-
全氟和多氟烷基物质(perfluoroalkyl and polyfluoroalkyl substances,PFASs)是一类人工合成的化学物质[1],因具有很好的热稳定性、较低的化学活性和良好的耐水性能而被广泛应用于防污/防水织物、消防泡沫、保护涂层以及食品包装等领域[2]。近年来,含PFASs的工业废水排放导致PFASs在环境基质[3]、野生动物体内[4]、人体组织和血清中[5]被普遍检出,同时,PFASs具有神经毒性、免疫毒性[6]、内分泌干扰作用及对胎儿发育的多种不良影响[7],其健康风险引起人们的广泛关注。
全氟辛酸(perfluorooctanoic acid,PFOA)是检出频率较高的一种PFASs[8],PFOA及其盐类于2019年在《斯德哥尔摩公约》中被列为新的持久性有机污染物[9](persistent organic pollutants,POPs)。PFOA具有高键能的碳氟键(C—F,552.0 kJ·mol−1),因此,化学稳定性高[10]。常规水处理方法如吸附、混凝沉淀和微生物处理等很难将其降解[11]。高级氧化工艺作为目前重要的深度处理工艺,其主要活性基团是具有强氧化能力的羟基自由基(HO·),其可以将大分子难降解有机物氧化为低毒或无毒的小分子物质。但是,目前由于PFOA与HO·的反应活性很低,所以多数高级氧化工艺也无法有效处理PFOA[12-14]。因此,研发高效处理工艺降解PFOA对于保护水质安全和人类健康十分重要。
近年来,紫外(UV)活化过硫酸盐(PDS)的工艺已被用于许多有机物的氧化降解中[15]。PDS光解可以产生2个硫酸根自由基(SO4−·)(式(1)),SO4−·是一种具有强氧化性的自由基[16]。
SO4−·的氧化还原电势为2.3 V,是一种非常亲电的自由基,在降解有机化合物方面比HO·更有效[17]。现有研究[18]表明,SO4−·可有效降解PFOA。QIAN等[19]通过UV/PDS工艺降解PFOA,在平均光强为2.88×10−7 einstein·(L·s)−1的条件下,在单独UV辐照下PFOA不发生降解,在UV/PDS工艺下其降解率为85.6%。YIN等[20]在pH为2的条件下活化PDS降解PFOA,PFOA降解率达89.9%。
光源是UV高级氧化的重要影响因素,相对于传统的低压汞灯(输出254 nm单波长UV),新型光源真空UV/UV(VUV/UV)汞灯可在不增加电能输入的情况下,额外输出185 nm VUV[21],在水处理应用中具有广阔的前景。VUV可以通过光解水产生强氧化性的HO·(式(2))降解有机污染物,同时VUV光子具有较高的能量,可直接光解有机污染物,目前已有研究表明VUV直接光解可有效降解PFOA[22]。因此,采用VUV/UV汞灯作为UV/PDS的光源,即VUV/UV与UV/PDS相结合,形成VUV/UV/PDS工艺具有高效降解PFOA的潜力。
本课题组前期开发了细管流光反应系统,可配装VUV/UV汞灯同时输出UV和VUV辐照,以探讨VUV/UV对水中污染物的降解效果。其可在保证UV剂量准确测定的基础上,输出接近实际工程的UV辐照强度,且具有实验样品量少、操作简单等优点。因此,本研究基于细管流光反应系统,探究了VUV/UV/PDS工艺对PFOA的降解效果,通过分析UV和VUV光子吸收分布以及活性基团降解贡献,探究了VUV/UV和UV/PDS工艺协同降解PFOA的效果和机理,以期为VUV/UV/PDS工艺的实际应用提供参考。
-
所有溶液均采用超纯水(Milli-Q Advanrtage)配置,PFOA和乙腈分别购自Aladdin Bio-Chem公司和Fisher scientific公司、尿苷、N,N-二乙基-p-苯二胺(DPD)、过氧化物酶(POD)、乙酸铵、过硫酸钾、过氧化氢(H2O2)、磷酸氢二钾和磷酸二氢钾均购自北京国药试剂公司。
DPD及POD溶液配制:准确量取24 mL 0.5 mol·L−1 K2HPO4溶液加入到376 mL 0.5 mol·L−1 KH2PO4溶液配制成pH等于6.0的缓冲溶液。准确量取103 μL 9.7 mol·L−1的H2O2溶液加入到1 L超纯水中配置成1 mmol·L−1母液,再分别稀释成2,5,10,15,20 μmol·L−1的标准溶液备用。准确称取1.0 g DPD药品溶于100 mL 0.05 mol·L−1 H2SO4溶液中,配置成10 g·L−1的DPD溶液;准确称取25 mg的POD药品溶于25 mL超纯水中配成1 g·L−1的POD溶液,在5 ℃下避光保存。
-
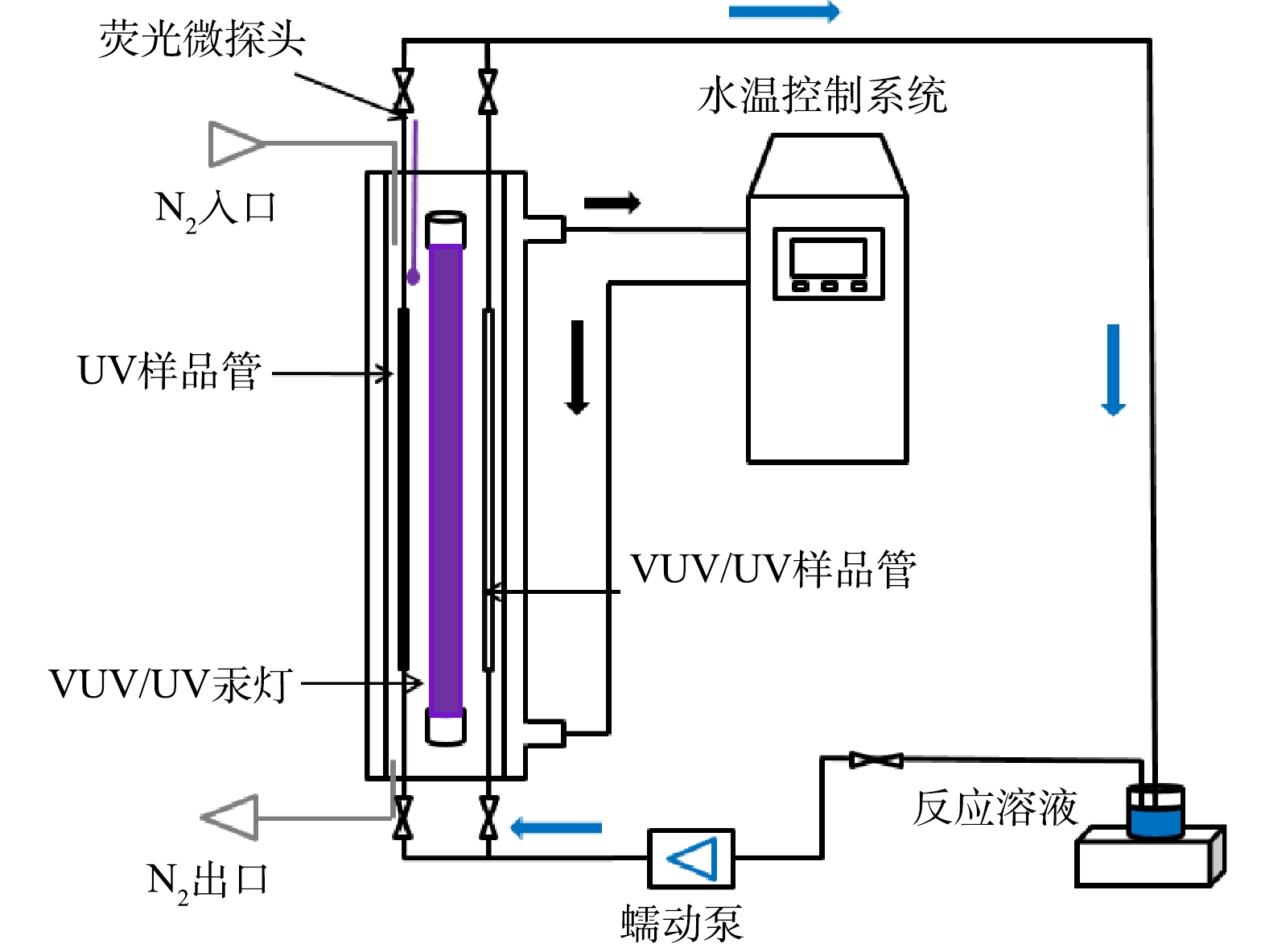
细管流光反应系统主体由双层石英管壁、中心处的VUV/UV汞灯、平行于VUV/UV汞灯安装的UV样品管和VUV样品管及水温控制器组成,如图1所示。反应溶液从样品管中流过接受UV(或VUV/UV)辐照,冷却水(25 ℃)从双层石英管壁间自下而上流过,保持系统恒温,从而保证辐照的稳定性。VUV/UV汞灯(浙江硖石万华,8 W)可同时输出185 nm VUV和254 nm UV 2种波长的光子。UV样品管为掺钛石英管,可滤过VUV/UV汞灯发射的185 nm波长的VUV,而对254 nm UV光子的吸收可忽略不计。VUV/UV样品管为高纯石英管,对VUV和UV光子的吸收均可忽略不计。UV和VUV/UV样品管的内径(r)和长度(h)均为0.34 cm和35 cm。由于两管与灯表面的距离相同(5.0 mm),因此,管内接收UV辐射剂量相同,便于比较额外VUV辐照的强化效果。
本研究采用循环模式进行实验,水样由蠕动泵输送通过细管流光反应系统的曝光部分并返回锥形瓶中,然后再循环以接受额外的曝光,直至达到所需的光子通量。需要指出的是,在一定的曝光时间内,只有一部分水样可以接受UV(或VUV和UV)辐照,而其余部分不接受辐照。因此,需要定义等效辐照时间(reduction equivalent exposure time,tree,s),其可根据式(3)进行计算。
式中:V为样品总体积,mL;t为总反应时间,s。可理解为全部水样同时接受辐照(相同辐照强度),达到过流模式下相同的反应效果所需要的反应时间。
-
本实验在细管流光反应系统中进行,开启VUV/UV汞灯预热10 min。在溶液瓶中加入70 mL初始质量浓度为100 μg·L−1、pH为7.5的PFOA溶液(0.5 mmol L−1磷酸盐缓冲溶液),开启蠕动泵使反应溶液流过VUV/UV或UV样品管,采用循环实验,按照设定时间进行取样,样品经0.22 μm针孔滤膜过滤后用超高效液相/质谱联用仪测定PFOA浓度。
-
PFOA浓度通过超高效液相/质谱联用(Agilent 1290,UPLC-MS/MS)进行测定,检测器为Agilent 6420 Triple Quad LC/MS,色谱柱为Acquity UPLC®CSHTM C18 column(2.1 mm×100 mm,1.7 µm)。流动相为2.0 mmol·L−1乙酸铵溶液(A)和乙腈(B)混合物,流速为0.2 mL·min−1。梯度洗脱程序如下:0~1 min(A相为80%);1~5 min(A相由80%匀速降至10%并于10%保持1 min);随后经过3 min的后运行,A相由10%回到80%。质谱操作条件如下:负离子电喷雾离子化模式,毛细管电压为2.0 kV,干燥气温度为350 ℃,干燥气流速为11 L·min−1,喷雾气压为40 psi。母离子(m/z)为412.9;子离子(m/z)分别为168.9和368.8,对应碰撞能量分别为10 eV和11 eV;碎裂电压为105 V。
H2O2浓度采用DPD-POD分光光度法[23]测定,取2 mL反应样品,依次加入0.2 mL pH等于6.0的缓冲溶液、10 μL POD溶液、100 μL DPD溶液,反应显色40 s,使用分光光度计在551 nm波长下测定吸光度。
-
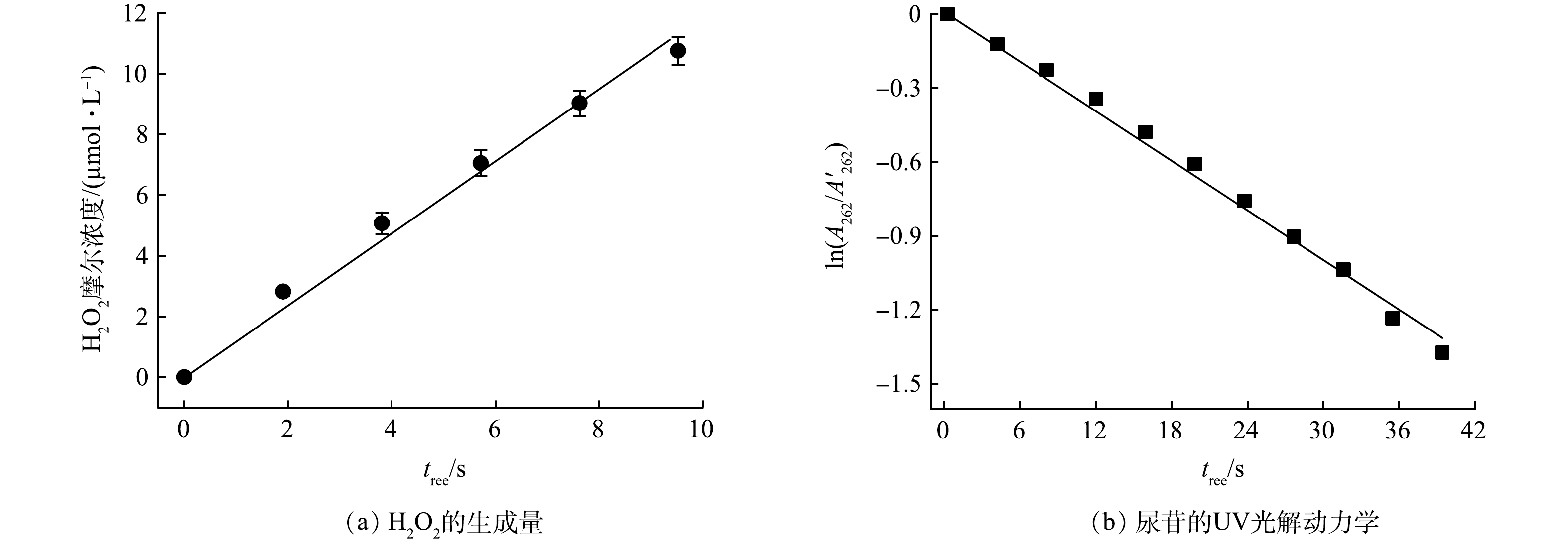
VUV辐照剂量测定采用研究组前期研发的微量H2O2生成法[24]。由于水分子在水溶液中浓度极高(55.6 mol·L−1),185 nm VUV辐照时,水分子会吸收绝大多数VUV光子,解离生成HO·和H·(式(2)),光量子产率(Φ)为0.33[25-26]。VUV汞灯表面高浓度的HO·会复合生成微量H2O2,该反应的二级反应速率常数为4×109 mol·(L·s)−1(式(4))。尽管H2O2会被UV辐照光解(Φ = 0.55)及HO·所消耗(式(5)~(6)),但由于VUV/UV光解水反应前期生成H2O2浓度较低,UV辐照及HO·对H2O2的消耗可忽略,H2O2产率(
${{r}}_{{\text{H}}_{\text{2}}{\text{O}}_{\text{2}}}$ )与VUV辐射剂量成正比例关系[27]。因此,通过不同时间采样,测定生成微量H2O2浓度并计算H2O2产率,即可实现VUV辐照剂量的测定。方法中微量H2O2浓度可通过DPD-POD分光光度法测定,加入POD试剂可以使溶液中发光氧化物的发光强度增强1 000倍,从而检测溶液中形成的微量H2O2。图2(a)反映了水(1 mmol·L−1的磷酸缓冲溶液)在细管流光反应系统中H2O2生成量与VUV辐照时间之间的关系。通过
${{r}}_{{\text{H}}_{\text{2}}{\text{O}}_{\text{2}}}$ (μmol·s−1)与VUV剂量的关系(式(7)),计算细管流光反应系统中VUV辐照的光子通量。式中:
${{q}}_{\text{VUV}}$ 为VUV光子通量,einstein·s−1;V为反应体积,L;${\textit{Φ}}_{{\text{H}}_{\text{2}}{\text{O}}_{\text{2}}}$ 为VUV辐照生成H2O2的量子产率($ {\textit{Φ}}_{{\text{H}}_{\text{2}}{\text{O}}_{\text{2}}} $ = 0.024)[24]。在细管流光反应系统中,${{q}}_{\text{VUV}}$ 与细管内VUV的辐照强度(EVUV)和细管的截面积(r = 3.4 mm,h = 350 mm)的乘积近似相等。因此,求得EVUV为1.16×10−4 einstein·(m2·s)−1。VUV的辐照剂量(FVUV)可以通过式(8)计算。 -
采用化学感光法测定细管流光反应系统的254 nm UV的辐照强度(EUV)。采用尿苷(0.012 mmol·L−1)作为感光剂(尿苷在UV辐照下的光解速率常数为1.84×10−2 s–1)[28],其吸收峰位于262 nm,根据式(9)计算EUV。
式中:EUV为254 nm处 UV的辐照强度,einstein·(m2·s)−1;
$ \epsilon_{\text{254}} $ 为254 nm处尿苷的摩尔吸光系数,8 740 L·(mol·cm)−1;Φu为尿苷光解反应的量子产率,0.02 mol·einstein−1;$ {\text{A}}_{\text{262}}^{\text{'}} $ 和$ {\text{A}}_{\text{262}} $ 分别为尿苷在262 nm处UV辐照前后的吸光度,cm−1。图2(b)为尿苷在UV细管中254 nm辐照下的光解结果,通过拟合可得一级光解速率常数为3.44×10−2。代入公式计算得到UV管中UV辐照强度(EUV)为1.39×10−3 einstein·(m·s)−1。UV的辐照剂量(FUV)可以通过式(10)进行计算。
-
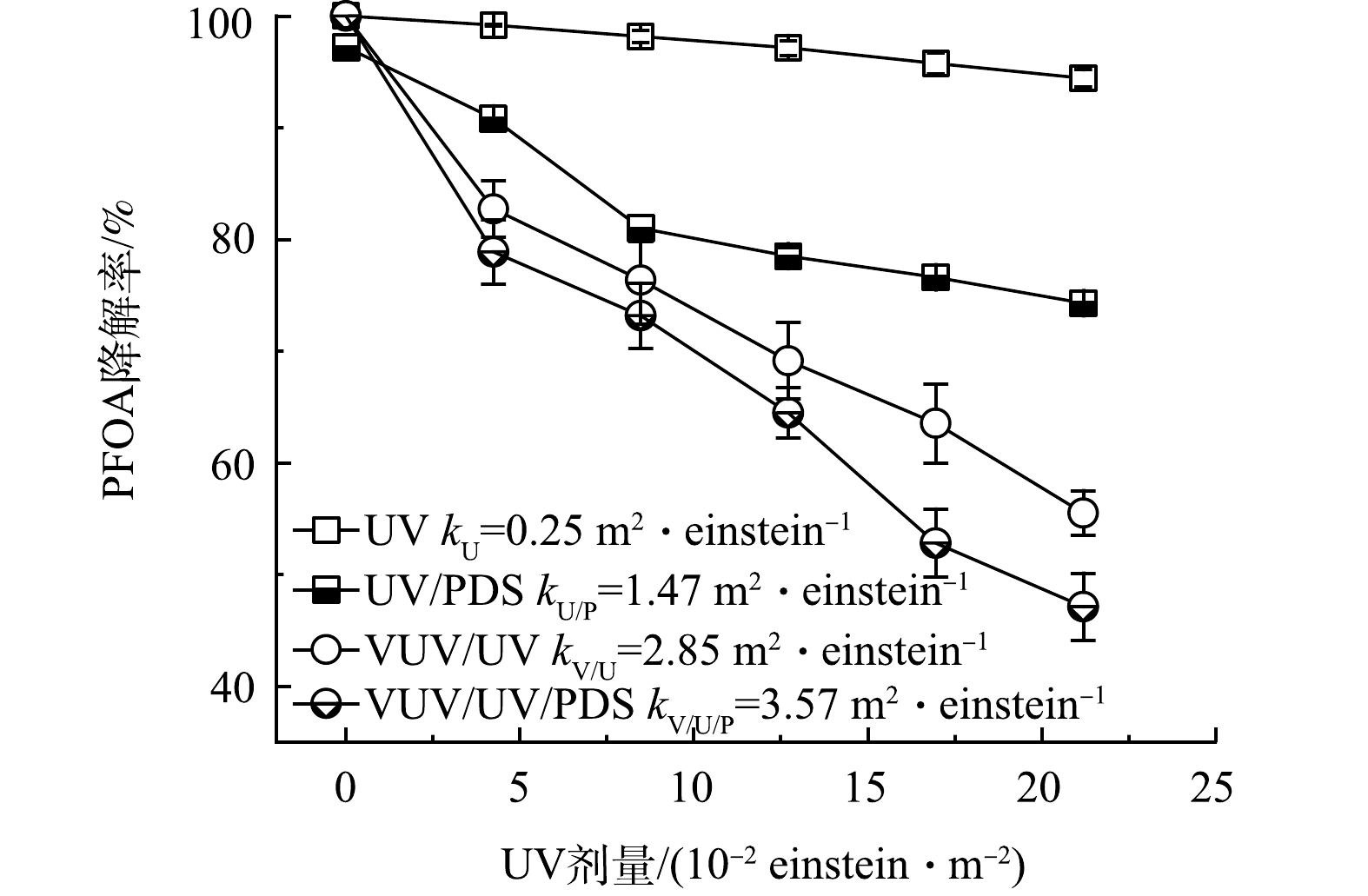
图3为UV和VUV/UV光源单独辐照及其与PDS(0.9 mmol·L−1)结合下对PFOA的降解效果。PFOA的降解率由高到低依次为VUV/UV/PDS、VUV/UV辐照、UV/PDS和UV辐照。PFOA在单独UV辐照下基本不发生降解反应[29],这是由于PFOA对254 nm波长下的光基本不吸收。VUV/UV工艺的降解效果较为显著,主要降解机制为VUV直接光解和被VUV光解水产生的HO·氧化降解。由于PDS光解生成SO4−·,可有效降解PFOA,因此,PFOA在UV/PDS工艺下发生明显降解。随着VUV/UV光源强化,VUV/UV/PDS工艺表现出最高的降解效果,准一级降解速率常数为3.57 m2·einstein−1。
-
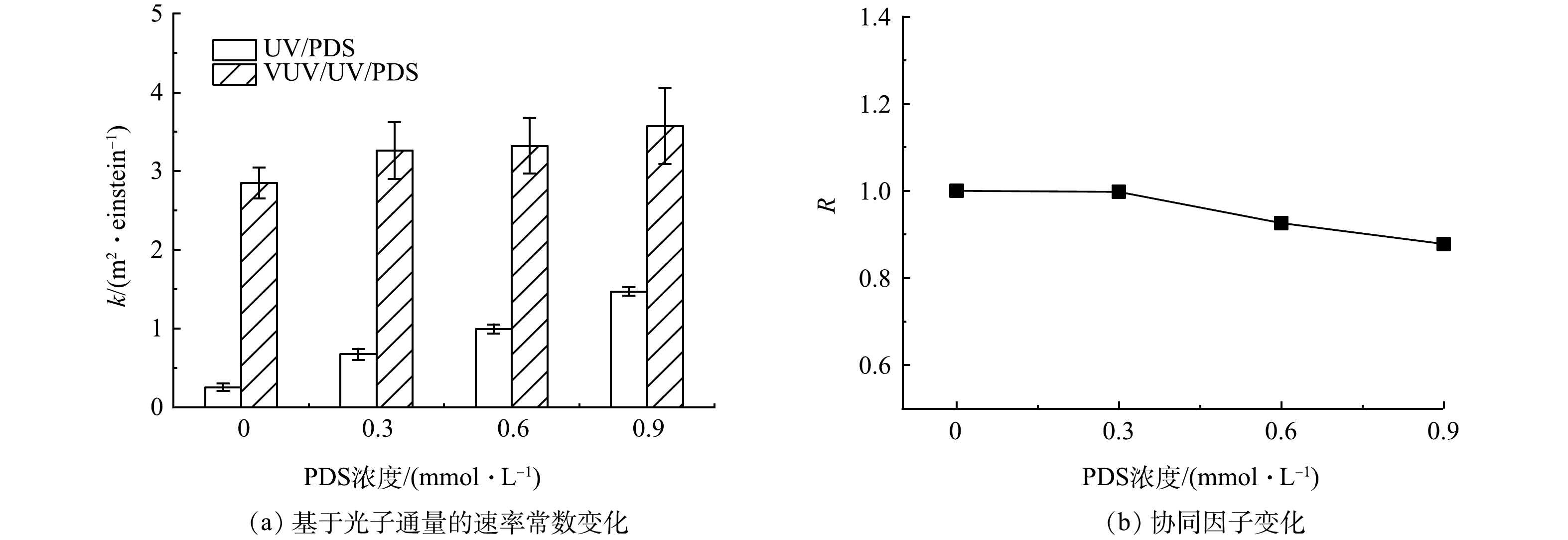
图4(a)为PDS浓度对UV/PDS和VUV/UV/PDS工艺对PFOA降解的影响。当PDS浓度为0、0.3、0.6和0.9 mmol·L−1时,UV/PDS和VUV/UV/PDS降解FPOA的速率常数也有所增加。VUV/UV/PDS工艺降解PFOA主要有以下3种途径:UV(254 nm)和VUV(185 nm)活化PDS产生的SO4−·和HO·的氧化降解、VUV(185 nm)直接光解、VUV光解水产生的HO·的氧化降解。由于在细管流光反应系统的VUV/UV和UV辐照中,UV剂量相同,因此,理论上,VUV/UV/PDS工艺对PFOA降解速率常数(kV/U/P),应该等于UV/PDS和VUV/UV工艺降解PFOA的速率常数之和(kU/P+kV/U)。如果联用工艺(VUV/UV/PDS)效果大于2个单独工艺,这说明发生协同降解作用。为了对联用工艺降解PFOA的强化效果进行定量评估,提出协同因子(R),其根据式(11)进行计算。
式中:kV/U/P,kU/P,kV/U分别为VUV/UV/PDS,UV/PDS,VUV/UV工艺降解PFOA的基于光子通量的伪一级速率常数。
如图4(b)所示,当PDS浓度为0、0.3、0.6和0.9 mmol·L−1时,R分别为1、0.99、0.93和0.88,呈现逐渐下降的趋势。这说明尽管VUV/UV/PDS工艺相对于UV/PDS有强化效果,但从上述3个主要的反应机制角度看,本工艺并未发生明显的协同强化效应,且随着PDS药剂投加量的增加,发生了一定的抑制效应。抑制效应主要源于2个方面。首先,由式(12)可知,PDS与SO4−·反应会生成氧化能力较弱的次生自由基,从而抑制SO4−·的活性[30]。其次,增加的PDS浓度会产生更高的VUV(185 nm)吸光度,竞争更多VUV光子,减弱VUV对PFOA的直接光解作用。
-
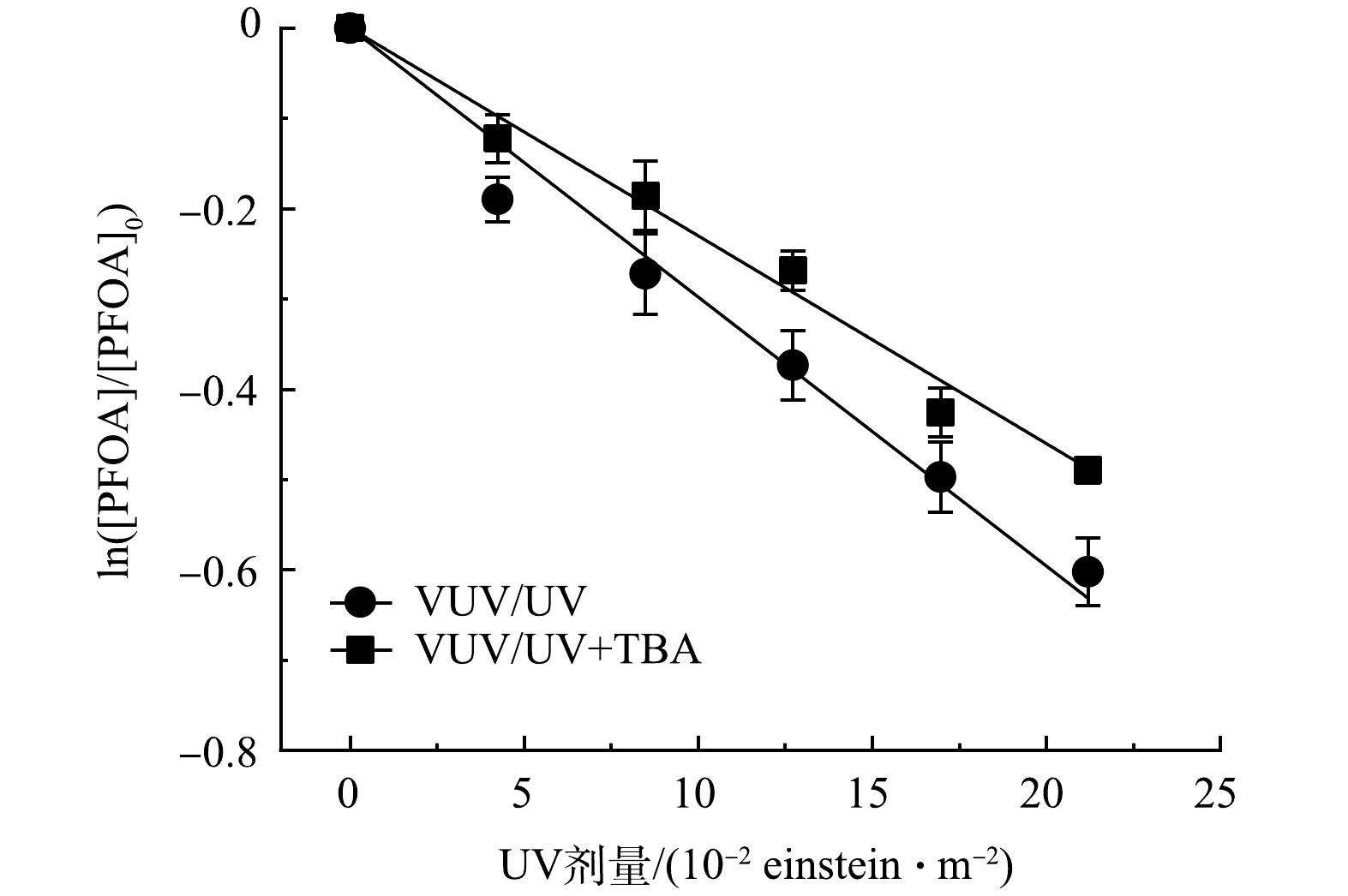
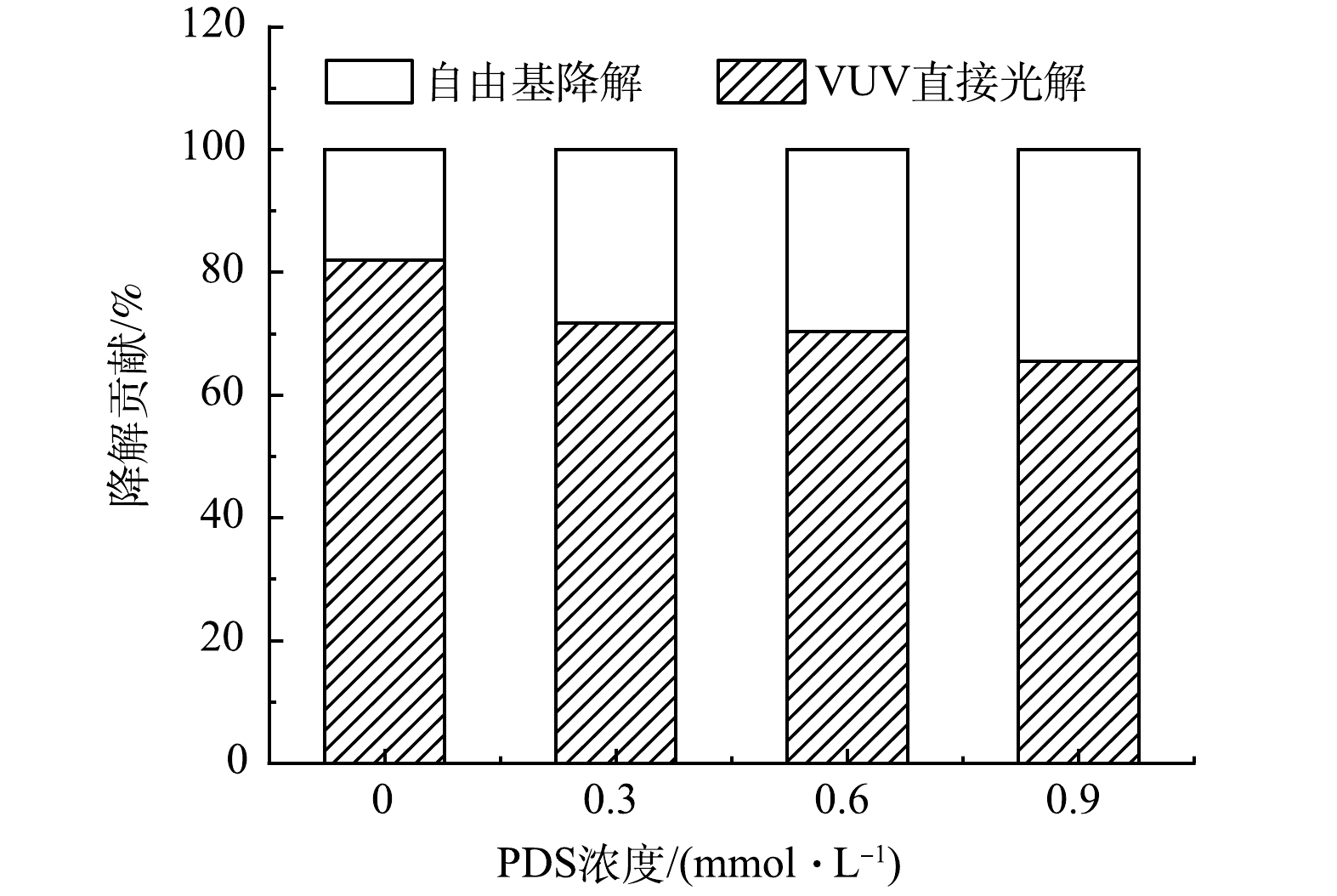
为定量评估VUV直接光解及自由基(HO·和SO4−·)间接氧化对PFOA降解的贡献,通过添加叔丁醇(10 mmol·L−1 TBA)对HO·进行淬灭[31]。如图5所示,VUV/UV工艺降解PFOA过程中,降解速率常数由2.85 m2·einstein−1降至2.34 m2·einstein−1。速率常数减弱表明HO·氧化对PFOA的降解贡献较少,仅占18%。而由于VUV光子较高的能量,可有效降解PFOA,其在VUV/UV直接辐照工艺中占主导作用(82%)。
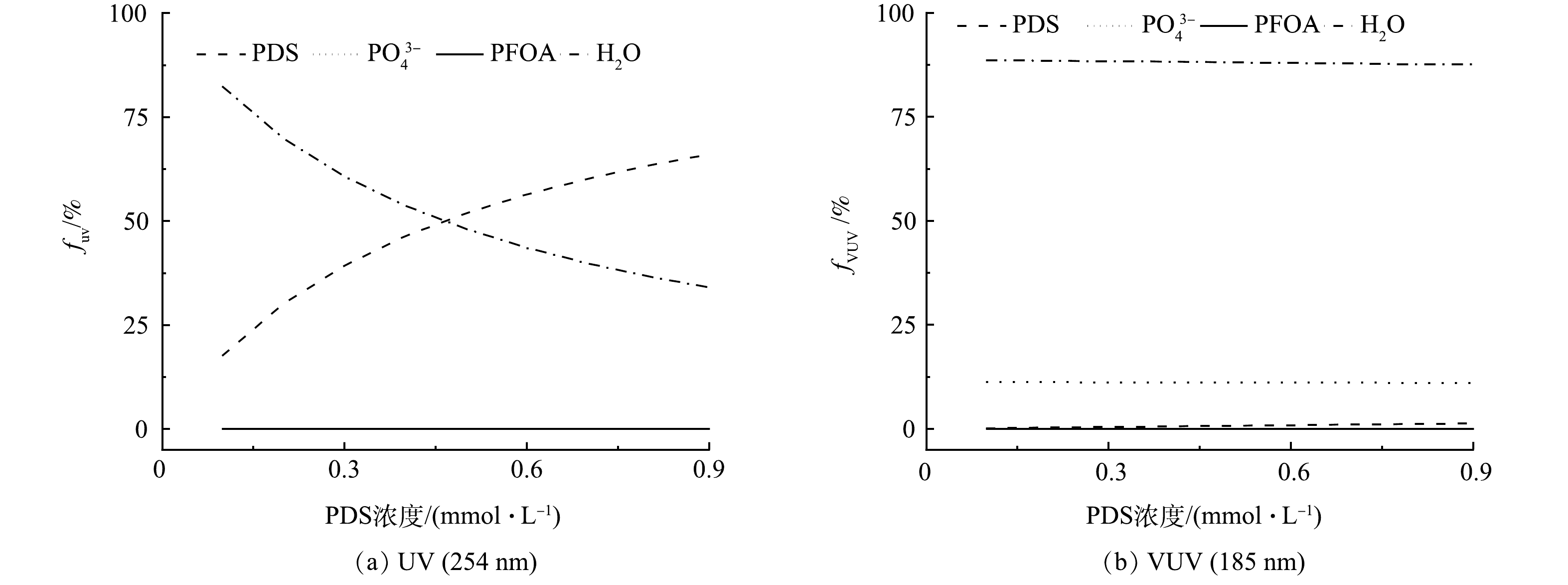
PFOA降解贡献结果表明(图6),随着PDS投加量的增加,自由基(HO·和SO4−·)对PFOA的降解贡献逐渐增大。为了深入探讨VUV/UV/PDS工艺对PFOA的降解机制,对UV和VUV光子在工艺溶液中各组分(磷酸盐(PO43-)、H2O、PFOA和PDS)的吸收分布(fUV和fVUV)进行了分析,结果见图7(a)。随着PDS投加量的增加,PDS对UV光子吸收比例由17.73%上升到65.99%,而对VUV光子吸收比例由0.14%上升到1.33%,SO4−·的生成量提高,从而强化SO4−·对PFOA的降解的贡献。同时,随着PDS浓度的增大,一部分VUV光子被PDS竞争吸收,导致PFOA对VUV光子的吸收率逐渐下降(吸收比例由0.23%降至0.19%),VUV对PFOA的直接光解作用逐渐减小。由于VUV直接光解的效率和贡献较高,因此,导致了协同R值的降低。综上所述,相对于UV/PDS工艺,VUV/UV/PDS工艺可在不增加能耗的情况下,通过额外VUV辐照,明显强化PFOA的降解效果。然而,由于作为主要活性基团HO·对PFOA的降解效果较差,工艺整体的效果仍有提高的潜力。
-
1)通过DPD-POD分光光度法和化学感光剂尿苷测定细管流光反应系统中VUV和UV的辐照强度分别为1.16×10−4 einstein·(m2·s)−1和1.39×10−3 einstein·(m2·s)−1。
2)通过比较不同工艺对PFOA的降解效果,发现PFOA降解率由高到低的工艺排序为VUV/UV/PDS、VUV/UV辐照、UV/PDS和UV辐照。相对于单独UV和VUV/UV辐照,PDS的投加会生成SO4−·,可强化对PFOA的降解。由于额外的185 nm VUV光子辐照,VUV/UV/PDS工艺相对于UV/PDS工艺对PFOA降解具有明显的强化作用。
3)当PDS浓度为0、0.3、0.6和0.9 mmol·L−1时,协同因子R均低于1(从1降低至0.88),表明尽管VUV/UV汞灯可明显强化UV/PDS工艺对PFOA的降解去除,但PDS和VUV/UV的联用并没有明显协同作用。主要作用机制在于PDS浓度的提升会增加SO4−·的生成,但同时PDS竞争吸收VUV光子导致PFOA的直接VUV降解作用减弱,导致PFOA降解的协同因子有所降低。
4)相对于UV/PDS工艺,VUV/UV/PDS工艺在不增加能耗的情况下,可通过VUV直接光解和VUV光解PDS生成的SO4−·,强化PFOA降解。本研究将为新型光源VUV/UV汞灯应用于PFASs的去除提供理论和应用支持。
真空紫外/紫外/过硫酸盐工艺降解水中全氟辛酸
Aquatic perfluorooctanoic acid degradation by a vacuum-ultraviolet/ ultraviolet/ persulfate process
-
摘要: 全氟辛酸(PFOA)是一种水中检出频率较高的全氟和多氟烷基物质,常规水处理工艺难以有效去除。紫外(UV)/过硫酸盐(PDS)工艺对PFOA具有较好的处理效果。相对于传统低压汞灯,新型光源真空UV/UV(VUV/UV)汞灯,可在不增加电能输入下,额外输出185 nm VUV光子有效光解PFOA。因此,采用VUV/UV汞灯驱动UV/PDS工艺具有高效降解PFOA的潜力。选用前期研发的配装VUV/UV汞灯的细管流光反应系统开展研究,通过微量过氧化氢生成法和化学感光剂(尿苷)测定VUV和UV辐照强度分别为1.16×10−4 einstein·(m2·s)−1和1.39×10−3 einstein·(m2·s)−1。结果表明,相对于单独UV和VUV/UV辐照,PDS的投加会生成SO4−·,进而强化PFOA的降解。由于额外的185 nm VUV光子辐照,VUV/UV/PDS工艺相对于UV/PDS工艺具有明显强化降解作用。当PDS浓度在0~0.9 mmol·L−1时,协同因子(R)均低于1,表明尽管VUV/UV汞灯可强化UV/PDS工艺对PFOA的去除,但PDS和VUV/UV的联用并没有明显的协同作用。PDS浓度的提升会增加SO4−·的生成,强化自由基降解的贡献(18%上升为35%),但同时竞争吸收VUV光子导致PFOA的直接VUV降解作用减弱(82%下降为65%),总体PFOA降解的协同效果有所减弱。以上研究结果表明VUV的加入可强化UV/PDS工艺去除PFOA的效率,为VUV/UV/PDS工艺应用于水中PFOA高效去除提供参考。
-
关键词:
- VUV/UV/PDS工艺 /
- 全氟辛酸 /
- 剂量测定 /
- 光子吸收 /
- 降解贡献
Abstract: Perfluorooctanoic acid (PFOA) is a kind of perfluoroalkyl and polyfluoroalkyl substance with high detection frequency in water, which cannot be effectively removed by the conventional water treatment processes. The ultraviolet (UV) /persulfate (PDS) process has a good treatment effect on PFOA. Compared with traditional low-voltage mercury lamps, the new light source of vacuum UV/UV (VUV/UV) mercury lamp can effectively photolyze PFOA by outputting additional 185 nm VUV photons without increasing the electric power input. Therefore, the UV/PDS process driven by VUV/UV mercury lamp has the potential to degrade PFOA efficiently. The mini-fluidic photoreaction system equipped with VUV/UV mercury lamp developed previously was used in this research. The VUV and UV irradiation intensities determined by micro hydrogen peroxide generation method and chemical actinometry (uridine) were 1.16×10−4 and 1.39×10−3 einstein·m−2·s−1, respectively. According to the results of PFOA degradation by different processes, the addition of PDS could generate SO4−· to enhance the degradation of PFOA comparing to individual UV or VUV/UV processes. Because of additional 185 nm VUV photon irradiation, the VUV/UV/PDS process could significantly enhance the PFOA degradation than the UV/PDS process. When the concentrations of PDS were 0~0.9 mmol·L−1, the values of synergistic factor R were all below 1, indicating that although the VUV/UV mercury lamp could enhance the removal of PFOA by UV/PDS process, the combination of PDS and UV/UV has no obvious synergistic effect. The increase of PDS concentration could increase the generation of SO4−· and strengthen the contribution of free radical degradation (18% to 35%), but PDS could also absorb VUV photons, which weakened the direct VUV degradation of PFOA (82% to 65%) and the overall synergistic effect of PFOA degradation. This study shows that the addition of VUV strengthens the UV/PDS process and significantly improves the removal efficiency of PFOA, which provides a theoretical reference for the application of the VUV/UV/PDS process to efficiently remove PFOA in water. -

-
-
[1] RAHMAN M F, PELDSZUS S, ANDERSON W B. Behaviour and fate of perfluoroalkyl and polyfluoroalkyl substances (PFASs) in drinking water treatment: A review[J]. Water Research, 2014, 50: 318-340. [2] ESFAHANI E B, MOHSENI M. Fluence-based photo-reductive decomposition of PFAS using vacuum UV (VUV) irradiation: Effects of key parameters and decomposition mechanism[J]. Journal of Environmental Chemical Engineering, 2022, 10(1). [3] ZAREITALABAD P, SIEMENS J, HAMER M, et al. Perfluorooctanoic acid (PFOA) and perfluorooctanesulfonic acid (PFOS) in surface waters, sediments, soils and wastewater: A review on concentrations and distribution coefficients[J]. Chemosphere, 2013, 91(6): 725-732. doi: 10.1016/j.chemosphere.2013.02.024 [4] GIESY J P, KANNAN K. Global distribution of perfluorooctane sulfonate in wildlife[J]. Environmental Science & Technology, 2001, 35(7): 1339-1342. [5] KANNAN K, CORSOLINI S, FALANDYSZ J, et al. Perfluorooctane sulfonate and related fluorochemicals in human blood from several countries[J]. Environmental Science & Technology, 2004, 38(17): 4489-4495. [6] DEWITT J C, PEDENADAMS M M, KELLER J M, et al. Immunotoxicity of perfluorinated compounds: Recent developments[J]. Toxicologic Pathology, 2012, 40(2): 300-311. doi: 10.1177/0192623311428473 [7] LEE Y J, KIM M K, BAE J, et al. Concentrations of perfluoroalkyl compounds in maternal and umbilical cord sera and birth outcomes in Korea[J]. Chemosphere, 2013, 90(5): 1603-1609. doi: 10.1016/j.chemosphere.2012.08.035 [8] FENTON S E, DUCATMAN A, BOOBIS A, et al. Perfluoroalkyl and polyfluoroalkyl substance toxicity and human health review: Current state of knowledge and strategies for informing future research[J]. Environmental Toxicology and Chemistry, 2020, 40(3): 606-630. [9] BAABISH A, SOBHANEI S, FIEDLER H, et al. Priority perfluoroalkyl substances in surface waters: A snapshot survey from 22 developing countries [J]. Chemosphere, 2021, 273. [10] GIRI R R, OZAKI H, OKADA T, et al. Factors influencing UV photodecomposition of perfluorooctanoic acid in water[J]. Chemical Engineering Journal, 2012, 180: 197-203. doi: 10.1016/j.cej.2011.11.049 [11] LOGANATHAN B G, SAJWAN K S, SINCLAIR E, et al. Perfluoroalkyl sulfonates and perfluorocarboxylates in two wastewater treatment facilities in Kentucky and Georgia[J]. Water Research, 2007, 41(20): 4611-4620. doi: 10.1016/j.watres.2007.06.045 [12] VECITIS C D, PARK H, CHENG J, et al. Treatment technologies for aqueous perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA)[J]. Frontiers of Environmental Science & Engineering in China, 2009, 3(2): 129-151. [13] JAVED H, LYU C, SUN R N, et al. Discerning the inefficacy of hydroxyl radicals during perfluorooctanoic acid degradation[J]. Chemosphere, 2020, 247: 125-130. [14] HORI H, HAYAKAWA E, EINAGA H, et al. Decomposition of environmentally persistent perfluorooctanoic acid in water by photochemical approaches[J]. Environmental Science & Technology, 2004, 38(22): 6118-6124. [15] WACLAWEKS S, LUTZE H V, GRUBEL K, et al. Chemistry of persulfates in water and wastewater treatment: A review[J]. Chemical Engineering Journal, 2017, 330: 46-62. [16] DOGLIOTTI L, HAYON E. Flash photolysis of persulfate ions in aqueous solutions. Study of the sulfate and ozonide radical anions[J]. Journal of Physical Chemistry, 1967, 71(8): 2511-2516. doi: 10.1021/j100867a019 [17] DAVIES M J, GILBERT B C, THOMAS C B, et al. Electron spin resonance studies. Part 69. Oxidation of some aliphatic carboxylic acids, carboxylate anions, and related compounds by the sulphate radical anion (SO4Ÿ−)[J]. Journal of the Chemical Society Perkin Transactions, 1985, 8(2): 1199-1204. [18] HORI H, YAMAMOTO A, HAYAKAWA E, et al. Efficient decomposition of environmentally persistent perfluorocarboxylic acids by use of persulfate as a photochemical oxidant[J]. Environmental Science & Technology, 2005, 39(7): 2383-2388. [19] QIAN Y J, GUO X, ZHANG Y L, et al. Perfluorooctanoic acid degradation using UV-persulfate process: Modeling of the degradation and chlorate formation[J]. Environmental Science & Technology, 2016, 50(2): 772-781. [20] YIN P H, HU Z H, SONG X, et al. Activated persulfate oxidation of perfluorooctanoic acid (PFOA) in groundwater under acidic conditions [J]. International Journal of Environmental Research and Public Health, 2016, 13(6). [21] ZOSCHKE K, BORNICK H, WORCH E. Vacuum-UV radiation at 185 nm in water treatment - A review[J]. Water Research, 2014, 52: 131-145. doi: 10.1016/j.watres.2013.12.034 [22] CHEN J, ZHANG P Y, LIU J. Photodegradation of perfluorooctanoic acid by 185 nm vacuum ultraviolet light[J]. Journal of Environmental Sciences, 2007, 19(4): 387-390. doi: 10.1016/S1001-0742(07)60064-3 [23] BADER H, STURZENEGGER V, HOIGNÉ J. Photometric method for the determination of low concentrations of hydrogen peroxide by the peroxidase catalyzed oxidation of N, N-diethyl-p-phenylenediamine (DPD)[J]. Water Research, 1988, 22(9): 1109-1115. doi: 10.1016/0043-1354(88)90005-X [24] YANG L X, LI M K, LI W T, et al. A green method to determine VUV (185nm) fluence rate based on hydrogen peroxide production in aqueous solution[J]. Photochemistry and Photobiology, 2018, 94(4): 821-824. doi: 10.1111/php.12913 [25] GONZALEZ M G, OLIVEROS E, WORNER M, et al. Vacuum-ultraviolet photolysis of aqueous reaction systems[J]. Journal of Photochemistry and Photobiology C-Photochemistry Reviews, 2004, 5(3): 225-246. doi: 10.1016/j.jphotochemrev.2004.10.002 [26] OPPENLANDER T, SCHWARZWALDE R. Vacuum-UV oxidation (H2O-VUV) with a xenon excimer flow-through lamp at 172 nm: Use of methanol as actinometer for VUV intensity measurement and as reference compound for OH-radical competition kinetics in aqueous systems[J]. Journal of Advanced Oxidation Technologies, 2002, 5(2): 155-163. [27] 杨腊祥. 真空紫外/紫外降解饮用水中典型农药的机理和应用研究[D]. 北京: 中国科学院生态环境研究中心, 2018. [28] LI M K, QIANG Z M, HOU P, et al. VUV/UV/chlorine as an enhanced advanced oxidation process for organic pollutant removal from water: Assessment with a novel mini-fluidic VUV/UV photoreaction system (MVPS)[J]. Environmental Science & Technology, 2016, 50(11): 5849-5856. [29] 李垣皓. 真空紫外/热活化过硫酸盐对全氟辛酸的脱氟反应研究[D]. 杭州: 浙江大学, 2020. [30] BUXTON G V, GREENSTOCK C L, HELMAN W P, et al. Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (·OH/·O) in aqueous solution[J]. Journal of Physical and Reference Data, 1988, 17(2): 513-886. doi: 10.1063/1.555805 [31] HUANG J Y, WANG X, PAN Z Q, et al. Efficient degradation of perfluorooctanoic acid (PFOA) by photocatalytic ozonation[J]. Chemical Engineering Journal, 2016, 296: 329-334. doi: 10.1016/j.cej.2016.03.116 -



 下载:
下载: