-
印染行业是用水量和废水排放量均较大的行业之一。印染加工中预处理、染色、印花和整理等工序产生的废水混合而成印染废水,这个过程中残余的有机染料会随之进入到废水中[1]。有机染料种类繁多,按分子结构分类可分为偶氮染料、蒽醌染料、靛系染料、酞菁染料、硫化染料及硝基和亚硝基染料等[2]。因有机染料分子中含有芳香结构而具有化学稳定性强和生物可降解性差的特性,甚至一些有机染料具有生物毒性,导致传统的生物处理工艺难以将其脱色和去除[3]。印染废水生化出水中含有未降解的有机染料,使其色度难以达到生产回用要求或排放标准,已经成为印染废水处理的一大难题[4]。
针对上述问题,基于羟基自由基的高级氧化技术被认为是可以有效降解有机染料的处理方法并得到了广泛研究[5]。光催化氧化、Fenton氧化和电化学氧化等高级氧化技术被用于降解水中的有机染料,并显示出较好的处理效果[6-8]。然而对于印染废水而言,其生化出水具有水量大、污染物含量低和仅需脱色的特点,体系复杂及能耗高的高级氧化技术难以胜任。因此,印染废水的处理需要一种流程简单、脱色能力强且无二次污染的高级氧化技术。真空紫外高级氧化技术可满足上述全部要求并在近年来得到了广泛关注,逐渐成为研究热点。真空紫外是指波长在100~200 nm的紫外光,相对于其他波段的紫外光具有更高的能量。低压汞灯是典型的真空紫外光源之一,当灯管的材质为超纯石英时可同时辐射出波长为185 nm的真空紫外光和254 nm的UV-C波段紫外光,即为双波长紫外光源(UV185/UV254)。185 nm真空紫外光的光子能量达6.70 eV,可以将水分子直接光解生成∙OH、∙H和eaq−等高活性的氧化和还原物种[9]。UV185辐照的光子能量高于部分有机污染物分子化学键的键能,有机污染物可通过化学键直接断裂而被降解[10]。已有研究表明,双波长紫外可通过直接光氧化有效降解乐果[11]、1,4-二恶烷[12]和邻氯苯酚[13]等有机污染物。
针对不同有机染料在双波长紫外辐照体系中直接光氧化降解的对比研究,目前还鲜有报道。本研究以低压汞灯为双波长紫外光源,选取MB、RhB和AO7等典型有机染料为模型污染物,考察了UV185辐照强度、有机染料初始质量浓度和初始pH等因素对有机染料降解效能的影响,确定了主要的活性物种,并结合模型污染物光降解过程中紫外-可见光吸收光谱分析结果,推测出有机染料可能的降解机制。
-
实验中所用试剂纯度均为分析纯,亚甲基蓝(MB)、酸性橙7(AO7)、罗丹明B(RhB)、碘化钾(KI)和钼酸铵(H8MoN2O4)等购自成都科龙化工试剂厂,碳酸氢钠(NaHCO3)购自重庆博艺化学试剂有限公司,过氧化氢(H2O2)、氢氧化钠(NaOH)、盐酸(HCl)、对苯醌(p-BQ)和邻苯二甲酸氢钾(C8H5KO4)购自国药集团化学试剂有限公司。实验中所用的超纯水由Direct-Pure UP型(RephiLe)超纯水器制取。
-
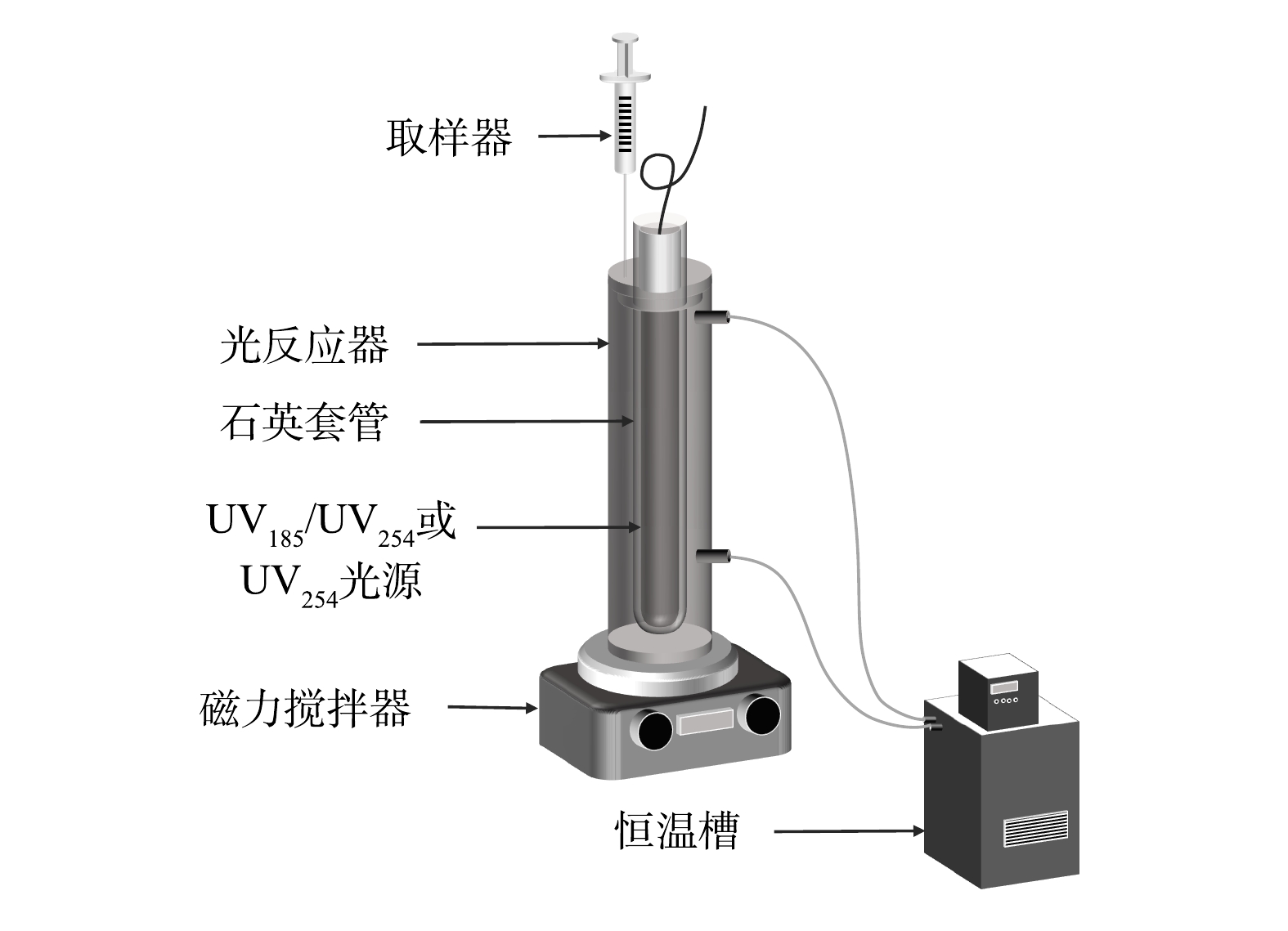
如图1所示,光反应器为一个自制的圆柱形玻璃管,有效容积为300 mL。光反应器外侧有水浴套,连接低温恒温槽(DC1006,宁波新芝生物科技股份有限公司)来保持反应体系温度稳定。实验光源为一个功率为8 W的双波长紫外灯(型号:10-08100,实验中紫外光源均来自北京航天宏达光电技术股份有限公司),可以同时发出UV185和UV254两种波长的紫外线,紫外灯置于石英套管中并位于光反应器中轴位置。实验中不同真空紫外强度分别由UV185辐照强度为10%、25%、50%和100%(以双波长紫外光源中UV185完全辐照强度计为100%,约1.3 mW·cm−2[14])的双波长紫外灯获得,采用仅辐照UV254(即UV185辐照强度为0%,型号:10-08100)的单波长紫外灯作为双波长紫外的对比光源。初始pH采用浓度为0.1 mol·L−1的盐酸和氢氧化钠调节。实验中将预定质量浓度的有机染料溶液加入反应器中,待溶液温度稳定到设定值时开启预热过的双波长紫外灯,依设定好的时间间隔取样并进行相应指标检测。
-
有机染料的浓度采用紫外-可见光分光光度计(SPECORD® 200 PLUS,德国耶拿)进行测定,MB、AO7和RhB的特征吸收波长分别为664、485和554 nm,比色皿光程为1 cm。纯水体系中H2O2的浓度采用改进的KI法测定[15]:分别配置试剂A(0.1 mol·L−1邻苯二甲酸氢钾)和试剂B(0.4 mol·L−1碘化钾、0.06 mol·L−1氢氧化钠和0.1 mmol·L−1钼酸铵);测定过程中取水样3.0 mL,然后加入1.5 mL试剂A和1.5 mL试剂B,混合均匀后静置3 min,然后采用1 cm光程比色皿在波长352 nm处测定H2O2浓度。有机染料溶液的pH采用台式酸度计(FE28,瑞士梅特勒-托利多)测定。
-
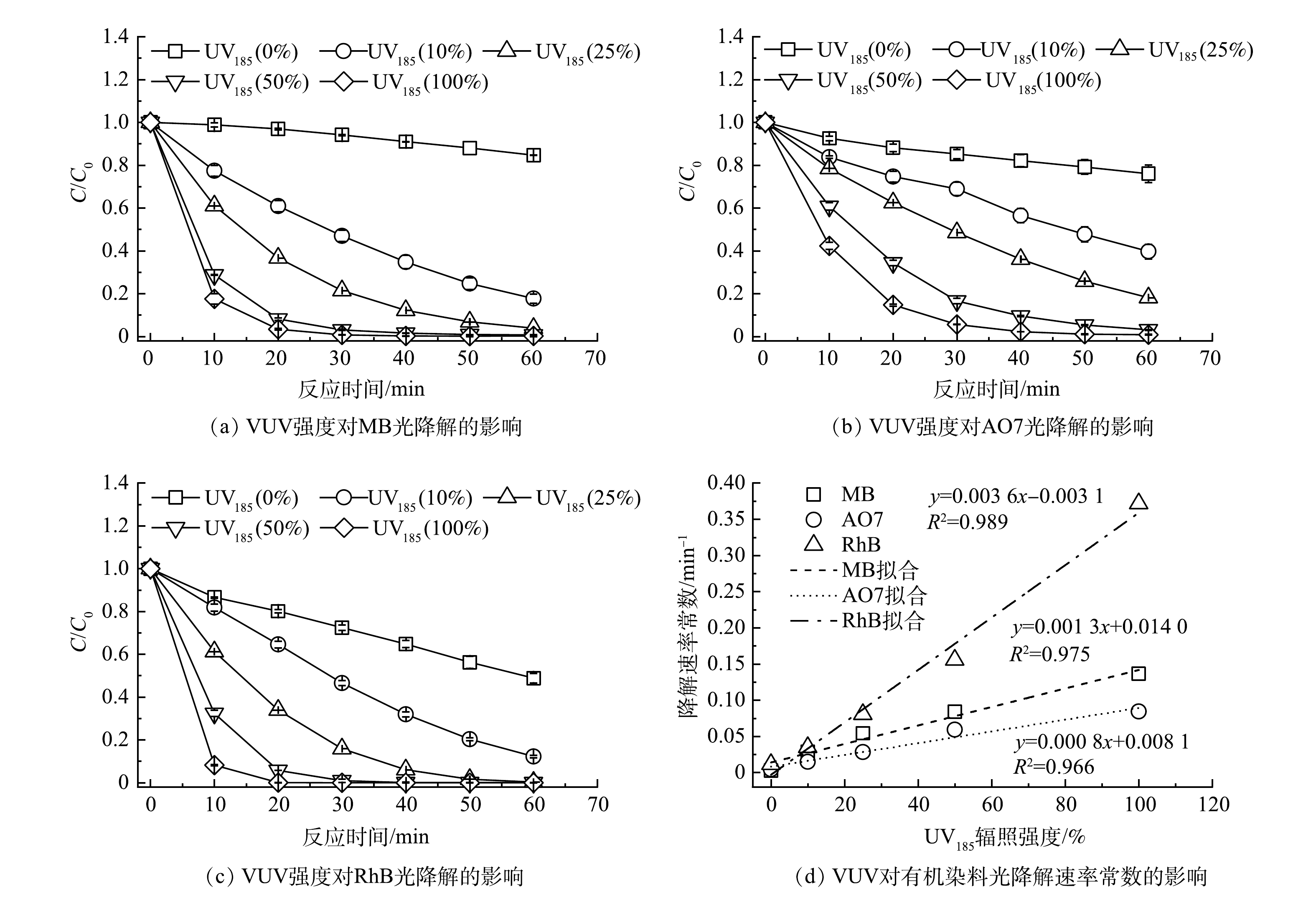
以UV185/UV254为光源,在有机染料初始质量浓度为30 mg·L−1、反应温度为25 ℃和不调节原水pH的条件下,考察了不同UV185辐照强度对有机染料降解效能的影响,结果如图2(a)~(c)所示。UV254单波长紫外辐照条件下MB、AO7和RhB在60 min后去除率分别为15.3%、24.0%和51.1%。当有UV185存在时,3种有机染料的去除率明显增加。在UV185辐照强度为100%的条件下,水溶液中的MB、AO7和RhB在反应60 min后的去除率分别达到100.0%、99.0%和100.0%。3种有机染料的紫外光降解过程均符合伪一级反应动力学模型,动力学参数列于表1中。如图2(d)所示,UV185辐照强度从10%增加到100%时,MB、AO7和RhB的降解反应速率常数分别升高了0.13、0.08和0.36 min−1。3种有机染料的降解速率均随着UV185辐照强度增加而提高,表明UV185辐照是加速有机染料降解的关键因素。同时,可以看到UV185辐照对3种有机染料降解的加速程度不同,顺序为RhB>MB>AO7,UV185辐照对RhB光降解速率的影响最为显著。
3种有机染料的降解均包含UV185和UV254 2种紫外光降解过程。实验中所选择的有机染料均具有光敏性,在UV254紫外光辐照下被激发为单重激发态,然后通过系间窜越转化为三重激发态[16-18]。当水中含有溶解氧时,处在激发态的有机染料分子将能量转移给基态的分子氧,生成具有氧化能力的单线态氧(1O2)并将有机染料降解(式(1))。
水分子在UV185辐照时可以被直接光解生成·OH(式(2)和式(3))[19],随着辐照强度的增加,体系中·OH的生成量也随之增加。
有研究表明,MB[20]、AO7[21]和RhB[22]等3种有机染料均可以被·OH攻击而氧化降解,因此,相较于UV254光解,UV185/UV254体系中产生的·OH可加速3种有机染料的降解过程。体系中UV185辐照强度的提高还可以增加水分子光解原生的还原物种·H/eaq−以及次生的活性氧物种HO2·/O2·−,因而可进一步提高有机染料的降解速率。另外,图2(d)表明UV185的辐照强度与有机染料降解速率常数的关系为线性正相关,说明UV185/UV254光降解有机染料过程中UV185光解水是限速步骤。
-
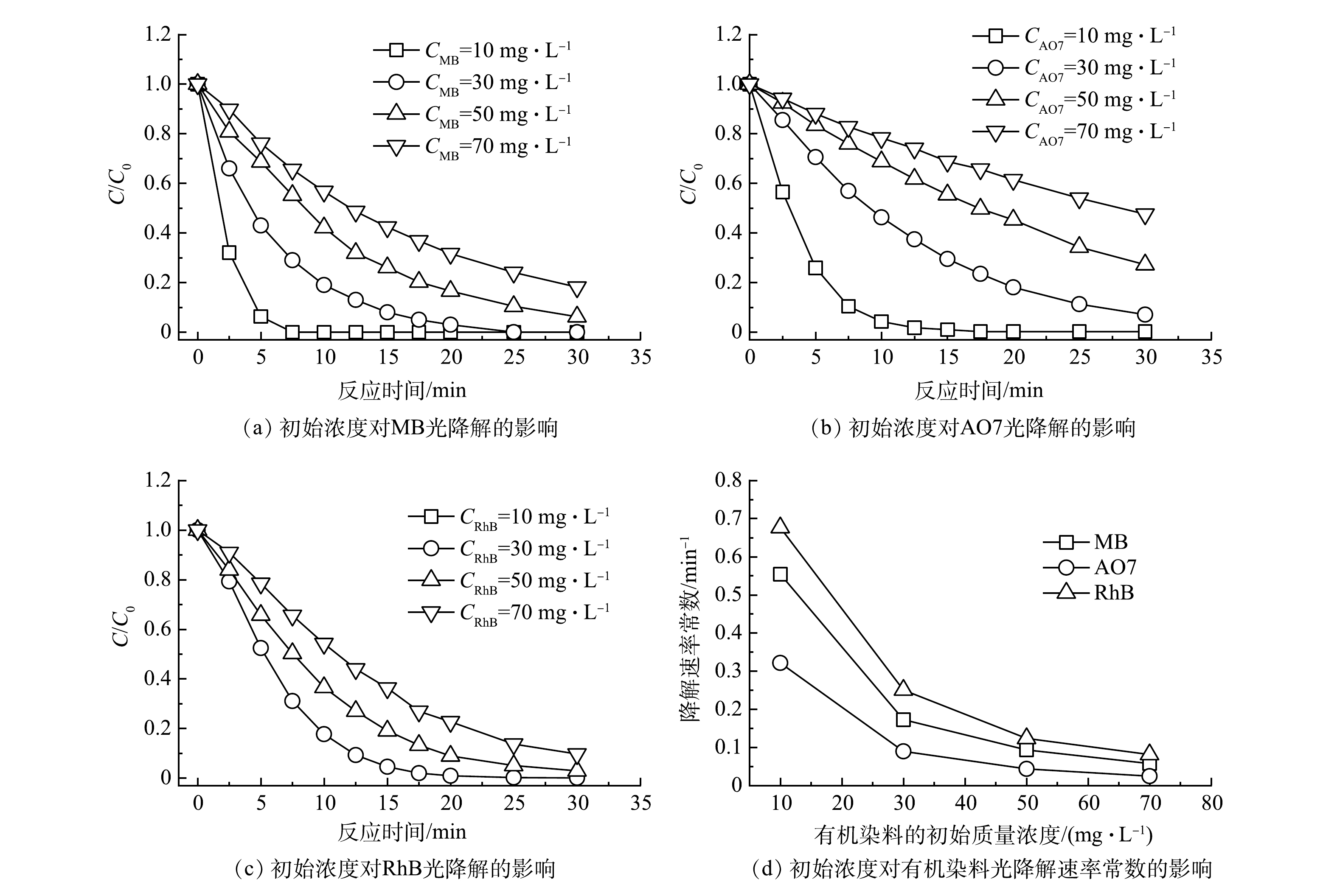
以UV185辐照强度为100%的UV185/UV254为光源,在反应温度为25 ℃和原水pH不调节的条件下,考察了不同有机染料初始质量浓度对有机染料降解效能的影响,结果如图3(a)~(c)所示。当MB、AO7和RhB等3种有机染料初始质量浓度分别为10、30、50和70 mg·L−1时,降解速率随着初始质量浓度的升高而降低。当有机染料质量浓度为10 mg·L−1时,MB、AO7和RhB等3种有机染料分别经过7.5、17.5和10 min即可完全去除。而有机染料初始质量浓度增加到70 mg·L−1时,MB、AO7和RhB等3种有机染料在反应30 min后的去除率分别为81.9%、53.4%和90.3%。不同初始质量浓度条件下有机染料的降解过程均符合伪一级反应动力学模型,动力学参数列于表2中。不同有机染料初始质量浓度条件对MB、AO7和RhB等3种有机染料降解速率影响规律一致,降解速率均随着初始质量浓度的增加而降低,即较低的初始质量浓度有利于有机染料的光氧化降解。
初始质量浓度对有机染料光氧化降解效能的影响机制可以从以下3个方面来讨论。首先,较高浓度的有机染料分子间对活性物种产生更强的竞争反应。在相同光源条件下发射的光子总量是固定的,在无有机染料存在的情况下,通过光辐照产生的氧化活性物种数量也是固定的。由前节可知,UV185/UV254体系中UV185光解水反应是有机染料光降解的限速步骤,即活性物种生成量相对有机染料是缺乏的。当体系中有机染料存在时开始消耗氧化活性物种,其数量显著超过氧化活性物种时就开始出现对活性物种的竞争。较高浓度的有机染料降解也会导致更多中间产物的生成,加剧了对活性物种的竞争。其次,有机染料及其降解产物不仅对活性物种具有竞争作用,对紫外光同样具有类似竞争作用的屏蔽作用。以MB为例,在185 nm和254 nm处MB的摩尔吸光系数分别为7 229 L·(mol·cm)−1和5 562 L·(mol·cm)−1[16],对2种波长的紫外均具有强吸收。因此,随着MB初始浓度的升高,越来越多的紫外光会被面向光源的MB屏蔽,导致背向光源的MB和水分子光解速率降低。如图3(d)所示,有机染料的初始质量浓度由10 mg·L−1升高到70 mg·L−1,MB、AO7和RhB的降解反应速率常数分别下降了0.50、0.30和0.59 min−1,可见初始质量浓度对RhB的降解速率影响最大。这是由于直接光解作用对RhB的贡献较大,低初始质量浓度条件下引起的光屏蔽作用更低而有利于RhB的直接光降解。最后,真空紫外光在水中的辐照范围有限,有机染料分子向有效辐照区域的传质成为限制因素。UV254在水中辐照的距离相对较长,超过40 mm,然而UV185有效辐照距离仅为5.5 mm[23]。UV185辐照对有机染料的降解有着更重要的作用,但辐照距离的限制使得有效反应局限于光源表面。因此,在UV185/UV254体系中,有机染料分子向UV185辐照区域传输的过程成为限速步骤之一,在更高的有机染料初始浓度下表观降解速率降低。
-
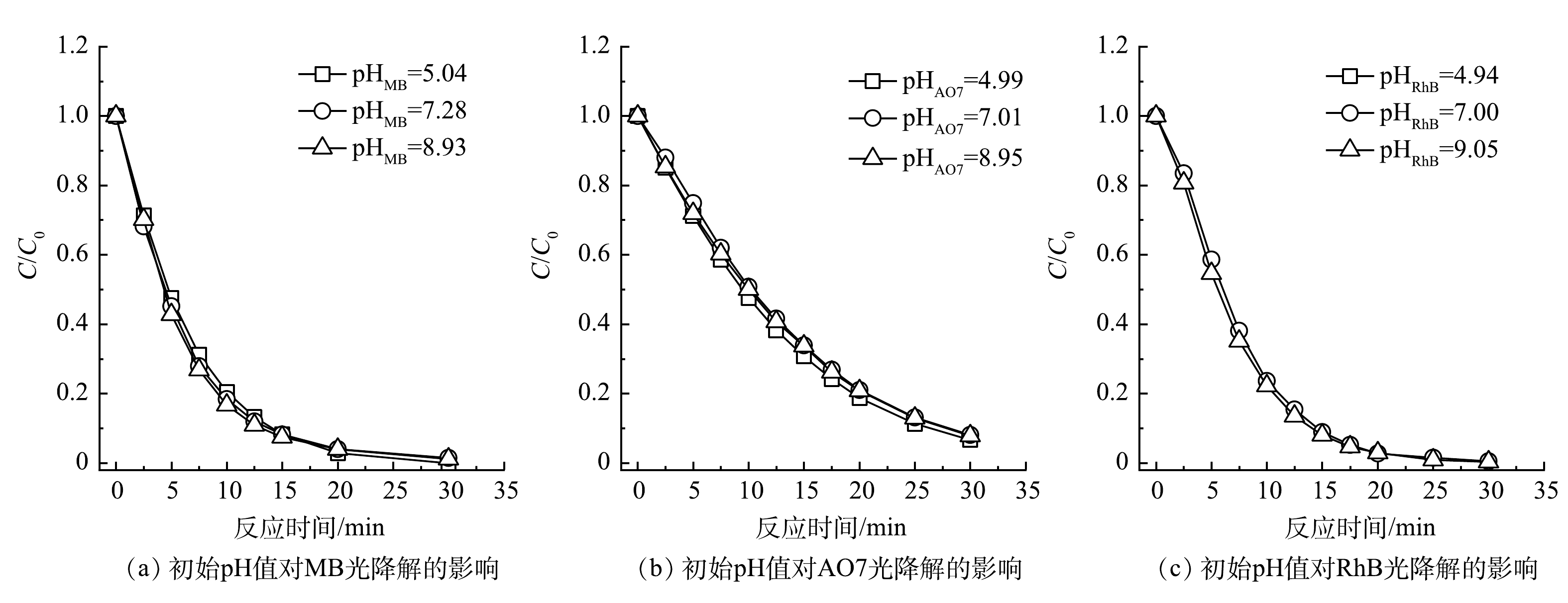
初始pH是影响光降解反应最重要的参数之一,研究其对有机染料的降解效率的影响有重要意义。以UV185辐照强度为100%的UV185/UV254为光源,在有机染料初始质量浓度为30 mg·L−1和反应温度为25 ℃条件下,考察了不同初始pH对3种有机染料光氧化降解效能的影响(图4所示)。不同初始pH下MB、AO7和RhB等3种有机染料在光降解反应30 min后均可以基本去除,去除率为91.9%~100.0%,且初始pH对降解速率没有显著影响。这表明在本实验条件下UV185/UV254光氧化有机染料过程可以适应较宽的pH范围,有更强的水质适应性。
在碱性条件下水中的OH−与·OH可以发生淬灭反应(式(4))[24],当水中存在大量OH−时,·OH被快速消耗导致其氧化攻击有机染料的概率减小。另外,·OH在不同pH下具有不同的氧化活性,在酸性和碱性条件下·OH的标准电位分别为2.8 V和1.55 V(vs NHE),这也可导致污染物降解速率有所不同[25]。GU等[26]认为,·OH是VUV光辐照降解三氯乙烷主要的氧化活性物种,并发现其降解速率受溶液的酸碱性影响显著,在pH为3的条件下最高,在pH为11的条件下最低。在本研究中,不同初始pH对有机染料的光氧化降解速率没有显著影响,表明除了·OH之外还存在其他活性物种或降解作用贡献于有机染料的降解。
-
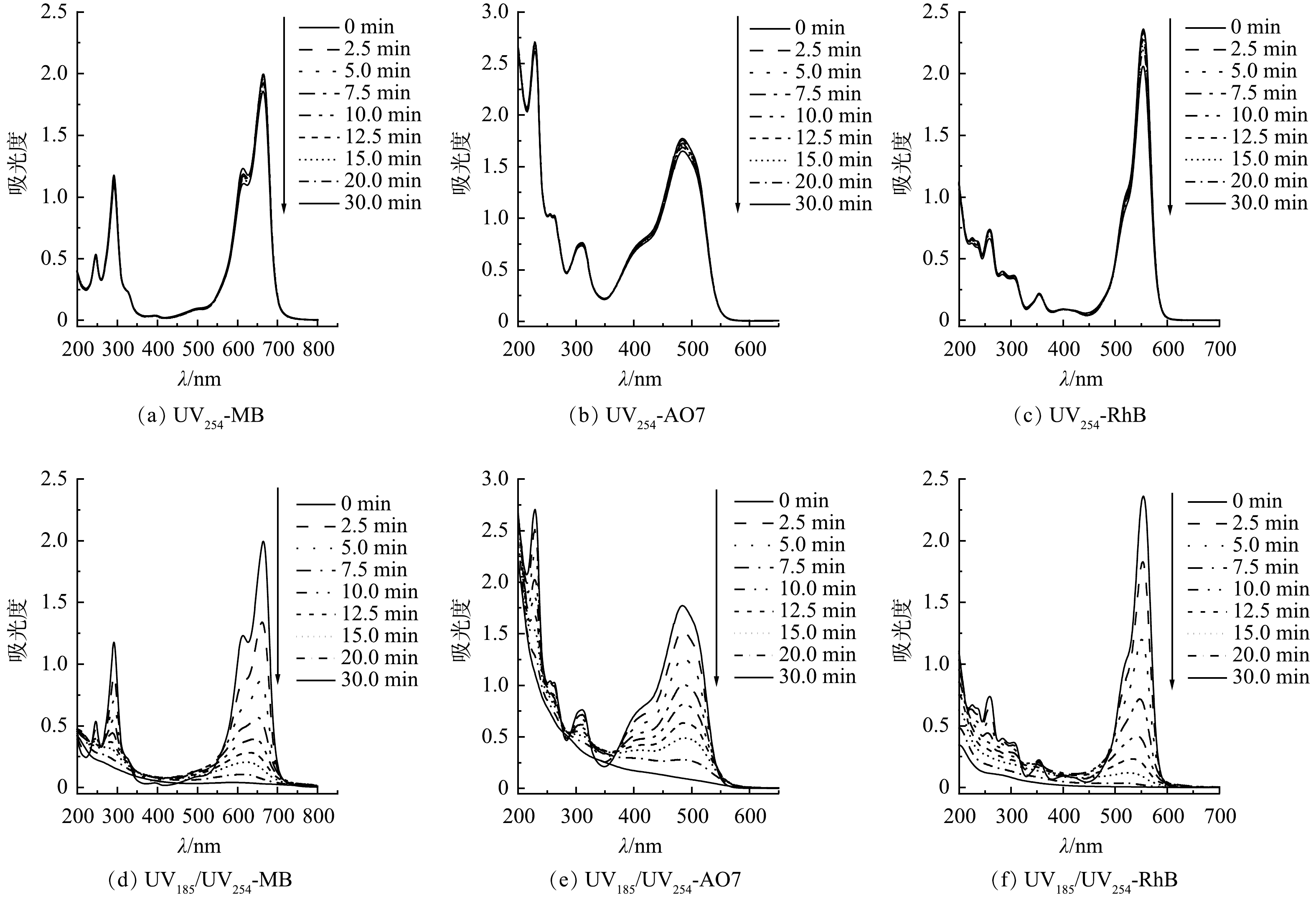
为了探明3种有机染料在双波长紫外光降解过程中的反应机制,分别对UV254和UV185/UV254光辐照条件下的MB、AO7和RhB溶液进行了UV-Vis吸收光谱分析。如图5(a)~(f)所示,MB、AO7和RhB分别在波长为664、485和554 nm处具有特征吸收峰。在UV254辐照条件下,3种有机染料的图谱没有显著变化,特征峰强度随光辐照时间的延长稍有降低,表明UV254单波长直接光解难以有效破坏有机染料分子结构(图5(a)~(c))。UV185/UV254光辐照时,3种有机染料的图谱均发生了较大变化且特征峰强度均有大幅降低,表明有机染料分子结构遭到破坏(图5(d)~(f))。对于MB的UV-Vis吸收光谱,600~700 nm的吸收峰可归因于包含共轭π体系的发色团,而292 nm处的吸收峰可归因于芳香结构[27]。在UV254单波长紫外辐照作用下,600~700 nm的吸收峰强度略有降低,这可能是由直接光解和光敏化产生的活性物种导致的,但这种作用对MB的降解贡献不大。而在UV185/UV254光辐照时,MB的UV-Vis吸收光谱两处的吸收峰强度均显著降低,表明发色基团和芳香环结构被UV185光辐照产生的活性物种攻击而破坏。AO7的特征吸收峰321 nm和242 nm分别对应分子结构中的萘基和苯基[28]。而485 nm处的吸收峰则对应腙形式的n→π*跃迁[29]。与MB类似,UV254单波长辐照对AO7的降解贡献较少,UV185/UV254光辐照时AO7的分子结构才能够被有效破坏。RhB的UV-Vis吸收光谱有2个主要的特征吸收峰:在554 nm处的特征吸收峰(C=N,C=O基团的跃迁)与染料发色有关;在259 nm处的特征吸收峰与RhB的芳香结构有关[30]。在UV254单波长辐照作用下,RhB在554 nm处的吸收峰强度有明显的降低,表明C=N和C=O基团在UV254单波长直接光解作用下即可破坏,并且对AO7降解的贡献不可忽略。在UV185/UV254光辐照时,RhB的吸收峰强度均显著降低直至完全消失。该区域吸收峰强度的大幅下降,说明RhB的发色基团和芳香结构被强烈破坏。值得注意的是,MB和RhB的特征峰随着光辐照时间的延长发生了蓝移。这是因为MB和RhB的分子结构中的二甲氨基和二乙胺基被破坏。二甲氨基和二乙胺基均为助色基团,当氮原子上的甲基或乙基被破坏时,其助色作用减弱,导致特征峰向短波长的方向移动[31-32]。特征峰的蓝移表明MB和RhB的降解过程发生脱甲基和脱乙基反应。
-
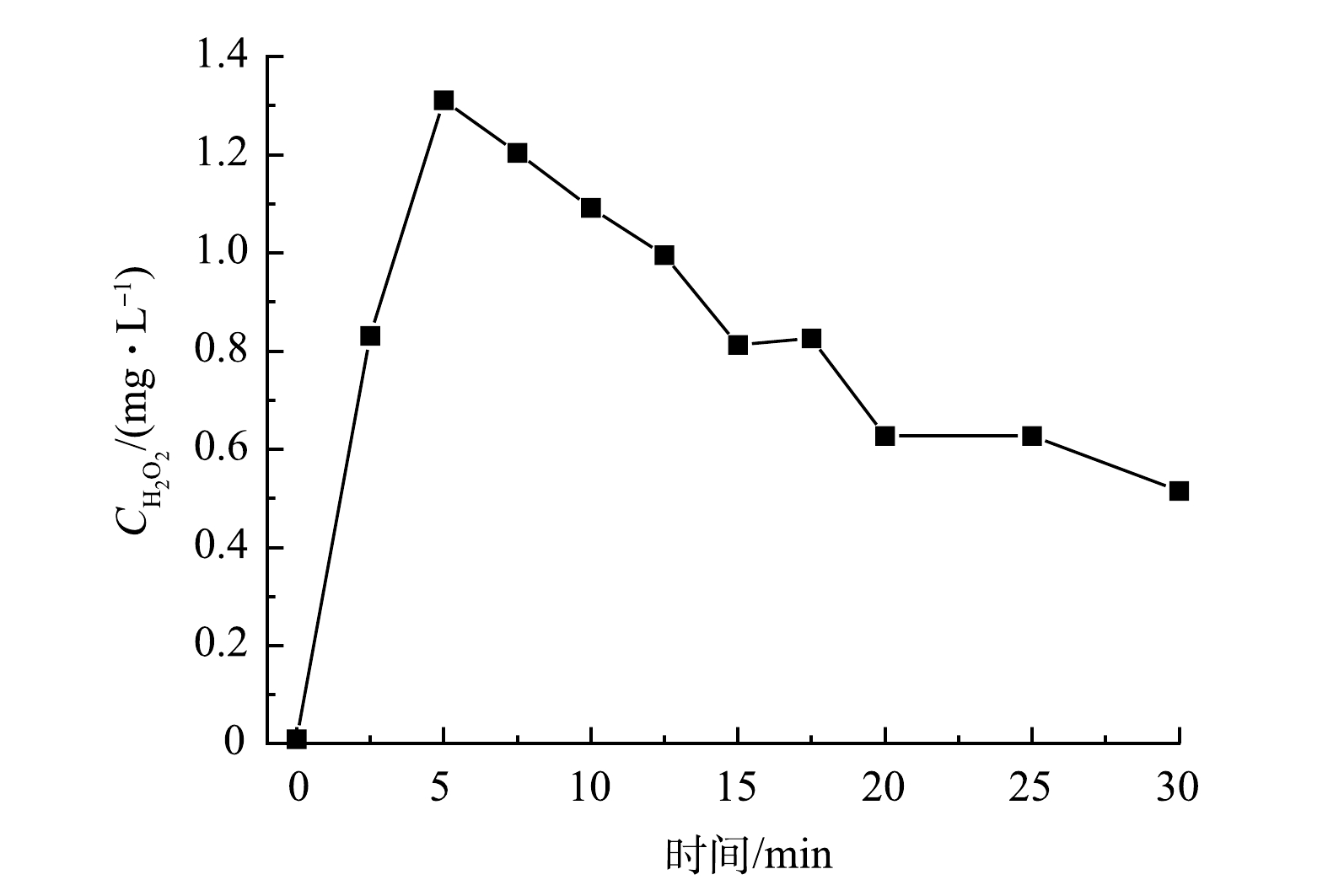
以UV185辐照强度为100%的UV185/UV254为光源,在纯水中生成的H2O2浓度的变化情况见图6。H2O2的质量浓度随光辐照时间的延长先升高后降低,在光辐照5 min时达到最高,为1.3 mg·L−1。CHEN等[33]指出,在双波长紫外辐照下纯水体系中主要通过的·OH复合生成H2O2,并且水中的溶解氧会提高水中H2O2的稳态浓度。MOUSSAVI等[34]研究表明:在连续流真空紫外光(UV185)反应器中检测到H2O2的质量浓度在4 min时达到约18 mg·L−1。水分子在UV185辐照下生成的·OH经反应后生成了H2O2(式(5)),另外一条H2O2的生成路径(式(7)~式(15))相对反应速率慢且路径更长,因此,式(5)为H2O2主要的生成路径。在UV254存在的条件下,H2O2又会分解产生·OH(式(6)),在反应初期式(6)、式(7)和式(8)等H2O2分解过程的速率低于H2O2生成速率,导致了H2O2浓度升高。随着反应的进行,水分子光解产生的eaq−增多,通过式(10)所示的反应可加速H2O2分解,因而导致光辐照5 min后H2O2的浓度开始降低。
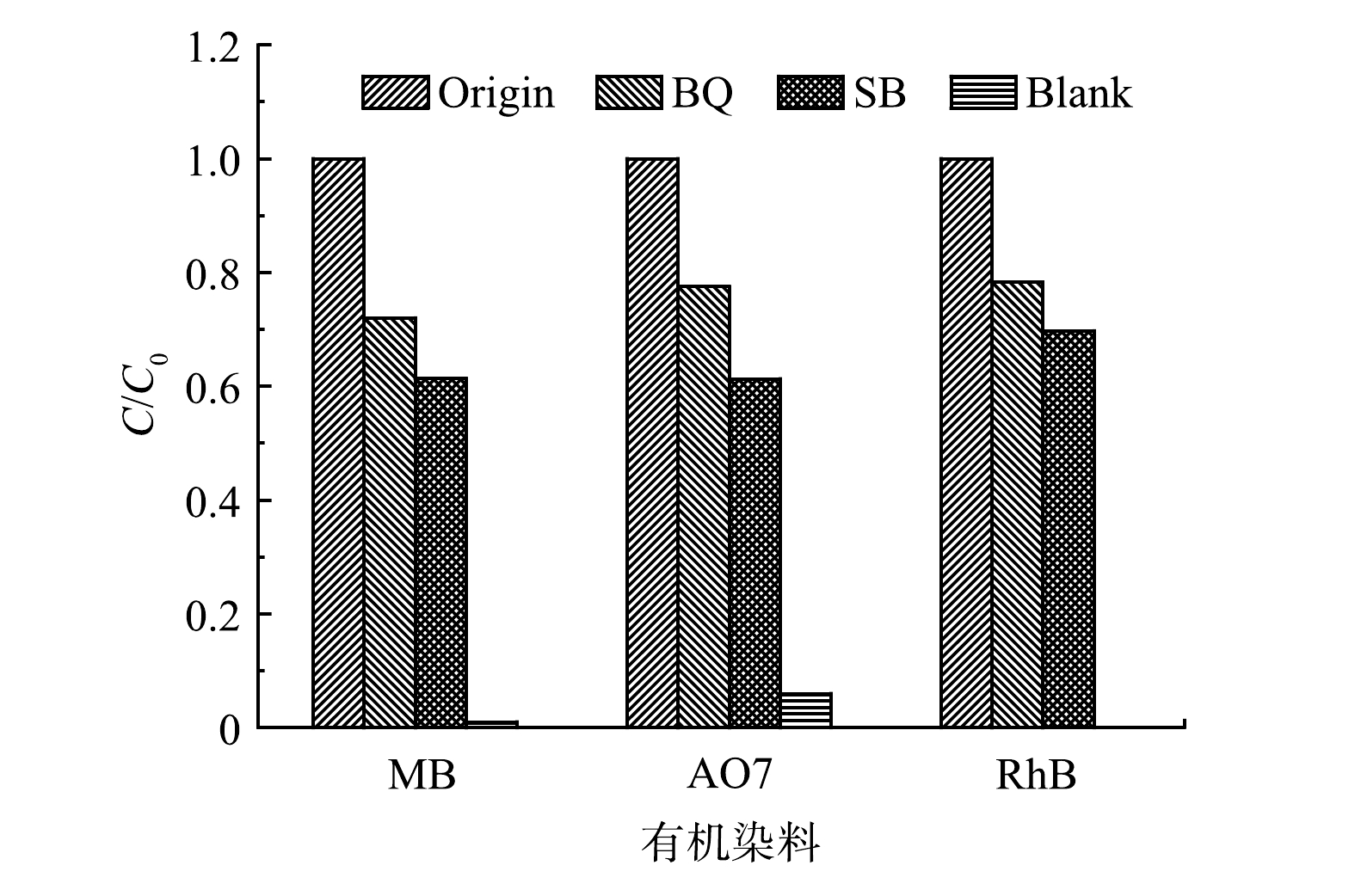
溶解氧的存在及H2O2的生成使得UV185/UV254光降解体系中活性物种的转化更加复杂。为确定UV185/UV254光氧化降解有机染料的活性物种的种类,采用对苯醌(BQ)作为HO2·/O2·−和·OH的淬灭剂,采用碳酸氢钠(SB)作为·OH的淬灭剂。如图7所示,在加入SB后,MB、AO7和RhB的去除率均大幅降低至38.6%、38.7%和30.3%。这表明·OH在3种有机染料的降解过程中均有显著贡献。在UV185/UV254光氧化体系中加入BQ后,MB、AO7和RhB的去除率分别进一步降至27.9%、22.5%和21.7%,但相较于添加SB体系降低幅度变小。BQ对有机染料的降解抑制作用大于SB,表明除·OH外还存在HO2·/O2·−的作用,但·OH的作用更大。
·OH和HO2·的寿命分别为2 ns和0.38 s[16],虽然在反应体系中HO2·存在的时间更长,但其氧化能力相对·OH更弱。此外,·OH是UV185光解水产生的初级活性物种,而HO2·是经过一系列转化过程产生的次级活性物种,生成量相对·OH更少。由此可以推断,·OH为UV185/UV254光降解有机染料的主要活性物种。
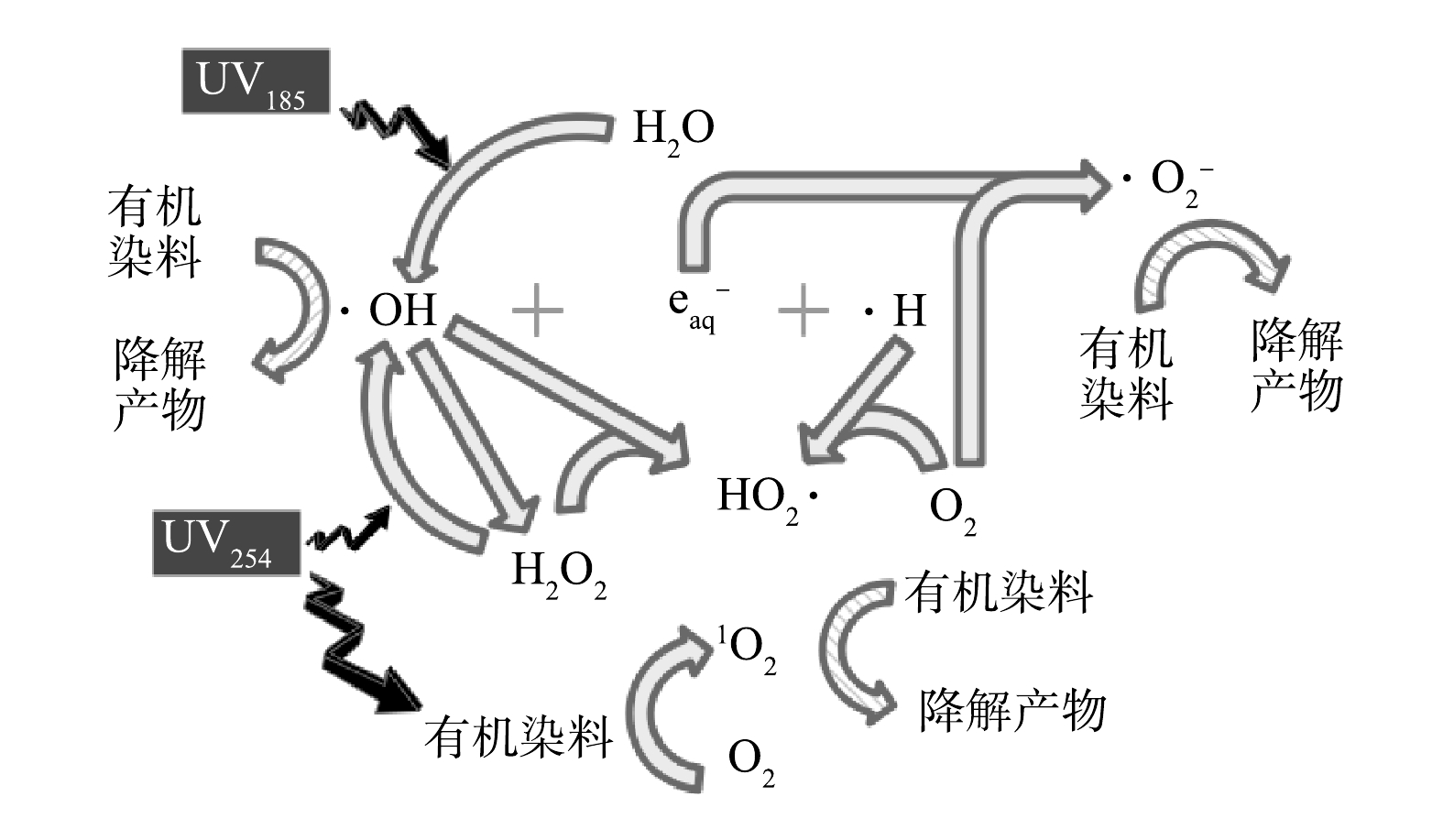
基于以上讨论,双波长紫外光降解水中有机染料的机制如图8所示。在UV185/UV254辐照下,有机染料可通过UV254的辐照作用转变为激发态,再与水中的溶解氧反应生成1O2,进而将部分有机染料氧化降解。水分子在UV185的辐照下生成初级活性物种·OH、eaq−和·H,通过他们之间以及水中溶解氧参与的反应,进一步生成HO2·和O2·−等次级活性物种。·OH通过复合反应生成H2O2,然后在UV254的光解作用下再次产生·OH。上述过程生成的·OH、HO2·和O2·−等活性物种通过氧化反应降解有机染料。
-
1) MB、AO7和RhB等3种有机染料在双波长紫外(UV185/UV254)辐照下均可以有效降解,反应60 min后的去除率分别达到100.0%、99.0%和100.0%,并且降解过程均符合伪一级反应动力学模型。
2)高UV185辐照强度和低有机染料初始质量浓度可提高有机染料的降解速率,且有机染料降解动力学常数与UV185辐照强度的关系为线性正相关;初始pH在5~9内时,其数值变化对有机染料的降解速率没有显著影响。
3) UV254对有机染料的光解仅能少量破坏发色基团,UV185光降解有机染料除了可以大幅破坏其发色基团外,还可以深度破坏分子内的芳香结构。
4)有机染料的降解包含UV254直接光解及UV185光辐照产生活性物种间接光解2种机制,其中UV254直接光解对RhB整体降解的贡献大于MB和AO7,达到了51.1%,间接光解的主要活性物种为·OH。
双波长紫外(UV185/UV254)直接光降解有机染料的效能及反应机制
Efficacy and mechanism of direct photodegradation of organic dyes by dual-wavelength ultraviolet (UV185/UV254) irradiation
-
摘要: 印染废水生化出水中残留的有机染料会导致色度超标,已经成为印染废水处理的一大难题。以双波长紫外(UV185/UV254)为光源,用于对水中亚甲基蓝(MB)、酸性橙7(AO7)和罗丹明B(RhB)等3种典型有机染料的光降解去除。考察了UV185辐照强度、有机染料初始质量浓度和初始pH等参数对3种有机染料降解动力学的影响,并对降解机理进行了探究。结果表明:当UV185辐照强度为100%、有机染料初始质量浓度为30 mg·L−1和温度为25 ℃时,光降解60 min后MB、AO7和RhB的去除率分别达到100.0%、99.0%和100.0%,且降解过程均符合伪一级反应动力学模型;UV254单波长紫外光辐照对有机染料有部分降解作用,其中对RhB降解的贡献达到了51.1%;UV185辐照强度与有机染料表观降解速率常数的关系为线性正相关;间接光降解的主要活性物种为·OH、HO2·和O2·−,其中·OH的贡献最大;UV185/UV254光降解可破坏有机染料的发色基团和芳香结构。以上研究结果可为新型双波长紫外高级氧化体系的开发及其在印染废水深度处理中的应用提供参考。Abstract: Residual organic dyes in the biotreated effluent of printing and dyeing wastewater leading to excessive chromaticity has become a major problem. A dual-wavelength ultraviolet (UV185/UV254) light source was used for the direct photodegradation of three typical organic dyes in water including methylene blue (MB), acid orange 7 (AO7) and rhodamine B (RhB). The effects of UV185 irradiation intensity, initial concentration, and initial pH on the degradation kinetics of these three types of organic dyes were investigated, and the degradation reaction mechanism was studied. The results showed that the removal rates of MB, AO7 and RhB reached 100.0%, 99.0% and 100.0%, respectively, after 60 min irradiation under the following conditions: 100% of UV185 irradiation intensity, 30 mg·L−1 initial concentration of organic dyes and 25 ℃, the degradation processes were in accordance with the pseudo-first order reaction kinetical model. The organic dyes were partially degraded by UV254 direct photolysis, of which UV254 direct photolysis accounted for 51.1% RhB degradation. The UV185 irradiation intensity was linearly correlated with the apparent degradation rate of the organic dyes; the main active species of indirect photodegradation were ·OH, HO2·and O2·−, of which ·OH presented the largest contribution. The UV-Vis absorption spectra indicated that the UV185/UV254 photodegradation destroyed the chromophores and aromatic structures of organic dyes. This study can provide a reference for the development of new dual-wavelength UV-AOPs and their application in the deep treatment of printing and dyeing wastewater.
-
Key words:
- dual-wavelength ultraviolet /
- organic dyes /
- AOPs /
- degradation pathway
-

-
表 1 3种有机染料在不同UV185辐照强度下光氧化降解过程反应动力学参数
Table 1. Kinetic parameters of photooxidation degradation of three organic dyes under different UV185 irradiation intensity
有机
染料不同UV185辐照强度下光氧化降解反应动力学参数 0% 10% 25% 50% 100% k/min−1 R2 k/min−1 R2 k/min−1 R2 k/min−1 R2 k/min−1 R2 MB 0.003 0.970 3 0.029 0.993 8 0.054 0.998 9 0.084 0.942 2 0.136 0.964 9 AO7 0.004 0.975 5 0.015 0.985 7 0.028 0.990 6 0.059 0.998 0 0.084 0.978 9 RhB 0.012 0.991 2 0.035 0.970 3 0.081 0.960 1 0.156 0.985 8 0.372 0.930 7 表 2 3种有机染料在不同初始质量浓度下光氧化降解过程反应动力学参数
Table 2. Kinetic parameters of photooxidation degradation of three organic dyes at different initial concentrations
有机
染料不同有机染料初始浓度下光氧化降解反应动力学参数 10 mg·L−1 30 mg·L−1 50 mg·L−1 70 mg·L−1 k/min−1 R2 k/min−1 R2 k/min−1 R2 k/min−1 R2 MB 0.554 0.978 8 0.173 0.998 1 0.093 0.998 3 0.058 0.999 6 AO7 0.321 0.995 3 0.090 0.997 3 0.043 0.995 4 0.025 0.999 5 RhB 0.676 0.945 7 0.251 0.985 4 0.123 0.995 1 0.082 0.991 6 -
[1] NIDHEESH P V, ZHOU M, OTURAN M A. An overview on the removal of synthetic dyes from water by electrochemical advanced oxidation processes[J]. Chemosphere, 2018, 197: 210-227. doi: 10.1016/j.chemosphere.2017.12.195 [2] GÜRSES A, AÇIKYILDIZ M, GÜNEŞ K, et al. Classification of Dye and Pigments[M]. Dyes and Pigments, Springer International Publishing, Cham, 2016. [3] AI Z H, LI J P, ZHANG L Z, et al. Rapid decolorization of azo dyes in aqueous solution by an ultrasound-assisted electrocatalytic oxidation process[J]. Ultrasonics Sonochemistry, 2010, 17(2): 370-375. doi: 10.1016/j.ultsonch.2009.10.002 [4] 李新, 刘勇弟, 孙贤波, 等. UV/H2O2法对印染废水生化出水中不同种类有机物的去除效果[J]. 环境科学, 2012, 33(8): 2728-2734. [5] NIDHEESH P V, GANDHIMATHI R, RAMESH S T, Degradation of dyes from aqueous solution by Fenton processes: A review[J]. Environmental Science and Pollution Research, 2013, 20(4): 2099-2132. [6] 饶砚迪, 盛义平, 刘琦, 等. TiO2-Ca(OH)2-石墨的制备与光催化降解染料废水[J]. 工业水处理, 2018, 38(2): 48-51. doi: 10.11894/1005-829x.2018.38(2).048 [7] 李丽华, 马明明, 任庆军, 等. Fe3O4/三维石墨烯非均相Fenton催化降解酸性红B[J]. 工业水处理, 2017, 37(8): 25-29. doi: 10.11894/1005-829x.2017.37(8).025 [8] 朱应良, 万金泉, 马邕文, 等. 电化学协同过硫酸盐法氧化处理橙黄G染料废水[J]. 水处理技术, 2016, 42(8): 48-51. [9] ZHANG Y L, WANG W L, LEE M Y, et al. Promotive effects of vacuum-UV/UV (185/254 nm) light on elimination of recalcitrant trace organic contaminants by UV-AOPs during wastewater treatment and reclamation: A review[J]. Science of the Total Environment, 2021: 151776. [10] HUANG H B, LEUNG D Y C, KWONG P C W, et al. Enhanced photocatalytic degradation of methylene blue under vacuum ultraviolet irradiation[J]. Catalysis Today, 2013, 201: 189-194. doi: 10.1016/j.cattod.2012.06.022 [11] WU Z D, YANG L X, TANG Y B, et al. Dimethoate degradation by VUV/UV process: Kinetics, mechanism and economic feasibility[J]. Chemosphere, 2021, 273: 129724. doi: 10.1016/j.chemosphere.2021.129724 [12] SHI G, NISHIZAWA S, MATSUSHITA T, et al. Computational fluid dynamics-based modeling and optimization of flow rate and radiant exitance for 1, 4-dioxane degradation in a vacuum ultraviolet photoreactor[J]. Water Research, 2021, 197: 117086. doi: 10.1016/j.watres.2021.117086 [13] HE X, CHI H Z, HE M R, et al. Efficient removal of halogenated phenols by vacuum-UV system through combined photolysis and ·OH oxidation: Efficiency, mechanism and economic analysis[J]. Journal of Hazardous Materials, 2021, 403: 123286. doi: 10.1016/j.jhazmat.2020.123286 [14] 包瑞格, 真空紫外光解去除水中抗生素头孢哌酮效能与机理研究[D]. 北京: 北京交通大学, 2018. [15] KORMANN C, BAHNEMANN D W, HOFFMANN M R, Photocatalytic production of hydrogen peroxides and organic peroxides in aqueous suspensions of titanium dioxide, zinc oxide, and desert sand[J]. Environmental Science & Technology, 1988, 22(7): 798. [16] LI M K, QIANG Z M, PULGARIN C, et al. Accelerated methylene blue (MB) degradation by Fenton reagent exposed to UV or VUV/UV light in an innovative micro photo-reactor[J]. Applied Catalysis B:Environmental, 2016, 187: 83-89. doi: 10.1016/j.apcatb.2016.01.014 [17] STYLIDI M, KONDARIDES D I, VERYKIOS X E, Visible light-induced photocatalytic degradation of Acid Orange 7 in aqueous TiO2 suspensions[J]. Applied Catalysis B: Environmental, 2004, 47(3): 189-201. [18] HORIKOSHI S, SAITOU A, HIDAKA H, et al. Environmental remediation by an integrated microwave/UV illumination method. V. Thermal and nonthermal effects of microwave radiation on the photocatalyst and on the photodegradation of Rhodamine-B under UV/Vis radiation[J]. Environmental Science & Technology, 2003, 37(24): 5813-5822. [19] GETOFF N, SCHENCK G O, Primary products of liquid water photolysis at 1236, 1470 and 1849 Å[J]. Photochemistry and Photobiology, 1968, 8(3): 167-178. [20] ČEHOVIN M, MEDIC A, KOMPARE B, et al. The enhancement of H2O2/UV AOPs for the removal of selected organic pollutants from drinking water with hydrodynamic cavitation[J]. Acta Chimica Slovenica, 2016, 2759: 837-849. [21] ZHONG X, XIANG L J, ROYER S, et al. Degradation of CI Acid Orange 7 by heterogeneous Fenton oxidation in combination with ultrasonic irradiation[J]. Journal of Chemical Technology and Biotechnology, 2011, 86(7): 970-977. doi: 10.1002/jctb.2608 [22] HOU M F, LIAO L, ZHANG W D, et al. Degradation of rhodamine B by Fe(0)-based Fenton process with H2O2[J]. Chemosphere, 2011, 83(9): 1279-1283. doi: 10.1016/j.chemosphere.2011.03.005 [23] ZOSCHKE K, BORNICK H, WORCH E, Vacuum-UV radiation at 185 nm in water treatment: A review[J]. Water Research, 2014, 52: 131-145. [24] BUXTON G V, GREENSTOCK C L, HELMAN W P, et al. Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (·OH/·O-) in aqueous solution[J]. Journal of Physical and Chemical Reference Data, 1988, 17(2): 513-886. doi: 10.1063/1.555805 [25] WANG J L, XU L J. Advanced oxidation processes for wastewater treatment: formation of hydroxyl radical and application[J]. Critical Reviews in Environmental Science and Technology, 2012, 42(3): 251-325. doi: 10.1080/10643389.2010.507698 [26] GU X G, LU S G, QIU Z F, et al. Photodegradation performance of 1, 1, 1-trichloroethane in aqueous solution: In the presence and absence of persulfate[J]. Chemical Engineering Journal, 2013, 215: 29-35. [27] SOLTANI T, ENTEZARI M H. Photolysis and photocatalysis of methylene blue by ferrite bismuth nanoparticles under sunlight irradiation[J]. Journal of Molecular Catalysis A:Chemical, 2013, 377: 197-203. doi: 10.1016/j.molcata.2013.05.004 [28] OON Y S, ONG S A, HO L N, et al. Microbial fuel cell operation using monoazo and diazo dyes as terminal electron acceptor for simultaneous decolourisation and bioelectricity generation[J]. Journal of Hazardous Materials, 2017, 325: 170-177. doi: 10.1016/j.jhazmat.2016.11.074 [29] PARK H, CHOI W. Visible light and Fe(III)-mediated degradation of Acid Orange 7 in the absence of H2O2[J]. Journal of Photochemistry and Photobiology A:Chemistry, 2003, 159(3): 241-247. doi: 10.1016/S1010-6030(03)00141-2 [30] RASHEED T, BILAL M, IQBAL H M N, et al. TiO2/UV-assisted rhodamine B degradation: Putative pathway and identification of intermediates by UPLC/MS[J]. Environmental technology, 2018, 39(12): 1533-1543. doi: 10.1080/09593330.2017.1332109 [31] YOGI C, KOJIMA K, WADA N, et al. Photocatalytic degradation of methylene blue by TiO2 film and Au particles-TiO2 composite film[J]. Thin Solid Films, 2008, 516(17): 5881-5884. doi: 10.1016/j.tsf.2007.10.050 [32] BEHNAJADY M A, MODIRSHAHLA N, TABRIZI S B, et al. Ultrasonic degradation of Rhodamine B in aqueous solution: Influence of operational parameters[J]. Journal of Hazardous Materials, 2008, 152(1): 381-386. doi: 10.1016/j.jhazmat.2007.07.019 [33] LI J, ZHANG Q, CHEN B, et al. Hydrogen peroxide formation in water during the VUV/UV irradiation process: Impacts and mechanisms of selected anions[J]. Environmental Research, 2021, 195: 110751. doi: 10.1016/j.envres.2021.110751 [34] MOUSSAVI G, REZAEI M, POURAKBAR M. Comparing VUV and VUV/Fe2+ processes for decomposition of cloxacillin antibiotic: Degradation rate and pathways, mineralization and by-product analysis[J]. Chemical Engineering Journal, 2018, 332: 140-149. doi: 10.1016/j.cej.2017.09.057 -



 下载:
下载: