-
药品和个人护理品(pharmaceuticals and personal care products,PPCPs)作为自然环境中具有潜在生态风险的一类新兴污染物,自身及代谢产物具有高水溶性、难降解性、高稳定性及难挥发性等特点,使用常规水处理手段难以去除,能通过不同途径大量流入自然水体,严重危害水环境质量和生态系统安全[1]。目前水中典型的PPCPs去除方法主要有物理法、生物法以及基于不同自由基去除的高级氧化法[2]。UV-LED/NaClO方法是一种基于氯自由基的高级氧化技术。已有研究[3-4]表明,UV-LED/NaClO方法可产生羟基自由基(HO·)、氯自由基(Cl·)、氯氧自由基(ClO·)、氧自由基(O−·)及次氯酸根自由基(ClOH·)等多种氧化性自由基,其中HO·为无选择性降解,含氯自由基(RCS)为选择性降解。因此,探明UV-LED/NaClO方法中不同自由基的去除贡献、水中阴离子等其他物质的影响对优化去除方法具有重要意义。
UV-LED/NaClO方法主要通过光解、氯化和自由基氧化等方式来降解污染物,其中RCS在取代或氧化反应过程中可能诱导氯化副产物形成。据报道[5],UV/氯方法能够促进一些毒性较强的卤代乙腈(HANs)卤代酮(HKs)和三氯硝基甲烷(TCP)消毒副产物(DBP)产生。因此,在使用UV-LED/NaClO方法去除污染物的过程中,溶液毒性的变化及有毒副产物的生成同样受到关注。但由于毒性产物作用机制复杂,结构特性各不相同,导致产物与毒性效应间的关系难以准确对应。因此,进一步厘清UV-LED/NaClO方法降解污染物过程中生成的毒性增强产物具有重要意义。美国EPA提出一种基于生物毒性实验的毒性鉴别(toxicity identification evaluation,TIE)评估技术体系,该方法能够确定疑似有毒物质的种类和浓度,并阐明有毒物质与生物效应之间的关系[6]。
作为一种典型酚类PPCPs,邻苯基苯酚(o-phenylphenol,OPP)在2017年被世界卫生组织国际癌症研究机构列为3类致癌物之一[7]。赖成然等[3]采用UV-LED/NaClO方法初步确定了HO·、Cl·和ClO·与OPP的二级反应速率常数。阴离子CO32−、NO3−以及天然有机质(NOM)等水质参数对不同组分贡献的影响却鲜见报道。
本研究以OPP为目标污染物,采用UV-LED/NaClO去除方法,分别考察CO32−、NO3−以及HA等因素对OPP降解过程中不同物种贡献的影响,研究环境水样中OPP去除时不同组分的贡献,推测OPP在UV-LED/NaClO方法中的降解路径,评估降解OPP过程中溶液的毒性变化;基于毒性鉴定评估 (TIE) 实验,筛选毒性效应增强产物,探讨产物与溶液毒性的关系,以期为全面评估UV-LED/NaClO方法降解水环境中微量污染物提供理论支撑和基础数据。
-
OPP(德国Dr.Ehrenstorfer公司,纯度>99.9%);沙丁胺醇(Salbutamol,SAL)(德国Dr.Ehrenstorfer公司,纯度>99.8%);乙酸铵(C2H7NO2)、磷酸二氢钾(KH2PO4)(HPLC级,中国aladdin);乙腈(HPLC级,德国Merck)、硝基苯(nitrobenzene,NB,99%)、苯甲酸(benzoic acid,BA,99.5%)、对二甲氧基苯(1,4-dimethoxybenzene,DMOB)、叔丁醇(tert-butanol,TBA)(HPLC级,上海安谱实验科技有限股份公司);次氯酸钠(CP,活性氯≥5.2%);BioFix® Lumi Multi-Shot冻干细菌及激活液;腐殖酸(humic acid,HA)(黄腐酸FA≥90%)、Na2S2O3·5H2O、K2HPO4·3H2O、NaHCO3、Na2CO3、NaNO3、H2O2、HCl和NaOH均为分析纯;实验室用水均为Mill-Q超纯水(≤18.2 MΩ)。
LC-20A高效液相色谱仪(shimadzu,日本),SPD-20A液相检测器(shimadzu,日本);LC-MS液相色谱质谱联用仪(Waters 2489,美国);发光细菌毒性检测仪(LumiFox6800,深圳朗石科学仪器);真空干燥箱(DZF-6050,上海精宏);纯水机(Milipore,美国);磁力恒温搅拌器(HJ-6A,江苏金坛峥嵘仪器);pH计(Eutevch,美国);UV-LED灯(深圳微紫科技,λ=278 nm,单个100 mW)。
-
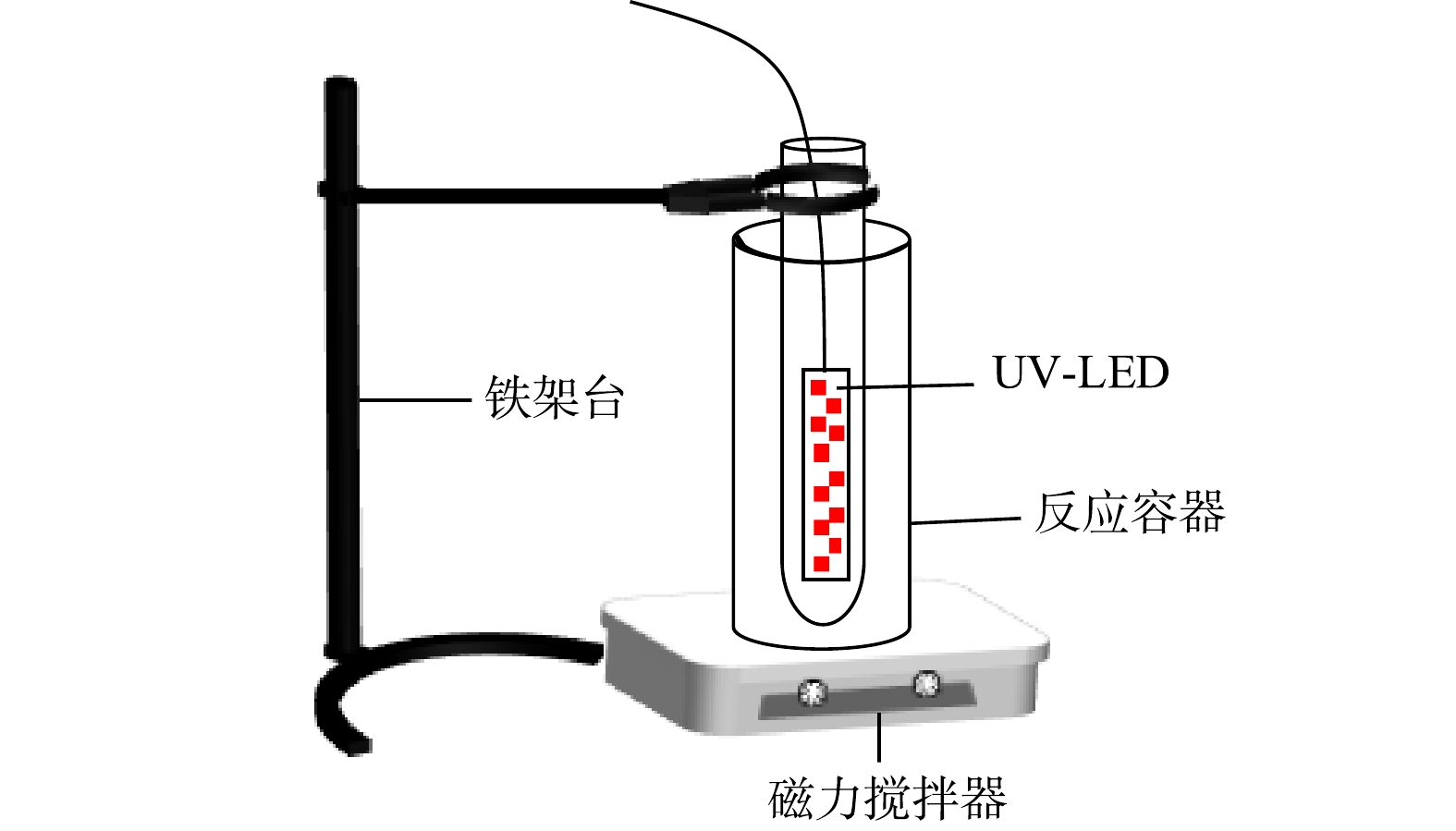
实验在置于磁力搅拌器上的光化学反应容器中进行。反应容器由特制圆筒形容器组成,紫外发生器由置于反应容器内8个并联UV-LED小灯珠(光强为0.33 mW∙cm−2)组成。实验装置见图1。
溶液pH采用0.1 mol·L−1的HCl或NaOH调节。实验时,先投加定量的NaClO溶液(1~7 mg·L−1),再开启UV-LED灯,在设定的反应时间点,准确吸取3 mL水样,经0.1 mol·L−1的Na2S2O3淬灭并过膜后,及时测定剩余OPP浓度。实验中所有反应均在相同条件下重复实验3次,结果取平均值。在遗传毒性实验中,待测水样经固相萃取后,将洗脱液置于微弱的氮气流下吹干,再用二甲基亚砜(DMSO)定容至100 μL,最后通过固相萃取将中间产物富集于活化后的HLB小柱(6 mL,500 mg,CNW,USA)中,并用10 mL丙酮洗脱。
-
1)动力学实验。据报道[3],NB与HO·具有专一性反应(二级反应速率常数为3.9×109 L·(mol·s)−1),因此实验以NB作为HO·的探针化合物。由于UV-LED/H2O2工艺中仅有HO·自由基生成,且单独H2O2无法去除NB和OPP,但UV-LED可以直接光解去除OPP[3]。因此,在反应体系中,NB和OPP的去除表达式[3]见式(1)和式(2)。联立式(1)和式(2) ,可得HO·的稳态浓度及HO·与OPP的二级反应速率常数。
式中:kNB和kOPP为UV-LED/H2O2降解NB和OPP过程中的拟一级动力学常数;kUV-LED,NB和kUV-LED,OPP为单独UV-LED降解NB和OPP的拟一级动力学常数;kHO·,NB和kHO·,OPP为HO·与NB、OPP的二级反应速率常数;C[HO·]ss为HO·在溶液中的稳态浓度。
由于UV-LED照射NaClO过程中会生成HO·、Cl·、ClO·及Cl2−·等氧化性自由基[3-4],故同时添加TBA和NaHCO3淬灭HO·、Cl·、ClO·及Cl2−·。NaHCO3能与HO·、Cl·和Cl2−·反应生成CO3−·(式(3)~式(5))[8],而CO3−·是一种高选择性自由基,可与含有富电子基团的化合物快速反应[9],故实验同时加入SAL以排除CO3−·对OPP去除的影响[10]。
在采用UV-LED/NaClO方法去除OPP过程中,单独投加NaHCO3(50 mmol·L−1)与同时投加TBA(100 mmol·L−1)和NaHCO3(50 mmol·L−1)的OPP和SAL的降解动力学[3]分别表示为式(6)、式(7)及式(8)、式(9)。
式中:kother,OPP和kother,SAL为其他未被淬灭的活性组分对OPP和SAL去除的拟一级动力学速率常数;kother为其他组分(Cl2−·、O−·、ClOH·)对OPP的降解贡献;C[OPP]t和C[OPP]0为OPP在t时刻及初始的浓度;k1,obs、k2,obs、k3,obs和k4,obs分别为SAL和OPP在不同条件下的拟一级动力学常数;kUV-LED,OPP、kUV-LED,SAL和kNaClO,OPP、kNaClO,SAL分别为单独UV-LED和单独NaClO降解OPP和SAL的拟一级动力学速率常数。
假设单独投加NaHCO3与同时投加TBA和NaHCO3 2种情形时的溶液环境相一致,则联立式(6)~式(9),得式(10)和式(11),从而求得ClO·与OPP的二级反应速率常数。
将式(11)计算出的二级反应速率常数带入含NB、BA、DMOB、OPP的UV-LED/NaClO方法中,便可通过竞争动力学计算出HO·、Cl·和ClO·的稳态浓度及其与OPP的二级反应速率常数。
-
配制3 μmol·L−1 OPP、NB、BA和DMOB溶液,建立竞争动力学模型,NaClO浓度为40.3 nmol·L−1。在阴离子、NOM的影响实验中, 初始OPP和NaClO的浓度分别为3 μmol·L−1和40.3 nmol·L−1,采用磷酸缓冲盐将pH控制在7.0±0.2,分别投加NaNO3、NaCO3、HA以考察NO3−、CO32−和NOM的影响。在不同实际水体下,不同组分对去除OPP的贡献实验条件相同,初始OPP和NaClO的浓度分别为3 μmol·L−1和40.3 nmol·L−1, pH=7.0±0.2。
-
采用HPLC测定OPP、NB、SAL、BA和DMOB,检测波长分别为254、262、223、230和225 nm。流动相为乙腈/水(体积比)=60∶40,SAL和BA的水相分别为7.5 mmol·L−1 KH2PO4、0.02 mmol·L−1 C2H7NO2,流速为1.0 mL·min−1,柱温为40 ℃, 进样量为10 μL。
采用LC-MS检测OPP降解生成的中间产物。LC-MS条件:色谱柱Insertsil®C-18(4.6 mm×250 mm,5 μm),流动相为乙腈/水(体积比)=60∶40,流速为0.5 mL·min−1,等梯度洗脱;柱温为30 ℃,进样体积为10 μL;质谱分析采用正/负离子模式,质荷比m/z为50~1 000。全扫描模式设置条件:离子源ESI,电喷雾电压为4 kV;雾化气压为275.84 kPa,辅助气压为206.88 kPa,中间碰撞气氮气压力为41.376 kPa,离子源温度为500 ℃。
-
毒性鉴定评估[6](toxicity identification evaluation,TIE)是美国EPA提出的毒性评估技术体系。该方法以生物毒性实验为基础,不仅能评估具有毒性的物种、浓度,还能阐明有毒物质浓度与生物效应间的关系。急毒性采用发光细菌实验方法,利用LumiFox 6800发光细菌毒性监测仪检测各样品的发光强度。该方法将明亮发光杆菌活化后的生物荧光与接种菌液后的水样的发光强度进行对比,利用公式计算发光强度被抑制的情况,以相对抑制率来表示水体毒性高低,计算方法见式(12)。
式中:I为发光强度的相对抑制率;I0、Isample分别为空白组和混合水样的发光强度。
生物遗传毒性测试方法(SOS/umu)是利用化学物质损害生物的 DNA,诱导产生应急反应(SOS response),产生 β-半乳糖苷酶基因并与UmuC基因相融合,从而根据细菌生成的 β-半乳糖苷酶的数量判断 DNA受损的程度的方法。SOS/umu的测试可参照文献中的方法[11]。在本实验中,首先,将冻存的菌液置于30 ℃下的含氨苄青霉素和氯霉素的LB培养基中,振荡培养;然后,用TGA培养基稀释溶液,直至A595 nm为0.05~0.09,再于37 ℃下振荡培养;1.5 h后,将培养菌液与经浓缩的水样混合,并于37 ℃下振荡培养;2 h后,测定该菌液的A595 nm,同时将该菌液与Z-buffer溶液(0.06 mol·L−1 Na2HPO4·12H2O、0.04 mol·L−1 NaH2PO4·H2O、0.01 mol·L−1 KCl、0.001 mol·L−1 MgSO4溶于1 000 mL水)、十二烷基苯磺酸钠(SDS)、氯仿混合摇匀,并添加邻硝基酚β-D-半乳糖苷(ONPG)缓冲溶液,于30 ℃下静置20 min,再加入Na2CO3溶液,终止反应,用酶标仪测定上清液在415、570 nm处的吸光度,计算β-半乳糖苷酶活性(式(13) )。
式中:β为半乳糖苷酶活性值;t为酶促反应时间,min;V为反应菌液在显色过程中的稀释倍率;A595 nm、A415 nm、A570 nm分别为595、415、570 nm波长的吸光度值。
采用XAD-8树脂柱分离OPP及其降解产物,XAD-8树脂柱为中等极性的大孔树脂柱,对极性和非极性有机物均有吸附能力,水样经树脂柱富集后,洗脱分离。根据TIE实验结果,有毒水样经树脂柱富集后,分别以乙腈/水(体积分数)=0%、20%、50%、80%和100% 进行洗脱分离,分离得到的5个组分分别标记为D1、D2、D3、D4和D5,每组分5个柱体积(200 mL)。
-
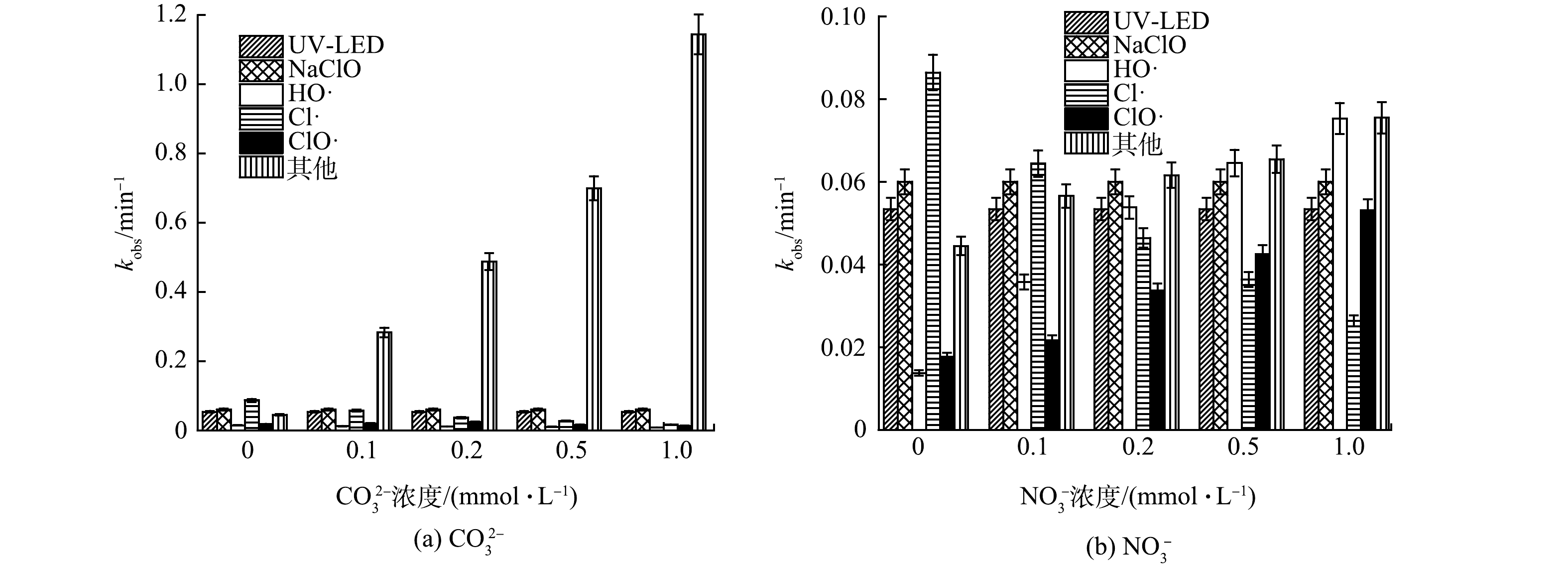
1)阴离子对OPP去除过程中不同组分贡献的影响。CO32−是常见的自由基清除剂,可与HO·发生反应,生成活性低于HO·的高选择性CO3−·自由基,从而可能会降低污染物降解效率,因此,本研究考察了CO32−对OPP去除的影响。如图2(a)所示,当CO32−浓度分别为0、0.1、0.2、0.5和1.0 mmol·L−1时,kobs分别为0.275、0.483、0.675、0.867和1.297 min−1,kobs随CO32−浓度的增加而增大,故CO32−对OPP去除具有促进作用, 但HO·、Cl·和ClO·的相对贡献却分别降低了4.400%、30.06%和5.400%。CO32−浓度为0.1 mmol·L−1时,HO·和Cl·的相对贡献仅占0.60%和1.2%,其他组分(CO3−·、Cl2−·、O−·、ClOH·、CO3−·等)的相对贡献则由最初的16.1%增至88.2%。这是因为,在UV-LED辐照下,CO32−分别以8.5×106 L·(mol·s)−1和2.2×108 L·(mol·s)−1[12]的反应速率与溶液中的HO·和Cl·反应,生成CO3−·,导致溶液中HO·和Cl·的浓度下降,CO3−·的浓度上升,实验中其他组分的贡献上升。
NO3−是一种光敏物质,在紫外线辐照下,光解为NO2−或NO2·,进而清除自由基(式(14)和式(15)),同时产生的单线态氧可生成HO·(式(16))。GHANBARI等[13]认为:产生的HO·可以补偿消耗的HO·,从而促进了污染物的降解;另一方面,NO3−可能与污染物竞争紫外光,从而抑制污染物直接光解。如图2(b)所示,当溶液中NO3−的浓度分别为0、0.1、0.2、0.5和1.0 mmol·L−1时,kobs分别为0.275、0.292、0.308、0.322和0.343 min−1,即kobs随NO3−浓度的增加而增大,表明NO3−可以促进OPP降解。Cl·的相对贡献随着NO3−浓度的增加而降低,其余各组分的贡献则有较为明显的增加,其中ClO·和HO·的贡献分别增至最高值15.4%和21.9%,HO·的增长幅度又高于ClO·。这是因为:NO3−被UV-LED辐射后,会生成大量O−·等含氧自由基(ROS)和NO2·等含氮自由基(RNS),而O−·能与H2O反应,生成HO·(式(16)),NO3−易被HO·氧化,生成RNS;同时少部分的HO·与氧化剂水解生成的HOCl进一步反应,生成ClO·(式(17)),NO3−不仅可以增加溶液中HO·和ClO·的浓度,还可以生成RNS和ROS,从而有效促进了OPP的降解。这与XU等[14]在UV/NaClO去除卡马西平实验中得出的NO3−可促进卡马西平去除的结果类似。
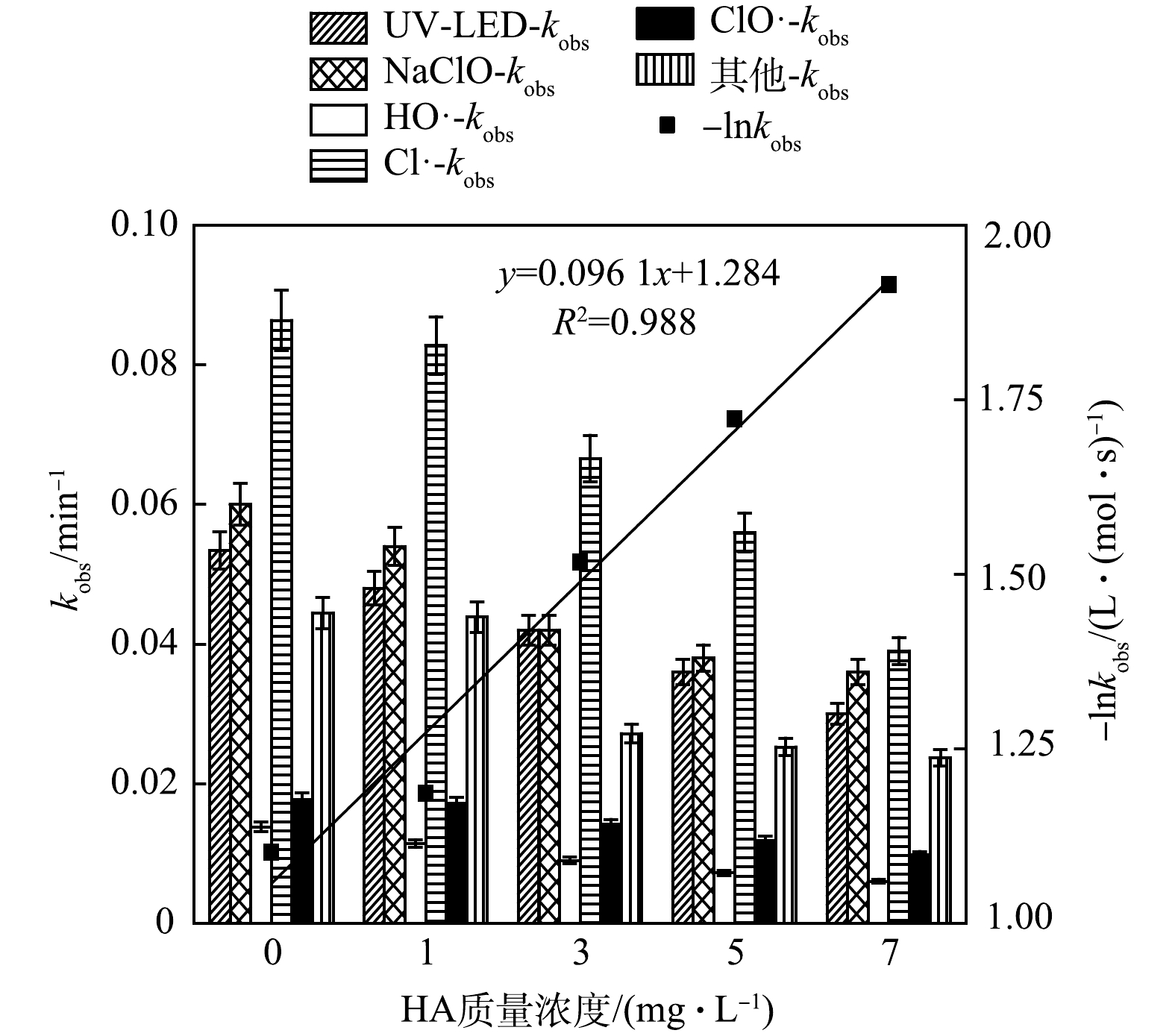
2) NOM对OPP去除过程中不同组分贡献的影响。腐殖酸(HA)是水体中天然有机质(NOM)的主要成分,故采用HA模拟水体中的NOM,考察其对OPP去除的影响,结果如图3所示。
当HA质量浓度由0增至1、3、5及7 mg·L−1时,kobs由0.275降至0.257、0.201、0.170和0.146 min−1,分别降低了6.470%、26.96%、38.08%和46.80%,表明HA对OPP的去除具有明显的抑制作用。各组分去除OPP的kobs均随HA质量浓度的增加而降低,其中HA对Cl·的抑制效果最为明显,kobs由0.086 4下降至0.039 min−1,相对贡献从31.4%降低至26.6%。此外,-lnkobs拟合曲线具明显线性相关性(R2=0.988), 拟二级反应速率常数为0.096 1 L·(mol·s)−1,说明OPP降解速率常数 kobs随HA质量浓度的增大而减小, OPP降解速率减慢。这是因为,HA会抑制紫外光对溶液的透光率,使UV-LED对OPP溶液的辐照效果变弱,降低了NaClO对光子的吸收[15],从而导致UV-LED和NaClO对OPP降解的相对贡献明显下降。另一方面,HA对HO·和Cl·具有淬灭作用(kHA,HO·=2.5×104 L·(mol·s)−1、kHA,Cl·=1.3×104 L·(mol·s)−1),而HA与ClO·及Cl2·−等反应速度较慢[16],因此,与其他组分相比,ClO·相对贡献率随HA质量浓度增加而引起的变化较小。CHOW等[17]利用UV/Cl工艺降解乙酰磺胺时也发现,随着HA质量浓度的增高,乙酰磺胺去除效果降低的幅度较小。
-
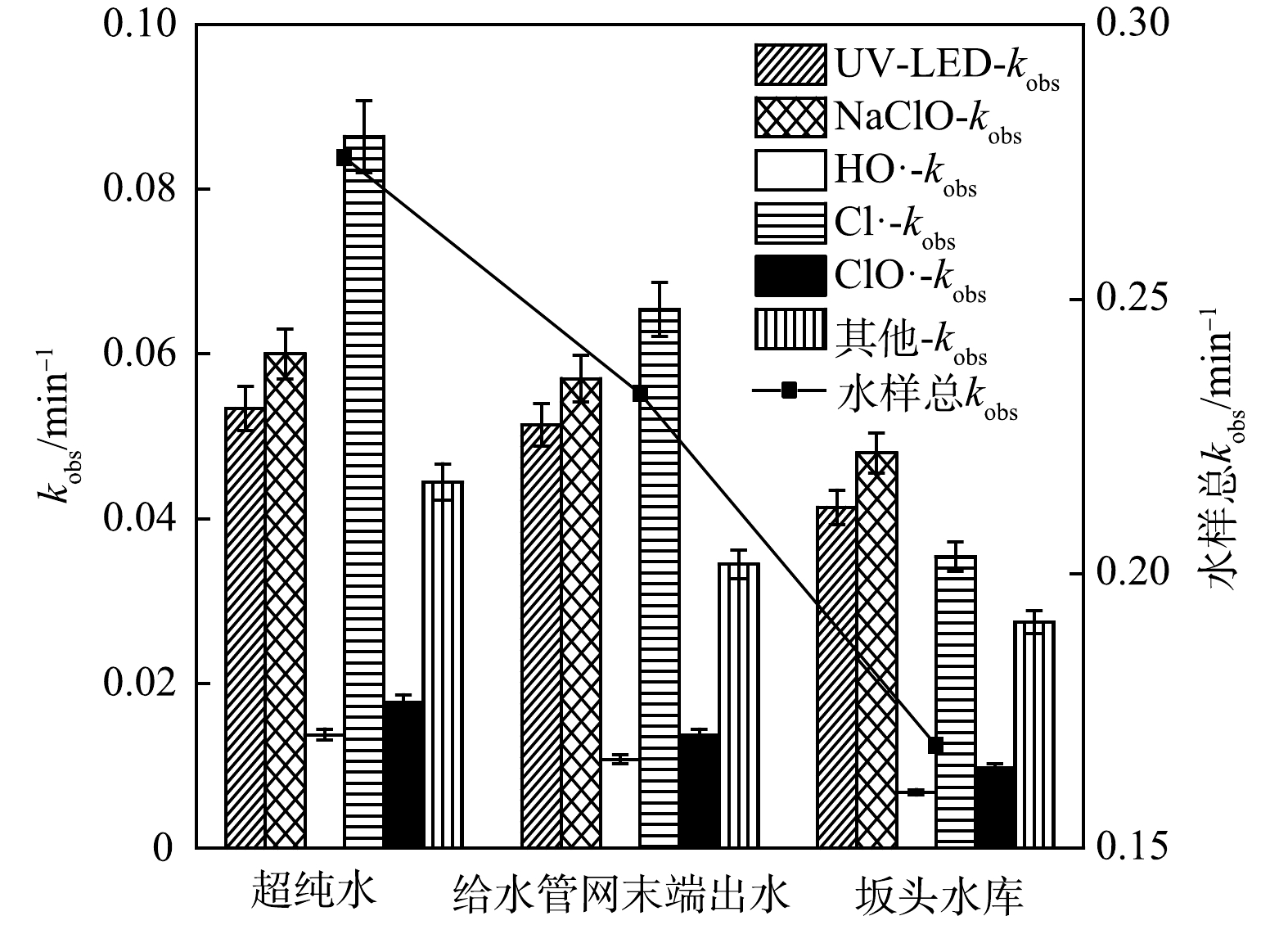
实验选取3种不同水样,考察不同环境水样对UV-LED/NaClO方法去除OPP的影响,水质参数见表1。通过OPP及在自由基探针下的剩余浓度建立了一级反应动力学模型,并计算了kobs和不同组分的相对贡献。
如图4所示,3种水样的kobs分别为0.275、0.232和0.168 min−1,纯水中OPP的降解效率最高,给水管网末端出水与坂头水库中OPP的去除均有不同程度的降低,HO·和Cl·对OPP降解的相对贡献分别降低4.640%和28.09%(给水管网末端出水)、4.030%和20.97%(坂头水库),UV-LED和NaClO的kobs也分别降至0.051和0.057 min−1(给水管网末端出水)、0.041和0.048 min−1(坂头水库)。不同环境水体中OPP去除率的差异可能与水中NOM浓度有较高的关联性:一方面,实际水样中NOM和其他杂质的存在抑制了活性组分(HO·、RCS)的产生,从而抑制OPP去除[18];另一方面,基于NaClO光降解的反应速率也会因Cl−的存在受到影响,Cl−可以与HO·和Cl·反应,生成活性较低的ClOH−·和Cl2−·[19],从而降低Cl−浓度,进而降低降解效率。这与KONG等[20]利用UV/NaClO分别在实际水体中降解碘帕醇和吡虫啉时得到的结论一致。
-
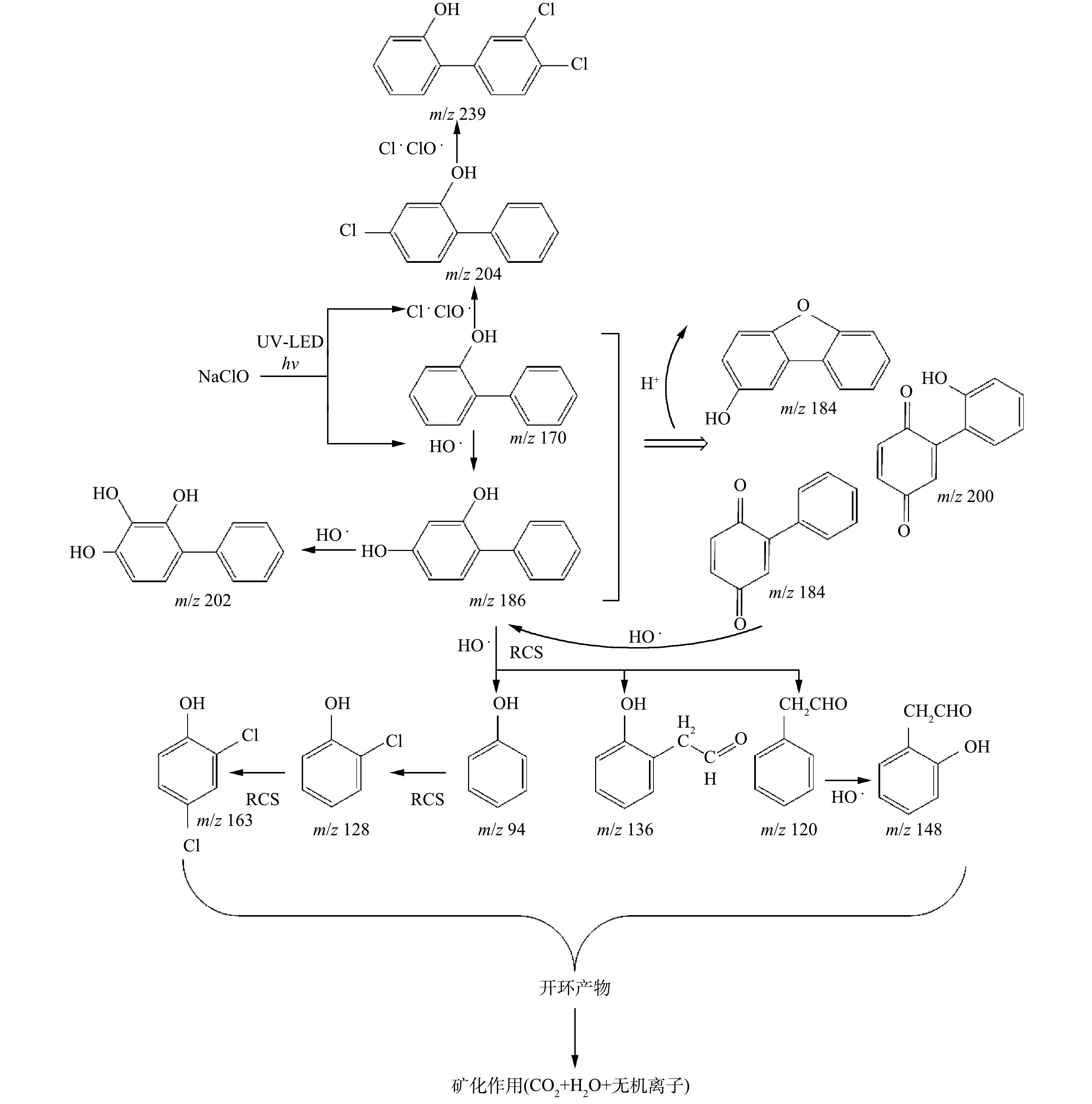
为研究UV-LED/NaClO方法降解OPP的机理,采用LC-MS对降解产物进行鉴定,主要产物有14种,分别包括P1(C12H10O2,3.67 min)、P2(C12H6ClO,3.32 min)、P3(C12H8OCl2,4.51 min)、P4(C6H6O,6.53 min)、P5(C6H6O2,3.12 min)、P6(C12H10O3,3.92 min)、P7(C12H8O2,5.88 min)、P8(C5H10OCl,6.56 min)、P9(C5H10OCl2,6.30 min)、P10(C8H8O,7.21 min)、P11(C9H8O2,7.63 min)、P12(C12H8O3,9.19 min)、P13(C12H8O2,5.11 min)、P14(C8H8O2,10.25 min)。由于UV-LED/NaClO方法对OPP的降解主要是HO·和RCS的攻击以及NaClO的氧化作用,因此,这14种中间产物大部分是通过羟基化、加成和取代等氧化反应产生,未开环时的主要产物为二羟基联苯异构体及氯代联苯异构体。主要氯代产物P2可能是NaClO氧化和Cl·攻击苯环发生加氯反应生成的;RCS继续攻击P2的同分异构体,便生成P3;而OPP被氧化脱去一个苯环后,生成P4(m/z 94,苯酚);接着再次加氯反应,生成苯酚氯代产物P8和P9;HO·也会与OPP的苯环发生加成反应,生成P1和P6。此外,还检测到P7和P12这2种产物。这可能是HO·被夺氢后,生成的超氧自由基与脱氢后的OPP再次发生取代作用,导致H转为O,从而生成P7和P12。OLAK-KUCHARCZYK等[21]利用UV/H2O2降解OPP时同样检测到了P7、P12和P13的生成,推测可能是由于HO·作用导致羟基化后O—O键断裂,生成苯醌类产物。芳香族联苯有机物在一定条件下与化合物发生氧化反应,导致脱环。实验中检测到P4、P10、P11、P14和P10这5种仅含一个苯环的中间产物,这归因于HO·、Cl·和ClO·的强氧化作用。此外,转化产物与自由基的进一步反应可能导致开环产物的形成和矿化[22],基于此,推测出OPP可能的降解路径(图5)。
可以看出,由于NaClO、HO·、Cl·和ClO·具有强氧化性,导致在UV-LED/NaClO去除OPP过程中产生多种氯代联苯、卤代苯酚和苯醌联苯类产物。楚文海等[23]指出,AOPs工艺与后续消毒处理过程中DBPs的产生量存在联系。当溶解性有机物(DOM)与消毒剂反应活性较低时,AOPs促进DBPs生成;而当反应活性高时,AOPs抑制DBPs生成;但即使AOPs生成的自由基量足够矿化,并有效控制有机DBPs,却仍无法有效控制无机DBPs的生成。此外, AOPs控制消毒副产物前体物时,可能会导致更多溴酸盐、醛类物质以及卤代有机物等氧化副产物的生成。因此,进一步确定消毒副产物的毒性效应,对探明降解过程中水质毒性变化规律具有重要意义。
-
1)急毒性变化。将UV-LED/NaClO方法处理后的溶液利用发光细菌实验方法鉴定降解过程中水质急性毒性变化,毒性效应检测结果见图6。反应开始时,溶液的相对抑制率为6%,表明母体物质OPP对发光细菌具有毒性效应。而在0~1.5 min,3种方法的相对抑制率均随着反应的进行而上升,表明在降解过程中生成了比母体污染物毒性更强的产物。随着反应的继续进行,与单独NaClO和组合方法相比,单独UV-LED方法的相对抑制率呈波动趋势,最高为12%,但急毒性未增加且小于另外2种方法。这是由于没有氯和RCS参与形成连锁反应生成氯代产物,从而减少了中间产物的生成,进而降低了消毒副产物的生成[9],因此单独UV-LED辐照OPP过程中溶液的毒性未增加。此外,有研究[24]表明,与单独NaClO和UV-LED/NaClO方法相比,单独UV方法降解PPCPs时能够产生较少的消毒副产物(DBPs)且不会产生的氯代中间产物,因此毒性更低,而单独NaClO方法处理后的溶液的急毒性和相对抑制率均先增加后略微下降并趋于稳定。这可能是反应开始时氯化产物生成且浓度增加,随着反应的继续进行,主要中间氯代产物浓度趋于稳定。LI等[25]利用UV/NaClO降解对乙酰氨基酚时发现,溶液急性毒性在60 min内基本稳定,这与本研究结果一致。
实验发现,UV-LED/NaClO方法处理后的溶液在反应初始阶段溶液相对抑制率增长缓慢,但在3 min时,相对抑制率突增至49%,而后呈下降趋势,直至反应结束。这是因为反应开始时生成一些具有毒性效应的产物,而后HO·和ClO·的攻击及UV的直接光解使其转化为毒性较低的产物,这与PAN等[26]观察到的在UV/Cl工艺降解萘普生过程中样品毒性先增加后减少的现象类似。此外,将反应时间延长至30 min时,OPP几乎完全降解,且3种方法的相对抑制率均有所下降(单独UV-LED、单独NaClO和UV-LED/NaClO方法分别为53%、7%和13%),急性毒性也更低。原因可能是,随着反应时间的延长,NaClO和Cl·浓度下降,氯代产物生成减少(如P2、P3、P8和P9),取而代之生成一些毒性较低的中间产物,并且部分被逐步破坏从而降低整体毒性[27]。结合去除率和急性毒性,UV-LED/NaClO方法可以有效去除OPP,但其水质整体毒性情况复杂,其变化规律尚未可知,因此需要做进一步的毒性评估来确保其方法安全。
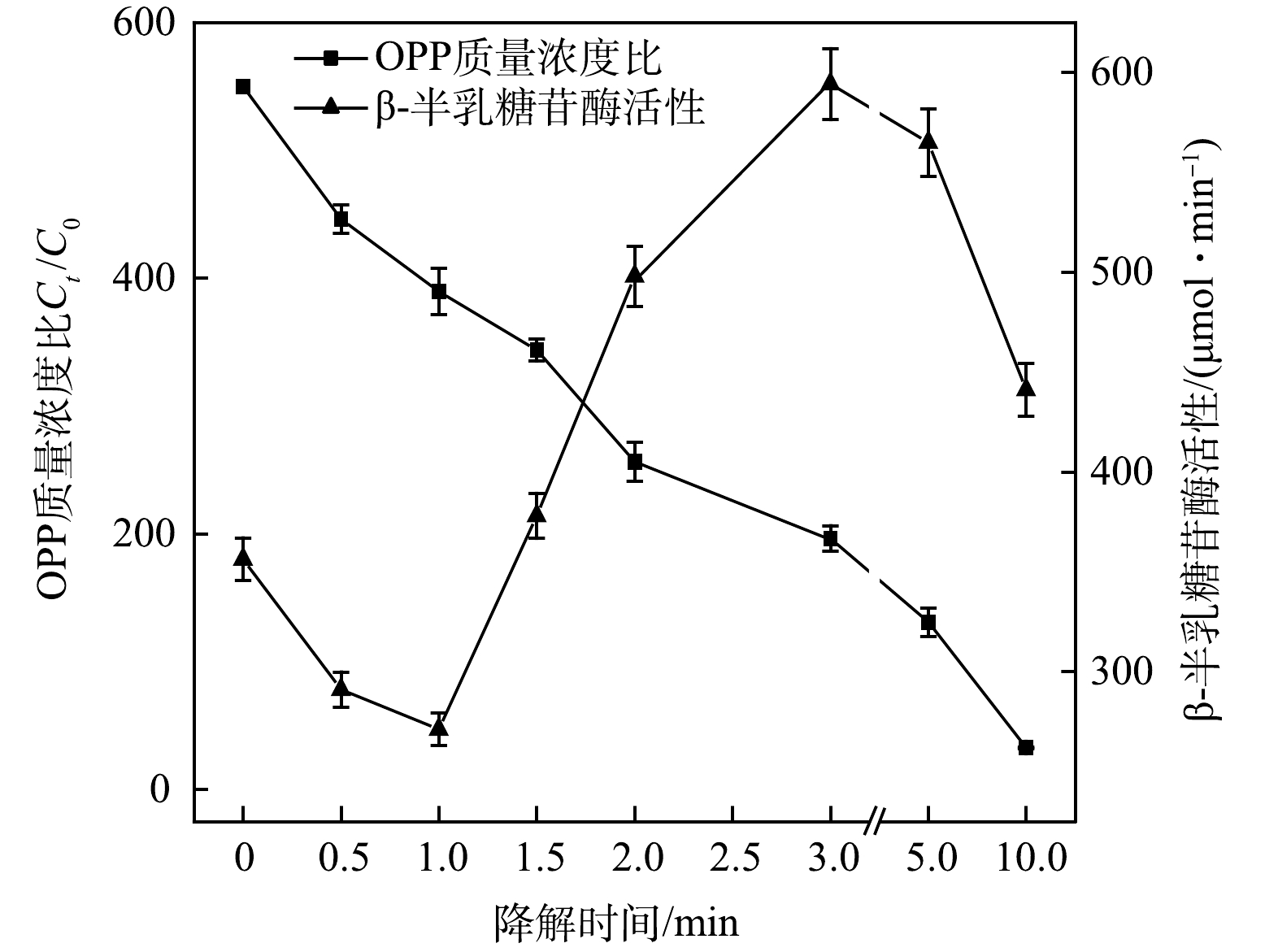
2)遗传毒性变化。除急性毒性外,基于生物DNA表达的遗传毒性同样是评价水质毒性的重要标准之一。在急性毒性实验条件下,采用SOS/umu 实验方法对UV-LED/NaClO方法降解OPP过程中的遗传毒性进行检测。如图7所示,溶液β-半乳糖苷酶活性值随OPP的降解呈现先下降再上升再下降的趋势。反应开始前,溶液的酶活性值为356.34,反应1 min后降至251.14。原因可能是,OPP本身具有一定的遗传毒性[28],在反应初期被快速降解,同时具有毒性的中间产物尚未生成,因此遗传毒性降低,β-半乳糖苷酶活性值下降。反应3 min时,酶活性值达到最大值594.19,随后不断降低,待反应结束时,仅为281.35,低于初始值。这表明,UV-LED/NaClO方法降解OPP过程中生成了比OPP本身遗传毒性更大的中间产物,但随反应的进行,可能被矿化生成毒性更小的中间产物。这与LI等[29]利用UV/NaClO降解双氯芬酸钠时发现遗传毒性先升后降的规律类似。反应溶液的急性毒性和遗传毒性不仅取决于中间产物,还取决于它们的特性,因此,需要进一步考察方法产生的中间产物与毒性之间的关系,筛选出毒性效应增强的消毒副产物并制定针对性的控制措施。
3)毒性组分分离。由图6和图7可知,UV-LED/NaClO方9法降解OPP在反应3 min时溶液的急性毒性和遗传毒性均为最大值。因此,以反应3 min的水样为代表,进行树脂分离得到5种极性不同的组分(D1、D2、D3、D4、D5),再用LC-MS分析得到5种组分中所含的中间产物,但由于缺乏标准物质以及组分难以完全分离。因此,以LC-MS峰面积间接表示中间产物的相对浓度并绘制组分分离结果(表2)以及部分组分毒性检测结果(图8)。
由表2可知,OPP去除3 min时检测到7种物质,其中D4组分内检测到了一种氯代产物P3和2种醛类产物P7及P12。由图8可看出,这3种产物使溶液的相对抑制率和β-半乳糖苷酶活性值较其他组分都处于高点,分别为20%和423.61,而具有较高β-半乳糖苷酶活性值的D3和D5组分分别检测到了P7和P12这2种醛类产物,这可能是溶液遗传毒性上升的原因。此外,从整体毒性规律来看,极性较弱的物质对毒性增长的贡献更大,但各组分的相对抑制率和β-半乳糖苷酶活性值均未超过分离前的溶液,这可能是分级分离方法使产物浓度变低或是产物之间的毒性拮抗效应导致的。
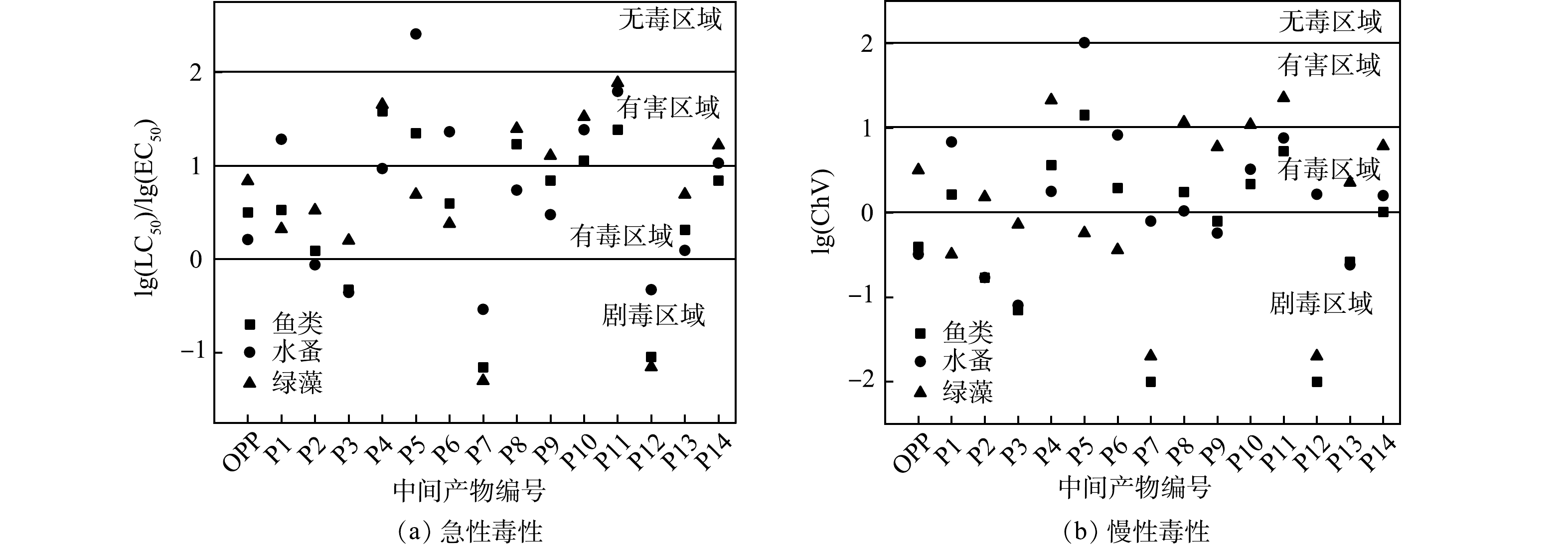
4) ECOSAR生态毒性预测。发光细菌实验和SOS/umu遗传毒性实验难以准确定位毒性效应增强的中间产物,树脂虽然可以根据极性大小分离中间产物,但仍无法对单一产物进行毒性评估。因此,利用ECOSAR软件模拟了OPP及其降解产物对鱼类、水蚤和绿藻的急性毒性LC50(对水生动物的半致死浓度)、EC50(对水生植物的半致死浓度)及ChV (慢性毒性值) 的影响,并根据GHS毒性分级[30]绘制了图9。
由图9可知,OPP及其降解产物对鱼类、水蚤和绿藻的急性毒性分别为3.16、1.62和6.84 mg·L−1,慢性毒性值分别为0.39、0.32和3.16 mg·L−1,其中慢性毒性最高达到了剧毒等级,与前文遗传毒性实验的结论相同。P2、P3和P7、P12的急性毒性和慢性毒性也都达到剧毒等级,这可能是溶液2种毒性上升的主要原因。此外,还观察到P4和P5苯酚类产物毒性相对较低,这可能是反应后期OPP及其中间体脱环后生成的主要产物。随着反应的进行,反应初期生成的P7和P12被自由基攻击而生成毒性较低的P4和P5,这有可能降低溶液的毒性,在发光细菌实验和SOS/umu遗传毒性实验也均得到了类似的结论。
UV-LED/NaClO方法降解OPP时的降解产物几乎都具有毒性且多数达到了有毒或剧毒等级,但同时也发现溶液毒性会随反应的进行而降低。因此,在实际降解过程中,应注意中间产物的生成和溶液的毒性变化,适当延长降解时间,既保证降解又保证水质安全。
-
1) UV-LED/NaClO方法具有协同作用,拟一级动力学常数为0.275 min−1 (R2=0.993),OPP降解过程中不同组分贡献大小顺序为 Cl·>NaClO>UV-LED>其他组分(Cl2−·、O−·、ClOH·等) >HO·>ClO·。在使用UV-LED/NaClO方法降解OPP过程中,kobs随着CO32−和NO3−浓度的增加而增大,CO32−对其他组分相对贡献的促进作用由16.1%增至88.2%, HO·和Cl·的相对贡献分别降低4.400%和30.06%;NO3−降低了Cl·的相对贡献,但提高了其余自由基的相对贡献;HA的自由基淬灭作用导致kobs和组分整体贡献下降;实际水样存在NOM等可溶性有机物和离子化合物,所以OPP的降解效率低于纯水。
2) OPP降解过程中主要产物为氯代产物、苯酚类产物和羟基化类产物,产物的生成主要与RCS和HO的加成、氧化和取代反应有关。
3) UV-LED/NaClO方法降解OPP过程中溶液的急性毒性和遗传毒性均呈现先上升后下降的趋势,表明降解过程产生具有毒性的产物。ECOSAR软件对产物的生态毒性预测结果表明,OPP降解过程中的产物毒性多数大于母体污染物,但延长反应时间可降解有毒物质。树脂分级分离进一步表明,急性毒性和遗传毒性最高的组分为D3和D4,推测具有毒性效应的产物可能是P7(C12H8O2)和P12(C12H8O3)2种醛类产物。
UV-LED/NaClO方法降解邻苯基苯酚过程中水质对自由基去除贡献的影响及产物毒性效应分析
Influence of water quality on contribution of free radical removal in the degradation of o-phenylphenol by UV-LED/NaClO method and its product toxicity effect analysis
-
摘要: 为去除水中的邻苯基苯酚(OPP),以UV-LED/NaClO方法为降解手段,考察了CO32−、NO3−、腐殖酸(HA)对OPP去除的影响,探究了HO·、UV-LED、NaClO和含氯自由基(Cl·、ClO·)等对去除贡献的影响,结合降解产物提出可能的OPP降解途径,采用毒性鉴定评估方法,筛选出毒性效应增强产物。结果表明:在pH=7.0±0.2、OPP初始浓度为3 μmol·L−1、NaClO=40.3 nmol·L−1条件下,不同组分的去除贡献大小顺序为Cl·>NaClO>UV-LED>其他组分(CO3−·、Cl2−·、O−·等)>HO·>ClO·,Cl·对OPP去除贡献最大为31.13%;当CO32−和NO3−浓度由0增至1.0 mmol·L−1时,拟一级反应速率常数(kobs)逐渐增大,Cl·的相对贡献分别降低30.05%和23.60%,其他组分的相对贡献分别增加72.10%和5.889%;HO·和ClO·的相对贡献随CO32−投加量的增加,分别降低4.4%和5.4%,随NO3−投加量的增加,相对贡献分别增加16.9%和9.00%;随着HA质量浓度增加,OPP去除的抑制作用增强,不同组分的相对贡献率均不断降低,Cl·抑制效果最为明显,相对贡献率由31.3%降至25.4%;降解产物急性毒性和遗传毒性均呈现先上升后下降的趋势,并于3 min时达到最毒。由此可知:UV-LED/NaClO方法可以有效去除OPP,CO32−和NO3−可促进OPP去除,而HA会抑制OPP去除;降解产物多数大于母体污染物,但延长反应时间可降解有毒物质;具有毒性效应增强的产物可能为P7(C12H8O2)和P12(C12H8O3)2种醛类产物。本研究结果可为进一步探究UV-LED/NaClO方法降解水环境中微污染物提供参考。
-
关键词:
- UV-LED/NaClO /
- 邻苯基苯酚 /
- 相对贡献 /
- 毒性鉴别 /
- 自由基
Abstract: In order to remove o-phenylphenol (OPP) from water, the UV-LED/NaClO method was used to degrade it. The influences of CO32−, NO3−, humic acid (HA) on OPP removal were studied, and the contributions of HO·, UV-LED, NaClO and chlorine radicals (Cl·、ClO·) on OPP decomposition were investigated. Furthermore, the degradation products were identified and the possible degradation pathways of OPP was proposed. In addition, the toxicity identification evaluation method was used to screen the degradation products with increased toxic effects. The result showed that at pH=7.0±0.2, OPP=3 μmol·L−1, NaClO=40.3 nmol·L−1, the contribution order of different species to OPP removal was Cl·>NaClO>UV-LED> other components (CO3−·、Cl2−·、O−·et al.)>HO·>ClO·, and the highest contributed of Cl· to OPP removal was 31.13%. The corresponding kobs increased with the dosages of CO32−and NO3− increasing from 0 to 1.0 mmol·L−1, then the relative contribution of Cl· decreased by 30.05% and 23.60%, respectively, the relative contributions of other components increased by 72.10% and 5.889% , respectively. The relative contribution of HO· and ClO· decreased by 4.4% and 5.4% with the increase of CO32− concentration, respectively, while increased by 16.9% and 9.00% with the increase of NO3− concentration, respectively. The inhibited effect for OPP removal increased with the increasing of HA concentration, the relative contribution of different species decreased accordingly, and the most inhibitory effects of Cl· occurred, the relative contribution decreased from 31.3% to 25.4%. The acute toxicity and genotoxicity of degradation products showed a increase trend at first, and then a decreased one, and reached the most toxic at 3 min. It can be seen that the UV-LED/NaClO method can effectively remove OPP, CO32− and NO3− can promote OPP removal, while HA inhibits OPP removal; some intermediate products possessed higher toxicity than their parent pollutants, but prolonging the reaction time can degrade toxic substances; the product with increased toxic effects may be aldehyde products P7(C12H8O2) and P12(C12H8O3). The results of this study can provide a reference for further study on the degradation of micro-pollutants in water by UV-LED/NaClO method. -

-
表 1 不同水样的主要水质参数
Table 1. Main water quality parameters of different water samples
水样 pH TOC/ (mg·L−1) UV254 /cm−1 Cl− /(mmol·L−1) 超纯水 6.79 0.120 0.0010 0.001 给水管网末端出水 7.13 1.38 0.018 0.002 坂头水库 6.32 2.62 0.063 0.05 表 2 XAD-8树脂分离OPP降解产物的参数
Table 2. Parameters of OPP degradation products separated by XAD-8 resin column
组分 质荷比m/z 中间产物编号 分子式 气质峰面积大或小 D1 170 OPP C12H10O6 小 94 P4 C6H6O 小 110 P5 C6H6O2 大 D2 94 P4 C6H6O 大 202 P6 C12H10O3 小 D3 184 P7 C12H8O2 大 148 P11 C12H8O2 小 D4 184 P7 C12H8O2 小 200 P12 C12H8O3 大 239 P3 C12H8OCl2 大 D5 239 P3 C12H8OCl2 小 200 P12 C12H8O3 小 -
[1] 王丹丹, 张婧, 杨桂朋. 药物及个人护理品的污染现状, 分析技术及生态毒性研究进展[J]. 环境科学研究, 2018, 31(12): 2013-2020. [2] GAN G Q, FAN S Y, LI X Y, et al. Adsorption and membrane separation for removal and recovery of volatile organic compounds[J]. Journal of Environmental Sciences, 2022, 123: 96-115. [3] 赖成然, 李青松, 马晓雁, 等. UV-LED/NaClO工艺降解水中邻苯基苯酚的活性组分贡献和影响因素[J]. 高校化学工程学报, 2022, 36(5): 766-774. [4] 李博强, 马晓雁, 李青松, 等. UV-LED/NaClO工艺对水中对乙酰氨基酚的降解[J]. 中国环境科学, 2019, 39(11): 4681-4688. doi: 10.3969/j.issn.1000-6923.2019.11.025 [5] 林英姿, 王高琪, 张代华, 等. 典型高级氧化技术控制抗生素氯化消毒副产物的研究进展[J]. 吉林师范大学学报(自然科学版), 2021, 42(1): 76-81. doi: 10.16862/j.cnki.issn1674-3873.2021.01.014 [6] 石志芳, 姜霞, 程积民, 等. 毒性鉴别评估(TIE)在沉积物毒性评估中的应用[J]. 环境科学与技术, 2010, 33(5): 89-95. doi: 10.3969/j.issn.1003-6504.2010.05.021 [7] TANI S, YONEZAWA Y, MORISAWA S, et al. Development of a new e. Coli strain to detect oxidative mutation and its application to the fungicide o-phenylphenol and its metabolites[J]. Mutation Research/Genetic Toxicology and Environmental Mutagenesis, 2007, 628(2): 123-128. doi: 10.1016/j.mrgentox.2006.12.006 [8] FANG J, YUN F, SHANG C. The roles of reactive species in micropollutant degradation in the UV/free chlorine system[J]. Environmental Science & Technology, 2014, 48(3): 1859-1868. [9] GUO K H, WU Z H, SHANG C I, et al. Radical chemistry and structural relationships of PPCP degradation by UV/chlorine treatment in simulated drinking water[J]. Environmental Science & Technology, 2017, 51(18): 10431-10439. [10] ZHOU Y J, CHEN C Y, GUO K H, et al. Kinetics and pathways of the degradation of PPCPS by carbonate radicals in advanced oxidation processes[J]. Water Research, 2020, 185: 116231. doi: 10.1016/j.watres.2020.116231 [11] 言野, 李娜, 刘楠楠, 等. 利用改进的SOS/umu方法检测水处理过程中污染物的遗传毒性效应[J]. 生态毒理学报, 2013, 8(6): 909-916. doi: 10.7524/AJE.1673-5897.20121017001 [12] GAO J, DUAN X, O SHEA K, et al. Degradation and transformation of bisphenol a in UV/sodium percarbonate: Dual role of carbonate radical anion[J]. Water Research, 2020, 171: 115394. doi: 10.1016/j.watres.2019.115394 [13] GHANBARI F, GIANNAKIS S, LIN K A, et al. Acetaminophen degradation by a synergistic peracetic acid/UVC-LED/Fe(Ⅱ) advanced oxidation process: Kinetic assessment, process feasibility and mechanistic considerations[J]. Chemosphere, 2021, 263: 128119. doi: 10.1016/j.chemosphere.2020.128119 [14] XU M Y, DENG J, CAI A, et al. Synergistic effects of UVC and oxidants (PS vs. chlorine) on carbamazepine attenuation: Mechanism, pathways, DBPS yield and toxicity assessment[J]. Chemical Engineering Journal, 2021, 413: 127533. doi: 10.1016/j.cej.2020.127533 [15] YIN, R, LING L, SHANG C, et al. Wavelength-dependent chlorine photolysis and subsequent radical production using UV-LEDS as light sources[J]. Water Research, 2018, 142: 452-485. doi: 10.1016/j.watres.2018.06.018 [16] NAWROCKI J, DĄBROWSKA A, RACZYK-STANISŁAWIAK U, et al. Reactivity of natural organic matter fractions with chlorine dioxide and ozone[J]. Water Research, 2004, 38(3): 547-558. doi: 10.1016/j.watres.2003.10.034 [17] CHOW C H, SZE-YIN LEUNG K. Transformations of organic micropollutants undergoing permanganate/bisulfite treatment: Kinetics, pathways and toxicity[J]. Chemosphere, 2019, 237: 124524. doi: 10.1016/j.chemosphere.2019.124524 [18] GUO K H, WU Z H, YAN S W, et al. Comparison of the UV/chlorine and UV/H2O2 processes in the degradation of PPCPS in simulated drinking water and wastewater: Kinetics, radical mechanism and energy requirements[J]. Water Research, 2018, 147: 184-194. doi: 10.1016/j.watres.2018.08.048 [19] ZHAO X, JIANG J, PANG S, et al. Degradation of iopamidol by three UV-based oxidation processes: Kinetics, pathways, and formation of iodinated disinfection byproducts[J]. Chemosphere, 2019, 221: 270-277. doi: 10.1016/j.chemosphere.2018.12.162 [20] KONG X J, JIANG J, MA J, et al. Comparative investigation of X-ray contrast medium degradation by UV/chlorine and UV/ H2O2[J]. Chemosphere, 2018, 193: 655-663. doi: 10.1016/j.chemosphere.2017.11.064 [21] OLAK-KUCHARCZYK M, LEDAKOWICZ S. Advanced oxidation of preservative agents in H2O2/UVC system: Kinetics study, transformation products and toxicity assessment[J]. Journal of Hazardous Materials, 2017, 333: 348-357. doi: 10.1016/j.jhazmat.2017.03.047 [22] WANG J L, WANG S Z. Reactive species in advanced oxidation processes: formation, identification and reaction mechanism[J]. Chemical Engineering Journal, 2020, 401: 126158. doi: 10.1016/j.cej.2020.126158 [23] 楚文海, 肖融, 丁顺克, 等. 饮用水中的消毒副产物及其控制策略[J]. 环境科学, 2021, 42(11): 5059-5074. doi: 10.13227/j.hjkx.202103034 [24] YEOM Y J, HAN J R, ZHANG X R, et al. A review on the degradation efficiency, DBP formation, and toxicity variation in the UV/chlorine treatment of micropollutants[J]. Chemical Engineering Journal, 2021, 424(18): 130053. [25] LI J Q, ZHOU S Q, LI M, et al. Mechanism insight of acetaminophen degradation by the UV/chlorine process: kinetics, intermediates, and toxicity assessment[J]. Environmental Science and Pollution Research, 2019, 26(24): 25012-25025. doi: 10.1007/s11356-019-05747-1 [26] PAN M W, WU Z H, TANG C Y, et al. Emerging investigators series: comparative study of naproxen degradation by the UV/chlorine and the UV/H2O2 advanced oxidation processes[J]. Environmental Science Water Research & Technology, 2018, 4(9): 1219-1230. [27] HUANG N, WANG T, WANG W L, et al. UV/chlorine as an advanced oxidation process for the degradation of benzalkonium chloride: Synergistic effect, transformation products and toxicity evaluation[J]. Water Research, 2017, 114: 246-253. doi: 10.1016/j.watres.2017.02.015 [28] YE Y, JIANG W W, et al. Assessing of genotoxicity of 16 centralized source-waters in China by means of the SOS/umu assay and the micronucleus test: Initial identification of the potential genotoxicants by use of a GC/MS method and the QSAR toolbox 3.0[J]. Mutation Research/Genetic Toxicology and Environmental Mutagenesis, 2014, 763: 36-43. doi: 10.1016/j.mrgentox.2013.11.003 [29] LI Q S, LAI C R, YU J W, et al. Degradation of diclofenac sodium by the UV/chlorine process: Reaction mechanism, influencing factors and toxicity evaluation[J]. Journal of Photochemistry and Photobiology A:Chemistry, 2022, 425: 113667. doi: 10.1016/j.jphotochem.2021.113667 [30] 付知雨, 卢玲, 孙锦业, 等. 化学品GHS急性毒性分类探讨[J]. 毒理学杂志, 2013, 27(1): 34-37. doi: 10.16421/j.cnki.1002-3127.2013.01.011 -



 下载:
下载: