10%。利用MFB磁性Fe2O3催化剂去除NO,可实现催化剂的循环再生,其反应过程见图3,具体机理如式(5)~式(12)所示。
进一步研究磁场对MFB中Fe2O3催化剂上NH3和NO的影响,可将Fe2O3催化剂上的反应归结为2个部分[35-36]:1)磁场增强了NO在Fe2O3表面的浓度以及吸附作用。NO易吸附在Fe和Fe2O3上,Fe2O3表面磁场受外加磁场和Fe2O3磁化强度的影响,其表面存在梯度磁场。由于NO是顺磁性的,从Fe2O3的环境空间转移到表面,直到表面周围的化学势相等,NO在表面的浓度增加;2)外加磁场加速了NH2活性自由基的生成和NH2与NO之间自由基的反应。GRZYBEK[37]证实了在473~533 K真空条件下,吸附在Fe2O3表面的NH3形成了稳定的NH2自由基。外加磁场会影响自由基中未配对电子的电子自旋,改变反应体系的顺序,从而改变Fe2O3催化剂上NO的SCR总速率。张雯等[38]利用外加磁场光催化降解Pt/TiO2也证实磁场增加了薄膜表面羟基自由基的生成速率。磁场可控制自由基对的系间迁跃,使自由基对尽可能保持在三重态,可有效抑制单重态自由基对的重结合,增加磁场作用下“笼”外反应的可能性[39]。通过将NO磁吸附在Fe2O3上,NO提供N原子的孤对电子,也通过配位吸附在相同或相邻Fe3+上,并保持其自由基活性。配位吸附氨上的一个H键通过脱氢分裂,生成活性表面NH2等自由基物质。Fe3+通过从分裂的H中获得一个电子而被还原。而H+与邻近的表面晶格氧离子迅速结合形成表面OH−,增加NH2自由基的形成以及NH2自由基与NO的反应,提高NO的转化率。
-
由于MFB中气相接触的改善,SCR发生了额外磁效应。磁性能可以控制床层结构、气固接触系数和NO在磁性Fe2O3催化剂上的SCR迁移率。通过提高相对气固速度,抑制和消除床内气泡,可实现床内粒子的良好诱导和混合[38]。除此之外,一些反应物分子与Fe2O3催化剂活性表面相互作用形成表面中间体NH2NO,迅速分解为N2和H2O。这些中间酸盐化合物提供了施加较小激活屏障的替代反应路径,因此,降低了脱硝反应所需的活化能,提高了反应速率。
-
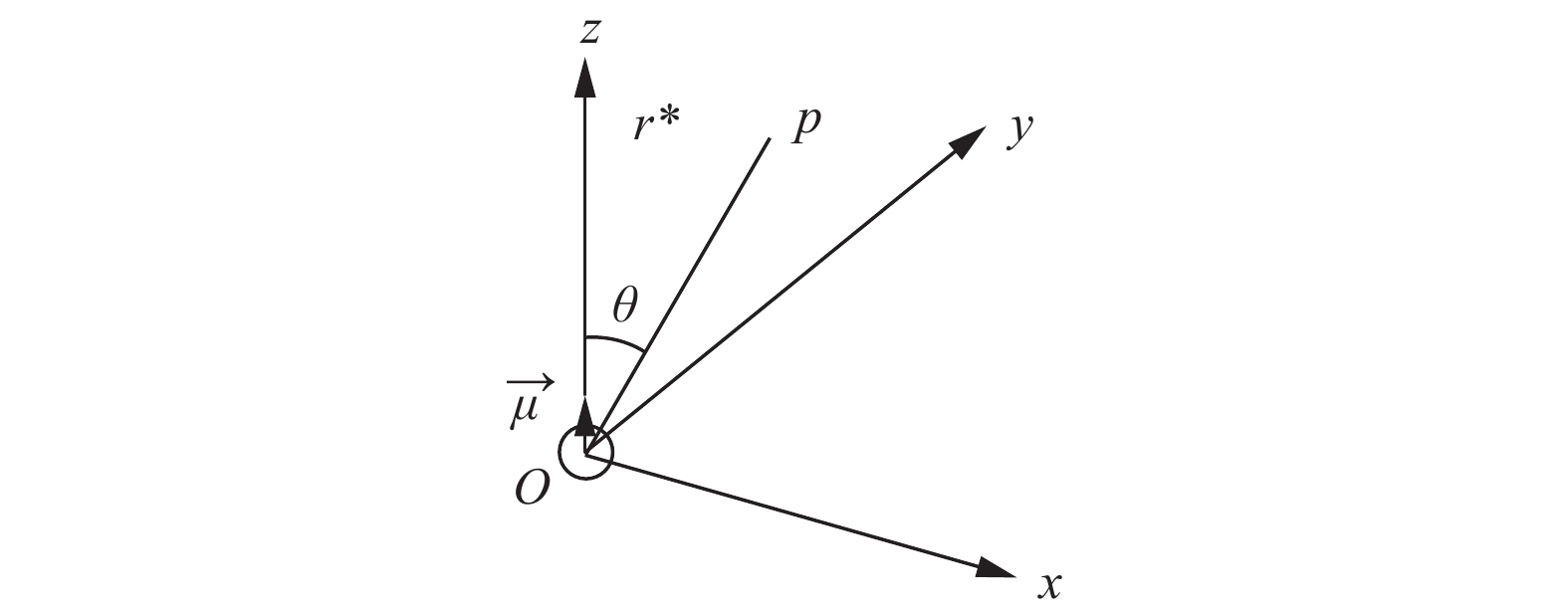
从磁场方面看,铁磁材料磁化产生的感应磁场比外加磁场强100~10 000倍,因此,粒子内部磁场强度很强,在磁化粒子的表面边界上有很大的磁场梯度。磁化后,催化剂颗粒周围的局部梯度磁场由外向内增大,NO分子被驱动力沿梯度磁场增加的方向强迫移动到铁磁粒子表面。磁化粒子外部磁场[38]如图4所示。g-Fe2O3是铁磁性的,Fe3+磁化八面体配位是沿着外磁场的,磁化坐标与外部磁场成反比,以Fe3+-N-O形式吸附在具有高自旋的磁性Fe3+上,在八面体配位下更容易与Fe3+反应和吸收。铁磁g-Fe2O3催化剂颗粒在外加磁场的作用下被磁化,产生一个比颗粒周围外加磁场强的局部磁场。因此,对NO的非均相吸附会产生磁场效应,迫使NO向g-Fe2O3表面移动,增强了NO在磁性Fe3+上的吸附,进而促进NO的选择性催化还原[40]。
-
由于铁磁催化剂颗粒被均匀磁化后,在顺磁性NO分子上会产生法拉第力,导致NO向颗粒表面移动产生边界效应,从而增强Fe3+磁性位。铁磁铁基材料与磁性能还有协同作用,可促进逆磁性反应物转化为顺磁性产物,以及电子反应中的转运。除了激活NH3、NO选择性催化还原反应,具有重要吸附作用,金属表面分子的化学吸附也不可忽视。过渡金属元素的磁性与其d带结构有关。d带结构的差异影响过渡金属表面位置分子的化学吸附[41-43]。DELBECQ等[41]指出,NO在磁性表面的化学吸附是由于NO分子上的2p自旋轨道与磁性表面上的空d自旋轨道相互作用而加速的。因此,磁性能增加NO在磁性Fe2O3催化剂表面的化学吸附。在低温、低成本、低污染下,利用含MFB的铁基催化剂可实现NO的高效去除。
2.1. 磁场对自由基的影响
2.2. 磁场对反应床的影响和中间体的形成
2.3. 局部磁场叠加效应
2.4. 顺磁性效应
-
燃煤电站锅炉是汞的最大排放源之一。汞是一种重要污染物,对人类和环境健康具有威胁,由于其波动性、持续性和生物积累性,已引起全世界关注[44]。由于高成本、废催化剂/吸附剂无法回收利用和灰分利用等负面影响,现有的Hg0脱除技术,如催化氧化法[45-52]和改性吸附剂法[53-55],无法在工业上实现大规模应用。利用外加磁场的引入,开发可回收可再生的磁性吸附剂/催化剂来克服这些局限性,可能是一个可行的研究方向。
磁场对工业废气中汞的净化有一定积极作用,磁性催化剂由于其反应的高效率和磁响应特征已被广泛关注。磁性催化剂在外加磁场的作用下,被磁化而拥有磁性,进而在磁场力作用下,分散催化剂到体系中,形成了各种微小磁场源。随着外磁场旋转及磁性微粒的运动,大小磁场产生的洛伦兹力不断变换,反应物汞分子不断受到扰动,促进反应进行[56-57]。DONG等[58]利用负载银纳米颗粒的磁性沸石复合材料研究对Hg0的去除影响,复合MagZ-Ag0(加磁)由磁铁矿(Fe3O4)绑定到沸石薄二氧化硅涂层。结果表明:加磁去除效果较好,5 min内Z-Ag0(不加磁)、MagZ-Ag0、2MagZ-Ag0吸附汞分别为98、139、148 mg·m−3;2MagZ-Ag0中可能会受到可用银吸附位点饱和或在随后的洗涤步骤中附着银离子损失的限制,且在400 ℃下进行累积或延长热处理可以提高汞捕集能力,使得吸附剂可以多次再生和再循环而不会出现性能下降。
近年来,利用磁性催化剂去除Hg0的机理研究已有一些突破。YANG等[59]利用粉煤灰负载可再生钴系磁层催化剂,用浸渍和热分解法制备了Co-MF催化剂,在150 ℃时,负载5.8%Co-MF催化剂对Hg0的去除率达到95%。随着温度的增加,300 ℃时,Hg0去除率从94.7%下降到56.5%,这可能是由于吸附在催化剂表面的汞出现解吸。在此基础上,YANG等[60]利用改性CuCl2探究Hg0的去除机理。基于CuCl2改性的煤灰磁珠(CuCl2-MF)磁性催化剂处理Hg0,用粉煤灰磁球浸渍法制备了CuCl2-MF催化剂,在150 ℃时,最佳负载量为6%的CuCl2-MF催化剂对Hg0的去除率高达90.6%。分析其原因,可能是由于不同铜负载量的催化剂存在不同的铜配位,或催化剂表面吸附位点的化学吸附作用。除此之外,热解温度为600 ℃, FeCl3生物质浸渍质量比为1.5 g·g−1时,木屑活化磁化磁性生物炭在较宽的反应温度窗口(120~250 ℃)仍表现出优良的除汞性能[61]。新型磁性生物炭(magnetic biochar carbon,MBC)表面可以沉积高度分散的Fe3O4颗粒。随着FeCl3的活化,MBC上形成了更多的富氧官能团,尤其是C=O基团。MBC中Fe3O4和C=O基团中的Fe3+(t)配位和晶格氧均可作为Hg0的活性吸附/氧化位点。而C=O基团可作为电子受体,促进Hg0氧化的电子转移。以上负载磁性Ag、Co、Cu、Fe处理气态汞的研究,揭示了部分磁性催化剂对Hg0的去除机理[58-61](见表1),为进一步研究提供参考。
不同气体组分的存在也会干扰利用外加磁场辅助去除Hg0的反应过程。不同气体的存在对Co-MF催化剂干扰Hg0去除的作用机理及反应式[59]见表2,故不同气体组成对磁助去除汞的影响效果主要是抑制、促进和双面效应。部分顺磁性气体,如O2,在磁性作用下可促进Hg0去除,而H2O的存在则相反。另外,还需进一步探究顺磁性气体组分在Hg0去除过程中的反应机理,以及磁场除汞的其他影响因素。
-
1)磁场对脱硫脱氮除汞的效果有明显提升作用,磁助脱硫改善吸附剂传质性能、提高催化活性、改变催化剂材料的物理性质和反应体系的熵,而使有效反应温度降低;磁助脱硝可造成局部磁场叠加,促进自由基和中间产物生成速率、控制自由基对的系间迁跃、抑制单重态自由基对的重结合以促进反应进行,通过磁化力增强了运输效应和化学吸附;磁助脱汞可在体系里形成各种微小磁场源,加速反应中的电子传递、增强磁性位点上的活化反应来提高转化率。
2)利用外加磁场脱硫脱硝脱汞,能够提高效率、快速便捷分离催化剂、多次催化剂再生和再循环而不会导致性能下降。现有研究大都处于实验阶段,还有许多问题亟待解决。首先,磁场作用于自由基活化反应以及对反应机制的影响等问题的机理不明确,受限条件及作用范围有待研究,具有一定的复杂性,尤其对于气体方面研究较少;其次,磁场对于单一顺磁性污染气体的处理效果较好,但用于含有顺磁性和逆磁性的多组分气体,作用效果还需进一步研究。开发出具有高吸附选择性、高稳定性的功能化磁性复合材料,利用磁场对顺磁性气体组分的促进作用,达到分开处理顺磁性和逆磁性气体,也是一个发展方向。
3)磁场应用于环境污染处理的重难点在于,针对不同污染物类型,受限于顺磁逆磁材料、磁场种类、强度和共存气体组分影响,还受限于温度、磁场梯度和磁场范围的影响。除此之外,实验室条件下磁场参数与工业化应用有所区别。如何将反应器及材料有效布局最为经济合理,也是值得讨论的问题。研究均匀磁化、磁场梯度机理以及磁场对材料性能的影响机制,并将其放大应用到工业化生产中,对材料进行有效分选回收,治理环境污染,做到环境友好型循环,是今后的重要研究方向。



 下载:
下载: