-
铅作为一种有毒重金属,对人体心血管系统、神经系统、肾和肝、生殖系统、呼吸系统、骨骼有不良影响[1]. 根据GB 25466-2010铅、锌工业污染物排放标准,排放污水的总铅含量应低于1 mg·L−1. 超标含Pb2+废水应在处理达标后方可排放,避免危害生态和环境. 目前,含铅废水的处理方法包括膜分离法、化学沉淀法、吸附法、离子交换法和电化学法等[2]. 其中,吸附法由于操作简单、成本低和效率高等优点,被视为最有前途的重金属废水处理方法[3]. 研究表明,碳基材料如碳纳米管、石墨烯、生物炭非常适合作为重金属废水处理的吸附剂[4]. 生物炭作为一种利用生物质原料,在无氧或限氧条件下通过热解制备的吸附剂,具备良好的比表面积、发达的多孔结构以及丰富的羟基、羧基等官能团,对废水中的重金属离子有良好的吸附性能,且处理成本和操作费用较低,已广泛应用于污水净化处理领域[5]. 例如,黄菲等[3]以不同的菌糠废弃物为原料制备生物炭,其中平菇菌糠生物炭对Cu2+最大吸附容量达到77.32 mg·g−1;香菇菌糠生物炭对Cd2+最大吸附量为74.26 mg·g−1,菌糠生物炭可作为一种廉价高效的吸附剂应用于水体中Cu2+、Cd2+的去除. Wang等[6]以废弃柠条制备生物炭,对Pb2+和Cd2+的最大吸附量分别达到220.94 mg·g−1和42.43 mg·g−1,经粗略估算,生产1 t生物炭的成本约为70美元,制备成本低吸附效率高.
不同生物质原料制备的生物炭具备不同特性,对重金属吸附能力也存在差异,寻找廉价且高效的生物炭材料,是废水中重金属吸附脱除的研究热点[2]. 我国是水产大国,根据2022年《中国渔业统计年鉴》,中国水产甲壳类总产量超过800万t. 其中虾蛄海洋捕捞量为219709 t. 除鲜食外,主要加工成虾仁、罐头等产品,过程中会产生约30%—40%的加工副产物(主要为虾壳),虾壳有的用于提取钙质、甲壳素、虾青素、虾油等,有的被直接废弃,如不能加以合理利用,会造成一定的环境污染和大量资源的浪费[7]. 甲壳类废弃物中含有大量CaCO3和甲壳素/壳聚糖,有报道证明CaCO3和甲壳素/壳聚糖是很好的重金属吸附剂[8]. Ma等[8]以龙虾壳为原料制备生物炭,用于吸附水体中的Cu2+和Cd2+,对Cu2+和Cd2+的吸附容量分别达到74.1 mg·g−1、126 mg·g−1. Hopkins等[9]利用蟹肉加工副产品制备的生物炭去除硫酸盐溶液中的Cu2+,吸附量达到184.8 mg·g−1,性能超过了商用活性炭和木质纤维素生物炭. 目前还未见以虾蛄壳为原料制备生物炭作为吸附剂用于吸附Pb2+的报道. 因此,以量大、价格低且易获取的虾蛄壳为原料制备生物炭,开发高效的吸附剂,用于处理重金属污染水体,可为甲壳类废弃物资源化利用提供有效途径,同时减少对环境的影响,有着巨大的潜力和重要的实践意义.
本研究以虾蛄壳为原料制备生物炭,采用扫描电镜(SEM)、X射线能谱分析仪(EDS)、傅里叶变换红外光谱仪(FTIR)、X射线衍射仪(XRD)和Zeta电位分析仪等对生物炭进行分析表征. 研究了虾蛄壳生物炭吸附Pb2+的影响因素、吸附动力学和等温吸附曲线,并探讨了吸附机理,为吸附水中的Pb2+以及大量甲壳类废弃物的资源化利用提供一种可行的方法.
-
虾蛄(Oratosquilla oratoria)壳收集于位于中国浙江省舟山市的舟山国际水产城. 硝酸铅、氢氧化钠、盐酸、氯化钠、氯化钾、碳酸氢钠、硫酸钠、硝酸钠为分析纯,购自国药集团化学试剂有限公司. Pb2+标准溶液(1000 mg·L−1)由国家标准物质中心提供. 所有化学溶液均使用超纯水制备.
-
虾蛄壳用超纯水洗涤以除去杂质,在65 ℃烘箱中干燥24 h,采用振动式细胞级超微粉碎机(XDW-6J,济南达微机械有限公司,中国)进行粉碎,制得的虾蛄壳粉末标记为MSS,储存于干燥器中备用.
利用激光粒度分析仪(Bettersize,丹东百特仪器有限公司,中国)测得MSS的平均粒径为30.70 μm,测得MSS的蛋白质、脂肪、水分、灰分、甲壳素含量分别为34.57%±0.84%、0.52%±0.02%、5.99%±0.12%、42.00%±0.05%、17.08%±0.73%.
-
将MSS装入石英管中,用石英棉从石英管两端塞入堵住MSS,防止粉末飞溅. 将石英管置于管式加热炉(RP-100P/1 kW,宝应县瑞普电气设备厂,中国),N2气体以10 mL·min−1的流速通过石英管,设定升温速率为10 ℃·min−1,热解温度达到700 ℃后保持2.5 h. 冷却至室温后,从石英管中取出虾蛄壳生物炭(标记为MSSB),密封保存. 吸附Pb2+后的MSSB标记为MSSB-Pb.
-
采用X射线衍射仪(Mini Flex600,日本理学公司,日本)、扫描电子显微镜(Sigma 300,德国蔡司公司,德国)、X射线能谱分析仪(Sigma 300,德国蔡司公司,德国)、傅里叶变换红外线光谱仪(IRAffinity-1S,日本岛津公司,日本)对MSS、MSSB和MSSB-Pb的晶形结构、表面形貌、元素组成及表面官能团进行分析. 采用比表面积及孔径分析仪(NOVA 2000e,美国康塔仪器公司,美国)分析MSSB的比表面积、孔径以及孔体积. 采用Zeta电位仪(Nano-ZS90,英国马尔文公司,英国)测定MSSB在不同pH水溶液中的Zeta电位,并确定零点电荷. 将MSSB与超纯水以1:20(g:mL)固液比混合,搅拌1 h,室温下沉降1 h,用pH计(pH-3e,上海仪电科学仪器仪器股份有限公司,中国)测定上清液的pH值[10]. 采用马弗炉(KSL-1200,合肥科晶材料科技有限公司,中国)高温处理(800 ℃,2 h)测定MSSB的灰分含量[8]. 采用Boehm滴定法测定MSSB表面官能团的含量[2].
在探究吸附机理的过程中为测定离子含量的变化,将10 mg的MSSB分别添加到100 mL超纯水和100 mL的Pb2+浓度为200 mg·L−1的溶液中,置于摇床中,设定温度为25 ℃,转速140 r·min−1,振荡时间为24 h. 用0.22 μm的针头过滤器分离出生物炭,通过离子色谱仪(ICS-1100,赛默飞世尔科技有限公司,美国)测定2组样品滤液中SO42−浓度. 用电感耦合等离子体质谱仪(iCAP6300,珀金埃尔默仪器有限公司,美国)测定K+、Na+、Ca2+和Mg2+的浓度.
-
吸附过程:将一定量MSSB和100 mL的Pb2+溶液混合于250 mL锥形瓶,置于恒温摇床中,25 ℃和140 r·min−1下振荡24 h,除pH影响的实验外,所有实验的初始pH值均设置为5.5.
为了研究初始溶液pH的影响,用HCl和NaOH溶液将200 mg·L−1的Pb2+溶液调节至不同的初始pH值(2—5.5),并添加10 mg的MSSB.
为了研究MSSB添加量的影响,准确称量3、5、7、10、13、15、17、20、25 mg的MSSB,分别加到浓度为200 mg·L−1的Pb2+溶液中.
在共存离子对MSSB吸附Pb2+的影响实验中,共存离子的浓度为0.05 mmol·L−1. MSSB的添加量为10 mg,Pb2+的初始浓度为200 mg·L−1. 在共存污染物浓度的影响实验中,Pb2+的初始浓度为200 mg·L−1,共存的污染物浓度从10 mg·L−1增加到200 mg·L−1,MSSB的添加量为10 mg.
吸附平衡后,用0.22 µm针头过滤器过滤,分离出MSSB,使用原子吸收光谱仪(240FS AA,安捷伦科技有限公司,美国)测定滤液中最终的Pb2+浓度. 所有吸附实验均重复3次,并报告这些结果的平均值以解释结果.
使用以下公式计算MSSB的Pb2+吸附容量和去除效率.
Pb2+吸附容量表示为(公式1):
式中,Qe(mg·g−1)为Pb2+在MSSB上的吸附容量;C0(mg·L−1)和Ce(mg·L−1)分别为Pb2+的初始浓度和平衡浓度;V(L)为溶液体积;m(g)是MSSB的添加量.
Pb2+去除效率表示为(公式2):
式中,Re(%)为Pb2+去除效率,C0(mg·L−1)和Ce(mg·L−1)分别为Pb2+的初始浓度和平衡浓度.
-
在吸附动力学实验中,Pb2+初始浓度为200 mg·L−1,MSSB的添加量为10 mg,在0—24 h内连续测量Pb2+浓度随吸附时间的变化. 采用拟一级、拟二级动力学模型进行拟合与分析[2].
拟一级动力学模型计算公式为:
拟二级动力学模型计算公式为:
式中,qt(mg·g−1)和qe(mg·g−1)分别为t时刻(min)和平衡时刻(min)的吸附容量;K1(min−1)和K2(g·(mg·min)−1)分别代表拟一阶和拟二阶模型中的速率常数.
在吸附等温实验中,Pb2+初始浓度为50—1000 mg·L−1,MSSB的添加量为10 mg. 采用Langmuir模型和Freundlich模型进行拟合与分析[2].
Langmuir模型的计算公式为:
Freundlich模型的计算公式为:
式中,Qe(mg·g−1)和Ce(mg·L−1)分别为Pb2+的吸附量和平衡时Pb2+的浓度;kL(L·mg−1)为与吸附自由能有关的Langmuir模型常数;qm(mg·g−1)为Langmuir模型中吸附在平衡状态下的最大单分子吸附量;kF(L·mg−1)和n分别为与吸附量和吸附强度有关的Freundlich常数.
通过Langmuir模型中的KL计算RL,用RL值来判断吸附过程的难易程度. 引入RL来估计吸附过程的趋势,与Langmuir等温线模型互补,可表示为:
-
采用Origin Pro 8.0软件对吸附动力学和吸附等温线进行拟合. 采用IBM SPSS Statistics 26.0软件进行统计分析,数据分析采用单因素方差分析(ANOVA),采用Tukey检验,P<0.05为差异有统计学意义. 采用Jade 6.0软件进行XRD数据处理、检索和分析.
-
MSSB产率为38.71%±1.45%,与蟹壳生物炭的产率(38.74%)相近[11]. 1 t虾蛄壳可以生产虾蛄壳生物炭387.1 kg. MSSB的理化性质见表1. MSSB表现出良好比表面积和孔隙结构,这是因为高温有助于生物质孔隙中挥发性有机物的释放和孔隙结构的形成. MSSB具有较高的灰分含量(69.29%±0.12%)和较高的pH值(13.29±0.05),经过高温热解,虾蛄壳中的大部分有机物质分解,矿物主要残留在其中,因此灰分较高. 同时,热解的过程可以去除生物质中—OH、—COOH等酸性官能团,促进KOH、NaOH、MgCO3、CaCO3等矿物从生物炭中分离,从而获得较高的pH值[12]. 值得一提的是,MSSB的平均粒径仅为34.55 μm,显著低于文献报道的结果[13],Jin等[14]指出生物炭的吸附性能随粒径的减小而增大,MSSB较小的粒径在吸附过程中起到一定的助益. 采用Boehm滴定法来确定MSSB表面的酸性官能团,结果表明MSSB含有丰富的官能团. 在吸附的过程中官能团可与Pb2+相互作用,起到吸附Pb2+的效果.
-
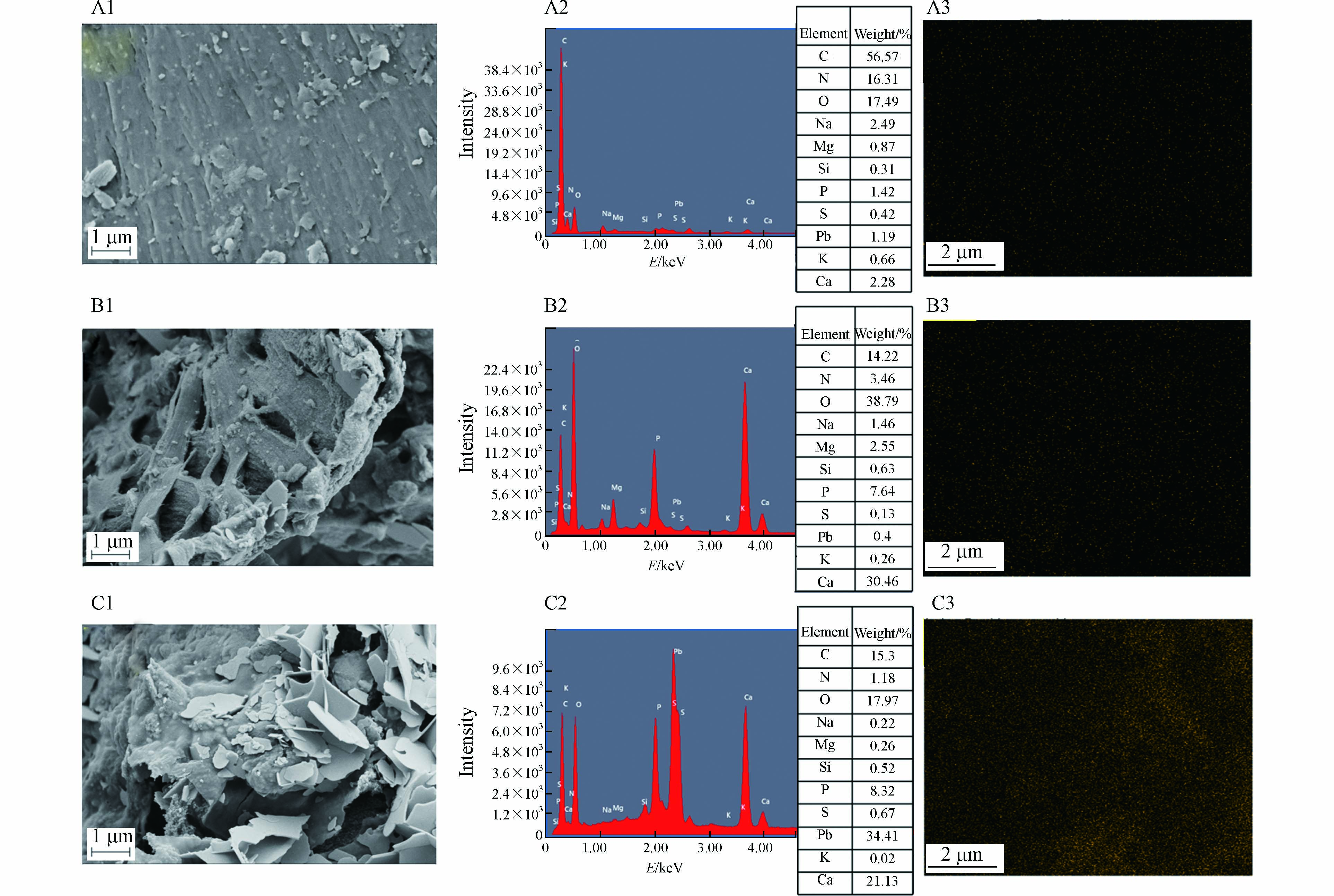
MSS、MSSB、MSSB-Pb的表观形貌利用SEM进行分析,结果见图1. 由图1(A1)可见,未经热解的MSS表面平整光滑并且致密,几乎没有孔隙结构的存在. 而经过在700 ℃下热解2.5 h的MSSB(B1)的表面变得粗糙,形成了清晰可见的一层层排列整齐的蜂窝状孔隙结构. 这样的孔隙结构可为MSSB吸附Pb2+提供大量可利用的吸附位点,起到吸附Pb2+的作用. MSSB-Pb的SEM图像(C1)与MSSB相比,观察到大量白色片状物质的存在,原有的孔隙结构被覆盖,有的甚至嵌入到MSSB孔隙结构的内部. 这与Zhang等[15]的SEM结果相似,在吸附Pb2+之后都出现了片状的物质.
用EDS-Mapping分析了MSSB吸附Pb2+前后的元素组成和Pb2+的分布,结果表明,扫描区域(C3)的Pb含量为34.41% wt,于未吸附前相比增加了34.01% wt. 这些现象说明Pb2+成功的被吸附到了MSSB上面. MSSB-Pb中、K、Na、Ca、Mg元素含量的减少可能归因于MSSB在吸附过程中的释放和Pb2+的吸附,O元素含量的减少可能是由于MSSB中含有的碳酸盐类或者金属氧化物参与了吸附,由此可推测,离子交换可能在吸附的过程中发挥了作用[15].
-
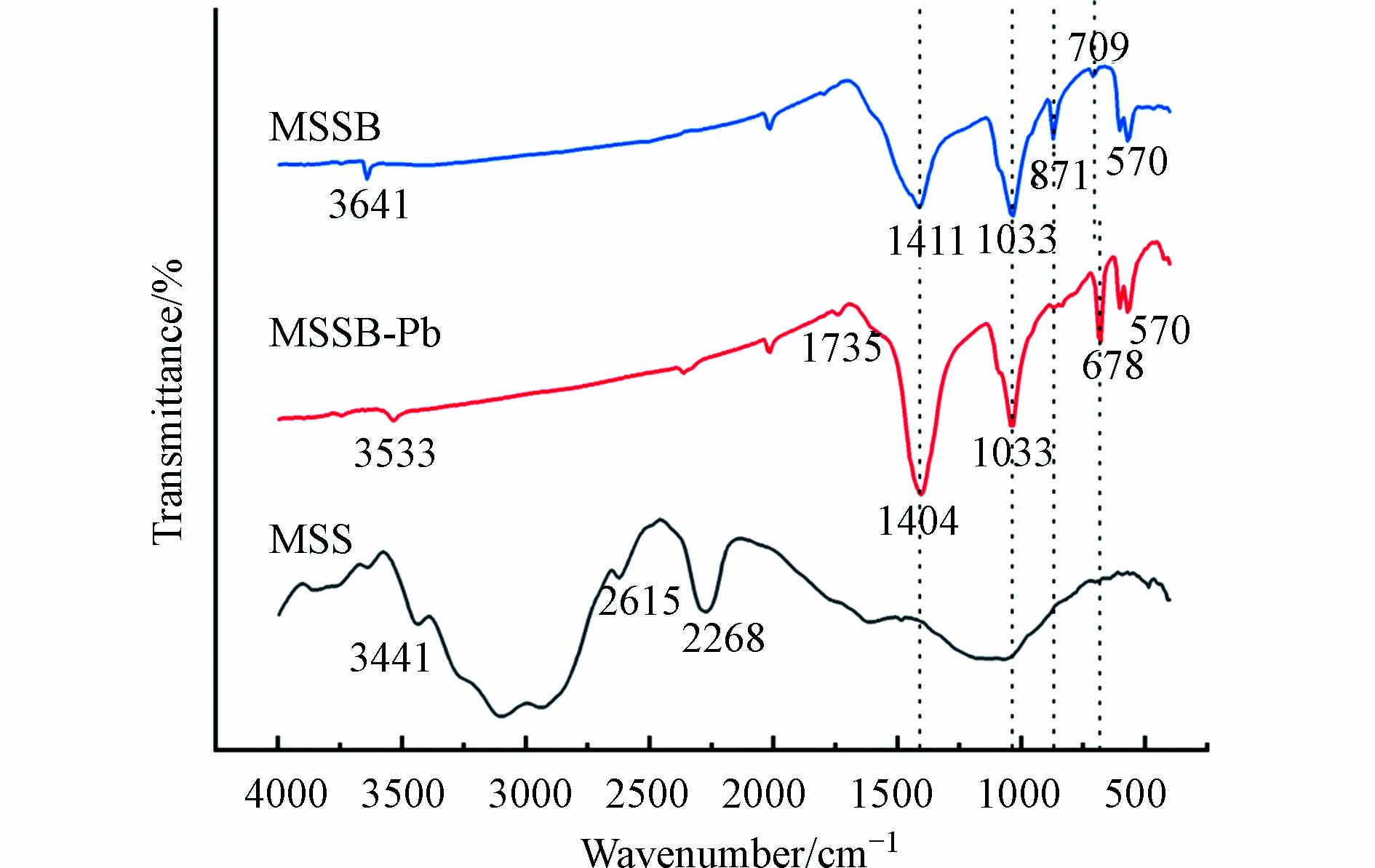
图2是MSS、MSSB、MSSB-Pb的FTIR谱图. MSS样品在3633 cm−1、3448 cm−1处的峰归因于O—H的伸缩振动. 由于虾蛄壳中存在源自脂类的脂肪族结构,2931 cm−1处的吸附峰归因于C—H不对称拉伸振动. 1620 cm−1附近的谱带归因于氨基中酰胺II中N—H的弯曲振动. 在1049 cm−1处显示出较宽的强度,这归因于C—O—C拉伸振动[8].
MSSB样品在1441 cm−1处产生的宽频带的新峰可归因于碳酸盐中C=O/C—O的存在. MSSB在1033 cm−1处显示出较强的强度,这归因于C—O—C拉伸振动. MSSB在871 cm−1的峰,是典型的碳酸钙结构[16],在606—570 cm−1处的条带属于磷酸盐中的O—P—O弯曲振动. MSS样品经过高温热解之后, 2931 cm−1、1789 cm−1、1620 cm−1和1049 cm−1处的峰均消失,可以理解为经过长时间的高温热解,MSS样品中的脂肪族化合物、蛋白以及糖类被分解[17],挥发性物质被去除,并且增加了MSSB的比表面积和孔隙率.
在MSSB吸附Pb2+之后,3641、1411、709 cm−1处的谱带分别移动到3533、1404、678 cm−1处,说明O—H、C=O/C—O、O—P—O键参与了MSSB对Pb2+的吸附[18]. 原本在871 cm−1处归属于碳酸钙结构的峰在吸附之后消失,说明碳酸钙参与了对Pb2+的吸附.
-
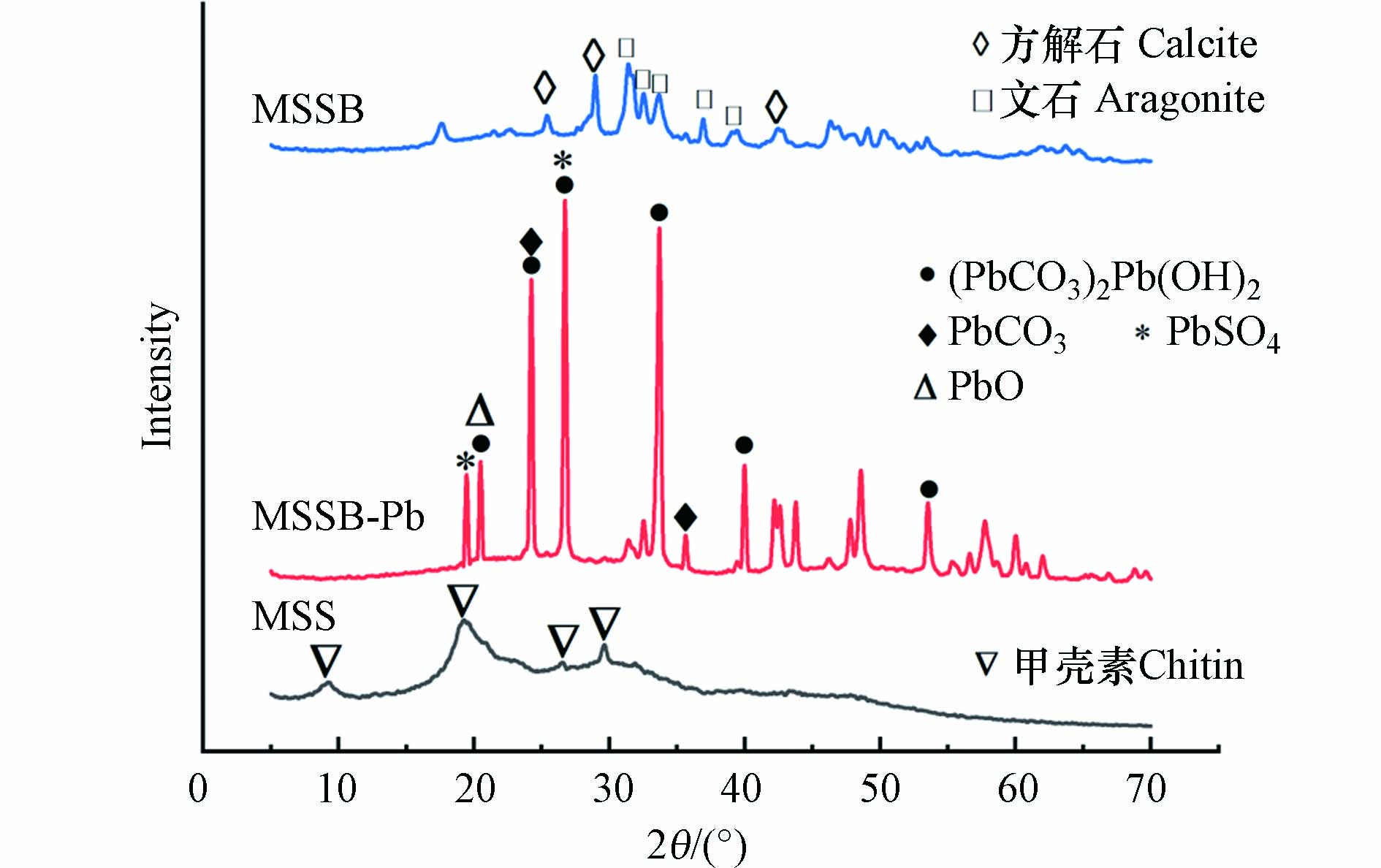
利用XRD对MSS、MSSB、MSSB-Pb的晶体结构进行分析,结果见图3. 从MSS的衍射图上可以观察到2θ=9°、19°、26°、29°的峰,这是甲壳素典型的晶体结构[19]. 经过高温热解之后,MSSB的XRD衍射图显示了方解石(2θ=24°、29°、39°、42°)和文石的特征峰(2θ=32°、33°、36°、39°),它们是CaCO3的晶体多晶型物[20].
MSSB-Pb的衍射图上出现了许多新的峰,主要包括Pb3(CO3)2(OH)2(2θ=20°、24°、27°、34°、40°和54°)以及少量的PbO(2θ=20°)、PbSO4(2θ=19°、27°)[21]和PbCO3(2θ=24°、36°)[20]. 结合SEM分析,证实白色的片状物质为含铅的沉淀物,也证实了Pb2+被MSSB成功的吸附,以及沉淀作用是MSSB吸附Pb2+的吸附机理之一.
-
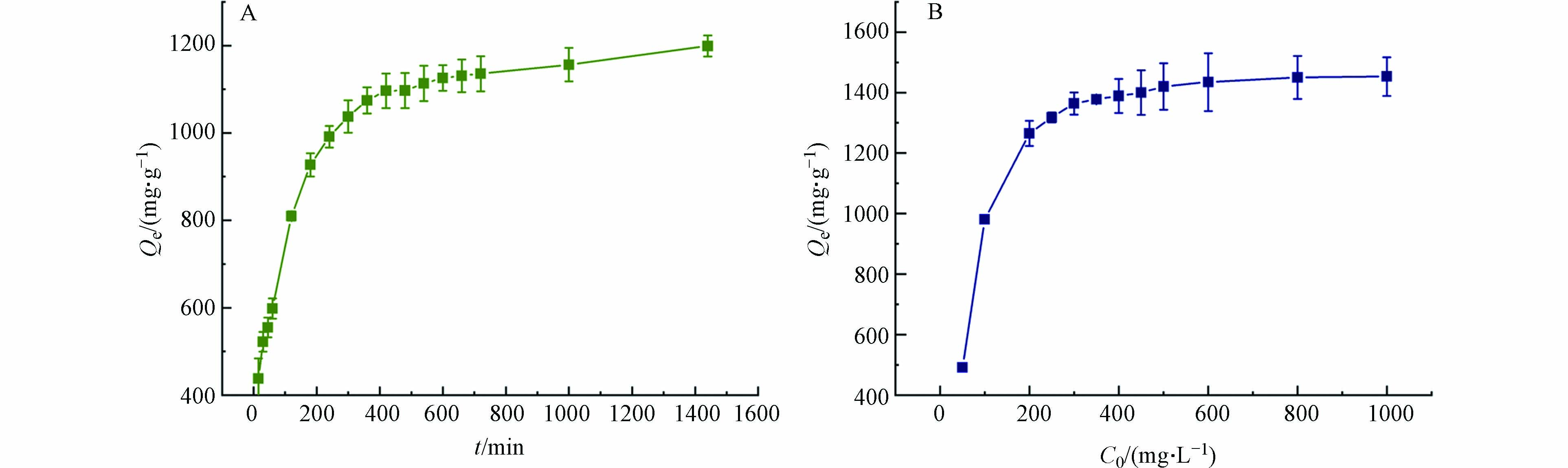
为了确定MSSB上Pb2+吸附的平衡时间,研究了吸附时间(5—1440 min)对吸附的影响,结果如图4A所示. MSSB对Pb2+的吸附大致可以划分为3个阶段,第一阶段为快速吸附阶段即随着吸附时间的增加,吸附容量快速增加,在约0—240 min内,吸附量达到了吸附总量的82.64%. 第二阶段,随着吸附时间的增加,吸附容量呈现缓慢增加的趋势,在约240—660 min内,吸附容量占总吸附量的11.63%,第三阶段为相对平衡阶段,及随着吸附时间的大量增加,吸附容量的变化平缓,达到相对平衡的阶段. 在约660—1440 min,长达780 min的吸附,吸附容量只占吸附总量5.73%. 可以解释为,在最初的240 min内,Pb2+与MSSB表面的吸附位点或者官能团等发生了强烈的相互作用,从而吸附容量快速增加,随着吸附时间的延长,吸附位点被Pb2+逐渐填满,官能团等参与吸附的物质逐渐被占用,因此,吸附容量的上升趋势也逐渐变得缓慢直至一个相对平衡的状态.
溶液中Pb2+的初始浓度同样也会影响MSSB对Pb2+的吸附,结果见图4B. 随着溶液的初始浓度从50 mg·L−1增加到400 mg·L−1,吸附容量快速增加. 随后变得缓慢直至平缓状态. 由于溶液中的MSSB的添加量固定,其可吸附位点有限,在低Pb2+浓度下,MSSB能将Pb2+完全吸附;而在高浓度下,过量的Pb2+使得MSSB的吸附位点饱和,使得MSSB对Pb2+的吸附容量逐渐达到平缓状态.
-
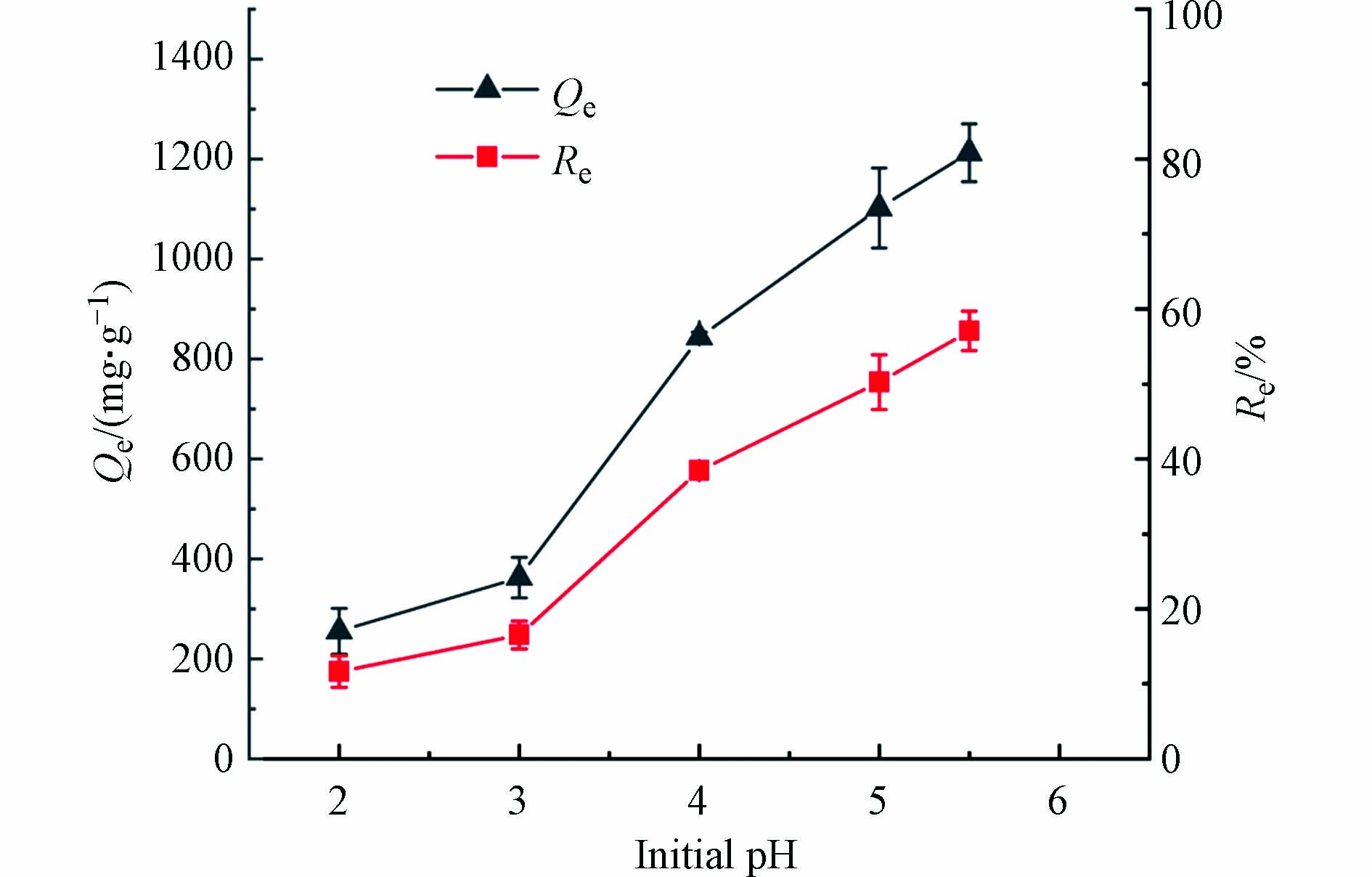
生物炭的表面电荷、矿物组分的溶解等都会受到pH的影响,而这些因素会影响生物炭对重金属的吸附性能. 因此,有必要研究溶液初始pH对MSSB吸附Pb2+的影响. 当溶液的pH值高于6时,Pb2+将以金属氢氧化物如Pb(OH)2等的形式存在[22]. 为了有效避免在吸附前溶液形成沉淀,影响测定结果,本研究仅在pH值为2—5.5时进行测定. 实验结果如图5,在pH=2.0时,对Pb2+的吸附容量非常低,仅为255.33 mg·g−1. 吸附容量随着初始pH值的增加而显著增加,在pH=5.5时,吸附容量达到1152.00 mg·g−1. 由于Pb2+和H+之间在吸附位点上的的竞争,在低pH条件下吸附容量相对较低[4],当pH增加时,Pb2+作为阳离子被有利的吸附. 另一方面,pH的升高更有利于重金属离子的水解以及MSSB表面的有机官能团的电离,促使MSSB与金属离子形成稳定的络合物[23].
-
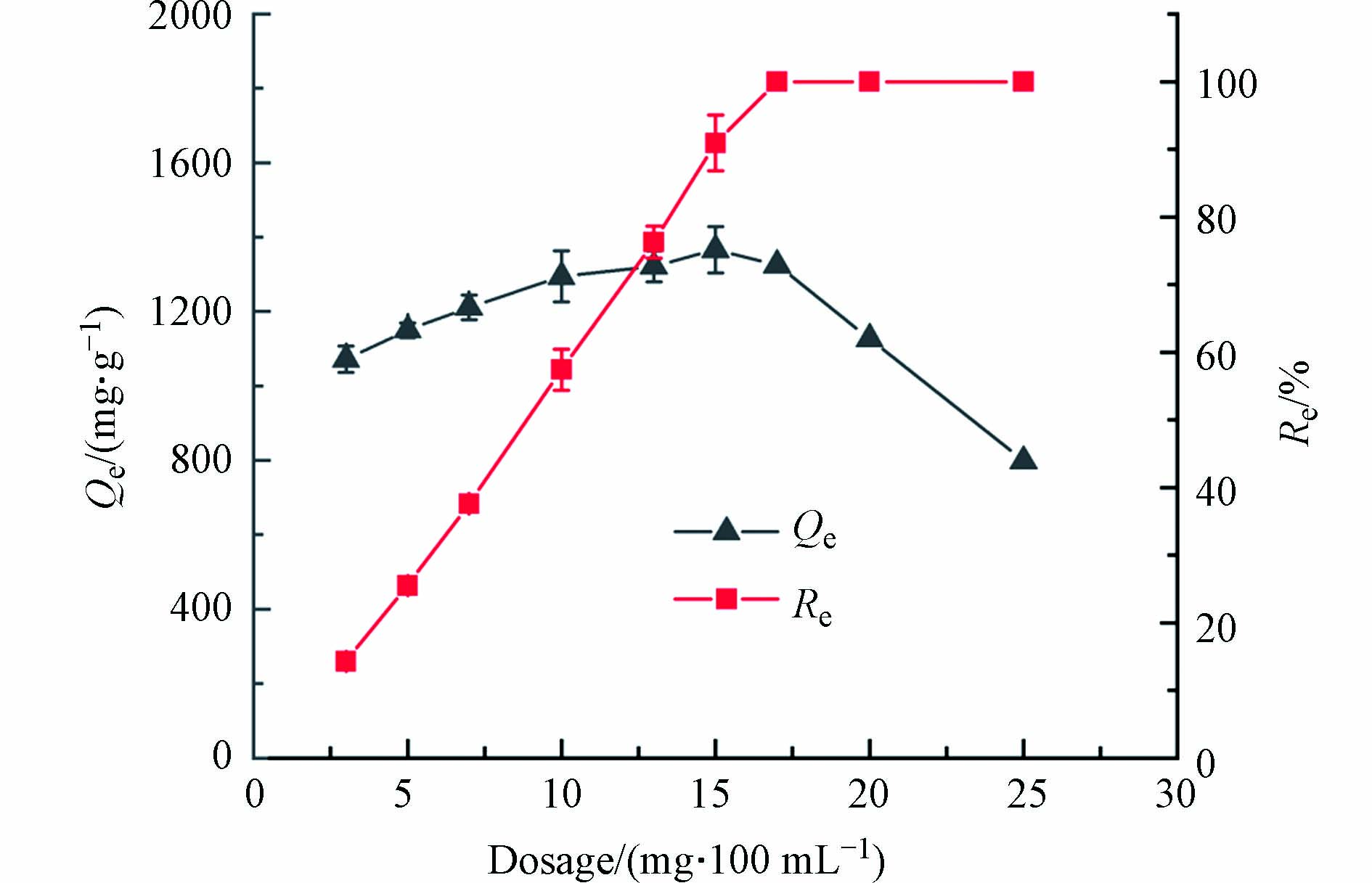
吸附剂添加量过少时,吸附不完全污染物残留较多;吸附剂添加量过多,分离吸附剂的成本会增加,也会导致吸附剂的浪费[24]. 图6为MSSB添加量对Pb2+吸附容量及去除率的影响. 随着MSSB添加量的增加,吸附容量表现出先升高后降低的趋势,而去除率呈现出逐渐升高直至平稳的趋势. 当MSSB的添加量大于到15 mg·100 mL−1时,吸附量开始下降,说明吸附系统中有未被占用的吸附剂,成本会增加. 因此MSSB添加量的临界点为15 mg·100 mL−1. MSSB的添加量增加时,去除率增大,但吸附容量降低,这可能是由于随着吸附剂添加量的增加,总吸附位点增多,在一定的Pb2+浓度下,吸附位点不能被完全占据,导致吸附容量降低. 当MSSB的添加量为15 mg·100 mL−1时,对Pb2+的吸附量最大,为1366.00 mg·g−1,此时去除率为90.92%. 综合考虑,最佳添加量为15 mg·100 mL−1.
-
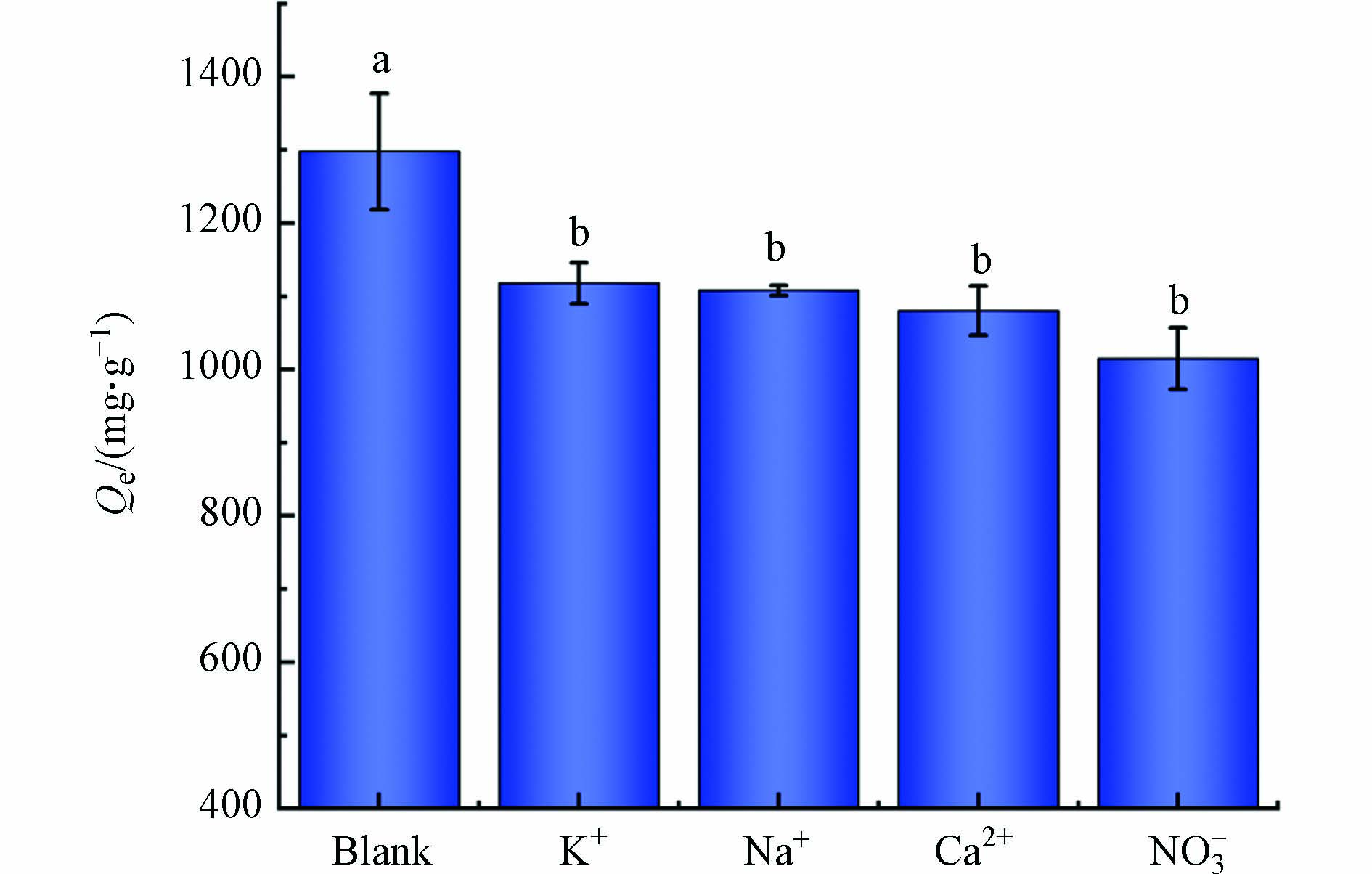
废水中存在多种离子,可能影响Pb2+的吸附. 因此,通过考虑不同的共存离子来评价MSSB对Pb2+吸附容量的影响. 结果见图7,实验表明,当溶液中存在K+、Ca2+、Na+、NO3−时,MSSB对Pb2+的吸附容量呈现不同程度的降低. 说明这些离子均会与Pb2+在生物炭上发生竞争吸附,可归因于生物炭的吸附位点被共存离子占用或堵塞. 同为阳离子,Ca2+的抑制作用比Na+和K+更明显,可能是因为Ca2+与Pb2+同为二价阳离子,在相同的活性位点上Ca2+更容易与Pb2+产生竞争. 此外,有研究表明Ca2+相较于K+和Na+具有更大的电荷密度,且对某些官能团有一定的络合能力,因此会占据更多的吸附位点[25]. Ca2+的电负性(1.00)大于Na+(0.93)和K+(0.82),具有更强的水合能力,能更有效地占据生物炭表面的活性位点[26]. 袁广翔等[27]研究了Ca2+与Na+对浆料Zeta电位的影响,发现与Na+相比,Ca2+更容易使吸附剂吸附过程的Zeta电位值过高甚至达到正值,从而影响吸附剂的性能. 其中NO3−的存在对MSSB吸附Pb2+的抑制作用相对明显,可能是NO3−的加入与Pb2+在吸附位点上产生了比K+、Ca2+、Na+更加激烈的竞争吸附或NO3−能在更大程度上干扰MSSB对Pb2+吸附. 此外有研究指出阴离子与生物炭的亲和力不同会导致吸附容量下降,推测NO3−的加入使吸附容量下降也与亲和力有关[28]. 共存离子对MSSB吸附Pb2+的抑制能力顺序为NO3−>Ca2+>Na+=K+.
-
在复杂的水体中,染料和重金属作为水体中常见的污染物之一,在吸附的过程中可能会影响生物炭对Pb2+的吸附,为了进一步探索MSSB在混合体系中的应用可行性,选择常见的阴离子偶氮染料甲基橙(MO)和Cu2+、Cd2+和Ni2+作为共存的污染物,以评估MSSB在二元体系中吸附Pb2+的能力.
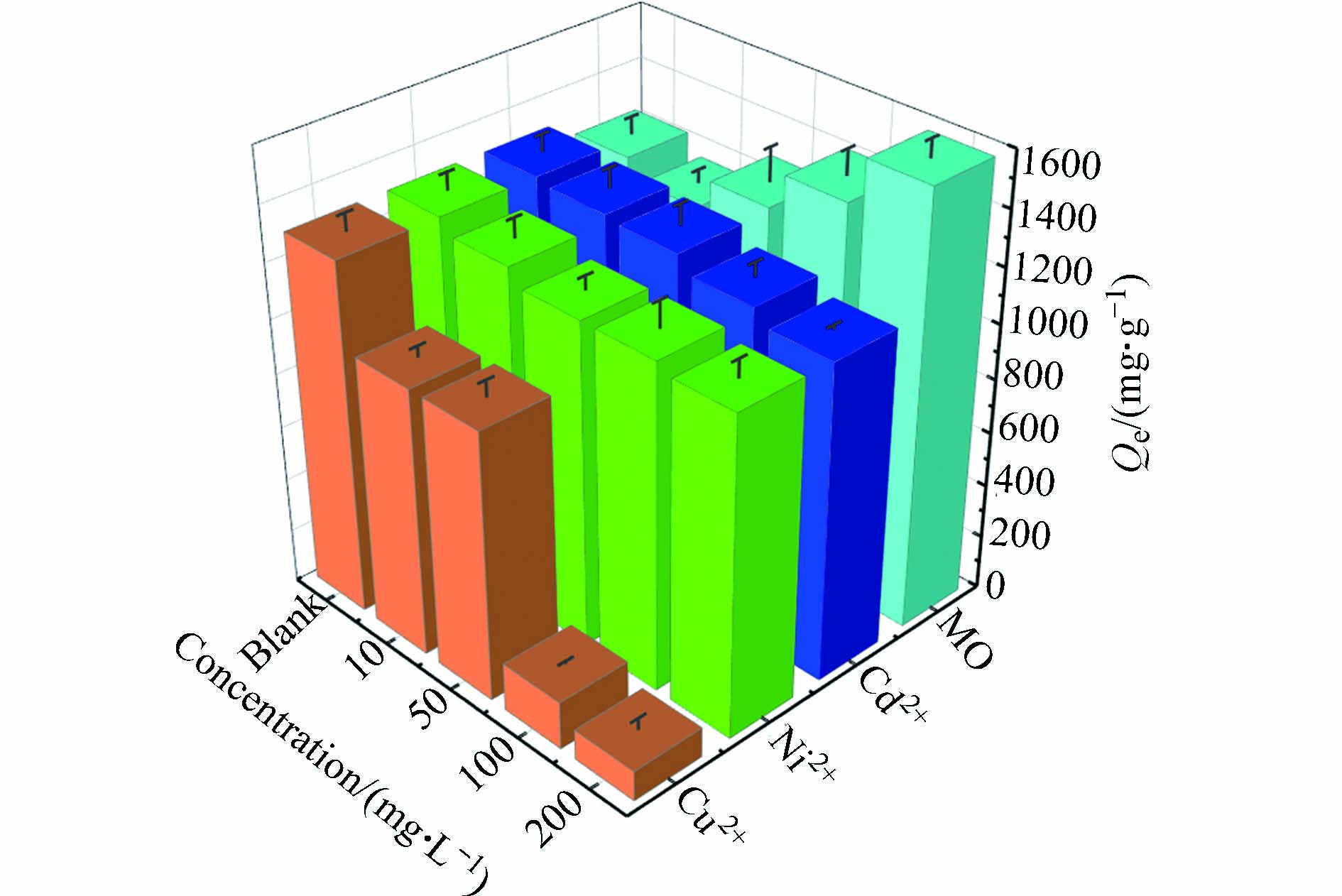
如下图8所示,Cu2+、Cd2+和Ni2+的存在都会在不同程度上抑制MSSB对Pb2+的吸附,并且随着共存金属离子的浓度的增加,抑制作用逐渐增强. 这其中Cu2+的抑制作用最明显,当共存的Cu2+浓度从10 mg·L−1增加到200 mg·L−1,吸附容量由1278.83 mg·g−1降低至112.17 mg·g−1. 这是因为MSSB提供的总吸附点位是一定的,在共存体系中,共存的金属离子与Pb2+在MSSB上产生了竞争吸附,会抢占MSSB表面的吸附位点,导致吸附容量降低. 在低浓度的条件下,共存金属离子对吸附位的竞争小,高浓度时离子强度增加了竞争,从而使MSSB对Pb2+的吸附量降低[29]. 从实验结果可知抑制程度为:Cu2+>Ni2+>Cd2+. 类似的,汪怡等[30]在研究中指出,在Cu-Pb复合体系中,吸附过程存在相互抑制作用,致使生物炭对Cu2+和Pb2+的吸附量较单一吸附均有下降. 相反的,张丹等[31]在研究中指出,在Cu2+和Pb2+共存的情况下,Cu2+的存在促进了Pb2+的吸附.
与共存金属离子不同的是,随着共存体系中MO浓度的不断增加,MSSB对Pb2+的吸附容量也在不断增加. 吸附容量从MO浓度为10 mg·L−1时的1162 mg·g−1增加到MO浓度为200 mg·g−1时的1582.67 mg·g−1,增加了36.20%. 这也证实了MO的存在会促进MSSB吸附Pb2+. 可能是MO与Pb2+这两种污染物在同一体系中产生协同作用,促进MSSB对污染物的吸附. Tovar-Gómez等[32]也报导了类似结果,制备吸附剂同时吸附酸性蓝25染料和Ni2+、Cd2+、Zn2+,酸性蓝25染料的存在显著有利于重金属的去除,吸附容量与单金属体系相比提高6倍.
-
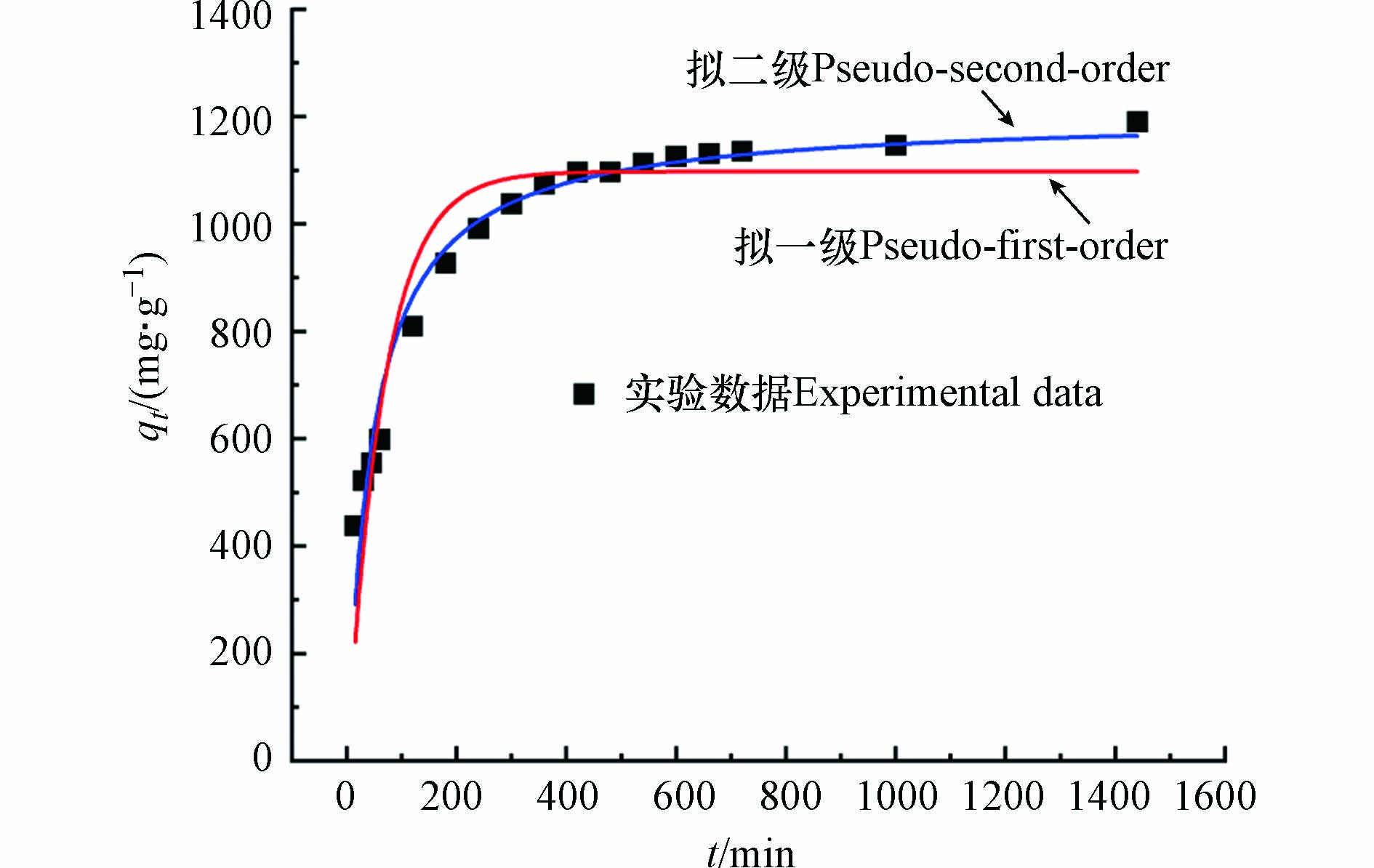
分别采用拟一级动力学模型、拟二级动力学模型对实验数据进行拟合. 吸附动力学模型拟合趋势线见图9,拟合结果见表2. 拟二级动力学方程的相关系数(R2=0.9646)高于拟一级动力学方程的(R2=0.8942)值,由拟二级动力学方程得到Pb2+的平衡吸附量为1202.28 mg·g−1,与实验结果(qe,exp)1199.49 mg·g−1的差距更小. 表明MSSB对Pb2+的吸附行为更符合准二级动力学模型. MSSB对Pb2+的吸附是化学吸附为主,即Pb2+与MSSB之间可能通过共用作用或交换电子形作用成化学键[4].
-
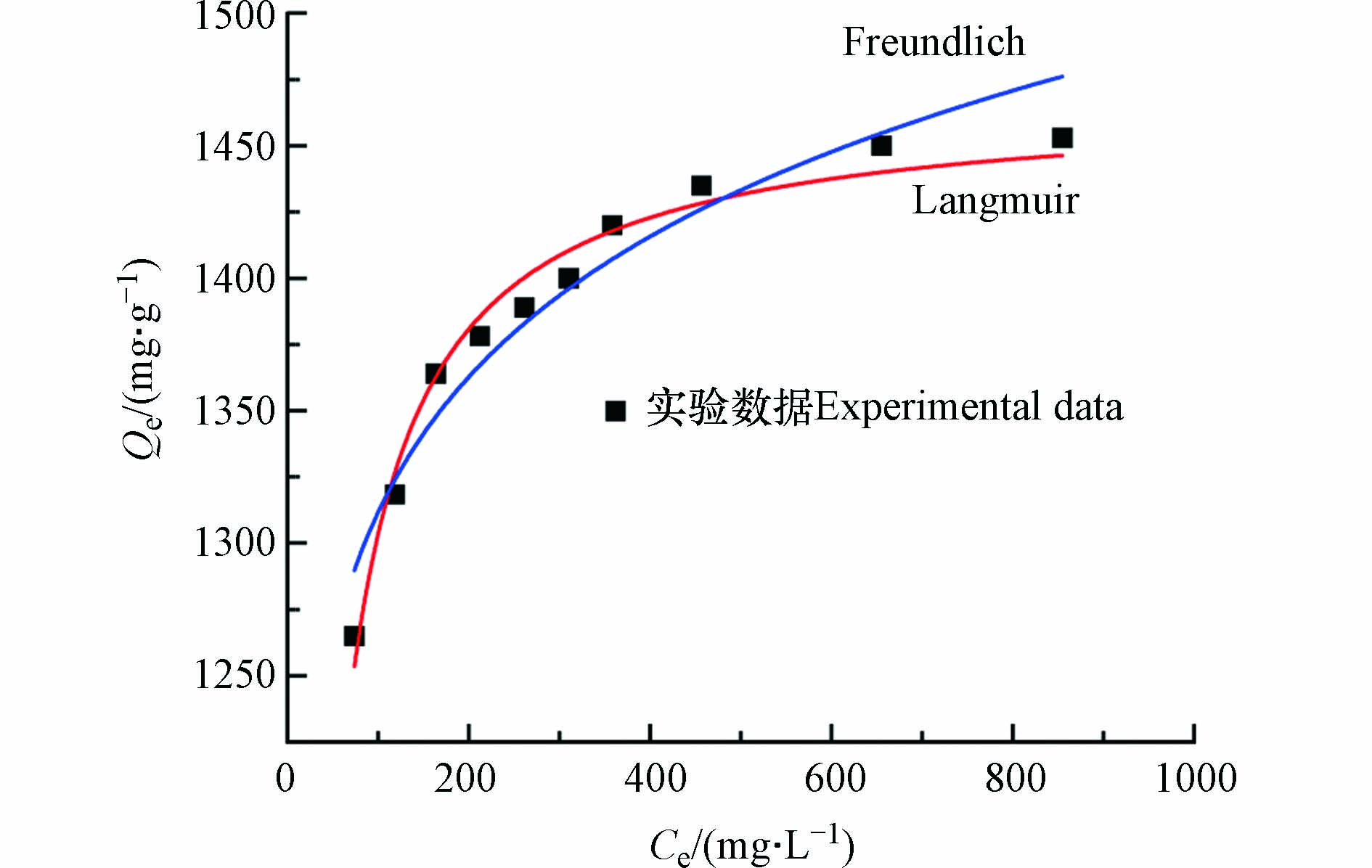
Langmuir模型和Freundlich模型对MSSB吸附Pb2+的等温吸附模型的拟合结果如图10,相关参数见表3所示. 从R2看,Pb2+的等温去除更符合Langmuir模型,表明MSSB对Pb2+吸附主要为单分子层吸附. 用分离因子RL可以进一步说明MSSB的吸附性能,Langmuir模型中的常数RL值来判断吸附剂在吸附过程的难易程度. RL的取值有4种情况:RL=0;0<RL<l;RL=1和RL>1,分别表示不可逆、有利、线性和不利[30]. 经计算,MSSB吸附Pb2+的分离系数RL的范围分别为0.01—0.20,说明MSSB对Pb2+的去除是有利的.
由各种原材料制备的生物炭及其对Pb2+的最大吸附量(qm,cal)见表4. 通过比较可以看出,相比已经报道的文献,利用虾蛄壳制备的生物炭对水中Pb2+的吸附容量虽然不是最高的,但是具有明显的优势. 首先虾蛄壳生物炭对Pb2+的吸附容量比已经报导的大部分的生物炭要高. 且与部分文献报道的结果相比,未经改性处理的虾蛄壳生物炭对Pb2+的吸附容量比其他文献中改性后的吸附容量还要大. 由此可见,就吸附容量而言,虾蛄壳生物炭是具有一定优势的. 因此,MSSB作为一种有效的吸附剂应用于废水处理领域有着巨大的前景.
-
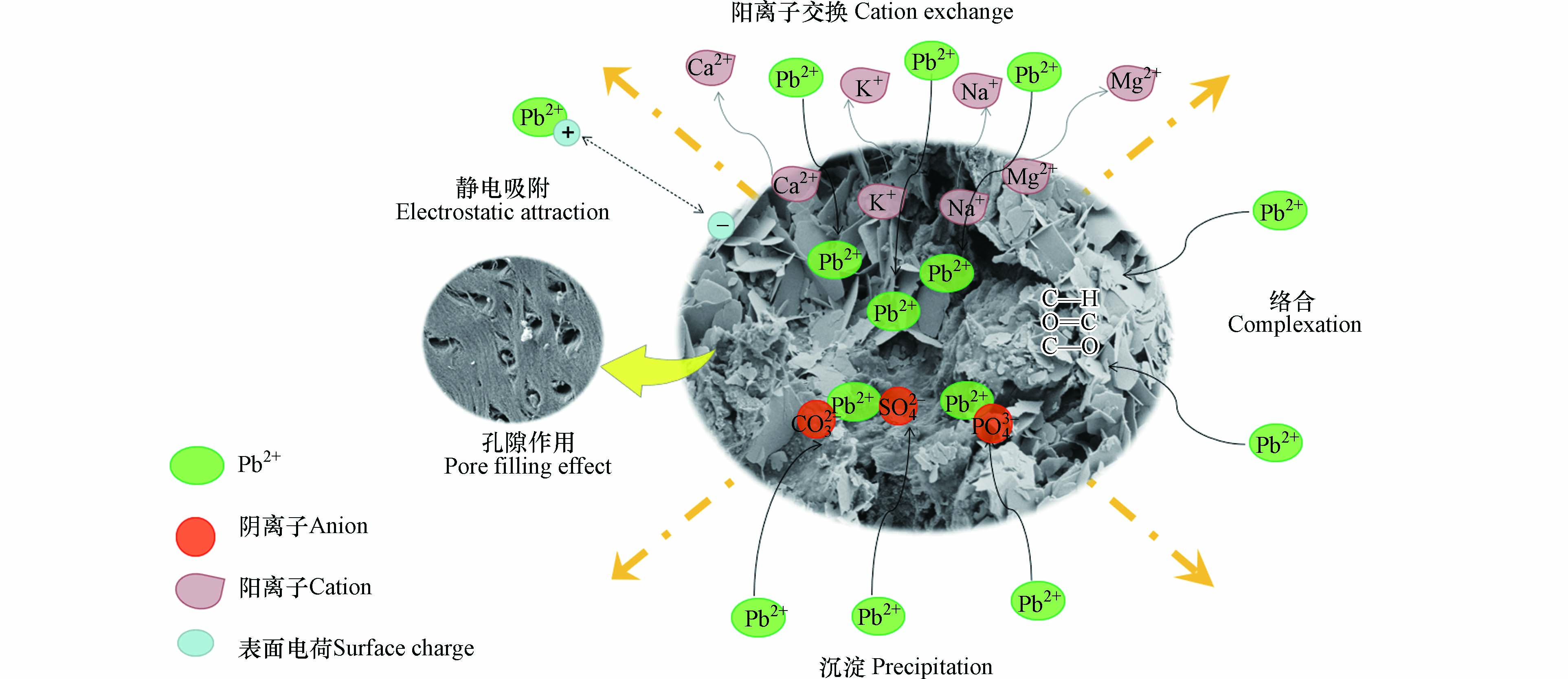
生物炭去除重金属涉及多种机制,包括重金属与阴离子(如CO32−、SO42−和OH−)的沉淀,重金属与阳离子(如K+、Na+、Ca2+和Mg2+)之间的离子交换,以及金属离子与官能团的络合作用等. 例如,Yang等[33]以微藻残渣为原料制备生物炭用于吸附Pb2+,并且对附机理进行了定量分析,指出矿物质的相互作用(沉淀与矿物和金属离子交换)占主导地位,其次是官能团的贡献. Du等[48]使用玉米秸秆和废铁制备了多孔磁性生物炭,以去除溶液中的Pb2+,并揭示还原、络合和沉淀是主要吸附机制.
-
首先,EDS-Mapping结果表示,MSSB-Pb的K、Na、Ca、Mg含量较MSSB均下降. 为了进一步说明离子交换机理,测量MSSB在吸附Pb2+前后溶液中的离子含量. 由表5可见,吸附Pb2+后,Mg2+、K+、Ca2+、Na+浓度都比未吸附时增加. 实验结果表明,在吸附的过程中Mg2+、K+、Ca2+、Na+从MSSB中释放到溶液中,再与Pb2+发生离子交换作用,从而使Pb2+顺利的被吸附到MSSB上.
-
通过对比FTIR,MSSB在871 cm−1处归属于碳酸钙结构的峰在吸附Pb2+之后消失了,这说明碳酸钙参与了对Pb2+的吸附. 其次,通过XRD检测到MSSB-Pb出现许多新的峰,这些峰主要对应的是PbCO3·Pb(OH)2、PbCO3、PbSO4与PbO. 这些成分的存在可归因于Pb2+与MSSB在吸附过程中释放的可溶性阴离子结合而产生沉淀[33]. 例如,PbCO3的产生,是因为CaCO3 (Ksp=3.36×10−9)具有比和PbCO3 (Ksp=7.40×10−14)更大的溶解度,揭示CaCO3可能在Pb2+溶液中转化为和PbCO3,而PbCO3与OH−结合,又可以产生(PbCO3)2Pb(OH)2[16]. 此外,采用离子色谱仪测定了MSSB在吸附Pb2+后滤液中相应离子的SO42−浓度. 如表5,在MSSB吸附Pb2+之后,滤液中的SO42−的含量有所下降,这也证实了阴离子SO42−参与了沉淀作用.
-
FTIR说明Pb2+在生物炭表面的络合机理. 在MSSB吸附Pb2+之后,3641、1411、709 cm−1处的谱带分别移动到3533、1404、678 cm−1处,证实O—H、C=O/C—O、O—P—O键参与了MSSB对Pb2+的吸附. 此外,与使用超纯水的空白对照相比(pH=5.82±0.04),吸附Pb2+后(pH=5.60±0.02)溶液的pH值更低,这可能是由于Pb2+与MSSB中存在的—COOH和—OH等官能团络合,产生了络合作用促进了吸附的发生[49].
-
孔隙作用,是指Pb2+扩散到生物炭的孔隙中的物理作用,首先,通过比表面积分析的结果可知MSSB具有良好的比表面积空隙结构,可为Pb2+的吸附提供吸附位点,其次通过对比MSSB与MSSB-Pb的SEM图像,吸附过后孔隙结构不复存在,而大量的铅散布在其中,可知孔隙在吸附过程中发挥了一定作用. 其次,实验在初始pH为5.5的条件下进行,经测定MSSB的零点电荷为4.4,当pH高于4.4时,MSSB的表面带负电荷,能够与重金属离子所带的正电荷之间产生强烈的静电吸引作用,从而起到吸附效果[45].
综上所述MSSB吸附Pb2+的吸附机理涉及离子交换作用、沉淀作用、络合作用、静电吸附作用以及孔隙作用,示意图见图11.
-
生物炭的成本在很大程度上取决于原料、生产过程与生产方式. 本研究中生产生物炭的原料是虾蛄壳,是作为渔业及食品行业加工业废弃物或下脚料收集使用的,原料成本低廉,且对虾蛄壳的处理方式简单仅需要清洗、干燥和磨粉三个步骤,并且虾蛄壳生物炭的制备,采用的是一步热解法制备,没有对其进行改性或复合处理,使得制备过程用时短更加简单,因此能耗也相对较低. 例如,Zhang等[50]制得的磁改性生物炭成本,改性材料的花费就占其总成本的68%. 综合考虑,经过粗略估算,生产1 kg虾蛄壳生物炭的成本约为13.7元人民币. 由于对原料的处理与热解是在实验室中小规模进行的,如果大规模生产将进一步降低成本. 相比常用的处理重金属废水的碳质吸附剂活性炭,研究中指出生产生物炭的费用相当于活性炭成本的2%—13%[51]. 由此可见,生物炭作为吸附剂,成本较低,具有一定的优势.
一旦吸附完成,就必须考虑吸附后的吸附剂的命运. 从吸附剂的能源回收方面考虑,燃料可以是一个可行的选择. 在此背景下,利用微型量热仪(FTT0001,英国FTT公司,英国)对吸附后的虾蛄壳生物炭的热值进行了测量,其热值为4.3 MJ·kg−1,与生物质花生壳的热值6.3 MJ·kg−1相近[52]. 废弃的生物炭可以用作燃料使用. Manisha等[2]在研究中也指出,吸附后的生物炭可作为能源用于电厂、水泥厂、钢铁制造业等行业. 此外,有研究指出,将生物质炭用于炼焦的研究最为常见. 在焦炭配煤中加入生物炭,可以增加焦煤的黏性,降低焦煤的流动度,焦炭的质量和反应性也得以提高[53].
-
利用废弃虾蛄壳为原料和简便的热解法,成功地合成了一种成本低且对Pb2+具有高吸附容量的吸附剂MSSB. 基于Langmuir模型,MSSB对Pb2+的最大吸附容量为1467.6 mg·g−1,较其他文献报道的生物炭具有明显的优势. MSSB对Pb2+的去除是一个pH依赖的过程,共存的离子以及污染物会在不同程度对吸附的过程产生影响. 拟二级动力学和Langmuir模型可以很好地描述MSSB对Pb2+的吸附动力学和等温线,MSSB对Pb2+的吸附以化学吸附和单分子层吸附为主. 此外,对吸附机理的深入探究揭示MSSB对Pb2+的良好吸附性能源于离子交换、沉淀、络合以及孔隙作用和静电吸附作用. 综上所述,MSSB是一种对Pb2+具有高吸附容量、低成本、支持甲壳类废物资源化利用以及有着广阔应用前景的吸附剂.
虾蛄壳生物炭吸附Pb2+的特性及机理研究
Study on the adsorption properties and mechanism of Pb2+ by the biochar prepared from mantis shrimp shells
-
摘要: 以废弃虾蛄壳为原料制备生物炭(MSSB),利用SEM、BET、FTIR和XRD等技术对MSSB进行了表征. 探讨了吸附时间、Pb2+浓度、初始pH、MSSB添加量、共存离子以及共存污染物等对MSSB吸附Pb2+的影响. 利用吸附动力学模型与等温吸附模型以及测定吸附前后滤液中离子含量的变化探究吸附机理. MSSB对Pb2+的吸附动力学更符合拟二级动力学模型,吸附等温线则更符合Langmuir模型,根据Langmuir模型计算得出MSSB对Pb2+的最大吸附容量为1467.6 mg·g−1. 吸附以化学吸附为主,具体涉及孔隙作用、沉淀作用、络合作用、静电吸引、离子交换等多重作用机制. MSSB有潜力用于高效去除水体中的Pb2+.Abstract: The biochar named MSSB was prepared from mantis shrimp shells in this study. MSSB was characterized by SEM, BET, FTIR and XRD. Effects of adsorption time, Pb2+ concentration, initial pH, MSSB dosage, coexisting ions and coexisting contaminants on Pb2+ adsorption by MSSB were investigated. The adsorption mechanism was further explored by the adsorption kinetics model, the isothermal adsorption model, and the changes in ion concentrations in filtrates before and after adsorption. The pseudo-second-order kinetic model better fitted the adsorption kinetics of Pb2+ by MSSB, and the adsorption isotherm was better fitted with the Langmuir model. The maximum adsorption capacity of Pb2+ by MSSB was calculated to be 1467.6 mg·g−1 based on the Langmuir model. The adsorption process was mainly chemical adsorption, involving multiple mechanisms such as pore action, precipitation, complexation, electrostatic attraction and ion exchange. MSSB could be potentially used for the high-efficient removal of Pb2+ in water.
-
Key words:
- biochar /
- Pb2+ /
- adsorption /
- mantis shrimp shell /
- mechanism /
- coexisting ions.
-

-
图 1 MSS(A1)、MSSB(B1)、MSSB-Pb(C1)放大30 K的SEM图像. MSS(A2)、MSSB(B2)、MSSB-Pb(C2)的EDS图. EDS-Mapping获得的 MSS(A3)、MSSB(B3)和MSSB-Pb(C3)的铅元素分布图.
Figure 1. SEM image with MSS(A1), MSSB(B1)and MSSB-Pb(C1)enlarged at 30 K. EDS of MSS(A2), MSSB(B2), and MSSB-Pb(C2). Distribution of lead elements in MSS(A3), MSSB(B3)and MSSB-Pb(C3)obtained by EDS-Mapping.
表 1 MSSB的理化性质
Table 1. Physicochemical properties of MSSB
测试指标
Indexes数值
Value比表面积 30.77 m2·g−1 孔体积 0.016 cm3·g−2 孔径 10.16 nm 平均粒径 34.55 μm pH 13.29±0.05 灰分 69.29%±0.12% 羧基 (1.123±0.06)mmol·g−1 内酯基 (0.420±0.04)mmol·g−1 酚羟基 (0.147±0.01)mmol·g−1 总酸性官能团 (1.727±0.11)mmol·g−1 表 2 吸附动力学拟合参数
Table 2. Adsorption kinetics fitting parameters
拟一级动力学模型
Pseudo-first-order kinetic model拟二级动力学模型
Pseudo-second-order kinetic modelqe exp/(mg·g−1) Qe cal/(mg·g−1) k1/min−1 R2 qe cal/(mg·g−1) K2/(g·(mg·min)−1) R2 1199.49 1097.63 0.015 0.8942 1202.28 1.77×10−5 0.9646 表 3 吸附等温线模型的拟合参数
Table 3. The fitting parameters of adsorption isotherm models
Langmuir模型
Langmuir isothermFreundlich模型
Freundlich isothermqm cal/(mg·g−1) kL/(L·mg−1) R2 kF/(L·mg−1) 1/n R2 1467.6 0.0797 0.9785 1018.29 0.055 0.9414 表 4 以不同原料制备的生物炭对Pb2+的最大吸附容量
Table 4. Maximum adsorption capacity of Pb2+ for biochar prepared with different raw materials
原料
Raw material吸附容量/(mg·g−1)
Adsorption capacity改性方法
Method of modification参考文献
References小球藻 131.41 — [33] 螺旋藻 154.56 — [33] 小龙虾虾壳 87.4 — [34] 蟹壳 75.26 NaOH [35] 牡蛎壳 1553.042 — [16] 小龙虾虾壳 208.5 — [36] 小龙虾虾壳 183.5 — [37] 小龙虾虾 1334.6 MgCl2·6H2O [37] 小龙虾虾壳 101.65 — [15] 小龙虾虾壳 119.16 MgCl2 [15] 鱼骨 714.24 — [38] 草鱼骨头 774.748 — [39] 茶渣 84.03 — [40] 茶渣 188.68 H3PO4 [40] 马缨丹 75.74 — [41] 芦苇秸秆 24.06 — [10] 稻草 60.24 — [42] 稻草 307.85 纳米羟基磷灰石 [42] 蚕粪 9.33 壳聚糖和C10H2O6 [43] 柚子果皮 92.13 — [44] 棉杆 146.78 — [45] 竹子 168.21 (NH4)2S2O8 [46] 污水污泥 7.56 — [47] 污水污泥 57.48 KOH [47] 污水污泥 47.59 CH3COOK [47] 污水污泥 22.40 CO2 [47] 虾蛄壳 1467.6 — 本研究 —表示未经改性处理;吸附容量指在25 ℃或298K或293K下,Langmuir模型的计算值 表 5 MSSSB在吸附Pb2+前后滤液中离子的含量
Table 5. Contents of ions in the filtrate before and after adsorption of Pb2+ by MSSB
K+/(mg·L−1) Ca2+/(mg·L−1) Na+/(mg·L−1) Mg2+/(mg·L−1) SO42-/(mg·L−1) 吸附前 0.3164 13.2477 0.2610 2.6523 0.0654 吸附后 0.3852 15.5775 0.3049 2.6565 0.0042 -
[1] 乔增运, 李昌泽, 周正, 等. 铅毒性危害及其治疗药物应用的研究进展 [J]. 毒理学杂志, 2020, 34(5): 416-420. doi: 10.16421/j.cnki.1002-3127.2020.05.016 QIAO Z Y, LI C Z, ZHOU Z, et al. Research progress on toxic harm of lead and its therapeutic drug application [J]. Journal of Toxicology, 2020, 34(5): 416-420(in Chinese). doi: 10.16421/j.cnki.1002-3127.2020.05.016
[2] CHOUDHARY M, KUMAR R, NEOGI S. Activated biochar derived from Opuntia ficus-indica for the efficient adsorption of malachite green dye, Cu+2 and Ni+2 from water [J]. Journal of Hazardous Materials, 2020, 392: 122441. doi: 10.1016/j.jhazmat.2020.122441 [3] 黄菲, 闫梦, 常建宁, 等. 不同菌糠生物炭对水体中Cu2+、Cd2+的吸附性能 [J]. 环境化学, 2020, 39(4): 1116-1128. doi: 10.7524/j.issn.0254-6108.2019091604 HUANG F, YAN M, CHANG J N, et al. Adsorption performance of Cu2+ and Cd2+ in water by different biochars derived from spent mushroom substrate [J]. Environmental Chemistry, 2020, 39(4): 1116-1128(in Chinese). doi: 10.7524/j.issn.0254-6108.2019091604
[4] 张凤智, 王敦球, 曹星沣, 等. 高锰酸钾改性椰壳生物炭对水中 Cd(II)和 Ni(II)的去除性能及机制[J]. 环境科学 2023, 44(6): 3278-3287 ZHANG F Z, WANG D Q, CAO X F, et al. Removal performance and mechanism of potassium permanganate modified coconut shell biochar for Cd(II) and Ni(II) in aquatic environment[J]. Environmental Science, 2023, 44(6): 3278-3287 (in Chinese).
[5] HOPKINS D, HAWBOLDT K. Biochar for the removal of metals from solution: A review of lignocellulosic and novel marine feedstocks [J]. Journal of Environmental Chemical Engineering, 2020, 8(4): 103975. doi: 10.1016/j.jece.2020.103975 [6] WANG T T, ZHENG J Y, LIU H T, et al. Adsorption characteristics and mechanisms of Pb2+ and Cd2+ by a new agricultural waste–Caragana korshinskii biomass derived biochar [J]. Environmental Science and Pollution Research, 2021, 28(11): 13800-13818. doi: 10.1007/s11356-020-11571-9 [7] 刘宇, 方国宏, 戎素红, 等. 虾、蟹壳利用的研究进展 [J]. 食品安全质量检测学报, 2018, 9(3): 461-466. LIU Y, FANG G H, RONG S H, et al. Research progress on the utilization of shrimp and crab shells [J]. Journal of Food Safety & Quality, 2018, 9(3): 461-466(in Chinese).
[8] MA J C, HUANG W, ZHANG X S, et al. The utilization of lobster shell to prepare low-cost biochar for high-efficient removal of copper and cadmium from aqueous: Sorption properties and mechanisms [J]. Journal of Environmental Chemical Engineering, 2021, 9(1): 104703. doi: 10.1016/j.jece.2020.104703 [9] HOPKINS D T, MacQUARRIE S, HAWBOLDT K A. Removal of copper from sulfate solutions using biochar derived from crab processing by-product [J]. Journal of Environmental Management, 2022, 303: 114270. doi: 10.1016/j.jenvman.2021.114270 [10] 唐登勇, 胡洁丽, 胥瑞晨, 等. 芦苇生物炭对水中铅的吸附特性 [J]. 环境化学, 2017, 36(9): 1987-1996. doi: 10.7524/j.issn.0254-6108.2017012001 TANG D Y, HU J L, XU R C, et al. Adsorption of lead onto reed biochar in aqueous solution [J]. Environmental Chemistry, 2017, 36(9): 1987-1996(in Chinese). doi: 10.7524/j.issn.0254-6108.2017012001
[11] DAI L C, TAN F R, LI H, et al. Calcium-rich biochar from the pyrolysis of crab shell for phosphorus removal [J]. Journal of Environmental Management, 2017, 198: 70-74. [12] XU X Y, ZHAO Y H, SIMA J K, et al. Indispensable role of biochar-inherent mineral constituents in its environmental applications: A review [J]. Bioresource Technology, 2017, 241: 887-899. doi: 10.1016/j.biortech.2017.06.023 [13] VAKILI M, RAFATULLAH M, SALAMATINIA B, et al. Application of chitosan and its derivatives as adsorbents for dye removal from water and wastewater: A review [J]. Carbohydrate Polymers, 2014, 113: 115-130. doi: 10.1016/j.carbpol.2014.07.007 [14] JIN Z L, XIAO S J, DONG H R, et al. Adsorption and catalytic degradation of organic contaminants by biochar: Overlooked role of biochar’s particle size [J]. Journal of Hazardous Materials, 2022, 422: 126928. doi: 10.1016/j.jhazmat.2021.126928 [15] ZHANG J Q, HU X L, YAN J P, et al. Crayfish shell biochar modified with magnesium chloride and its effect on lead removal in aqueous solution [J]. Environmental Science and Pollution Research, 2020, 27(9): 9582-9588. doi: 10.1007/s11356-020-07631-9 [16] LIAN W L, LI H Y, YANG J H, et al. Influence of pyrolysis temperature on the cadmium and lead removal behavior of biochar derived from oyster shell waste [J]. Bioresource Technology Reports, 2021, 15: 100709. doi: 10.1016/j.biteb.2021.100709 [17] ZAZYCKI M A, BORBA P A, SILVA R N F, et al. Chitin derived biochar as an alternative adsorbent to treat colored effluents containing methyl violet dye [J]. Advanced Powder Technology, 2019, 30(8): 1494-1503. doi: 10.1016/j.apt.2019.04.026 [18] YAN Y B, SARKAR B, ZHOU L, et al. Phosphorus-rich biochar produced through bean-worm skin waste pyrolysis enhances the adsorption of aqueous lead [J]. Environmental Pollution, 2020, 266: 115177. doi: 10.1016/j.envpol.2020.115177 [19] CÁRDENAS G, CABRERA G, TABOADA E, et al. Chitin characterization by SEM, FTIR, XRD, and 13 cross polarization/mass angle spinning NMR [J]. Journal of Applied Polymer Science, 2004, 93(4): 1876-1885. doi: 10.1002/app.20647 [20] KAZAK O, TOR A. Characteristics and mechanisms for highly efficient adsorption of Pb(II) from aqueous solutions by engineered vinasse biochar with cold oxygen plasma process [J]. Chemical Engineering and Processing - Process Intensification, 2022, 171: 108766. doi: 10.1016/j.cep.2021.108766 [21] 苏德仁, 陈凤鸣, 李汇春, 等. 改性西瓜皮生物炭对废水中Pb2+的吸附研究 [J]. 新能源进展, 2021, 9(6): 496-505. doi: 10.3969/j.issn.2095-560X.2021.06.006 SU D R, CHEN F M, LI H C, et al. Study on the high-efficiency adsorption of Pb2+ by the modified watermelon peel biochar [J]. Advances in New and Renewable Energy, 2021, 9(6): 496-505(in Chinese). doi: 10.3969/j.issn.2095-560X.2021.06.006
[22] 朱俊波, 赵建兵, 周世萍, 等. 花生壳生物炭去除水中铅镉离子的性能及吸附机理研究 [J]. 西南林业大学学报(自然科学), 2022, 42(5): 78-86. ZHU J B, ZHAO J B, ZHOU S P, et al. Study on adsorption performance and mechanism of peanut shell biochar for Pb2+ and Cd2+ in water [J]. Journal of Southwest Forestry University (Natural Sciences), 2022, 42(5): 78-86(in Chinese).
[23] 韩剑宏, 郭金越, 张连科, 等. 生物炭/铁酸锰对Zn2+和Cu2+的吸附性能试验 [J]. 水资源保护, 2020, 36(2): 59-64. HAN J H, GUO J Y, ZHANG L K, et al. Adsorption test of biochar-MnFe2O4 to Zn2+ and Cu2+ [J]. Water Resources Protection, 2020, 36(2): 59-64(in Chinese).
[24] ZHAO N, LI B, HUANG H M, et al. Modification of kelp and sludge biochar by TMT-102 and NaOH for cadmium adsorption [J]. Journal of the Taiwan Institute of Chemical Engineers, 2020, 116: 101-111. doi: 10.1016/j.jtice.2020.10.036 [25] YANG H I, LOU K Y, RAJAPAKSHA A U, et al. Adsorption of ammonium in aqueous solutions by pine sawdust and wheat straw biochars [J]. Environmental Science and Pollution Research, 2018, 25(26): 25638-25647. doi: 10.1007/s11356-017-8551-2 [26] 姜晶, 黄晓月, 白金龙, 等. 高锰酸钾改性生物炭对水中噻虫胺吸附性能及机理 [J]. 环境工程学报, 2022, 16(4): 1175-1185. JIANG J, HUANG X Y, BAI J L, et al. Adsorption of clothianidin by potassium permanganate modified biochar in aqueous solution [J]. Chinese Journal of Environmental Engineering, 2022, 16(4): 1175-1185(in Chinese).
[27] 袁广翔, 张玉娟, 戴红旗, 等. 钙离子与钠离子对浆料Zeta电位的影响 [J]. 南京林业大学学报(自然科学版), 2011, 35(3): 119-123. YUAN G X, ZHANG Y J, DAI H Q, et al. Effects of calcium and sodium ions on the Zeta potential of pulp [J]. Journal of Nanjing Forestry University (Natural Sciences Edition), 2011, 35(3): 119-123(in Chinese).
[28] 仝海娟, 刘丽晓, 范方方, 等. 壳聚糖改性桔子皮生物炭吸附Cr(Ⅵ)的研究 [J]. 化工技术与开发, 2022, 51(9): 54-57. TONG H J, LIU L X, FAN F F, et al. Adsorption of Cr(Ⅵ) with chitosan modified orange peel biochar [J]. Technology & Development of Chemical Industry, 2022, 51(9): 54-57(in Chinese).
[29] SAHA U K, TANIGUCHI S, SAKURAI K. Simultaneous adsorption of cadmium, zinc, and lead on hydroxyaluminum- and hydroxyaluminosilicate-montmorillonite complexes [J]. Soil Science Society of America Journal, 2002, 66(1): 117. doi: 10.2136/sssaj2002.1170 [30] 汪怡, 李莉, 宋豆豆, 等. 玉米秸秆改性生物炭对铜、铅离子的吸附特性 [J]. 农业环境科学学报, 2020, 39(6): 1303-1313. WANG Y, LI L, SONG D D, et al. Copper and lead ion adsorption characteristics of modified corn stalk biochars [J]. Journal of Agro-Environment Science, 2020, 39(6): 1303-1313(in Chinese).
[31] 张丹, 高健伟, 马培, 等. 溶液中多种金属离子共存对毛木耳生物吸附能力的影响 [J]. 生态环境, 2008, 17(5): 1822-1827. ZHANG D, GAO J W, MA P, et al. Effect of competitive interference on the metal ions biosorption by Auricularia polytricha mycelial [J]. Ecology and Environment, 2008, 17(5): 1822-1827(in Chinese).
[32] TOVAR-GÓMEZ R, RIVERA-RAMÍREZ D A, HERNÁNDEZ-MONTOYA V, et al. Synergic adsorption in the simultaneous removal of acid blue 25 and heavy metals from water using a Ca(PO3)2-modified carbon [J]. Journal of Hazardous Materials, 2012, 199/200: 290-300. doi: 10.1016/j.jhazmat.2011.11.015 [33] YANG Z J, HOU J, WU J, et al. The effect of carbonization temperature on the capacity and mechanisms of Pb(II) adsorption by microalgae residue-derived biochar [J]. Ecotoxicology and Environmental Safety, 2021, 225: 112750. doi: 10.1016/j.ecoenv.2021.112750 [34] 李杨, 冯涛, 王乔兵. 虾壳生物炭对水溶液中铅离子的吸附研究 [J]. 工业安全与环保, 2020, 46(11): 93-96. doi: 10.3969/j.issn.1001-425X.2020.11.023 LI Y, FENG T, WANG Q B. Adsorption of lead in aqueous solution by crayfish shell-derived biochar [J]. Industrial Safety and Environmental Protection, 2020, 46(11): 93-96(in Chinese). doi: 10.3969/j.issn.1001-425X.2020.11.023
[35] 刘雪平, 杨治广, 王红强, 等. 甲壳素生物炭质对水体中Pb2+的吸附特性 [J]. 湖北农业科学, 2014, 53(3): 549-552. LIU X P, YANG Z G, WANG H Q, et al. Adsorptive behavior of Pb2+ in aqueous solution by chitin-char [J]. Hubei Agricultural Sciences, 2014, 53(3): 549-552(in Chinese).
[36] XIAO Y L, XUE Y W, GAO F, et al. Sorption of heavy metal ions onto crayfish shell biochar: Effect of pyrolysis temperature, pH and ionic strength [J]. Journal of the Taiwan Institute of Chemical Engineers, 2017, 80: 114-121. doi: 10.1016/j.jtice.2017.08.035 [37] 高菲. 小龙虾壳生物炭对水溶液中Pb(Ⅱ)的吸附性能研究[D]. 武汉: 武汉大学, 2017. GAO F. Removal of Pb(Ⅱ) from aqueous solutions by crayfish shell biochar[D]. Wuhan: Wuhan University, 2017 (in Chinese).
[38] PICCIRILLO C, MOREIRA I S, NOVAIS R M, et al. Biphasic apatite-carbon materials derived from pyrolysed fish bones for effective adsorption of persistent pollutants and heavy metals [J]. Journal of Environmental Chemical Engineering, 2017, 5(5): 4884-4894. doi: 10.1016/j.jece.2017.09.010 [39] WANG W, LIU Y Y, SONG S X, et al. Facile pyrolysis of fishbone charcoal with remarkable adsorption performance towards aqueous Pb (II) [J]. Journal of Environmental Chemical Engineering, 2017, 5(5): 4621-4629. doi: 10.1016/j.jece.2017.08.052 [40] 刘爽, 汪东风, 徐莹. 磷酸活化茶渣生物炭对铅的吸附性能影响和吸附机理研究 [J]. 中国海洋大学学报(自然科学版), 2022, 52(1): 56-64. doi: 10.16441/j.cnki.hdxb.20210070 LIU S, WANG D F, XU Y. Studies on lead adsorption performance of phosphoric acid activated tea residue biochar and associating mechanism [J]. Periodical of Ocean University of China, 2022, 52(1): 56-64(in Chinese). doi: 10.16441/j.cnki.hdxb.20210070
[41] 张杰, 贺敏婕, 陈可欣, 等. 马缨丹生物炭对水中Pb(Ⅱ)污染的吸附研究 [J]. 现代化工, 2021, 41(7): 122-127. doi: 10.16606/j.cnki.issn0253-4320.2021.07.026 ZHANG J, HE M J, CHEN K X, et al. Preparation of lantana biochar material and its adsorption behavior to Pb2+ in water [J]. Modern Chemical Industry, 2021, 41(7): 122-127(in Chinese). doi: 10.16606/j.cnki.issn0253-4320.2021.07.026
[42] AHMED W, XU T W, MAHMOOD M, et al. Nano-hydroxyapatite modified biochar: Insights into the dynamic adsorption and performance of lead (II) removal from aqueous solution[J]. Environmental Research, 2022, 214(Pt 2): 113827. [43] BIAN P Y, LIU Y X, ZHENG X Q, et al. Removal and mechanism of cadmium, lead and copper in water by functional modification of silkworm excrement biochar [J]. Polymers, 2022, 14(14): 2889. doi: 10.3390/polym14142889 [44] DINH V P, NGUYEN D K, LUU T T, et al. Adsorption of Pb(II) from aqueous solution by pomelo fruit peel-derived biochar [J]. Materials Chemistry and Physics, 2022, 285: 126105. doi: 10.1016/j.matchemphys.2022.126105 [45] GAO L, LI Z H, YI W M, et al. Impacts of pyrolysis temperature on lead adsorption by cotton stalk-derived biochar and related mechanisms [J]. Journal of Environmental Chemical Engineering, 2021, 9(4): 105602. doi: 10.1016/j.jece.2021.105602 [46] SHEN Y, GUO J Z, BAI L Q, et al. High effective adsorption of Pb(II) from solution by biochar derived from torrefaction of ammonium persulphate pretreated bamboo [J]. Bioresource Technology, 2021, 323: 124616. doi: 10.1016/j.biortech.2020.124616 [47] ZHANG J J, SHAO J G, JIN Q Z, et al. Sludge-based biochar activation to enhance Pb(II) adsorption [J]. Fuel, 2019, 252: 101-108. doi: 10.1016/j.fuel.2019.04.096 [48] DU Q, ZHANG S S, SONG J P, et al. Activation of porous magnetized biochar by artificial humic acid for effective removal of lead ions [J]. Journal of Hazardous Materials, 2020, 389: 122115. doi: 10.1016/j.jhazmat.2020.122115 [49] 刘凌沁, 黄亚继, 胡华军, 等. 流化床制备玉米秸秆生物炭的Pb2+吸附特性及机理 [J]. 东南大学学报(自然科学版), 2022, 52(4): 666-675. LIU L Q, HUANG Y J, HU H J, et al. Pb2+ adsorption characteristics and mechanism of corn stalk biochar produced by fluidized bed [J]. Journal of Southeast University (Natural Science Edition), 2022, 52(4): 666-675(in Chinese).
[50] ZHANG R Y, ZHENG X X, CHEN B H, et al. Enhanced adsorption of sulfamethoxazole from aqueous solution by Fe-impregnated graphited biochar [J]. Journal of Cleaner Production, 2020, 256: 120662. doi: 10.1016/j.jclepro.2020.120662 [51] KEARNS J P, WELLBORN L S, SUMMERS R S, et al. 2, 4-D adsorption to biochars: Effect of preparation conditions on equilibrium adsorption capacity and comparison with commercial activated carbon literature data [J]. Water Research, 2014, 62: 20-28. doi: 10.1016/j.watres.2014.05.023 [52] 张淑会, 邵建男, 兰臣臣, 等. 生物质能在炼铁领域应用的研究现状及展望 [J]. 钢铁, 2022, 57(12): 13-22. doi: 10.13228/j.boyuan.issn0449-749x.20220329 ZHANG S H, SHAO J N, LAN C C, et al. Application status and prospect of biomass energy in ironmaking process [J]. Iron & Steel, 2022, 57(12): 13-22(in Chinese). doi: 10.13228/j.boyuan.issn0449-749x.20220329
[53] 魏汝飞, 朱玉龙, 龙红明, 等. 生物质铁矿球团研究现状与展望 [J]. 烧结球团, 2022, 47(1): 29-37. doi: 10.13403/j.sjqt.2022.01.005 WEI R F, ZHU Y L, LONG H M, et al. Research status and prospect of biomass iron ore pellets [J]. Sintering and Pelletizing, 2022, 47(1): 29-37(in Chinese). doi: 10.13403/j.sjqt.2022.01.005
-



 下载:
下载: